1. Introduction
Wound infections are a major problem in veterinary medicine, especially in equine patients. Advancements in wound care have been made including the use of medicinal Manuka honey as an antimicrobial and anti-bioflim agent in chronic wound management. The goal of this research was to provide veterinary practitioners with comparative data on the use of medical-grade, commercial, food-grade, and raw local honey as antimicrobial dressings for inhibiting growth of common bacterial species in wounds. We ascertained bacterial susceptibility in vitro to gather information for possible future in vivo utilization.
There is a high incidence of traumatic wound infections in equine patients and an increasing prevalence of antimicrobial resistant bacterial species in wounds [
1,
2]. A 2005 report published by the USDA APHIS indicated that 19% to 24% of equine euthanasias in the United States were the result of skin wounds or trauma [
3]. Local treatments reduce the development of resistance to systemic antibiotics, are cost-effective, and decrease morbidity. One commonly used topical antiseptic is polyhexamethylene biguanide (PHMB) with and without hypertonic saline (20%). PHMB is a broad-spectrum yet safe antimicrobial used as a post-surgical dressing.
1 Silver sulfadiazine (SSC) preparations are also commonly used for topical wound treatment. Recently, the use of medical-grade Manuka honey for the treatment of resistant bacterial infections has shown promising success [
4]. A better understanding of topical antimicrobial agents in animals is important to provide the most successful wound healing.
Polyhexamethylene biguanide (PHMB) antimicrobial dressing is commercially available as a gauze or foam dressing. PHMB is effective against a broad spectrum of Gram-positive and Gram-negative bacterial species. PHMB has shown to be efficacious against methicillin-resistant Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and other bacterial species. As an antimicrobial, PHMB is bactericidal, reduces bacterial colonization and invasion, while maintaining a balance of normal skin flora.a
Hypertonic saline (20%) dressings are used to debride infected or exudative wounds. Hypertonic saline (20%) dressings work by removing fluid from bacteria and necrotic cells using osmotic gradients, allowing bacteria and debris to be lifted off a wound with dressing change [
5].
Topical application of 1% SSC to incisional wounds in rats is associated with faster wound healing versus that in control wounds [
3]. Treatment with 1% silver sulfadiazine cream (SSC) resulted in improved neovascularization and epithelialization compared with results for 0.25% sodium hypochlorite and 10% povidone-iodine solutions in mice with ear injuries [
3]. Silver sulfadiazine has proven to reduce microbial counts in horses with metatarsal and metacarpal injuries, however, it also resulted in exuberant granulation tissue that required surgical resection [
3].
Medical-grade Manuka honey has proven in-vitro bactericidal activity against
Bacillus subtilis,
Escherichia coli, Pseudomonas aeruginosa, methicillin-resistant
Staphylococcus aureus. Manuka honey also has demonstrated potent in-vitro activity against antibiotic-resistant bacteria and has shown success in treatment of wound infections not responding to antibiotic therapy [
4,
6]. The efficacy of honey for post-surgical wounds can be attributed to its bactericidal compounds, moist environment that promotes healing, and high viscosity that maintains an effective barrier [
4]. Manuka-type honey has been shown to have anti-biofilm activity as well [
7]. Within chronic wounds, bacteria within a biofilm are protected from host defenses and harbor antimicrobial resistance [
8]. New Zealand Manuka-type honeys in wound dressings prevent and eradicate
Staphylococcus aureus biofilms. Methylglyoxal (MGO), di-hydroxyacteone (DHA), and hydrogen peroxide are the antimicrobial and antibiofilm components in Manuka honey [
7]. Medical-grade honey has proven immunomodulatory properties that facilitate wound repair [
7].
Our hypothesis is that medical grade Manuka honey would have comparable bactericidal effect to PHMB (+/- hypertonic saline) and would have better bactericidal effect when compared to commercial, food-grade honey and raw, local honey.
