1. Introduction
Steel as a building material has been widely used worldwide[1], but steel is prone to corrosion in many cases, such as atmospheric corrosion, soil corrosion, water environment corrosion, etc[2-4]. Since steel corrosion can cause significant damage to human life and property every year[5], it is necessary to implement effective measures to reduce steel corrosion. Pure Zn coatings have advantages such as corrosion resistance, affordability, and ease of production. For decades, zinc coatings have been widely used to protect steel. These coatings can be applied through electrodeposition or hot dip methods[6, 7]. Compared to the hot dip method, the zinc coating obtained by the electrodeposition method is thinner and has a better surface finish. By selecting appropriate electrodeposition parameters, the surface of the Zn coating can be prepared to have no pores, resulting in higher corrosion resistance and improved mechanical properties[8, 9]. However, the corrosion resistance of pure zinc coatings is limited under conditions such as high temperatures and acidic and alkaline environments. Therefore, in order to enhance its performance, it is necessary to modify the Zn coating.
Numerous researchers have discovered that composite coatings can act as viable substitutes for zinc coatings. At present, composite coatings have been widely studied. Introducing ceramic materials gives coatings higher hardness, high temperature, and corrosion resistance, such as Zn-ZrO2, Ni-W-SiC, and Zn-Al2O3[10-12]. However, when the introduced materials are reduced to the nanoscale, they exhibit entirely different characteristics. Nanoparticles located at grain boundaries can hinder dislocation slip and recrystallization in crystals, thereby improving the performance of composite coatings[13]. Therefore, nanocomposite coatings have better application prospects. Adding nanocomposite materials to traditional coatings can enhance the corrosion resistance, thermal stability, wear resistance, lubricity, and biocompatibility of the coatings[14-18].
Alumina nanoparticles are gaining growing interest because of their high hardness, stability at high temperatures, and exceptional corrosion resistance. Compared with the pure Zn coating, incorporating nano-Al2O3 particles improves the corrosion resistance of the composite coating[19]. Abdulwahab et al.[20] placed Zn-Ni and Zn-Ni-Al2O3, obtained by electrodeposition, in a 200°C oven for heat treatment. The hardness value of the Zn-Ni-Al2O3 composite coating increased by 2.89%. The hardness of the Zn-Ni alloy plating layer decreased by 26.67%. The results show that the nanometer-sized Al2O3 particles can enhance thermal stability and resistance to high-temperature oxidation. Blejan et al.[18] found that the addition of nano-alumina to Zn-Ni significantly improved the corrosion resistance of the composite coating.
In recent years, rare earth materials have gradually been used in new materials and in transforming traditional industries[21]. Rare earth yttrium oxide nanoparticles are widely used in industries such as catalysts, biomedical, electronic devices, high-temperature superconductors, and surface modification due to their excellent corrosion resistance and high-temperature oxidation resistance.[22]. Zhang et al.[23] added Y2O3 to the Zn matrix through filler bonding. The results show that the addition of Y2O3 improves the corrosion resistance of the alloy. When the Y2O3 content is increased to 4wt%, the thickest coating shows the highest level of corrosion resistance. Li et al.[24] used the pulsed electrodeposition method to prepare a nanometer composite plating of Ni-W/TiN-Y2O3. The studies indicate that nanoparticles of TiN/Y2O3 are dispersed in the coating. Nanocrystalline particles with a particle size ranging from 14 to 17 nm can be obtained, significantly enhancing the corrosion and wear resistance of the coating.
There are few reports on the study of incorporating nano Y2O3 into the zinc matrix to enhance the performance of the coating. Meanwhile, the preparation of composite coatings by incorporating nanoscale Y2O3 and Al2O3 particles into a Zn matrix has not yet been published. In this study, we prepared Zn-Y2O3-Al2O3 nanocomposite coatings on Q235 steel plates using the co-deposition method. Throughout the entire experimental process, the concentrations of nano Y2O3 and Al2O3 particles were used as independent variables to investigate the effects of these concentrations on the morphology, corrosion behavior, and mechanical properties of Zn-Y2O3- Al2O3 nanocomposite coatings.
2. Materials and Methods
2.1. Preparation of electrolyte solution
The composition of the electroplating bath is listed in
Table 1. All agents are of analytical purity levels. Add HCl or NaOH to the plating solution to decrease its pH to 3.5. Add Al
2O
3 (50 nm) and Y
2O
3 (20 nm) particles to the plating solution and use a magnetic agitator to stir at 1000 rpm for 18 hours to ensure the uniform dispersion of the nanopowder.
2.2. Pretreatment of substrate
In this work, the anode is a 15×10×1 mm3 zinc sheet (purity 99.999 %), and the substrate (cathode) is Q235 carbon steel, the size of 10×10×1 mm3. Since the purchased carbon steel surface has scratches, rusting, and other defects, it is necessary to preserve its surface. Use 400, 800, 1200, 1600, 2000, and 3000 grit emery papers to grind the surface of carbon steel, remove surface impurities and oxidation layer, then polish with w3.5 abrasive paste; after achieving a flawless surface, place the samples in the drier. In order to remove carbon steel surface grease, place it in ethanol ultrasonic cleaning 15min. The washed samples are soaked for 15 seconds in a 1 M HNO3 solution. This activation step aims to eliminate the oxidation layer formed on the surface of the carbon steel and enhance the coating quality. After activation, rinse the substrate with deionized water and then leave it to air dry.
2.3. Preparation of composite coatings
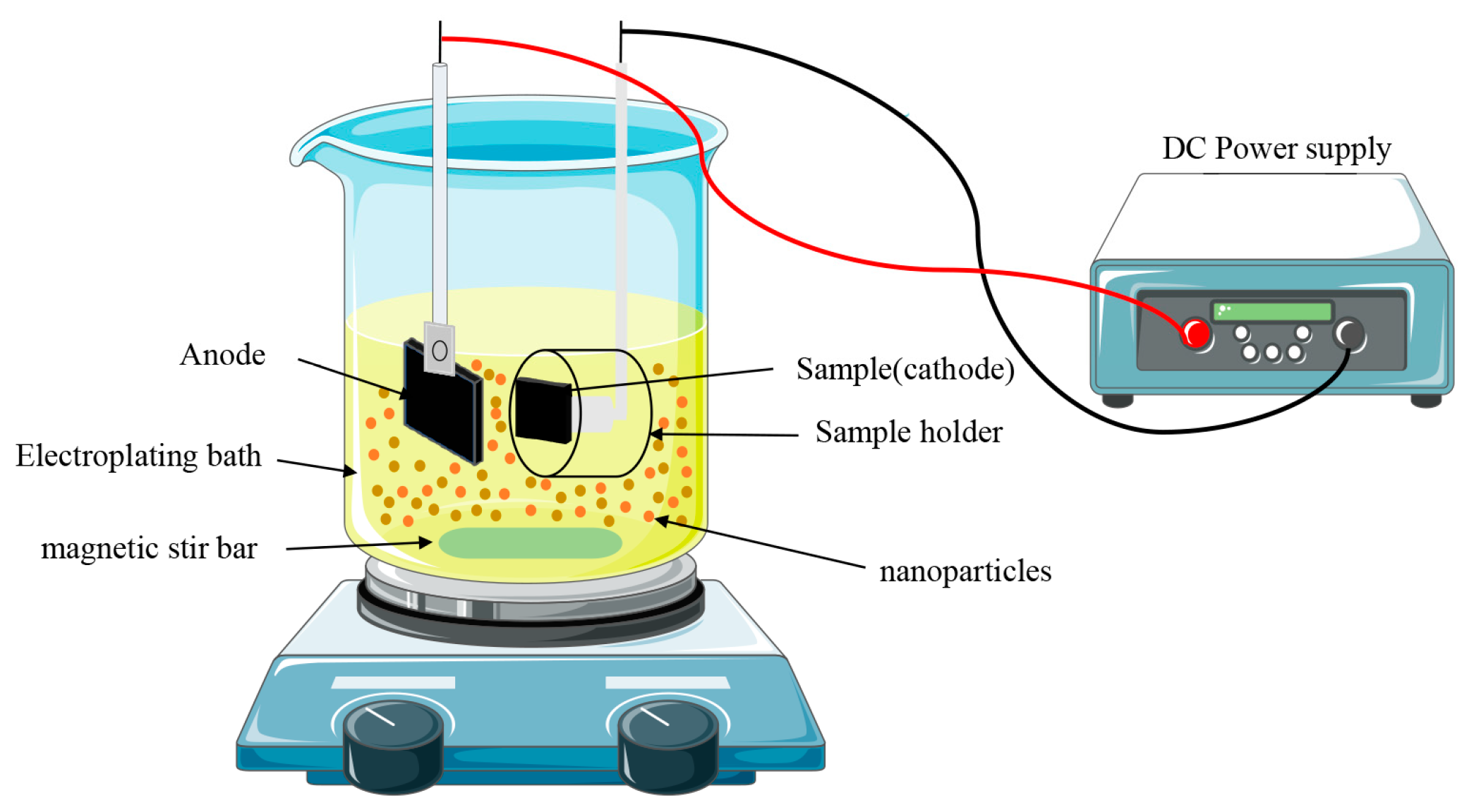
Figure 1 shows a schematic diagram of the experimental setup for electrodeposition. In this experiment, the anode used epoxy resin to embed the five sides of the carbon steel sheet, leaving only a 1 cm
2 area exposed, and it was connected with a copper wire. The electrodeposition experiment co-deposited one h with 30 mA/cm
2 current density at 35°C. The electroplating solution was stirred constantly with a magnetic agitator at a rate of 550 rpm during the experiment to keep the nanoparticles suspended in the solution at all times. Once the experiment is complete, flush the sample with deionized water for 10 seconds and then allow it to dry. The content of Y
2O
3 and Al
2O
3 in the plating solution determines the composition of the deposited Zn-Y
2O
3-Al
2O
3 composite coating. The coating description is shown in
Table 2.
