Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Determination of the Proximate Composition
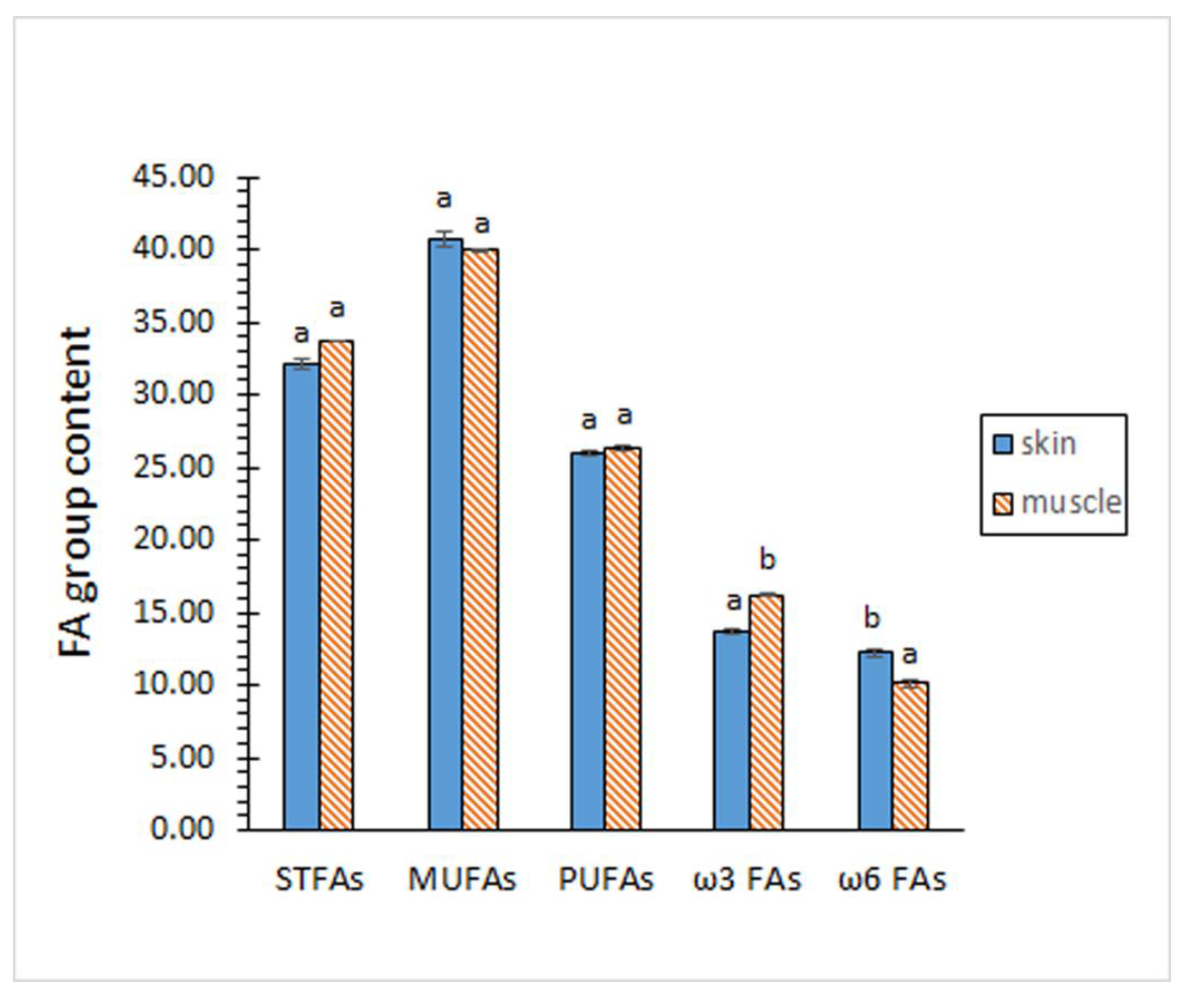
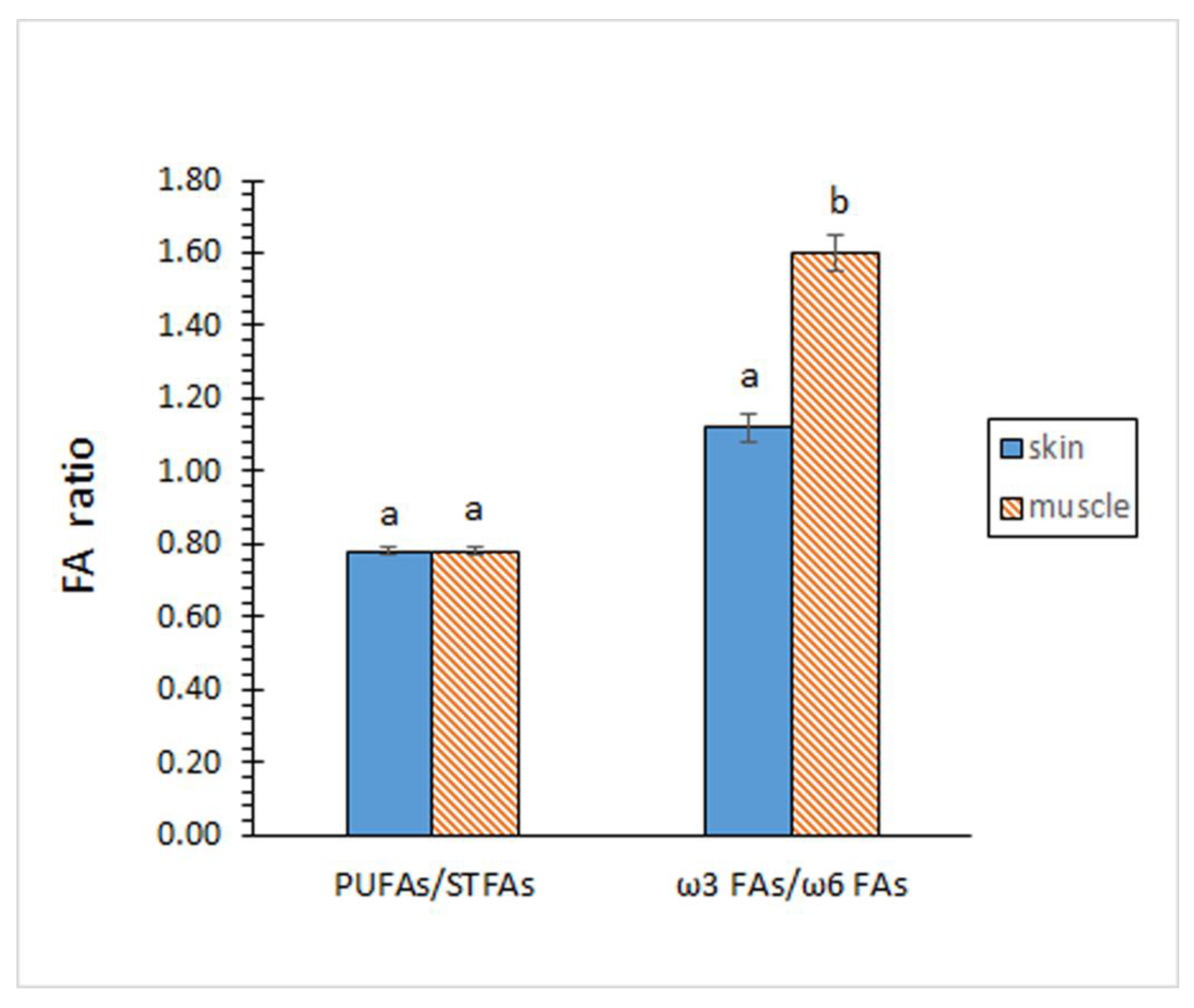
2.2. Analysis of the FA Composition
2.3. Lipid Class Composition
3. Materials and Methods
3.1. Fish Material and Sampling
3.2. Proximate Composition Analysis
3.3. FA Analysis
3.4. Analysis of Lipid Classes
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilami, S.K.; Sampels, S. Nutritional Value of Fish: lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. 2018, 26, 242–253. [Google Scholar] [CrossRef]
- Xu, H.; Turchini, G.M.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.; Armah, C.; Miles, E.; Madden, J.; Clark, A.; Caslake, M.; Calder, P. Consumption of fish oil providing amounts of eicosapentaenoic acid and docosahexaenoic acid that can be obtained from the diet reduces blood pressure in adults with systolic hypertension: A retrospective analysis. J. Nutr. 2016, 146, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Schunck, W.; Konkel, A.; Fischer, R.; Weylandt, K. Therapeutic potential of omega-3 fatty acid-derived epoxy eicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, Article 3. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A. Antioxidants. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2010; pp. 309–326. [Google Scholar] [CrossRef]
- Kim, Y.N. Vitamins. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press, Francis and Taylor Group: Boca Raton, FL, USA, 2010; pp. 327–350. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Özyurt, G.; Özkütük, S. Advances in discard and by-product processing. In Innovative Technologies in Seafood Processing; Özogul, Y., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 323–350. [Google Scholar] [CrossRef]
- Rustad, T.; Storro, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–152. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduiña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef]
