Submitted:
20 January 2024
Posted:
22 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General experimental procedures
2.2. Fungal lineage reactivations
2.3. Obtaining the extracts and determining the minimum inhibitory concentration - MIC
2.4. Large-scale extraction and isolation of compounds 1 and 2
3. Results
3.1. Determination of the Minimum Inhibitory Concentration - MIC of P. purpurogenum extracts
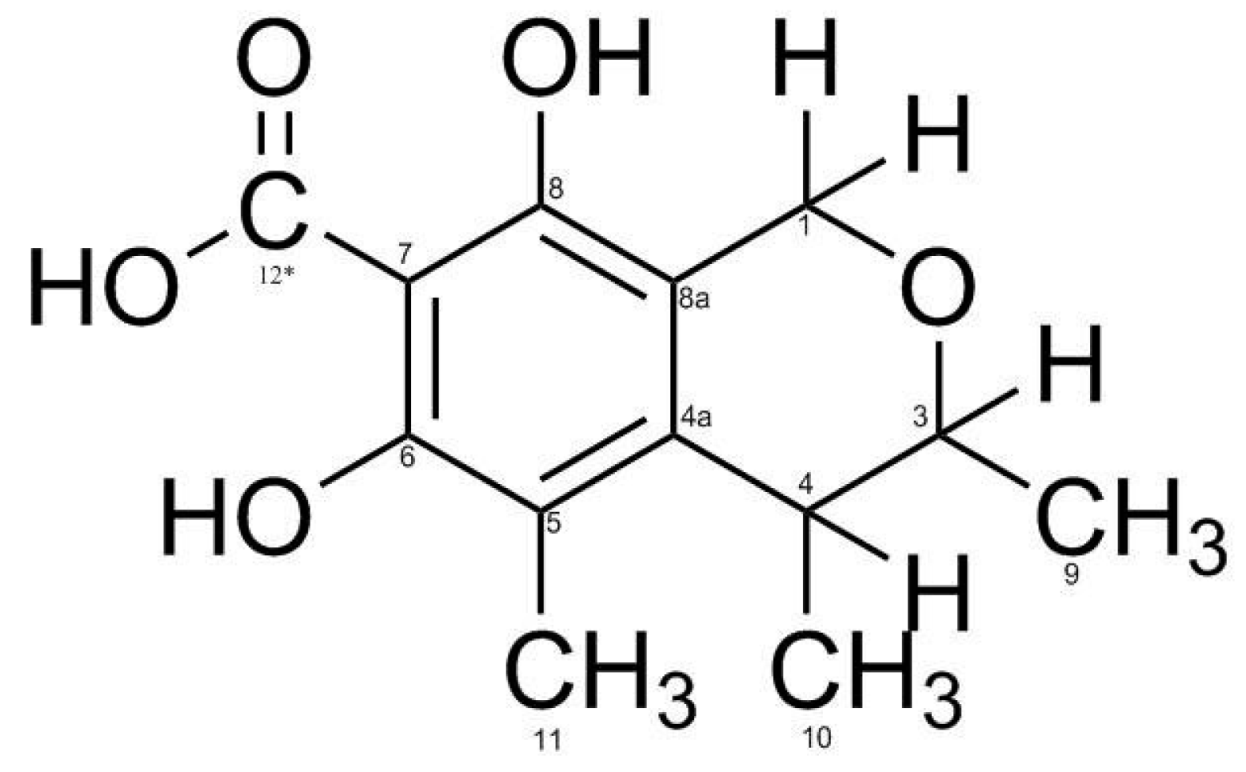
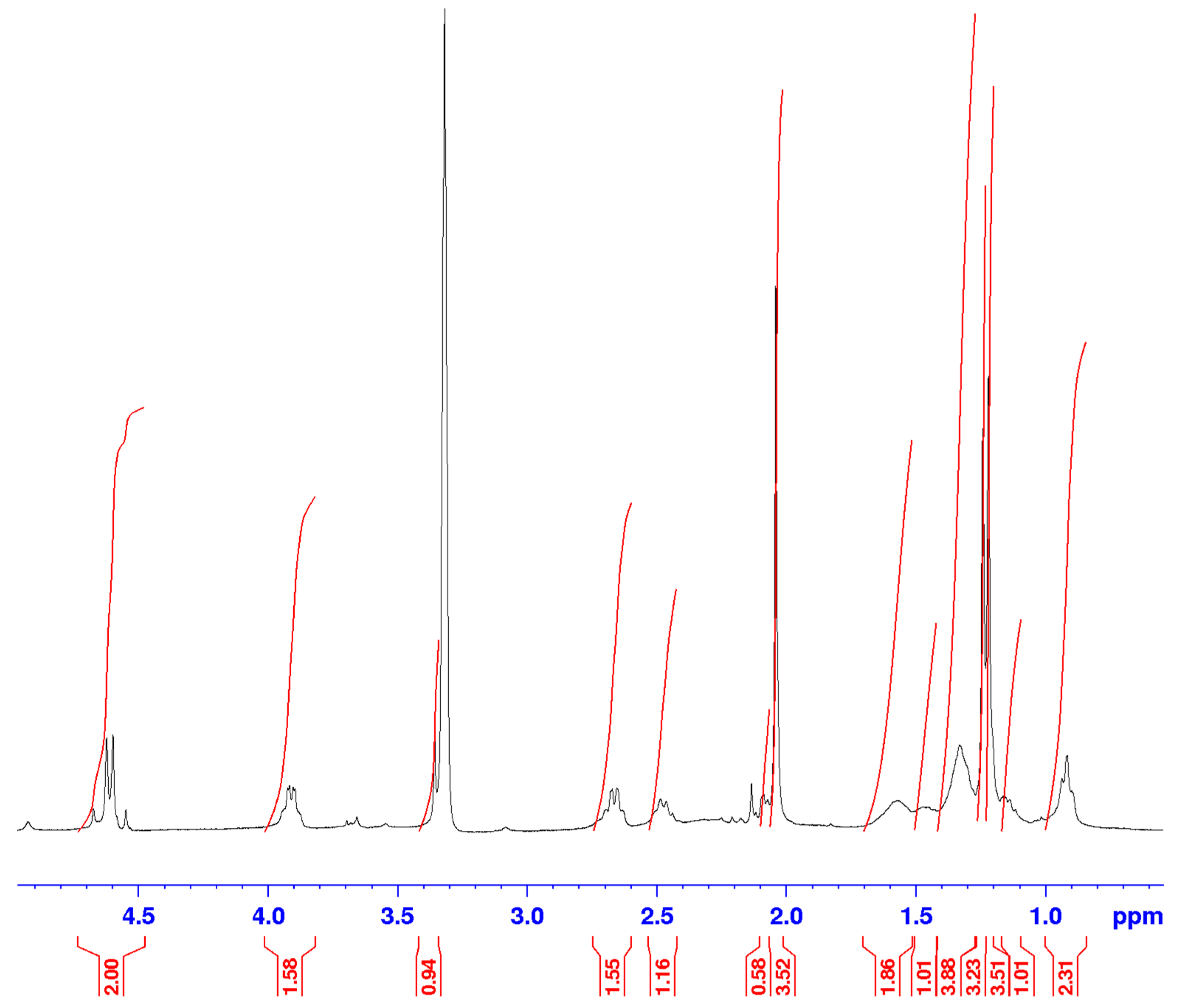
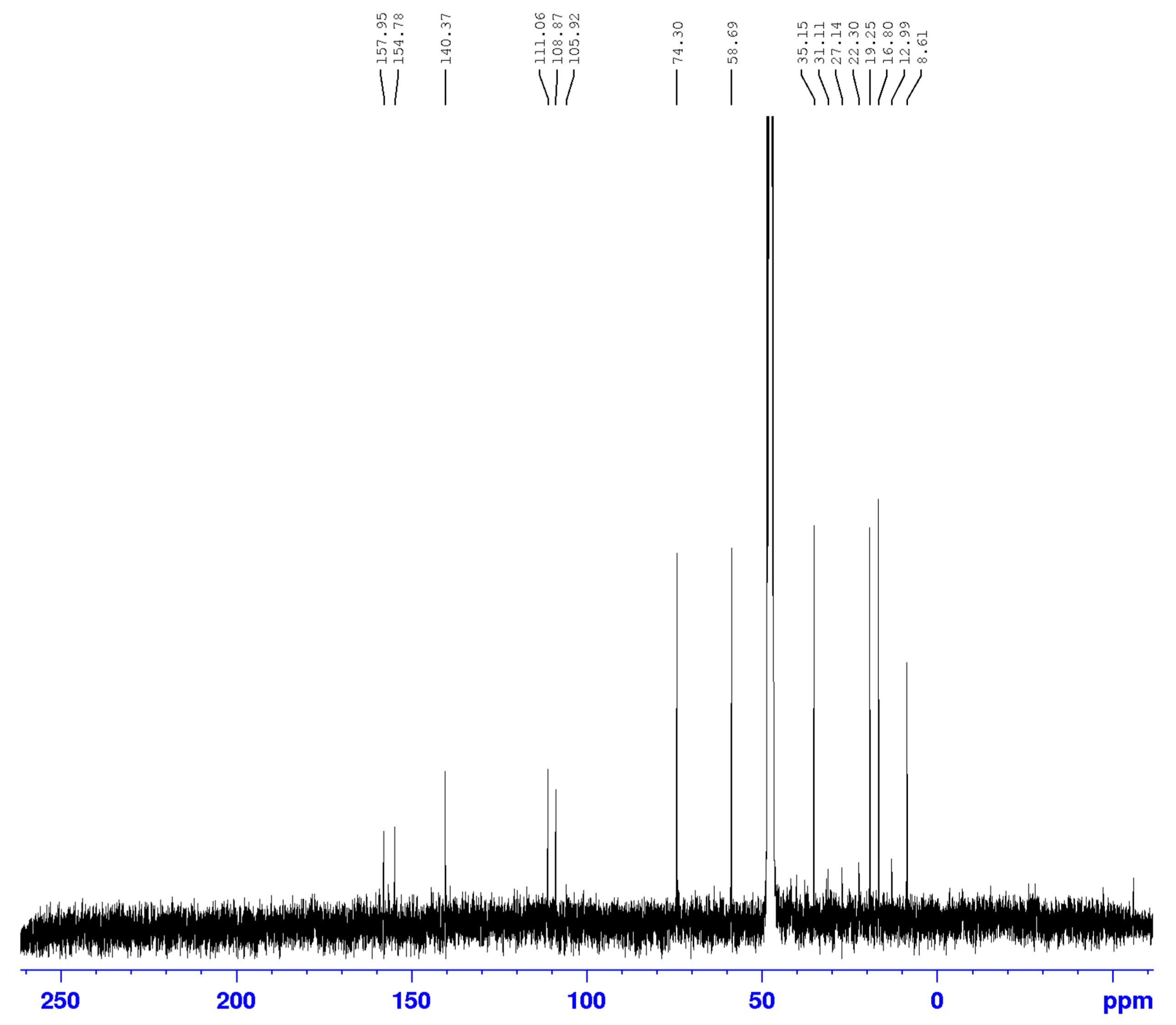
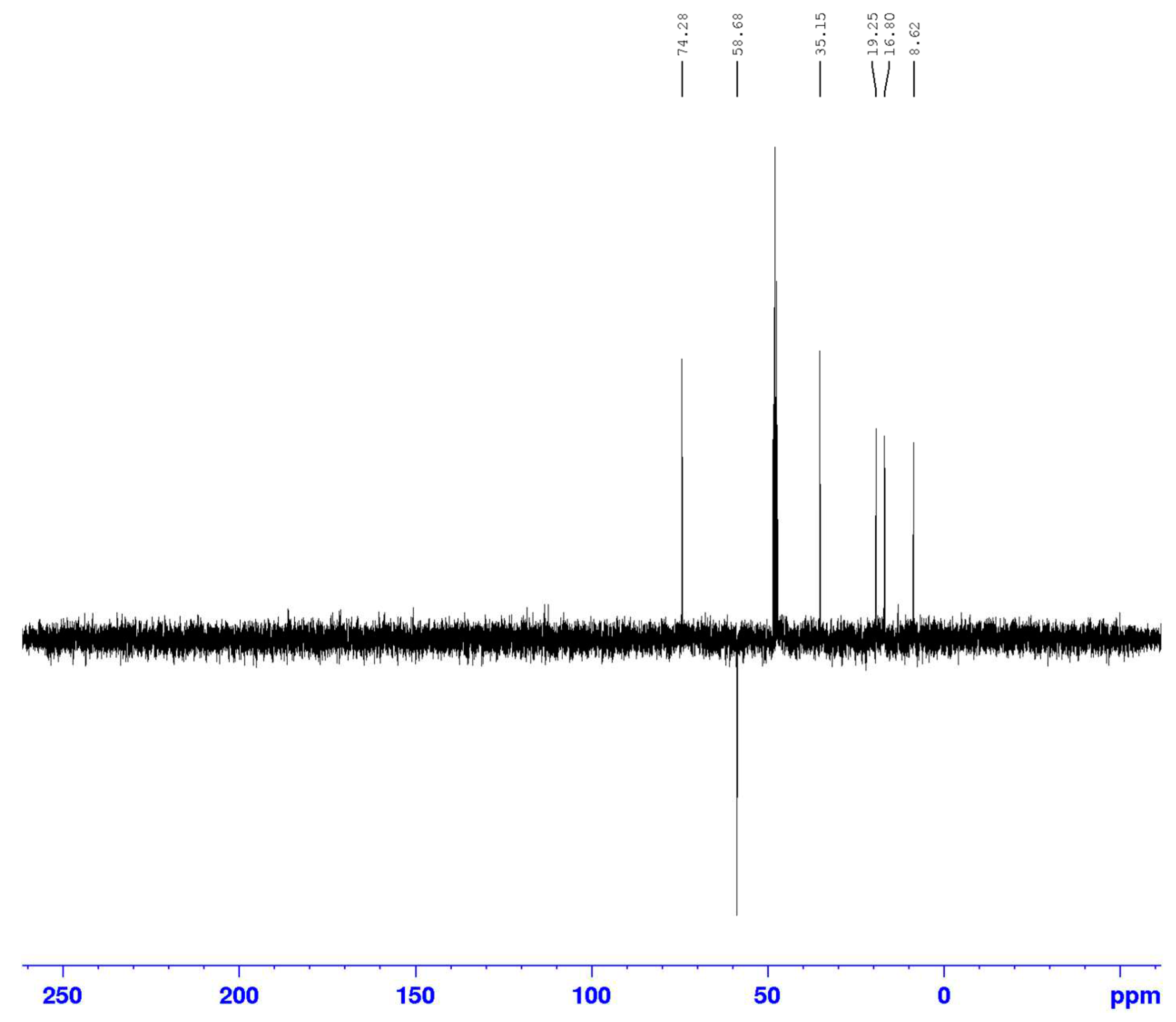
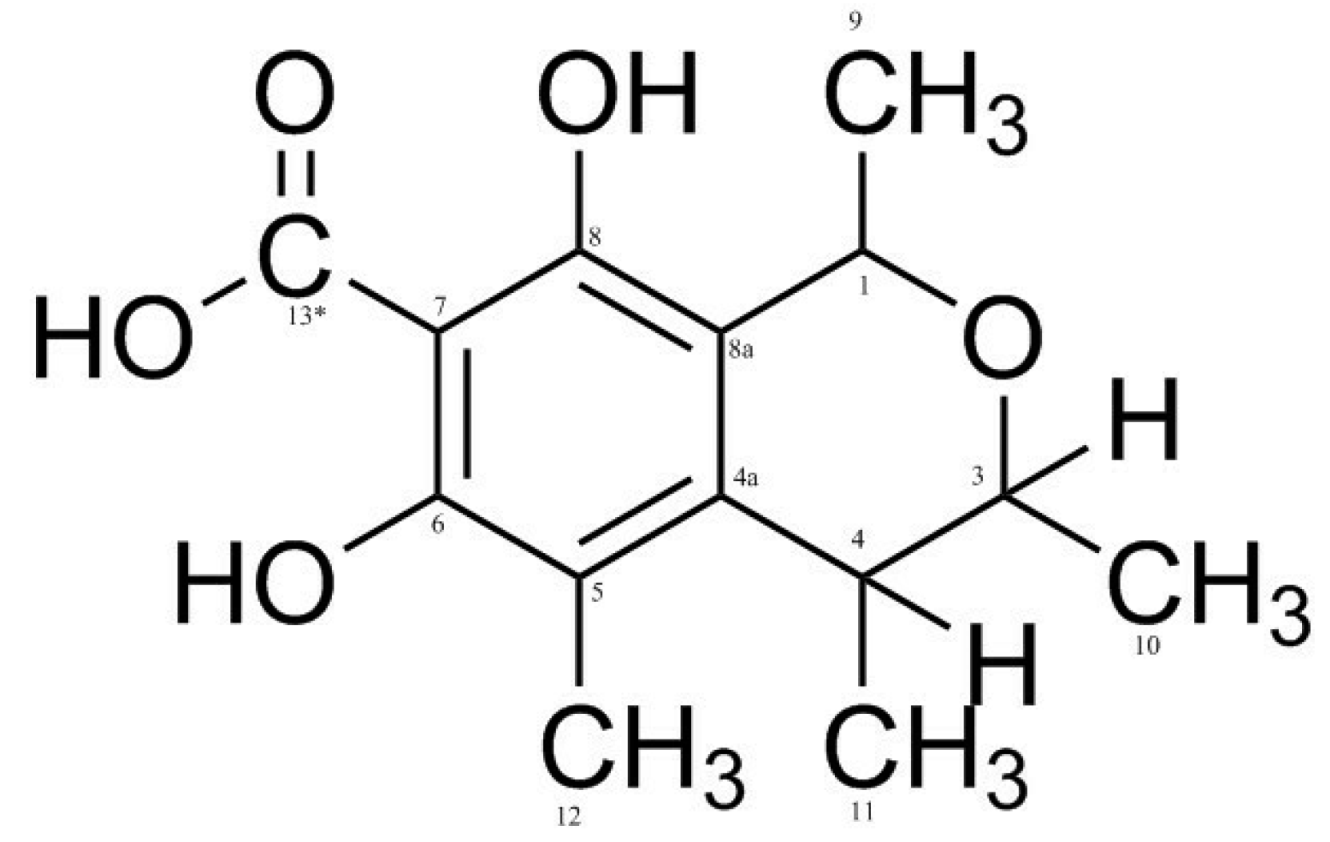
