Submitted:
27 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
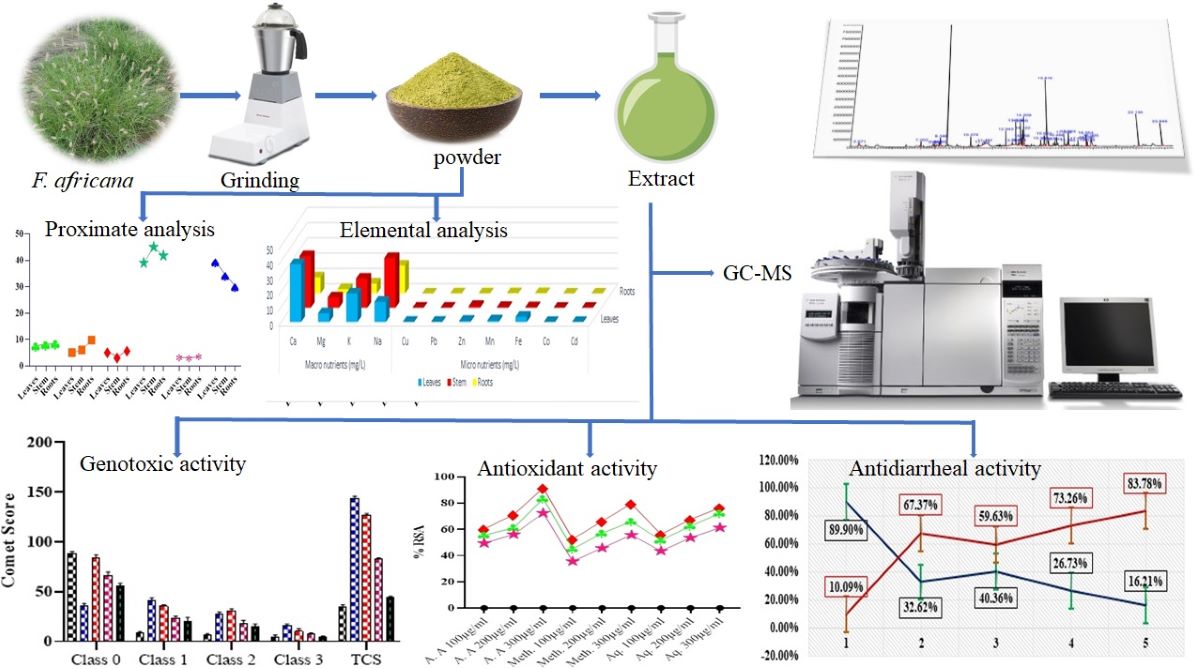
2.1. GC-MS Analysis
2.2. Macro and Micro-Nutrients Composition
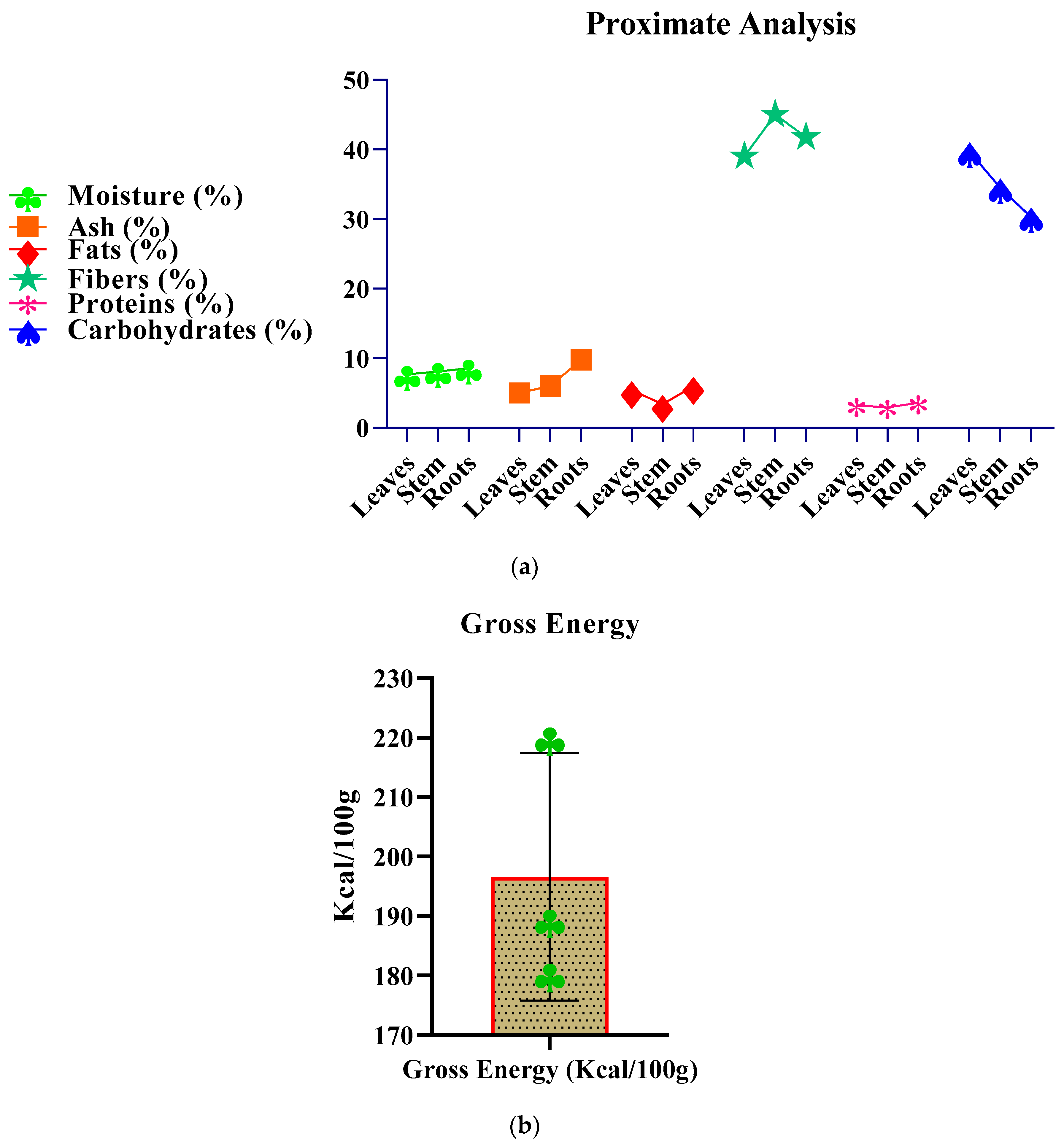
2.3. Proximate/Nutritional Analysis
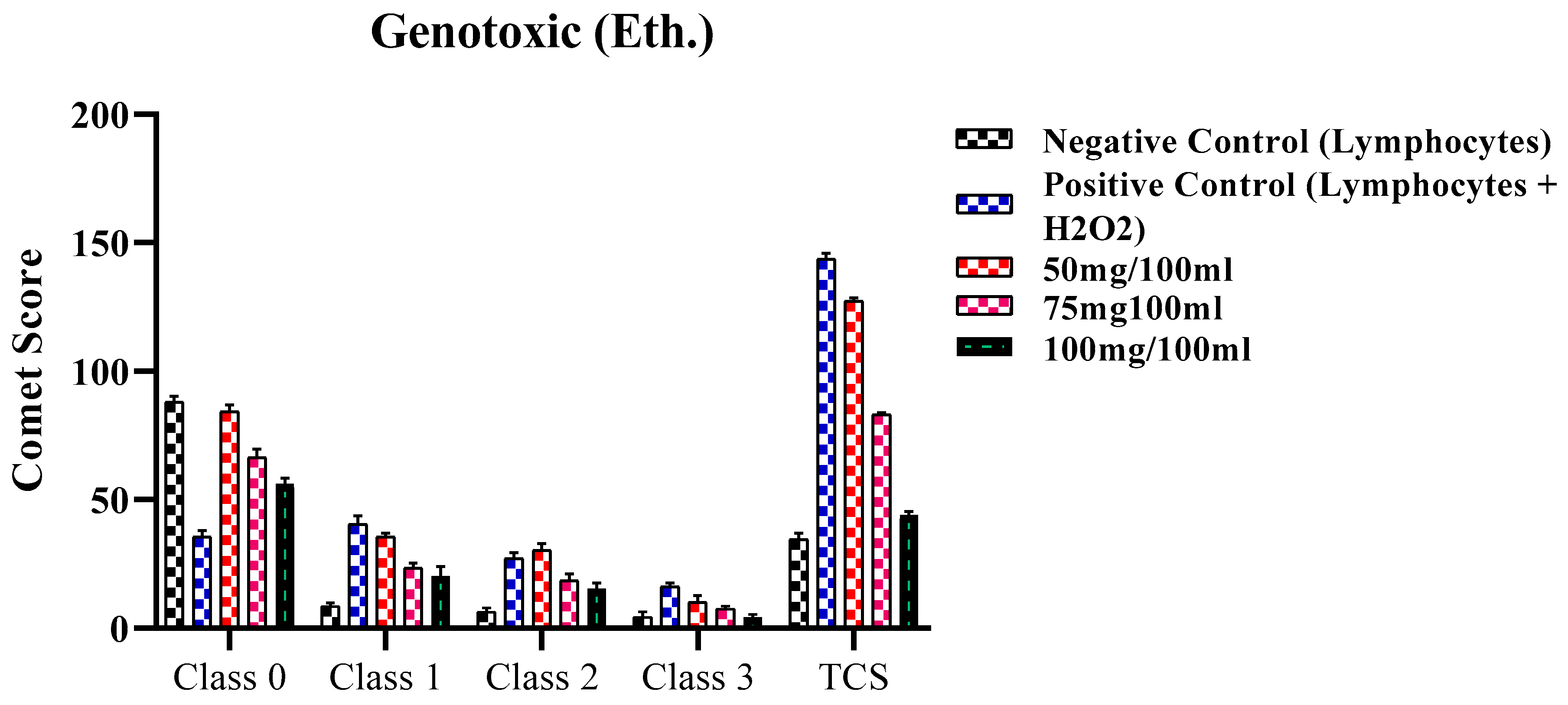
2.4. Genotoxic Activity
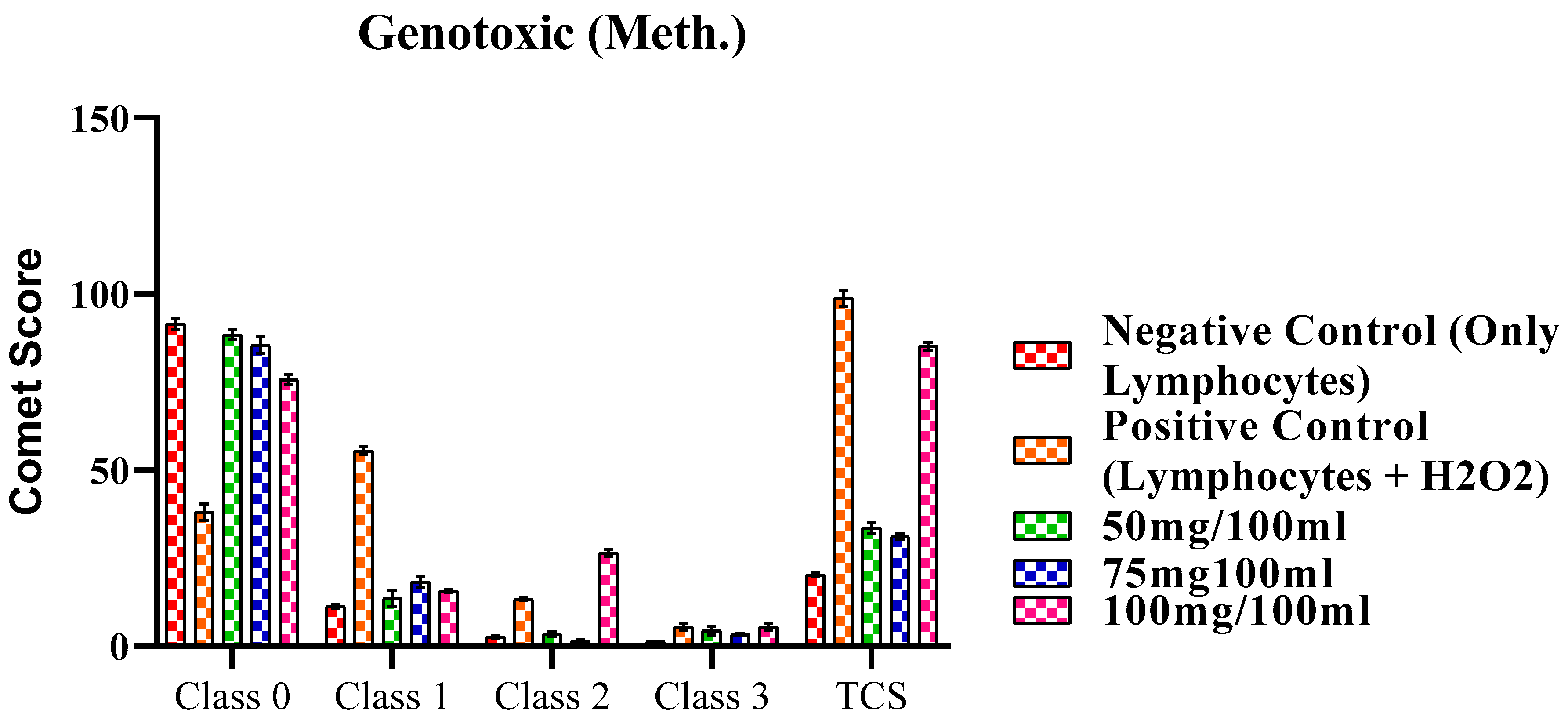
2.4.1. Genotoxic Activity of methanol extract of F. africana.
2.4.2. Genotoxic Activity of ethanolic extract of F. africana
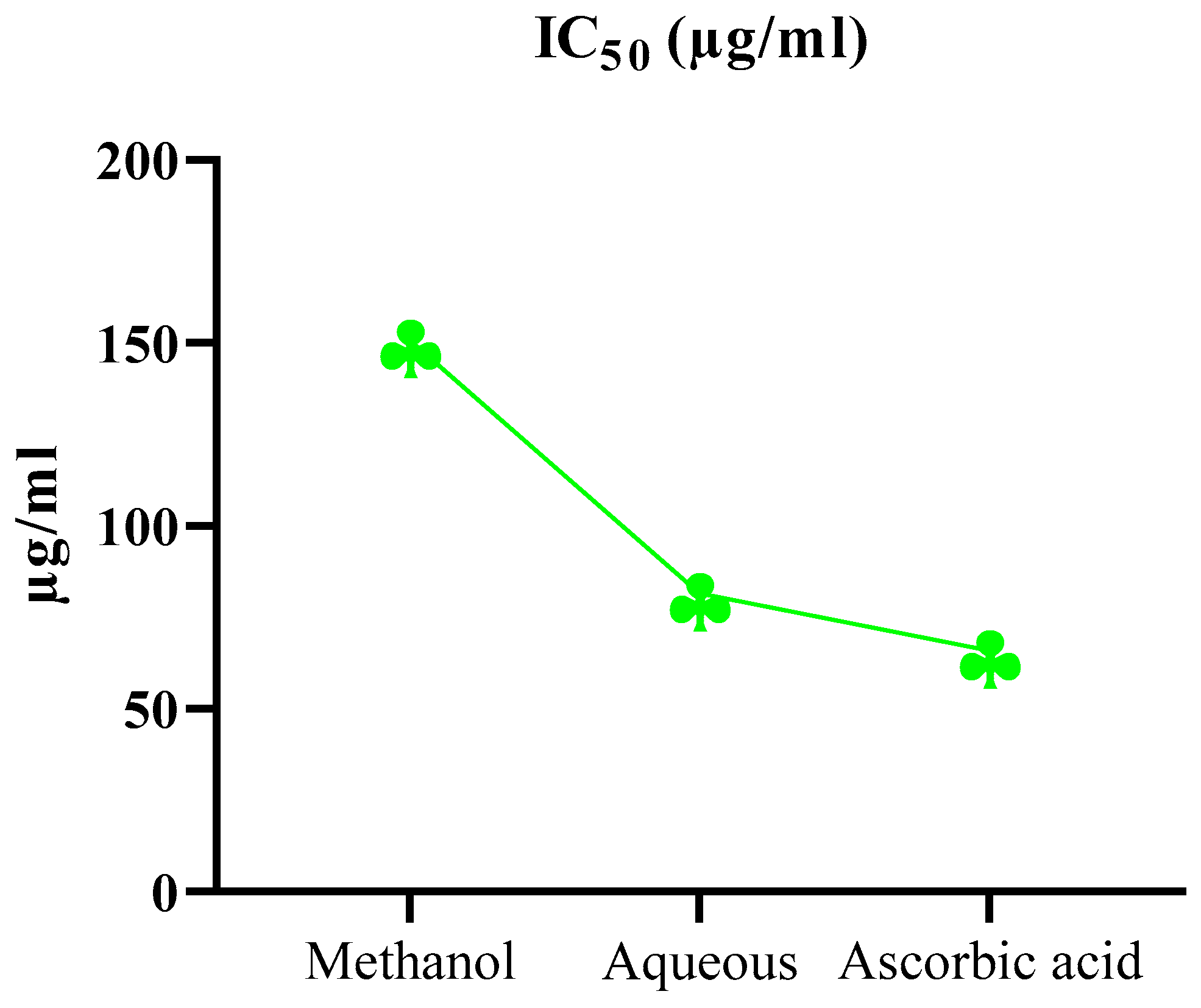
2.5. Antioxidant Activity via DPPH
2.6. Antidiarrheal Activity
3. Discussion
4. Materials and Methods
4.1. Collection and Identification of Plant
4.2. Extraction procedure
4.3. GC-MS Analysis
4.4. Elemental Analysis
4.5. Proximate/Nutritional Analysis
4.5.1. Determination of Moisture Content
4.5.2. Determination of Ash Content
4.5.3. Determination of Crude lipid
4.5.4. Determination of Crude protein
4.5.5. Determination of Crude Fiber
4.5.6. Determination of carbohydrates contents and gross energy
4.6. Genotoxic Activity
4.7. Antioxidant Activity via DPPH
4.8. Antidiarrheal Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, K.; Mashwani, Z.U.R.; Khan, M.A.; Ullah, Z.; Chaudhary, H.J. An Ethno Botanical Perspective of Traditional Medicinal Plants from the Khattak Tribe of Chonthra Karak, Pakistan. J. Ethnopharmacol., 2015, 165, 251–259. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, S.; Pandey, C.A. Concept of Standardization, Extraction and Pre Phytochemical Screening Strategies for Herbal Drug. J. Pharmacogn. Phytochem., 2014, 2, 115–119. [Google Scholar]
