Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and materials
2.2. Clarias gariepinus
2.3. Ethics statement
2.4. Experimental design
2.5. Sample collection
2.5.1. Blood samples
2.5.2. Tissue samples
2.6. Oxidative stress biomarkers measurements
2.6.1. Determination of serum malondialdehyde (MDA) activity:
2.6.2. Determination of serum catalase enzyme activity
2.6.3. Determination of serum glutathione peroxidase (GPx) activity
2.7. Genotoxicity assays
2.7.1. Detection of DNA damage by Single cell gel electrophoresis (Comet assay)
- a)
- Preparation of slides
- b) Lysis and electrophoresis
- c) Slide examination
2.7.2. Micronucleus and Erythrocyte morphological alterations
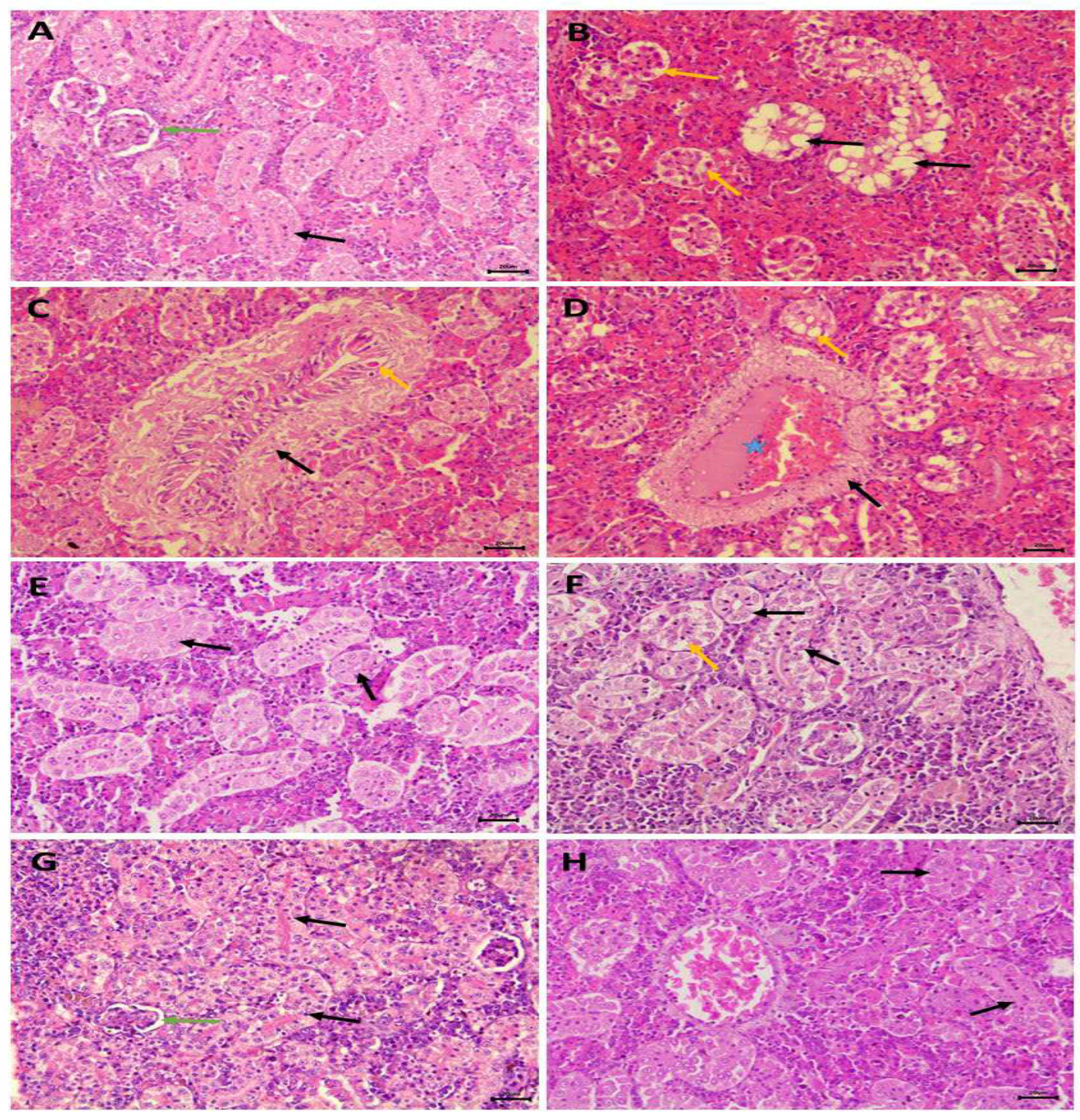
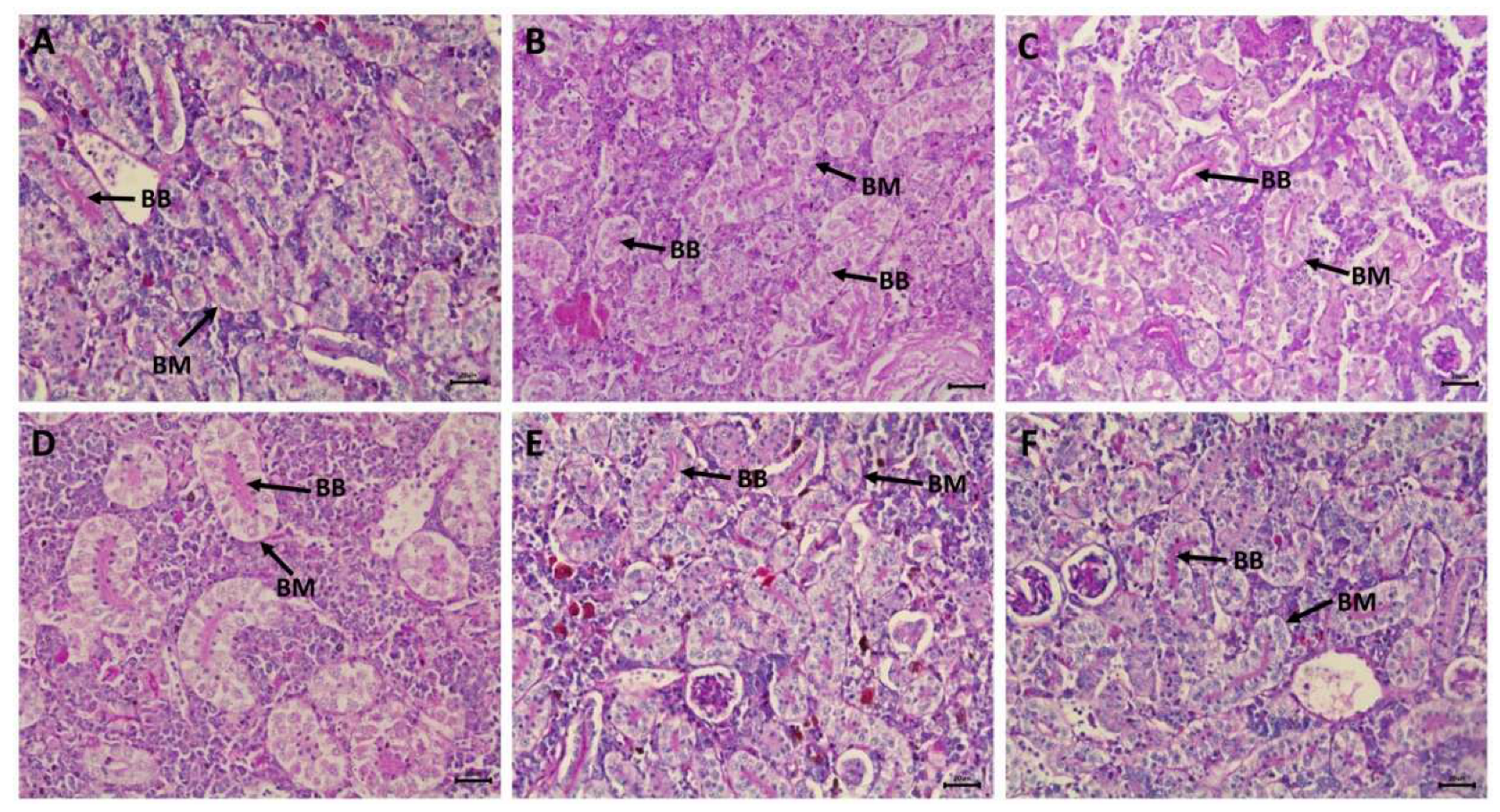
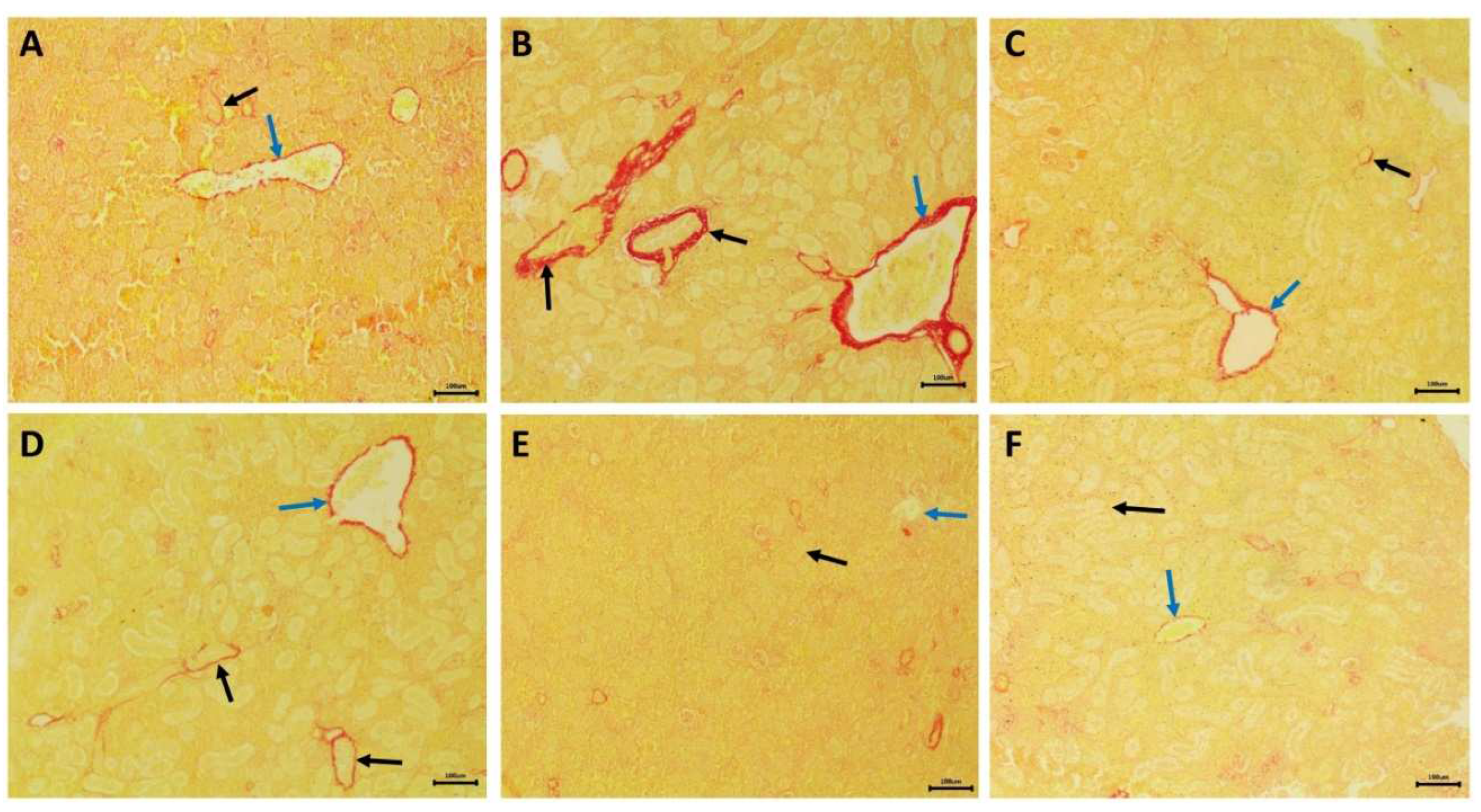
2.8. Histopathological examination of the kidney
2.9. Statistical analysis
3. Results
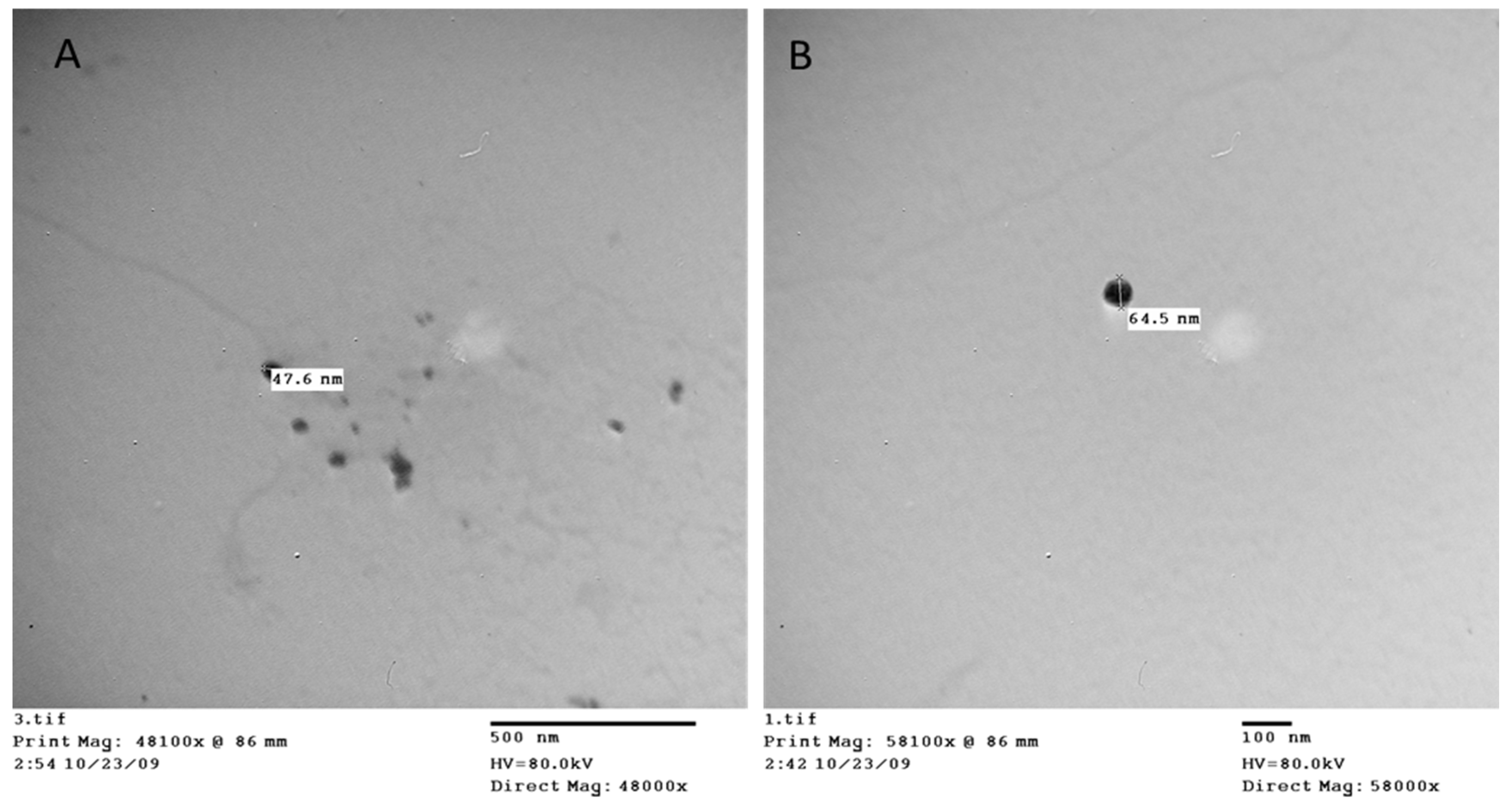