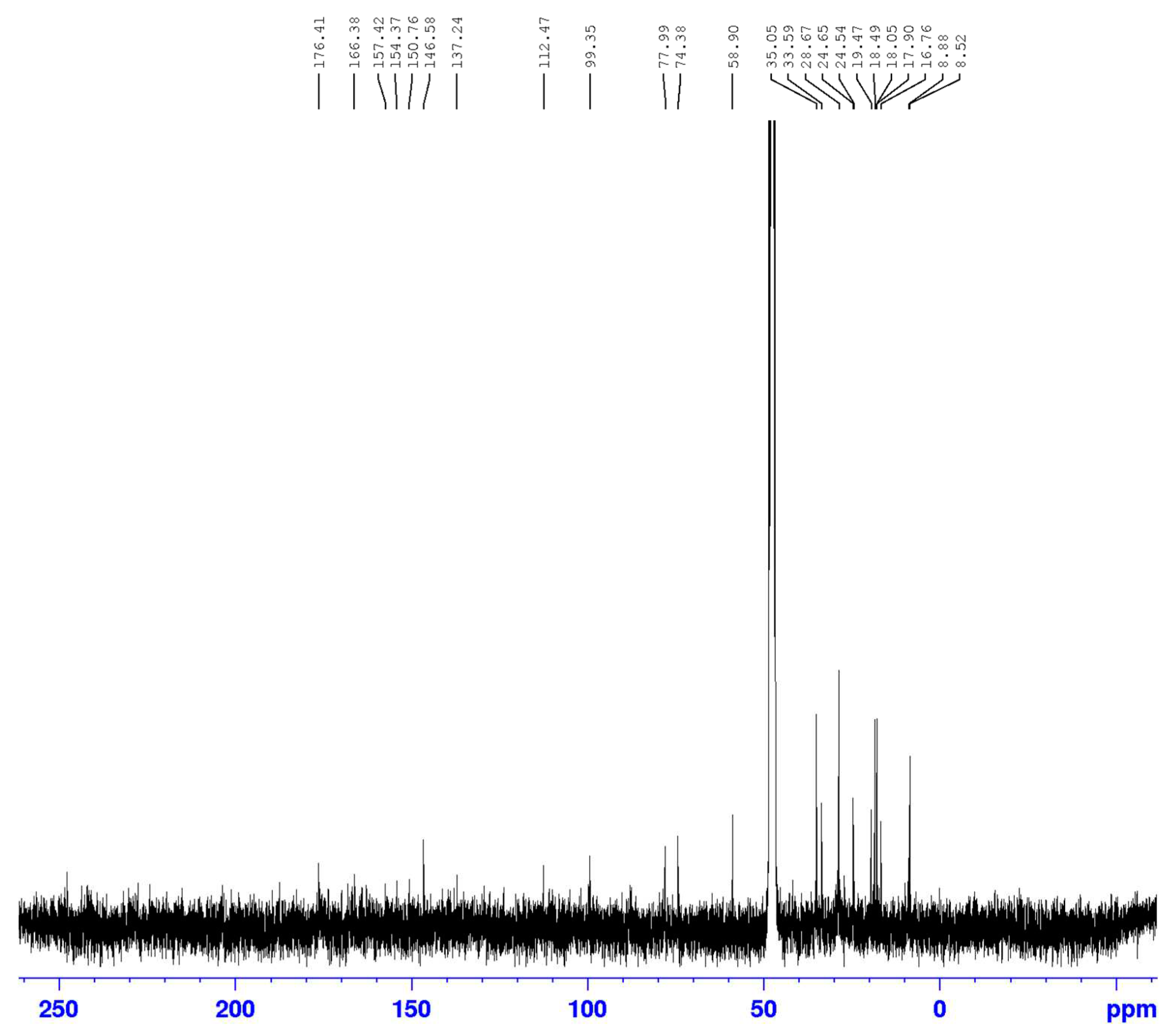
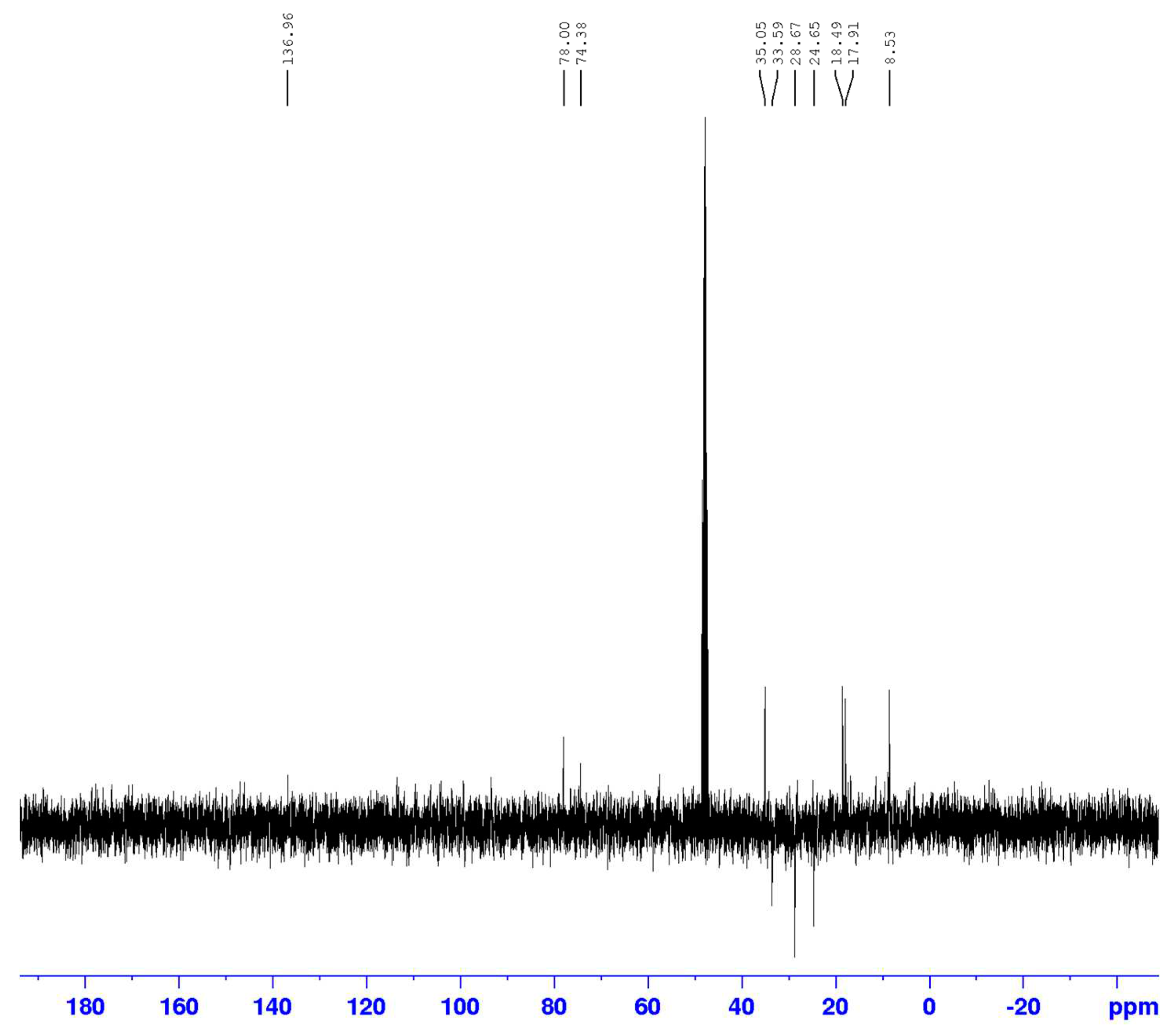
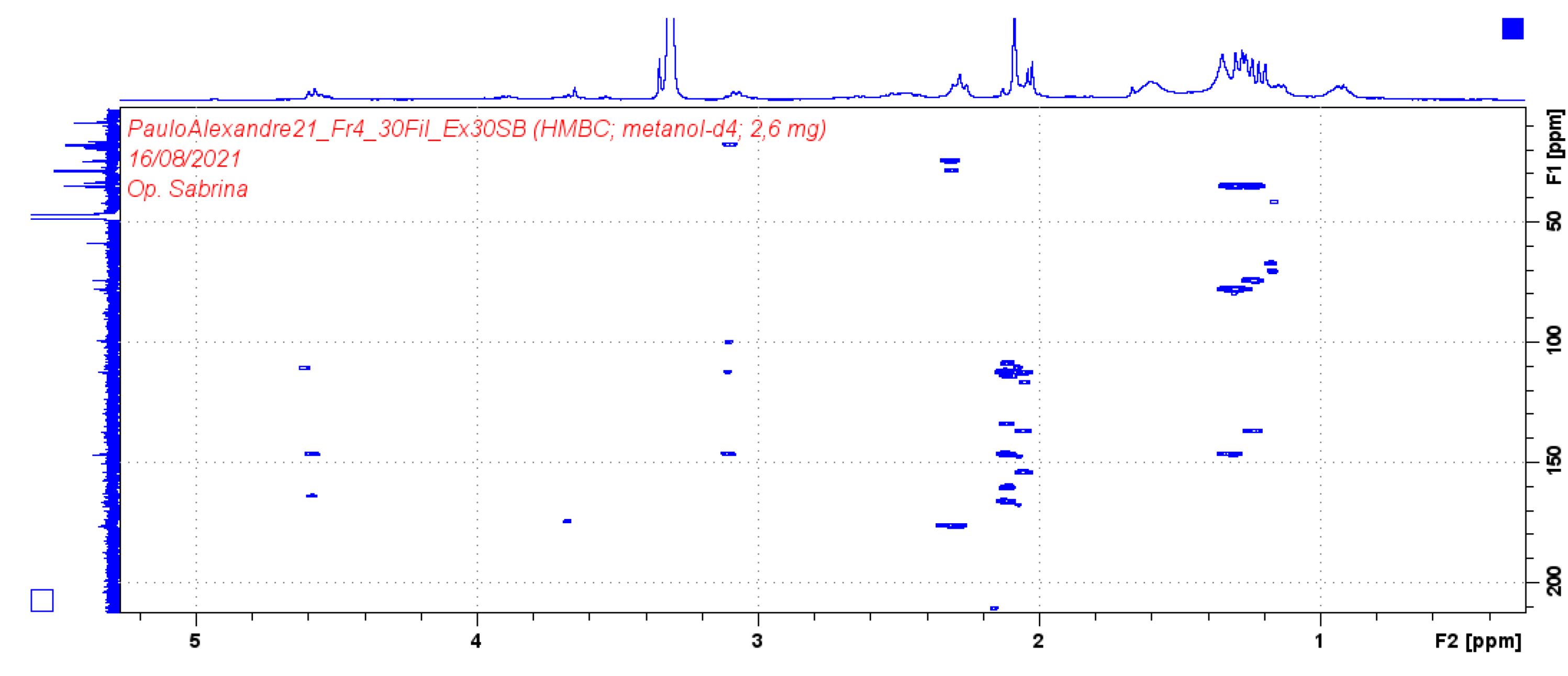
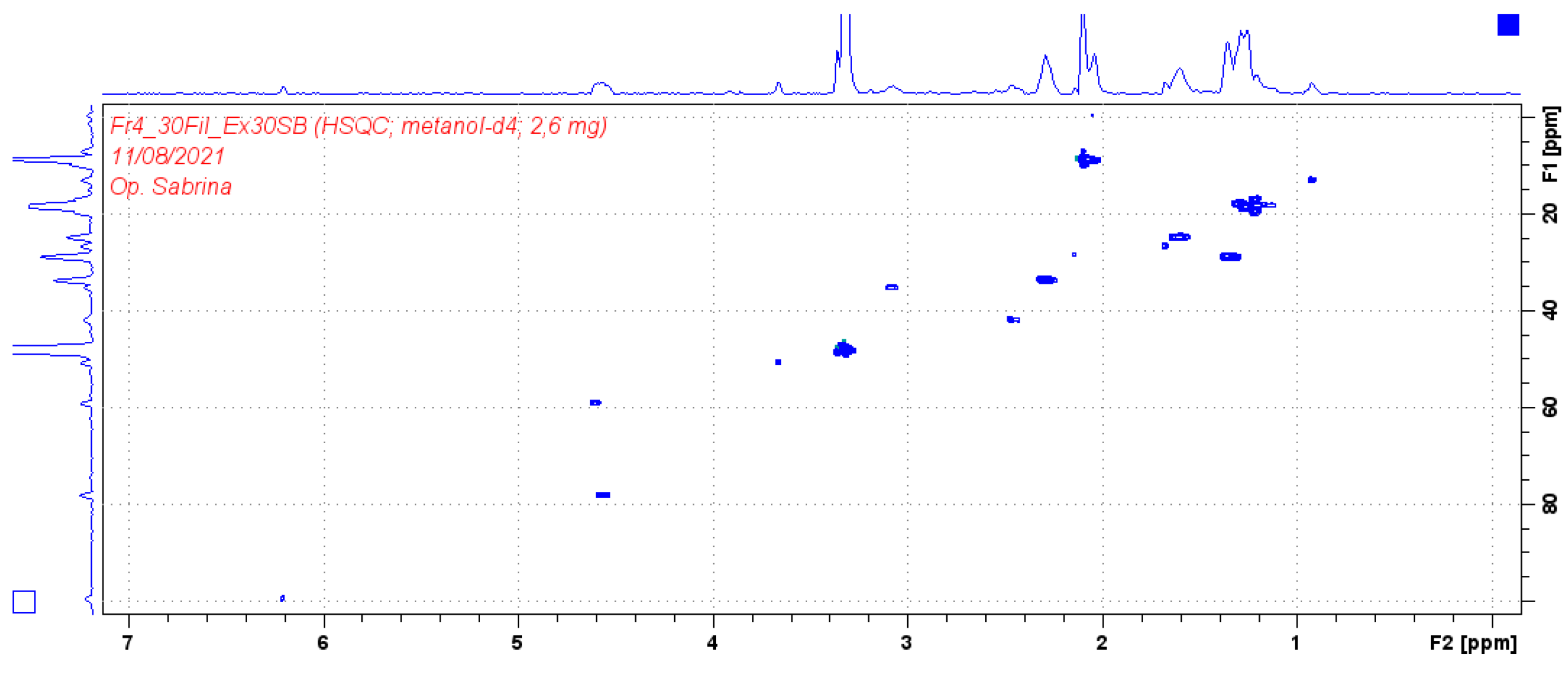
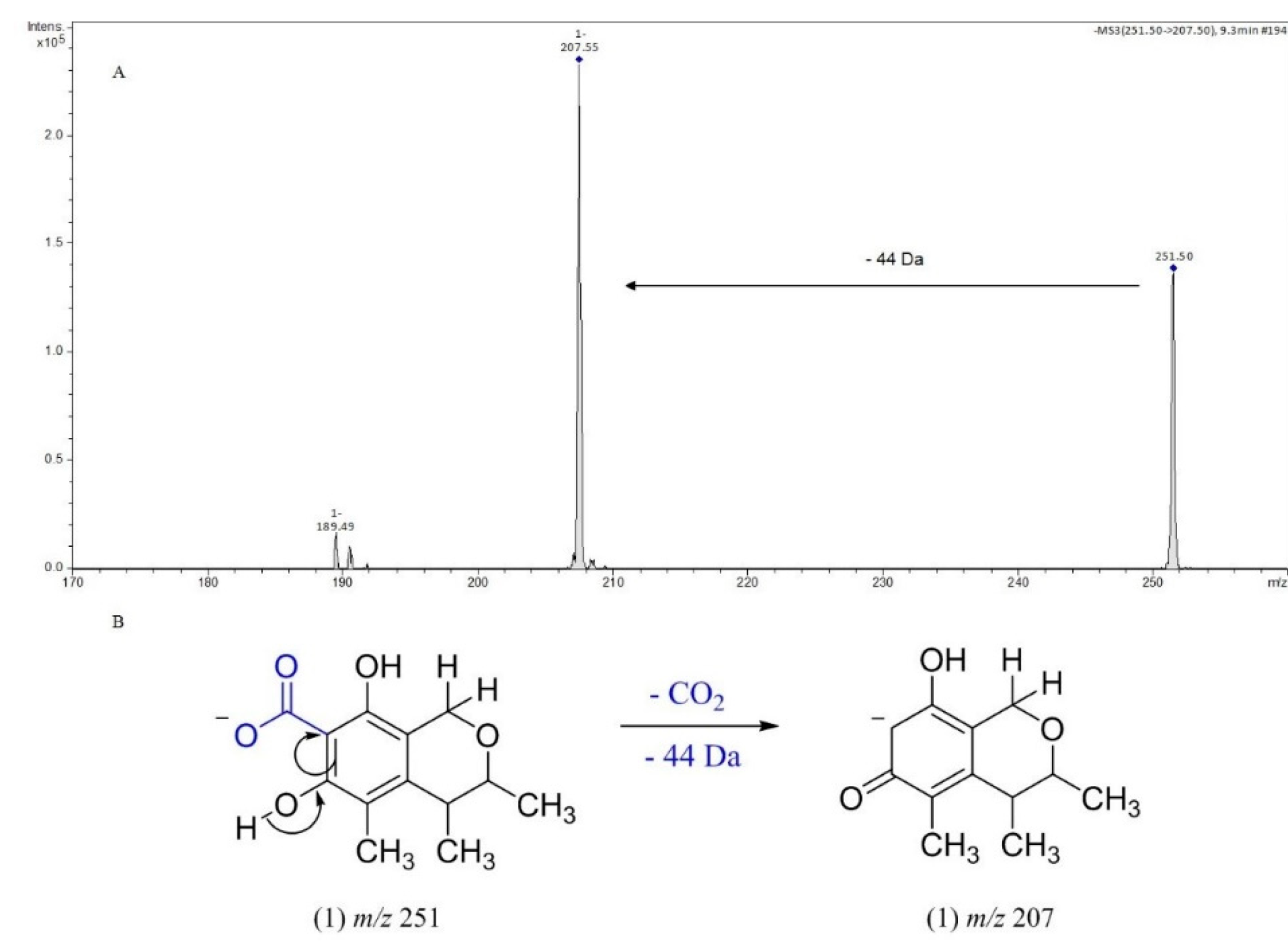
