Submitted:
10 January 2024
Posted:
11 January 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
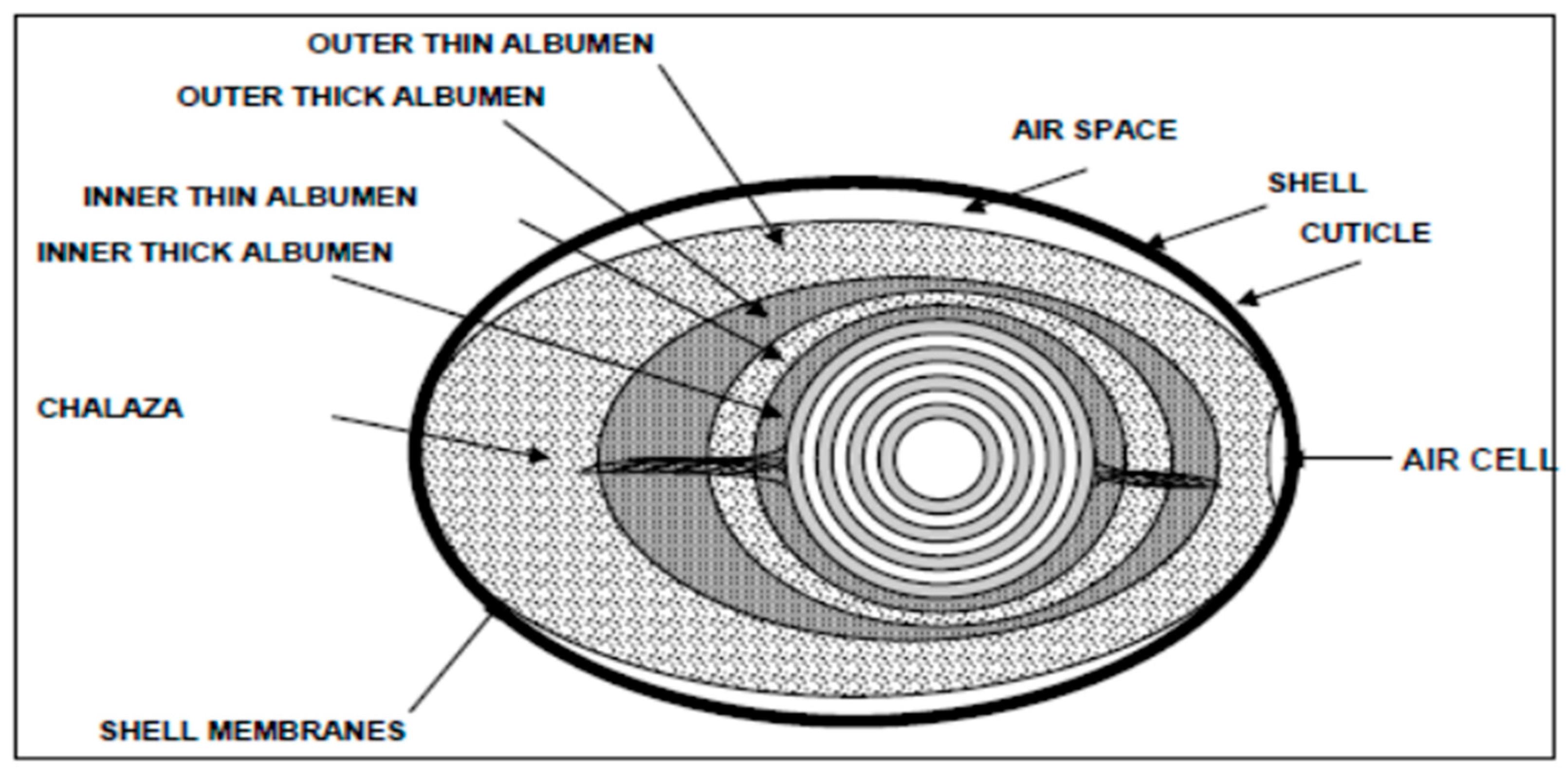
2. Egg as a nutrient for companion animals
2.1. Egg as a protein source
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Protein | g | 12.4±0.4 | 12.9±0.8 | 48.9±1.8 | 3.7 | 5.7 | 6.2 | 25.0 | 15 | 23 | 25 |
| Arginine | mg | 763±31 | 785±23 | 3144±84 | 229 | 345 | 396 | 880 | 26 | 39 | 45 |
| Histidine | mg | 297±11 | 307±12 | 1210±10 | 89 | 135 | 152 | 480 | 19 | 28 | 32 |
| Isoleucine | mg | 591±86 | 648±9 | 2670±425 | 177 | 285 | 336 | 950 | 19 | 30 | 35 |
| Leucine | mg | 974±122 | 1070±29 | 4246±190 | 292 | 471 | 535 | 1700 | 17 | 28 | 31 |
| Lysine | mg | 863±23 | 906±25 | 3446±72 | 259 | 399 | 431 | 880 | 29 | 45 | 49 |
| Methionine | mg | 378±60 | 413±23 | 1575±62 | 113 | 182 | 198 | 830 | 14 | 22 | 24 |
| Methionine + Cysteine |
mg | 701±81 | 717±45 | 2740±139 | 210 | 316 | 345 | 1630 | 13 | 19 | 21 |
| Phenylealanine | mg | 613±79 | 669±14 | 2629±3184 | 184 | 294 | 331 | 1130 | 16 | 26 | 29 |
| Phenylealanine + Tyrosine | mg | 1075±144 | 1198±38 | 4730±206 | 323 | 527 | 596 | 1850 | 17 | 28 | 32 |
| Threonine | mg | 8640±139 | 599±32 | 2318±184 | 192 | 263 | 292 | 1080 | 18 | 24 | 27 |
| Tryptophan | mg | 169±21 | 191±10 | 764±57 | 51 | 84 | 96 | 350 | 14 | 24 | 27 |
| Valine | mg | 727±125 | 810±11 | 3220±372 | 218 | 357 | 406 | 1230 | 18 | 29 | 33 |
| Energy | kcal | 139±7 | 140±9 | 562±38 | 42 | 61 | 71 | 1000 | 4 | 6 | 7 |
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Protein | g | 12.4±0.4 | 12.9±0.8 | 48.9±1.8 | 3.7 | 5.7 | 6.2 | 12.5 | 30 | 45 | 49 |
| Arginine | mg | 763±31 | 785±23 | 3144±84 | 229 | 345 | 396 | 480 | 48 | 72 | 83 |
| Histidine | mg | 297±11 | 307±12 | 1210±10 | 89 | 135 | 152 | 160 | 56 | 84 | 95 |
| Isoleucine | mg | 591±86 | 648±9 | 2670±425 | 177 | 285 | 336 | 270 | 66 | 106 | 125 |
| Leucine | mg | 974±122 | 1070±29 | 4246±190 | 292 | 471 | 535 | 640 | 46 | 74 | 84 |
| Lysine | mg | 863±23 | 906±25 | 3446±72 | 259 | 399 | 431 | 210 | 123 | 190 | 205 |
| Methionine | mg | 378±60 | 413±23 | 1575±62 | 113 | 182 | 198 | 110 | 103 | 165 | 180 |
| Methionine + Cysteine |
mg | 701±81 | 717±45 | 2740±139 | 210 | 316 | 345 | 210 | 100 | 150 | 164 |
| Phenylealanine | mg | 613±79 | 669±14 | 2629±3184 | 184 | 294 | 331 | 250 | 74 | 118 | 133 |
| Phenylealanine + Tyrosine | mg | 1075±144 | 1198±38 | 4730±206 | 323 | 527 | 596 | 960 | 34 | 55 | 62 |
| Threonine | mg | 8640±139 | 599±32 | 2318±184 | 192 | 263 | 292 | 320 | 60 | 82 | 91 |
| Tryptophan | mg | 169±21 | 191±10 | 764±57 | 51 | 84 | 96 | 80 | 63 | 105 | 120 |
| Valine | mg | 727±125 | 810±11 | 3220±372 | 218 | 357 | 406 | 320 | 68 | 111 | 127 |
| Energy | kcal | 139±7 | 140±9 | 562±38 | 42 | 61 | 71 | 250 | 4 | 25 | 28 |
2.1.1. Bioactive proteins
| Protein | Bioactive properties |
|---|---|
| Ovalbumin | Antihypertensive, antibacterial, antioxidant, immunomodulating, anticancer |
| Ovotransferrin | Bacteriostatic and bactericidal, antiviral , antifungal, immunomodulating |
| Ovomucoid | Immunomodulating, trypsin inhibitor |
| Ovomucin | Antiviral, antiadhesive and antibacterial, anticancer, immunomodulating, hypercholesterolemic |
| Lysozyme | Antibacterial, antiviral, anticancer, antioxidant, immunomodulating |
| Ovoinhibitor | Antimicrobial (serine protease inhibitor) |
| Ovoglycoprotein | Activation of inflammatory cell lines |
| Ovomacroglobulin | Antibacterial (trypsin and papain inhibitor) and anti-inflammatory, treatment of keratitis |
| Ovoflavoprotein | Antimicrobial (riboflavin binding) |
| Avidin | Antimicrobial (biotin binding), anticancer, immunomodulating |
| Cystatin | Anticancer, antibacterial, immunomodulating, protease inhibitor |
| Main Components | Main Biochemical Functions |
|---|---|
| Collagens | Optimum mechanical strength, Thermal stability, Wound healing, Osteocompatibity, Anchorage to nanohydroxyapatite, Biomineralization |
| Osteopontin | Affinity binding for hydroxyapatite and osteoblasts, Inhibitor of mineralization, Modulation of osteoclast differentiation, Recruitment of macrophages, Regulation of cytokine production, Inhibition of vascular calcification, Regulation of apatite crystal size and growth, Tissue remodeling |
| Fibronectin | Promotion of cell adhesion, Improving cell growth, migration, and differentiation, Wound healing |