2. Materials and Methods
Bacterial isolates for testing were from the American Type Culture Collection (ATCC) (Pseudomonas aeruginosa, Escherichia coli, and methicillin-resistant Staphylococcus aureus) and from an equine sample submitted to the Colorado State Veterinary Diagnostic Laboratory (Streptococcus equi ssp. zooepidemicus (Streptococcus zooepidemicus)). Isolates were grown on trypticase soy agar plates with 5% sheep red blood cells overnight at 37°C and isolated colonies were suspended in phosphate buffered saline to a 0.5 McFarland standard using the BBL™ Prompt™ inoculation system (BD). This resulted in a concentration of bacteria at approximately 1.5 x 108 colony forming units per ml. Test materials included sterile gauze with 20% hypertonic saline, hypertonic saline with PHMB gauze, silver sulfadiazine cream, PHMB gauze, PHMB foam, raw local honey, commercial, food-grade honey, and medical-grade Manuka honey. A solution of 20% hypertonic sugar was used as control for the honey to assess if hypertonicity was responsible for any antimicrobial activity that might be observed.
Antimicrobial susceptibility testing. Two dilutions of each bacterial species were used to determine the MIC and MBC with challenge doses of bacteria. The 0.5 McFarland solution was used undiluted and was diluted with Mueller Hinton Broth to reach approximately 105 CFU/ml. To determine the minimum inhibitory concentration (MIC) a constant volume of 50 microliters of the bacterial dilution was added to doubling dilutions of each antimicrobial treatment at a constant volume of 100 microliters (diluted in 0.9% saline) in a 96 well plate. The plates were covered and incubated overnight at 37°C. After incubation, the plates were read visually for the minimum concentration of the drug that was required to inhibit visual growth of the organism. To determine the minimum bactericidal