Submitted:
28 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Requirements for Osteochondral Tissue Regeneration
3. Peptides for Bone Regeneration
3.1. Osteo-Inducers
3.1.1. Collagen-Mimetic/Derived Peptides
3.1.2. BMP-Mimetic/Derived Peptides
3.1.3. Hormone-Derived Peptides
3.1.4. Circulating Peptides
3.1.5. Other ECM-Derived Peptides
3.2. Angiogenic
4. Peptides for Cartilage Regeneration
4.1. Chondroinductive/Chondrogenic Peptides
4.1.1. TGF-β Mimetic Peptides
4.1.2. BMP2-Derived/Mimetic Peptides
5. Other Supporting Peptides
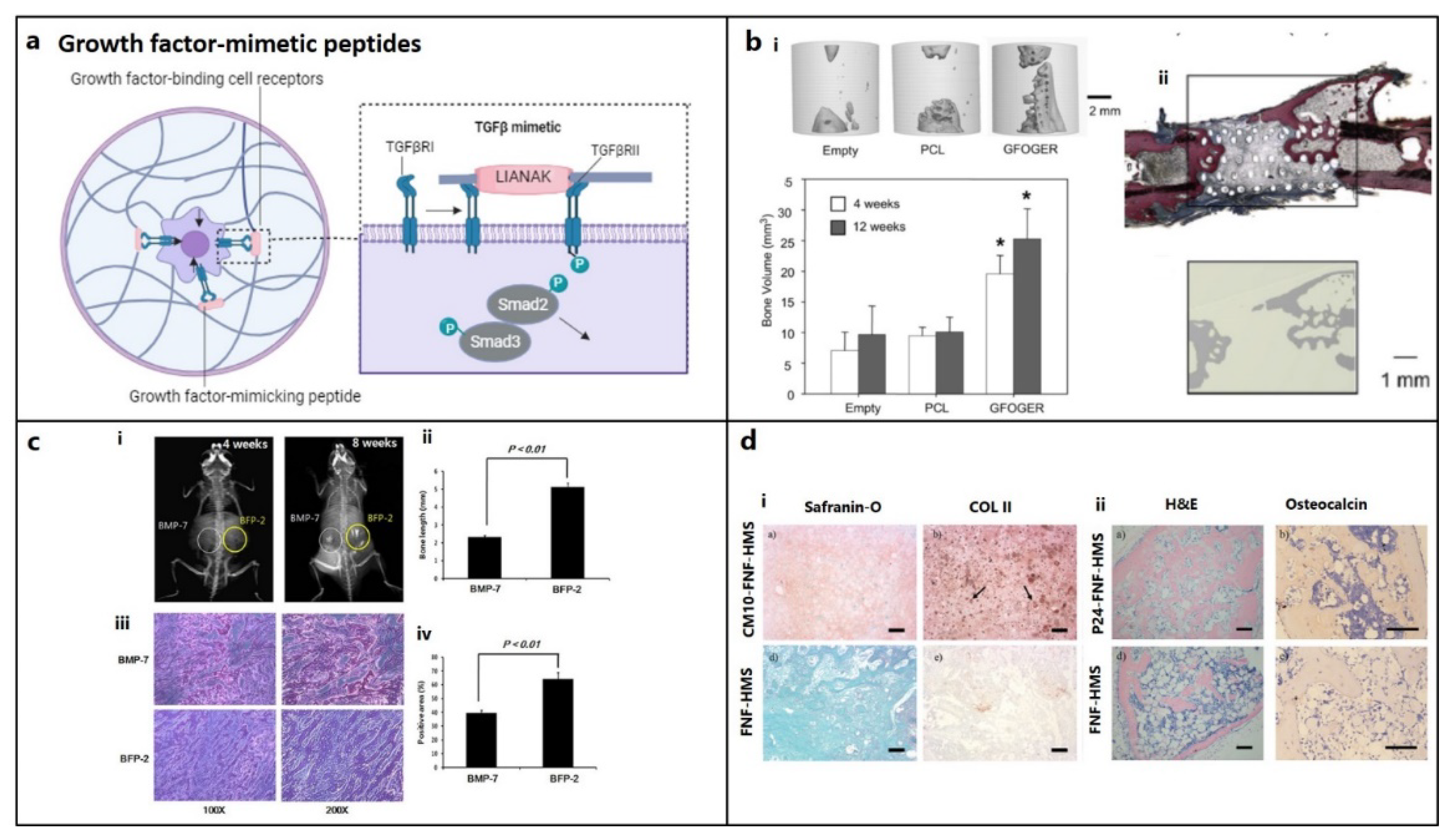
5.1. Adhesion, Binding, or Affinity Peptides
5.2. Cell Penetrating Peptides (CPPs)
5.3. Peptides Promoting Cell Migration
5.4. Self-Assembly (SA) Peptides
5.5. Degradable Peptides
5.6. Antimicrobial and Immunomodulatory Peptides
6. Peptide-Conjugated Biomaterials
6.1. Osteo-Inductive Scaffold
6.2. Chondro-Inductive Scaffolds
6.3. Multifunctional Scaffolds
7. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Yildirim, N.; Amanzhanova, A.; Kulzhanova, G.; Mukasheva, F.; Erisken, C. Osteochondral interface: regenerative engineering and challenges. ACS Biomaterials Science & Engineering 2023, 9, 1205–1223. [Google Scholar]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Rheumatology 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Liao, Q.; Gee, C.W. Surgical management of osteochondral defects of the knee: an educational review. Current Reviews in Musculoskeletal Medicine 2021, 14, 60–66. [Google Scholar] [CrossRef]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioactive materials 2022, 9, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Madry, H. Surgical therapy in osteoarthritis. Osteoarthritis and cartilage 2022, 30, 1019–1034. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioactive materials 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nature Reviews Rheumatology 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Chen, L.; Wei, L.; Su, X.; Qin, L.; Xu, Z.; Huang, X.; Chen, H.; Hu, N. Preparation and Characterization of Biomimetic Functional Scaffold with Gradient Structure for Osteochondral Defect Repair. Bioengineering 2023, 10, 213. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Palermo, N.; D’Amora, U.; Oddo, S.; Guglielmino, S.P.P.; Conoci, S.; Szychlinska, M.A.; Calabrese, G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. International Journal of Molecular Sciences 2022, 23, 7388. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Moreira, A.; Weber, A.; Williams, G.R.; Costa, P.F. Osteochondral tissue engineering: The potential of electrospinning and additive manufacturing. Pharmaceutics 2021, 13, 983. [Google Scholar] [CrossRef]
- Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic peptides and attachment methods determine tissue regeneration in modified bone graft substitutes. Journal of Functional Biomaterials 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. Journal of controlled release 2016, 244, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: a systematic review. BMC medicine 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Ai, C.; Lee, Y.H.D.; Tan, X.H.; Tan, S.H.S.; Hui, J.H.P.; Goh, J.C.-H. Osteochondral tissue engineering: perspectives for clinical application and preclinical development. Journal of Orthopaedic Translation 2021, 30, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Le, H.; Wang, Y.; Liu, H.; Li, Z.; Yang, X.; Wang, C.; Ding, J.; Chen, X. Instructive cartilage regeneration modalities with advanced therapeutic implantations under abnormal conditions. Bioactive Materials 2022, 11, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-dimensional printing of multilayered tissue engineering scaffolds. Materials Today 2018, 21, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Rameshbabu, A.P.; Maity, P.P.; Mandal, A.; Bankoti, K.; Dutta, J.; Das, D.K.; Dey, G.; Mandal, M.; Dhara, S. Osteochondral defects healing using extracellular matrix mimetic phosphate/sulfate decorated GAGs-agarose gel and quantitative micro-CT evaluation. ACS Biomaterials Science & Engineering 2018, 5, 149–164. [Google Scholar]
- Bittner, S.M.; Guo, J.L.; Mikos, A.G. Spatiotemporal control of growth factors in three-dimensional printed scaffolds. Bioprinting 2018, 12, e00032. [Google Scholar] [CrossRef]
- Gibson, J.D.; O’Sullivan, M.B.; Alaee, F.; Paglia, D.N.; Yoshida, R.; Guzzo, R.M.; Drissi, H. Regeneration of articular cartilage by human ESC-derived mesenchymal progenitors treated sequentially with BMP-2 and Wnt5a. Stem cells translational medicine 2017, 6, 40–50. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.E.; Carpio, L.R.; Bradley, E.W.; Dudakovic, A.; Lian, J.B.; van Wijnen, A.J.; Kakar, S.; Hsu, W.; Westendorf, J.J. Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice. Bone 2014, 66, 277–286. [Google Scholar] [CrossRef]
- Du, X.; Xie, Y.; Xian, C.J.; Chen, L. Role of FGFs/FGFRs in skeletal development and bone regeneration. Journal of cellular physiology 2012, 227, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Diaz-Gomez, L.; Xie, V.Y.; Bittner, S.M.; Jiang, E.Y.; Wang, B.; Mikos, A.G. Three-dimensional printing of click functionalized, peptide patterned scaffolds for osteochondral tissue engineering. Bioprinting 2021, 22, e00136. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: mechanisms and interventions. Nature Reviews Rheumatology 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Pozos-Guillén, A.; Molina, G.; Soviero, V.; Arthur, R.A.; Chavarria-Bolaños, D.; Acevedo, A.M. Management of dental caries lesions in Latin American and Caribbean countries. Brazilian Oral Research 2021, 35, e055. [Google Scholar] [CrossRef]
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Advanced drug delivery reviews 2012, 64, 1257–1276. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature medicine 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Albisetti, W.; Agus, A.; Iori, S.; Tagliabue, L. Risk factors contributing to fracture non-unions. Injury 2007, 38, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef]
- Connelly, J.; Petrie, T.; García, A.; Levenston, M. Fibronectin-and Collagen-Mimetic Ligands Regulate BMSC Chondrogenesis in 3D Hydrogels. European cells & materials 2011, 22, 168. [Google Scholar]
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E.; Dupont, K.M.; Templeman, K.L.; Hutmacher, D.W.; Guldberg, R.E.; García, A.J. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 2010, 31, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; García, A.J.; Guldberg, R.E. Nanofiber orientation and surface functionalization modulate human mesenchymal stem cell behavior in vitro. Tissue Engineering Part A 2014, 20, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Engineering 1999, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hestehave Pedersen, R.; Rasmussen, M.; Overgaard, S.; Ding, M. Effects of P-15 peptide coated hydroxyapatite on Tibial defect repair in vivo in Normal and osteoporotic rats. BioMed Research International 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Koepple, M.; Moest, T.; Neumann, K.; Weisel, T.; Schlegel, K.A. In vivo evaluation of biofunctionalized implant surfaces with a synthetic peptide (P-15) and its impact on osseointegration. A preclinical animal study. Clinical Oral Implants Research 2016, 27, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 2014, 15, 1484–1490. [Google Scholar] [PubMed]