| Keratin | Self-Assembly, Promotion of cell adhesion |
| Cysteine-rich eggshell membrane proteins (CREMPs) | Wound healing |
| Histones | Chromatin folding and compaction, Potent antimicrobial properties |
| Avian beta defensins | Promotion of innate defense system, Reinforcement of the antimicrobial defenses associated with the ESM |
| Ovocalyxin-36 | Potent antimicrobial properties, Positive immune-modulating effects |
| Apolipoproteins | Binding and transport of lipids |
| Protocadherin | Adhesion and differentiation functions |
| Chondroitin sulfate | Formation of porous hydrated gels Immuno-inhibition property for articular cartilage repair Binding Ca2+ Molecules’ migration through the matrix |
| Hyaluronic acid | Water-retaining property, Improving angiogenesis and tissue morphogenesis |
2.2. Egg as a lipid source
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Total fat | g | 9.6±0.5 | 9.6±0.7 | 38.4±4.5 | 4.6 | 4.7 | 5.6 | 13.8 | 34 | 34 | 40 |
| Linoleic acid (LA, 18:2, Ώ6) |
mg | 1248±349 | 1142±381 | 4327±922 | 605 | 554 | 630 | 2800 | 22 | 20 | 22 |
| Alpha-linolenic acid (ALA, 18:3, Ώ3) | mg | 101±55 | 110±49 | 471±229 | 49 | 53 | 68 | 110 | 44 | 49 | 62 |
| Arachidonic acid (AA, 20:4, Ώ6) | mg | 54±27 | 52±27 | 138±73 | 26 | 25 | 20 | ||||
| Eicosapentaenoic acid (EPA, 20:5, Ώ3) | mg | 16±21 | 12±13 | 210±99 | 8 | 6 | 31 | ||||
| Docosahexaenoic acid (DHA, 22:6, Ώ3) | mg | 75±39 | 77±33 | 204±61 | 36 | 38 | 30 | ||||
| EPA + DHA | mg | 71±37 | 70±35 | 248±133 | 34 | 34 | 36 | 110 | 31 | 31 | 33 |
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Total fat | g | 9.6±0.5 | 9.6±0.7 | 38.4±4.5 | 4.6 | 4.7 | 5.6 | 5.6 | 83 | 83 | 100 |
| Linoleic acid (LA, 18:2, Ώ6) |
mg | 1248±349 | 1142±381 | 4327±922 | 605 | 554 | 630 | 350 | 173 | 158 | 180 |
| Alpha-linolenic acid (ALA, 18:3, Ώ3) |
mg | 54±27 | 52±27 | 138±73 | 26 | 25 | 20 | ||||
| Arachidonic acid (AA, 20:4, Ώ6) |
mg | 101±55 | 110±49 | 471±229 | 49 | 53 | 68 | 3.75 | 1303 | 1426 | 1826 |
| Eicosapentaenoic acid (EPA, 20:5, Ώ3) |
mg | 16±21 | 12±13 | 210±99 | 8 | 6 | 31 | ||||
| Docosahexaenoic acid (DHA, 22:6, Ώ3) | mg | 75±39 | 77±33 | 204±61 | 36 | 38 | 30 | ||||
| EPA + DHA | mg | 71±37 | 70±35 | 248±133 | 34 | 34 | 36 | 6.25 | 551 | 546 | 576 |
2.3. Eggs and vitamins
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Fat soluble | |||||||||||
| Vitamin A | µg | 176±37 | 170±63 | 510±303 | 71 | 68 | 61 | 379 | 19 | 18 | 16 |
| Vitamin D | µg | 2.1±0.8 | 1.9±0.9 | 5.5±3.7 | 0.8 | 0.8 | 0.7 | 3.4 | 24 | 22 | 19 |
| Vitamin E | mg | 2.6±1.4 | 2.7±1.5 | 5.1±3.3 | 1.1 | 1.1 | 0.6 | 7.5 | 14 | 15 | 8 |
| Vitamin K | µg | 10±12 | 9±13 | 15±27 | 4.0 | 3.4 | 1.8 | 410 | 1.0 | 0.8 | 0.4 |
| Water soluble | |||||||||||
| Thiamine (B1) | µg | 75±10 | 64±11 | 280±84 | 30 | 26 | 34 | 560 | 5 | 5 | 6 |
| Riboflavin (B2) | µg | 431±52 | 407±52 | 1535±351 | 172 | 163 | 184 | 1300 | 13 | 13 | 14 |
| Niacin (B3) | µg | 81±27 | 77±32 | 283±119 | 32 | 31 | 34 | 4250 | 0.8 | 0.7 | 0.8 |
| Pantothenic acid (B5) | mg | 1.7±0.4 | 1.5±0.6 | 5.5±0.2 | 0.7 | 0.6 | 0.7 | 3.75 | 19 | 16 | 18 |
| Pyridoxine (B6) | µg | 124±87 | 122±98 | 352±142 | 50 | 49 | 42 | 375 | 13 | 13 | 11 |
| Biotin (B7) | µg | 23±3 | 23±5 | 86±15 | 9 | 9 | 10 | ||||
| Folic acid (B9) | µg | 58±18 | 49±18 | 159±27 | 23 | 20 | 19 | 67.5 | 34 | 29 | 28 |
| Cobalamin (B12) | µg | 1.4±0.6 | 1.2±0.5 | 5.7±3.5 | 0.6 | 0.5 | 0.7 | 87.5 | 0.6 | 0.6 | 0.8 |
| Lutein + Zeaxanthine |
µg | 395±65 | 409±109 | 835 | 158 | 164 | 100 | ||||
| Choline | mg | 318±24 | 294 | 1267 | 127 | 118 | 152 | 425 | 30 | 28 | 36 |
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Fat soluble | |||||||||||
| Vitamin A | µg | 176±37 | 170±63 | 510±303 | 71 | 68 | 61 | 62.5 | 113 | 109 | 98 |
| Vitamin D | µg | 2.1±0.8 | 1.9±0.9 | 5.5±3.7 | 0.8 | 0.8 | 0.7 | 0.4 | 207 | 189 | 165 |
| Vitamin E | mg | 2.6±1.4 | 2.7±1.5 | 5.1±3.3 | 1.1 | 1.1 | 0.6 | 2.5 | 42 | 44 | 24 |
| Vitamin K | µg | 10±12 | 9±13 | 15±27 | 4.0 | 3.4 | 1.8 | 82 | 4.9 | 4.2 | 2.2 |
| Water soluble | |||||||||||
| Thiamine (B1) | µg | 75±10 | 64±11 | 280±84 | 30 | 26 | 34 | 330 | 9 | 8 | 10 |
| Riboflavin (B2) | µg | 431±52 | 407±52 | 1535±351 | 172 | 163 | 184 | 270 | 64 | 60 | 68 |
| Niacin (B3) | µg | 81±27 | 77±32 | 283±119 | 32 | 31 | 34 | 2500 | 1.3 | 1.2 | 1.4 |
| Pantothenic acid (B5) | mg | 1.7±0.4 | 1.5±0.6 | 5.5±0.2 | 0.7 | 0.6 | 0.7 | 0.4 | 174 | 148 | 166 |