- Radha; Kumar, M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S.; Choudhary, P.; Parameswari, E.; Alhariri, A.; Samota, M.K.; Damale, R.D.; Singh, S.; Berwal, M.K.; Dhumal, S.; Bhoite, A.G.; Senapathy, M.; Sharma, A.; Bhushan, B.; Mekhemar, M. Evaluation of Nutritional, Phytochemical, and Mineral Composition of Selected Medicinal Plants for Therapeutic Uses from Cold Desert of Western Himalaya. Plants, 2021, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Gaonkar, V.P.; Chimagave, S.S.; Hullatti, K. Pharmacognostical and Biological Evaluation of Mayurshikha (Actiniopteries Dichotoma Bedd): An Ayurvedic Medicinal Plant. Int. J. Ayurvedic Med., 2022, 13, 338–344. [Google Scholar] [CrossRef]
- Chintamunnee, V.; Mahomoodally, M.F. Herbal Medicine Commonly Used against Non-Communicable Diseases in the Tropical Island of Mauritius. J. Herb. Med., 2012, 2, 113–125. [Google Scholar] [CrossRef]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev., 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Ullah, I.; Ullah, I.; Ali, M.; Durrani, F.; Khan, S.U.; Hussain, D.; Mehmood, S.; Khan, S.U.; Ullah, M.; Hussain, K.; Bahadur, S.; Aneva, I.Y.; Bussmann, R.W. Quantitative Study of Medicinal Plants and Biological Activities of Two Common Species Used by Inhabitants of District Bannu, Pakistan. Acta Ecol. Sin., 2023, 43, 271–287. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev., 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dagli, N.; Dagli, R.; Mahmoud, R.; Baroudi, K. Essential Oils, Their Therapeutic Properties, and Implication in Dentistry: A Review. J. Int. Soc. Prev. Community Dent., 2015, 5, 335–340. [Google Scholar] [CrossRef]