3.1. Polystyrene nanoplastics characterization
3.2. Mortality rate
3.3. Oxidative status
3.3.1. Lipid peroxide
3.3.2. Catalase and glutathione peroxidase activities:
3.4. Genotoxic biomarkers for DNA damage
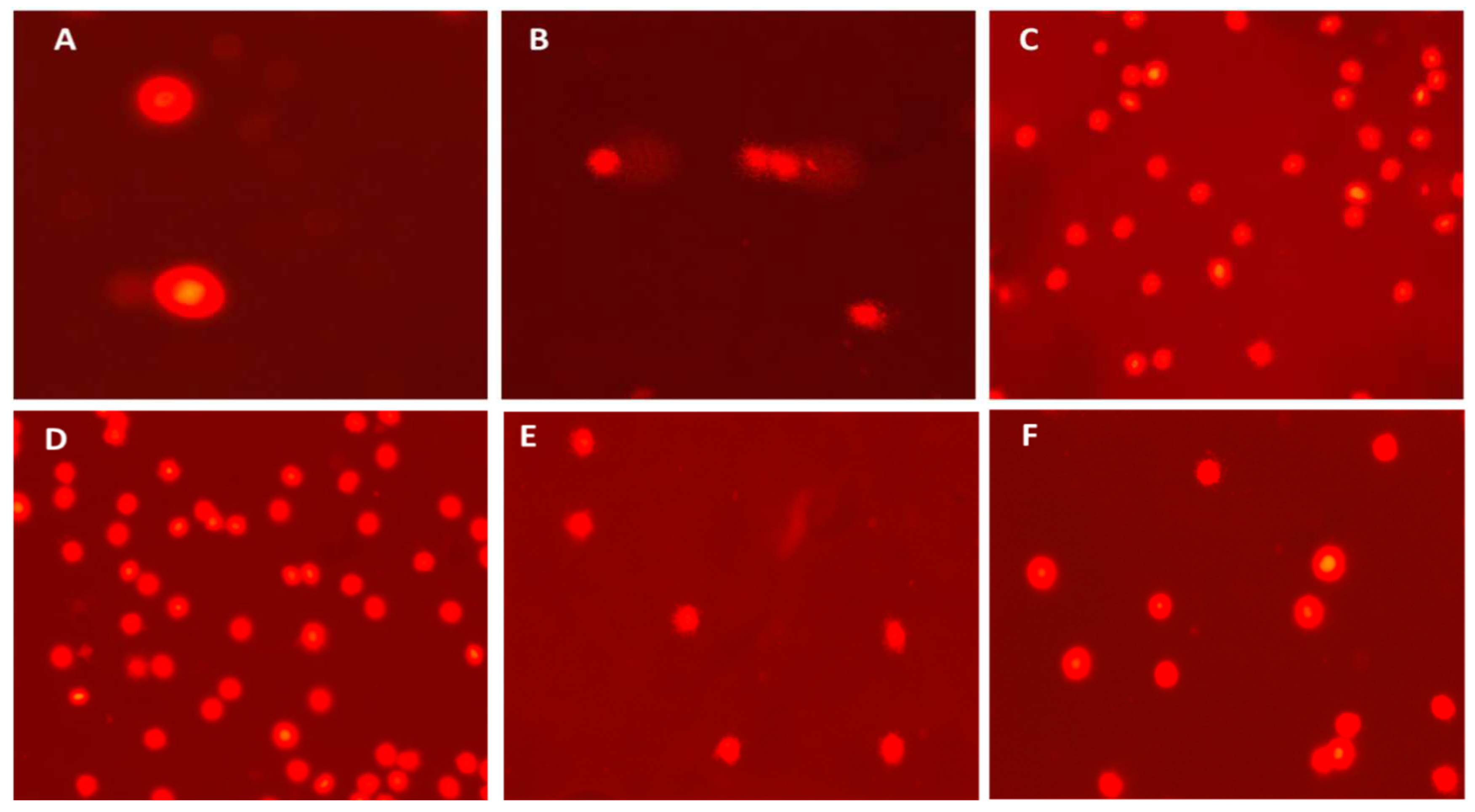
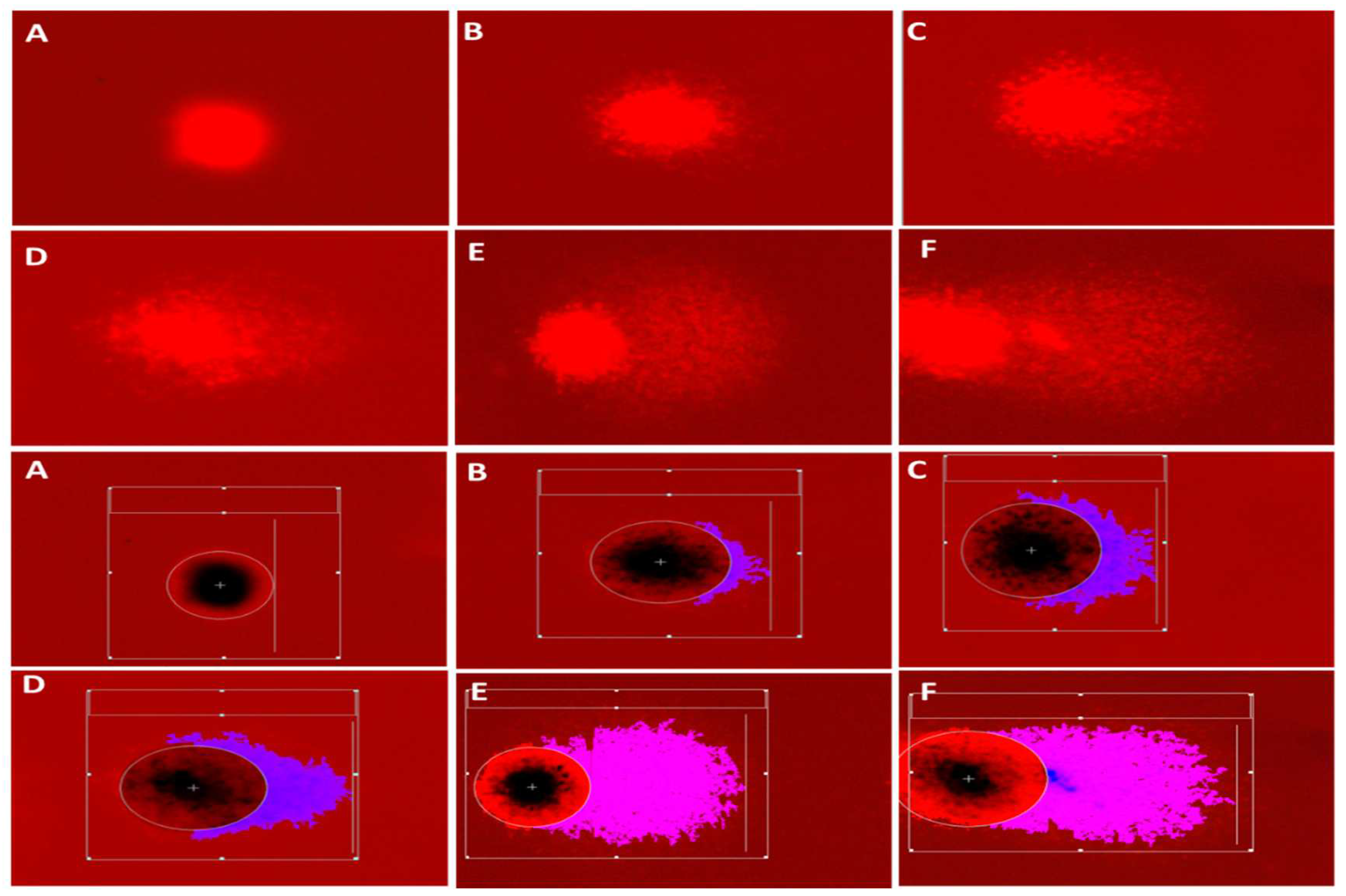
3.4.1. DNA damage parameters (Comet assay)
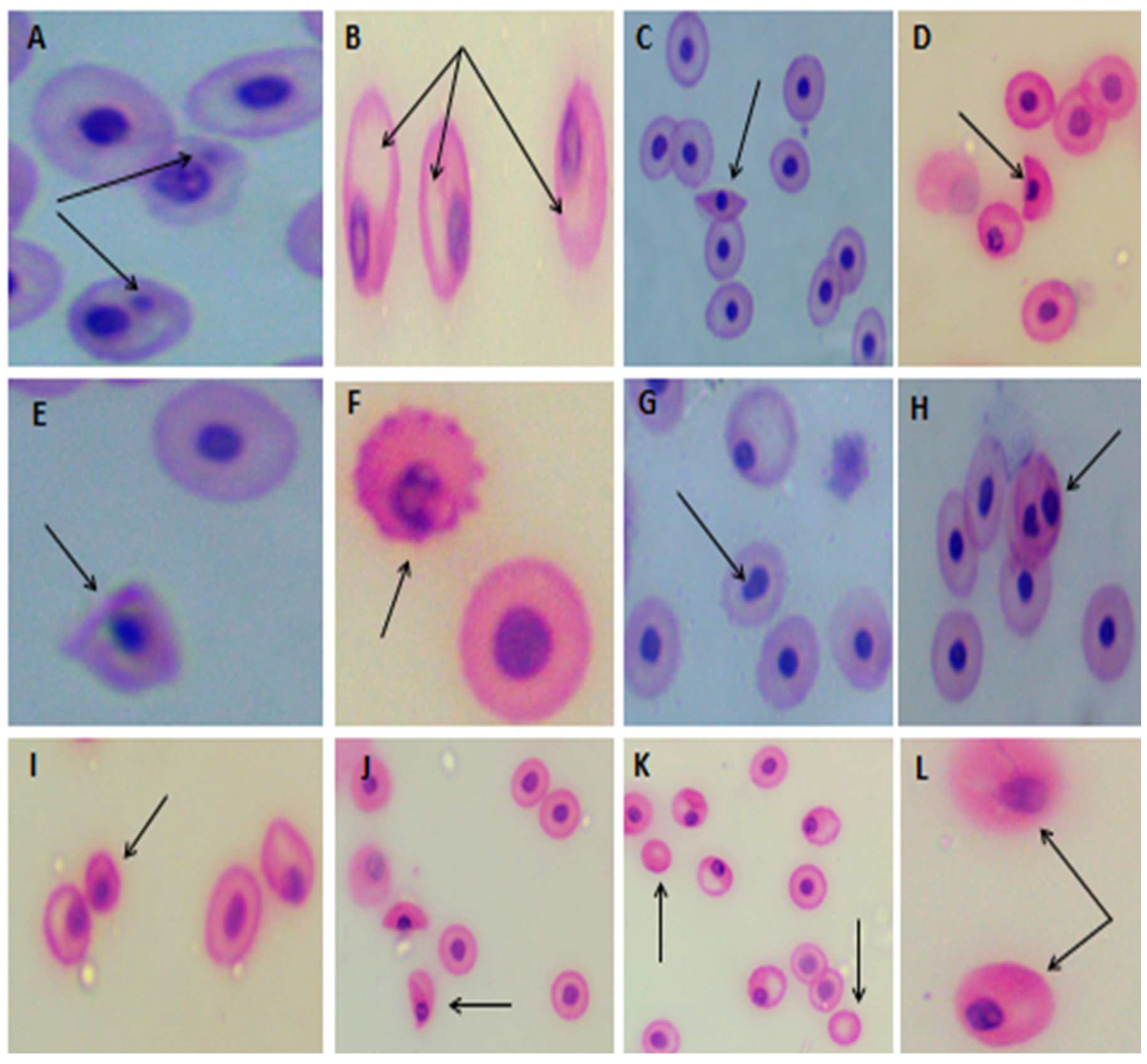
3.4.2. Erythrocyte micronucleus (EMN) and morphological alterations
3.5. Nephrotoxic effects
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Conflict of Interest
References
- Nag, A.; Baksi, A.; Ghosh, J.; Kumar, V.; Bag, S.; Mondal, B.; Ahuja, T.; Pradeep, T. Tribochemical degradation of polytetrafluoroethylene in water and generation of nanoplastics. ACS Sustain. Chem. Eng. 2019, 7, 17554–17558. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef]
