Submitted:
30 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
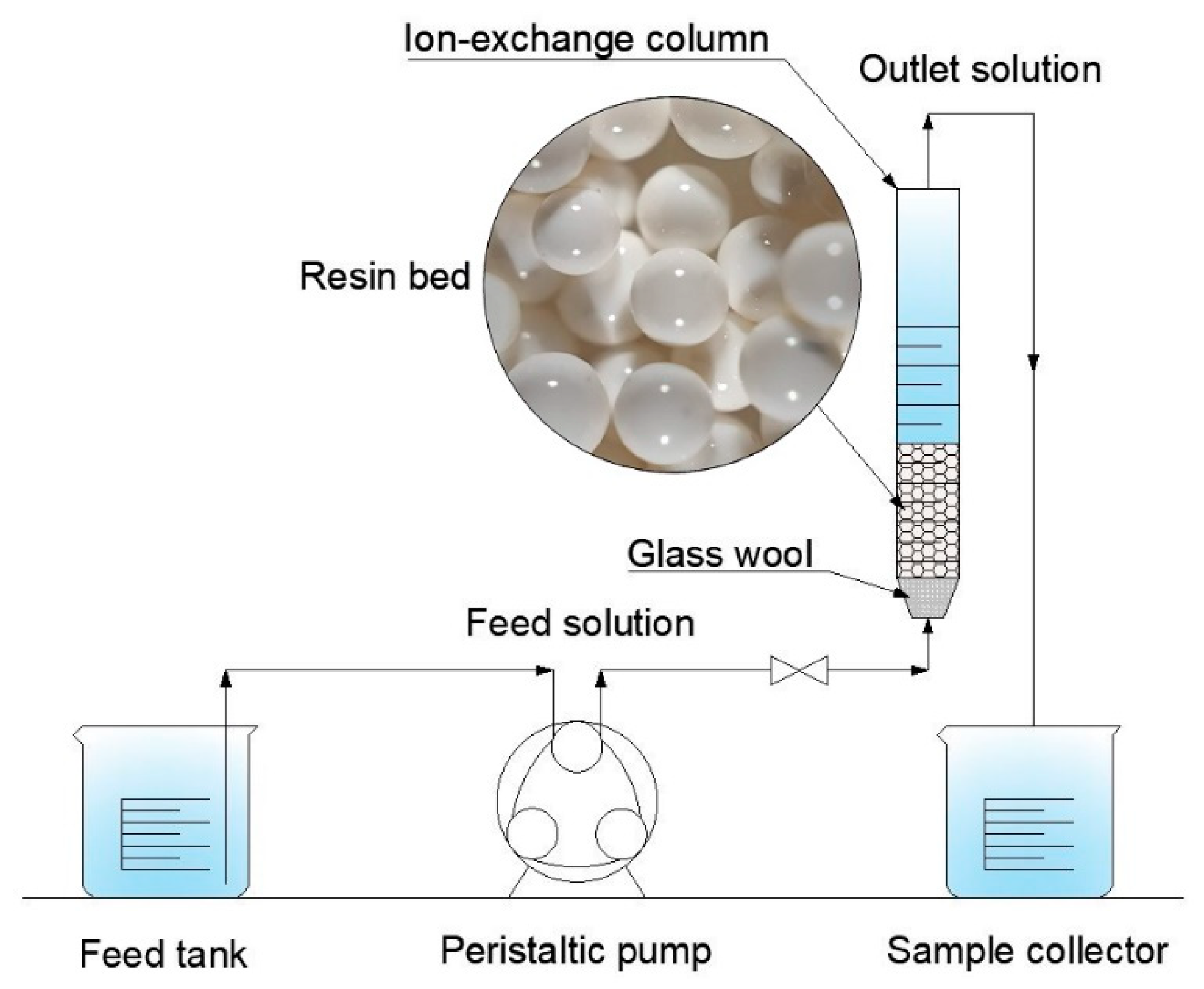
2.2. Experimental
2.3. Analysis
2.4. Calculating Methods