- Mahomoodally, M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evidence-based Complement. Altern. Med., 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Nisha, K.; Darshana, M.; Madhu, G.; Bhupendra, M.K. GC-MS Analysis and Anti-Microbial Activity of Psidium Guajava (Leaves) Grown in Malva Region of India. Int. J. Drug Dev. Res., 2011, 3, 237–245. [Google Scholar]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC–MS Analysis of Phytoconstituents from Amomum Nilgiricum and Molecular Docking Interactions of Bioactive Serverogenin Acetate with Target Proteins. Sci. Rep., 2020, 10, 16438. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Galanty, A.; Fołta, M.; Kryczyk-Kozioł, J.; Szlósarczyk, M.; Rubio, P.S.; de Carvalho, I.S.; Paśko, P. Determination of Essential Minerals and Trace Elements in Edible Sprouts from Different Botanical Families—Application of Chemometric Analysis. Foods, 2022, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes Nigrum l.), Raspberry (Rubus Idaeus), and Aronia (Aronia Melanocarpa). Nutrients, 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Gundorina, S.; Vergel, K.; Grozdov, D.; Ciocarlan, A.; Aricu, A.; Dragalin, I.; Ciocarlan, N. Elemental Analysis of Lamiaceae Medicinal and Aromatic Plants Growing in the Republic of Moldova Using Neutron Activation Analysis. Phytochem. Lett., 2020, 35, 119–127. [Google Scholar] [CrossRef]
- Riaz, I.; Bibi, Y.; Ahmad, N.; Nisa, S.; Qayyum, A. Evaluation of Nutritional, Phytochemical, Antioxidant and Cytotoxic Potential of Capsella Bursa-Pastoris, a Wild Vegetable from Potohar Region of Pakistan. Kuwait J. Sci., 2021, 48, 1–11. [Google Scholar] [CrossRef]