concentration (MBC) all wells of the incubated plate that had no visible bacterial growth were subcultured to trypticase soy agar plates with 5% sheep red blood cells and incubated overnight at 37 degrees Celsius. The minimum concentration of antimicrobial that allowed no bacterial growth on subculture was recorded.
Due to the opacity of SSC, it was not possible to interpret any reduction of bacterial growth in the 96 well plate, therefore, no MICs could be determined. To observe MBC based on 80% reduction of growth, a lawn of each bacterial species was made on individual sheep blood agar plates, 10 microliters of each dilution of SSC was pipetted onto the agar plates, and incubated overnight at 37°C. The zone of inhibition, a circular area around the antimicrobial where the bacteria does not grow, is used to measure susceptibility of the bacteria to the antimicrobial [
9]. The amount of growth in the zone of inhibition was recorded for each bacterial species.
3. Results
Total sample numbers are based on two dilutions of each bacteria, four organisms tested, 10 treatment antimicrobials and 10 replicates for MIC and MBC.
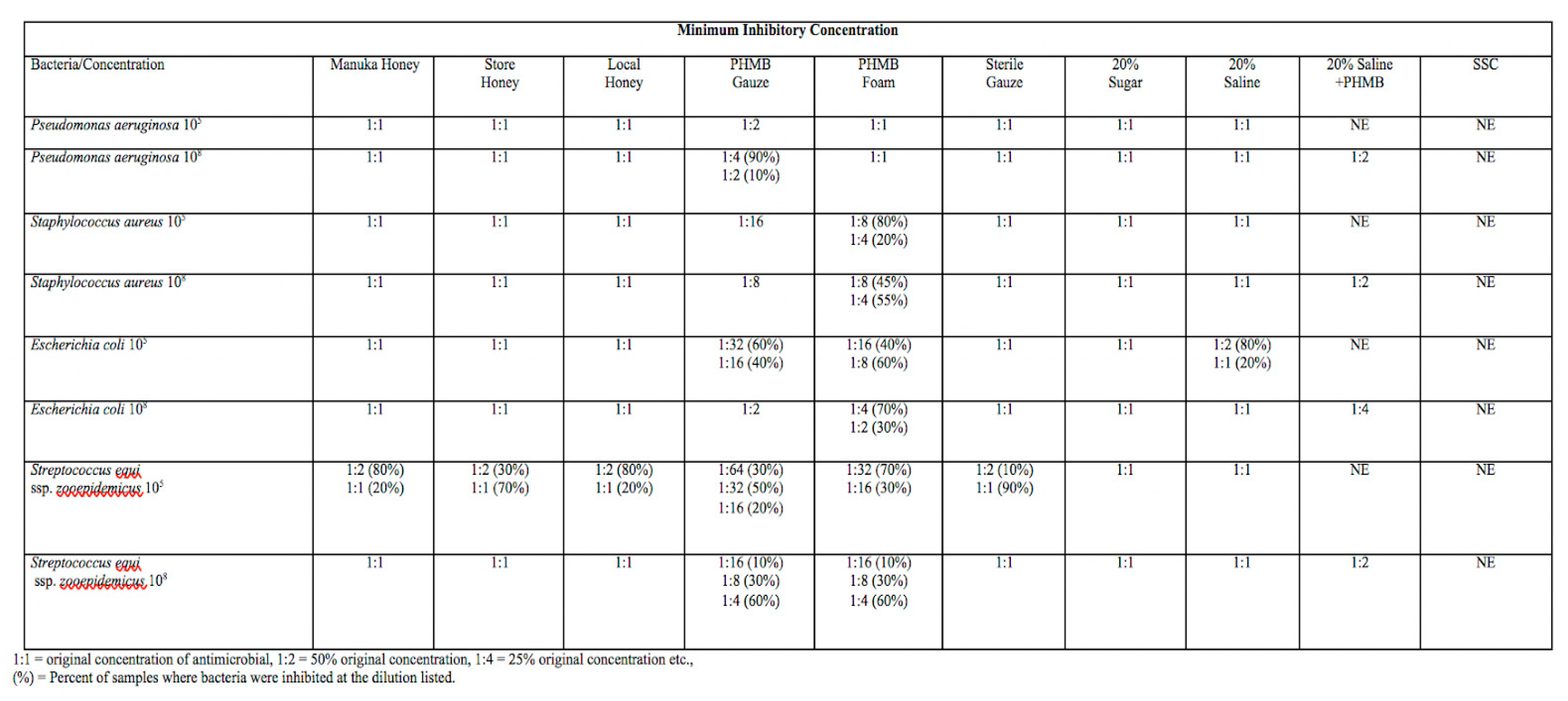
Table 1-Minimum inhibitory concentration of each wound dressing represented in the columns with each bacterial species at either 10
5 or 10
8 CFU/mL on the rows. The dilution of antimicrobial that resulted in no visible growth of bacteria in a 96 well plate is the minimum inhibitory concentration for that antimicrobial with a given bacterial species. 1:1= original concentration of antimicrobial, 1:2 = 50% of the original concentration, 1:4= 25% of the original concentration. The percentages next to dilutions are the percent of samples where bacterial growth was inhibited for a given dilution out of 10 replicates. NE= Not examined. MIC for 20% Saline + PHMB and SSC was examined for each bacterial species at 10