- Álvarez-Ruiz, R.; Picó, Y. Analysis of emerging and related pollutants in aquaticbiota. Trends Environ. Anal. Chem. 2020, 25, 00082. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A. ; Narayan, R; Law, K. L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar]
- Babayemi, J. O.; Nnorom, I. C.; Osibanjo, O.; Weber, R. Ensuring sustainability in plastics use in Africa: consumption, waste generation, and projections. Environ. Sci. Europe 2019, 31, 1–20. [Google Scholar] [CrossRef]
- Song, Y. K.; Hong, S. H.; Jang, M.; Han, G. M.; Jung, S. W.; Shim, W. J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. Integr. Environ. Assess. Manag. 2017, 13, 516–521. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T. S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef] [PubMed]
- Mason, S. A.; Welch, V. G.; Neratko, J. Synthetic polymer contamination in bottled water. Front. Chem. 2018, 407. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.; Halbert, G. W.; Langridge, J.; Florence, A. T. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A. P.; Kramer, E.; Hendriksen, P. J.; Tromp, P.; Helsper, J. P.; van der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology. 2015, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Prata, J. C.; da Costa, J. P.; Lopes, I.; Duarte, A. C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sarkar, A.; Yadav, O. P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano-and microplastics and knowledge gaps: a scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Yee, M. S. L.; Hii, L. W.; Looi, C. K.; Lim, W. M.; Wong, S. F.; Kok, Y. Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O.; Leong, C. O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Jeong, C. B.; Kang, H. M.; Lee, M. C.; Kim, D. H.; Han, J.; Hwang, D. S.; Souissi, S.; Lee, S. J.; Shin, K.H.; Park, H.G.; Lee, J. S. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Brandts, I.; Cánovas, M.; Tvarijonaviciute, A.; Llorca, M.; Vega, A.; Farré, M.; Teles, M. Nanoplastics are bioaccumulated in fish liver and muscle and cause DNA damage after a chronic exposure. Environ. Res. 2022, 113433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Balbi, T.; Salis, A.; Damonte, G.; Cortese, K.; Caratto, V.; Monopoli, M.P.; Dawson, K.; Bergami, E.; Corsi, I. Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: Role of soluble hemolymph proteins. Environ. Res. 2016, 150, 73–81. [Google Scholar] [CrossRef]
- Han, Y.; Lian, F.; Xiao, Z.; Gu, S.; Cao, X.; Wang, Z.; Xing, B. Potential toxicity of nanoplastics to fish and aquatic invertebrates: Current understanding, mechanistic interpretation, and meta-analysis. J. Hazard Mater. 2021, 127870. [Google Scholar] [CrossRef]
- Pienaar, U. D. V. The freshwater fished of the Kruger National Park. Koedoe 1968, 11, 1–82. [Google Scholar] [CrossRef]
- Skelton, P. A Complete Guide to the Freshwater Fishes of Southern Africa. Halfway House: Southern Book Publishers Ltd. 1993.