- Haleshappa, R.; Niketh, S.; Kolgi, R.R.; Patil, S.J.; Murthy, K.R.S. Phytochemicals, Anti-Nutritional Factors and Proximate Analysis of Simarouba Glauca Seeds. Int. Adv. Res. J. Sci. Eng. Technol., 2022, 9, 218–227. [Google Scholar]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant Properties, Phenolic Composition, Bioactive Compounds and Nutritive Value of Medicinal Halophytes Commonly Used as Herbal Teas. South African J. Bot., 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Albouchi, F.; Hassen, I.; Casabianca, H.; Hosni, K. Phytochemicals, Antioxidant, Antimicrobial and Phytotoxic Activities of Ailanthus Altissima (Mill.) Swingle Leaves. South African J. Bot., 2013, 87, 164–174. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H. Bin. Antioxidant Capacities and Total Phenolic Contents of Infusions from 223 Medicinal Plants. Ind. Crops Prod., 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, A.H.; Wani, Z.A.; Mohiuddin, T.; Shah, Z.; Abbas, N.; Ashraf, N. Phytochemical Analysis and Antioxidant Activity of Different Tissue Types of Crocus Sativus and Oxidative Stress Alleviating Potential of Saffron Extract in Plants, Bacteria, and Yeast. South African J. Bot., 2015, 99, 80–87. [Google Scholar] [CrossRef]
- Martinelli, E.; Granato, D.; Azevedo, L.; Gonçalves, J.E.; Lorenzo, J.M.; Munekata, P.E.S.; Simal-Gandara, J.; Barba, F.J.; Carrillo, C.; Rajoka, M.S.R. Current Perspectives in Cell-Based Approaches towards the Definition of the Antioxidant Activity in Food. Trends Food Sci. Technol., 2021, 116, 232–243. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Mohamed, H.I. Plant Responses to Induced Genotoxicity and Oxidative Stress by Chemicals. In; Springer, 2021; pp. 103–131.