8 CFU/mL only. The MIC for SSC was not able to be visualized due to the opacity of SSC.
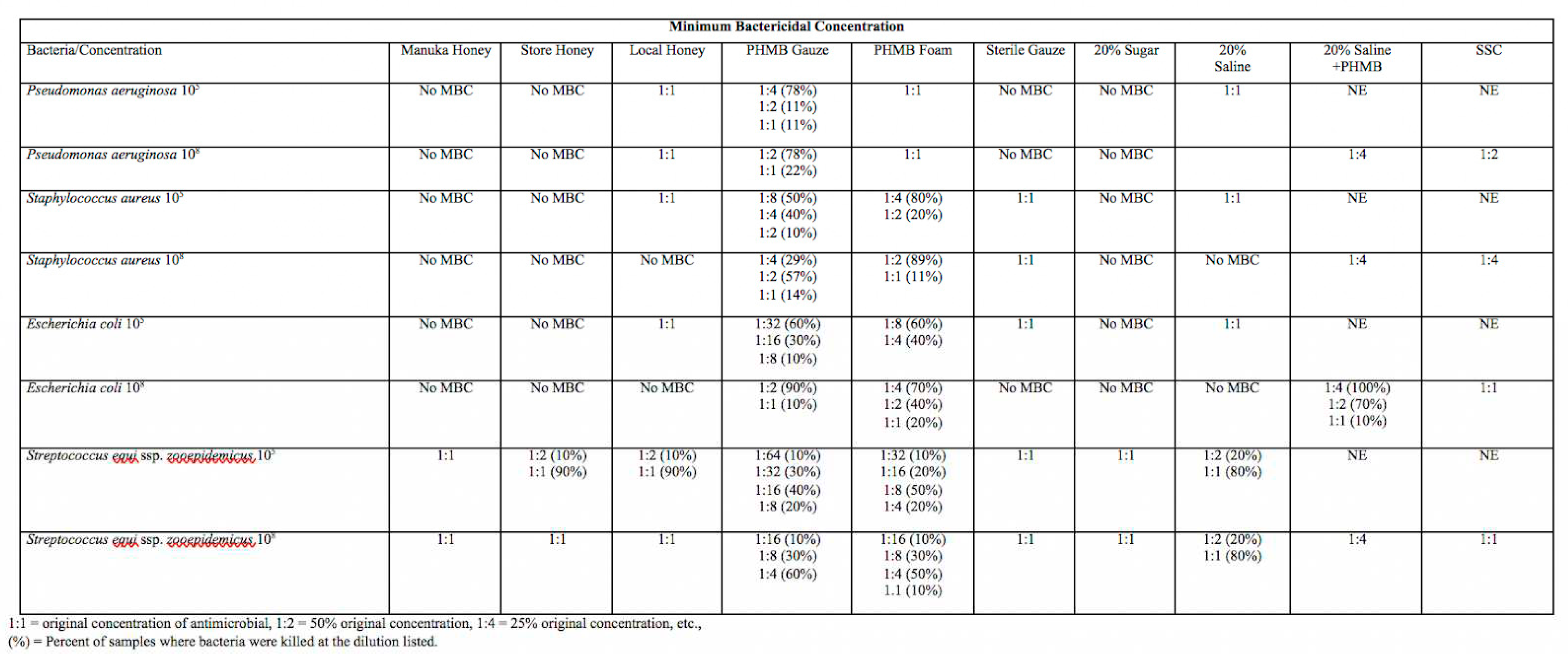
Table 2 - Minimum bactericidal concentration of each wound dressing represented in the columns with each bacterial species at either 10
5 or 10
8 CFU/mL on the rows. The dilution of antimicrobial that resulted in no visible bacterial growth when subcultured to trypticase soy agar plates with 5% sheep red blood cells is the minimum bactericidal concentration for that antimicrobial with a given bacterial species. 1:1= original concentration of antimicrobial, 1:2 = 50% of the original concentration, 1:4= 25% of the original concentration. The percentages next to dilutions are the percent of samples where bacterial growth was inhibited for a given dilution out of 10 replicates. NE= Not examined. MBC for 20% Saline + PHMB and SSC was examined for each bacterial species at 10
8 CFU/mL only. No MBC means that growth was visible on all agar plates when sub-cultured and incubated overnight at 37°C.
MIC and MBC for Manuka, store, and local honeys were comparable to those of sterile gauze, sugar, and hypertonic saline, at 1:1 to 1:2 for all bacterial species tested. Across bacterial species, local honey proved to have more bactericidal activity when compared to Manuka and commercial, food-grade honey. Local honey had an MBC at a dilution of 1:1 for Pseudomonas aeruginosa and Staphylococcus aureus (105 CFU/mL) while commercial, food-grade honey and Manuka honey had no MBC, which means there was visible growth on all subcultures. Manuka honey and commercial, food-grade honey had an MBC for Streptococcus zooepidemicus, ranging from 1:1 (90-100% of samples depending on isolate) to 1:2 (10% of samples).