2.4. Surface characterization
The surface morphology of the composite coating was analyzed using environmental scanning electron microscopy (ESEM, USA). An X-ray diffractometer (XRD, DMAX, Riken Instruments Japan) operated with Cu-Kα radiation was used to analyze the phase structure of the composite coatings. The scanning rate was set at 5°/min, and the scanning range was 10° < θ < 90°.
2.5. Electrochemical measurement
Using an electrochemical workstation (Admiral, USA), electrochemical impedance spectroscopy (EIS) and linear sweep voltammetry (LSV) tests were conducted in a three-electrode system to evaluate the corrosion behavior of composite coatings. The three-electrode system consists of a composite coating as the working electrode, Ag/AgCl as the reference electrode, and a platinum plate electrode with a working area of 1 cm2. Before conducting the testing study, the coating samples were immersed in a 3.5wt% NaCl solution for a specific period to obtain a stable open circuit potential (OCP). The linear sweep voltammetry test was conducted in a 3.5wt% NaCl electrolyte, with a potential range of -1.5 V~1.5 V and a scanning rate of 10 mV/s. The measurement of EIS ranges from 0.1 Hz to 100000 Hz, with a disturbance voltage of 10 mV.
2.6. Microhardness test
Use a microhardness tester (VH1102, China) to test the hardness of composite coatings. The applied load is 50 g, and the loading time is 10 seconds. Select 10 points on the surface of each composite coating for hardness testing and take the average value.
2.7. Contact angle text
The static contact angle of the coating is measured using a contact angle detector (JY-PHb, China). At 25°C, the contact angle is measured on the surface of the coating by using three μL of deionized water droplets. The surface coating is tested at ten different locations to minimize experimental errors, and the average value is calculated as the final data.
3. Results and discussion
3.1. Deposition mechanism of nanocomposite coatings
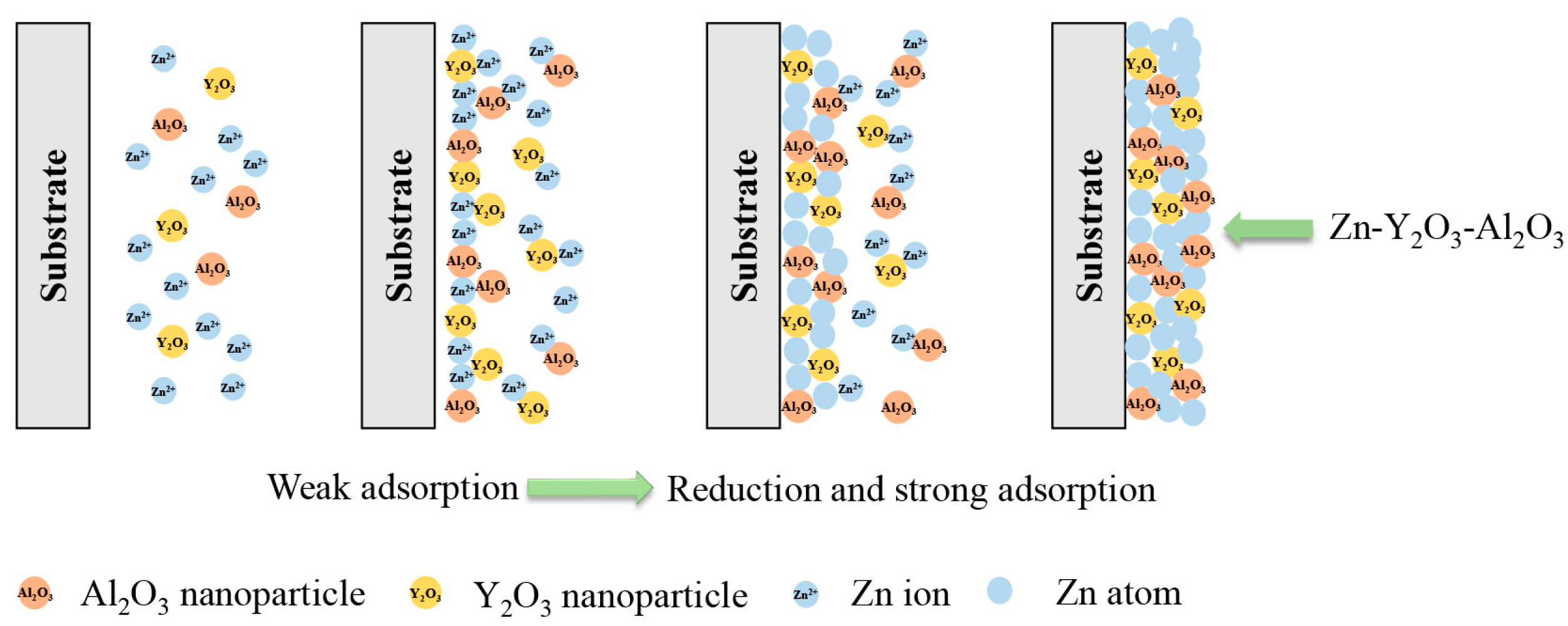
The formation mechanism of nanocomposite coatings is shown in
Figure 2. When a stable voltage is applied to both ends of the electrode, Zn
2+ in the solution migrates towards the cathode. At the cathode, electrons are gained, and an oxidation-reduction reaction occurs, forming Zn nuclei on the cathode's surface. At the same time, H
+ in the solution combines with electrons to form H
2, while OH
- near the anode (Zn sheet) loses electrons and produces O
2. During this process, the nanoparticles dispersed in the plating solution adsorb with Zn
2+ and migrate towards the cathode surface. Upon reaching the cathode, they adsorb onto the metal surface to form a diffusion layer. When Zn
2+ undergoes oxidation-reduction to form a Zn coating, the nanoparticles adsorbed by Zn
2+ are embedded into the Zn coating, ultimately forming a nanocomposite coating. The reaction equation is as follows[25]:
3.2. X-ray diffraction (XRD)
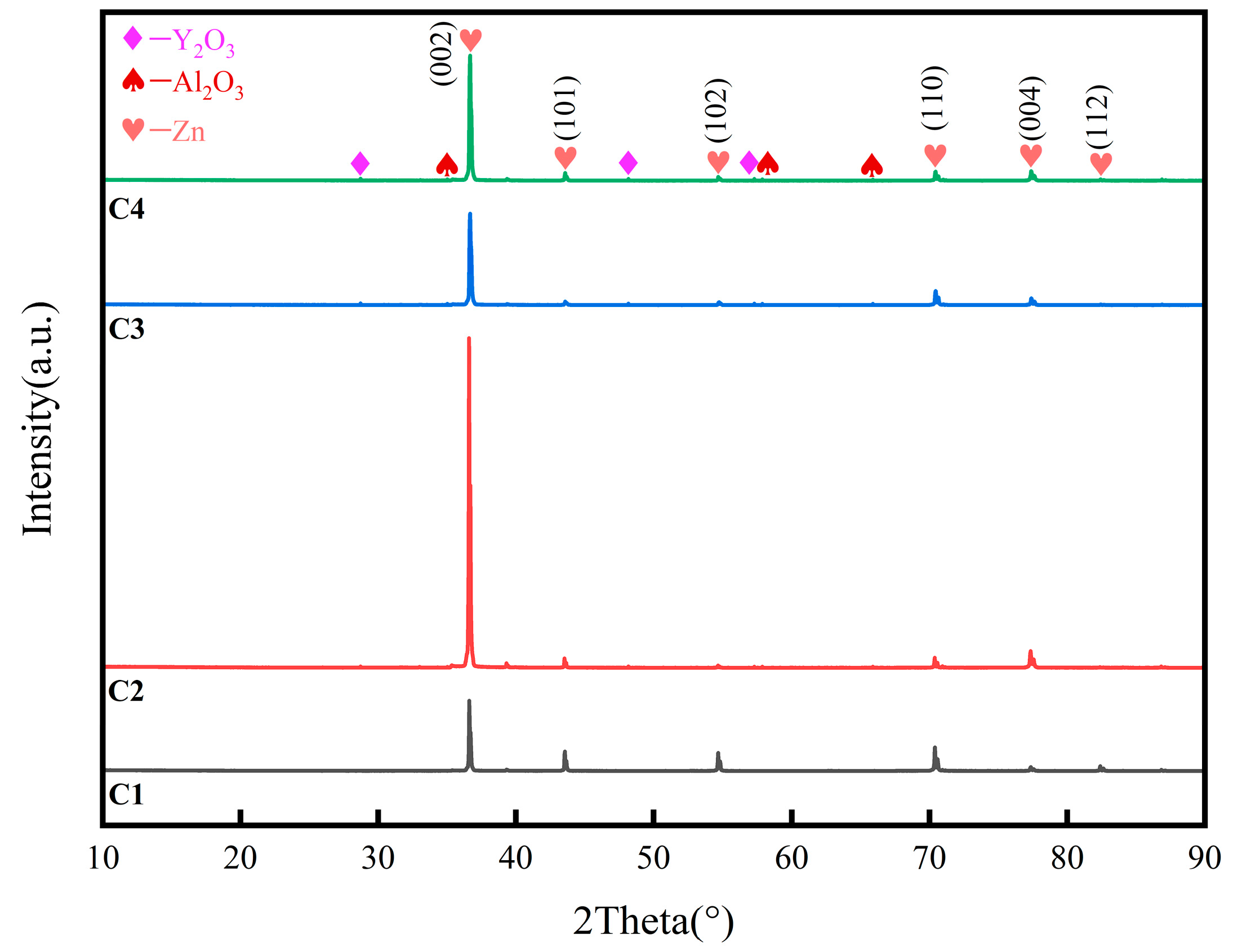
Figure 3 presents an X-ray diffraction (XRD) pattern of the Zn coating and the Zn-Y
2O
3- Al
2O