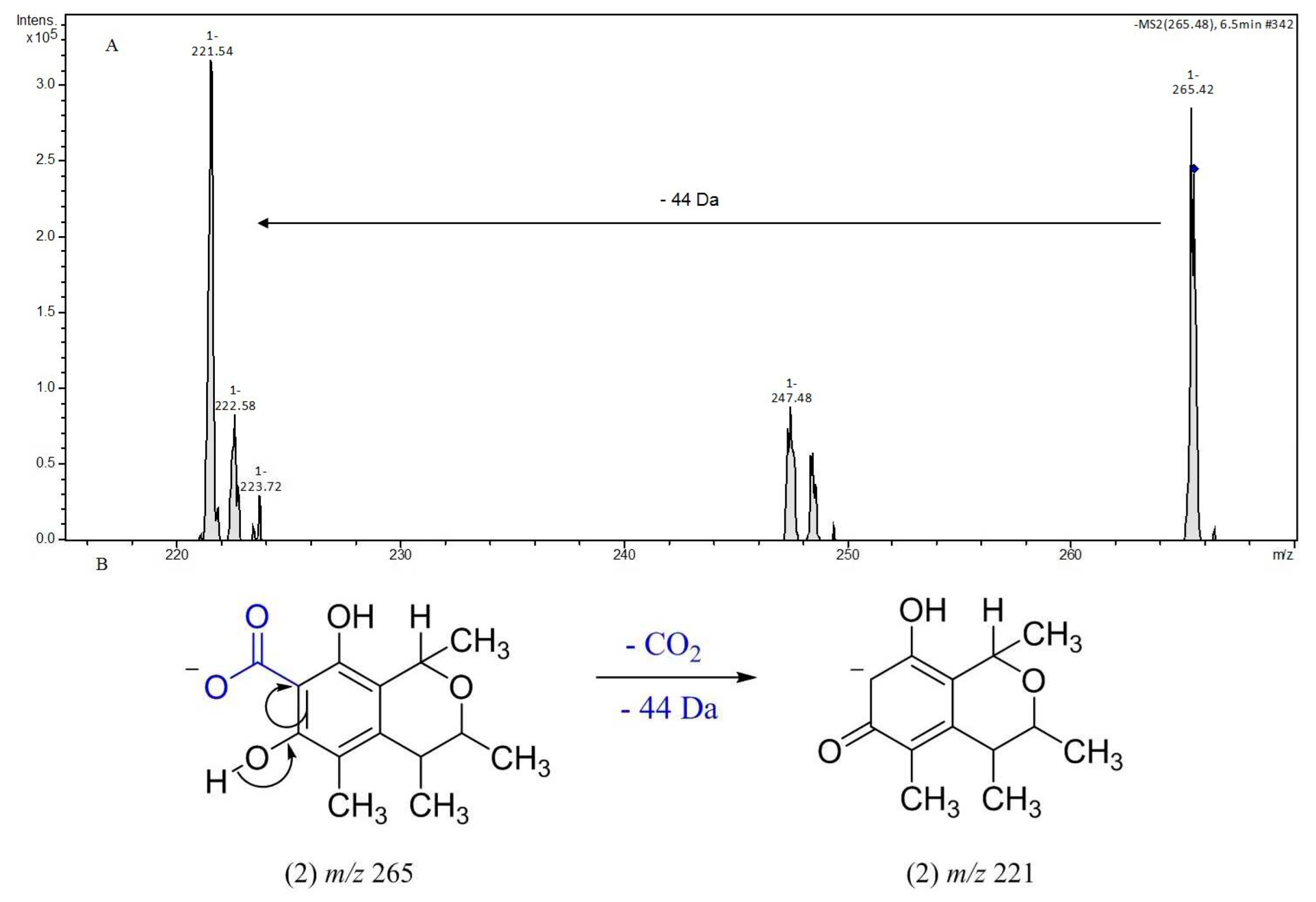
3.2. Identification of isolated compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- XU, J. Fungal species concepts in the genomics era. Genome, 2020, 459-468.
