Submitted:
10 January 2024
Posted:
11 January 2024
You are already at the latest version
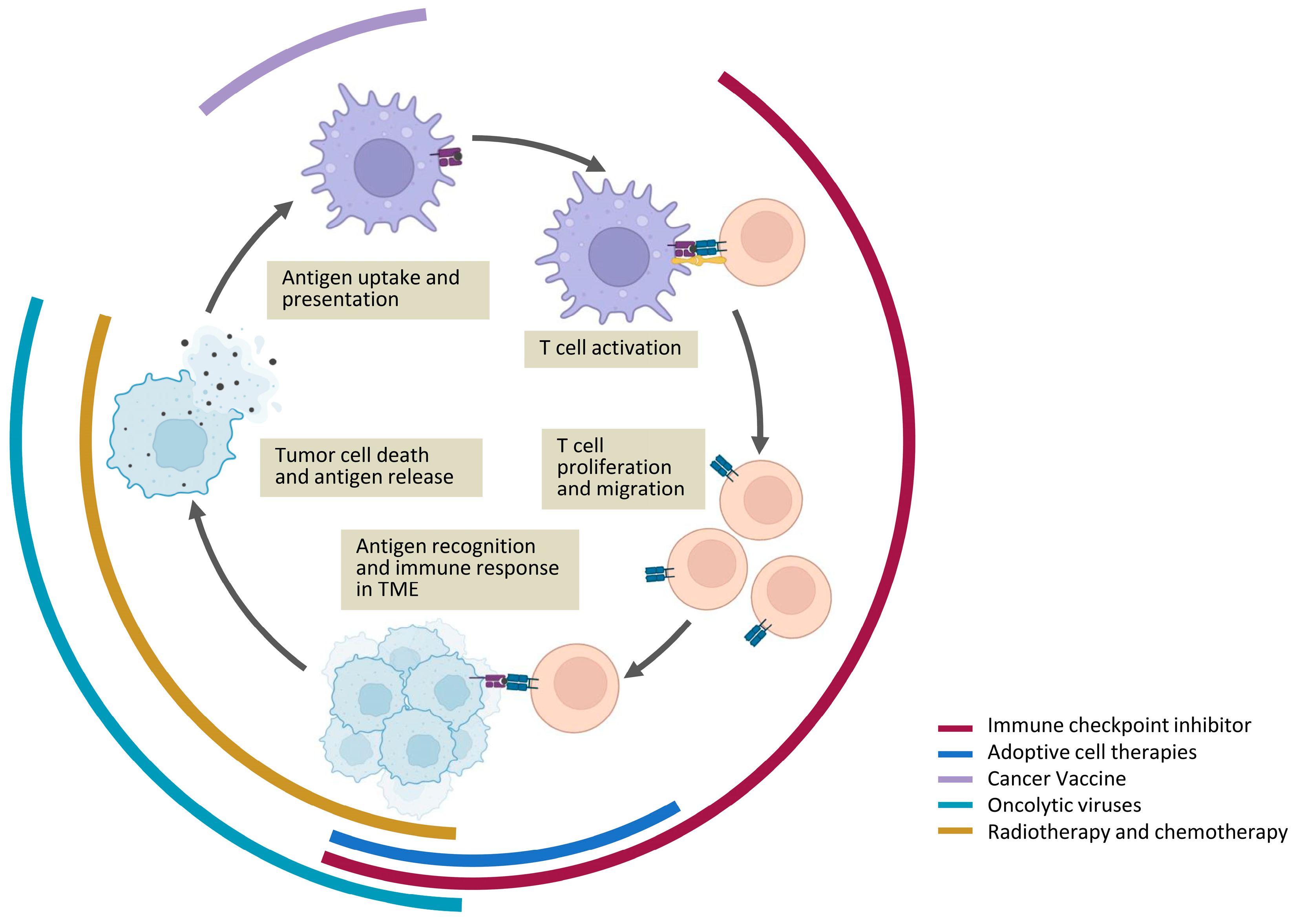
Abstract
Keywords:
1. Medulloblastoma
1.1. ICI for MB
1.2. Chimeric Antigen Receptor (CAR)-T Cell and TCR Modificed T Cell Therapy for MB
1.3. Naural Killer (NK) Cell Therapy for MB
1.4. Vaccine Therapy for MB
1.5. Oncolytic Virus Therapy for MB
2. Pediatric Glioma
2.1. ICI Therapy for Pediatric Glioma
2.2. CAR T Therapy for Pediatric Glioma
2.3. Vaccines for Pediatric Gliomas
2.4. NK cell Therapy for Pediatric Glioma
2.5. Oncolytic Virus Therapy for Pediatric Glioma
3. Ependymoma
3.1. ICI for Pediatric Ependymoma
3.2. CAR T Therapy for Pediatric Ependymoma
3.3. Vaccines for Pediatric Ependymoma
4. Tumor Immune Microenvironment (TIME)
4.1. Medulloblastoma TIME
4.2. Pediatric Glioma TIME
4.3. Ependymoma TIME
5. Future Directions and Prospective
Funding
Conflicts of Interest
References
- Heath, J.A., S. Zacharoulis, and M.W. Kieran, Pediatric neuro-oncology: current status and future directions. Asia Pac J Clin Oncol, 2012. 8(3): p. 223-31. [CrossRef]
- Lutz, K., S.T. Jünger, and M. Messing-Jünger, Essential Management of Pediatric Brain Tumors. Children (Basel), 2022. 9(4). [CrossRef]
- Chevignard, M., et al., Core deficits and quality of survival after childhood medulloblastoma: a review. Neurooncol Pract, 2017. 4(2): p. 82-97. [CrossRef]
- Lah, T.T., M. Novak, and B. Breznik, Brain malignancies: Glioblastoma and brain metastases. Semin Cancer Biol, 2020. 60: p. 262-273. [CrossRef]
- Aziz-Bose, R. and M. Monje, Diffuse intrinsic pontine glioma: molecular landscape and emerging therapeutic targets. Curr Opin Oncol, 2019. 31(6): p. 522-530. [CrossRef]
- Liu, C., et al., Clinical cancer immunotherapy: Current progress and prospects. Front Immunol, 2022. 13: p. 961805. [CrossRef]
- Seidel, J.A., A. Otsuka, and K. Kabashima, Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol, 2018. 8: p. 86. [CrossRef]
- Kalos, M. and C.H. June, Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity, 2013. 39(1): p. 49-60. [CrossRef]
- Lin, M.J., et al., Cancer vaccines: the next immunotherapy frontier. Nat Cancer, 2022. 3(8): p. 911-926. [CrossRef]
- Shalhout, S.Z., et al., Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol, 2023. 20(3): p. 160-177. [CrossRef]
- Louis, D.N., et al., The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol, 2021. 23(8): p. 1231-1251. [CrossRef]
- Louis, D.N., et al., The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol, 2016. 131(6): p. 803-20. [CrossRef]
- Fangusaro, J. and P. Bandopadhayay, Advances in the classification and treatment of pediatric brain tumors. Curr Opin Pediatr, 2021. 33(1): p. 26-32. [CrossRef]
- Ostrom, Q.T., et al., CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol, 2018. 20(suppl_4): p. iv1-iv86. [CrossRef]
- Rossi, A., et al., Medulloblastoma: from molecular pathology to therapy. Clin Cancer Res, 2008. 14(4): p. 971-6. [CrossRef]
- Northcott, P.A., et al., Medulloblastoma. Nature Reviews Disease Primers, 2019. 5(1): p. 11. [CrossRef]
- Smoll, N.R. and K.J. Drummond, The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci, 2012. 19(11): p. 1541-4. [CrossRef]
- Gajjar, A., et al., Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol, 2006. 7(10): p. 813-20. [CrossRef]
- Louis, D.N., et al., The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology, 2021. 23(8): p. 1231-1251. [CrossRef]
- Taylor, M.D., et al., Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol, 2012. 123(4): p. 465-72. [CrossRef]
- Cavalli, F.M.G., et al., Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell, 2017. 31(6): p. 737-754 e6. [CrossRef]
- Manoranjan, B., et al., Wnt activation as a therapeutic strategy in medulloblastoma. Nat Commun, 2020. 11(1): p. 4323. [CrossRef]
- Sursal, T., et al., Molecular Stratification of Medulloblastoma: Clinical Outcomes and Therapeutic Interventions. Anticancer Res, 2022. 42(5): p. 2225-2239. [CrossRef]
- Thompson, M.C., et al., Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol, 2006. 24(12): p. 1924-31. [CrossRef]
- Kool, M., et al., Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell, 2014. 25(3): p. 393-405. [CrossRef]
- Menyhart, O. and B. Gyorffy, Principles of tumorigenesis and emerging molecular drivers of SHH-activated medulloblastomas. Ann Clin Transl Neurol, 2019. 6(5): p. 990-1005. [CrossRef]
- Zhukova, N., et al., Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol, 2013. 31(23): p. 2927-35. [CrossRef]
- Ramaswamy, V., et al., Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol, 2016. 131(6): p. 821-31. [CrossRef]
- Northcott, P.A., et al., The whole-genome landscape of medulloblastoma subtypes. Nature, 2017. 547(7663): p. 311-317. [CrossRef]
- Choi, J.Y., Medulloblastoma: Current Perspectives and Recent Advances. Brain Tumor Res Treat, 2023. 11(1): p. 28-38. [CrossRef]
- Sharma, T., et al., Second-generation molecular subgrouping of medulloblastoma: an international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol, 2019. 138(2): p. 309-326. [CrossRef]
- Kumar, R., A.P.Y. Liu, and P.A. Northcott, Medulloblastoma genomics in the modern molecular era. Brain Pathol, 2020. 30(3): p. 679-690. [CrossRef]
- Vermeulen, J.F., et al., Prognostic relevance of tumor-infiltrating lymphocytes and immune checkpoints in pediatric medulloblastoma. Oncoimmunology, 2018. 7(3): p. e1398877. [CrossRef]
- Martin, A.M., et al., PD-L1 expression in medulloblastoma: an evaluation by subgroup. Oncotarget, 2018. 9(27): p. 19177-19191. [CrossRef]
- Bockmayr, M., et al., Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology, 2018. 7(9): p. e1462430. [CrossRef]
- Pham, C.D., et al., Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clin Cancer Res, 2016. 22(3): p. 582-95. [CrossRef]