3 coating with varying nanoparticle concentrations. Most of the diffraction lines in the X-ray diffraction pattern correspond to the hexagonal structure of Zn[11]. The Zn-Y
2O
3-Al
2O
3 nanocomposite coating has a structure similar to zinc coating, but the preferential orientation of Zn has changed. The diffraction diagram shows six distinct peaks at 36.6°, 43.5°, 54.6°, 70.3°, 77.3°, and 82.4° on standard PDF cards, corresponding to the Zn phase (002), (101), (102), (110), (004), and (112) planes. As depicted in the graph, the addition of Y
2O
3 and Al
2O
3 nanoparticles resulted in a significant increase in the (002) crystal diffraction peaks. The intensity of most of the remaining diffraction peaks weakened, indicating that the introduction of nanoparticles further enhanced the deposition orientation of the (002) crystal surface. Low-intensity Al
2O
3 peaks were observed in all Zn-Y
2O
3-Al
2O
3 coated XRD profiles. at 35.02 °, 57.86 ° and 65.89 ° respectively. This proves the successful co-deposition of Al
2O
3 nanoparticles with zinc matrix. At the same time, low-intensity peaks of Zn-Y
2O
3-Al
2O
3 were observed in all Y
2O
3-coated XRD profiles. In 28.70°, 48.19°, and 57.29°, respectively, it was also proven that Y
2O
3 nanoparticles were successfully added to the zinc matrix. In addition, the peak width of the Zn-Y
2O
3-Al
2O
3 nanocomposite coating is slightly wider than that of the Zn coating. This is because the nanoparticles inhibit the growth of the crystals, resulting in a refinement of the crystal size[10].
3.3. Surface morphology of composite coatings
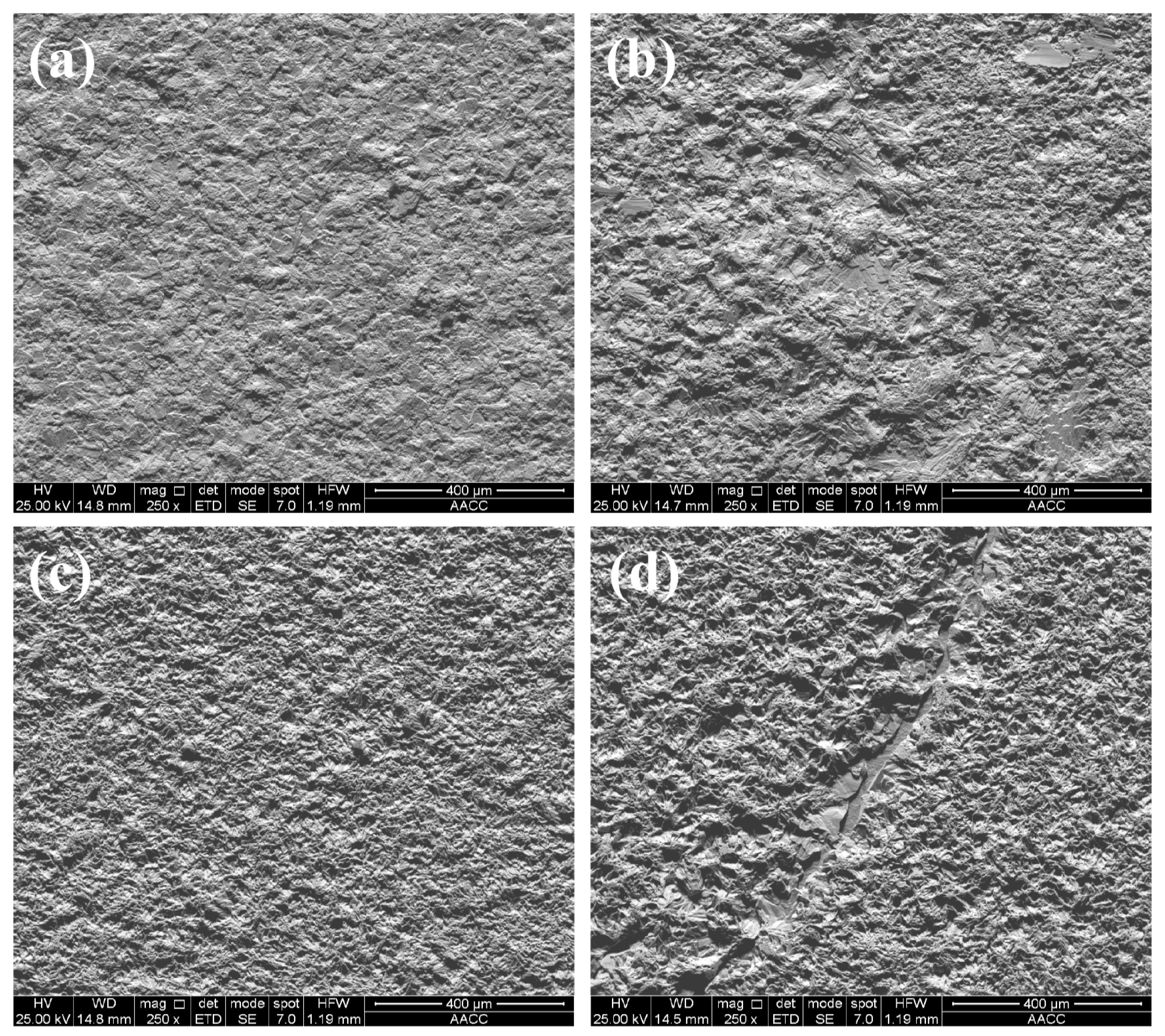
Figure 4 shows the surface shape of the Zn-Y
2O
3-Al
2O
3 coating obtained by adding different concentrations of nanometer Y
2O
3 and Al
2O
3 particles to the electrolyte. As can be seen from the diagram, the surface of the pure zinc coating exhibits a flake crystal structure, which is observed to be unevenly distributed. The microstructure of the C3 coating is significantly better than that of the pure C1, C2, and C4 coatings, mainly in its dense organization and small grain size. The microstructure refinement of the composite coatings is mainly due to the introduction of nanoparticles, which are successfully and uniformly dispersed and encapsulated in the Zn matrix. The nanoparticles bound to the Zn matrix increase the number of nucleation sites, impede the growth of the crystals, and lead to the formation of small-sized grains. The surface of the C2 coating exhibits a partially dense microstructure and smaller grains. This can be attributed to the low concentration of incorporated nanoparticles, which reduces nucleation sites on the coating surface[15]. The surface of the C4 coating is rough, the grain size is large, and there are cracks and holes. This may be due to an excessive concentration of added nanoparticles. This results in the agglomeration of nanoparticles and increased stress within the composite coating[26]. Reduced grain size and a dense microstructure are always ideal for composite coatings with high hardness and excellent corrosion resistance[27].