- Sandoval-Herrera, N.; Paz Castillo, J.; Herrera Montalvo, L.G.; Welch, K.C. Micronucleus Test Reveals Genotoxic Effects in Bats Associated with Agricultural Activity. Environ. Toxicol. Chem., 2021, 40, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Mamza, U.T.; Madziga, H.A.; Yakubu, J.; Sodipo, O.A.; Abdulrahman, F.I.; Khan, I.Z. Qualitative Phytochemical Evaluation and Antispasmodic Studies of the Stem Bark of Boswellia Dalzielii on Rabbit Jejunum. Int. J. Acad. Res. Dev., 2020, 5, 45–49. [Google Scholar]
- Kassim, D.; Ouattara, G.A.; David, A.D.S.; Paul, Y.A. The Antispasmodic Activity of Ethanol Extract of the Stem Bark of Piliostigma Reticulatum Horscht DC (Ceasalpiniaceae), and Its Dichloromethane Fraction Isolated. GSC Biol. Pharm. Sci., 2020, 11, 36–45. [Google Scholar] [CrossRef]
- Majeed, M.; Bhatti, K.H.; Amjad, M.S.; Abbasi, A.M.; Bussmann, R.W.; Nawaz, F.; Rashid, A.; Mehmood, A.; Mahmood, M.; Khan, W.M. Ethno-Veterinary Uses of Poaceae in Punjab, Pakistan. PLoS One, 2020, 15, e0241705. [Google Scholar] [CrossRef]
- Al-Sghair, F.G.; Mahklouf, M.H. Floristic Analysis of the Family Asteraceae in Libya Depending on Flora of Libya. Am. J. Life Sci. Res., 2017, 5, 170–183. [Google Scholar] [CrossRef]
- Fatima, I.; Kanwal, S.; Mahmood, T. Evaluation of Biological Potential of Selected Species of Family Poaceae from Bahawalpur, Pakistan. BMC Complement. Altern. Med., 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rawal Jatin R. and R. Priya Sonawani. Determination of Bioactive Components of Cynodon Dactylon by GC-MS Analysis & i t ’ s In Vitro Antimicrobial Activity. Int. J. Pharm. LIFE Sci. (Int., 2016, 7, 4880–4885.
- Fatima, I.; Akhtar, W.; Bangash, N.K.; Kanwal, S.; Rauf, N.; Malik, T.S.S.; Mahmood, T. Volatile Profiling, Elemental Composition and Biological Activities of Aerial Parts of Seven Poaceae Species. Plant Biosyst., 2022, 156, 908–925. [Google Scholar] [CrossRef]
- Kabir, S.H.; Das, A.K.; Rahman, M.S.; Singh, M.S.; Morshed, M.; Marma, A.S.H. Effect of Genotype on Proximate Composition and Biological Yield of Maize (Zea Mays L.). Arch. Agric. Environ. Sci., 2019, 4, 185–189. [Google Scholar] [CrossRef]
- Cho, C.W.; Song, Y.R.; Lim, W.C.; Hwang, Y.H.; Rhee, Y.K.; Choi, J.W.; Lee, K.T.; Hong, H. Do. Acute Oral Toxicity and Genotoxicity of Polysaccharide Fraction from Young Barley Leaves (Hordeum Vulgare L.). Foods, 2020, 9, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Sultan, R.A.; Kabir, M.S.H.; Uddin, M.M.N.; Uddin, M.; Mahmud, Z. Al; Raihan, S.Z.; Qais, N. Ethnopharmacological Investigation of the Aerial Part of Phragmites Karka (Poaceae). J. Basic Clin. Physiol. Pharmacol., 2017, 28, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, H.A.; Ali, M.N. GC-MS Analysis and Evaluation of Mutagenic and Antimutagenic Activity of Ethyl Acetate Extract of Ajuga Bracteosa Wall Ex. Benth: An Endemic Medicinal Plant of Kashmir Himalaya, India. J. Clin. Toxicol., 2016, 06, 1–9. [Google Scholar] [CrossRef]
- Sumathi, B.M.; Uthayakumari, F. GC MS Analysis of Leaves of Jatropha Maheswarii Subram & Nayar. Sci. Res. Report., 2014, 4, 24–30. [Google Scholar]
- Jeeva, S.; Johnson, M.; Aparna, J.S.; Irudayaraj, V. Preliminary Phytochemical and Anti-Bacterial Studies on Flowers of Selected Medicinal Plants. Int. J. Med. Arom. Plants, 2011, 1, 107–114. [Google Scholar]
- Mohammed, G.J.; Al-Jassani, M.J.; Hameed, I.H. Anti-Bacterial, Antifungal Activity and Chemical Analysis of Punica Grantanum (Pomegranate Peel) Using GC-MS and FTIR Spectroscopy. Int. J. Pharmacogn. Phytochem. Res., 2016, 8, 480–494. [Google Scholar]
- Krause, B.M.; Bauer, B.; Neudörfl, J.M.; Wieder, T.; Schmalz, H.G. ItaCORMs: Conjugation with a CO-Releasing Unit Greatly Enhances the Anti-Inflammatory Activity of Itaconates. RSC Med. Chem., 2021, 12, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem., 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Hassan, S.B.; Gali-Muhtasib, H.; Göransson, H.; Larsson, R. Alpha Terpineol: A Potential Anticancer Agent Which Acts through Suppressing NF-ΚB Signalling. Anticancer Res., 2010, 30, 1911–1919. [Google Scholar]
- Saeed, H.M.; Ferdosi, M.F.H.; Khan, I.H.; Javaid, A. ANTIBACTERIAL ACTIVITY AND GC-MS ANALYSIS OF WHITE FLOWERS EXTRACT OF NERIUM OLEANDER L. Int. J. Biol. Biotechnol., 2023, 25, 163–168. [Google Scholar]
- Kamal, M.; Shakya, A.K.; Jawaid, T. Benzofurans: A New Profile of Biological Activities 15-Hydroxyprostaglandin Dehydrogenase (15-PGDH) Inhibitors View Project HPLC MV View Project. Int. J. Med. Pharm. Sci., 2011, 1, 1–15. [Google Scholar]
- Rautela, I.; Joshi, P.; Thapliyal, P.; Pant, M.; Dheer, P.; Bisht, S.; Sinha, V.B.; Sundriyal, S.; Sharma, M.D. Comparative GC-MS Analysis of Euphorbia Hirta and Euphorbia Milli for Therapeutic Potential Utilities. Plant Arch., 2020, 20, 3515–3522. [Google Scholar]
- Wang, W.; Jiang, Y.; Song, B.; Tang, X.; Wu, H.; Jin, Z.; Chen, L. Discovery of Quality Markers in the Rhizome of Atractylodes Chinensis Using GC–MS Fingerprint and Network Pharmacology. Arab. J. Chem., 2023, 16, 105114. [Google Scholar] [CrossRef]
- Javaid, A.; Khan, I.H.; Ferdosi, M.F.H. ANTIMICROBIAL AND OTHER BIOACTIVE CONSTITUENTS OF Cannabis Sativus ROOTS FROM PAKISTAN. J. Weed Sci. Res., 2021, 27, 359–368. [Google Scholar] [CrossRef]
- Mishra, A.K.; Tiwari, K.N.; Saini, R.; Chaurasia, J.K.; Mishra, S.K. Assessment of Antioxidant Potential in Seed Extracts of Nyctanthes Arbor-Tristis L. and Phytochemical Profiling by Gas Chromatography-Mass Spectrometry System. Brazilian J. Pharm. Sci., 2022, 58, 1–16. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Rayan, M.; Masalha, M.; Abu-Farich, B.; Al-Jaas, H.; Abu-Lafi, M.; Rayan, A. Phytochemical Composition and Biological Activities of Wild Scolymus Maculatus L. Medicines, 2019, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alvindia, D. de G.; Mangoba, M.A.A. Biological Activities of Moringa Oleifera Lam. against Anthracnose of Mango Caused by Colletotrichum Gloeosporioides Penz. Arch. Phytopathol. Plant Prot., 2020, 53, 659–672. [CrossRef]
- Riaz, M.; Rasool, N.; Bukhari, I.H.; Shahid, M.; Zubair, M.; Rizwan, K.; Rashid, U. In Vitro Antimicrobial, Antioxidant, Cytotoxicity and GC-MS Analysis of Mazus Goodenifolius. Molecules, 2012, 17, 14275–14287. [Google Scholar] [CrossRef]
- Njoya, H..; Onyeneke, C.E.; Okwuonu, C..; Al., E. Phytochemical, Proximate and Elemental Analysis of the African Mistletoe ( Tapinanthus Preussii ) Crude Aqueous and Ethanolic Leaf Extracts. J. Med. Plants Stud., 2018, 6, 162–170.