- Goymer, A.; Steele, K.; Jenkins, F.; Burgess, G.; Andrews, L.; Baumgartner, N.; Gubili, C.; Griffiths, A.M. For R-eel ?! Investigating international sales of critically endangered species in freshwater eel products with DNA barcoding. Food Cont. 2023, 150, 109752. [Google Scholar] [CrossRef]
- Heinsbroek, L.T.N.; Støttrup, J.G.; Jacobsen, C.; Corraze, G.; Kraiem, M.M.; Holst, L.K.; Tomkiewicz, J.; Kaushik, S.J. A review on broodstock nutrition of marine pelagic spawners: the curious case of the freshwater eels (Anguilla spp.). Aquacult. Nutr. 2013, 19, 1–24. [Google Scholar] [CrossRef]
- Støttrup, J.G.; Tomkiewicz, J.; Jacobsen, C.; Butts, I.A.E.; Holst, L.K.; Krüger-Johnsen, M.; Graver, C.; Lauesen, P.; Fontagne-Dicharry, S.; Heinsbroek, L.T.N.; Geneviève, C.; Kaushik, S. Development of a broodstock diet to improve developmental competence of embryos in European eel, Anguilla anguilla. Aquacult. Nutr. 2016, 22, 725–737. [Google Scholar] [CrossRef]
- Özogul, Y.; Ucar, Y.; Takadaş, F.; Durmus, M.; Köşker, A.R.; Polat, A. Comparison of green and conventional extraction methods on lipid yield and fatty acid profiles of fish species. Eur. J. Lipid Sci. Technol. 2018, 120, 1800107. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Cobas, N.; Martínez, S. Proximate composition, fatty acid profile and total amino acid contents in samples of the European eel (Anguilla anguilla) of different weights. Int. J. Gastr. Food Sci. 2021, 25, 100364. [Google Scholar] [CrossRef]
- Rudovica, V.; Bartkevics, V. Chemical elements in the muscle tissues of European eel (Anguilla anguilla) from selected lakes in Latvia. Environm. Monit. Assess. 2015, 87, 608. [Google Scholar] [CrossRef] [PubMed]
- Kucukgulmez, A.; Yanar, Y.; Gerçek, G.; Gülnaz, O.; Celik, M. Effects of chitosan on color, sensory and microbiological properties of European eel (Anguilla anguilla) fillets during refrigerated storage. J. Food Proc. Preserv. 2013, 37, 766–771. [Google Scholar] [CrossRef]
- Özogul, I.; Polat, A.; Özogul, Y.; Boga, E.K.; Özogul, F.; Ayas, D. Effects of laurel and myrtle extracts on the sensory, chemical and microbiological properties of vacuum-packed and refrigerated European eel (Anguilla anguilla) fillets. Int. J. Food Sci. Technol. 2014, 49, 847–853. [Google Scholar] [CrossRef]