3.4. Effect of particle loading on corrosion resistance
Open circuit potential refers to the potential difference between the soluble metal electrode and the electrolyte due to the spontaneous redox reaction that occurs when the electrode is immersed in the electrolyte. This potential difference is referred to as the open circuit potential. The system is in a dynamic equilibrium at an open circuit potential. This means that the redox reaction occurring on the electrode and the reaction in the electrolyte are in equilibrium, and the electrode does not undergo significant corrosion.
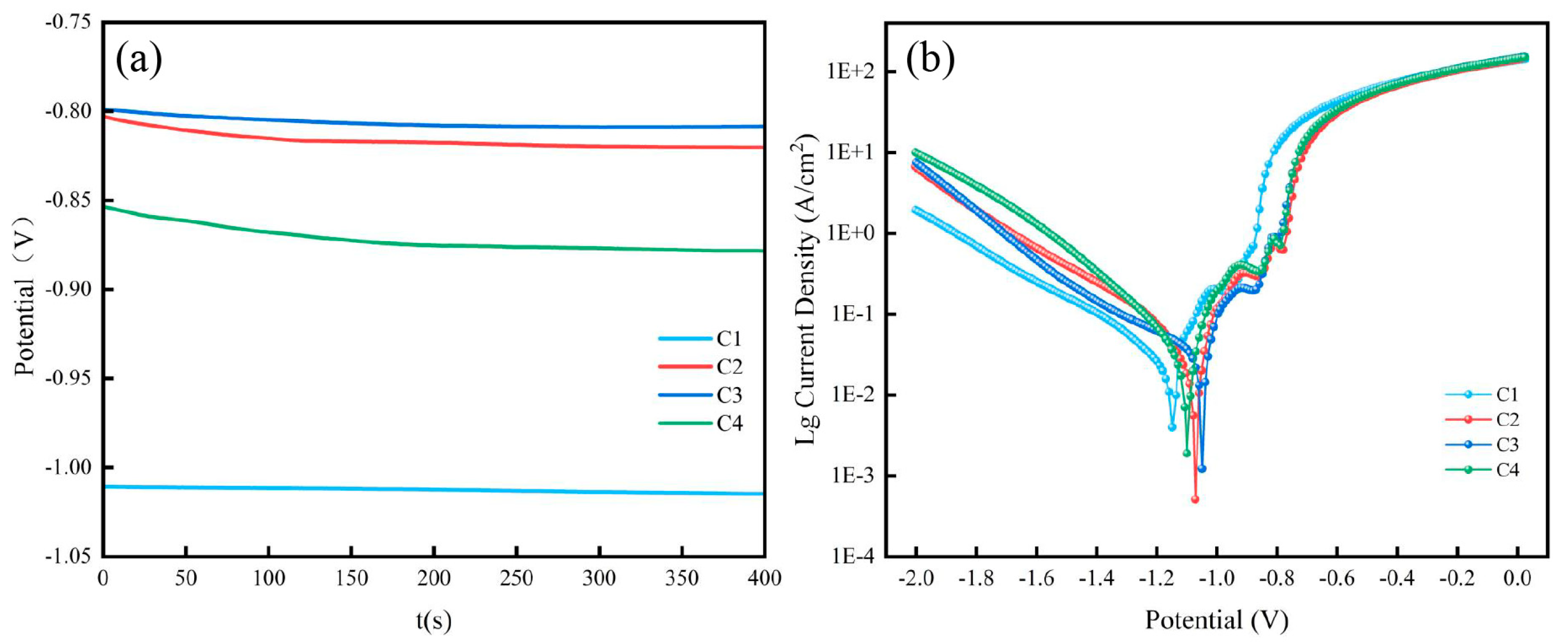
Figure 5a illustrates the change in the open circuit potential of pure Zn and Zn-Y
2O
3-Al
2O
3 composite coatings over time in a 3.5wt% NaCl solution. As shown in the figure, compared with pure Zn coating, the composite coating exhibits a higher potential. This suggests that the composite coating performs better because it suppresses the anodic reaction and enhances the composite coating's anti-corrosion properties.
The corrosion behavior of various composite coatings was studied using linear scanning voltammetry.
Figure 5b shows the polarization curve of the Zn-Y
2O
3-Al
2O
3 composite coating in a 3.5wt% NaCl solution at various concentrations of Y
2O
3 and Al
2O
3 nanoparticles. The self-corrosion current (I
coor) and corrosion voltage (E
coor) fitted according to Tafel extrapolation are listed in
Table 3. In general, the higher the positive corrosion potential of the coating, the lower the self-corrosion current density, indicating that the coating has a lower tendency to corrode[28]. As shown in
Table 3, the corrosion potential of composite coatings is higher than that of pure zinc coatings (E
coor=1.18 V). This demonstrates how effectively increasing the Zn coating's resistance to corrosion may be achieved by using nanoscale Y
2O
3 and Al
2O
3 particles. A plating solution containing 10 g/L of nano-Y
2O
3 and Al
2O
3 particles raises the composite coating's corrosion potential to 1.02 V. Meanwhile, the self-corrosion current density of the Zn-Y
2O
3-Al
2O
3 nanocomposite coating (I
coor=6.15×10
-6) is significantly smaller than that of other coatings. From the perspective of corrosion kinetics, the corrosion rate of nanocomposite coatings is slower than that of pure Zn coatings.