- Dunkel, I.J., et al., Nivolumab with or without ipilimumab in pediatric patients with high-grade CNS malignancies: Safety, efficacy, biomarker, and pharmacokinetics-CheckMate 908. Neuro Oncol, 2023. 25(8): p. 1530-1545. [CrossRef]
- Eisemann, T. and R.J. Wechsler-Reya, Coming in from the cold: overcoming the hostile immune microenvironment of medulloblastoma. Genes Dev, 2022. 36(9-10): p. 514-532. [CrossRef]
- Ahmed, N., et al., Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res, 2007. 67(12): p. 5957-64. [CrossRef]
- Gajjar, A., et al., Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol, 2004. 22(6): p. 984-93. [CrossRef]
- Nellan, A., et al., Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer, 2018. 6(1): p. 30. [CrossRef]
- Ivanov, D.P., et al., In vitro models of medulloblastoma: Choosing the right tool for the job. J Biotechnol, 2016. 236: p. 10-25. [CrossRef]
- Morgan, R.A., et al., Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther, 2010. 18(4): p. 843-51. [CrossRef]
- Epping, M.T. and R. Bernards, A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res, 2006. 66(22): p. 10639-42. [CrossRef]
- Orlando, D., et al., Adoptive Immunotherapy Using PRAME-Specific T Cells in Medulloblastoma. Cancer Res, 2018. 78(12): p. 3337-3349. [CrossRef]
- Li, S., et al., Pediatric medulloblastoma express immune checkpoint B7-H3. Clin Transl Oncol, 2022. 24(6): p. 1204-1208. [CrossRef]
- Haydar, D., et al., Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncol, 2021. 23(6): p. 999-1011. [CrossRef]
- Majzner, R.G., et al., CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res, 2019. 25(8): p. 2560-2574. [CrossRef]
- Donovan, L.K., et al., Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nature Medicine, 2020. 26(5): p. 720-731. [CrossRef]
- Donovan, L.K., et al., Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med, 2020. 26(5): p. 720-731. [CrossRef]
- Stastny, M.J., et al., Medulloblastomas Expressing IL13Rα2 are Targets for IL13-zetakine+ Cytolytic T Cells. Journal of Pediatric Hematology/Oncology, 2007. 29(10): p. 669-677. [CrossRef]
- Wu, S.Y., et al., Natural killer cells in cancer biology and therapy. Mol Cancer, 2020. 19(1): p. 120. [CrossRef]
- Castriconi, R., et al., Both CD133+ and CD133- medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. Eur J Immunol, 2007. 37(11): p. 3190-6. [CrossRef]
- Fernández, L., et al., In vitro Natural Killer Cell Immunotherapy for Medulloblastoma. Front Oncol, 2013. 3: p. 94. [CrossRef]
- Powell, A.B., et al., Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J Transl Med, 2019. 17(1): p. 321. [CrossRef]
- Kennis, B.A., et al., Monitoring of intracerebellarly-administered natural killer cells with fluorine-19 MRI. J Neurooncol, 2019. 142(3): p. 395-407. [CrossRef]
- Khatua, S., et al., Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro Oncol, 2020. 22(8): p. 1214-1225. [CrossRef]
- Vallera, D.A., et al., NK-Cell-Mediated Targeting of Various Solid Tumors Using a B7-H3 Tri-Specific Killer Engager In Vitro and In Vivo. Cancers (Basel), 2020. 12(9). [CrossRef]
- Ardon, H., et al., Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer, 2010. 54(4): p. 519-25. [CrossRef]
- Nair, S.K., et al., Ex vivo generation of dendritic cells from cryopreserved, post-induction chemotherapy, mobilized leukapheresis from pediatric patients with medulloblastoma. J Neurooncol, 2015. 125(1): p. 65-74. [CrossRef]
- Studebaker, A.W., et al., Treatment of medulloblastoma with a modified measles virus. Neuro Oncol, 2010. 12(10): p. 1034-42. [CrossRef]
- Lal, S., et al., An oncolytic measles virus–sensitive Group 3 medulloblastoma model in immune-competent mice. Neuro-Oncology, 2018. 20(12): p. 1606-1615. [CrossRef]
- Hutzen, B., et al., Treatment of medulloblastoma using an oncolytic measles virus encoding the thyroidal sodium iodide symporter shows enhanced efficacy with radioiodine. BMC Cancer, 2012. 12: p. 508. [CrossRef]
- Hutzen, B., et al., Treatment of medulloblastoma with oncolytic measles viruses expressing the angiogenesis inhibitors endostatin and angiostatin. BMC Cancer, 2014. 14: p. 206. [CrossRef]
- Friedman, G.K., et al., Herpes simplex virus oncolytic therapy for pediatric malignancies. Mol Ther, 2009. 17(7): p. 1125-35. [CrossRef]
- Mineta, T., et al., Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med, 1995. 1(9): p. 938-43. [CrossRef]
- Parker, J.N., et al., Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A, 2000. 97(5): p. 2208-13. [CrossRef]
- Friedman, G.K., et al., Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro Oncol, 2016. 18(2): p. 227-35. [CrossRef]
- Cassady, K.A., Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection. J Virol, 2005. 79(14): p. 8707-15. [CrossRef]
- Cassady, K.A., et al., Pre-clinical Assessment of C134, a Chimeric Oncolytic Herpes Simplex Virus, in Mice and Non-human Primates. Mol Ther Oncolytics, 2017. 5: p. 1-10. [CrossRef]
- Hedberg, J., et al., Oncolytic virus-driven immune remodeling revealed in mouse medulloblastomas at single cell resolution. Mol Ther Oncolytics, 2023. 30: p. 39-55. [CrossRef]
- Chase, M., R.Y. Chung, and E.A. Chiocca, An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nature Biotechnology, 1998. 16(5): p. 444-448. [CrossRef]
- Studebaker, A.W., et al., Oncolytic Herpes Virus rRp450 Shows Efficacy in Orthotopic Xenograft Group 3/4 Medulloblastomas and Atypical Teratoid/Rhabdoid Tumors. Mol Ther Oncolytics, 2017. 6: p. 22-30. [CrossRef]
- Gromeier, M., et al., Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A, 2000. 97(12): p. 6803-8. [CrossRef]
- Thompson, E.M., et al., Poliovirus Receptor (CD155) Expression in Pediatric Brain Tumors Mediates Oncolysis of Medulloblastoma and Pleomorphic Xanthoastrocytoma. J Neuropathol Exp Neurol, 2018. 77(8): p. 696-702. [CrossRef]