- Molinari, G. Natural Products in Drug Discovery: Present Status and Perspectives. Pharmaceutical Biotechnology, 2009, 13-27.
- Guimarães, D. O. Produtos naturais de fungos endofíticos associados a espécies de Asteraceae e ensaio antibiótico no modelo de infecção em "Caenorhabditis elegans". Universidade de São Paulo. Ribeirão Preto. 2010.
- Musalem, S. M.; Steiner, W. J.; Contreras, O. I. Produccion de celulasas por hungos aislados de madeira e suelos del sur de Chile. Boletín Micológico, 1984, 2, 17–25. [Google Scholar]
- Fritz, M.; Ranaval, M. C.; Braet, C.; Eyzaguirre, J. E. A. A family 51 a-L-arabinofuranosidase from Penicillium purpurogenum: purification, properties and amino acid sequence. Mycological research, 2008, 8, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Steiner, J.; Eyzaguirre, J. Beta-glucosidase from Penicillium purpurogenum: purification and properties. Biotechnology and Applied Biochemistry, 1992, 2, 185–191. [Google Scholar] [CrossRef]
- Chavez, R.; Navarro, C.; Calderón, I.; Pereira, A.; Bull, P.; Eyzaguirre, J. Secretion of endoxylanase A from Penicillium purpurogenum by Saccharomyces cerevisiae transformed with genomic fungal DNA. FEMS Microbiology Letters, 2002, 2, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Egana, L.; Gutiérrez, R.; Caputo, V.; Peirano, A.; Steiner, Eyzaguirre, J. Purification and characterization of two acetyl xylan esterases from Penicillium purpurogenum. Biotechnology and Applied Biochemistry, 1996, 1, 33–39. [Google Scholar] [CrossRef]
- Frisvad, J. C.; Smedsgaard, J.; Larsen, T. O.; Samson, R. A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Studies in Mycology, 2004, 49, 201–241. [Google Scholar]
- Mapari, S. A. S.; Meyer, A. S.; Thrane, U. Colorimetric Characterization for Comparative Analysis of Fungal Pigments and Natural Food Colorants. Journal of Agricultural and Food Chemistry, 2006, 19, 7027–7035. [Google Scholar] [CrossRef]
- Khethr, F. B. H.; Ammar, S. S.; Saïdana, D.; Daami, M.; Chariaa, J.; Liouane, K.; Mahjoub, M. A.; elal, A. N.; Mighri, Z. Chemical composition, antibacterial and antifungal activities of Trichoderma sp. growing in Tunisia. Annals of microbiology, 2008, 58, 303–308. [Google Scholar] [CrossRef]
- Sokovic, M. E. A.; Vukojević, J.; Marin, P. D.; Brkić, D. D.; Vajs, V.; Griensven, L. J. L. D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules, 2009, 1, 238–249. [Google Scholar] [CrossRef]
- Wang, H. E. A.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. Anti-influenza Virus Polyketides from the Acid-Tolerant Fungus Penicillium purpurogenum JS03-21. J. Nat. Prod, 2011, 9, 2014–1239. [Google Scholar] [CrossRef]
- Ghanbari, M. A. T.; Mohammadkhani, H. S.; Babaeizad, V. Identification of some secondary metabolites produced by four Penicillium species. Mycologia Iranica, 2014, 2, 107–113. [Google Scholar]
- XUE, J. E. A.; Wu, P.; Xu, L.; Wei, X. Penicillitone, a Potent in Vitro Anti-inflammatory and Cytotoxic Rearranged Sterol with an Unusual Tetracycle Core Produced by Penicillium purpurogenum. Organic Letters, 2014, 5, 1518–1521. [Google Scholar] [CrossRef]
- Murshid, S. S. A.; Badr, J. M.; Youssef, D. T. A. Penicillosides A and B: new cerebrosides from the marine-derived fungus Penicillium species. Revista Brasileira de Farmacognosia, 2016, 26, 29–33. [Google Scholar] [CrossRef]
- Pastre, R. E. A.; Marinho, A. M. R.; Rodrigues-filho, E. Diversidade de policetídeos produzidos por espécies de Penicillium isoladas de Melia azedarach e Murraya paniculata. Química Nova, 2007, 8, 1867–1871. [Google Scholar] [CrossRef]
- Samson, R. A.; Pitt, J. I. Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic, Amsterdam, 2000,pp 255-260.
- Damodaran, C.; Ramadoss, C. S.; Shanmugasundaram, E. R. B. A rapid procedure for the isolation, identification and estimation of citrinin. Analytical Biochemistry, 1973, 52, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, F. C.; Samson, R. A. Taxonomy of Penicillium section Citrina. Studies in Mycology, 2011, 1, 53–138. [Google Scholar] [CrossRef] [PubMed]
- Santos, C. M. C.; Costa, G. L.; Figueroa-Villar, J. D. ; Identification of citrinin as the defense metabolite of Penicillium corylophilum stressed with the antagonist fungus Beauveria bassiana. Natural Products Research, 2012, 24, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C. A. F. , Fernandes, B. C. Freire, R. B. Supressão da resposta imunitária humoral causada pela citrinina. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 2005, 2, 171–176. [Google Scholar] [CrossRef]
- de Lima, A. K.; Rodrigues. J. R.; Souza. S. I.; Rodrigues, J. C.; de Souza, T. C.; Maia, C. R.; Fernandes, O. C. C. Fungos isolados da água de consumo de uma comunidade ribeirinha do médio Rio Solimões, Amazonas-Brasil: potencial patogênico. Ambiente Água - An Interdisciplinary Journal of Applied Science, 2017, 12, 1017–1024. [Google Scholar] [CrossRef]
- P., W. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Informational Supplement. CLSI document M100-S30. 30º. ed. [S.l.]: Clinical and Laboratory Standards Institute, 2020.
- Batista, B. N.; Raposo, N. V.; Silva, I. R. Isolamento e avaliação da atividade antimicrobiana de fungos endofíticos de açaizeiro. Revista Fitos, 2018, 2, 161–174. [Google Scholar] [CrossRef]
- DA SILVA, J. C. Perfil da viabilidade celular e atividade antimicrobiana de espécies de Penicillium do acervo da coleção de culturas dpua. Universidade Federal do Amazonas - Programa de Pós Graduação em Diversidade Biológica. Manaus. 2008.