- Oyeyinka, B.O.; Afolayan, A.J. Comparative Evaluation of the Nutritive, Mineral, and Antinutritive Composition of Musa Sinensis l. (Banana) and Musa Paradisiaca l. (Plantain) Fruit Compartments. Plants, 2019, 8, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Kntapo, F.M.; Salisu, A.G.; Said, R.M. X-Ray Flourescence (XRF) Elemental Composition of Euphorbia Hirta Linn (Asthma Weed) as a Medicinal Plant. Asian J. Med. Biol. Res., 2018, 4, 49–54. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Kaushik, R.; Dhull, S.B. Synthesis, Characterization and Cellular Mineral Absorption of Nanoemulsions of Rhododendron Arboreum Flower Extracts Stabilized with Gum Arabic. J. Food Sci. Technol., 2019, 56, 5194–5203. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients, 2018, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U. Magnesium and Drugs. Int. J. Mol. Sci., 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Saxena, H.O.; Soni, A.; Mohammad, N.; Choubey, S.K. Phytochemical Screening and Elemental Analysis in Different Plant Parts of Uraria Picta Desv.: A Dashmul Species. J. Chem. Pharm. Res., 2014, 6, 756–760. [Google Scholar]
- Ekmekcioglu, C.; Wallner, P.; Kundi, M.; Weisz, U.; Haas, W.; Hutter, H.P. Red Meat, Diseases, and Healthy Alternatives: A Critical Review. Crit. Rev. Food Sci. Nutr., 2018, 58, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Solana, M.A.; Mejía-García, V.Y.; Téllez-Valencia, A.; García-Arenas, G.; Salas-Pacheco, J.; Alba-Romero, J.J.; Sierra-Campos, E. Nutritional Content and Elemental and Phytochemical Analyses of Moringa Oleifera Grown in Mexico. J. Chem., 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Saraf, A.; Samant, A. Evaluation of Some Minerals and Trace Elements in Achyranthes Aspera Linn. Int. J. Pharma Sci., 2013, 3, 229–233. [Google Scholar]
- D’Elia, L.; Galletti, F.; Fata, E. La; Sabino, P.; Strazzullo, P. Effect of Dietary Sodium Restriction on Arterial Stiffness: Systematic Review and Meta-Analysis of the Randomized Controlled Trials. J. Hypertens., 2018, 36, 734–743. [Google Scholar] [CrossRef]
- Kabra, A.; Baghel, U.S. Nutritional Value and Elemental Analysis of Katphala (Myrica Esculenta Buch-Ham). J. Biol. Chem. Chronicles, 2018, 4, 19–25. [Google Scholar]
- Ositadinma, I.M.; Martina, N.A. Copper, Magnesium and Selenium Levels in Serum Samples of Male Patients with Type 2 Diabetes Mellitus. Arch. Curr. Res. Int., 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Song, Y.; Pruden, A.; Edwards, M.A.; Rhoads, W.J. Natural Organic Matter, Orthophosphate, PH, and Growth Phase Can Limit Copper Antimicrobial Efficacy for Legionella in Drinking Water. Environ. Sci. Technol., 2021, 55, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Karahan, F.; Yalcin, I.E.; Hocaoglu-ozyigit, A.; Ilcim, A. Heavy Metals and Trace Elements Detected in the Leaves of Medicinal Plants Collected in the Southeast Part of Turkey. Arab. J. Geosci. 2022. [Google Scholar]
- Ali, M.; Nas, F.S. The Effect of Lead on Plants in Terms of Growing and Biochemical Parameters: A Review. MOJ Ecol. Environ. Sci., 2018, 3. [Google Scholar] [CrossRef]
- Barbeş, L.; Bărbulescu, A.; Stanciu, G. Statistical Analysis of Mineral Elements Content in Different Melliferous Plants from the Dobrogea Region, RomÂnia. Rom. Reports Phys., 2020, 72, 1–14. [Google Scholar]
- Fahad, S.M.; Islam, A.F.M.M.; Ahmed, M.; Uddin, N.; Alam, M.R.; Alam, M.F.; Khalik, M.F.; Hossain, M.S.; Hossain, M.L.; Abedin, M.J. Determination of Elemental Composition of Malabar Spinach, Lettuce, Spinach, Hyacinth Bean, and Cauliflower Vegetables Using Proton Induced X-Ray Emission Technique at Savar Subdistrict in Bangladesh. Biomed Res. Int., 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aquisman, A.E.; Klutse, C.K.; Droepenu, E.K. Phytochemical Screening for Antioxidant Activity and Elemental Analysis of the Leaves and Cracked - Barks of Mangifera Indica ( Mango ). Arch. Pharmacol. Pharm. Sci., 2021, 1, 1–10. [Google Scholar]
- Dénou, A.; Ahmed, A.; Dafam, D.G.; Yakubu, T.P.; Sanogo, R.; Diallo, D.; Alemika, T.E. Pharmacognostic, Physicochemical and Phytochemical Investigations on Aerial Parts of Argemone Mexicana L. Res. J. Pharmacogn., 2020, 7, 15–24. [Google Scholar]
- Mashkoor Hussein, H. Determination of Phytochemical Composition and Ten Elements Content (Cd, Ca, Cr, Co, Fe, Pb, Mg, Mn, Ni and Zn) Ofcardaria Draba By Gc-Ms, Ft-Ir and Aas Techniques. Int. J. Pharma Bio Sci., 2016, 7, 1009–1017. [Google Scholar]
- Achi, N.K.; Onyeabo, C.; Ekeleme-Egedigwe, C.A.; Onyeanula, J.C. Phytochemical, Proximate Analysis, Vitamin and Mineral Composition of Aqueous Extract of Ficus Capensis Leaves in South Eastern Nigeria. J. Appl. Pharm. Sci., 2017, 7, 117–122. [Google Scholar]