2.4.1. Adsorption efficiency calculations
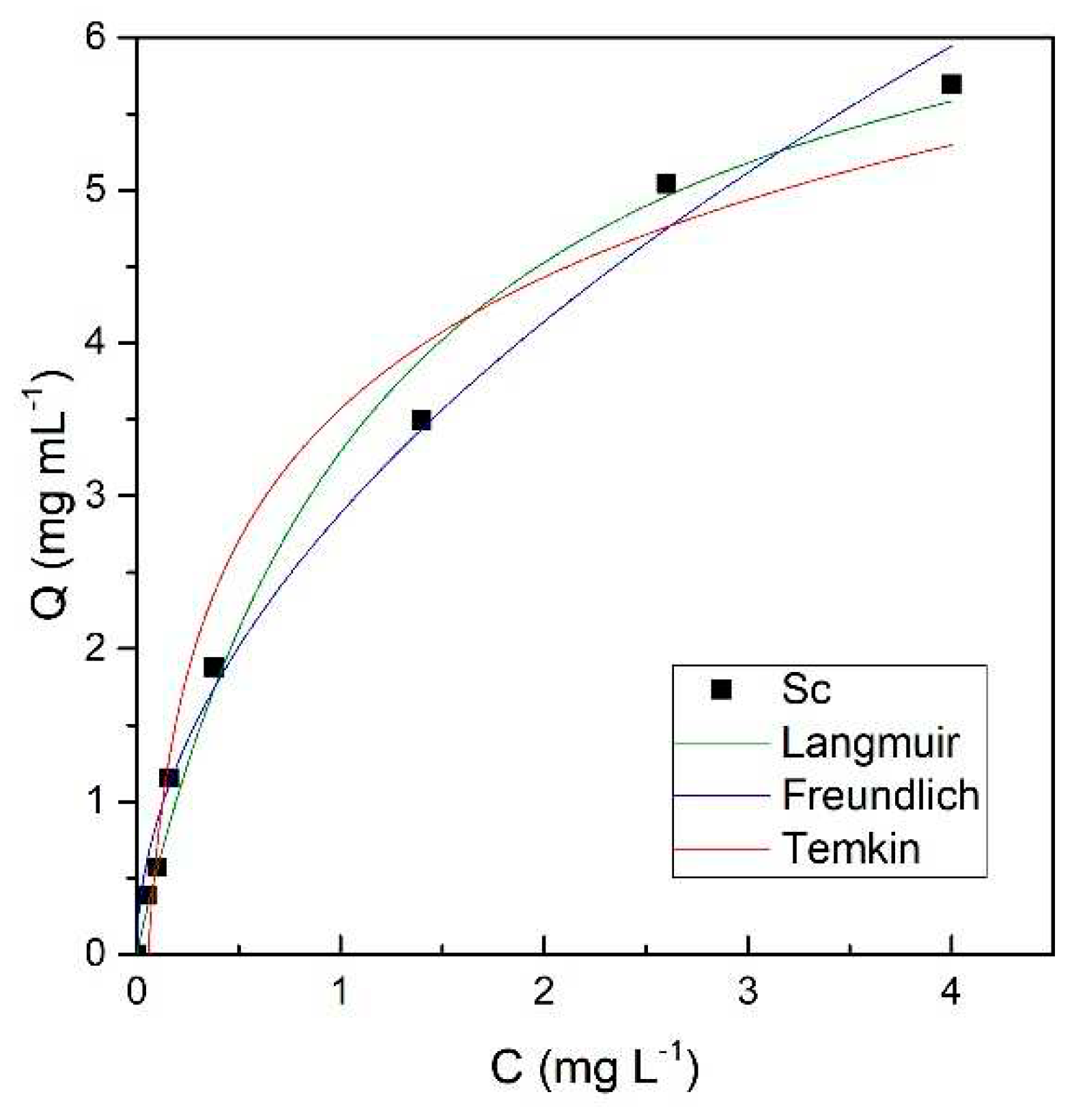
2.4.2. Adsorption Isotherm Studies
2.4.3. Adsorption Column Studies
3. Results and Discussion
3.1. Batch Sc adsorption