- Chapla, V. M.; Biasetto, C. R.; Araujo, A. R. Fungos Endofíticos: Uma Fonte Inexplorada e Sustentável de Novos e Bioativos Produtos Naturais. Rev. Virtual Quim, 2013, 3, 421–437. [Google Scholar]
- Yenn, T. W.; Ibrahim, D.; Chang, L. K.; Rashid, A.S.; Ring, L. C.; Nee, T. W.; Noor, M. I. M. Antimicrobial efficacy of endophytic Penicillium purpurogenum ED76 against clinical pathogens and its possible mode of action. Korean Journal of Microbiology, 2017, 3, 193–199. [Google Scholar]
- Xue, J.; Li, H; Wu, P. ; Xu, L.; Yuan, Y.; Wei, X. Bioactive Polyhydroxanthones from Penicillium purpurogenum. Journal of Natural Products, 2020, 5, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A. M. R.; Rodrigues-Filho, E.; Moitinho, M. L. R.; Santos, L. S. Biologically Active Polyketides Produced by Penicillium janthinellum Isolated as an Endophytic Fungus from Fruits of Melia azedarach. Journal of the Brazilian Chemical Society 2005, 208–283. [Google Scholar] [CrossRef]
- Haraguchi, H.; Taniguchi, T. T.; Oi, S.; Hashimoto, K. Citrinin, an Electron Acceptor Having Antifungal Activity. Agricultural and Biological Chemistry, 1989, 6, 1741–1742. [Google Scholar]
- Valente, A. M. M. P. O uso da RMN na caracterização e quantificação de metabólitos produzidos por microorganismos com potencial biotecnológico. Universidade Federal de São Carlos. São Carlos - SP, p. 183. 2007.
- Poupko, R.; Luz, Z.; Destro, R. Carbon-13 NMR of Citrinin in the Solid State and in Solutions. The Journal of Physical Chemistry A, 1997, 28, 5097–5102. [Google Scholar] [CrossRef]
- Marinho, A. M. R.; Rodrigues-Filho, E. Dicitrinol, a Citrinin Dimer Produced by Penicillium janthinellum. Helvetica Chimica Acta, 2011, 5, 835–841. [Google Scholar] [CrossRef]
- Deruiter, J.; Jacyno, J. M.; Davis, Cutler, H. G. Studies on aldose reductase inhibitors from fungi. I. Citrinin and related benzopyran derivatives. Journal of Enzyme Inhibition, 1992, 3, 201–210. [Google Scholar] [CrossRef]
- Clark, B. R.; Capon, R. J.; Lacey, E.; Tennant, S.; Gill, J. H. Citrinin revisited: from monomers to dimers and beyond. Organic Biomolecular Chemistry, 2006, 1520–1528. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, C. T.; Ting, H. S. Citrino pinacol. Science Record (China) 1950, 213–220. [Google Scholar]
- Warren, H. H.; Finkelstein, M.; Scola, D. A. The synthesis and antibiotic activity of analogs of citrinin and dihydrocitrinin. Journal of American Chemistry Society, 1957, 10, 1926–1928. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, F. K.; Hwang, F. T.; Fan, C. S. Citrinin as an antibiotic. Science 1947, 291–292. [Google Scholar] [CrossRef]