| Pyridoxine (B6) | µg | 124±87 | 122±98 | 352±142 | 50 | 49 | 42 | 160 | 31 | 31 | 26 |
| Biotin (B7) | µg | 23±3 | 23±5 | 86±15 | 9 | 9 | 10 | ||||
| Folic acid (B9) | µg | 58±18 | 49±18 | 159±27 | 23 | 20 | 19 | 47 | 49 | 42 | 41 |
| Cobalamin (B12) | µg | 1.4±0.6 | 1.2±0.5 | 5.7±3.5 | 0.6 | 0.5 | 0.7 | 1.4 | 41 | 36 | 49 |
| Lutein + Zeaxanthine |
µg | 395±65 | 409±109 | 835 | 158 | 164 | 100 | ||||
| Choline | mg | 318±24 | 294 | 1267 | 127 | 118 | 152 | 159 | 80 | 74 | 96 |
2.4. Eggs and minerals
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Ash | g | 0.9±0.1 | 0.9±0.1 | 3.7±0.3 | 0.4 | 0.4 | 0.4 | ||||
| Calcium (Ca) | mg | 52±9 | 49±8 | 218±19 | 21 | 20 | 26 | 1000 | 2 | 2 | 3 |
| Phosphorus (P) | mg | 188±26 | 186±34 | 761±89 | 75 | 74 | 91 | 750 | 10 | 10 | 12 |
| Magnesium (Mg) | mg | 13±3 | 12±2 | 42±6 | 5 | 5 | 5 | 150 | 3 | 3 | 3 |
| Potassium (K) | mg | 132±8 | 122±24 | 533±42 | 53 | 49 | 64 | 1000 | 5 | 5 | 6 |
| Sodium (Na) | mg | 136±11 | 163±75 | 514±37 | 55 | 65 | 62 | 200 | 27 | 33 | 31 |
| Chloride (Cl) | mg | 189±13 | 294±196 | 721±37 | 76 | 117 | 87 | 300 | 25 | 39 | 29 |
| Iron (Fe) | mg | 1.8±0.2 | 1.9±0.3 | 6.5±0.3 | 0.7 | 0.7 | 0.8 | 7.5 | 9 | 10 | 10 |
| Zinc (Zn) | mg | 1.2±0.1 | 1.3±0.1 | 4.1±1.1 | 0.5 | 0.5 | 0.5 | 15 | 3 | 3 | 3 |
| Copper (Cu) | µg | 59±10 | 68±8 | 212±53 | 24 | 27 | 25 | 1500 | 2 | 2 | 2 |
| Manganese (Mn) | µg | 32±11 | 35±11 | 90±36 | 13 | 14 | 11 | 1200 | 1.1 | 1.2 | 0.9 |
| Iodine (I) | µg | 36±13 | 34±15 | 186±65 | 14 | 14 | 22 | 220 | 6 | 6 | 10 |
| Selenium (Se) | µg | 24±9 | 24±10 | 94±44 | 10 | 10 | 11 | 87.5 | 11 | 11 | 13 |
| Nutrients / 100 g | Digestible portion / serving | RDA | RDA-% / serving | ||||||||
| Raw | Boiled | Powder | Raw (50 g) | Boiled (50 g) | Powder (15 g) | Raw | Boiled | Powder | |||
| Ash | g | 0.9±0.1 | 0.9±0.1 | 3.7±0.3 | 0.4 | 0.4 | 0.4 | ||||
| Calcium (Ca) | mg | 52±9 | 49±8 | 218±19 | 21 | 20 | 26 | 180 | 12 | 11 | 15 |
| Phosphorus (P) | mg | 188±26 | 186±34 | 761±89 | 75 | 74 | 91 | 160 | 47 | 46 | 57 |
| Magnesium (Mg) | mg | 13±3 | 12±2 | 42±6 | 5 | 5 | 5 | 25 | 20 | 19 | 20 |
| Potassium (K) | mg | 132±8 | 122±24 | 533±42 | 53 | 49 | 64 | 330 | 16 | 15 | 19 |
| Sodium (Na) | mg | 136±11 | 163±75 | 514±37 | 55 | 65 | 62 | 42 | 130 | 155 | 147 |
| Chloride (Cl) | mg | 189±13 | 294±196 | 721±37 | 76 | 117 | 87 | 60 | 126 | 196 | 144 |
| Iron (Fe) | mg | 1.8±0.2 | 1.9±0.3 | 6.5±0.3 | 0.7 | 0.7 | 0.8 | 5 | 14 | 15 | 16 |
| Zinc (Zn) | mg | 1.2±0.1 | 1.3±0.1 | 4.1±1.1 | 0.5 | 0.5 | 0.5 | 4.6 | 11 | 11 | 11 |
| Copper (Cu) | µg | 59±10 | 68±8 | 212±53 | 24 | 27 | 25 | 300 | 8 | 9 | 8 |
| Manganese (Mn) | µg | 32±11 | 35±11 | 90±36 | 13 | 14 | 11 | 300 | 4 | 5 | 4 |
| Iodine (I) | µg | 36±13 | 34±15 | 186±65 | 14 | 14 | 22 | 88 | 16 | 15 | 25 |
| Selenium (Se) | µg | 24±9 | 24±10 | 94±44 | 10 | 10 | 11 | 19 | 50 | 50 | 59 |
3. Disease-specific impacts of egg
3.1. Joint health
3.1. Skin health
3.3. Gastrointestinal
3.3.1. Adaptive immunity / Passive immunotherapy
3.3.2. Effect of bioactive egg compounds on gastrointestinal disorders
3.3.3. Metabolic syndrome and obesity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shoveller, A.K.; De Godoy, M.R.C.; Larsen, J.; Flickinger, E. Emerging Advancements in Canine and Feline Metabolism and Nutrition. Sci. World J. 2016, 2016, 2016, 9023781. [Google Scholar] [CrossRef]
- German, A.J. Style over substance: what can parenting styles tell us about ownership styles and obesity in companion animals? Brit. J. Nutr. 2015, 113, S72–S77. [Google Scholar] [CrossRef]
- German, A.J.; Ryan, V.H.; German, A.C.; Wood, I.S.; Trayhurn, P. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet. J. 2010, 85, 4–9. [Google Scholar] [CrossRef]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota–Gut–Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef]
- Xiao, H.; Kang, S. The Role of the Gut Microbiome in Energy Balance With a Focus on the Gut-Adipose Tissue Axis. Front. Genet. 2020, 11, 297. [Google Scholar] [CrossRef]
- Taylor, R.C.; Omed, H.; Edwards-Jones, G. The greenhouse emissions footprint of free-range eggs. Poultry Sci. 2014, 93, 231–237. [Google Scholar] [CrossRef]
- Seuss-Baum, I. Nutritional Evaluation of Egg Compounds. In Bioactive Egg Compounds; Huopalahti, R.; Lopez-Fandino, R.; Anton, M.; Schade, R., Eds.; Springer Publication, Berlin, Germany, 2007, pp. 117-144, ISBN 978-3-540-37883-9.