- Uysal, O.; Arslan, E.; Gulseren, G.; Kilinc, M.C.; Dogan, I.; Ozalp, H.; Caglar, Y.S.; Guler, M.O.; Tekinay, A.B. Collagen peptide presenting nanofibrous scaffold for intervertebral disc regeneration. ACS Applied Bio Materials 2019, 2, 1686–1695. [Google Scholar] [PubMed]
- Harbers, G.M.; Healy, K.E. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2005, 75, 855–869. [Google Scholar] [CrossRef]
- Anderson, J.M.; Vines, J.B.; Patterson, J.L.; Chen, H.; Javed, A.; Jun, H.-W. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta biomaterialia 2011, 7, 675–682. [Google Scholar]
- Sindrey, D.; Pugh, S.; Smith, T. Connective Tissue Stimulating Peptides. US20050288229A1, US Patent 2005/02888229 A1. 2005. [Google Scholar]
- Huang, W.; Yu, K.; Kang, M.; Wang, Q.; Liao, W.; Liang, P.; Liu, G.; Cao, Y.; Miao, J. Identification and functional analysis of three novel osteogenic peptides isolated from tilapia scale collagen hydrolysate. Food Research International 2022, 162, 111993. [Google Scholar] [CrossRef]
- Lam, H.; Li, S.; Lou, N.; Chu, J.; Bhatnagar, R. Synthetic peptides cytomodulin-1 (CM-1) and cytomodulin-2 (CM-2) promote collagen synthesis and wound healing in vitro. In Proceedings of the The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2004; pp. 5028–5030. [Google Scholar]
- Lin, Z.-Y.; Duan, Z.-X.; Guo, X.-D.; Li, J.-F.; Lu, H.-W.; Zheng, Q.-X.; Quan, D.-P.; Yang, S.-H. Bone induction by biomimetic PLGA-(PEG-ASP) n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. Journal of Controlled Release 2010, 144, 190–195. [Google Scholar] [CrossRef]
- Duan, Z.; Zheng, Q.; Guo, X.; Yuan, Q.; Chen, S. Experimental research on ectopic osteogenesis of BMP2-derived peptide P24 combined with PLGA copolymers. Journal of Huazhong University of Science and Technology 2007, 27, 179–182. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Ogata, S.I.; Ohtsuki, C.; Tanihara, M. Prolonged ectopic calcification induced by BMP-2–derived synthetic peptide. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2004, 70, 115–121. [Google Scholar] [CrossRef]
- Kang, E.-J.; Kim, S.-K.; Eom, T.-G.; Choi, K.-O.; Lee, T.-H. Evaluation of the osteogenic activity of the BMP-2 mimetic peptide, PEP7, in vitro and in vivo. International Journal of Oral & Maxillofacial Implants 2013, 28. [Google Scholar]
- Saito, A.; Suzuki, Y.; Ogata, S.-i.; Ohtsuki, C.; Tanihara, M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2003, 1651, 60–67. [Google Scholar] [CrossRef]
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta biomaterialia 2011, 7, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, J.; Jabbari, E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir 2008, 24, 12508–12516. [Google Scholar] [CrossRef]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. Journal of Controlled Release 2009, 134, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Yeo, S.-I.; Park, J.-W.; Shin, H.-I.; Bae, Y.-C.; Suh, J.-Y. The effects of synthetic peptide derived from hBMP-2 on bone formation in rabbit calvarial defect. Tissue Eng Regen Me 2008, 5, 488–497. [Google Scholar]
- Lee, J.S.; Lee, J.S.; Murphy, W.L. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta biomaterialia 2010, 6, 21–28. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Kitamura, M.; Ogata, S.I.; Yoshihara, Y.; Masuda, S.; Ohtsuki, C.; Tanihara, M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an α-tricalcium phosphate scaffold. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2006, 77, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, J.S.; Kim, J.H.; Seon, J.K.; Park, K.S.; Jeong, M.H.; Yoon, T.R. Bone-forming peptide-2 derived from BMP-7 enhances osteoblast differentiation from multipotent bone marrow stromal cells and bone formation. Experimental & molecular medicine 2017, 49, e328–e328. [Google Scholar]
- Patterson, J. Peptide-functionalized Biomaterials with Osteoinductive or Anti-biofilm Activity. Racing for the Surface: Antimicrobial and Interface Tissue Engineering, 2020; 129–168. [Google Scholar]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; Gurski, L.; Bonor, J.; Price, C.; Wang, L.; Beamer, W.G.; Nohe, A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 2011, 49, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2. 1, a novel peptide, induces articular cartilage formation in vivo. Journal of Orthopaedic Research 2017, 35, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic injection of CK2. 3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. Journal of Orthopaedic Research 2015, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Alkhiary, Y.M.; Gerstenfeld, L.C.; Krall, E.; Westmore, M.; Sato, M.; Mitlak, B.H.; Einhorn, T.A. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). JBJS 2005, 87, 731–741. [Google Scholar]
- Komrakova, M.; Stuermer, E.K.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Daub, F.; Martens, T.; Witzenhausen, P. Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 2010, 47, 480–492. [Google Scholar] [CrossRef]
- Mognetti, B.; Marino, S.; Barberis, A.; Bravo Martin, A.-S.; Bala, Y.; Di Carlo, F.; Boivin, G.; Portigliatti Barbos, M. Experimental stimulation of bone healing with teriparatide: histomorphometric and microhardness analysis in a mouse model of closed fracture. Calcified tissue international 2011, 89, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chintamaneni, S.; Finzel, K.; Gruber, B. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporosis international 2010, 21, 1059–1063. [Google Scholar] [CrossRef]
- Whitfield, J.F.; Motley, P.; Willick, G.E. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treatments in Endocrinology 2002, 1, 175–190. [Google Scholar] [CrossRef]
- Peggion, E.; Mammi, S.; Schievano, E.; Silvestri, L.; Schiebler, L.; Bisello, A.; Rosenblatt, M.; Chorev, M. Structure− Function Studies of Analogues of Parathyroid Hormone (PTH)-1− 34 Containing β-Amino Acid Residues in Positions 11− 13. Biochemistry 2002, 41, 8162–8175. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Lozano, D.; Portal-Núñez, S.; Maycas, M.; De la Fuente, M.; Caeiro, J.R.; Esbrit, P. Comparison of the skeletal effects induced by daily administration of PTHrP (1–36) and PTHrP (107–139) to ovariectomized mice. Journal of cellular physiology 2012, 227, 1752–1760. [Google Scholar] [CrossRef]
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regí, M.; García-Honduvilla, N.; Buján, J. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Mrak, E.; Guidobono, F.; Moro, G.; Fraschini, G.; Rubinacci, A.; Villa, I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by β-catenin stabilization. Journal of cellular physiology 2010, 225, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Melzi, R.; Pagani, F.; Ravasi, F.; Rubinacci, A.; Guidobono, F. Effects of calcitonin gene-related peptide and amylin on human osteoblast-like cells proliferation. European journal of pharmacology 2000, 409, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, X.; Zhao, R.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-κB activation, osteoclastogenesis and bone resorption. Bone 2010, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jiang, D. The role and mechanism of exogenous calcitonin gene-related peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell biochemistry and biophysics 2014, 69, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Calland, J.W.; Harris, S.E.; Carnes Jr, D.L. Human pulp cells respond to calcitonin gene-related peptide in vitro. Journal of endodontics 1997, 23, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Vignery, A.; McCarthy, T. The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone 1996, 18, 331–335. [Google Scholar] [CrossRef]