- Ersoy, B. Effects of cooking methods on the proximate, mineral and fatty acid composition of European eel (Anguilla anguilla). Int. J. Food Sci. Technol. 2011, 46, 522–527. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Carballo, J.; Rodríguez-González, M.; Martínez, S. Impact of the filling medium on the colour and sensory characteristics of canned European eels (Anguilla anguilla L.). Foods 2022, 11, 1115. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, H.; Capitani, F.; Krichen, F.; Mantovani, V.; Ben Amor, I.; Galeotti, F.; Maccari, F.; Nedjar, N.; Volpi, N.; Bougatef, A. Studies on European eel skin sulfated glycosaminoglycans: recovery, structural characterization and anticoagulant activity. Int. J. Biol. Macrom. 2018, 115, 891–899. [Google Scholar] [CrossRef]
- Taktak, W.; Nasri, R.; López-Rubio, A.; Chentir, I.; Gómez-Mascaraque, L.G.; Boughriba, S.; Nasri, M.; Karra-Chaabouni, M. Design and characterization of novel ecofriendly European fish eel gelatin-based electrospun microfibers applied for fish oil encapsulation. Process Biochem. 2021, 106, 10–19. [Google Scholar] [CrossRef]
- Teng, H.; Qian, Y.; Fan, X.; Cao, H.; Tian, Y.; Chen, L. Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells. Food Sci and Human Wellness 2022, 11, 1482–1490. [Google Scholar] [CrossRef]
- Piclet, G. Le poisson aliment. Composition-Intérêt nutritionnel. Cah. Nutr. Diét. 1987, XXII, 317–335. [Google Scholar]
- Prego, R.; Pazos, M.; Medina, I.; Aubourg, S.P. Comparative chemical composition of different muscle zones in angler (Lophius piscatorius). J. Food Compos. Anal. 2012, 28, 81–87. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Prego, R.; Fett, R.; Aubourg, S.P. The chemical composition of different edible locations (central and edge muscles) of flat fish (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2018, 53, 271–281. [Google Scholar] [CrossRef]
- Park, J.S.; Chandra Roy, V.; Kim, S.Y.; Lee, S.C.; Chun, B.S. Extraction of edible oils and amino acids from eel by-products using clean compressed solvents: an approach of complete valorization. Food Chem. 2022, 38, 132949. [Google Scholar] [CrossRef]