- Andersen, C.J. Bioactive Egg Components and Inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef] [PubMed]
- Pali-Schöll, I.; De Lucia, M.; Jackson, H.; Janda, J.; Mueller, R.S.; Jensen-Jarolim, E. Comparing immediate-type food allergy in humans and companion animals—revealing unmet needs. Allergy. 2017, 72, 1643–1656. [Google Scholar] [CrossRef]
- Burley, R.W.; Vadehra, D.V. The albumen: chemistry. In The Avian Egg: Chemistry and Biology; Burley, R.W.; Vadehra, D.V., Eds.; John Wiley & Sons, New York, NY, USA, 1989, pp. 65-128.
- Li-Chan, E.C.Y; Kim, H.-O. Structure and chemical composition of eggs. In Hen Eggs: Their Basic and Applied Science; Yamamoto, T., Juneja, L.R., Hatta, H., Kim, M., Eds.; CRC Press Inc., Boca Raton, FL, USA, 1997, pp. 1-95.
- Juneja, L.R ; Kim, M. Egg yolk proteins. In Hen Eggs: Their Basic and Applied Science; Yamamoto, T., Juneja, L.R., Hatta, H., Kim, M., Eds.; CRC Press Inc., Boca Raton, FL, USA, 1997, pp. 57 – 71.
- Aro, H. Fractionation of Hen Egg and Oat Lipids with supercritical Fluids Chemical and functional Properties of Fractions, Doctoral Dissertation, University of Turku, Turku, Finland, 2012.
- Sugino, H.; Nitoda, T.; Juneja, L.R. General chemical composition of hen eggs. In Hen Eggs: Their Basic and Applied Science; Yamamoto, T., Juneja, L.R., Hatta, H., Kim, M., Eds.; CRC Press Inc., Boca Raton, FL, USA, 1997, pp. 13-24, ISBN 9780849340055.
- DeVore, D.; Long, F.; Osborne, M.; Adams, R.; Franklin, M. Anti-inflammatory activity of eggshell membrane and processed eggshell membrane preparations. US Patent US 2007/0178170 A1, 2007.
- Vuong, T.T.; Rønning, S.B.; Suso, H.-P.; Schmidt, R.; Prydz, K.; Marlene Lundström, M.; Moen, A.; Pedersen, M.E. The extracellular matrix of eggshell displays anti-inflammatory activities through NF-κB in LPS-triggered human immune cells. J. Inflam. Res. 2017, 10, 83–962017. [Google Scholar] [CrossRef] [PubMed]
- Bayat, P., Rambaud, C., Priem, B.; Bourderioux, M.; Bilong, M.; Poyer, S.; Pastoriza-Gallego, M.; Oukhaled, A.; Mathé, J.; Daniel, R.. Comprehensive structural assignment of glycosaminoglycan oligo- and polysaccharides by protein nanopore. Nat. Commun. 2022, 13, 5113. [CrossRef] [PubMed]
- Sah, M.K.; Rath, S.N. Soluble eggshell membrane: A natural protein to improve the properties of biomaterials used for tissue engineering applications. Mater. Sci. Engin: C. 2016, 67, 807–821. [Google Scholar] [CrossRef]
- National Research Council (NRC). Fat and fatty acids. In Nutrient requirements of dogs and cats. The National Academy Press, Washington, DC, USA, 2006, pp. 81–110. [CrossRef]
- EuroFIR, European Food Information Resource. Available online: http://www.eurofir.org/ (accessed on 18th December 2023).
- Unece Standard Egg-1, United Nations New York and Geneva, 2010.
- Kempe, R.; Leppänen, M.; Mäki, K.; Saastamoinen, M.; Särkijärvi, S.; Tiira, K. Koiran ruokinta ja hoito. Saastamoinen, M.; Teräväinen, H. Eds.; Kariston Kirjapaino Oy, Hämeenlinna, Finland, 2010, ISBN 1799-4268.
- Grandjean, D.; Buckley, C.; Charlton, C.; Merrill, R.; Morris, P.; Stevenson, A. In WALTHAM Pocket Book of Essential Nutrition for Cats and Dogs; Grandjean, D., Butterwick, R., Eds.; Beyond Design Solutions Ltd., London, UK, 2009.
- Evenepoel, P.; Geypens, B.; Luypaerts, A.; Hiele, M.; Ghoos, Y.; Rutgeerts, P. Digestibility of Cooked and Raw Egg Protein in Humans as Assessed by Stable Isotope Techniques. J. Nutr. 1998, 128, 1716–1722. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Higashiguchi, S.; Juneka, L.R.; Kim, M.; Yamamoto, T. A structural phase of heat-denatured lysozyme with novel antimicrobial action. J. Agric. Food Chem. 1996, 44, 1418–1423. [Google Scholar] [CrossRef]
- Mine, Y.; Zhang, J.W. Comparative studies on antigenity and allergenicity of native and denatured egg white proteins. J. Agric. Food Chem. 2002, 50, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.; Wilhelm, S.; Linek, M.; Beco, L.; Fontaine, J.; Bergvall, K.; Favrot, C. Factors affecting allergen-specific IgE serum levels in cats. Can J Vet Res. 2012, 76, 45–51. [Google Scholar] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poultry Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L. Egg white proteins. Comp. Biochem. Physiol., 1991, 100B, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Basto, A.P.; Badenes, M.; Almeida, S.C.; Martins, C.; Duarte, A.; Santos, D.M.; Leitão, A. Immune response profile elicited by the model antigen ovalbumin expressed in fusion with the bacterial OprI lipoprotein. Mol. Immunol. 2015, 64, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Khueychai, S.; Jangpromma, N.; Choowongkomon, K.; Joompang, A.; Daduang, S.; Vesaratchavest, M.; Payoungkiattikun, W.; Tachibana, S.; Klaynongsruang, S. A novel ACE inhibitory peptide derived from alkaline hydrolysis of ostrich (Struthio camelus) egg white ovalbumin. Process Biochemist. 2018, 73, 235–245. [Google Scholar] [CrossRef]
- Legros, J.; Jan, S.; Bonnassie, S.; Gautier, M.; Croguennec, T.; Pezennec, S.; Cochet, M.-F.; Nau, F.; Andrews, S.C.; Baron, F. The Role of Ovotransferrin in Egg-White Antimicrobial Activity: A Review. Foods 2021, 10, 823. [Google Scholar] [CrossRef]
- Krkavcová, E.; Kreisinger, J.; Hyánková, L.; Hyršl, P.; Javůrková, V. The hidden function of egg white antimicrobials: egg weight-dependent effects of avidin on avian embryo survival and hatchling phenotype. Biol. Open. 2018, 9, bio031518. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Labas, V.; Helloin, E.; Hervé-Grépinet, V.; Slugocki, C.; Berges, M.; Bourin, M.C.; Brionne, A.; Poirier, J.C.; Gautron, J.; Coste, F.; Nys, Y. Ovalbumin-related protein X is a heparin-binding ov-serpin exhibiting antimicrobial activities. J. Biol. Chem. 2013, 14, 17285–17295. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Jeong, W.; Kim, J.; Yoshimura, Y.; Bazer, F.W.; Han, J.Y.; Song, G. Expression and regulation of beta-defensin 11 in the oviduct in response to estrogen and in ovarian tumors of chickens. Mol. Cell Endocrinol. 2013, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guyot, N.; Labas, V.; Harichaux, G.; Chessé, M.; Poirier, J.C.; Nys, Y.; Réhault-Godbert, S. Proteomic analysis of egg white heparin-binding proteins: towards the identification of natural antibacterial molecules. Sci. Rep. 2016, 6, 27974. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; D’Silva, I. Bioactive components in egg white. In Egg Bioscience and Biotechnology. Mine, Y. Ed.; John Wiley & Sons, Hoboken, NJ, USA, 2008, pp.141-184, ISBN 978-0-470-03998-4.