Figure 5b illustrates that the current density of the Zn coating reduces as the reaction progresses in the cathode polarization region, covering the range from -2.0 V to -1.18 V. This is because electrons are attracted from the solution to the electrode surface and react with oxygen in the following manner:
When located in the anode polarization region from -1.18 V to 0.7 V, the current density of the Zn coating increases as the reaction progresses. This occurs when the coating begins to react with the electrolyte, primarily through the following reactions:
However, as the reaction progresses, the change in current density stabilizes when the scanning potential increases above 0.7 V. The stability exhibited can be attributed to the creation of an oxide-based passivation film on the surface of the coating, which impedes the rate of coating dissolution[29]. The formation of this film primarily occurs as follows:
In addition, particle agglomeration reduces its usability, resulting in reduced co-deposition efficiency and the formation of low-quality coatings[30]. When the concentration of these nanoparticles in the electroplating solution increased from 0 g/L to 15 g/L, the corrosion current density of the composite coating followed a pattern of initially decreasing and then increasing. When the nanoparticles are incorporated at a concentration of 10 g/L, the nanocomposite coatings exhibit minimal corrosion current density, indicating optimal corrosion resistance. The optimal particle load for achieving this resistance is 10 g/L of nanometer Y2O3 and 10 g/L of nanometer Al2O3. The particles in the electroplating solution are dispersed at the optimum level at this concentration. By forming these nanoparticles, an inert physical barrier is established on the coating’s surface, which reduces the effective contact area between the corrosive medium and the substrate metal, improving the coating’s corrosion resistance[13].
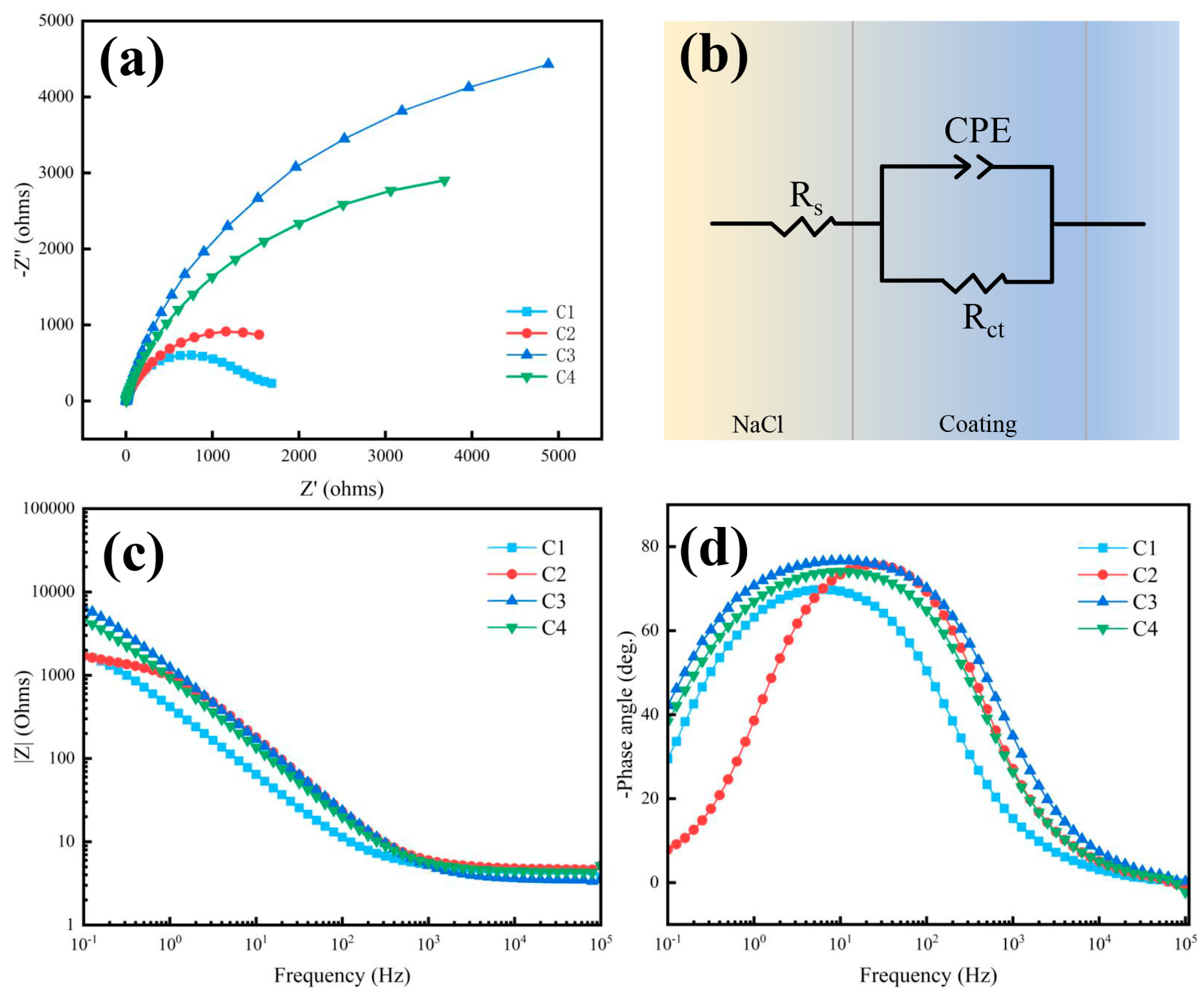
Electrochemical impedance spectroscopy is a powerful electrochemical auxiliary technique commonly used to evaluate the corrosion resistance of the prepared coating surface[31]. AC impedance testing was conducted on the prepared composite coating to investigate its corrosion resistance further in a 3.5wt% NaCl solution.
Figure 6a shows the Nyquist plots of different composite coatings obtained through EIS testing. It can be seen from the figure that a single capacitive ring with a circular arc shape is displayed from high frequency to low frequency. A relationship exists between the radius of the capacitive impedance arc and the charge transfer resistance, where a larger arc radius signifies a higher charge transfer resistance value and improved corrosion resistance performance of the coating.[32]. The impedance arc radius of the composite coating exhibits a trend of initially increasing and then decreasing as the doping concentrations of nano Y
2O
3 and Al
2O
3 are raised. The composite coating exhibits the largest impedance arc and superior corrosion resistance at a doping concentration of 10g/L for both nano Y2O3 and Al2O3. This finding aligns with the polarization curve test results of the composite coating. Using the Randles circuit model for fitting to explain the EIS results further, the equivalent circuit is shown in
Figure 6b, characterized by only one time constant[33]. Within this equivalent circuit, R
s denotes the resistance of the electrolyte. CPE is a constant phase angle element, and a lower CPE value signifies a higher surface quality of the composite coating[34]. R
ct stands for charge transfer resistance, which is associated with the corrosion rate and serves as a crucial parameter reflecting the corrosion resistance of composite coatings. A higher R
ct value indicates superior corrosion resistance of the composite coating. The fitting data for the coatings are presented in
Table 4, revealing that the maximum impedance modulus of all composite coatings exceeds that of pure Zn coatings (1385 Ω cm
2). The impedance modulus reaches a remarkable value of 10257 Ω cm
2 for the C3 coating, indicating its exceptional corrosion resistance.
Figure 6c shows the bode modulus diagram, and | Z | represents the impedance value of the composite coating. The impedance modulus in the low-frequency zone is frequently used to assess the corrosion resistance of the coating. Because the formation of a protective barrier, such as the corrosion product film, at the steel/coating interface, which effectively mitigates corrosion[35-37]. When the doping concentrations of nano Y
2O
3 and Al
2O
3 are both 10 g/L, the highest impedance value in
Figure 6c is 6592.84 Ω cm
2, indicating that the C3 coating has better corrosion resistance. In addition, at higher frequencies, a larger phase angle indicates the presence of a more stable dielectric film, and the stability of the coating is also better[38]. The maximum phase angle of C3 coating indicates that it has better stability and better barrier performance.