- Reddy, P.S., et al., Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst, 2007. 99(21): p. 1623-33. [CrossRef]
- Wadhwa, L., et al., Treatment of invasive retinoblastoma in a murine model using an oncolytic picornavirus. Cancer Res, 2007. 67(22): p. 10653-6. [CrossRef]
- Yu, L., et al., A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro Oncol, 2011. 13(1): p. 14-27. [CrossRef]
- McFadden, G., Poxvirus tropism. Nat Rev Microbiol, 2005. 3(3): p. 201-13. [CrossRef]
- Lun, X.Q., et al., Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res, 2007. 67(18): p. 8818-27. [CrossRef]
- Pollack, I.F., S. Agnihotri, and A. Broniscer, Childhood brain tumors: current management, biological insights, and future directions. J Neurosurg Pediatr, 2019. 23(3): p. 261-273. [CrossRef]
- Freeman, C.R., J.P. Farmer, and J. Montes, Low-grade astrocytomas in children: evolving management strategies. Int J Radiat Oncol Biol Phys, 1998. 41(5): p. 979-87. [CrossRef]
- Krishnatry, R., et al., Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer, 2016. 122(8): p. 1261-9. [CrossRef]
- Bandopadhayay, P., et al., Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer, 2014. 61(7): p. 1173-9. [CrossRef]
- Shaw, E.G. and J.H. Wisoff, Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol, 2003. 5(3): p. 153-60. [CrossRef]
- Ater, J.L., et al., Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children's Oncology Group. Cancer, 2016. 122(12): p. 1928-36. [CrossRef]
- de Blank, P., et al., Management of pediatric low-grade glioma. Curr Opin Pediatr, 2019. 31(1): p. 21-27. [CrossRef]
- Zhang, J., et al., Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet, 2013. 45(6): p. 602-12. [CrossRef]
- Jones, D.T., et al., Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene, 2009. 28(20): p. 2119-23. [CrossRef]
- Pfister, S., et al., BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest, 2008. 118(5): p. 1739-49. [CrossRef]
- Ryall, S., U. Tabori, and C. Hawkins, Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun, 2020. 8(1): p. 30. [CrossRef]
- Cole, B.L., Neuropathology of Pediatric Brain Tumors: A Concise Review. Neurosurgery, 2022. 90(1): p. 7-15. [CrossRef]
- Ostrom, Q.T., et al., CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol, 2021. 23(12 Suppl 2): p. iii1-iii105. [CrossRef]
- Blionas, A., et al., Paediatric gliomas: diagnosis, molecular biology and management. Ann Transl Med, 2018. 6(12): p. 251. [CrossRef]
- Jones, C. and S.J. Baker, Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nature Reviews Cancer, 2014. 14(10): p. 651-661. [CrossRef]
- Thorbinson, C. and J.P. Kilday, Childhood Malignant Brain Tumors: Balancing the Bench and Bedside. Cancers (Basel), 2021. 13(23). [CrossRef]
- Mackay, A., et al., Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell, 2017. 32(4): p. 520-537.e5. [CrossRef]
- Thorbinson, C. and J.-P. Kilday, Childhood Malignant Brain Tumors: Balancing the Bench and Bedside. Cancers, 2021. 13(23): p. 6099. [CrossRef]
- Damodharan, S., et al., Diffuse Intrinsic Pontine Glioma: Molecular Landscape, Evolving Treatment Strategies and Emerging Clinical Trials. J Pers Med, 2022. 12(5). [CrossRef]
- Cacciotti, C., et al., Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: a single institution experience. J Neurooncol, 2020. 149(1): p. 113-122. [CrossRef]
- Gorsi, H.S., et al., Nivolumab in the Treatment of Recurrent or Refractory Pediatric Brain Tumors: A Single Institutional Experience. J Pediatr Hematol Oncol, 2019. 41(4): p. e235-e241. [CrossRef]
- S. Johnson, T., et al., IMMU-04. FIRST-IN-CHILDREN PHASE 1B STUDY USING THE IDO PATHWAY INHIBITOR INDOXIMOD IN COMBINATION WITH RADIATION AND CHEMOTHERAPY FOR CHILDREN WITH NEWLY DIAGNOSED DIPG (NCT02502708, NLG2105). Neuro-Oncology, 2021. 23(Supplement_1): p. i27-i27. [CrossRef]
- Kim, H.M., et al., The epidermal growth factor receptor variant type III mutation frequently found in gliomas induces astrogenesis in human cerebral organoids. Cell Prolif, 2021. 54(2): p. e12965. [CrossRef]
- Bax, D.A., et al., EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res, 2009. 15(18): p. 5753-61. [CrossRef]
- Li, G., et al., Expression of epidermal growth factor variant III (EGFRvIII) in pediatric diffuse intrinsic pontine gliomas. J Neurooncol, 2012. 108(3): p. 395-402. [CrossRef]
- Johnson, L.A., et al., Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med, 2015. 7(275): p. 275ra22. [CrossRef]
- O'Rourke, D.M., et al., A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med, 2017. 9(399). [CrossRef]
- Ravanpay, A.C., et al., EGFR806-CAR T cells selectively target a tumor-restricted EGFR epitope in glioblastoma. Oncotarget, 2019. 10(66): p. 7080-7095. [CrossRef]
- Hegde, M., et al., Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest, 2016. 126(8): p. 3036-52. [CrossRef]
- Bielamowicz, K., et al., Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol, 2018. 20(4): p. 506-518. [CrossRef]
- Shen, L., et al., The efficacy of third generation anti-HER2 chimeric antigen receptor T cells in combination with PD1 blockade against malignant glioblastoma cells. Oncol Rep, 2019. 42(4): p. 1549-1557. [CrossRef]
- Wang, S.S., et al., HER2 chimeric antigen receptor T cell immunotherapy is an effective treatment for diffuse intrinsic pontine glioma. Neurooncol Adv, 2023. 5(1): p. vdad024. [CrossRef]
- Pule, M.A., et al., Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med, 2008. 14(11): p. 1264-70. [CrossRef]
- Ahmed, N., et al., HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol, 2017. 3(8): p. 1094-1101. [CrossRef]
- Vitanza, N.A., et al., Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med, 2021. 27(9): p. 1544-1552. [CrossRef]
- Zeng, J., et al., IL13RA2 is overexpressed in malignant gliomas and related to clinical outcome of patients. Am J Transl Res, 2020. 12(8): p. 4702-4714.