- Julien, L.A.; Baron, F.; Bonnassie, S.; Nau, F.; Guerin, C.; Jan, S.; Andrews, S.C. The anti-bacterial iron-restriction defence mechanisms of egg white; the potential role of three lipocalin-like proteins in resistance against Salmonella. Biometals. 2019, 32, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Huang, X.; Yan, N.; Jia, L.; Ma, M. Purification of hen egg white ovomacroglobulin using one-step chromatography. J Sep Sci. 2013, 36, 3717–22. [Google Scholar] [CrossRef] [PubMed]
- Hytönen, V.P.; Määttä, J.A.; Niskanen, E.A.; Huuskonen, J.; Helttunen, K.J.; Halling, K.K.; Nordlund, H. R:; Rissanen, K.; Johnson, M.S.; Salminen, T.A.; Kulomaa, M.S.; Laitinen, O.H.; Airenne, T.T. Structure and characterization of a novel chicken biotin-binding protein A (BBP-A). BMC Struct Biol. 2007, 7, 8. [Google Scholar] [CrossRef]
- Hegenauer, J.; Saltmann, P.; Nace, G. Iron (III)-phosphoprotein charts: Stoichiometric equilibrium constant for interaction of iron(III) and phosphoserine residues of phosvitin and casein. Biochemistry 1979, 18, 3865–3879. [Google Scholar] [CrossRef]
- Lu, C.L.; Baker, R.C. Characteristics of egg yolk phosvitin as an antioxidant for inhibiting metal-catalyzed phospholipid oxidation. Poultry Sci 1986, 65, 2065–2075. [Google Scholar] [CrossRef]
- Lu, C.L.; Baker, R.C. Effect of pH and food ingredients on the stability of egg yolk phopholipids and metal-chelator antioxidant activity of phosvitin. J. Food Sci. 1987, 52, 613–616. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sogo, N.; Iwao, R.; Miyamoto, T. Antioxidant effect of egg yolk on linoleate in emulsions. Agric. Biol. Chem. 1990, 54, 3099–3104. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Ishihara, N.; Juneja, L.R. Antioxidant activity of egg-yolk protein hydrolysates in alinoleic acid oxidation system. Food Chem. 2004, 86, 99–103. [Google Scholar] [CrossRef]
- Sattar Khan, M.A.; Nakamura, S.; Ogawa, M.; Akita, E.; Azakami, H.; Kato, A. Bactericidal action of egg yolk phosvitin against Escherichia coli under thermal stress. J. Agric. Food Chem. 2000, 48, 1503–1506. [Google Scholar] [CrossRef]
- Brady, D.; Gaines, S.; Fenelon, L.; McPartlin, J.; O’Farrelly, C. A lipo-protein derived antimicrobial factor from hen-egg yolk is active against Streptococcus species. J. Food Sci. 2002, 67, 3096–3103. [Google Scholar] [CrossRef]
- Mine, Y.; Oberle, C.; Kassaify, Z. Eggshell matrix proteins as defense mechanism of avian eggs. J Agric Food Chem. 2003, 51, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Park, K.; Yoo, Y.; Kim, J.; Yang, H.; Shin, Y. Effects of Egg Shell Membrane Hydrolysates on Anti-Inflammatory, Anti-Wrinkle, Anti-Microbial Activity and Moisture-Protection. Korean J. Food Sci. Anim. Resour. 2014, 34, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian Eggshell Membrane as a Novel Biomaterial A Review. Foods 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, G.C; Koppel, K. Pet Food Palatability Evaluation: A Review of Standard Assay Techniques and Interpretation of Results with a Primary Focus on Limitations. Animals 2015, 5, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.G.; Legette, L.L.; Ikonte, C.J.; Mitmesser, S.H. Choline and DHA in Maternal and Infant Nutrition: Synergistic Implications in Brain and Eye Health. Nutrients. 2019, 21, 1125. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, C.; Fascetti, A.J. Macronutrients in Feline Health. Vet. Clin. North Am: Small Anim. Pract. 2014, 44, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Ylikorpi, P. Kissanhoidon käsikirja: kissan käyttäytyminen, käsittely, perushoito ja ruokinta. Art House, Helsinki, Finland, 2013.
- Fakhoury, H.M.A.; Kvietys, P.R. , AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid. Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Moretti, A.; Paoletta, M.; Liguori, S.; Bertone, M.; Toro, G.; Iolascon, G. Choline: An Essential Nutrient for Skeletal Muscle. Nutrients. 2020, 18, 12–2144. [Google Scholar] [CrossRef] [PubMed]
- Sam, C.; Bordoni, B. Physiology, Acetylcholine. In: StatPearls [Internet]; StatPearls Publishing, Treasure Island, FL, USA; 2023.
- Neunzehn, J.; Szuwart, T.; Wiesmann, H.P. Eggshells as natural calcium carbonate source in combination with hyaluronan as beneficial additives for bone graft materials, an in vitro study. Head Face Med. 2015, 16, 12. [Google Scholar] [CrossRef]
- Nguyen, P.; Reynolds, B.; Zentek, J.; Paßlack, N.; Leray, V. Sodium in feline nutrition. J. Anim. Physiol. Anim. Nutr. (Berl). 2017, 101, 403–420. [Google Scholar] [CrossRef] [PubMed]
- McCown, J.L.; Specht, A.J. Iron Homeostasis and Disorders in Dogs and Cats: A Review. J. Am. Anim. Hosp. Assoc. 2011, 47, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Jugan, M.C. Anemia, iron deficiency, and cobalamin deficiency in cats with chronic gastrointestinal disease. J. Vet. Intern. Med. 2021, 35, 172–178. [Google Scholar] [CrossRef]
- Zentrichová, V.; Pechová, A.; Kovaˇríková, S. Selenium and Dogs: A Systematic Review. Animals 2021, 11, 418. [Google Scholar] [CrossRef]
- EFSA, Feed Material Register. Available online: www.feedmaterialsregister.eu/register (accessed on 10th October 2023).