- Ballica, R.; Valentijn, K.; Khachatryan, A.; Guerder, S.; Kapadia, S.; Gundberg, C.; Gilligan, J.; Flavell, R.A.; Vignery, A. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. Journal of bone and mineral research 1999, 14, 1067–1074. [Google Scholar] [CrossRef]
- Bab, I.; Gazit, D.; Chorev, M.; Muhlrad, A.; Shteyer, A.; Greenberg, Z.; Namdar, M.; Kahn, A. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. The EMBO journal 1992, 11, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Gabet, Y.; Müller, R.; Regev, E.; Sela, J.; Shteyer, A.; Salisbury, K.; Chorev, M.; Bab, I. Osteogenic growth peptide modulates fracture callus structural and mechanical properties. Bone 2004, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Brager, M.A.; Patterson, M.J.; Connolly, J.F.; Nevo, Z. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. Journal of Orthopaedic Research 2000, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Guo, C.; Xu, X.; Gao, J.; Zhang, J.; Chen, T.; Cui, D. Osteogenic growth peptide enhances the proliferation of bone marrow mesenchymal stem cells from osteoprotegerin-deficient mice by CDK2/cyclin A. Acta Biochim Biophys Sin 2010, 42, 801–806. [Google Scholar] [CrossRef]
- An, G.; Xue, Z.; Zhang, B.; Deng, Q.; Wang, Y.; Lv, S. Expressing osteogenic growth peptide in the rabbit bone mesenchymal stem cells increased alkaline phosphatase activity and enhanced the collagen accumulation. Eur Rev Med Pharmacol Sci 2014, 18, 1618–1624. [Google Scholar] [PubMed]
- Li, G.; Cui, Y.; McILmurray, L.; Allen, W.E.; Wang, H. rhBMP-2, rhVEGF165, rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. Journal of orthopaedic research 2005, 23, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vordemvenne, T.; Paletta, J.R.; Hartensuer, R.; Pap, T.; Raschke, M.J.; Ochman, S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC musculoskeletal disorders 2011, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Pazdrak, B.; Carney, D.H. Systemic administration of thrombin peptide TP508 enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia. Journal of vascular research 2013, 50, 186–196. [Google Scholar] [CrossRef]
- Hanratty, B.M.; Ryaby, J.T.; Pan, X.-H.; Li, G. Thrombin related peptide TP508 promoted fracture repair in a mouse high energy fracture model. Journal of Orthopaedic Surgery and Research 2009, 4, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Touma, E.; Qi, Y.; Rousseau, E.; Quigg, R.J.; Ryaby, J.T. TP508 accelerates fracture repair by promoting cell growth over cell death. Biochemical and biophysical research communications 2007, 364, 187–193. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tomin, E.; Doty, S.B.; Lane, J.M.; Carney, D.H.; Ryaby, J.T. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. Journal of orthopaedic research 2005, 23, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Park, Y.-J.; Lee, Y.-M.; Rhyu, I.-C.; Ku, Y. The biological effects of fibrin-binding synthetic oligopeptides derived from fibronectin on osteoblast-like cells. Journal of Periodontal & Implant Science 2012, 42, 113–118. [Google Scholar]
- Lee, J.-A.; Ku, Y.; Rhyu, I.-C.; Chung, C.-P.; Park, Y.-J. Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects. Journal of periodontal & implant science 2010, 40, 211–219. [Google Scholar]
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Science translational medicine 2011, 3, 100ra189. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choo, J.-E.; Park, H.-J.; Park, J.-B.; Lee, S.-C.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochemical and biophysical research communications 2007, 357, 68–74. [Google Scholar] [CrossRef]
- Shin, M.K.; Kim, M.-K.; Bae, Y.-S.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cellular signalling 2008, 20, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Park, J.-B.; Min, D.-S.; Lee, S.-J.; Rhyu, H.K.; Jo, I.-H.; Chung, C.-P.; Park, Y.-J. Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials 2007, 28, 4257–4267. [Google Scholar] [CrossRef]
- Egusa, H.; Kaneda, Y.; Akashi, Y.; Hamada, Y.; Matsumoto, T.; Saeki, M.; Thakor, D.K.; Tabata, Y.; Matsuura, N.; Yatani, H. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 2009, 30, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Yuki, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Hashida, M.K.; Harutsugu, K.; Nokihara, K.; Daito, M.; Matsuura, N. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dental materials journal 2004, 23, 650–655. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, Y.; Son, J.Y.; Bae, J.W.; Park, K.D. In situ SVVYGLR peptide conjugation into injectable gelatin-poly (ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjugate chemistry 2012, 23, 2042–2050. [Google Scholar] [CrossRef]
- Rezania, A.; Healy, K.E. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnology progress 1999, 15, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Stile, R.A.; Healy, K.E. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules 2001, 2, 185–194. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Benoit, D.S. Depot-based delivery systems for pro-angiogenic peptides: a review. Frontiers in bioengineering and biotechnology 2015, 3, 102. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Basile, A.; Capasso, D.; Di Gaetano, S.; Di Stasi, R.; Pascale, M.; Turco, C.M.; Ziche, M.; Morbidelli, L.; D’Andrea, L.D. Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis. Biochemical pharmacology 2012, 84, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Silva, E.A.; Yuen, W.W.; Mooney, D.J. Spatio–temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharmaceutical research 2007, 24, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-M.; Kang, Y.; Chun, H.J.; Jeong, J.-W.; Park, C. Evaluation of the in vitro and in vivo angiogenic effects of exendin-4. Biochemical and biophysical research communications 2013, 434, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Nokihara, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Matsuo, M.; Umakoshi, Y.; Takahashi, J.; Matsuura, N. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochemical and biophysical research communications 2003, 310, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Egusa, H.; Kaneda, Y.; Hirata, I.; Kawaguchi, N.; Hirao, T.; Matsumoto, T.; Yao, M.; Daito, K.; Suzuki, M. Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dental materials journal 2007, 26, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.F.; Iruela-Arispe, M.L.; Johnson, R.S.; Sage, E.H. SPARC is a source of copper-binding peptides that stimulate angiogenesis. The Journal of cell biology 1994, 125, 929–943. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Burke, K.; Antonienko, E.; Brown III, E.; Benoit, D.S. Enzymatically-responsive pro-angiogenic peptide-releasing poly (ethylene glycol) hydrogels promote vascularization in vivo. Journal of Controlled Release 2015, 217, 191–201. [Google Scholar] [CrossRef]
- Hardy, B.; Raiter, A.; Weiss, C.; Kaplan, B.; Tenenbaum, A.; Battler, A. Angiogenesis induced by novel peptides selected from a phage display library by screening human vascular endothelial cells under different physiological conditions. Peptides 2007, 28, 691–701. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Research Part C: Embryo Today: Reviews 2014, 102, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Qian, J.J. Peptide compositions with growth factor-like activity. 1997.