- Knudson, K.M., et al., Recent Advances in IL-13Rα2-Directed Cancer Immunotherapy. Front Immunol, 2022. 13: p. 878365. [CrossRef]
- Kahlon, K.S., et al., Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res, 2004. 64(24): p. 9160-6. [CrossRef]
- Krebs, S., et al., T cells redirected to interleukin-13Rα2 with interleukin-13 mutein--chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy, 2014. 16(8): p. 1121-31. [CrossRef]
- Brown, C.E., et al., Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res, 2015. 21(18): p. 4062-72. [CrossRef]
- Brown, C.E., et al., Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Mol Ther, 2018. 26(1): p. 31-44. [CrossRef]
- Brown, C.E., et al., Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med, 2016. 375(26): p. 2561-9. [CrossRef]
- Kim, K., et al., Chimeric Antigen Receptor T Cells With Modified Interleukin-13 Preferentially Recognize IL13Rα2 and Suppress Malignant Glioma: A Preclinical Study. Front Immunol, 2021. 12: p. 715000. [CrossRef]
- Krenciute, G., et al., Characterization and Functional Analysis of scFv-based Chimeric Antigen Receptors to Redirect T Cells to IL13Rα2-positive Glioma. Mol Ther, 2016. 24(2): p. 354-363. [CrossRef]
- Pituch, K.C., et al., Adoptive Transfer of IL13Rα2-Specific Chimeric Antigen Receptor T Cells Creates a Pro-inflammatory Environment in Glioblastoma. Mol Ther, 2018. 26(4): p. 986-995. [CrossRef]
- Gu, A., et al., IL13Rα2-targeted third-generation CAR-T cells with CD28 transmembrane domain mediate the best anti-glioblastoma efficacy. Cancer Immunol Immunother, 2023. 72(7): p. 2393-2403. [CrossRef]
- Nazha, B., C. Inal, and T.K. Owonikoko, Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front Oncol, 2020. 10: p. 1000. [CrossRef]
- Longee, D.C., et al., Disialoganglioside GD2 in human neuroectodermal tumor cell lines and gliomas. Acta Neuropathol, 1991. 82(1): p. 45-54. [CrossRef]
- Mount, C.W., et al., Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med, 2018. 24(5): p. 572-579. [CrossRef]
- de Billy, E., et al., Dual IGF1R/IR inhibitors in combination with GD2-CAR T-cells display a potent anti-tumor activity in diffuse midline glioma H3K27M-mutant. Neuro Oncol, 2022. 24(7): p. 1150-1163. [CrossRef]
- Gargett, T., et al., GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. J Immunother Cancer, 2022. 10(9). [CrossRef]
- Majzner, R.G., et al., GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature, 2022. 603(7903): p. 934-941. [CrossRef]
- Liu, Z., et al., Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma. Mol Cancer, 2023. 22(1): p. 3. [CrossRef]
- Maachani, U.B., et al., B7-H3 as a Prognostic Biomarker and Therapeutic Target in Pediatric central nervous system Tumors. Transl Oncol, 2020. 13(2): p. 365-371. [CrossRef]
- Tang, X., et al., B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol Ther Oncolytics, 2019. 14: p. 279-287. [CrossRef]
- Nehama, D., et al., B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine, 2019. 47: p. 33-43. [CrossRef]
- Vitanza, N.A., et al., Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety. Cancer Discov, 2023. 13(1): p. 114-131. [CrossRef]
- Arnone, C.M., et al., Oncolytic adenovirus and gene therapy with EphA2-BiTE for the treatment of pediatric high-grade gliomas. J Immunother Cancer, 2021. 9(5). [CrossRef]
- Yi, Z., et al., Optimizing EphA2-CAR T Cells for the Adoptive Immunotherapy of Glioma. Mol Ther Methods Clin Dev, 2018. 9: p. 70-80. [CrossRef]
- Chow, K.K., et al., T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther, 2013. 21(3): p. 629-37. [CrossRef]
- Lin, Q., et al., First-in-Human Trial of EphA2-Redirected CAR T-Cells in Patients With Recurrent Glioblastoma: A Preliminary Report of Three Cases at the Starting Dose. Front Oncol, 2021. 11: p. 694941. [CrossRef]
- Gu, J., et al., Clinical implications and prognostic value of EMMPRIN/CD147 and MMP2 expression in pediatric gliomas. Eur J Pediatr, 2009. 168(6): p. 705-10. [CrossRef]
- Maggs, L., et al., CAR T Cell-Based Immunotherapy for the Treatment of Glioblastoma. Front Neurosci, 2021. 15: p. 662064. [CrossRef]
- Lasky, J.L., 3rd, et al., Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res, 2013. 33(5): p. 2047-56.