- Mosley, C.I.; Edwards, T.; Romano, L.; Truchetti, G.; Dunbar, L.; Schiller, T.; Gibson, T.; Bruce, C.; Troncy, E. Proposed Canadian Consensus Guidelines on Osteoarthritis Treatment Based on OA-COAST Stages 1–4. Front. Vet. Sci. 2022, 9, 830098. [Google Scholar] [CrossRef]
- NASC, National Animal Supplements Council. Available online: www.nasc.cc (accessed on 10th October 2023).
- Martinek, V. Anatomie und physiologie des hyalinen knorpels. Dtsch. Z. Sportmed. 2003, 54, 166–170. [Google Scholar]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Hernandez, S.M.; Begum, L.; Keller, L.E.; Fu, Q.; Zhang, S.; Fortier, L.A. Synovium secretome as a disease-modifying treatment for equine osteoarthritis. Am. J. Vet. Res. 2022, 83, ajvr.22.05.0082. [Google Scholar] [CrossRef]
- Nakamura, H.; Vo, P.; Kanakis, I.; Liu, K.; Bou-Gharios, G. Aggrecanase-selective tissue inhibitor of metalloproteinase-3 (TIMP3) protects articular cartilage in a surgical mouse model of osteoarthritis. Sci.Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, I.; Figenschau, Y.; Olsen, E.; Bakkelund, W.; Smedsröd, B.; Sveinbjörnsson, B. Tumor necrosis factor related apoptosis-inducing ligand induces apoptosis in human articular chondrocytes in vitro. Biochem. Biophys. Res. Commun. 2002, 296, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zhang, Q.; Wang, Y.; Lee, B.; Betageri, G.V.; Chow, M.S.; Huang, M.; Zuo, Z. Bioavailability enhancement of glucosamine hydrochloride by chitosan. Int. J. Pharm. 2013, 455, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ikawa, N.; Ozimek, L. Extraction of Glycosaminoglycans from Chicken Eggshell. Poultry Sci. 2001, 80, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Jerosch, J. Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int. J. Rheumatol. 2011, 969012. [Google Scholar] [CrossRef] [PubMed]
- Casale, J.; Crane, J.S. Biochemistry, Glycosaminoglycans; In StatPearls. Available online: www.ncbi.nlm.nih.gov/books/NBK544295/ (accessed on 12th December 2023).
- Xiao, J.F.; Zhang, Y.N.; Wu, S.G.; Zhang, H.J.; Yue, H.Y.; Qi, G.H. Manganese supplementation enhances the synthesis of glycosaminoglycan in eggshell membrane: A strategy to improve eggshell quality in laying hens. Poultry Sci. 2014, 93, 380–388. [Google Scholar] [CrossRef]
- Ruff, K.J.; Durham, P.L.; O'Reilly, A.; Long, F.D. Eggshell membrane hydrolyzates activate NF-κB in vitro: possible implications for in vivo efficacy. J. Inflamm. Res. 2015, 9, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef]
- Serra Aguado, C.I.; Ramos-Plá, J.J.; Soler, C.; Segarra, S.; Moratalla, V.; Redondo, J.I. Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury. Animals 2021, 11, 1264. [Google Scholar] [CrossRef]
- Dodge, G.R.; Regatte, R.R.; Noyszewski, E.A.; Hall, J.O.; Sharma, A.V.; Callaway, D.A.; Reddy, R. The Fate of Oral Glucosamine Traced by (13)C Labeling in the Dog. Cartilage 2011, 2, 279–285. [Google Scholar] [CrossRef]
- Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Schunck, M.; Louton, H.; Oesser, S. (2017) The Effectiveness of Specific Collagen Peptides on Osteoarthritis in Dogs-Impact on Metabolic Processes in Canine Chondrocytes. Open J. Anim. Sci. 2017, 7, 254–266. [Google Scholar] [CrossRef]
- Kobayashi, K.; Jokaji, R.; Miyazawa-Hira, M.; Takatsuka, S.; Tanaka, A.; Ooi, K.; Nakamura, H.; Kawashiri, S. Elastin-derived peptides are involved in the processes of human temporomandibular disorder by inducing inflammatory responses in synovial cells. Mol. Med. Rep. 2017, 16, 3147–3154. [Google Scholar] [CrossRef]
- Ruff, K.J; Durham, P.L; O’Reilly, A.; Long, F.D. Eggshell membrane hydrolyzates activate NF-κB in vitro: possible implications for in vivo efficacy. J. Inflamm. Res. 2015, 8, 49–57. [Google Scholar] [CrossRef]
- Schunck, M.; Schulze, C.H.; Oesser, S. Collagen peptide supplementation stimulates proteoglycan biosynthesis and aggrecan expression of articular chondrocytes. Osteoarthritis and Cartilage 2009, 17, S1. [Google Scholar] [CrossRef]
- Schunck, M.; Oesser, S. Specific collagen peptides benefit the biosynthesis of matrix molecules of tendons and ligaments. J. Int. Soc. Sports Nutr. 2013, 10, P23, www.jissn.com/content/10/S1/P23. [Google Scholar] [CrossRef]
- Schunck, M.; Louton, H.; Oesser, S. The Effectiveness of Specific Collagen Peptides on Osteoarthritis in Dogs-Impact on Metabolic Processes in Canine Chondrocytes. Open J. Anim. Sci. 2017, 7, 254–266. [Google Scholar] [CrossRef]
- Ruff, K.J.; DeVore, D.P. Reduction of pro-inflammatory cytokines in rats following 7-day oral supplementation with a proprietary eggshell membrane-derived product. Mod. Res. Inflamm. 2014, 3, 19–25. [Google Scholar] [CrossRef]
- Wedekind, K.J.; Coverdale, J.A.; Hampton, T.R.; Atwell, C.A.; Sorbet, R.H.; Lunnemann, J.; Harrell, R.J.; Greiner, L.; Keith, N.K.; Evans, J.L.; Zhao, J.; Knight, C.D. Efficacy of an equine joint supplement, and the synergistic effect of its active ingredients (chelated trace minerals and natural eggshell membrane), as demonstrated in equine, swine, and an osteoarthritis rat model. Open Access Anim. Physiol. 2015, 7, 13–27. [Google Scholar] [CrossRef]
- Sim, B.Y.; Bak, J.W.; Lee, H.J.; Jun, J.A.; Choi, H.J.; Kwon, C.J.; Kim, H.Y.; Ruff, K.J.; Brandt, K.; Kim, D.H. Effects of natural eggshell membrane (NEM) on monosodium iodoacetate-induced arthritis in rats. J. Nutr. Health 2015, 48, 310–318. [Google Scholar] [CrossRef]
- Baird, R.K. The effect of Hydrolyzed Eggshell Membrane Powder on joint mobility in dog. Biova study report 2008.