3.5. Microhardness test
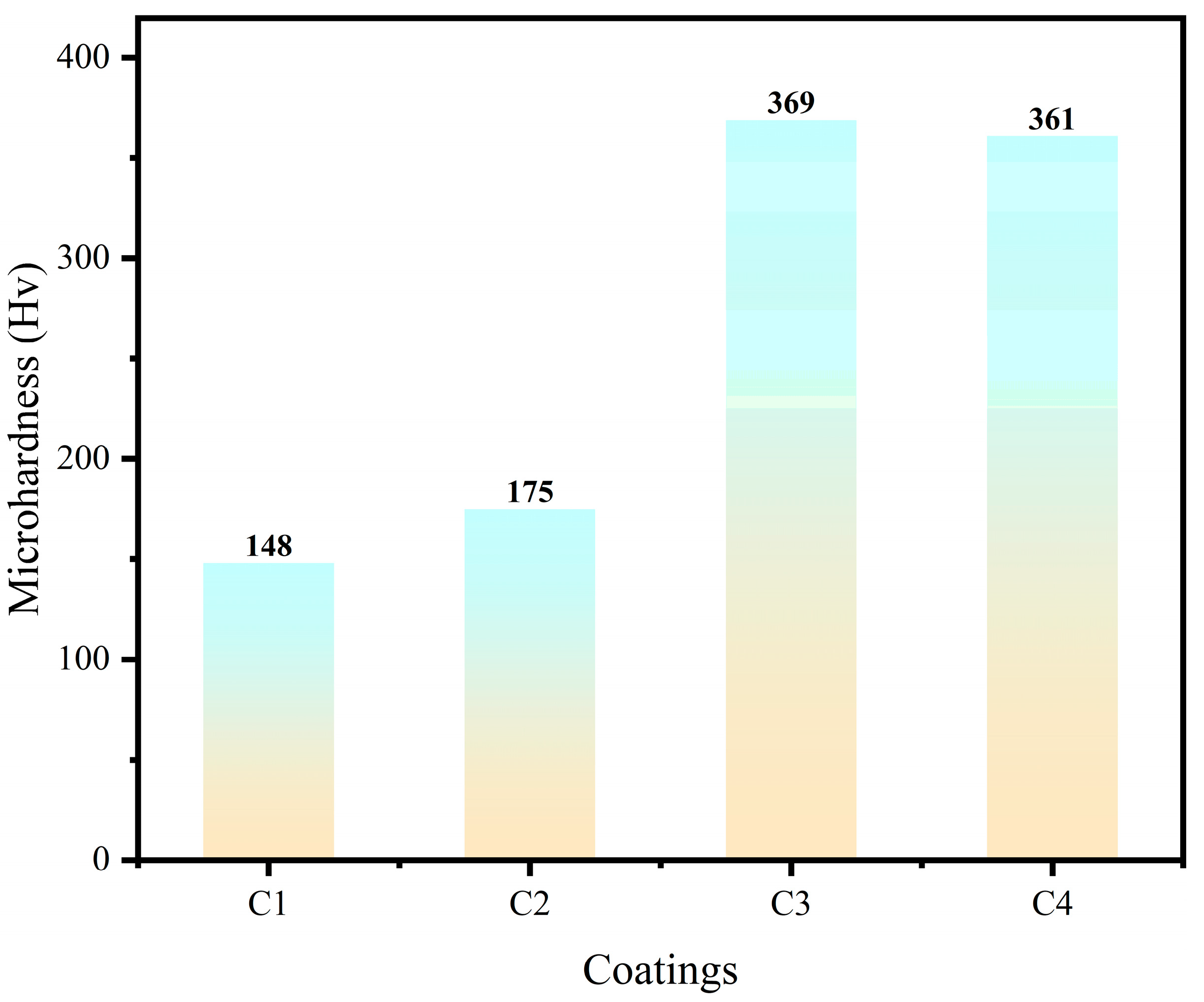
In practice, the coating not only requires excellent corrosion resistance but also excellent mechanical properties. Therefore, the mechanical properties of the Zn and Zn-Y2O3-Al2O3 coatings are evaluated by microhardness testing. To mitigate experimental error, ten points are chosen randomly from each sample for hardness testing. The average value of these points is considered the sample’s hardness result.
The hardness test results for the Zn and Zn-Y
2O
3-Al
2O
3 coatings are shown in
Figure 7. Based on the graph, it is observable that the composite coating exhibits higher microhardness than the pure Zn coating. As the content of Y
2O
3 and Al
2O
3 nanoparticles increases in the plating solution, the hardness of the composite coating initially rises and then declines. The pure zinc coating has a hardness value of 148 Hv. However, when 10 g/L of nanoscale Y
2O
3 and Al
2O
3 particles are added, the hardness of the composite coating can reach 369 Hv, indicating a 2.5-fold increase compared to the pure Zn coating. The inclusion of nanoparticles significantly enhances the microhardness of the composite coating[39]. On the one hand, this is due to the excellent mechanical properties of nano Y
2O
3 and Al
2O
3 particles, which are uniformly dispersed into the Zn matrix, playing a dispersion-strengthening role. On the other hand, nanoparticles bound to the Zn matrix increase the number of nucleation sites. In addition, it hinders crystal growth, forming small grain sizes and promoting a more significant number of grain boundaries[18, 40]. The coating surface is dense, and this grain refinement helps to increase the hardness value of the coating[41]. At a concentration of 15 g/L of Y
2O
3 and Al
2O
3 nanoparticles in the plating solution, the hardness of the coating decreases. This is due to the pronounced agglomeration behavior of the nanoparticles, leading to the formation of uneven areas, such as pores and cracks, on the surface of the composite coating. As a result, the dispersion-strengthening effect of nanoparticles within the coating diminishes, causing a reduction in the hardness value of the composite coating.
3.6. Contact angle text
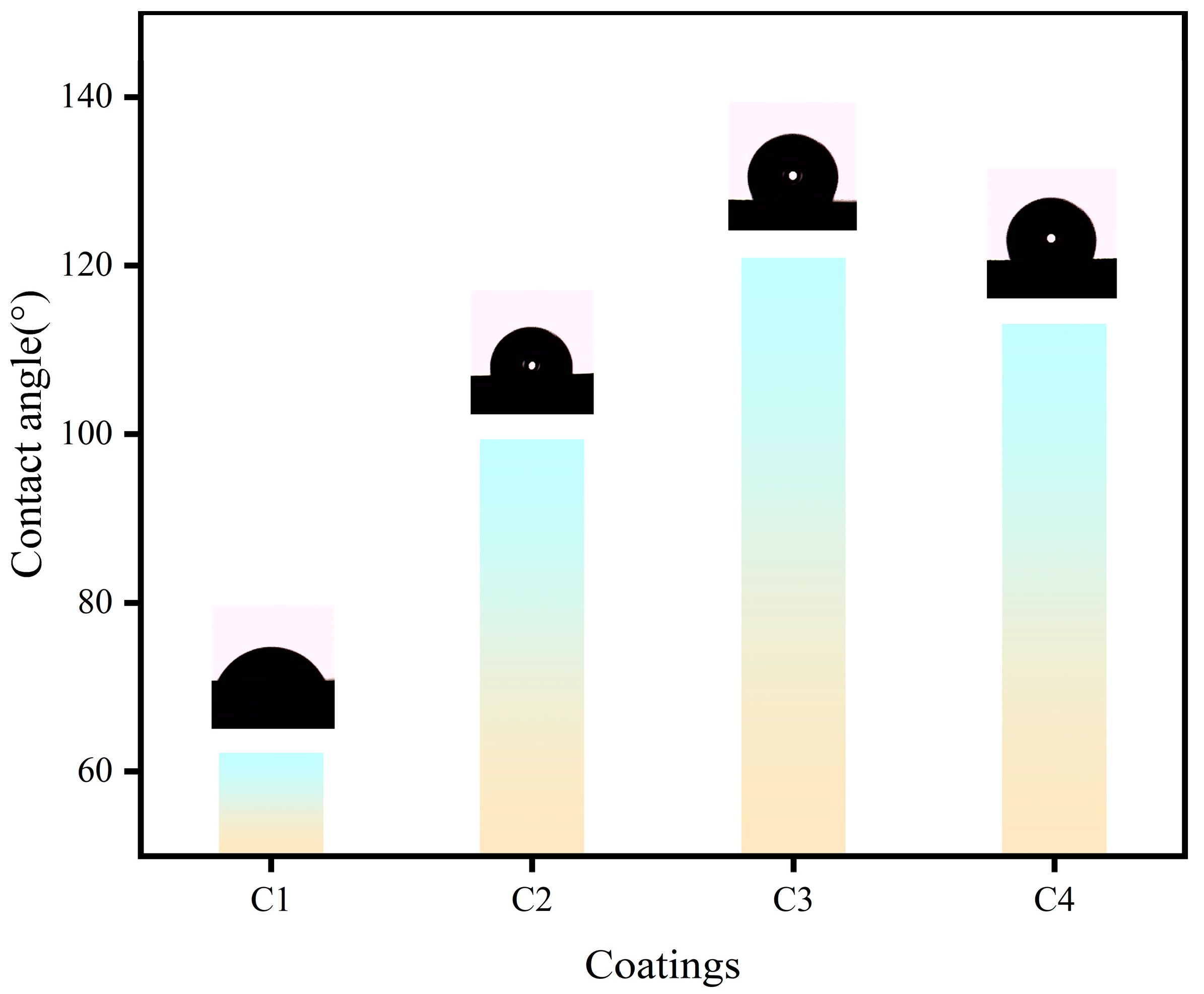
Variations in the surface morphology of Zn coatings can lead to differences in hydrophobic properties[42].