- Cruz, J. S.; Costa, L.; Figueroa-Villar, J. D. História, Aplicações, Atividade e Modificações da Citrinina. Revista Virtual de Química, 2016, 8, 650–664. [Google Scholar]
- Franco, C. M.; Fente, C. A.; Vazquez, B.; Cepeda, A.; Lallaoui, L.; Prognon, P.; Mahuzier, G. Simple and sensitive highperformance liquid chromatography fluorescence method for he determination of citrinin - Application to the analysis of fungal cultures and cheese extracts. Journal of Chromatography A, 1996, 1, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Betina, V.; Baráthová, H. Citrinin - an inducer of permeability changes in Eremothecium ashbyi. The Journal of Antibiotics, 1968, 10, 628–629. [Google Scholar] [CrossRef]
- Lu, Z. Y.; Lin, Z. J.; Wang, W. L.; Du, L.; Zhu, T. J.; Fang, Y. C.; Gu, Q. Q.; Zhu, W. M. Citrinin Dimers from the Halotolerant Fungus Penicillium citrinum B-57. Journal of Natural Products, 2008, 4, 543–546. [Google Scholar] [CrossRef]
| Pathogen | Extract | Growing medium | Concentration (µg. mL-1) |
|---|---|---|---|
| C. albicans | Acoet | SB | 31.25 |
| Acoet | ISP2 | 500 | |
| MeOH | ISP2 | 1000 | |
| Acoet | YES | 62.5 | |
| C. tropicalis | Acoet | SB | 250 |
| S. aureus | Acoet | SB | 125 |
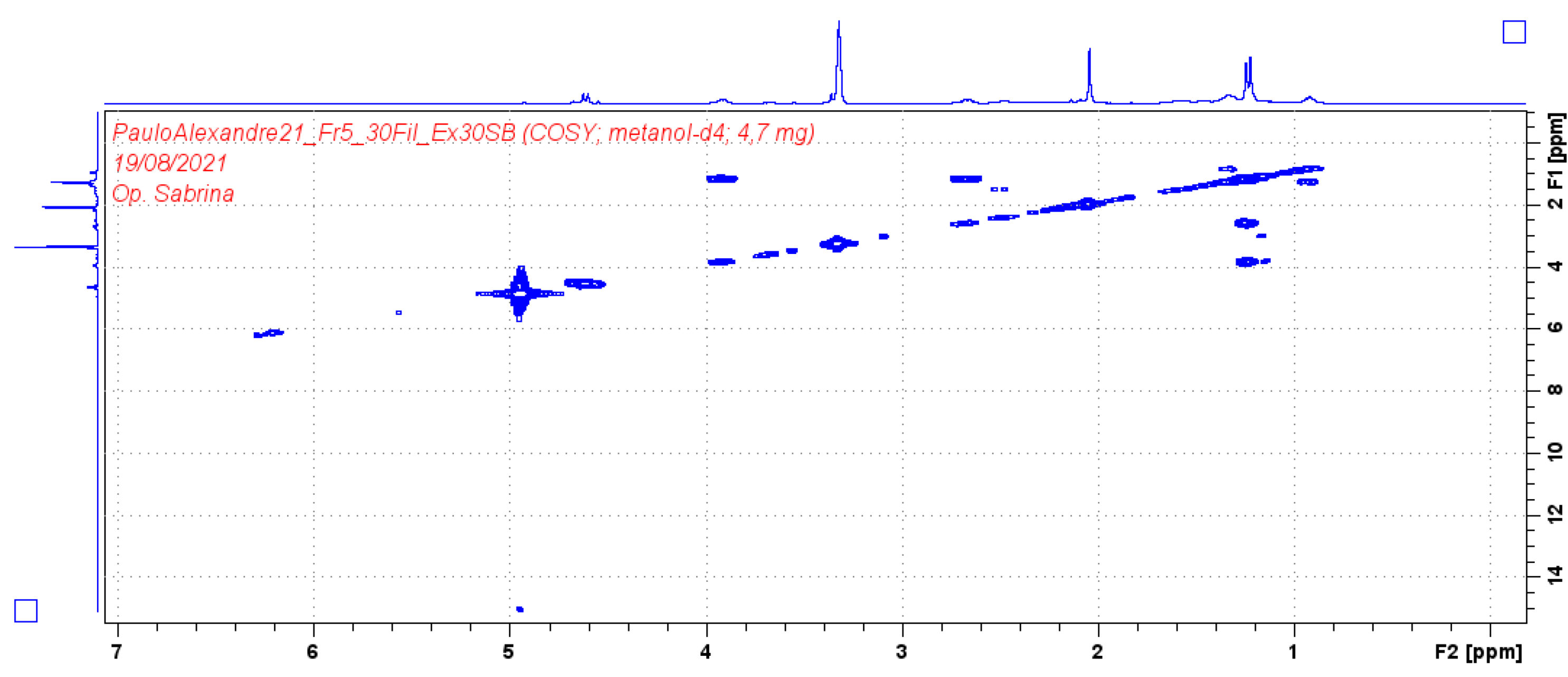
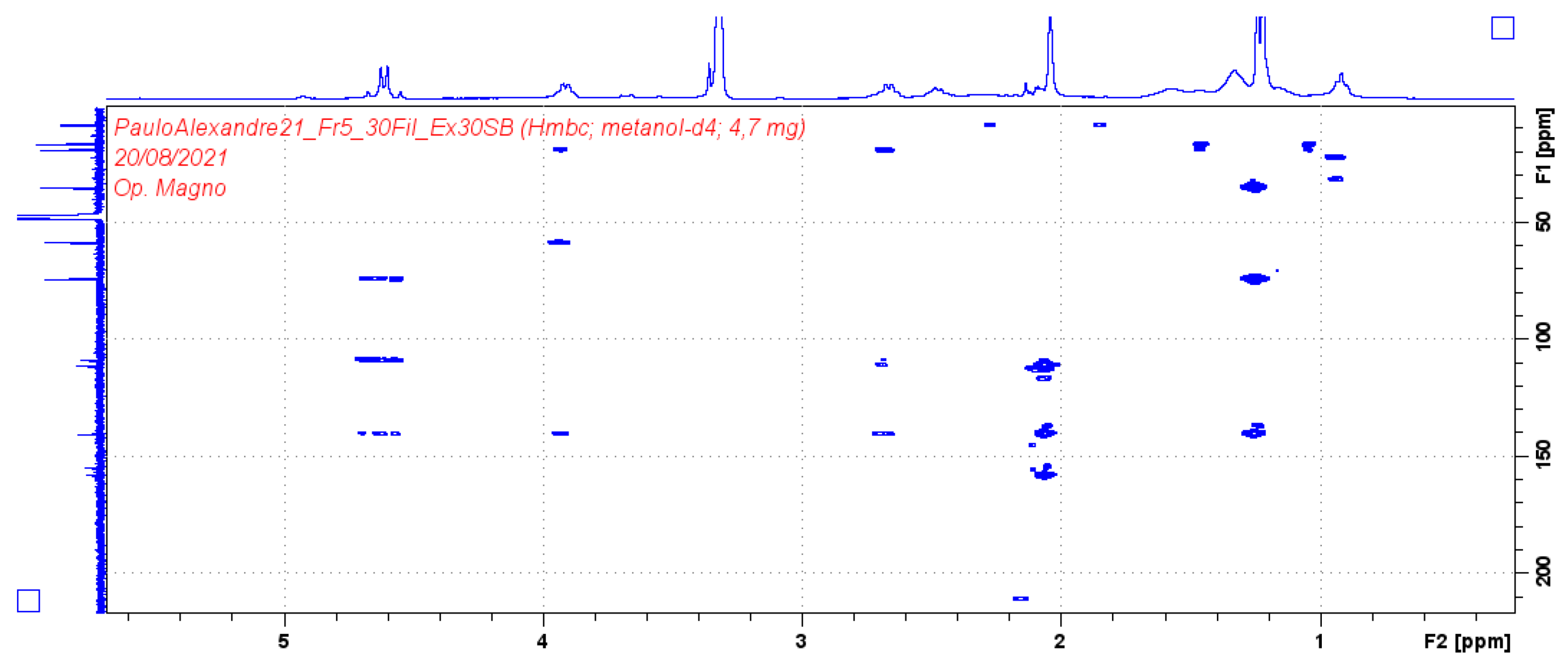
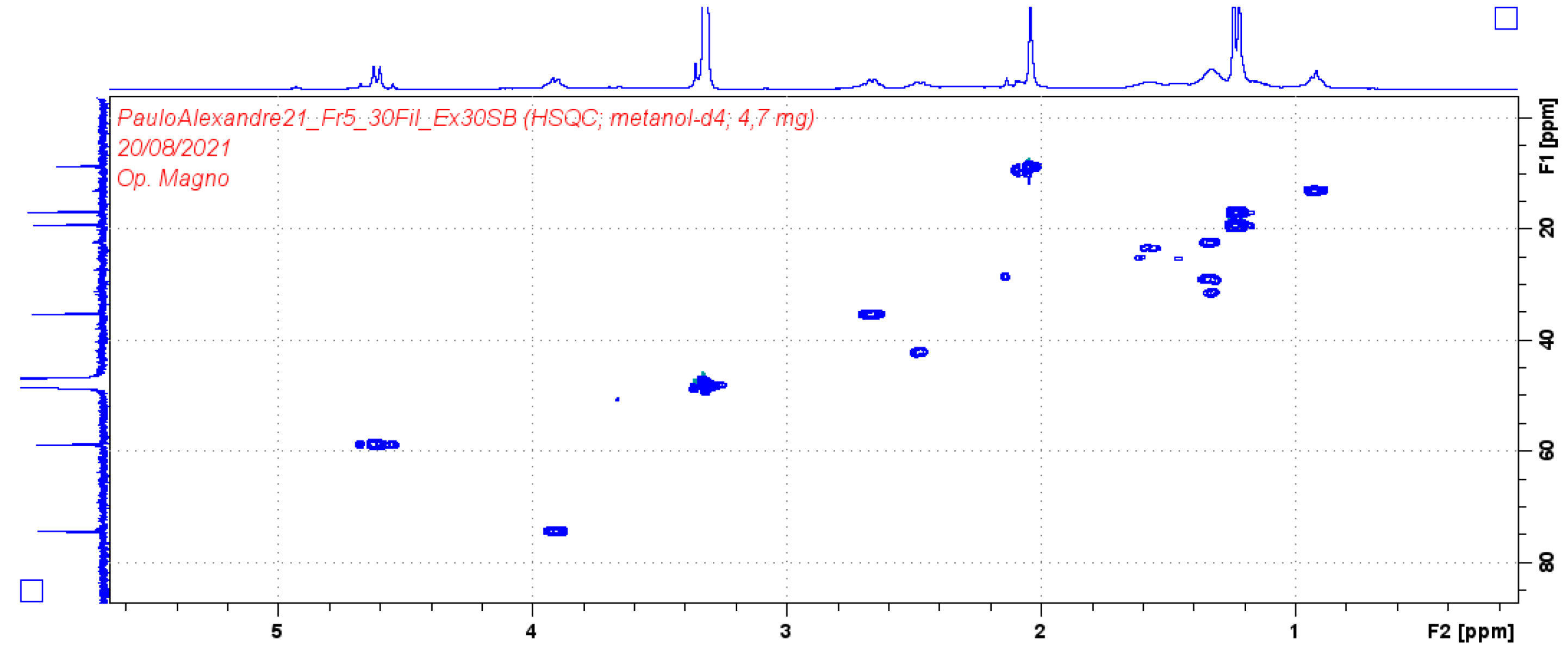
| Position | Compound (1) | |||
|---|---|---|---|---|
| δH (J in Hz) | δC | HMBC | COSY | |
| 1 | 4.61 (2H, d 7.2) | 58.69 | 74.2/108.9/140.4/154.8 | 2.05/2.66 |
| 3 | 3.91 (1H, d 6.4) | 74.2 | 19.42/58.69/140 | 1.27/2.66 |
| 4 | 2.66 (1H, q 6.4) | 35.1 | 19.42/108/111/140 | 1.23 |
| 4a | - | 140.0 | ||
| 5 | - | 111.0 | ||
| 6 | - | 157.0 | ||
| 7 | - | 105.9 | ||
| 8 | - | 154.8 | ||
| 8a | - | 108.8 | ||
| 9 | 1.21 (3H, d 6.4) | 16.7 | ||
| 10 | 1.23 (3H, d 6.4) | 19.4 | 35.1/74.2/140 | |
| 11 | 2.04 (3H, s) | 8.6 | 111/140/157 | |
| 12* | - | - | ||
| Position | Compound (2) | |||
|---|---|---|---|---|
| δH (J in Hz) | δC | HMBC | COSY | |
| 1 | 4.54 (1H, m) | 77.9 | 17.9/74.37/110.8/146.5/163.9 | 1.25 |
| 3 | 1.22 (1H, m) | 74.3 | ||
| 4 | 3.08 (1H, q 6.7) | 35.0 | 17.9/146.5 | 1.29 |
| 4a | - | 146.5 | ||
| 5 | - | 112.4 | ||
| 6 | - | 154.3 | ||
| 7 | - | - | ||
| 8 | - | 163.9 | ||
| 8a | - | 110.8 | ||
| 9 | 1.25 (3H, d 6.7) | 18.4 | ||
| 10 | 1.29 (3H, d 6.7) | 17.9 | 35.05/77.9/146.5 | |
| 11 | 1.21 (3H, d 6.7) | 16.8 | 35.05 | |
| 12 | 2.09 (3H, s) | 8.5 | 112.4/146.5 | |
| 13 | - | 178 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