- Ruff, K.J.; Kopp, K.J.; Von Behrens, P.; Lux, M.; Mahn, M.; Back, M. Effectiveness of NEM® brand eggshell membrane in the treatment of suboptimal joint function in dogs: a multicenter, randomized, double-blind, placebo-controlled study. Vet. Med. (Auckl) 2016, 7, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Gil-Quintana, E.; Fenaux, M.; Sanchez, N.; Torre, C. The efficacy of Ovopet® in the treatment of hip dysplasia in dogs. J. Vet. Med. Anim. Health 2018, 10, 198–207. [Google Scholar]
- Muller, C.; Enomoto, M.; Buono, A.; Steiner, J.M.; Lascelles, B.D.X. Placebo-controlled pilot study of the effects of an eggshell membrane-based supplement on mobility and serum biomarkers in dogs with osteoarthritis. Vet. J. 2019, 253, 105379. [Google Scholar] [CrossRef] [PubMed]
- Blasco, J.M.-I.; Aguirre, A.; Gil Quintana, E.; Fenaux, M. The effect of daily administration of 300 mg of Ovomet® for treatment of arthritis in elderly patients. Int. J. Clin. Rheumatol. 2016, 11, 077–081. [Google Scholar]
- Gil-Quintana, E.; Molero, A.; Aguirre, A. Ovopet® a new and effective treatment to decrease inflammation, pain and lameness in competing trotters. J. Vet. Med. Anim. Health 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Ruff, K.J.; DeVore, D.P.; Leu, M.D.; Robinson, M.A. Eggshell membrane: A possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin. Interv. Aging 2009a, 4, 235–240. [Google Scholar] [CrossRef]
- Ruff, K.J.; Winkler, A.; Jackson, R.W.; DeVore, D.P.; Ritz, B.W. Eggshell membrane in the treatment of pain and stiffness from osteoarthritis of the knee: a randomized, multicenter, double-blind, placebo-controlled clinical study. Clin. Rheumatol. 2009, 28, 907–914. [Google Scholar] [CrossRef]
- Danesch, U.; Seybold, M.; Rittinghausen, R.; Treibel, W.; Bitterlich, N. NEM Brand Eggshell Membrane Effective in the Treatment of Pain Associated with Knee and Hip Osteoarthritis: Results from a Six Center, Open Label German Clinical Study. J. Arthritis 2014, 3, 136. [Google Scholar] [CrossRef]
- Jensen, G.S.; Lenninger, M.R.; Beaman, J.L.; Taylor, R.; Benson, K.F. Support of joint function, range of motion, and physical activity levels by consumption of a water-soluble egg membrane hydrolyzate. J. Med. Food 2015, 8, 1042–1048. [Google Scholar] [CrossRef]
- Brunello, E.; Masini, A. NEM® Brand Eggshell Membrane Effective in the Treatment of Pain and Stiffness Associated with Osteoarthritis of the Knee in an Italian Study Population. Int. J. Clin. Med. 2016, 7, 169–175. [Google Scholar] [CrossRef]
- Ruff, K.J.; Morrison, D.; Duncan, S.A.; Back, M.; Aydogan, C.; Theodosakis, J. Beneficial effects of natural eggshell membrane versus placebo in exercise-induced joint pain, stiffness, and cartilage turnover in healthy, postmenopausal women. Clin. Interv. Aging 2018, 13, 285–295. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D.; Schneider, L.V. A Randomized, Double-Blind, Placebo-Controlled, Prospective Clinical Trial Evaluating Water-Soluble Chicken Eggshell Membrane for Improvement in Joint Health in Adults with Knee Osteoarthritis. J. Med. Food. 2019, 22, 875–884. [Google Scholar] [CrossRef]
- Kiers, J.L.; Bult, J.H.F. Mildly Processed Natural Eggshell Membrane Alleviates Joint Pain Associated with Osteoarthritis of the Knee: A Randomized Double-Blind Placebo-Controlled Study. J. Med. Food 2021, 24, 292–298. [Google Scholar] [CrossRef]
- Ahmed, T.A.E; Suso, H.-P.; Maqbool, A. , Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mat. Sci. Engin: C 2019, 95, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hou, Q,; Zhong, L.; Zhao, Y.; Li, M.; Fu, X. Bioactive Molecules for Skin Repair and Regeneration: Progress and Perspectives. Stem Cells Int. 2019, Article ID 6789823. [CrossRef]
- Al-Atif, H. Collagen Supplements for Aging and Wrinkles: A Paradigm Shift in the Fields of Dermatology and Cosmetics. Dermatol. Pract. Concept. 2022, 12, e2022018. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Buckley, C.; Murphy, E.J.; Montgomery, T.R.; Major, I. Hyaluronic Acid: A Review of the Drug Delivery Capabilities of This Naturally Occurring Polysaccharide. Polymers 2022, 14, 3442. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, X.; Li, L.; Yu, C.; Nie, L.; Tian, W.; Zhang, H.; Huang, S.; Zang, H. Understanding hyaluronic acid induced variation of water structure by near-infrared spectroscopy. Sci Rep. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Ogston, A.G.; Stanier, J.E. The physiological function of hyaluronic acid in synovial fluid; viscous, elastic and lubricant properties. J. Physiol. 1953, 119, 244–252. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid. A key molecule in skin aging. Dermatoendocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Bolke, L.; Schlippe, G.; Gerß, J.; Vos, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant'Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, 2. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.I.; Sotelo, C.G. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Mar. Drugs 2018, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Rupa, R.; Kovacs-Nolan, J.; Turner, P.V.; Matsui, T.; Mine, Y. Oral administration of hen egg white ovotransferrin attenuates the development of colitis induced by dextran sodium sulfate in mice. J. Agric. Food Chem. 2015, 63, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S.; Hewlings, S. The effect of oral hydrolyzed eggshell membrane on the appearance of hair, skin, and nails in healthy middle-aged adults: A randomized double-blind placebo-controlled clinical trial. J. Cosmet. Dermatol. 2020, 19, 1463–1472. [Google Scholar] [CrossRef]
- Sim, W.-J.; Ahn, J.; Lim, W.; Son, D.J.; Lee, E.; Lim, T.-G. Anti-skin aging activity of eggshell membrane administration and its underlying mechanism. Mol. Cell. Toxicol. 2023, 19, 165–176. [Google Scholar] [CrossRef]
- Kinsella, J.E. α-Linolenic acid: Functions and effects on linoleic acid metabolism and eicosanoid-mediated reactions. In Advances in food and nutrition research, Vol 35. Kinsella, J.E., Ed.; Academic Press, London 1991, pp. 1-160.
- Kirby, N.A.; Hester, S.L.; Rees, C.A.; Kennis, R.A.; Zoran, D.L.; Bauer, J.E. Skin surface lipids and skin and hair coat condition in dogs fed increased total fat diets containing polyunsaturated fatty acids. J. Anim. Physiol. Anim. Nutr. 2009, 93, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Heinze, C.R.; Linder, D.E.; Freeman, L.M. Evaluation of marketing claims, ingredients, and nutrient profiles of over-the-counter diets marketed for skin and coat health of dogs. J. Am. Vet. Med. Assoc 2015, 246, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Logas, D.; Kunkle, G.A. Double-blinded Crossover Study with Marine Oil Supplementation Containing High-dose icosapentaenoic Acid for the Treatment of Canine Pruritic Skin Disease. Vet. Dermatol. 1994, 5, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, T.R.; Lourenço, A.L.; Gregório, H.; Queiroga, F.L. Therapeutic Effect of EPA/DHA Supplementation in Neoplastic and Non-neoplastic Companion Animal Diseases: A Systematic Review. In vivo 2021, 35, 1419–1436. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Solano, F. Metabolism and Functions of Amino Acids in the Skin. Adv. Exp. Med. Biol. 2020, 1265, 187–199. [Google Scholar] [CrossRef]
- Zou, P.; Du, Y.; Yang, C.; Cao, Y. Trace element zinc and skin disorders. Front. Med. (Lausanne) 2023, 9, 1093868. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients. 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Hanate, M.; Aw, W.; Itoh, H.; Saito, K.; Kobayashi, S.; Hachimura, S.; Fukuda, S.; Tomita, M.; Hasebe, Y.; Kato, H. Eggshell membrane powder ameliorates intestinal inflammation by facilitating the restitution of epithelial injury and alleviating microbial dysbiosis. Sci. Rep. 2017, 7, 43993. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; de Wit, A.; Diedericks, C.F.; Venema, P.; van der Linden, E.; . Sagis, L. M-C. Foaming and emulsifying properties of extensively and mildly extracted Bambara groundnut proteins: A comparison of legumin, vicilin and albumin protein. Food Hydrocolloids 2022, 123, 107190. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Hatta, H.; Ozeki, M.; Tsuda, K. Egg yolk antibody IgY and its applications. In Hen Eggs: Their Basic and Applied Science; Yamamoto, T., Juneja, L.R., Hatta, H., Kim, M., Eds.; CRC Press Inc., Boca Raton, FL, USA, 1997, pp. 151-178.