- Renner, J.N.; Liu, J.C. Investigating the effect of peptide agonists on the chondrogenic differentiation of human mesenchymal stem cells using design of experiments. Biotechnology Progress 2013, 29, 1550–1557. [Google Scholar] [CrossRef]
- Zhang, Z.; Gupte, M.J.; Jin, X.; Ma, P.X. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Advanced functional materials 2015, 25, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kumar, R.; Parsad, D.; Kumar, N. Cytomodulin-functionalized porous PLGA particulate scaffolds respond better to cell migration, actin production and wound healing in rodent model. Journal of tissue engineering and regenerative medicine 2014, 8, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yang, D.; Wong, J.H.; Wang, J.; Yin, C.; Zhu, Y.; Fan, S.; Ng, T.B.; Xia, J.; Li, Z. A Thioether-Stabilized d-Proline–l-Proline-Induced β-Hairpin Peptide of Defensin Segment Increases Its Anti-Candida albicans Ability. ChemBioChem 2016, 17, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Ruczynski, J.; Wierzbicki, P.M.; Kogut-Wierzbicka, M.; Mucha, P.; Siedlecka-Kroplewska, K.; Rekowski, P. Cell-penetrating peptides as a promising tool for delivery of various molecules into the cells. Folia histochemica et cytobiologica 2014, 52, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Srinivasan, P.P.; Xu, X.; Jia, X.; Safran, C.B.K.; Nohe, A. CK2. 1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Research & Therapy 2017, 8, 1–11. [Google Scholar]
- Renner, J.N.; Kim, Y.; Liu, J.C. Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells. Tissue Engineering Part A 2012, 18, 2581–2589. [Google Scholar] [CrossRef]
- Scholz, M.; Schleicher, P.; Sewing, A.; Gelinsky, M.; Kandziora, F. Cyclic-RGD is as effective as rhBMP-2 in anterior interbody fusion of the sheep cervical spine. Spine 2013, 38, E59–E65. [Google Scholar] [CrossRef]
- Hennessy, K.M.; Pollot, B.E.; Clem, W.C.; Phipps, M.C.; Sawyer, A.A.; Culpepper, B.K.; Bellis, S.L. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials 2009, 30, 1898–1909. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Hennessy, K.M.; Bellis, S.L. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 2007, 28, 383–392. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel peptide-based platform for the dual presentation of biologically active peptide motifs on biomaterials. ACS applied materials & interfaces 2014, 6, 6525–6536. [Google Scholar]
- Paredes, V.; Salvagni, E.; Rodríguez-Castellon, E.; Gil, F.; Manero, J. Study on the use of 3-aminopropyltriethoxysilane and 3-chloropropyltriethoxysilane to surface biochemical modification of a novel low elastic modulus Ti–Nb–Hf alloy. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2015, 103, 495–502. [Google Scholar] [CrossRef]
- Benoit, D.S.; Anseth, K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 2005, 26, 5209–5220. [Google Scholar] [CrossRef]
- Schuler, M.; Hamilton, D.W.; Kunzler, T.P.; Sprecher, C.M.; de Wild, M.; Brunette, D.M.; Textor, M.; Tosatti, S.G. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2009, 91, 517–527. [Google Scholar] [CrossRef]
- Dee, K.C.; Andersen, T.T.; Bizios, R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. Journal of biomedical materials research 1998, 40, 371–377. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Romeo, J.D.; McGowan, K.A.; Gawalt, E.S. Increased osteoblast adhesion on physically optimized KRSR modified calcium aluminate. Journal of Biomedical Materials Research Part A 2012, 100, 1229–1238. [Google Scholar] [CrossRef]
- Sun, S.; Yu, W.; Zhang, Y.; Zhang, F. Increased preosteoblast adhesion and osteogenic gene expression on TiO 2 nanotubes modified with KRSR. Journal of Materials Science: Materials in Medicine 2013, 24, 1079–1091. [Google Scholar]
- Nelson, M.; Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. International journal of nanomedicine 2006, 1, 339. [Google Scholar]
- Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanograined Ti modified with KRSR. Journal of Biomedical Materials Research Part A 2007, 80, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored integrin–extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem cells and development 2012, 21, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Lai, T.-S.; Lin, H.-C. Substrate stiffness and sequence dependent bioactive peptide hydrogels influence the chondrogenic differentiation of human mesenchymal stem cells. Journal of Materials Chemistry B 2021, 9, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, J.; Wong, D.S.H.; Li, J.; Zhao, P.; Bian, L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials 2017, 145, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lin, S.; Sun, Y.; Feng, Q.; Li, G.; Bian, L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials 2016, 77, 44–52. [Google Scholar] [CrossRef]
- Li, W.; Yu, B.; Li, M.; Sun, D.; Hu, Y.; Zhao, M.; Cui, C.-B.; Hou, S. NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochemical and biophysical research communications 2010, 391, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Hirayama, T.; Abbas, S.; Abu-Amer, Y. The IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. Journal of Biological Chemistry 2004, 279, 37219–37222. [Google Scholar] [CrossRef]
- Ligorio, C.; Mata, A. Synthetic extracellular matrices with function-encoding peptides. Nature Reviews Bioengineering 2023, 1–19. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, J.; Shen, L.; Ruan, Y.; Dong, J.; Guo, C.; Chen, Z. Biotin-conjugated anti-CD44 antibody–avidin binding system for the improvement of chondrocyte adhesion to scaffolds. Journal of Biomedical Materials Research Part A 2014, 102, 1140–1148. [Google Scholar] [CrossRef]
- Cheung, C.S.; Lui, J.C.; Baron, J. Identification of chondrocyte-binding peptides by phage display. Journal of Orthopaedic research 2013, 31, 1053–1058. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Pi, Y.; Wang, X.; Jia, Z.; Zhu, J.; Dai, L.; Chen, W.; Yin, L.; Chen, H. Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo. Biomaterials 2012, 33, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Roadcap, D.W.; Dhakephalkar, A.; Gonias, S.L. A 16-amino acid peptide from human α2-macroglobulin binds transforming growth factor-β and platelet-derived growth factor-BB. Protein Science 2000, 9, 1986–1992. [Google Scholar] [CrossRef]
- Shah, R.N.; Shah, N.A.; Del Rosario Lim, M.M.; Hsieh, C.; Nuber, G.; Stupp, S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proceedings of the National Academy of Sciences 2010, 107, 3293–3298. [Google Scholar] [CrossRef]