MIC and MBC for PHMB gauze and foam was variable between different bacterial species and proved to have a stronger bactericidal and inhibitory effect on Escherichia coli and Streptococcus zooepidemicus than on Staphylococcus aureus and Pseudomonas aeruginosa. The MIC and MBC for PHMB gauze and foam was consistently at a higher dilution compared to the other antimicrobials. The majority of antimicrobials exhibited stronger inhibitory and bactericidal activity against a Streptococcus zooepidemicus isolate that was obtained from a wound compared to the other organisms that were ATCC lab acclimated. PHMB gauze had bactericidal effects against Streptococcus zooepidemicus (105 CFU/mL) at a dilution of 1:64 for 10% of samples, significant bactericidal effects were found in 40% of samples at a dilution of 1:16. MBC of other bacterial species tested was at dilutions of 1:1 to 1:8 for both PHMB gauze and PHMB foam. PHMB gauze and foam had MBCs ranging from 1:1 to 1:64 while the other antimicrobials had MBCs of 1:1 to 1:4 across bacterial species tested. PHMB gauze + 20% hypertonic saline had lower bactericidal effect than PHMB gauze or foam alone, with MBCs ranging from 1:1 to 1:4 depending on the isolate
4. Discussion
Results of the present study suggested that PHMB gauze and foam has superior bactericidal effect when compared to hypertonic saline, PHMB + hypertonic saline, SSC, Manuka honey, local honey, and commercial, food-grade honey. It is interesting that the PHMB + hypertonic saline did not perform as well as the PHMB gauze or foam, possibly due to further dilution of PHMB with the hypertonic saline. Most antimicrobials exhibited stronger inhibitory and bactericidal activity with Streptococcus zooepidemicus that was obtained from an equine wound compared to the other organisms that were ATCC lab acclimated. There could be differences between wild type and lab acclimated bacteria that result in greater antimicrobial susceptibility in wild-type bacteria, but the mechanism is unknown. A comparative study of bacterial reduction using ATCC and wild type bacteria from the same species could help answer this question. Each bacterial species was tested with PHMB gauze and foam, hypertonic saline, Manuka honey, local honey, and commercial, food-grade honey at 105 and 108 CFU/mL because these represent typical bacterial counts in contaminated wounds. Testing for SSC and PHMB + hypertonic saline wound dressings was only against bacteria at 108 CFU/mL.
Interestingly, local honey appeared to have improved bactericidal effect against all bacterial species compared to commercial, food-grade and Manuka honey. Perhaps there are antimicrobial properties in raw, local honey that are destroyed in the filtering and pasteurization process that commercial, food-grade and medical grade honey go through. The concern with using local, raw honey on a wound involves not knowing all of the components that are present. Local honey could have contamination with pesticides, antibiotics, and bacterial or fungal spores [
10]. These potential contaminants could impact the wound healing process and have negative side effects.
Antimicrobial properties of honey in general include hypertonicity, low pH, and hydrogen peroxide production, but these properties are negligible with dilution [
4]. Phenolics are compounds found in honey that act as antioxidants and contribute to anti-inflammatory and healing properties of honey [
11]. Honey aids autolytic debridement and promotes growth of healthy granulation tissue [
12]. This selective destruction of bacterial cells is what separates honey from many of the other topical antimicrobials, which have some inherent cytotoxic effects. By inadvertently destroying the healthy cells that are trying to re-populate in the wound bed, wound healing is actually slowed with some topical products. A study comparing SSC and honey in vivo found honey to result in reduction of early inflammatory signs, better control of infection, and quicker wound healing when compared to SSC [
12].