Experiments and Study of Sorption Mechanism
3.2. Column Adsorption and Breakthrough Modelling
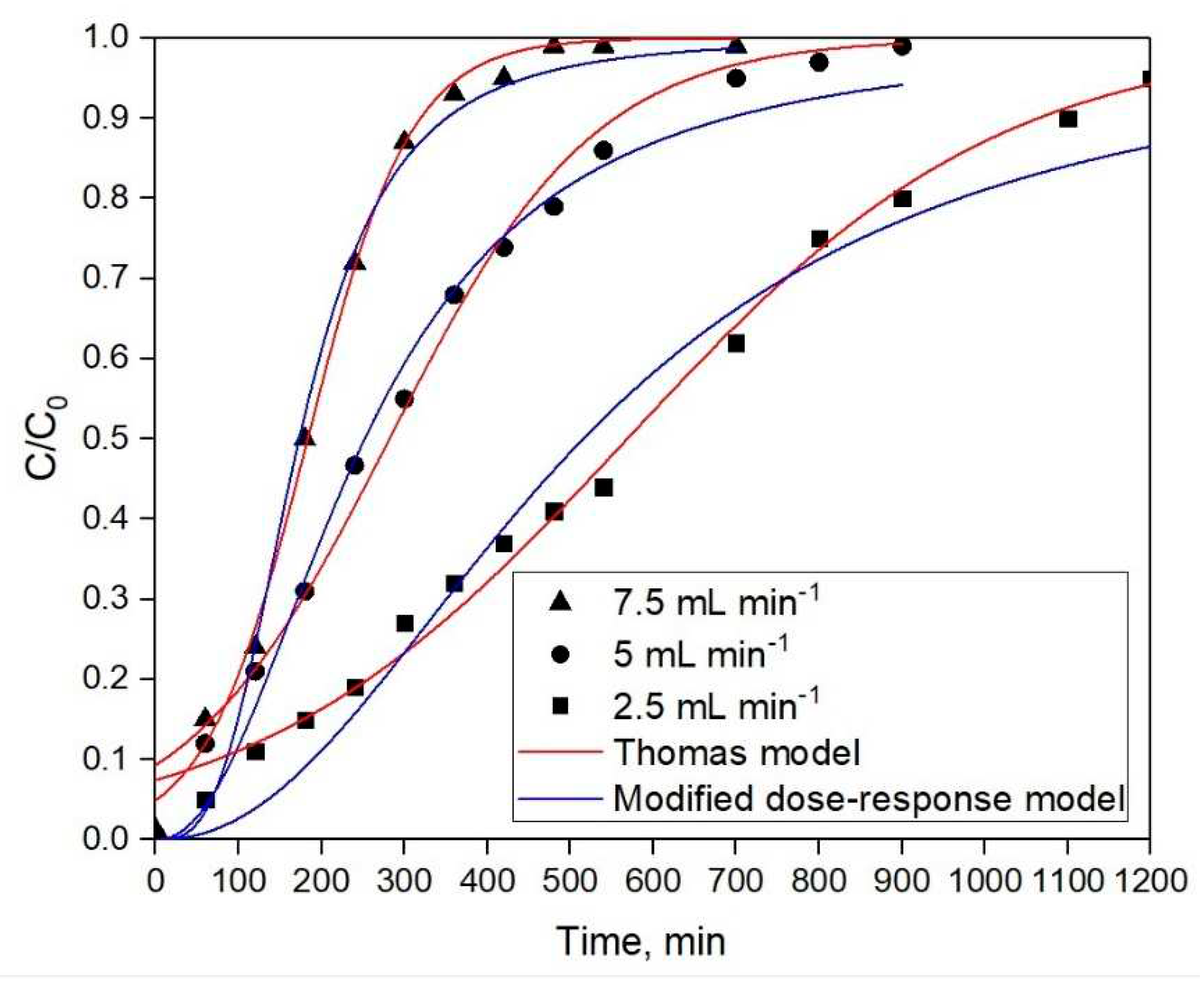
3.2.1. Adsorption study from simulated solution