- Teugels, G. A systematic revision of the African species of the genus Clarias (Pisces: Clariidae). Annales Musee Royal de l'Afrique Centrale 1986, 247, 1–199. [Google Scholar]
- Nwani, C. D.; Ifo, C. T.; Nwamba, H. O.; Ejere, V. C.; Onyishi, G. C.; Oluah, S. N.; Ikwuagwu, O.E.; Odo, G. E. Oxidative stress and biochemical responses in the tissues of African catfish Clarias gariepinus juvenile following exposure to primextra herbicide. Drug Chem. Toxicol. 2015, 38, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Kumar, M.; Vivekanand, V.; Pareek, N. Photoautotrophic microorganisms and bioremediation of industrial effluents: current status and future prospects. 3 Biotech. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Wagenen, J.V.; Moller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Marine Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M. P. The importance of selenium to human health. The lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Mohamed, S. A. K. S.; Upreti, S.; Rajendra, S. V.; Dang, R. Genotoxicity: Mechanisms, testing guidelines and methods. Int. J. Pharm. Pharm. Sci. 2017, 1, 133–138. [Google Scholar]
- Gontijo, A.M.M.C.; Barreto, R.E.; Speit, G.; Reyes, V.A.V.; Volpato, G.L.; Salvadori, D.M.F. Anesthesia of fish with benzocaine does not interfere with comet assay results. Mutat Res. 2003, 534, 165–172. [Google Scholar] [CrossRef]
- Bolognesi, C.; Hayashi, M. Micronucleus assay in aquatic animals. Mutagenesis 2011, 26, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Braham, R. P.; Blazer, V. S.; Shaw, C. H.; Mazik, P. M. Micronuclei and other erythrocyte nuclear abnormalities in fishes from the Great Lakes Basin, USA. Environ. Mol. Mutagen. 2017, 58, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): a case study with nanopolystyrene. Sci. Total Envi ron. 2018, 643, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Durigon, E.G.; Kunz, D.F.; Peixoto, N.C.; Uczay, J.; Lazzari, R. Diet selenium improves the antioxidant defense system of juveniles Nile tilapia (Oreochromis niloticus L.). Braz. J. Biol. 2019, 79, 527–532. [Google Scholar] [CrossRef]
- Bai, S.; Koo, J.; Kim, K.; Kim, S. Effects of Chlorella powder as a feed additive on growth performance in juvenile Korean rockfish, Sebastesschlegeli (Hilgendorf). Aquacult Res. 2001, 32, 92–98. [Google Scholar] [CrossRef]
- Wilson, J.M.; Bunte, R.M.; Carty, A.J. Evaluation of rapid cooling and tricaine methanesulfonate (ms222) as methods of euthanasia in zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 785–789. [Google Scholar]
- Bello, O. S.; Olaifa, F. E.; Emikpe, B. O. Haematological and blood biochemical changes in African catfish, Clarias gariepinus fed walnut (Tetracarpidium conophorum Mull Arg) leaf and onion (Allium cepa Linn) bulb supplemented diets. Am. J. Exp. Agric. 2014, 4, 1593–1603. [Google Scholar] [CrossRef]
- Kei, S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica. Chimica. Acta. 1978, 90, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in enzymology; Academic press, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Abd Ellah, M. R.; Niishimori, K.; Goryo, M.; Okada, K.; Yasuda, J. Glutathion peroxidase and glucose-6-phosphate dehydrogenase activities in bovine blood and liver. J. Vet. Med. Sci. 2004, 66, 1219–1221. [Google Scholar] [CrossRef]
- Villela, I.V.; Oliveira, I.M.; Silva, J.; Henriques, J.A.P. DNA damage and repair in haemolynph cells of golden mussel (Limnoperna fortunei) exposed to environmental contaminants. Mutat. Res. 2006, 605, 78–86. [Google Scholar] [CrossRef]
- Pascoe, S.; Gatehouse, D. The use of a simple haematoxylin and eosin staining procedure to demonstrate micronuclei within rodent bone marrow. Mutat. Res. 1986, 164, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, K.R.; Tilbury, K.L.; Myers, M.S. Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Can. Aquat. Sci. 1990, 47, 2123–2136. [Google Scholar] [CrossRef]
- Fischer, AH.; Jacobson, KA.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harbor. Protocol, pdb. Prot. 2008, 49, 86. [Google Scholar] [CrossRef] [PubMed]
- McManus, J.F.A. Histological demonstration of mucin after periodic acid. Nature 1946, 158, 202. [Google Scholar] [CrossRef] [PubMed]
- Bhutda, S.; Surve, M.V.; Anil., A.; Kamath, K.; Singh., N.; Modi, D; Banerjee, A. Histochemical Staining of Collagen and Identification of Its Subtypes by Picrosirius Red Dye in Mouse Reproductive Tissues. Bio-Protocol 2017, 7, 2592. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Naik, T. S. K.; Anil, A. G.; Dhiman, J.; Kumar, V.; Dhanjal, D. S.; Ramamurthy, P. C. Micro (nano) plastics in wastewater: A critical review on toxicity risk assessment, behaviour, environmental impact and challenges. Chemosphere 2022, 290, 133169. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.; Besson, M.; Oberhaensli, F.; Taylor, A.; Gillet, B.; Hughes, S.; Melvi, S.D.; Bustamante, P.; Swarzenski, P.W.; Lecchini, D.; Metian, M. A multifaceted assessment of the effects of polyethylene microplastics on juvenile gilthead seabreams (Sparus aurata). Aquat. Toxicol. 2021, 241, 106004. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Estrela, F.N.; Pereira, P.S.; de Andrade Vieira, J.E.; de Lima Rodrigues, A.S.; Silva, F.G.; Malafaia, G. Toxicity of polystyrene nanoplastics in Ctenopharyngodon idella juveniles: a genotoxic, mutagenic and cytotoxic perspective. Science of The Total Environment 2021, 752, 141937. [Google Scholar] [CrossRef]
- Lu, Y.F.; Zhang, Y.; Deng, Y.F.; Jiang, W.; Zhao, Y.P.; Geng, J.J.; Ding, L.L.; Zhao, Y. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Ai, Q.; Mai, K.; Zhang, W. Dietary selenium requirement for juvenile cobia, Rachycentron canadum L. Aquac. Res. 2010, 41, e594–e601. [Google Scholar] [CrossRef]
- Andrews, S.R.; Sahu, N.P.; Pal, A.K.; Kumar, S. Haematological modulation and growth of Labeo rohita fingerlings: effect of dietary mannan-oligosaccharide, yeast extract, protein hydrolysate and Chlorella sp. Aquacult. Res. 2009, 41, 61–69. [Google Scholar] [CrossRef]
- Eom, H.J.; Nam, S.E.; Rhee, J.S. Polystyrene Microplastics Induce Mortality through Acute Cell Stress and Inhibition of Cholinergic Activity in a Brine Shrimp. Mol. Cell. Toxicol. 2020, 16, 233–243. [Google Scholar] [CrossRef]
- Kim, J.; Rhee, J.S. Biochemospherical and Physiological Responses of the Water Flea Moina Macrocopa to Microplastics: A Multigenerational Study. Mol. Cell. Toxicol. 2021 17, 523–532.