It was discovered that Manuka honey maintains its antimicrobial effect even with dilution and has “non-peroxide activity” due to a compound called methyl glyoxal (MGO) [
11]. MGO results from the spontaneous dehydration of its precursor dihydroxyacetone (DHA), a naturally occurring phytochemical found in the nectar of flowers of Leptospermum scoparium, Leptospermum polygalifolium, and some related Leptospermum species native to New Zealand and Australia [
11]. There is no evidence of damage to host cells, however the mechanism behind this selective toxicity to bacterial cells is not understood [
11]. Manuka honey has been tested in vitro against many types of skin and wound pathogens, and notably, shows efficacy against multi-drug resistance (MDR) bacterial phenotypes and bacteria in biofilms [
11]. Medical grade Manuka honey has proven efficacy and is tested to be free of contaminants and then pasteurized to ensure the product is safe for medical use [
13].
This study had some inherent limitations, one of which is that a wound bed is a complex environment that is not easily replicated in vitro. The wound microenvironment contains peripheral blood (red blood cells, lymphocytes, macrophages, neutrophils), growth factors, fibroblasts, necrotic tissue, contamination with debris and multiple types of bacteria, fungus, and yeast [
14]. The many components that are present in a wound cannot be easily replicated in a laboratory setting. If the natural wound environment were replicated, this would result in more variables when completing a study of bacterial reduction and would naturally confound results. While it is understood that infection delays wound healing, bacterial reduction cannot be directly correlated with faster wound healing due to the dynamic nature of the wound microenvironment. One major benefit to honey is that it maintains a moist wound environment. Moisture is known to help prevent tissue dehydration, cell death, accelerates angiogenesis, increases breakdown of necrotic tissue and fibrin, and potentiates interaction of growth factors with target cells [
15]. These positive properties cannot be observed in vitro. Honey also provides a protective barrier with high viscosity that could help prevent new infections and associated inflammation [
4]. These properties of moisture and viscosity contribute to the positive wound healing effects of honey, but these properties could not be fully considered in this study.
5. Conclusions
Proper topical wound management is essential to reduce side effects of systemic antimicrobials, speed healing, and reduce antimicrobial resistance. We were interested in studying different types of honey when compared to more commonly used wound dressings (PHMB, SSC, Hypertonic saline). Honey seems to have fewer cytotoxic effects and has bactericidal properties that would improve wound healing. Honey also maintains a moist wound environment, which we know is vital to proper healing. Our data showed that MIC and MBC for Manuka, store, and local honeys were comparable to those of sterile gauze, sugar, and hypertonic saline, at 1:1 to 1:2 for all bacterial species tested. Local honey proved to have more bactericidal activity when compared to Manuka and commercial, food-grade honey across all bacterial species tested. The MIC and MBC for PHMB gauze and foam was consistently at a higher dilution compared to the other antimicrobials. The majority of antimicrobials exhibited stronger inhibitory and bactericidal activity against a Streptococcus zooepidemicus isolate obtained from a wound compared to other bacteria that were ATCC lab acclimated.
The data we gathered showed that PHMB gauze and foam outperformed other wound dressings when it comes to MBC and MIC. The surprising findings were that local honey also out-performed store-bought and medical grade Manuka honey. We cannot recommend using local honey or store-bought honey in-vivo due to the risk for contamination. We hope the data we gathered can help spark additional research regarding efficacy of wound dressings for different bacterial isolates. Additional research in the chemical composition and contaminants in local honey compared to store-bought or medical grade Manuka honey would be interesting. Additional research comparing wild type and ATCC lab acclimated bacterial isolates is needed to discern whether differences in antimicrobial susceptibility exist. Based on our data, honey is similar in efficacy to hypertonic saline so depending on the wound environment and need for debridement, then practitioners may be able to replace hypertonic saline with medical-grade Manuka honey in some circumstances. An in vivo study would help determine the wound healing capabilities of honey compared to commonly used wound dressings like PHMB gauze and foam, SSC, and hypertonic saline. While a prospective controlled study would provide less variability, the animal welfare concerns associated with a prospective wound study would not be acceptable to equine practitioners in the field.