- Al-Fartusie, F.S.; Mohssan, S.N. Essential Trace Elements and Their Vital Roles in Human Body Analytical Biochemistry View Project Enzymology View Project Essential Trace Elements and Their Vital Roles in Human Body. Indian J. Adv. Chem. Sci., 2017, 5, 127–136. [Google Scholar]
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.U.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M.; Shaheen, Z. Accumulation of Heavy Metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the Soil, Water and Plants and Analysis of Physico-Chemical Parameters of Soil and Water Collected from Tanda Dam Kohat. J. Pharm. Sci. Res., 2015, 7, 89–97. [Google Scholar]
- Hameed, I.; Hussain, F. Proximate and Elemental Analysis of Five Selected Medicinal Plants of Family Solanaceae. Pak. J. Pharm. Sci., 2015, 28, 1203–1215. [Google Scholar] [PubMed]
- Asomugha, R.N.; Udowelle, N.A.; Offor, S.J.; Njoku, C.J.; Ofoma, I.V.; Chukwuogor, C.C.; Orisakwe, O.E. Heavy Metals Hazards from Nigerian Spices. Rocz. Panstw. Zakl. Hig., 2016, 67, 309–314. [Google Scholar] [PubMed]
- Sara, G, Y.; Barde, L, Y.; Mohammed, M.; Kabiru, M. Elemental and Phytochemical Analysis of Diospyros Mespiliformis Leaves and Stem Bark in Nangere Local Government Area of Yobe State, Nigeria. GSC Adv. Res. Rev., 2022, 11, 051–055. [CrossRef]
- Debanovic, V.; Soskic, M.; Durovic, D.; Mugosa, B. Investigation of Heavy Metals Content in Selected Tea Brands Marketed in Podgorica, Montenegro. Int. J. Pharm. Sci. Res., 2016, 12, 4798–4804. [Google Scholar]
- Omolola, T.O. Phytochemical, Proximate and Elemental Composition of Tithonia Diversifolia (Hemsley) A. Gray Leaves. Int. Ann. Sci., 2020, 8, 54–61. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem., 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Kayitesi, E.; Moteetee, A.N. Proximate Analyses and Amino Acid Composition of Selected Wild Indigenous Fruits of Southern Africa. Plants, 2021, 10, 1–20. [Google Scholar] [CrossRef]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Comparison of the Proximate Composition, Vitamins (Ascorbic Acid, α-Tocopherol and Retinol), Anti-Nutrients (Phytate and Oxalate) and the GC-MS Analysis of the Essential Oil of the Root and Leaf of Rumex Crispus L. Plants, 2019, 8, 1–15. [Google Scholar] [CrossRef]
- Hammond, S.T.; Brown, J.H.; Burger, J.R.; Flanagan, T.P.; Fristoe, T.S.; Mercado-Silva, N.; Nekola, J.C.; Okie, J.G. Food Spoilage, Storage, and Transport: Implications for a Sustainable Future. Bioscience, 2015, 65, 758–768. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, B.K.; Bhattacharjee, S.; Seal, T. Nutritional Composition, Mineral Content, Antioxidant Activity and Quantitative Estimation of Water Soluble Vitamins and Phenolics by RP-HPLC in Some Lesser Used Wild Edible Plants. Heliyon, 2019, 5, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Mohammad, Z.; Hussain, W.; Ali, N.; Ali, A.; Hussain, J.; Hussain, D. Evaluation of Mineral, Proximate Compositions and Anti-Oxidant Activities of Some Wild Edible Vegetables of District Kurram Khyber Pakhtunkhwa, Pakistan. Plant Sci. Today, 2022, 9, 301–311. [Google Scholar] [CrossRef]
- Ezeabara, C.A.; Egwuoba, G.C. Comparative Screening of Phytochemical and Proximate Constituents of Leaf, Stem and Root of Oldenlandia Corymbosa L. and Oldenlandia Herbacea (L.) Roxb. Am. J. Life Sci. Res., 2016, 4, 114–120. [Google Scholar] [CrossRef]
- Sambo, H.S.; Olatunde, A.; Kiyawa, A.S. Phytochemical, Proximate and Mineral Analyses of Solanum Incanum Fruit. Int. J. Chem., Mater. Environ. Res., 2016, 3, 8–13. [Google Scholar]
- Okafor, U.I.; Omemu, A.M.; Obadina, A.O.; Bankole, M.O.; Adeyeye, S.A.O. Nutritional Composition and Antinutritional Properties of Maize Ogi Cofermented with Pigeon Pea. Food Sci. Nutr., 2018, 6, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, U.M.-M.; Nwodo, O.F.C.; Anosike, C.A. Phytochemical and Proximate Composition of Cucumber (Cucumis Sativus) Fruit from Nsukka, Nigeria. African J. Biotechnol., 2018, 17, 1215–1219. [Google Scholar]
- Ogidi, O.; Esie, N.; And, O.D.-J. of P.; 2019, U. Phytochemical, Proximate and Mineral Compositions of Bryophyllum Pinnatum (Never Die) Medicinal Plant. Researchgate.Net, 2019, 8, 629–635. [Google Scholar]
- Sodamade, A.B.; Bolaji, O.S.; Owonikoko, A.D. The Nutritive Value and Amino Acid Characteristics of Solanum Aethiopicum Leaf Protein Concentrates. Int. J. Adv. Res. Chem. Sci., 2015, 2, 28–33. [Google Scholar]
- Ezeabara, C.A.; Nwafulugo, S.N. Comparison of Phytochemical and Proximate Compositions of Parts of Cleome Ciliata Schum. & Thonn. and Cleome Viscosa L. World J. Biomed. Pharm. Sci., 2015, 1, 1–5. [Google Scholar]