- Oku, T.; Sugawara, A.; Choudhury, M.; Komatsu, M.; Yamada, S.; Ando, S. Lipid and fatty acid compositions differentiate between wild and cultured Japanese eel (Anguilla japonica). Food Chem. 2009, 115, 436–440. [Google Scholar] [CrossRef]
- Nurfaidah; Metusalach; Sukarno; Mahendradatta, M. Protein and albumin contents in several freshwater fish species of Makassar, South Sulawesi, Indonesia. Int. Food Res. J. 2021, 28, 745–751. [CrossRef]
- Lee, K.; Kim, Y.J.; Hong, Y.K.; Song, M.Y.; Lee, W.O.; Hwang, K.T. Lipid content and fatty acid composition of freshwater eels Anguilla japonica caught in different seasons and locations in South Korea. Fish. Sci. 2020, 86, 573–580. [Google Scholar] [CrossRef]
- Saito, H.; Kurogi, H.; Chow, S.; Mochioka, N. Variation of lipids and fatty acids of the Japanese eel, Anguilla japonica, during spawning migration. J. Oleo Sci. 2015, 64, 603–616. [Google Scholar] [CrossRef]
- Magalhães, J.P.; Müller, M.; Rainger, G.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578–1582. [Google Scholar] [CrossRef]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef]
- Swanson, S.; Block, R.; Mousa, S. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.M.; Lee, B.H.; Yu, X. Current trends and future perspectives on omega-3 fatty acids. Res. J. Biol. 2017, 5, 11–20. [Google Scholar]
- Uauy, R.; Valenzuela, A. Marine oils: The health benefits of n-3 fatty acids. Nutrition 2000, 16, 680–684. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Mekinić, I.G.; Čagalj, M.; Hamed, I.; Skroza, D. Production and characterization of crude oils from seafood processing by-products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Cantor, A.H.; Decker, E.A.; Collins, V.P. Fatty acids in poultry and egg products. In Fatty Acids in Foods and their Health Implications; Chow, C.K., Ed.; 3rd Ed., CRC Press, Boca Raton, FL, USA, 2008; pp. 127–154. [CrossRef]
- Palmquist, D.L.; Jensen, R.G. Fatty acids in milk fat. In Fatty Acids in Foods and their Health Implications; Chow, C.K., Ed.; 3rd Ed., CRC Press, Boca Raton, FL, USA, 2008; pp. 109–125.