- Thakkar, S.; Fernandes, H.; Moroni, L. Decellularized extracellular matrix scaffolds for cartilage regeneration. Cartilage Tissue Engineering: Methods and Protocols, 2015; 133–151. [Google Scholar]
- Masters, K.S.; Shah, D.N.; Leinwand, L.A.; Anseth, K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 2005, 26, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.-P.; Horejs, C.-M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp: a versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.-Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and Arg-Gly-Asp–modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Engineering Part A 2015, 21, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, B.; Meyer, J.; Jonczyk, A.; Kessler, H.; Adamietz, P.; Meenen, N.M.; Kantlehner, M.; Goepfert, C.; Nies, B. RGD-peptides for tissue engineering of articular cartilage. Biomaterials 2002, 23, 3455–3463. [Google Scholar] [CrossRef]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef]
- Homandberg, G.A.; Hui, F. Arg-Gly-Asp-Ser peptide analogs suppress cartilage chondrolytic activities of integrin-binding and nonbinding fibronectin fragments. Archives of biochemistry and biophysics 1994, 310, 40–48. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.H.; Ge, Z.; Tan, L.P.; Hui, J.H.P.; Yang, Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kurashima, K.; Tustusmi, Y.; An, C.-H.; Suh, J.-Y.; Doi, H.; Nomura, N.; Noda, K.; Hanawa, T. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly (ethylene glycol) in rabbit cancellous bone. Acta Biomaterialia 2011, 7, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Tavella, S.; Raffo, P.; Tacchetti, C.; Cancedda, R.; Castagnola, P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Experimental cell research 1994, 215, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proceedings of the National Academy of Sciences 2013, 110, 10117–10122. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Wang, C.-C. Surface modification with peptide for enhancing chondrocyte adhesion and cartilage regeneration in porous scaffolds. Colloids and Surfaces B: Biointerfaces 2011, 84, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Song, H.; Park, S.G.; Lee, S.H.; Ko, J.J.; Park, J.H.; Jeong, J.; Cheon, Y.P.; Lee, D.R. Regulation of Differentiation Potential of Human Mesenchymal Stem Cells by Intracytoplasmic Delivery of Coactivator-Associated Arginine Methyltransferase 1 Protein Using Cell-Penetrating Peptide. Stem Cells 2012, 30, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Lee, J.Y.; Choi, Y.J.; You, H.K.; Hong, S.-D.; Chung, C.P.; Park, Y.J. Intracellular delivery of cell-penetrating peptide-transcriptional factor fusion protein and its role in selective osteogenesis. International journal of nanomedicine 2014, 1153–1166. [Google Scholar]
- Park, S.; Doh, J.; Park, S.; Lim, J.; Kim, S.; Youn, J.; Jin, H.; Seo, S.; Song, M.; Sung, S. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene therapy 2010, 17, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chu, X.; Li, W.; Pan, Q.; You, H. Chondrogenic effect of precartilaginous stem cells following NLS-TAT cell penetrating peptide-assisted transfection of eukaryotic h TGF β3. Journal of Cellular Biochemistry 2013, 114, 2588–2594. [Google Scholar] [CrossRef]
- Pan, Q.; Li, W.; Yuan, X.; Rakhmanov, Y.; Wang, P.; Lu, R.; Mao, Z.; Shang, X.; You, H. Chondrogenic effect of cell-based scaffold of self-assembling peptides/PLGA-PLL loading the hTGFβ3 plasmid DNA. Journal of Materials Science: Materials in Medicine 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Comprehensive Physiology 2012, 2, 2369. [Google Scholar] [PubMed]
- Shen, W.; Chen, X.; Chen, J.; Yin, Z.; Heng, B.C.; Chen, W.; Ouyang, H.-W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials 2010, 31, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, A.; Cohen, R.I.; Faulknor, R.; Schloss, R.; Yarmush, M.L.; Berthiaume, F. The development and characterization of SDF1α-elastin-like-peptide nanoparticles for wound healing. Journal of controlled release 2016, 232, 238–247. [Google Scholar] [CrossRef]
- Torres, P.; Díaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. The FASEB Journal 2017, 31, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Xia, C.J.; Wei, Y.; Yao, Y.; Dong, M.W.; Lin, K.Z.; Yu, L.S.; Gao, Y.; Fan, Y.Y. Annexin A1-derived peptide Ac2-26 facilitates wound healing in diabetic mice. Wound Repair and Regeneration 2020, 28, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Imanishi, A.; Mastrofrancesco, A.; Picardo, M.; Paus, R.; Mangoni, M.L. The frog skin-derived antimicrobial peptide esculentin-1a (1-21) NH2 promotes the migration of human HaCaT keratinocytes in an EGF receptor-dependent manner: a novel promoter of human skin wound healing? PloS one 2015, 10, e0128663. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Frontiers in oncology 2015, 5, 144. [Google Scholar] [CrossRef]
- Rubert Pérez, C.M.; Álvarez, Z.; Chen, F.; Aytun, T.; Stupp, S.I. Mimicking the bioactivity of fibroblast growth factor-2 using supramolecular nanoribbons. ACS Biomaterials Science & Engineering 2017, 3, 2166–2175. [Google Scholar]
- Zhang, S. Emerging biological materials through molecular self-assembly. Biotechnology advances 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polymer Chemistry 2012, 3, 18–33. [Google Scholar] [CrossRef]
- Avinash, M.; Govindaraju, T. Nanoarchitectonics of biomolecular assemblies for functional applications. Nanoscale 2014, 6, 13348–13369. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choo, J.E.; Choi, Y.S.; Lee, K.Y.; Min, D.S.; Pi, S.H.; Seol, Y.J.; Lee, S.J.; Jo, I.H.; Chung, C.P. Characterization of the surface immobilized synthetic heparin binding domain derived from human fibroblast growth factor-2 and its effect on osteoblast differentiation. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2007, 83, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Inagaki, A.; Khan, M.; Mori, K.; Penninger, J.M.; Nakamura, M.; Udagawa, N.; Aoki, K.; Ohya, K.; Uchida, K. Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-κB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity. Journal of Biological Chemistry 2013, 288, 5562–5571. [Google Scholar] [CrossRef]
- Ikeno, M.; Hibi, H.; Kinoshita, K.; Hattori, H.; Ueda, M. Effects of self-assembling peptide hydrogel scaffold on bone regeneration with recombinant human bone morphogenetic protein-2. Oral & Craniofacial Tissue Engineering 2011, 1. [Google Scholar]