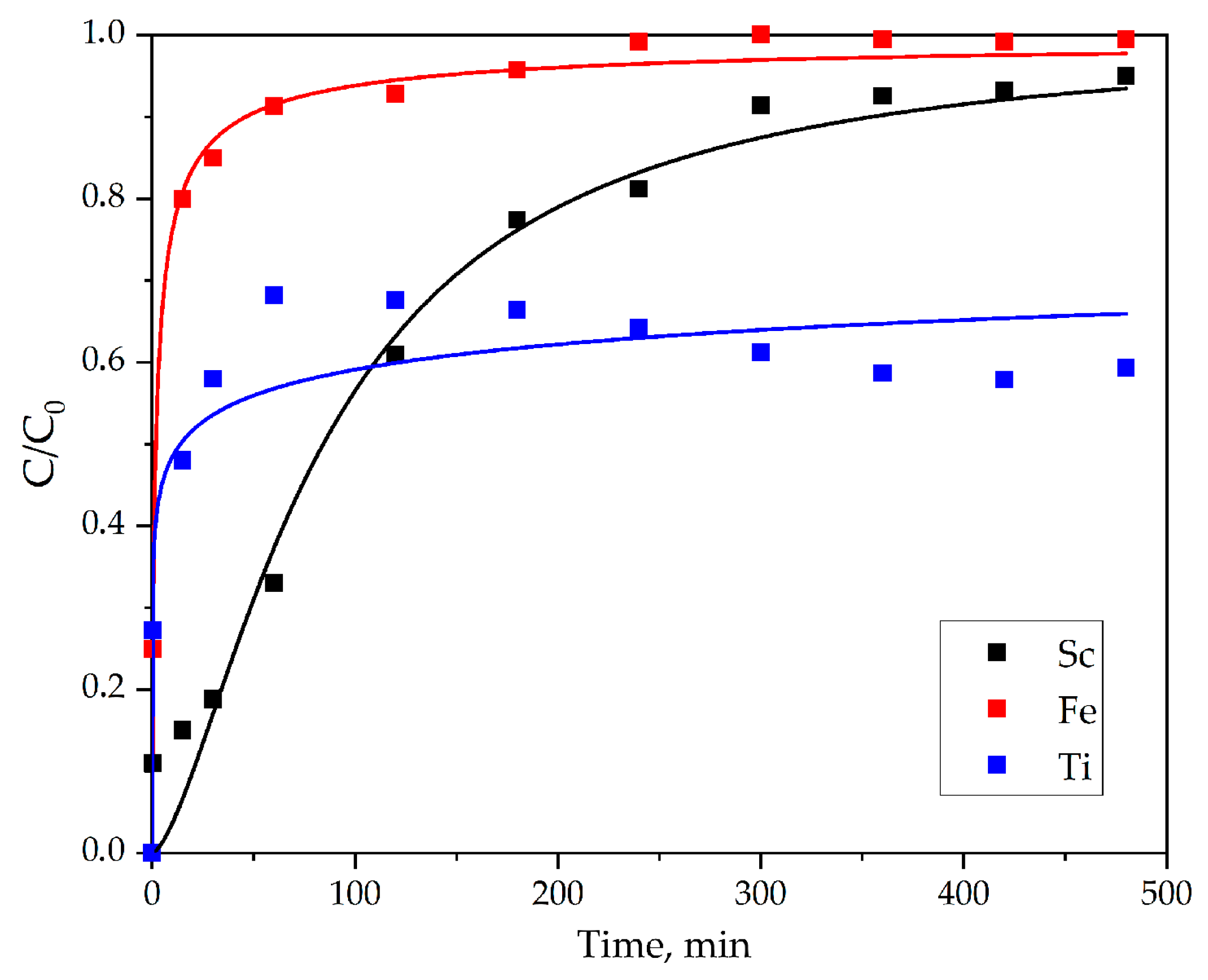
3.2.1. Adsorption study from PLS
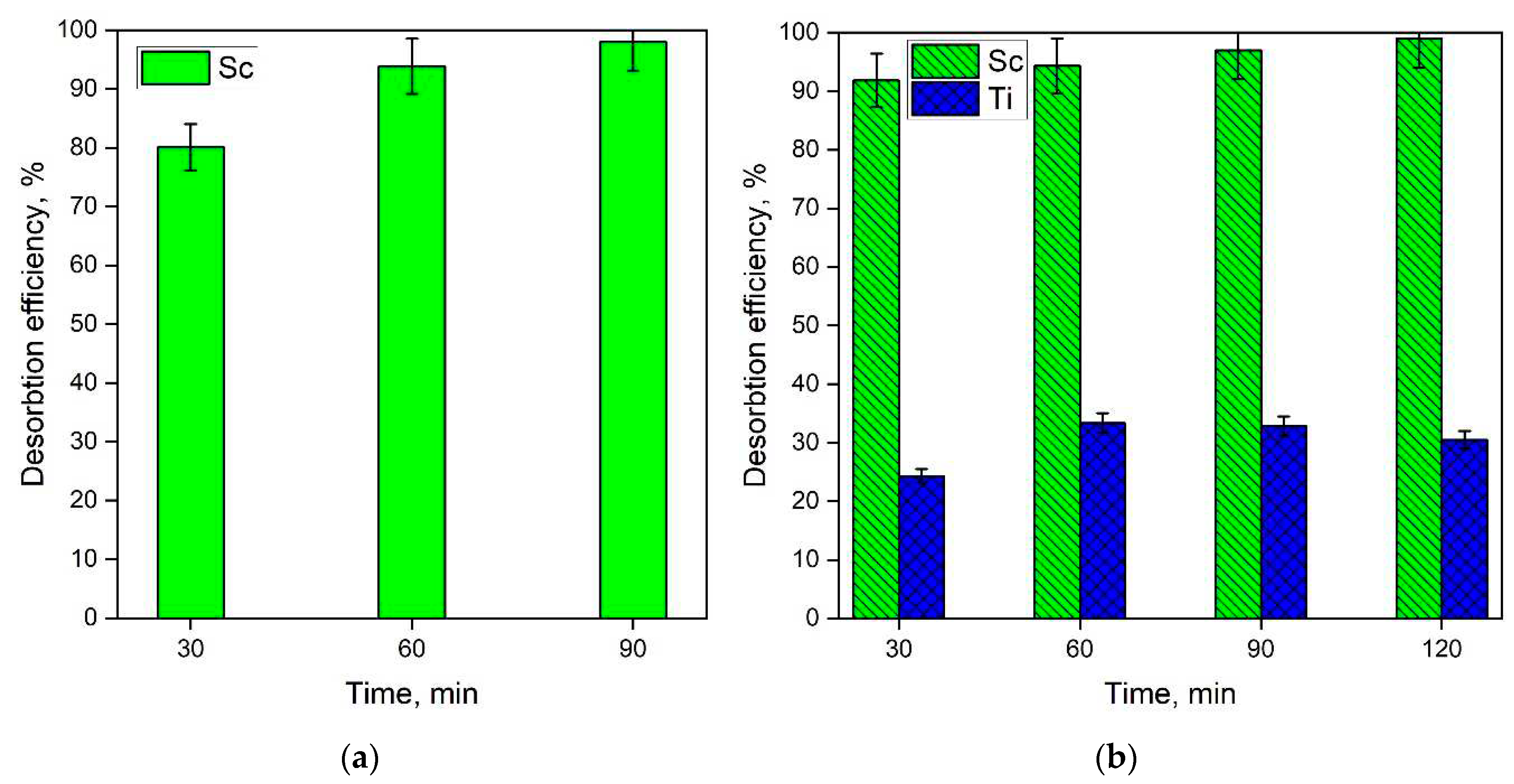
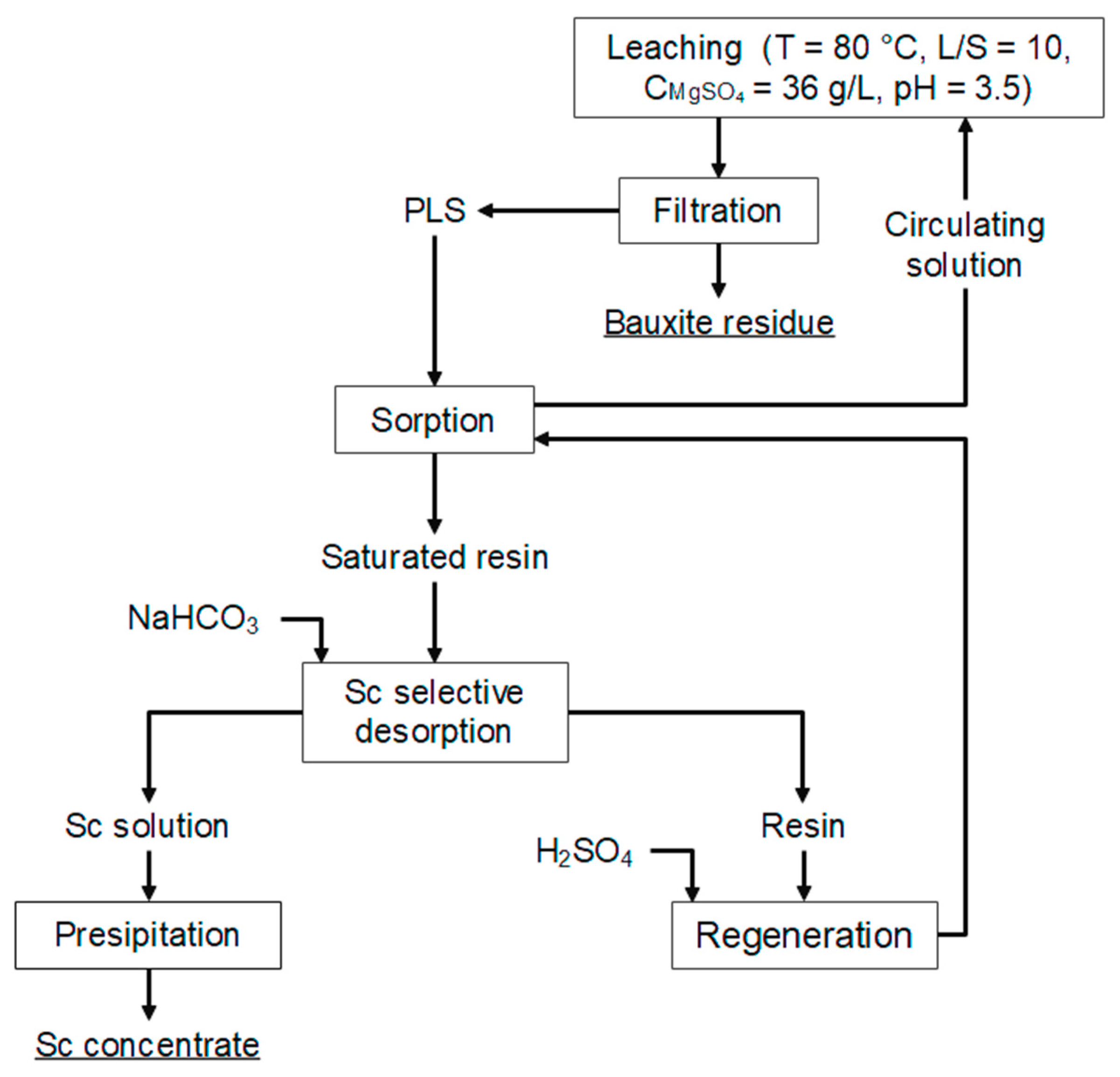
3.3. Desorption Studies
4. Conclusions
- For the adsorption of Sc on resin, Langmuir equations adequately describe the sorption isotherms under batch conditions. This indicates the chemisorption process on the chelating resin.