- Benitez-Ribas, D., et al., Immune Response Generated With the Administration of Autologous Dendritic Cells Pulsed With an Allogenic Tumoral Cell-Lines Lysate in Patients With Newly Diagnosed Diffuse Intrinsic Pontine Glioma. Front Oncol, 2018. 8: p. 127. [CrossRef]
- Okada, H., et al., Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol, 2008. 88(3): p. 245-50. [CrossRef]
- Pollack, I.F., et al., Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol, 2014. 32(19): p. 2050-8. [CrossRef]
- Pollack, I.F., et al., Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol, 2016. 18(8): p. 1157-68. [CrossRef]
- Pollack, I.F., et al., Antigen-specific immunoreactivity and clinical outcome following vaccination with glioma-associated antigen peptides in children with recurrent high-grade gliomas: results of a pilot study. J Neurooncol, 2016. 130(3): p. 517-527. [CrossRef]
- Chheda, Z.S., et al., Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med, 2018. 215(1): p. 141-157. [CrossRef]
- Immisch, L., et al., Response to: Correspondence on 'H3.3K27M mutation is not a suitable target for immunotherapy in HLA-A2+ patients with diffuse midline glioma' by Chheda et al. J Immunother Cancer, 2023. 11(3). [CrossRef]
- Mueller, S., et al., Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J Clin Invest, 2020. 130(12): p. 6325-6337. [CrossRef]
- Kmiecik, J., J. Zimmer, and M. Chekenya, Natural killer cells in intracranial neoplasms: presence and therapeutic efficacy against brain tumours. J Neurooncol, 2014. 116(1): p. 1-9. [CrossRef]
- Fares, J., et al., Advances in NK cell therapy for brain tumors. NPJ Precis Oncol, 2023. 7(1): p. 17. [CrossRef]
- Chu, Y., et al., Combinatorial immunotherapy of N-803 (IL-15 superagonist) and dinutuximab with ex vivo expanded natural killer cells significantly enhances in vitro cytotoxicity against GD2(+) pediatric solid tumors and in vivo survival of xenografted immunodeficient NSG mice. J Immunother Cancer, 2021. 9(7). [CrossRef]
- Shida, Y., et al., Ex Vivo Expanded and Activated Natural Killer Cells Prolong the Overall Survival of Mice with Glioblastoma-like Cell-Derived Tumors. Int J Mol Sci, 2021. 22(18). [CrossRef]
- Yvon, E.S., et al., Cord blood natural killer cells expressing a dominant negative TGF-β receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy, 2017. 19(3): p. 408-418. [CrossRef]
- Shaim, H., et al., Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest, 2021. 131(14). [CrossRef]
- Liu, Z., et al., Intravenous injection of oncolytic picornavirus SVV-001 prolongs animal survival in a panel of primary tumor-based orthotopic xenograft mouse models of pediatric glioma. Neuro Oncol, 2013. 15(9): p. 1173-85. [CrossRef]
- Tayeb, S., Z. Zakay-Rones, and A. Panet, Therapeutic potential of oncolytic Newcastle disease virus: a critical review. Oncolytic Virother, 2015. 4: p. 49-62. [CrossRef]
- Lam, H.Y., et al., Safety and clinical usage of newcastle disease virus in cancer therapy. J Biomed Biotechnol, 2011. 2011: p. 718710. [CrossRef]
- Csatary, L.K., et al., MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol, 2004. 67(1-2): p. 83-93. [CrossRef]
- Wagner, S., et al., Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. Apmis, 2006. 114(10): p. 731-43. [CrossRef]
- Markert, J.M., et al., A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther, 2014. 22(5): p. 1048-55. [CrossRef]
- Markert, J.M., et al., Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther, 2009. 17(1): p. 199-207. [CrossRef]
- Markert, J.M., et al., Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther, 2000. 7(10): p. 867-74. [CrossRef]
- Ring, E., et al., PCM-09COMPARISON OF THE SENSITIVITIES OF PEDIATRIC HIGH-GRADE BRAIN TUMOR VERSUS ADULT GLIOBLASTOMA XENOGRAFTS TO ENGINEERED ONCOLYTIC HERPES SIMPLEX VIROTHERAPY. Neuro-Oncology, 2016. 18(suppl_3): p. iii141-iii141. [CrossRef]
- Friedman, G.K., et al., Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N Engl J Med, 2021. 384(17): p. 1613-1622. [CrossRef]
- Cockle, J.V., et al., Oncolytic Herpes Simplex Virus Inhibits Pediatric Brain Tumor Migration and Invasion. Mol Ther Oncolytics, 2017. 5: p. 75-86. [CrossRef]
- Martínez-Vélez, N., et al., The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nature Communications, 2019. 10(1): p. 2235. [CrossRef]
- Fueyo, J., et al., Preclinical Characterization of the Antiglioma Activity of a Tropism-Enhanced Adenovirus Targeted to the Retinoblastoma Pathway. JNCI: Journal of the National Cancer Institute, 2003. 95(9): p. 652-660. [CrossRef]
- Lang, F.F., et al., Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol, 2018. 36(14): p. 1419-1427. [CrossRef]
- Martinez-Velez, N., et al., Delta-24-RGD combined with radiotherapy exerts a potent antitumor effect in diffuse intrinsic pontine glioma and pediatric high grade glioma models. Acta Neuropathologica Communications, 2019. 7(1): p. 64. [CrossRef]
- Gállego Pérez-Larraya, J., et al., Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N Engl J Med, 2022. 386(26): p. 2471-2481. [CrossRef]
- Aguilar, L.K., B.W. Guzik, and E. Aguilar-Cordova, Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem, 2011. 112(8): p. 1969-77. [CrossRef]
- Kieran, M.W., et al., Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol, 2019. 21(4): p. 537-546. [CrossRef]
- Vitanza, N.A. and S. Partap, Pediatric Ependymoma. J Child Neurol, 2016. 31(12): p. 1354-66. [CrossRef]
- Ostrom, Q.T., et al., Alex's Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol, 2015. 16 Suppl 10(Suppl 10): p. x1-x36. [CrossRef]
- McGuire, C.S., K.L. Sainani, and P.G. Fisher, Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg, 2009. 110(4): p. 725-9. [CrossRef]
- Elsamadicy, A.A., et al., Comparison of epidemiology, treatments, and outcomes in pediatric versus adult ependymoma. Neurooncol Adv, 2020. 2(1): p. vdaa019. [CrossRef]
- Merchant, T.E., et al., Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol, 2009. 10(3): p. 258-66. [CrossRef]
- Pajtler, K.W., et al., Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell, 2015. 27(5): p. 728-43. [CrossRef]
- Witt, H., et al., DNA methylation-based classification of ependymomas in adulthood: implications for diagnosis and treatment. Neuro Oncol, 2018. 20(12): p. 1616-1624. [CrossRef]
- Larrew, T., et al., Molecular Classification and Therapeutic Targets in Ependymoma. Cancers (Basel), 2021. 13(24). [CrossRef]
- Mascarenhas, L., et al., Phase 1 clinical trial of durvalumab in children with solid and central nervous system tumors. Journal of Clinical Oncology, 2022. 40(16_suppl): p. 10029-10029. [CrossRef]