- Lee, D.H.; Lee, S.; Rhee, J.S. Consistent exposure to microplastics induces age-specific physiological and biochemical changes in a marine mysid. Mar. Pollut. Bull. 2021, 162, 111850. [Google Scholar] [CrossRef]
- Varó, I.; Perini, A.; Torreblanca, A.; Garcia, Y.; Bergami, E.; Vannuccini, M. L.; Corsi, I. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physiological, biochemical and molecular levels. Sci. Total Environ. 2019, 675, 570–580. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Mythili Meera, M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep. 2019, 9, 1–15. [Google Scholar]
- Shah, N.; Khan, A.; Habib Khan, N.; Khisroon, M. Genotoxic consequences in common grass carp (Ctenopharyngodon idella Valenciennes, 1844) exposed to selected toxic metals. Biol. Trace Elem. Res. 2020, 199, 305–314. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A. R.; Pereira, B. P.; Fonseca, M.; Mestre, N. C.; Fonseca, T. G.; Bebianno, M. J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Palić, D. Micro-and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A. A.; Chelomin, V. P.; Zhuravel, E. V.; Kukla, S. P.; Slobodskova, V. V.; Dovzhenko, N. V. Genotoxicity of polystyrene (PS) microspheres in short-term exposure to gametes of the sand dollar Scaphechinus mirabilis (Agassiz, 1864)(Echinodermata, Echinoidea). J. mar. sci. eng. 2021, 9, 1088. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Marciano, K.; Downs, C.A. Migration of nonylphenol from food-grade plastic is toxic to the coralreef fish species Pseudochromis fridmani. Chemosphere 2015, 139, 223–228. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Chronic toxicity of polystyrene nanoparticles in the marine mussel Mytilus galloprovincialis. Chemosphere 2022, 287, 132356. [Google Scholar] [CrossRef]
- Estrela, F.N.; Batista Guimarães, A.T.; Silva, F.G.; Marinho da Luz, T.; Silva, A.M.; Pereira, P.S.; Malafaia, G. Effects of polystyrene nanoplastics on Ctenopharyngodon idella (grass carp) after individual and combined exposure with zinc oxide nanoparticles. J. Hazard Mater. 2021, 403, 123879. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, S.; Domenech, J.; Barguilla, I.; Cortés, C.; Marcos, R.; Hernández, A. Genotoxic and immunomodulatory effects in human white blood cells after ex vivo exposure to polystyrene nanoplastics. Environ. Sci Nano. 2020, 7, 3431–3446. [Google Scholar] [CrossRef]
- Magni, S.; Gagné, F.; André, C.; Della Torre, C.; Auclair, J.; Hanana, H.; Parenti, C.C.; Bonasoro, F.; Binelli, A. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 2018, 631–632, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhang, J.; Zhao, X.; Feng, J.; Teng, Y.; Chen, B.; Sun, X.; Zhu, L.; Sun, X.; Qu, K. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020, 258, 113657. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L. The comet assay in nanotoxicology research. Anal Bioanal Chemosphere 2010, 398, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Barría, C.; Martins, M.A.; Franco-Martínez, L.; Barreto, A.; Tvarijonaviciute, A.; Tort, L.; Oliveira, M.; Teles, M. Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J. Hazard. Mater. 2021, 644, 123590. [Google Scholar] [CrossRef]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal micronucleus cytome assay. Nat. Protoc. 2009, 4, 825–837. [Google Scholar] [CrossRef]
- Jacob, H.; Besson, M.; Swarzenski, P.W.; Lecchini, D.; Metian, M. Effects of virgin micro- and nanoplastics on fish: trends, meta-analysis, and perspectives. Environ. Sci. Technol. 2020, 54, 4733–4745. [Google Scholar] [CrossRef]
- Ateeq, B.; Ali, M. N.; Ahmad, W. Induction of micronuclei and erythrocyte alterations in the catfish Clarias batrachus by 2, 4-dichlorophenoxyacetic acid and butachlor. Mutat. Res. 2002, 518, 135–144. [Google Scholar] [CrossRef]
- Brown, L. Aquaculture for veterinarians: fish husbandry and medicine; Pergamon press: Oxford, North Chicago, USA, 1993; 440 pp. [Google Scholar]
- Sayed, A. H.; Hamed, M.; Badrey, A. E.A.; Soliman, H.A.M. Bioreme diation of hemotoxic and oxidative stress induced by polyeth ylene microplastic in Clarias gariepinus using lycopene, citric acid, and Chlorella. Comp. Biochem. Physiol. 2021, Part C 250, 109189. [Google Scholar]
- Da Costa Araujo, A.P.; de Andrade Vieira, J.E; Malafaia, G. Toxicity and trophic transfer of polyethylene microplastics from Poecilia reticulata to Danio rerio. Sci. Total Environ. 2020, 742, 140217. [Google Scholar] [CrossRef]