- The high values of R2 for the equations of the breakthrough models indicate that the Thomas model is applicable to describing the Sc adsorption from simulated Mg-containing solutions. According to Langmuir model, the maximum calculated capacity for batch process was 8.576 g L−1, while the maximum capacity for column sorption obtained using the Thomas model was 7.013 mg mL−1.
- The results of the column adsorption study using PLS showed that significant sorption of Ti in addition to Sc was observed. This is apparently due to the properties of Ti being close to the properties of REEs.
- Sc can be efficiently desorbed (>98%) by NaHCO3 solution (200 g L−1) from both simulated and real solutions within a duration of 1.5 h. After 1.5 h of desorption, the concentration of Sc in the desorption solution was 562 mg L−1, while the concentration of Mg and Ti was lower than 200 mg L−1 and 50 mg L−1, respectively. This indicates that the resin is very selective towards Sc or rare earth elements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vind, J.; Alexandri, A.; Vassiliadou, V.; Panias, D.; Vind, J.; Alexandri, A.; Vassiliadou, V.; Panias, D. Distribution of Selected Trace Elements in the Bayer Process. Metals 2018, 8, 327. [Google Scholar] [CrossRef]
- Phoung, S.; Williams, E.; Gaustad, G.; Gupta, A. Exploring Global Supply and Demand of Scandium Oxide in 2030. Journal of Cleaner Production 2023, 401, 136673. [Google Scholar] [CrossRef]
- Ray, A.R.; Mishra, S. Hydro Metallurgical Technique as Better Option for the Recovery of Rare Earths from Mine Tailings and Industrial Wastes. Sustainable Chemistry and Pharmacy 2023, 36, 101311. [Google Scholar] [CrossRef]
- Salman, A.D.; Juzsakova, T.; Mohsen, S.; Abdullah, T.A.; Le, P.-C.; Sebestyen, V.; Sluser, B.; Cretescu, I. Scandium Recovery Methods from Mining, Metallurgical Extractive Industries, and Industrial Wastes. Materials 2022, 15, 2376. [Google Scholar] [CrossRef] [PubMed]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of Valuable Elements from Red Mud with a Focus on Using Liquid Media—A Review. Recycling 2021, 6, 38. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, Th.; Ochsenkühn, K.M.; Parissakis, G. Recovery of Lanthanides and Yttrium from Red Mud by Selective Leaching. Analytica Chimica Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of Scandium from Synthetic Red Mud Leach Solutions by Solvent Extraction with D2EHPA. Separation and Purification Technology 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Abhilash; Sinha, S.; Sinha, M.K.; Pandey, B.D. Extraction of Lanthanum and Cerium from Indian Red Mud. International Journal of Mineral Processing 2014, 127, 70–73. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Jing, Q.; Zhang, M. Recovery of Scandium from Leachate of Sulfation-Roasted Bayer Red Mud by Liquid–Liquid Extraction. JOM 2017, 69, 2373–2378. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Chen, J.; Zou, D.; Deng, Y.; Li, D. Application of P507 and Isooctanol Extraction System in Recovery of Scandium from Simulated Red Mud Leach Solution. Journal of Rare Earths 2019, 37, 1002–1008. [Google Scholar] [CrossRef]
- Das, S.; Behera, S.S.; Murmu, B.M.; Mohapatra, R.K.; Mandal, D.; Samantray, R.; Parhi, P.K.; Senanayake, G. Extraction of Scandium(III) from Acidic Solutions Using Organo-Phosphoric Acid Reagents: A Comparative Study. Separation and Purification Technology 2018, 202, 248–258. [Google Scholar] [CrossRef]
- Hérès, X.; Blet, V.; Di Natale, P.; Ouaattou, A.; Mazouz, H.; Dhiba, D.; Cuer, F. Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins. Metals 2018, 8, 682. [Google Scholar] [CrossRef]