Author Contributions
M. Simpson- first author, writing-original draft preparation, investigation/ data curation, D. Hendrickson-corresponding author/senior author, conceptualization, funding acquisition, writing-review and editing D. Hyatt-second author, methodology, writing-review and editing S. Rao-third author, methodology, writing-review and editing.
Funding
This research was funded by Colorado State University Young Investigator’s Grant and the Hadley and Marion Stuart Foundation.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
PHMB - Polyhexamethylene biguanide
MIC- Minimum inhibitory concentration
MBC- Minimum bactericidal concentration
SSC- Silver sulfadiazine cream
CFU- Colony forming units
ATCC- American Type Culture Collection
References
- MacDonald D, Morley PS, Bailey SM, et. al. An examination of the occurence of surgical wound infection following equine orthopaedic surgery (1981-1990). Equine vet J 1994, 26, 323-326. [CrossRef]
- Weese JS, Archambault M, Dick H, et. al. Methicillin-resistant Staphylococcus aureus in Horses and Horse Personnel, 2000-2002. Emerg Infect Dis. 2005. [CrossRef]
- Harmon CC, Hawkins JF, Li J, et. al. Effects of topical application of silver sulfadiazine cream, triple antimicrobial ointment, or hyperosmolar nanoemulsion on wound healing, bacterial load, and exuberant granulation tissue formation in bandaged full-thickness equine skin wounds. American Journal of Veterinary Research. 2017, 78, 638-646 https://doi.org/10.2460/ajvr.78.5.638. [CrossRef]
- Mandal MD, Mandal S. Honey: Its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011, 154-160. [CrossRef]
- Hendrickson, D. Wound Care Management for the Equine Practitioner. Teton NewMedia, Incorporated. 2005.
- Kwakman P, te Velde A, de Boer L, et. al. (Two major medicinal honeys have different mechanisms of bactericidal activity. Amsterdam. PLoS One. 2011; 6(3): e17709. [CrossRef]
- Lu J, Turnbull L, Burke CM, et. al. Manuka-type honeys can eradicated biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. Australia, 2014. Peer J; 2: e326. [CrossRef]
- Edwards R, Harding K. Bacteria and wound healing. Current Opinion in Infectious Disease. 2004, 17, 91-96. [CrossRef]
- Bhargav HS, Shastri SD, Poorn SP, et. al. Measurement of the Zone of Inhibition of an Antibiotic. 2016 IEEE International Conference on Advanced Computing (IACC). 2016, 27-28. [CrossRef]
- Al-Waili N, Salom K, Al-Ghamdi A, et. al. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. The Scientific World Journal. 2012. [CrossRef]
- 11. Carter DA, Blair SE, Cokcetin NN, et. al. Therapeutic Manuka honey: No longer so alternative. Front Microbiol. 2016, 7, 569. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4837971/. [CrossRef]
- Subrahmanyam M. (1998). A prospective randomized clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. Volume 24, Issue 2. 157-161. https://www.ncbi.nlm.nih.gov/pubmed/9625243. [CrossRef] [PubMed]
- Manuka Health New Zealand. Quality Fact Sheet. 2017. https://www.manukahealth.co.nz/media/1340/022-92-quality-fact-sheet-fa-v2-digital.pdf.
- Junker J, Kamel RA, Caterson EJ et. al. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Care 2013, 348-356. 0412. [CrossRef]
- 15. Visweswara RP, Lakhsmi S, Ramesh N, et. al. (2017). Honey, Propolis, and Royal Jelly: A comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5549483/. [CrossRef]
Note
| 1 |
Covidien Product Insert Data. 710 Medtronic Parkway, Minneapolis, MN 55432-5604 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).