- Yeung, J.T., et al., Increased expression of tumor-associated antigens in pediatric and adult ependymomas: implication for vaccine therapy. J Neurooncol, 2013. 111(2): p. 103-11. [CrossRef]
- Hwang, E.I., et al., Why haven't we solved intracranial pediatric ependymoma? Current questions and barriers to treatment advances. Neoplasia, 2023. 39: p. 100895. [CrossRef]
- Sahu, A., et al., In vivo tumor immune microenvironment phenotypes correlate with inflammation and vasculature to predict immunotherapy response. Nat Commun, 2022. 13(1): p. 5312. [CrossRef]
- Grabovska, Y., et al., Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nature Communications, 2020. 11(1): p. 4324. [CrossRef]
- Andersen, J.K., H. Miletic, and J.A. Hossain, Tumor-Associated Macrophages in Gliomas-Basic Insights and Treatment Opportunities. Cancers (Basel), 2022. 14(5). [CrossRef]
- Maximov, V., et al., Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma. Nature Communications, 2019. 10(1): p. 2410. [CrossRef]
- Tan, I.L., et al., CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedgehog-Medulloblastoma model. Oncogene, 2021. 40(2): p. 396-407. [CrossRef]
- Dang, M.T., et al., Macrophages in SHH subgroup medulloblastoma display dynamic heterogeneity that varies with treatment modality. Cell Rep, 2021. 34(13): p. 108917. [CrossRef]
- Plant, A.S., et al., Immunophenotyping of pediatric brain tumors: correlating immune infiltrate with histology, mutational load, and survival and assessing clonal T cell response. J Neurooncol, 2018. 137(2): p. 269-278. [CrossRef]
- Lieberman, N.A.P., et al., Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol, 2019. 21(1): p. 83-94. [CrossRef]
- Robinson, M.H., et al., Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J Immunother Cancer, 2020. 8(2). [CrossRef]
- Ross, J.L., et al., Platelet-derived growth factor beta is a potent inflammatory driver in paediatric high-grade glioma. Brain, 2021. 144(1): p. 53-69. [CrossRef]
- Lin, G.L., et al., Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun, 2018. 6(1): p. 51. [CrossRef]
- Bailey, C.P., et al., Computational immune infiltration analysis of pediatric high-grade gliomas (pHGGs) reveals differences in immunosuppression and prognosis by tumor location. Comput Syst Oncol, 2021. 1(3). [CrossRef]
- Griesinger, A.M., et al., Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol, 2013. 191(9): p. 4880-8. [CrossRef]
- Ritzmann, T., et al., EPEN-23. A COMPUTATIONAL ANALYSIS OF THE TUMOUR IMMUNE MICROENVIRONMENT IN PAEDIATRIC EPENDYMOMA. Neuro-Oncology, 2020. 22(Supplement_3): p. iii312-iii312. [CrossRef]
- Hoffman, L.M., et al., Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol, 2014. 127(5): p. 731-45. [CrossRef]
- Griesinger, A.M., et al., Multi-omic approach identifies hypoxic tumor-associated myeloid cells that drive immunobiology of high-risk pediatric ependymoma. iScience, 2023. 26(9): p. 107585. [CrossRef]
- Budhiraja, S., et al., Immunobiology and Cytokine Modulation of the Pediatric Brain Tumor Microenvironment: A Scoping Review. Cancers, 2023. 15(14): p. 3655. [CrossRef]
- van Bree, N. and M. Wilhelm, The Tumor Microenvironment of Medulloblastoma: An Intricate Multicellular Network with Therapeutic Potential. Cancers (Basel), 2022. 14(20). [CrossRef]
- Wang, S.S., P. Bandopadhayay, and M.R. Jenkins, Towards Immunotherapy for Pediatric Brain Tumors. Trends Immunol, 2019. 40(8): p. 748-761. [CrossRef]
- Lu, D.Y., et al., HAART in HIV/AIDS Treatments: Future Trends. Infect Disord Drug Targets, 2018. 18(1): p. 15-22. [CrossRef]
- DeSelm, C., et al., Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape. Mol Ther, 2018. 26(11): p. 2542-2552. [CrossRef]
- Hwang, J.K., J. Hong, and C.O. Yun, Oncolytic Viruses and Immune Checkpoint Inhibitors: Preclinical Developments to Clinical Trials. Int J Mol Sci, 2020. 21(22). [CrossRef]
- Tian, M., et al., An optimized bicistronic chimeric antigen receptor against GPC2 or CD276 overcomes heterogeneous expression in neuroblastoma. J Clin Invest, 2022. 132(16). [CrossRef]
| ICI therapies | ||||||||
| NCT number | Tumor | Title | Phase | Treatment | Ages | Status | Time | Results |
| NCT02359565 | Constitutional Mismatch Repair Deficiency Syndrome Lynch Syndrome Malignant Glioma Recurrent Brain Neoplasm Recurrent Childhood Ependymoma Recurrent Diffuse Intrinsic Pontine Glioma Recurrent Medulloblastoma Refractory Brain Neoplasm Refractory Diffuse Intrinsic Pontine Glioma Refractory Ependymoma Refractory Medulloblastoma |
Pembrolizumab in Treating Younger Patients With Recurrent, Progressive, or Refractory High-Grade Gliomas, Diffuse Intrinsic Pontine Gliomas, Hypermutated Brain Tumors, Ependymoma or Medulloblastoma | Phase I | Pembrolizumab | 1 Year to 30 Years | Recruiting | 05/2015-12/2026 | |
| NCT02502708 | Glioblastoma Multiforme Glioma Gliosarcoma Malignant Brain Tumor Ependymoma Medulloblastoma Diffuse Intrinsic Pontine Glioma Primary CNS Tumor |
Study of the IDO Pathway Inhibitor, Indoximod, and Temozolomide for Pediatric Patients With Progressive Primary Malignant Brain Tumors | Phase I | indoximod | 3 Years to 21 Years | Completed | 10/2015-02/2020 | Johnson et. al. 2021 |
| NCT02793466 | Solid Tumor Lymphoma Central Nervous System Tumors |
Durvalumab in Pediatric and Adolescent Patients | Phase I | Durvalumab | 1 Year to 17 Years | Completed | 07/2016-04/2023 | Mascarenhas et. al 2022 |
| NCT03130959 | Various Advanced Cancer Diffuse Intrinsic Pontine Glioma non-brainstem High Grade Glioma medulloblastoma |
A Study to Evaluate the Safety and Efficacy of Nivolumab Monotherapy and Nivolumab in Combination With Ipilimumab in Pediatric Participants With High Grade Primary Central Nervous System (CNS) Malignancies (CheckMate 908) | Phase II | Nivolumab, Ipilimumab | 6 Months to 21 Years | Completed | 06/2017-01-2022 | Dunkel et.al. 2023 |
| NCT04049669 | Glioblastoma Medulloblastoma Ependymoma Diffuse Intrinsic Pontine Glioma |
Pediatric Trial of Indoximod With Chemotherapy and Radiation for Relapsed Brain Tumors or Newly Diagnosed DIPG | Phase I | indoximod | 3 Years to 21 Years | Recruiting | 10/2019-10/2027 | |
| NCT04323046 | Glioblastoma Malignant Glioma Recurrent Glioblastoma Recurrent Malignant Glioma Recurrent Grade III Glioma Grade III Glioma |
Immunotherapy Before and After Surgery for Treatment of Recurrent or Progressive High Grade Glioma in Children and Young Adults | Phase I | nivolumab | 6 Months to 25 Years | Recruiting | 10/2020-03/2029 | |