- Korovin, V.; Shestak, Y.; Pogorelov, Y.; Cortina, J.L. Solid Polymeric Extractants (TVEX). Synthesis, Extraction Characterization, and Applications for Metal Extraction Processes. ChemInform 2009, 40, chin.200927276. [Google Scholar] [CrossRef]
- Kabay, N.; Cortina, J.L.; Trochimczuk, A.; Streat, M. Solvent-Impregnated Resins (SIRs) – Methods of Preparation and Their Applications. Reactive and Functional Polymers 2010, 70, 484–496. [Google Scholar] [CrossRef]
- Kauczor, H.W.; Meyer, A. Structure and Properties of Levextrel Resins. Hydrometallurgy 1978, 3, 65–73. [Google Scholar] [CrossRef]
- Korovin, V.; Shestak, Y. Scandium Extraction from Hydrochloric Acid Media by Levextrel-Type Resins Containing Di-Isooctyl Methyl Phosphonate. Hydrometallurgy 2009, 95, 346–349. [Google Scholar] [CrossRef]
- Mostajeran, M.; Bondy, J.M.; Reynier, N.; Cameron, R. Mining Value from Waste: Scandium and Rare Earth Elements Selective Recovery from Coal Fly Ash Leach Solutions. Minerals Engineering 2021, 173, 107091. [Google Scholar] [CrossRef]
- Bao, S.; Hawker, W.; Vaughan, J. Scandium Loading on Chelating and Solvent Impregnated Resin from Sulfate Solution. Solvent Extraction and Ion Exchange 2018, 36, 100–113. [Google Scholar] [CrossRef]
- Sharaf, M.; Yoshida, W.; Kubota, F.; Goto, M. A Novel Binary-Extractant-Impregnated Resin for Selective Recovery of Scandium. Journal of Chemical Engineering of Japan 2019, 52, 49–55. [Google Scholar] [CrossRef]
- Ghosh, A.; Dhiman, S.; Gupta, A.; Jain, R. Process Evaluation of Scandium Production and Its Environmental Impact. Environments 2022, 10, 8. [Google Scholar] [CrossRef]
- Orlandini, K.A. Cation Exchange Separation of Scandium from Rare Earths in Oxalic Acid Media. Inorganic and Nuclear Chemistry Letters 1969, 5, 325–331. [Google Scholar] [CrossRef]
- Molchanova, T.V.; Akimova, I.D.; Tatarnikov, A.V. Ion-Exchange Methods of Scandium Recovery from the Ores of the Tomtor Deposit. Russian Metallurgy (Metally) 2019, 2019, 674–679. [Google Scholar] [CrossRef]
- Page, M.J.; Soldenhoff, K.; Ogden, M.D. Comparative Study of the Application of Chelating Resins for Rare Earth Recovery. Hydrometallurgy 2017, 169, 275–281. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Virolainen, S.; Massima Mouele, E.S.; Laatikainen, M.; Repo, E.; Laatikainen, K. The Recent Progress of Ion Exchange for the Separation of Rare Earths from Secondary Resources – A Review. Hydrometallurgy 2023, 218, 106047. [Google Scholar] [CrossRef]
- Felipe, E.C.B.; Batista, K.A.; Ladeira, A.C.Q. Recovery of Rare Earth Elements from Acid Mine Drainage by Ion Exchange. Environmental Technology 2021, 42, 2721–2732. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylenko, M. Development and Screening of Resins to Recover REE and Scandium from Different Sources. In Extraction 2018; Davis, B.R., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Alvear Flores, G.R., Jak, E., Goodall, G., Free, M.L., Asselin, E., Chagnes, A., Dreisinger, D., Jeffrey, M., Lee, J., Miller, G., Petersen, J., Ciminelli, V.S.T., Xu, Q., Molnar, R., Adams, J., Liu, W., Verbaan, N., Goode, J., London, I.M., Azimi, G., Forstner, A., Kappes, R., Bhambhani, T., Eds.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, 2018; pp. 2113–2122. ISBN 978-3-319-95021-1. [Google Scholar]
- Koodynska, D.; Hubicki, Z. Investigation of Sorption and Separation of Lanthanides on the Ion Exchangers of Various Types. In Ion Exchange Technologies; Kilislioglu, A., Ed.; InTech, 2012; ISBN 978-953-51-0836-8. [Google Scholar]
- Virolainen, S.; Repo, E.; Sainio, T. Recovering Rare Earth Elements from Phosphogypsum Using a Resin-in-Leach Process: Selection of Resin, Leaching Agent, and Eluent. Hydrometallurgy 2019, 189, 105125. [Google Scholar] [CrossRef]