- Butkovic, V.; Simpraga, M.; Sehic, M.; Stanin, D.; Susic, V.; Capak, D.; Kos, J. Dental disease of dogs: a retrospective study of radiological data. Acta Vet. Brno 2001, 70, 203–208. [Google Scholar] [CrossRef]
- Kyllar, M.; Witter, K. Prevalence of dental disorders in pet dogs. Vet. Med.-Czech 2005, 50, 496–505. [Google Scholar] [CrossRef]
- Kortegaard, H.E.; Eriksen, T.; Baelum, V. Periodontal disease in research beagle dogs – an epidemilogical study. J. Small Anim. Pract. 2008, 49, 610–616. [Google Scholar] [CrossRef]
- U.K. Study Explores Prevalence of Feline Dental Disease. Available online: todaysveterinarybusiness.com/feline-dental-disease (accessed 24th September 2023).
- Reiter, A.M. Periodontal Disease in Small Animals. In The Merck Veterinary Manual, 2022.
- Shafiqur, R.A.K.M.; Ibrahim, E.-S.M.; Isoda, R.; Umeda, K.; Nguyen, V.S.; Kodama, Y. Effect of passive immunization. by anti-gingipain IgY on periodontal heath of dogs. Vet. Sci. Dev. 2011, 1:e8, 35-39. [CrossRef]
- Davis, I.J.; Wallis, C.; Deusch, O.; Colyer, A.; Milella, L.; Loman, N.; Harris, S. A cross-sectional survey of bacterial species in plaque from client owned dogs with healthy gingiva, gingivitis or mild periodontitis. PLoS ONE, 2013, 8, e83158. [CrossRef]
- Diraviyam, T.; Zhao, B.; Wang, Y.; Schade, R.; Michale, A.; Zhang, X. (2014) Effect of cheicken egg yolk antibodies (IgY) against diarrhea in domestical animals: a systematic review and meta-analysis. PLoS ONE 2014, 9, e97716. [Google Scholar] [CrossRef]
- Van Nguyen, S.; Umeda, K.; Yokoyama, H.; Tohya, Y.; Kodama, Y. (2006), Passive protection of dogs against clinical disease due to canine parvovirus-2 by specific antibody from chicken egg yolk. Can. J. Vet. Res. 2006, 70, 62–64. [Google Scholar]
- Mine, Y.; Kovacs-Nolan, J. Chicken Egg Yolk Antibodies as Therapeutics in Enteric Infectious Disease: A Review. J. Med. Food 2002, 5, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Hlokoe, V.R.; Tyasi, T.L.; Gunya, B. Chicken ovarian follicles morphology and growth differentiation factor 9 gene expression in chicken ovarian follicles: review. Heliyon 2022, 8, e08742. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Vernerova, K.; Kizek, R.; Bozzi, R.; Kadlec, J.; Curn, V.; Kouba, F.; Fernandez, C.; Machander, V.; Horna, H. Associations Between IGF1, IGFBP2 and TGFß3 Genes Polymorphisms and Growth Performance of Broiler Chicken Lines. Animals 2020, 10, 800. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal. Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Jia H.; Lyu, W.; Hirota, K.; Saito. E.; Miyoshi, M.; Hohjoh, H.; Furukawa, K.; Saito, K.; Haritani, M.; Taguchi, A.; Hasebe, Y.; Kato, H. Eggshell membrane modulates gut microbiota to prevent murine pre-cachexia through suppression of T helper cell differentiation. J. Cachexia, Sarcopenia and Muscle. 2022. [CrossRef]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Sutoyo, D.A.; Atmaka, D.A.; Sidabutar, L.M.G.B. Dietary factors affecting Firmicutes and Bacteroidetes ratio in solving obesity problem: a literature review. Media Gizi Indonesia. 2020, 15, 94–109. [Google Scholar] [CrossRef]
- Biozyme. Available online: biozymeinc.com/additive/eqe/ (accessed on 2nd November 2023).
- Ramli, N.S.; Jia, H.; Sekine, A.; Lyu, W.; Furukawa, K.; Saito, K.; Hasebe, Y.; Kato, H. Eggshell membrane powder lowers plasma triglyceride and liver total cholesterol by modulating gut microbiota and accelerating lipid metabolism in high-fat diet-fed mice. Food Sci. Nutr. 2020, 8, 2512–2523. [Google Scholar] [CrossRef]
- Algya, K.M.; Cross, T.-W.L.; Leuck, K.N.; Kastner, M.E.; Baba, T.; Lye, L.; de Godoy, M.R.C.; Swanson, K.S. Apparent total-tract macronutrient digestibility, serum chemistry, urinalysis, and fecal characteristics, metabolites and microbiota of adult dogs fed extruded, mildly cooked, and raw diets. J. Anim. Sci. 2018, 96, 3670–3683. [Google Scholar] [CrossRef]
- Jewell, D.E.; Jackson, M.I.; Cochrane, C.-Y.; Badri, D.V. Feeding Fiber-Bound Polyphenol Ingredients at Different Levels Modulates Colonic Postbiotics to Improve Gut Health in Cats. Animals 2022, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, X.; Liu, X.; Wang, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective From Gut Microbiota. Front. Nutr. 2021, 8, 693412. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.B.; Clifton, P.M. Energy Intake and Satiety Responses of Eggs for Breakfast in Overweight and Obese Adults-A Crossover Study. Int. J. Environ. Res. Public Health. 2020, 3, 17–5583. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Beerda, B.; Hendriks, W.H.; van der Poel, A.F.; Verstegen, M.W. Impact of nutrition on canine behaviour: current status and possible mechanisms. Nutr. Res. Rev. 2007, 20, 180–94. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.P; Wakshlag, J.J. Canine and feline obesity: a review of pathophysiology, epidemiology, and clinical management. Vet Med: Res. and Rep 2015, 49–60. [Google Scholar] [CrossRef]

| Water (%) | Protein (%) | Lipid (%) |
Carbohydrate (%) | Ash (%) |
|
|---|---|---|---|---|---|
| Whole egg (100 %) | 66.1 | 12.8-13.4 | 10.5-11.8 | 0.3-1.0 | 0.8-1.0 |
| Yolk (28-29 %) | 48.7 | 15.7-16.6 | 31.8-35.5 | 0.2-1.0 | 1.1 |
| Egg white (60-63 %) | 87.6 | 9.7-10.6 | 0.03 | 0.4-0.9 | 0.5-0.6 |
| Eggshell and membranes (9-11 %) | 1.6 | 6.2-6.4 | 0.03 | Traces | 91-92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