| NCT04167618 | Medulloblastoma, Childhood | 177Lu-DTPA-Omburtamab Radioimmunotherapy for Recurrent or Refractory Medulloblastoma | Phase I/II | 177Lu-DTPA-omburtamab | 3 Years to 19 Years | Terminated | 09/2021-08/2022 | |
| CAR T cell therapies | ||||||||
| NCT number | Tumor | title | Phase | Treatment | Ages | Status | Time | Results |
| NCT01109095 | Glioblastoma Multiforme (GBM) | CMV-specific Cytotoxic T Lymphocytes Expressing CAR Targeting HER2 in Patients With GBM (HERT-GBM) | Phase I | HER2-CAR CMV-T cell | Child, Adult, Older Adult | Completed | 10/2010-03/2018 | Badhiwala et.al. 2013 |
| NCT02271711 | Recurrent Childhood Medulloblastoma Recurrent Ependymoma Recurrent Medulloblastoma |
Expanded Natural Killer Cell Infusion in Treating Younger Patients With Recurrent/Refractory Brain Tumors | Phase I | Autologous NK cells | up to 21 Years | Completed | 03/2015-08/2020 | Khatua et.al. 2020 |
| NCT02208362 | Recurrent Glioblastoma Recurrent Malignant Glioma Recurrent WHO Grade II Glioma Recurrent WHO Grade III Glioma Refractory Glioblastoma Refractory Malignant Glioma Refractory WHO Grade II Glioma Refractory WHO Grade III Glioma |
Genetically Modified T-cells in Treating Patients With Recurrent or Refractory Malignant Glioma | Phase I | IL13Rα2-Specific CAR T cell | 12 Years to 75 Years | Active, Not recruiting | 05/2015-12/2023 | Brown et.al. 2016 |
| NCT02442297 | Brain Tumor, Recurrent Brain Tumor, Refractory |
T Cells Expressing HER2-specific Chimeric Antigen Receptors(CAR) for Patients With HER2-Positive CNS Tumors (iCAR) | Phase I | HER2 CAR T cell | 3 Years and older | Active, Not recruiting | 04/2016-04/2037 | |
| NCT03500991 | Central Nervous System Tumor, Pediatric Glioma Ependymoma Medulloblastoma Germ Cell Tumor Atypical Teratoid/Rhabdoid Tumor Primitive Neuroectodermal Tumor Choroid Plexus Carcinoma Pineoblastoma |
HER2-specific CAR T Cell Locoregional Immunotherapy for HER2-positive Recurrent/Refractory Pediatric CNS Tumors | Phase I | HER2-Specific CAR T Cell | 1 Year to 26 Years | Active, Not recruiting | 07/2018-07/2039 | Vitanza et.al. 2021 |
| NCT03652545 | Brain Tumor | Multi-antigen T Cell Infusion Against Neuro-oncologic Disease (REMIND) | Phase I | multi-antigen associated specific cytotoxic T lymphocytes (TAA-T) | 6 Months to 80 Years | Recruiting | 12/2018-03/2026 | |
| NCT03638167 | Central Nervous System Tumor, Pediatric Glioma Ependymoma Medulloblastoma Germ Cell Tumor Atypical Teratoid/Rhabdoid Tumor Primitive Neuroectodermal Tumor Choroid Plexus Carcinoma Pineoblastoma |
EGFR806-specific CAR T Cell Locoregional Immunotherapy for EGFR-positive Recurrent or Refractory Pediatric CNS Tumors | Phase I | EGFR806-specific CAR T cell |
1 Year to 26 Years | Active, Not recruiting | 03/2019-03/2040 | |
| NCT04185038 | Central Nervous System Tumor Diffuse Intrinsic Pontine Glioma Diffuse Midline Glioma Ependymoma Medulloblastoma, Childhood Germ Cell Tumor Atypical Teratoid/Rhabdoid Tumor Primitive Neuroectodermal Tumor Choroid Plexus Carcinoma Pineoblastoma, Childhood Glioma |
Study of B7-H3-Specific CAR T Cell Locoregional Immunotherapy for Diffuse Intrinsic Pontine Glioma/Diffuse Midline Glioma and Recurrent or Refractory Pediatric Central Nervous System Tumors | Phase I | B7-H3-Specific CAR T cell | 1 Year to 26 Years | Recruiting | 12/2019-05/2041 | |
| NCT04099797 | Diffuse Intrinsic Pontine Glioma High Grade Glioma Embryonal Tumor Ependymal Tumor |
C7R-GD2.CAR T Cells for Patients With GD2-expressing Brain Tumors (GAIL-B) | Phase I | C7R-GD2.CAR T Cell | 12 Months to 21 Years | Recruiting | 02/2020-02/2039 | |
| NCT03170141 | Glioblastoma Multiforme of Brain Glioblastoma Multiforme |
Immunogene-modified T (IgT) Cells Against Glioblastoma Multiforme | Phase I | GD2-specific 4SCAR T cells | 1 Year to 80 Years | Enrolling by Invitation | 05/2020-12/2023 | Liu et.al. 2023 |
| NCT04196413 | Glioma of Spinal Cord Glioma of Brainstem |
GD2 CAR T Cells in Diffuse Intrinsic Pontine Gliomas(DIPG) & Spinal Diffuse Midline Glioma(DMG) | Phase I | GD2 CAR T cell | 2 Years to 50 Years | Recruiting | 06/2020-07/2043 | |
| NCT04510051 | Malignant Brain Neoplasm Recurrent Malignant Brain Neoplasm Refractory Malignant Brain Neoplasm |
CAR T Cells After Lymphodepletion for the Treatment of IL13Rα2 Positive Recurrent or Refractory Brain Tumors in Children | Phase I | IL13(EQ)BBzeta/CD19t+ T cells | 4 Years to 25 Years | Recruiting | 12/2020-08/2024 | |
| NCT04903080 | Ependymoma | HER2-specific Chimeric Antigen Receptor (CAR) T Cells for Children With Ependymoma | Phase I | HER2 CAR T cell | 1 Year to 22 Years | Active, Not recruiting | 07/2022-02/2043 | |
| NCT05835687 | Central Nervous System Neoplasms Atypical Teratoid/Rhabdoid Tumor Diffuse Midline Glioma, H3 K27M-Mutant Ependymoma High Grade Glioma Glioblastoma Medulloblastoma |
Loc3CAR: Locoregional Delivery of B7-H3-CAR T Cells for Pediatric Patients With Primary CNS Tumors | Phase I | B7-H3-CAR T cell | up to 21 Years | Recruiting | 04/2023-03/2028 | |
| NCT05768880 | Diffuse Intrinsic Pontine Glioma Diffuse Midline Glioma Recurrent CNS Tumor, Adult Recurrent, CNS Tumor, Childhood Refractory Primary Malignant Central Nervous System Neoplas |
Study of B7-H3, EGFR806, HER2, And IL13-Zetakine (Quad) CAR T Cell Locoregional Immunotherapy For Pediatric Diffuse Intrinsic Pontine Glioma, Diffuse Midline Glioma, And Recurrent Or Refractory Central Nervous System Tumors | Phase I | SC-CAR4BRAIN, an autologous CD4+ and CD8+ T cells expressing combinations of B7-H3, EGFR806, HER2, and IL13-zetakine CAR | 1 Year to 26 Years | Recruiting | 05/2023-12/2043 | |
| NCT05298995 | Brain Tumor, Pediatric Medulloblastoma, Childhood Embryonal Tumor High Grade Glioma Diffuse Midline Glioma Diffuse Intrinsic Pontine Glioma Brain Tumor Adult |
GD2-CAR T Cells for Pediatric Brain Tumours | Phase I | iC9-GD2-CAR T-cell | 6 Months to 30 Years | Recruiting | 11/2023-11/2038 | |
| Vaccine therapies | ||||||||
| NCT number | Tumor | title | Phase | Treatment | Ages | Status | Time | Results |
| NCT00107185 | Brain and Central Nervous System Tumors | Vaccine Therapy in Treating Young Patients Who Are Undergoing Surgery for Malignant Glioma | Phase I | autologous tumor lysate-pulsed DC vaccine | 1 Year to 18 Years | Completed | 01/2005-03/2010 | Lasky et.al. 2013 |
| NCT01130077 | Newly Diagnosed Pediatric Pontine Glioma Newly Diagnosed Pediatric High Grade Glioma Recurrent Pediatric High Grade Glioma Recurrent Pediatric Low Grade Glioma |