- Shoppert, A.; Loginova, I.; Napol’skikh, J.; Valeev, D. High-Selective Extraction of Scandium (Sc) from Bauxite Residue (Red Mud) by Acid Leaching with MgSO4. Materials 2022, 15, 1343. [Google Scholar] [CrossRef] [PubMed]
- Shoppert, A.; Loginova, I.; Napol’skikh, J.; Kyrchikov, A.; Chaikin, L.; Rogozhnikov, D.; Valeev, D. Selective Scandium (Sc) Extraction from Bauxite Residue (Red Mud) Obtained by Alkali Fusion-Leaching Method. Materials 2022, 15, 433. [Google Scholar] [CrossRef]
- Chaikin, L.; Shoppert, A.; Valeev, D.; Loginova, I.; Napol’skikh, J. Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust. Minerals 2020, 10, 500. [Google Scholar] [CrossRef]
- Hubicki, Z. Studies on Selective Separation of Sc(III) from Rare Earth Elements on Selective Ion-Exchangers. Hydrometallurgy 1990, 23, 319–331. [Google Scholar] [CrossRef]
- Marhol, M.; Svehla, G.; Wilson, C.L.; Wilson, D.W.; Veselý, V. Ion Exchangers in Analytical Chemistry: Their Properties and Use in Inorganic Chemistry; Wilson and Wilson’s comprehensive analytical chemistry; Elsevier: Amsterdam Oxford New York, 1982; ISBN 978-0-444-99717-3. [Google Scholar]
- Hamaguchi, H.; Ohuchi, A.; Onuma, N.; Kuroda, R. Anion Exchange Behavior of Rare Earth Elements in Potassium Sulfate Medium. Journal of Chromatography A 1964, 16, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Smyshlyaev, D.; Kirillov, E.; Kirillov, S.; Bunkov, G.; Rychkov, V.; Botalov, M.; Taukin, A.; Yuldashbaeva, A.; Malyshev, A. Recovery and Separation of Sc, Zr and Ti from Acidic Sulfate Solutions for High Purity Scandium Oxide Production: Laboratory and Pilot Study. Hydrometallurgy 2022, 211, 105889. [Google Scholar] [CrossRef]
- Wood, S.A. The Aqueous Geochemistry of the Rare-Earth Elements and Yttrium. Chemical Geology 1990, 82, 159–186. [Google Scholar] [CrossRef]
- Lin, P.; Yang, X.; Werner, J.M.; Honaker, R.Q. Application of Eh-pH Diagrams on Acid Leaching Systems for the Recovery of REEs from Bastnaesite, Monazite and Xenotime. Metals 2021, 11, 734. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A New Model for Heavy Metal Removal in a Biosorption Column. Adsorption Science & Technology 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Biosorption of Phenol by Immobilized Activated Sludge in a Continuous Packed Bed: Prediction of Breakthrough Curves. Process Biochemistry 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Calero, M.; Hernáinz, F.; Blázquez, G.; Tenorio, G.; Martín-Lara, M.A. Study of Cr (III) Biosorption in a Fixed-Bed Column. Journal of Hazardous Materials 2009, 171, 886–893. [Google Scholar] [CrossRef]
| Element | Mg | Na | Ca | Si | Al | K | Fe | Sc | Ti |
|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | 7270.0 | 1809.38 | 621.12 | 286.3 | 274.10 | 281.3 | 50.74 | 12.31 | 1.58 |
| Model | Langmuir | Freundlich | Temkin | ||||
|---|---|---|---|---|---|---|---|
| Parameter | R2 | Qm, g L−1 | KL, L mg−1 | R2 | KF, L mg−1 | R2 | KT, L mg−1 |
| Value | 0.983 | 8.576 | 0.985 | 0.975 | 3.440 | 0.963 | 1615 |
| Thomas model | 2.5 mL min−1 | 5 mL min−1 | 7.5 mL min−1 | |
| Kt (L min−1 mg−1) | 0.00142 | 6.834×10-4 | 3.65×10-4 | |
| Q0 (mg mL−1) | 7.013 | 6.776 | 6.383 | |
| R2 | 0.99 | 0.989 | 0.996 | |
| Modified dose-response model | 2.5 mL min−1 | 5 mL min−1 | 7.5 mL min−1 | |
| a | 2.197 | 2.184 | 3.115 | |
| q0 (mg mL−1) | 10.214 | 4.845 | 3.213 | |
| R2 | 0.973 | 0.987 | 0.988 |
| MDR model | Sc | Fe | Ti |
| a | 0.679 | 1.532 | 0.187 |
| q0 (mg mL−1) | 3.888 | 0.343 | 0.082 |
| R2 | 0.984 | 0.997 | 0.905 |
| Element | Sc | Ti | Fe |
|---|---|---|---|
| βSc/E | - | 0.25 | 6.90 |
| Kd (mL g−1) | 0.40 | 1.61 | 0.06 |
| Qd, mg mL−1 | 3.77 | 1.14 | 2.81 |
| Element | Sc | Mg | Ti | Fe | Y | La | Ce | Nd | Sm | Th |
| mg L−1 | 461.5 | 195.9 | 48.8 | 16.1 | 9.4 | 4.3 | 6.2 | 5.0 | 1.9 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