- Wood, J.D.; Enser, M.; Richardson, R.I.; Whittington, F.M. Fatty acids in meat and meat products. In Fatty Acids in Foods and their Health Implications; Chow, C.K., Ed.; 3rd Ed., CRC Press, Boca Raton, FL, USA, 2008; pp. 87–107. [CrossRef]
- Achouri, A.; Kharrat, N.; Smichi, N.; Miled, N.; Gargouri, Y.; Fendri, A. Nutritional properties, oxidative stability, and in vitro digestibility of oils extracted from muscles of wild and breeding eels (Anguilla anguilla). J. Food Process. Preserv. 2018, 42, e13519. [Google Scholar] [CrossRef]
- Medina, I.; Sacchi, R.; Aubourg, S.P. A 13C-NMR study of lipid alterations during fish canning: Effect of filling medium. J. Sci. Food Agric. 1995, 69, 445–450. [Google Scholar] [CrossRef]
- Aubourg, S.P; Quitral, V.; Larraín, M.A.; Rodríguez, A.; Gómez, J.; Maier, L.; Vinagre, J. Autolytic degradation and microbiological activity in farmed Coho salmon (Oncorhynchus kisutch) during chilled storage. Food Chem. 2007, 104, 369–375. [Google Scholar] [CrossRef]
- Vázquez, M.; Torres, J.A.; Gallardo, J.M.; Saraiva, J.A.; Aubourg, S.P. Lipid hydrolysis and oxidation development in frozen mackerel (Scomber scombrus): Effect of a high hydrostatic pressure pre-treatment. Innov. Food Sci. Emer. Technol. 2013, 18, 24–30. [Google Scholar] [CrossRef]
- Takahashi, K.; Inoue, Y. Marine by-product phospholipids as booster of medicinal compounds. Adv. Food Nutr. Res. 2012, 65, 31–46. [Google Scholar] [PubMed]
- Köhler, A.; Sarkinnen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects–a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Campos, R.M.; Batista, I.; Nunes, M.L.; Empis, J.M. Antioxidant synergy of alpha-tocopherol and phospholipids. J. Am Oil Chem. Soc. 1999, 76, 905–913. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Leon, M.M.; Zamora, R. Antioxidative activity of amino phospholipids and phospholipid/amino acid mixtures in edible oils as determined by the rancimat method. J. Agric. Food Chem. 2006, 54, 5461–5467. [Google Scholar] [CrossRef]
- Medina, I.; Aubourg, S.P.; Pérez-Martín, R. Composition of phospholipids of white muscle of six tuna species. Lipids 1995, 30, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Van Ginneken, V.; Hekman, M.; Verheij, E. The lipid composition and biochemistry of the migrating European eel (Anguilla anguilla L.): A LCMS-study following a lipidomics based systems biology approach. Adv. Biochem Biotechnol. 2018, 3, 165. [Google Scholar]
- AOAC. Official Methods for Analysis of the Association of Analytical Chemistry, 15th ed.; Association of Official Chemists, Inc.: Arlington, VA, USA, 1990; pp. 931–937. [Google Scholar]
- Bligh, E.; Dyer, W. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R. Polyunsaturated fatty acids in tuna phospholipids: Distribution in the sn-2 location and changes during cooking. J. Agric. Food Chem. 1996, 44, 585–589. [Google Scholar] [CrossRef]