A Pilot Study of Glioma Associated Antigen Vaccines in Conjunction With Poly-ICLC in Pediatric Gliomas | Phase I | HLA-A2 restricted GGAs combine with poly-ICLC | 12 Months to 21 Years | Active, Not recruiting | 02/2009-12/2025 | Pollack et. al. 2014 |
| NCT01326104 | Medulloblastoma Neuroectodermal Tumor |
Vaccine Immunotherapy for Recurrent Medulloblastoma and Primitive Neuroectodermal Tumor (Re-MATCH) | Phase II | DC vaccine | up to 30 Years | Active, Not recruiting | 09/2010-12/2023 | |
| NCT01795313 | Ependymoma | Immunotherapy for Recurrent Ependymomas in Children Using Tumor Antigen Peptides With Imiquimod | Phase I | IL-13Rα2, EphA2, Survivin | 12 Months to 21 Years | Recruiting | 08/2012-12/2025 | |
| NCT02840123 | Diffuse Intrinsic Pontine Glioma | Safety Study of DIPG Treatment With Autologous Dendritic Cells Pulsed With Lysated Allegenic Tumor Lines | Phase I | autologous DC pulsed with lysated allegenic tumor lines | 3 Years to 18 Years | Unknown | 06/2016-03/2019 | Benitez-Ribas et.al. 2018 |
| NCT02960230 | Diffuse Intrinsic Pontine Glioma Glioma Diffuse Midline Glioma, H3 K27M-Mutant |
H3.3K27M Peptide Vaccine With Nivolumab for Children With Newly Diagnosed DIPG and Other Gliomas | Phase I/II | H3.3K27M Specific Peptide Vaccine Combined With Poly-ICLC With and Without PD-1 Inhibition | 3 Years to 21 Years | Active, Not recruiting | 11/2016-11/2024 | Muller et. al. 2022 |
| NCT03334305 | Malignant Glioma High Grade Glioma |
Adoptive Cellular Therapy in Pediatric Patients With High-grade Gliomas (ACTION) | Phase I | DC vaccine | 3 Years to 21 Years | Active, Not recruiting | 05/2018-05/2026 | |
| NCT03396575 | Diffuse Intrinsic Pontine Glioma (DIPG) Brain Stem Glioma |
Brain Stem Gliomas Treated With Adoptive Cellular Therapy During Focal Radiotherapy Recovery Alone or With Dose-intensified Temozolomide (Phase I) (BRAVO) | Phase I | DC vaccine | 3 Years to 30 Years | Active, Not recruiting | 07/2018-06/2025 | |
| NCT04749641 | Diffuse Intrinsic Pontine Glioma | Neoantigen Vaccine Therapy Against H3.3-K27M Diffuse Intrinsic Pontine Glioma (ENACTING) | Phase I | H3.3-K27M Neoantigen Vaccine | 5 Years and older | Recruiting | 03/2021-12/2024 | |
| NCT04911621 | High Grade Glioma Diffuse Intrinsic Pontine Glioma |
Adjuvant Dendritic Cell Immunotherapy for Pediatric Patients With High-grade Glioma or Diffuse Intrinsic Pontine Glioma (ADDICT-pedGLIO) | Phase I/II | WT1 mRNA-loaded autologous monocyte-derived DCs | 12 Months to 17 Years | Active, Not recruiting | 09/2021-06/2027 | |
| NCT04573140 | Adult Glioblastoma | A Study of RNA-lipid Particle (RNA-LP) Vaccines for Newly Diagnosed Pediatric High-Grade Gliomas (pHGG) and Adult Glioblastoma (GBM) (PNOC020) | Phase I | RNA-LP vaccines | 21 Years and older | Recruiting | 10/2021-07/2027 | |
| NCT04978727 | Medulloblastoma Glioblastoma Multiforme Anaplastic Astrocytoma High-grade Astrocytoma NOS Anaplastic Oligodendroglioma Anaplastic Ependymoma Ependymoma Diffuse Intrinsic Pontine Glioma |
A Pilot Study of SurVaxM in Children Progressive or Relapsed Medulloblastoma, High Grade Glioma, Ependymoma and Newly Diagnosed Diffuse Intrinsic Pontine Glioma | Phase I | SurVaxM | 1 Year to 21 Years | Recruiting | 07/2022-06/2028 | |
| Oncolytic virus therapies | ||||||||
| NCT number | Tumor | title | Phase | Treatment | Ages | Status | Time | Results |
| NCT00634231 | Malignant Glioma Recurrent Ependymoma |
A Phase I Study of AdV-tk + Prodrug Therapy in Combination With Radiation Therapy for Pediatric Brain Tumors | Phase I | AdV-tk followed by valacyclovir | 3 Years to 21 Years | Completed | 10/2010-06/2021 | Kieran et.al. 2019 |
| NCT02457845 | Supratentorial Neoplasms, Malignant Malignant Glioma Glioblastoma Anaplastic Astrocytoma PNET Cerebral Primitive Neuroectodermal Tumor Embryonal Tumor |
HSV G207 Alone or With a Single Radiation Dose in Children With Progressive or Recurrent Supratentorial Brain Tumors | Phase I | HSV G207 | 3 Years to 18 Years | Active, Not recruiting | 05/2016-01/2024 | Friedman et.al. 2021 |
| NCT02962167 | Medulloblastoma, Childhood, Recurrent Atypical Teratoid/Rhabdoid Tumor Medulloblastoma Recurrent |
Modified Measles Virus (MV-NIS) for Children and Young Adults With Recurrent Medulloblastoma or Recurrent ATRT | Phase I | modified measles virus (MV-NIS) | 12 Months to 39 Years | Completed | 02/2017-05/2023 | |
| NCT03178032 | Brainstem Glioma | Oncolytic Adenovirus, DNX-2401, for Naive Diffuse Intrinsic Pontine Gliomas | Phase I | DNX-2401 | 1 Year to 18 Years | Completed | 05/2017-1/2021 | Gállego Pérez-Larraya et.al. 2022 |
| NCT03043391 | Malignant Glioma Anaplastic Astrocytoma Anaplastic Oligoastrocytoma Anaplastic Oligodendroglioma Glioblastoma Gliosarcoma Atypical Teratoid/Rhabdoid Tumor of Brain Medulloblastoma Ependymoma Pleomorphic Xanthoastrocytoma of Brain Embryonal Tumor of Brain |
Phase 1b Study PVSRIPO for Recurrent Malignant Glioma in Children | Phase I | oncolytic poliovirus (PVSRIPO) | 12 Years to 21 Years | Unkown | 12/2017-03/2022 | |
| NCT04482933 | Neoplasms High Grade Glioma Glioblastoma Multiforme Malignant Glioma of Brain Anaplastic Astrocytoma of Brain High-grade Glioma Anaplastic Glioma Giant Cell Glioblastoma |
HSV G207 With a Single Radiation Dose in Children With Recurrent High-Grade Glioma | Phase II | HSV G207 combined with a single 5 Gy dose of radiation | 3 Years to 21 Years | Active, Not recruiting | 12/2023-12/2028 | |
| NCT03911388 | Recurrent or Refractory Cerebellar Brain Tumors | HSV G207 in Children With Recurrent or Refractory Cerebellar Brain Tumors | Phase I | HSV G207 | 3 Years to 21 Years | Recruiting | 09/2019-09/2026 | |
| NCT04758533 | Diffuse Intrinsic Pontine Glioma Medulloblastoma, Childhood, Recurrent |
Clinical Trial to Assess the Safety and Efficacy of AloCELYVIR With Newly Diagnosed Diffuse Intrinsic Pontine Glioma (DIPG) in Combination With Radiotherapy or Medulloblastoma in Monotherapy (AloCELYVIR) | Phase I/II | bone marrow-derived allogenic mesenchymal stem cells loaded with an oncolytic Adenovirus (ICOVIR-5) | 1 Year to 21 Years | Recruiting | 04/2021-04/2026 | |
| Location | Molecular grouping | WHO grade |
|---|---|---|
| Supratentorial | ZFTA fusion positive | |
| YAP1 fusion positive | ||
| Supratentorial ependymoma (other) | 2, 3 | |
| Posterior fossa | Posterior fossa group A | |
| Posterior fossa group B | ||
| Posterior fossa ependymoma (other) | 2, 3 | |
| Spine | MYCN amplified | |
| Spinal ependymoma | 2, 3 | |
| Spinal cord | Myxopapillary ependymoma | 2 |
| CNS | Subependymoma | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