- Recha-Sancho, L.; Semino, C.E. Heparin-based self-assembling peptide scaffold reestablish chondrogenic phenotype of expanded de-differentiated human chondrocytes. Journal of Biomedical Materials Research Part A 2016, 104, 1694–1706. [Google Scholar] [CrossRef]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.i. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2006, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proceedings of the National Academy of Sciences 2002, 99, 9996–10001. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Advanced healthcare materials 2015, 4, 702–713. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. International journal of molecular sciences 2021, 22, 11691. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, R.; Chai, C.; Wang, Y.; Chen, T.; Li, H.; Hu, Y.; Feng, Q.; Li, J. Antimicrobial peptides for bone tissue engineering: Diversity, effects and applications. Frontiers in Bioengineering and Biotechnology 2022, 10, 1030162. [Google Scholar] [CrossRef]
- Sun, Y.; Chan, J.; Bose, K.; Tam, C. Simultaneous control of infection and inflammation with keratin-derived antibacterial peptides targeting TLRs and co-receptors. Science Translational Medicine 2023, 15, eade2909. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, E.; Li, C.; Zeng, P.; Li, C.; Diepeveen-de Buin, M.; Lu, W.-Y.; Breukink, E.; Lu, W. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS letters 2010, 584, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proceedings of the National Academy of Sciences 2014, 111, E1409–E1418. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochemical and biophysical research communications 1998, 244, 253–257. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials 2018, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against bone infection. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-W.; Yang, C.-Y.; Chang, H.-T.; Lan, C.-Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PloS one 2011, 6, e17755. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrobial agents and chemotherapy 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. The FEBS journal 2017, 284, 3662–3683. [Google Scholar] [CrossRef]
- Luca, V.; Stringaro, A.; Colone, M.; Pini, A.; Mangoni, M.L. Esculentin (1-21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cellular and molecular life sciences 2013, 70, 2773–2786. [Google Scholar] [CrossRef]
- Chen, S.; Lu, Z.; Wang, F.; Wang, Y. Cathelicidin-WA polarizes E. coli K88-induced M1 macrophage to M2-like macrophage in RAW264. 7 cells. International Immunopharmacology 2018, 54, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Annals of the rheumatic diseases 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bian, A.; Jia, F.; Lan, J.; Yang, H.; Yan, K.; Xie, L.; Qiao, H.; Chang, X.; Lin, H. “Dual-functional” strontium titanate nanotubes designed based on fusion peptides simultaneously enhancing anti-infection and osseointegration. Biomaterials Advances 2022, 133, 112650. [Google Scholar] [CrossRef]
- Zhou, L.; Han, Y.; Ding, J.; Chen, X.; Huang, S.; Xing, X.; Wu, D.; Chen, J. Regulation of an antimicrobial peptide GL13K-modified titanium surface on osteogenesis, osteoclastogenesis, and angiogenesis base on osteoimmunology. ACS Biomaterials Science & Engineering 2021, 7, 4569–4580. [Google Scholar]
- Liu, H.-W.; Wei, D.-X.; Deng, J.-Z.; Zhu, J.-J.; Xu, K.; Hu, W.-H.; Xiao, S.-H.; Zhou, Y.-G. Combined antibacterial and osteogenic in situ effects of a bifunctional titanium alloy with nanoscale hydroxyapatite coating. Artificial cells, nanomedicine, and biotechnology 2018, 46, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumar, M.; Chansuria, J.; Singh, T.B.; Bhatnagar, R.; Shukla, V.K. Effect of Cytomodulin-10 (TGF-ß1 analogue) on wound healing by primary intention in a murine model. International Journal of Surgery 2009, 7, 460–465. [Google Scholar] [CrossRef]
- Boda, S.K.; Wang, H.; John, J.V.; Reinhardt, R.A.; Xie, J. Dual delivery of alendronate and E7-BMP-2 peptide via calcium chelation to mineralized nanofiber fragments for alveolar bone regeneration. ACS biomaterials science & engineering 2020, 6, 2368–2375. [Google Scholar]
- Laboy-López, S.; Méndez Fernández, P.O.; Padilla-Zayas, J.G.; Nicolau, E. Bioactive Cellulose Acetate Electrospun Mats as Scaffolds for Bone Tissue Regeneration. International Journal of Biomaterials 2022, 2022. [Google Scholar] [CrossRef]
- Lukasova, V.; Buzgo, M.; Sovkova, V.; Dankova, J.; Rampichova, M.; Amler, E. Osteogenic differentiation of 3D cultured mesenchymal stem cells induced by bioactive peptides. Cell Proliferation 2017, 50, e12357. [Google Scholar] [CrossRef]
- Gentile, P.; Ferreira, A.M.; Callaghan, J.T.; Miller, C.A.; Atkinson, J.; Freeman, C.; Hatton, P.V. Multilayer nanoscale encapsulation of biofunctional peptides to enhance bone tissue regeneration in vivo. Advanced healthcare materials 2017, 6, 1601182. [Google Scholar] [CrossRef]
- Gentile, P.; Ghione, C.; Tonda-Turo, C.; Kalaskar, D. Peptide functionalisation of nanocomposite polymer for bone tissue engineering using plasma surface polymerisation. RSC advances 2015, 5, 80039–80047. [Google Scholar] [CrossRef]
- Shekaran, A.; García, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. Journal of biomedical materials research Part A 2011, 96, 261–272. [Google Scholar] [CrossRef]
- Hasenbein, M.; Andersen, T.; Bizios, R. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials 2002, 23, 3937–3942. [Google Scholar] [CrossRef]
- Posadowska, U.; Storm, I.; de Wolf, F.; van den Beucken, J.; Leeuwenburgh, S. Nanofibrillar hydrogel scaffolds from recombinant protein-based polymers with integrin-and proteoglycan-binding domains. Production of protein-based polymers in Pichia pastoris 1, 135.
- Reyes, C.D.; García, A.J. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2004, 69, 591–600. [Google Scholar] [CrossRef]
- Yukna, R.A.; Callan, D.P.; Krauser, J.T.; Evans, G.H.; Aichelmann-Reidy, M.E.; Moore, K.; Cruz, R.; Scott, J.B. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. Journal of periodontology 1998, 69, 655–663. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Srour, S.; Wiltfang, J.; Neukam, F.W.; Schlegel, K.A. Enhanced bone regeneration with a synthetic cell-binding peptide—in vivo results. Biochemical and biophysical research communications 2005, 329, 789–795. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Kessler, P.; Srour, S.; Wiltfang, J.; Schlegel, K.A. Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials 2005, 26, 5648–5657. [Google Scholar] [CrossRef]
- Matos, S.; Guerra, F.; Krauser, J.T.; Figueiredo, H.; Marcelino, J.P.; Sanz, M. Evaluation of an anorganic bovine-derived mineral with P-15 hydrogel bone graft: preliminary study in a rabbit cranial bone model. Clinical Oral Implants Research 2012, 23, 698–705. [Google Scholar] [CrossRef]