Figure 8 presents the static contact angle (CA) of the Zn-Y
2O
3-Al
2O
3 composite coating in deionized water at various doping concentrations of nano-Y
2O
3 and Al
2O
3 particles. The figure illustrates that the CA of the pure zinc coating is 62.2°, while the CA of the composite coating surpasses that of the pure Zn coating. As the doping concentration of nanometer Y
2O
3 and Al
2O
3 particles increases, the CA of the composite coating initially exhibits an increment, followed by a decrement. In addition, the composite coating obtained when the additions of nanometer Y
2O
3 and Al
2O
3 particles in the plating solution were 10 g/L, respectively, had a maximum CA of about 120.9°, indicating excellent hydrophobic properties. The surface hydrophobicity test results for the composite coating align with the findings of the corrosion resistance assessment. This occurs because the non-uniform nanostructure present on the composite coating surface traps a significant volume of air within the material, leading to a considerable reduction in the contact area and contact duration between the coating surface and the corrosive medium. When the coating is submerged in a corrosive medium, the gas trapped on the coating’s surface creates a protective gas film between the corrosive medium and the metal surface, thereby enhancing the corrosion resistance of the hydrophobic coating within the corrosive environment[43].
4. Conclusions
The Zn-Y2O3-Al2O3 nanocomposite coating was successfully prepared on a Q235 steel plate using electrodeposition technology. This was done by adding nanoparticles to the plating solution. The research compared the impact of nanoparticles on the surface morphology, micro-hardness, hydrophobicity, corrosion, and resistance of composite materials. Findings suggest that the incorporation of nanoparticles augments the number of nucleation sites on the coating surface, impedes crystal growth, accomplishes grain refinement, and diminishes surface roughness. Due to grain refinement and dispersion strengthening, the hardness of Zn-Y2O3-Al2O3 nanocomposite coatings is significantly improved. According to research, the composite coating demonstrates favorable hydrophobicity and exceptional corrosion resistance at a nanoparticle dosage of 10 g/L. This can be attributed to the formation of an inert physical barrier on the coating surface by the nanoparticles. This barrier significantly reduces the contact time and surface area between the coating and the corrosive medium, effectively isolating the corrosive medium from the Q235 steel plate.
Author Contributions
Conceptualization, X.F.; methodology, X.F., F.Q., S.M.C. and C.D.; validation, F.Q.; formal analysis, F.Q.; investigation, X.F., F.Q., S.M.C. and C.D.; resources, X.F.; data curation, X.F. and F.Q.; writing–original draft, F.Q.; writing–review and editing, X.F. and S.M.C.; visualization, F.Q.; supervision, X.F.; project administration, X.F. and C.D.; funding acquisition, X.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2021YFC2902100) and the Postgraduate Research & Practice Innovation Program of Jiangsu province (Grant No. KYCX23_2810) and the Graduate Innovation Program of China University of Mining and Technology (Grant No. 2023WLJCRCZL039).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviation
The following abbreviations are used in this manuscript:
| LSV |
Linear Sweep Voltammetry |
| EIS |
Electrochemical Impedance Spectroscopy |
| OCP |
Open Circuit Potential |
| CA |
Contact Angle |
References
- Zou, Y.; Wang, J.; Zheng, Y. Y. Zou, Y.; Wang, J.; Zheng, Y. Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, (1), 208-216. [CrossRef]
- Lin, C. C.; Wang, C. X. Correlation between accelerated corrosion tests and atmospheric corrosion tests on steel. J. Appl. Electrochem. 2005, 35, (9), 837-843. [CrossRef]
- Choi, Y. S.; Nesic, S.; Young, D. Effect of impurities on the corrosion behavior of CO2 transmission pipeline steel in supercritical CO2-water environments. Environ. Sci. Technol. 2010, 44, (23), 9233-9238. [CrossRef]
- Wang, S.; Du, C.; Li, X.; Liu, Z.; Zhu, M.; Zhang, D. Field corrosion characterization of soil corrosion of X70 pipeline steel in a red clay soil. Prog. Nat. Sci.: Mater. Int. 2015, 25, (3), 242-250. [CrossRef]
- Chen, B.; Wu, Q.; Li, J.; Lin, K.; Chen, D.; Zhou, C.; Wu, T.; Luo, X.; Liu, Y. A novel and green method to synthesize a epoxidized biomass eucommia gum as the nanofiller in the epoxy composite coating with excellent anticorrosive performance. Chem. Eng. J. 2020, 379. [Google Scholar] [CrossRef]
- Basavanna, S.; Naik, Y. A. Electrochemical studies of Zn-Ni alloy coatings from acid chloride bath. J. Appl. Electrochem. 2009, 39, (10), 1975-1982. [CrossRef]
- Popoola, A. P. I.; Fayomi, O. S. Performance evaluation of zinc deposited mild steel in chloride medium. Int. J. Electrochem. Sci. 2011, 6, (8), 3254-3263. [CrossRef]
- Elsherief, A. E.; Shoeib, M. A. Characterization of electro-deposited Zn-Ni alloy from an all-chloride solution. Corros. Prev. Control 2003, 50, (1), 25-34.