- Álvarez, V.; Medina, I.; Prego, R.; Aubourg, S.P. Lipid and mineral distribution in different zones of farmed and wild blackspot seabream (Pagellus bogaraveo). Eur. J. Lipid Sci. Technol. 2009, 111, 957–966. [Google Scholar] [CrossRef]
- Vioque, E.; Holman, R. Quantitative estimation of esters by thin-layer chromatography. J. Am. Oil Chem. Soc. 1962, 39, 63–66. [Google Scholar] [CrossRef]
- Lowry, R.; Tinsley, I. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef]
- Raheja, R.; Kaur, C.; Singh, A.; Bhatia, A. New colorimetric method for the quantitative determination of phospholipids without acid digestion. J. Lipid Res. 1973, 14, 695–697. [Google Scholar] [CrossRef]
- Huang, T.; Chen, C.; Wefler, V.; Raftery, A. A stable reagent for the Liebermann-Buchardt reaction. Anal. Chem. 1961, 33, 1405–1407. [Google Scholar] [CrossRef]
- Cabrini, L.; Landi, L.; Stefanelli, C.; Barzanti, V.; Sechi, A. Extraction of lipid and lipophilic antioxidants from fish tissues: A comparison among different methods. Comp. Biochem. Physiol. Biochem. Molec. Biol. 1992, 101, 383–386. [Google Scholar] [CrossRef] [PubMed]
| Chemical constituent | Tissue | |
|---|---|---|
| Skin | Muscle | |
| Moisture | 677.0 ± 7.0 a | 783.3 ± 3.5 b |
| Proteins | 271.6 ± 7.2 b | 166.4 ± 1.8 a |
| Lipids | 38.0 ± 0.9 b | 28.6 ± 1.6 a |
| Ash | 27.7 ± 2.1 b | 9.9 ± 1.0 a |
| FA | Tissue | |
|---|---|---|
| Skin | Muscle | |
| 14:0 | 2.70 ± 0.03 a | 3.09 ± 0.04 b |
| 15:0 | 0.70 ± 0.06 a | 0.65 ± 0.01 a |
| 16:0 | 22.37 ± 0.15 a | 22.39 ± 0.09 a |
| 17:0 | 1.48 ± 0.05 b | 1.05 ± 0.02 a |
| 18:0 | 5.97 ± 0.13 a | 5.91 ± 0.20 a |
| 16:1 ω7 | 6.63 ± 0.12 a | 6.92 ± 0.15 b |
| 18:1 ω7 | 6.93 ± 0.06 a | 6.84 ± 0.16 a |
| 18:1 ω9 | 25.34 ± 0.40 a | 25.09 ± 0.47 a |
| 20:1 ω9 | 1.25 ± 002 b | 1.12 ± 0.01 a |
| 22:1 ω9 | 0.16 ± 0.01 b | 0.13 ± 0.01 a |
| 24:1 ω9 | 0.45 ± 0.03 b | 0.15 ± 0.02 a |
| 18:2 ω6 | 2.35 ± 0.05 a | 2.25 ± 0.14 a |
| 20:2 ω6 | 1.25 ± 0.05 b | 1.05 ± 0.08 a |
| 20:4 ω6 | 5.45 ± 0.09 a | 4.25 ± 0.13 b |
| 22:4 ω6 | 3.23 ± 0.14 a | 2.44 ± 0.10 b |
| 20:5 ω3 | 5.83 ± 0.14 a | 6.74 ± 0.07 b |
| 22:5 ω3 | 4.82 ± 0.18 a | 5.14 ± 0.08 b |
| 22:6 ω3 | 3.06 ± 0.08 a | 4.23 ± 0.16 b |
| Lipid class | Tissue | |
|---|---|---|
| Skin | Muscle | |
| Triacylglycerols | 400.6 ± 28.0 a (15.2 ± 3.0 a) |
411.6 ± 7.3 a (11.8 ± 0.6 a) |
| Free fatty acids | 43.6 ± 1.4 b (1.7 ± 0.4 b) |
30.9 ± 0.8 a (0.9 ± 0.1 a) |
| Phospholipids | 111.1 ± 5.5 b (4.2 ± 0.6 b) |
93.4 ± 5.5 a (2.7 ± 0.6 a) |
| Sterols | 104.7 ± 5.8 b (4.0 ± 0.6 b) |
24.2 ± 1.1 a (0.7 ± 0.2 a) |
| Alpha-tocopherol | 274.0 ± 14.7 b (10.4 ± 1.8 b) |
178.0 ± 38.8 a (5.1 ± 1.9 a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