- Culpepper, B.K.; Phipps, M.C.; Bonvallet, P.P.; Bellis, S.L. Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain. Biomaterials 2010, 31, 9586–9594. [Google Scholar] [CrossRef]
- Wang, V.; Misra, G.; Amsden, B. Immobilization of a bone and cartilage stimulating peptide to a synthetic bone graft. Journal of Materials Science: Materials in Medicine 2008, 19, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Shuqiang, M.; Kunzheng, W.; Xiaoqiang, D.; Wei, W.; Mingyu, Z.; Daocheng, W. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. Journal of plastic, reconstructive & aesthetic surgery 2008, 61, 1558–1560. [Google Scholar]
- Jung, R.E.; Hämmerle, C.H.; Kokovic, V.; Weber, F.E. Bone regeneration using a synthetic matrix containing a parathyroid hormone peptide combined with a grafting material. International Journal of Oral & Maxillofacial Implants 2007, 22. [Google Scholar]
- Sheller, M.R.; Crowther, R.S.; Kinney, J.H.; Yang, J.; Di Jorio, S.; Breunig, T.; Carney, D.H.; Ryaby, J.T. Repair of rabbit segmental defects with the thrombin peptide, TP508. Journal of orthopaedic research 2004, 22, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.; Moodie, G.; Dimond, P.; Giorani, C.; Ehrlich, M.; Valentini, R. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials 1999, 20, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, W.; Reinstorf, A.; Pompe, W.; Grass, R.; Biewener, A.; Holch, M.; Zwipp, H.; Rammelt, S. Effect of modification of hydroxyapatite/collagen composites with sodium citrate, phosphoserine, phosphoserine/RGD-peptide and calcium carbonate on bone remodelling. Bone 2007, 40, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hemraz, U.D.; Fenniri, H.; Webster, T.J. Tuning cell adhesion on titanium with osteogenic rosette nanotubes. Journal of biomedical materials research Part A 2010, 95, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Shimpi, T.M.; Sanow, W.R.; Storey, D.M.; Kitchell, B.S.; Webster, T.J. Molecular plasma deposited peptides on anodized nanotubular titanium: an osteoblast density study. Journal of Biomedical Materials Research Part A 2011, 98, 192–200. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.H.; Jung, Y. In situ chondrogenic differentiation of bone marrow stromal cells in bioactive self-assembled peptide gels. Journal of bioscience and bioengineering 2015, 120, 91–98. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, B.; Yang, J.; Heng, B.C.; Yang, Z.; Ge, Z.; Lin, J. TGF-β1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration. Journal of Materials Chemistry B 2018, 6, 675–687. [Google Scholar] [CrossRef]
- Ju, X.; Liu, X.; Zhang, Y.; Chen, X.; Chen, M.; Shen, H.; Feng, Y.; Liu, J.; Wang, M.; Shi, Q. A photo-crosslinked proteinogenic hydrogel enabling self-recruitment of endogenous TGF-β1 for cartilage regeneration. Smart Materials in Medicine 2022, 3, 85–93. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Hsieh, M.-F.; Fang, C.-H.; Jiang, C.-P.; Lin, B.; Lee, H.-M. Osteochondral regeneration induced by TGF-β loaded photo cross-linked hyaluronic acid hydrogel infiltrated in fused deposition-manufactured composite scaffold of hydroxyapatite and poly (ethylene glycol)-block-poly (ε-caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, J.; Yu, X.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; Ding, J. 3D-printed porous scaffolds of hydrogels modified with TGF-β1 binding peptides to promote in vivo cartilage regeneration and animal gait restoration. ACS applied materials & interfaces 2022, 14, 15982–15995. [Google Scholar]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, J.; Zhang, Y.; Tan, Y.; Zhou, J.; Zhou, D. Immobilization of RGD peptide onto the surface of apatite-wollastonite ceramic for enhanced osteoblast adhesion and bone regeneration. Journal of Wuhan University of Technology-Mater. Sci. Ed. 2014, 29, 626–634. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X.; Wu, B.; Zou, Z.; Duan, Z. A novel peptide-modified and gene-activated biomimetic bone matrix accelerating bone regeneration. Journal of Biomedical Materials Research Part A 2014, 102, 2864–2874. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Huang, W.; Lin, X.; Xie, X.; He, Z.; He, X.; Liu, S.; Bai, L.; Lu, B. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration. Biofabrication 2020, 12, 025030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, X.; Liu, T.; Huang, Y.; Li, J. The effects of Peptide Mel4-coated titanium plates on infection rabbits after internal fixation of open fractures. Archives of Orthopaedic and Trauma Surgery 2022, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Wu, D.; Huang, W.; Lin, Y.; Zhou, B.; Chen, J. The effects of titanium surfaces modified with an antimicrobial peptide GL13K by silanization on polarization, anti-inflammatory, and proinflammatory properties of macrophages. BioMed Research International 2020, 2020. [Google Scholar] [CrossRef]
- Fischer, N.G.; Chen, X.; Astleford-Hopper, K.; He, J.; Mullikin, A.F.; Mansky, K.C.; Aparicio, C. Antimicrobial and enzyme-responsive multi-peptide surfaces for bone-anchored devices. Materials Science and Engineering: C 2021, 125, 112108. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogues, M.; Velasco, F.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: the blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Applied Materials & Interfaces 2017, 9, 21618–21630. [Google Scholar]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-inspired multifunctional hydrogel coating for prevention of infections and enhanced osteogenesis. ACS applied materials & interfaces 2017, 9, 11428–11439. [Google Scholar]
- Zhongxing, L.; Shaohong, W.; Jinlong, L.; Limin, Z.; Yuanzheng, W.; Haipeng, G.; Jian, C. Three-dimensional printed hydroxyapatite bone tissue engineering scaffold with antibacterial and osteogenic ability. Journal of Biological Engineering 2021, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, X.; Liu, M.; Fernandes, G.; Zhang, Y.; Yang, S.; Ionita, C.N.; Yang, S. Antimicrobial peptide combined with BMP2-modified mesenchymal stem cells promotes calvarial repair in an osteolytic model. Molecular Therapy 2018, 26, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, J.; Wang, W.; Liang, X.; Zhang, W.; Li, W.; Lu, L.; Xiao, L.; Xu, Y.; Wang, Z. Facile and versatile surface functional polyetheretherketone with enhanced bacteriostasis and osseointegrative capability for implant application. ACS Applied Materials & Interfaces 2021, 13, 59731–59746. [Google Scholar]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta Biomaterialia 2019, 86, 323–337. [Google Scholar] [CrossRef]