- Amuda, M. O. H.; Subair, O. W.; Obitayo, O. W. Study of optimum conditions for zinc plating on mild steel. Int. J. Eng. Res. Afr. 2010, 2, 31-39. [CrossRef]
- Vathsala, K.; Venkatesha, T. V. Zn-ZrO2 nanocomposite coatings: Elecrodeposition and evaluation of corrosion resistance. Appl. Surf. Sci. 2011, 257, (21), 8929-8936. [CrossRef]
- Malatji, N.; Popoola, A. P. I.; Fayomi, O. S. I.; Loto, C. A. Multifaceted incorporation of Zn-Al2O3/Cr2O3/SiO2 nanocomposite coatings: anti-corrosion, tribological, and thermal stability. Int. J. Adv. Manuf. Technol. 2016, 82, (5-8), 1335-1341. [CrossRef]
- Zhang, P.; Zhao, Y.; Huang, J.; Li, J.; Cao, L.; Liu, J.; Han, G.; Du, W.; Chen, L.; Xiao, L.; Wang, Q.; Yang, Y.; Zhu, S.; Li, W. Enhanced mechanical and wear properties of Ni-W-SiC composite coatings by synergistic influence of micro-nano SiC mixture. Surf. Coat. Technol. 2023, 467. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Wang, S.; Cao, Y.; Fang, A.; Wang, Q.; Gong, J.; Dai, Y. Fabrication of Pb-Co-ZrO2 nanocomposite coatings and correlation of corrosion mechanisms with electronic work functions. Mater. Today Commun. 2023, 37. [Google Scholar] [CrossRef]
- Fustes, J.; Gomes, A.; da Silva Pereira, M. I. Electrodeposition of Zn-TiO2 nanocomposite films-effect of bath composition. J. Solid State Electrochem. 2008, 12, (11), 1435-1443. [CrossRef]
- Praveen, B. M.; Venkatesha, T. V. Electrodeposition and properties of Zn-nanosized TiO2 composite coatings. Appl. Surf. Sci. 2008, 254, (8), 2418-2424. [CrossRef]
- Zheng, H.-Y.; An, M.-Z. Electrodeposition of Zn-Ni-Al2O3 nanocomposite coatings under ultrasound conditions. J. Alloys Compd. 2008, 459, (1-2), 548-552. [CrossRef]
- Ranganatha, S.; Venkatesha, T. V.; Vathsala, K.; Kumar, M. K. P. Electrochemical studies on Zn/nano-CeO2 electrodeposited composite coatings. Surf. Coat. Technol. 2012, 208, 64–72. [Google Scholar] [CrossRef]
- Blejan, D.; Muresan, L. M. Corrosion behavior of Zn-Ni-Al2O3 nanocomposite coatings obtained by electrodeposition from alkaline electrolytes. Mater. Corros. 2013, 64, (5), 433-438. [CrossRef]
- Malatji, N.; Popoola, A. P. I.; Fayomi, O. S. I. The effect of nanoparticulate loading on the fabrication and characterization of multi-doped Zn-Al2O3-Cr2O3 hybrid coatings on mild steel. Int. J. Adv. Manuf. Technol. 2017, 90, (9-12), 2443-2452. [CrossRef]
- Abdulwahab, M.; Fayomi, O. S. I.; Popoola, A. P. I.; Dodo, M. R. In-situ hybrid study of thermal behaviour of Zn-Ni and Zn-Ni-Al2O3 nanocrystallite thin films induced TEA/MEA by electrocodeposition. Results Phys. 2017, 7, 213–215. [Google Scholar] [CrossRef]
- Harvey, T. G. Cerium-based conversion coatings on aluminium alloys: A process review. Corros.Eng.Sci.Technol. 2013, 48, (4), 248-269. [CrossRef]
- Xing, S.; Zhu, W.; You, S.; Yu, W.; Jiang, C.; Ji, V. Investigation on microstructure and tribological performances of electrodeposited Ni-W-Y2O3 composite coatings. J. Alloys Compd. 2023, 965. [Google Scholar] [CrossRef]
- Zhang, Y. J.; Wang, Z. X.; Yu, R. P.; Zhao, H. Effect of Adding Y2O3 on Property of Zn Coatings via Pack Cementation. Surf. Eng. Appl. Electrochem. 2023, 59, (2), 192-198. [CrossRef]
- Li, B.; Li, D.; Zhang, J.; Chen, W.; Zhang, W. Electrodeposition of Ni-W/TiN-Y2O3 nanocrystalline coating and investigation of its surface properties and corrosion resistance. J. Alloys Compd. 2019, 787, 952–962. [Google Scholar] [CrossRef]
- Safavi, M. S.; Tanhaei, M.; Ahmadipour, M. F.; Ghaffari Adli, R.; Mahdavi, S.; Walsh, F. C. Electrodeposited Ni-Co alloy-particle composite coatings: A comprehensive review. Surf. Coat. Technol. 2020, 382. [Google Scholar] [CrossRef]
- Wu, T.; Ma, M.; Ding, K.; Nan, X.; Wang, Z.; Wei, X.; Zhu, X. Effect of Y2O3 nanoparticles on the microstructure and corrosion resistance of electrodeposited Ni-Mo-Y2O3 nanocomposite coatings. Int. J. Electrochem. Sci. 2023, 18, (6). [CrossRef]
- Kumar, C. M. P.; Chandrashekarappa, M. P. G.; Kulkarni, R. M.; Pimenov, D. Y.; Giasin, K. The Effect of Zn and Zn-WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate. MATERIALS 2021, 14, (9). [CrossRef]
- Jin, W.; Xiao, S.; Kou, Q.; Ding, D.; Zhang, J.; Fang, X.; Ge, C.; Zhong, C.; Zhu, H.; Haarberg, G. M. Preparation of diboride coatings by electrophoretic deposition in nanoparticle-containing molten inorganic salts. Mater. Lett. 2022, 306. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A.; Nasirpouri, F.; Hosseini, M. G. Corrosion resistance of Ni-Co alloy and Ni-Co/SiC nanocomposite coatings electrodeposited by sediment codeposition technique. Appl. Surf. Sci. 2014, 307, 351–359. [Google Scholar] [CrossRef]
- Ridosic, M.; Salicio-Paz, A.; Garcia-Lecina, E.; Zabinski, P.; Zivkovic, L. S.; Bajat, J. B. The effect of the ultrasound agitation and source of ceria particles on the morphology and structure of the Zn-Co-CeO2 composite coatings. J.MATER.RES.TECHNOL. 2021, 13, 1336–1349. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- He, X.; Song, R. G.; Kong, D. J. Microstructure and corrosion behaviours of composite coatings on S355 offshore steel prepared by laser cladding combined with micro-arc oxidation. Appl. Surf. Sci. 2019, 497. [Google Scholar] [CrossRef]
- Du, Y.; Wang, D.; Si, P.; Wei, L.; Wang, Y.; Yu, B.; Zhang, X.; Ye, S. Electrodeposition of a Ni-P-Ti3C2Tx/MoS2 coating incorporating MoS2 intercalated Ti3C2Tx particles. Surf. Coat. Technol. 2018, 354, 119–125. [Google Scholar] [CrossRef]
- Ren, A.; Kang, M.; Fu, X. Corrosion behaviour of Ni/WC-MoS2 composite coatings prepared by jet electrodeposition with different MoS2 doping concentrations. Appl. Surf. Sci. 2023, 613. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liu, J.; Ge, Y.; Yan, X.; Sun, Y.; Wu, J.; Zhang, P. GO-modified double-walled polyurea microcapsules/epoxy composites for marine anticorrosive self-healing coating. Mater. Des. 2020, 189. [Google Scholar] [CrossRef]
- Calado, L. M.; Taryba, M. G.; Carmezim, M. J.; Montemor, M. F. Self-healing ceria-modified coating for corrosion protection of AZ31 magnesium alloy. Corros. Sci. 2018, 142, 12–21. [Google Scholar] [CrossRef]
- Cambon, J. B.; Ansart, F.; Bonino, J. P.; Turq, V. Effect of cerium concentration on corrosion resistance and polymerization of hybrid sol-gel coating on martensitic stainless steel. Prog. Org. Coat. 2012, 75, (4), 486-493. [CrossRef]
- Della Rovere, C. A.; Alano, J. H.; Silva, R.; Nascente, P. A. P.; Otubo, J.; Kuri, S. E. Characterization of passive films on shape memory stainless steels. Corros. Sci. 2012, 57, 154–161. [Google Scholar] [CrossRef]
- Fayomi, O. S. I.; Abdulwahab, M.; Popoola, A. P. I. Properties evaluation of ternary surfactant-induced Zn-Ni-Al2O3 films on mild steel by electrolytic chemical deposition. J. Ovonic Res. 2013, 9, (5), 123-132.
- Tuaweri, T. J.; Wilcox, G. D. Behaviour of Zn-SiO2 electrodeposition in the presence of N,N-dimethyldodecylamine. Surf. Coat. Technol. 2006, 200, (20-21), 5921-5930. [CrossRef]
- Popoola, A. P. I.; Fayomi, O. S. I.; Aigbodion, V. S.; Abdulwahab, M. Surface modification, strengthening effect and electrochemical comparative study of Zn-Al2O3-CeO3 and Zn-TiO2-CeO3 coating on mild steel. Int. J. Adv. Manuf. Technol. 2016, 85, (5-8), 1419-1427. [CrossRef]
- Shen, X.; Sheng, J.; Zhang, Q.; Xu, Q.; Cheng, D. The Corrosion Behavior of Zn/Graphene Oxide Composite Coatings Fabricated by Direct Current Electrodeposition. J. Mater. Eng. Perform. 2018, 27, (7), 3750-3761. [CrossRef]
- Alagi, P.; Ghorpade, R.; Choi, Y. J.; Patil, U.; Kim, I.; Baik, J. H.; Hong, S. C. Carbon Dioxide-Based Polyols as Sustainable Feedstock of Thermoplastic Polyurethane for Corrosion-Resistant Metal Coating. ACS Sustainable Chem. Eng. 2017, 5, (5), 3871-3881. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).