- Ashouri, S.; Keyvanshokooh, S.; Salati, A. P.; Johari, S. A.; Pasha-Zanoosi, H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S. A.; Pasha-Zanoosi, H. J. A. N. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac. Nutr. 2017, 23, 611–617. [Google Scholar] [CrossRef]
- Bera, S.; Rosa, V. D.; Rachidi, W.; Diamond, A. M. Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis 2013, 28, 127–134. [Google Scholar] [CrossRef]
- Abdelhamid, F. M.; Elshopakey, G. E.; Aziza, A. E. Ameliorative effects of dietary Chlorella vulgaris and β-glucan against diazinon-induced toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 96, 213–222. [Google Scholar] [CrossRef]
- Bengwayan, P.T.; Laygo, J.C.; Pacio, A.E.; Poyaoan, J.L.Z.; Rebugio, J.F.; Yuson, A.L.L. A comparative study on the antioxidant property of Chlorella (Chlorella sp) tablet and glutathione tablet. Int. J. Sci. Res. 2010, 2, 25–35. [Google Scholar]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wang, W.; Gonzalez-Gil, G.; Vrouwenvelder, J. S.; Li, Z. Effects of nano-and microplastics on kidney: Physicochemospherical properties, bioaccumulation, oxidative stress and immunoreaction. Chemosphere 2022, 288, 132631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, K.F.; Lin, K.Y.A.; Chen, J.K.; Jiang, X.Y.; Lin, C.H. The nephrotoxic potential of polystyrene microplastics at realistic environmental concentrations. J. Hazard. Mat. 2022, 427, 127871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, B.; Wang, M.; Tong, J.; Pan, J.; Wang, N.; Gong, P.; Long, M. Selenium protects against zearalenone-induced oxidative stress and apoptosis in the mouse kidney by inhibiting endoplasmic reticulum stress. Oxidative Med. Cell. Longev 2020. [Google Scholar] [CrossRef]
- Latif, A. A.E.; Assar, D.H.; Elkaw, E.M.; Hamza, H.A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Hamouda, R.A. Protective role of Chlorella vulgaris with Thiamine against Paracetamol induced toxic effects on haematological, biochemical, oxidative stress parameters and histopathological changes in Wistar rats. Sci. Rep. 2021, 11, 3911. [Google Scholar] [CrossRef]

| Ingredients | % |
|---|---|
| Fish meal (65%) | 9 |
| Soybean meal (46%) | 36.85 |
| Corn gluten (60%) | 12.2 |
| Yellow corn | 19.25 |
| Wheat bran | 5.7 |
| Fish oil | 6.00 |
| Starch | 7.00 |
| Mineral premix (without se) | 2.00 |
| Vitamin premix | 2.00 |
| Total | 100 |
|
Proximate composition % dry matter (DM) |
% |
| Crude protein (CP) | 30 |
| Crude fiber (CF) | 4.8 |
| Ash | 8.2 |
| Ether extract (EE) | 6.5 |
| *Nitrogen free extract (NFE) | 50.5 |
| Se | Ch | NPs+Ch | NPs+Se | NPs | Control | Groups |
|---|---|---|---|---|---|---|
| Parameters | ||||||
| 4.02±0.9b | 3.72±0.8b | 4.98± 0.7b | 5.56±0.5b | 10.22± 2.4 a | 3.71± 0.7b | MDA |
| 352.9±33.4a | 232.93±11.2b | 202.43±40.5b | 235±62.3b | 201.43±5.2b | 225.9±13.6b | CAT |
| 32.43±8.0a | 24.33±2.99a | 25.57±4.2a | 33.7± 10.4a | 19.1± 6.4a | 27.5± 5.6a | GPX |
| Parameters | Control | NPs | NP+ Se | NP+ Ch | Ch | Se |
|---|---|---|---|---|---|---|
| Tail DNA% | 0.57±0.12bc | 3.43 ±0.53a | 1.26±0.19b | 0.98±0.17b | 0.56±0.16b c | 0.08±0.03c |
| Tail Length(µm) | 8.24 ±0.62c | 32.14 ±2.60a | 15.42±1.21 b | 9.77±0.60c | 7.50 ±0.45cd | 4.22 ±0.28 d |
| Tail moment | 0.19±0.05b | 3.60±0.88a | 0.44±0.07b | 0.20±0.06b | 0.15±0.03b | 0.09±0.01b |
| Olive tail moment | 0.46±0.09b | 4.58±0.66a | 1.15±0.15b | 0.59±0.11b | 0.30±0.05b | 0.11±0.02b |
| Se | Ch | NPs+Ch | NPs+Se | NPs | Control | Groups |
|---|---|---|---|---|---|---|
| Parameters | ||||||
| 3.0±0.57b | 2.3±0.33b | 3.3±1.2b | 4.3±1.20b | 22.3±2.00a | 2.3±0.88b | Mn |
| 5.3±0.88b | 6.0±1.52b | 8.3±2.6b | 9.3±1.45b | 47.3±5.78a | 7.0 ±0.57b | Vc |
| 4.3±1.20b | 5.0±1.15b | 6±0.57b | 7.6±0.88b | 14.3±1.45a | 5.0±1.50b | Tc |
| 3.3±0.88b | 3.0±1.00b | 4.3±0.88b | 5.0±1.15b | 11.3±1.45a | 4.3±1.20b | Ac |
| 4.0±1.5b | 4.3±1.20b | 5.3±0.88b | 5.6±1.20b | 12.6±1.76a | 3.6±0.88b | Cr |
| 2.3±0.88b | 2.0±0.57b | 3.6±1.20b | 4.6±0.80b | 13.3±0.88a | 2.0±0.57b | Sk |
| 2.0±0.57b | 1.6±0.66b | 2.6±1.20b | 2.3±0.80b | 7.3±1.45a | 1.6±0.33b | Sp |
| 3.6±0.88b | 2.6±1.20b | 5.6±1.70b | 5.3±1.20b | 19.0±1.15a | 4.6±1.20b | Mc |
| 4.6±0.88d | 6.3±1.2bc | 7.0±2.00bc | 12.0±2.50b | 50.0 ±3.20a | 7.6±1.76bc | Ecn |
| 1.3±0.33b | 1.3±0.33b | 4.0±0.57b | 4.3±1.40b | 10±1.50a | 3.0±0.57b | Bin |
| 1.6±0.33bc | 1.0±0.33d | 2.3±0.88bc | 3.6±0.80b | 8.3±1.20a | 1.3±0.33bc | Notn |
| 2.0±0.57b | 1.3±0.33b | 2.0±0.57b | 3.0±0.57b | 9.0±1.15a | 2.6±0.88b | Enn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