- Trzcińska, Z.; Bruggeman, M.; Ijakipour, H.; Hodges, N.J.; Bowen, J.; Stamboulis, A. Polydopamine linking substrate for amps: Characterisation and stability on ti6al4v. Materials 2020, 13, 3714. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Chen, J.; Nie, B.; Yue, B.; Zhang, W.; Lyu, Z.; Long, T.; Wang, Y. KR-12 coating of polyetheretherketone (PEEK) surface via polydopamine improves osteointegration and antibacterial activity in vivo. Journal of Materials Chemistry B 2020, 8, 10190–10204. [Google Scholar] [CrossRef]

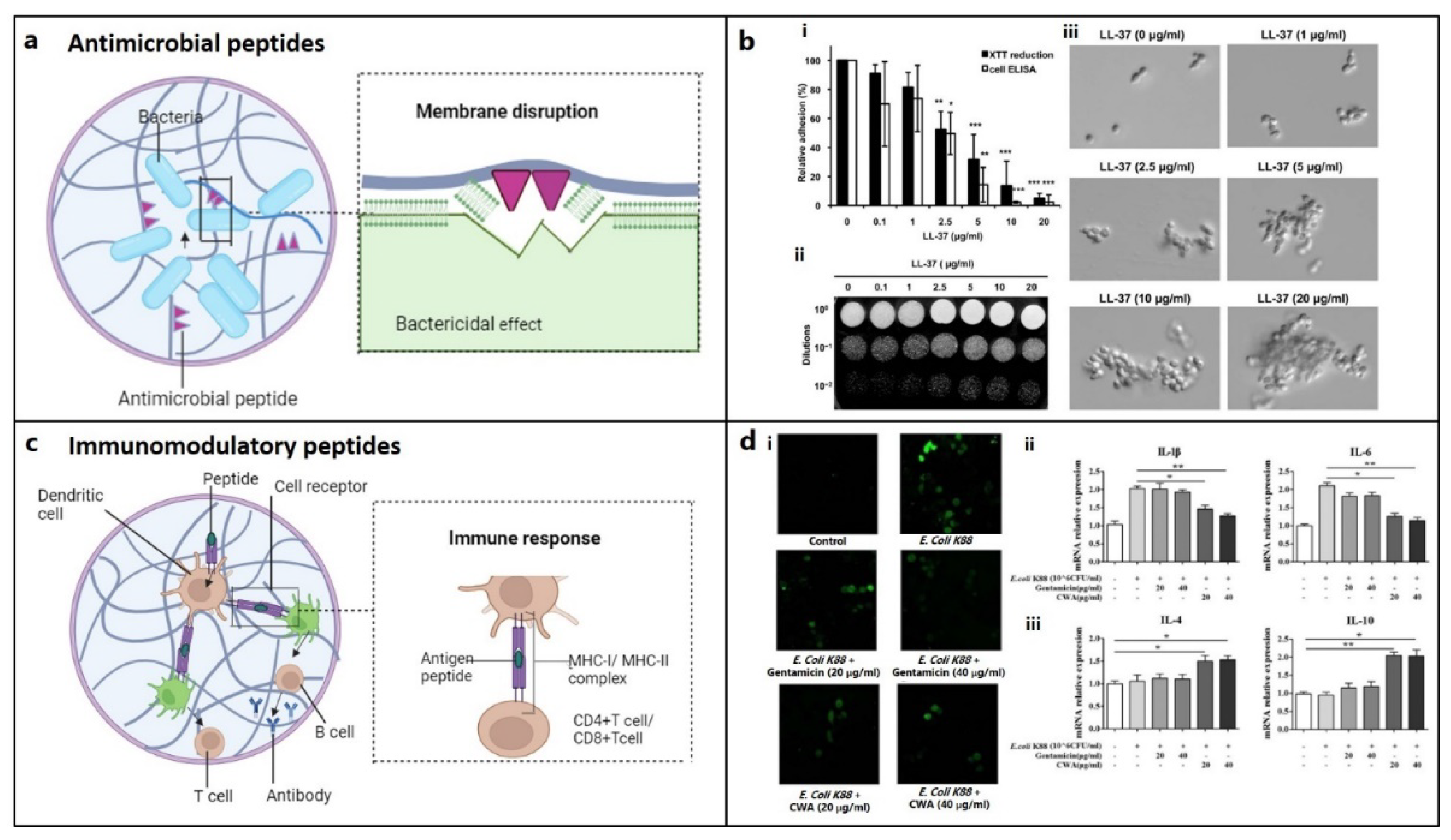
| Types | Source | Function | Ref |
|---|---|---|---|
| a. Osteogenic peptides | |||
| (i) Osteo-inductive peptides | |||
| GFOGER | Col | ↑ α2β1 integrin binding, osteoblastic differentiation | [28] |
| GTPGQGIAGQRGVV (P15 peptide) |
↑ osteogenic differentiation | [33] | |
| ((PKG)4-(POG)4-(DOG)4) (KOD peptide) |
↑ECM deposition | [37] | |
| DGEA | ↑expression of osteogenic markers (ALP, RUNX2, OCN). | [39] | |
| NGLPGPIGP (BCSP™-1) |
↑ mineralization | [40] | |
| GPAGPHGPVG, APDPFRMY, TPERYY | ↑ALP activity, mineralization, osteogenic gene expression | [41] | |
| KIPKASSVPTELSAISTLYL | BMP-2 | ↑ALP activity | [42] |
| SKIPKASSVPTGLSAISTLYLAAA (P24) | ↑ bone formation | [43,44,45] | |
| CKIPKPSSVP-TELSAISMLYL (PEP7) | ↑ bone formation | [46] | |
| KIPKASSVPTELSAISTLYL | ↑ALP activity, osteogenic marker | [47] | |
| NSVNSKIPKACCVPTELSAI, KIPKASSVPTELSAISTLYL, DWIVA | ↑Osteogenic differentiation, in vivo bone formation | [12] | |
| RKKNPNCRRH | BMP-4 | ↑Osteogenic differentiation | [55] |
| VEHDKEFFHPRYHH (BFP-2) | BMP-7 | ↑Osteogenic differentiation | [54] |
| TVPKPSSAPTQLNAISTLYF, GQGFSYPYKAVFSTQ, ETLDGQSINPKLAGL | ↑Osteogenic differentiation | [55] | |
| KVGKACCVPTKLSPISVLY | BMP-9 | ↑Osteogenic differentiation | [55] |
| CK2.2, CK2.3 | synthetic | ↑ BMP signaling | [57] |
| PTHrP1–34, PTHrP1–36, PTHrP107–111 | PTH | ↑ Osteoblast differentiation, bone formation | [64,65,66] |
| CGRP–α and β-CGRP | CGRP | ↑ Osteogenic differentiation, bone formation | [67,68,69,70] |
| ALKRQGRTLYGFGG (OGP) | Mammalian blood | ↑ Osteogenic differentiation, ALP, OCN and collagen secretion, mineralization | [76,77,78] |
| AGYKPDEGKRGDACEGDSGGPFV (TP508) | Thrombin | ↑ chemotaxis, proliferation, osteogenic differentiation | [79,80] |
| FN III9-10/12-14 | FN | ↑ osteoblast activity, mineralization | [85] |
| CBM | OPN | ↑ osteogenic differentiation, bone formation | [90] |
| SVVYGLR | OPN | ↑ osteogenesis, neovascularization | [91,92,93] |
| FHRRIKA | BSP | ↑ osteoblast activity, mineralization | [94] |
| (ii) Angiogenic peptides | |||
| QK | VEGF | ↑EC migration, proliferation | [97] |
| PBA2-1c | PDGF-BB | Blood vessels formation | [98] |
| Exendin-4 | Exendin-4 | ↑ tube formation of HUVECs | [99] |
| SPARC113, SPARC118 | OPN | ↑ in vivo angiogenesis | [103] |
| TP508 | Thrombin | ↑ neo-angiogenesis | [84] |
| RoY | Synthetic | ↑ EC proliferation, sprouting, in vivo angiogenesis | [104] |
| b. Chondroinductive peptides | |||
| CMs | TGF-β | ↑Chondrogenic differentiation | [107,108] |
| CK2.1 | synthetic | ↑Chondrogenesis | [112] |
| c. Other supporting peptides | |||
| (i) Adhesion, binding, affinity peptides | |||
| RGD | Col, FN, VN | ↑Cellular adhesion/ or differentiation | [114] |
| PHSRN | FN | ↑Cell adhesion | [30,117] |
| FHRRIKA | BSP | ↑Cell proliferation, spreading, mineralization | [116,120] |
| KRSR | BSP, FN, VN, OPN, thrombospondin | ↑Osteoblast adhesion, osteogenic gene expression | [122,123,124,125] |
| HAV | N-Cadherin | ↑Cell adhesion | [126] |
| NEMO-binding domain (NBD) peptide | synthetic | ↑Osteoblast diferentiation ↓bone resorption, osteoclastogenesis | [130] [131] |
| CDPGYIGSR | Laminin | ↑Cell adhesion, ECM deposition | [150] |
| (ii) Peptides supporting cell migration | |||
| SDF1-ELP | SDF-1 | EC migration, vascularization | [158] |
| Histatin-1 | Saliva | EC adhesion, migration, angiogenesis | [159] |
| Ac2-26 | Annexin A1 | Cell migration | [160] |
| Esculentin 1-21 | Frog-skin | Cell migration | [161] |
| LL-37 | Human | EC migration | [162] |
| (ii) Cell penetrating peptides (CPPs) | |||
| NLS-TAT | HIV-1 Tat protein | ↑Chondrogenic differentiation | [154,155] |
| (iii) Self-assembly (SA) peptides | |||
| AcN-RADARADARADARADA-CONH2 (RADA16-I) | synthetic | ↑ ALP, OCN, Runx2 | [169] |
| (iv) Degradable peptides | |||
| KCGPQGIWGQCK | MMP-derived | ↑GAGs, collagen deposition | [140,173] |
| Antimicrobial and immunomodulatory peptides | |||
| TLISWIKNKRKQRPRVSRRRRRRGGRRRR (Melamine) | synthetic | destabilization of cell membrane | [175] |
| LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES (LL-37) | human cathelicidin | inhibit cell wall synthesis | [179] |
| GIGKFLKKAKKFGKAFVKILKK (Pexiganan) | synthetic analogue of magainin2 | disrupts membrane | [175] |
| GIGKFLHSAGKFGKAFVGEIMKS (Magainin-1) |
frog skin | destabilizes or disrupts bacterial membrane | [175] |
| RRWVRRVRRWVRRVVRVVRRWVRR (PLG0206) | synthetic | disrupts cell membrane | [175] |
| RGGRLCYCRRRFCVCVGR (Protegrin-1) | porcine leukocytes | disrupts cell membrane | [175] |
| RLCRIVVIRVCR (Bactenecin) | bovine neutrophils | inhibit protein synthesis | [175] |
| VRVRVRVRVDPPTRVRVRVRV (PEP8R) | synthetic | disrupts bacterial cell membrane | [175] |
| ILPWKWPWWPWRR (Indolicidin) | bovine neutrophil | Increase cell membrane permeability | [175] |
| ILRWPWWPWRRK (Omiganan) | synthetic | destabilizes cell membrane | [175] |
| RAIGGGLSSVGGGSSTIKY (KAMP-19) | keratin | pore formation in bacterial cell membra | [176] |
| HNP-1 | human neutrophil | destabilizes cell wall integrity | [177] |
| RWRWRW-NH2 | synthetic | delocalizes peripheral membrane proteins | [178] |
| AMP 1037 | synthetic | ↓swimming and motility of bacteria, gene expression | [183] |
| Piscidin and sculentin 1–21 | fish | degrades biofilm | [184,185] |
| Histatin-1, 38 | saliva | antimicrobial | [159] |
| β defensin 3 | human | immunomodulator | [189] |
| GKIIKLKASLKLL (GL13K) | salivary protein | delocalizes cell membrane lipids | [190] |
| Abbreviations: BMP: Bone morphogenic protein, ALP: Alkaline Phosphatase, OCN: Osteocalcin, Col: Collagen, CK: Calmodulin Complexes, PTH: Parathyroid hormone, CGRP: Calcitonin gene-related peptide, OGP: Osteogenic growth peptide, FN: Fibronectin, VN: Vironectin, OPN: Osteopontin, CBM: Collagen-binding motif, BSP: bone sialoprotein, VEGF: Vascular endothelial growth factor, PDGF-BB, EC: Endothelial cell; CMs: Cytomodulins, GAG: Glycosaminoglycan; SDF-1: Stromal cell-derived factor 1, SDF1-ELP: SDF1 elastin-like peptide, CK2: casein kinase II | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