- Zihad, S.M.N.K.; Gupt, Y.; Uddin, S.J.; Islam, M.T.; Alam, M.R.; Aziz, S.; Hossain, M.; Shilpi, J.A.; Nahar, L.; Sarker, S.D. Nutritional Value, Micronutrient and Antioxidant Capacity of Some Green Leafy Vegetables Commonly Used by Southern Coastal People of Bangladesh. Heliyon, 2019, 5, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Adnan, M. Nutritional Potential of Pakistani Medicinal Plants and Their Contribution to Human Health in Times of Climate Change and Food Insecurity. Pakistan J. Bot., 2018, 50, 287–300. [Google Scholar]
- Akhtar, M.F.; Saleem, A.; Sharif, A.; Akhtar, B.; Nasim, M. Bin; Peerzada, S.; Raza, M.; Ijaz, H.; Ahmed, S.; Shabbir, M.; Ali, S.; Akbar, Z.; Ul Hassan, S.S. Genotoxic and Cytotoxic Action Potential of Terminalia Citrina, a Medicinal Plant of Ethnopharmacological Significance. EXCLI J., 2016, 15, 589–598. [Google Scholar] [PubMed]
- Akolade, O.R.; Chinwe, A.S.; Olalekan, B.T.; Halima, A.T.; Fatima, A.A.; Emuejevoke, T.T.; Herbert, C.A.B. Haematological and Genotoxicity Evaluations of Phytochemical Compounds from N-Hexane Extract of Uvaria Chamae Stem on Selected Organs in Mice. Ann. Sci. Technol., 2018, 3, 28–34. [Google Scholar] [CrossRef]
- Sabahi, Z.; Soltani, F.; Moein, M. Insight into DNA Protection Ability of Medicinal Herbs and Potential Mechanisms in Hydrogen Peroxide Damages Model. Asian Pac. J. Trop. Biomed., 2018, 8, 120–129. [Google Scholar]
- Mattana, C.M.; Cangiano, M.A.; Alcaráz, L.E.; Sosa, A.; Escobar, F.; Sabini, C.; Sabini, L.; Laciar, A.L. Evaluation of Cytotoxicity and Genotoxicity of Acacia Aroma Leaf Extracts. Sci. World J., 2014, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.F.; Alves, J.M.; Corrêa, M.B.; Cunha, W.R.; Veneziani, R.C.S.; Tavares, D.C. In Vitro Cytotoxicity, Genotoxicity and Antigenotoxicity Assessment of Solanum Lycocarpum Hydroalcoholic Extract. Pharm. Biol., 2016, 54, 2786–2790. [Google Scholar] [CrossRef]
- Al-Faifi, Z.I.A.; Masrahi, Y.S.; Aly, M.S.; Al-Turki, T.A.; Dardeer, T. Evaluation of Cytotoxic and Genotoxic Effects of Euphorbia Triaculeata Forssk. Extract. Asian Pacific J. Cancer Prev., 2017, 18, 771–777. [Google Scholar]
- Nwozo, O.S.; Effiong, E.M.; Aja, P.M.; Awuchi, C.G. Antioxidant, Phytochemical, and Therapeutic Properties of Medicinal Plants: A Review. Int. J. Food Prop., 2023, 26, 359–388. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans Regia l.) Fruit and Tree. Molecules, 2019, 24. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr., 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Del Nobile, M.A. Antioxidant Compounds from Vegetable Matrices: Biosynthesis, Occurrence, and Extraction Systems. Crit. Rev. Food Sci. Nutr., 2016, 56, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Birru, E.M.; Asrie, A.B.; Adinew, G.M.; Tsegaw, A. Antidiarrheal Activity of Crude Methanolic Root Extract of Idigofera Spicata Forssk.(Fabaceae). BMC Complement. Altern. Med., 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Degu, A.; Kefale, B.; Alemayehu, D.; Tegegne, G.T. Evaluation of the Antidiarrheal Activity of Hydromethanol Crude Extracts of Ruta Chalepensis and Vernonia Amygdalina in Mice. Evidence-based Complement. Altern. Med., 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Ferede, Y.A.; Zewdu, W.S.; Zeleke, M.M.; Alemu, M.A. Evaluation of Antidiarrheal Activity of 80% Methanolic Extract of the Leaves of Cordia Africana (Lamiaceae) in Mice. Evidence-based Complement. Altern. Med., 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.D.; Kidanu, B.B.; Tadesse, T.Y.; Belachew, T.F.; Atnafie, S.A. Evaluation of in Vivo Antidiarrheal Activity of Solvent Fractions of Hagenia Abyssinica (Rosaceae) in Swiss Albino Mice. Evidence-Based Complement. Altern. Med., 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Ayalew, M.; Bekele, A.; Mengistie, M.G.; Atnafie, S.A. Evaluation of the Antidiarrheal Activity of 80% Methanol Extract and Solvent Fractions of the Leaf of Bersama Abyssinica Fresen (Melianthaceae) in Mice. BMC Complement. Med. Ther., 2022, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abdela, J. Evaluation of In Vivo Antidiarrheal Activities of Hydroalcoholic Leaf Extract of Dodonaea Viscosa L.(Sapindaceae) in Swiss Albino Mice. J. Evidence-Based Integr. Med., 2019, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Asrie, A.B.; Abdelwuhab, M.; Shewamene, Z.; Gelayee, D.A.; Adinew, G.M.; Birru, E.M. Antidiarrheal Activity of Methanolic Extract of the Root Bark of Cordia Africana. J. Exp. Pharmacol., 2016, 8, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nasir, E.; Ali, S.I. Flora of West Pakistan. Pakistan Agricultural Research Council, 1972.
- Wali, M.; Sajjad, A.S.; Sumaira, S.; Muhammad, N.; Safia, H.; Muhammad, J. Green Synthesis of Gold Nanoparticles and Their Characterizations Using Plant Extract of Papaver Somniferum. Nano Sci. Nano Technol, 2017, 11, 118. [Google Scholar]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Zengin, G.; Jaradat, N.; Messaoudi, M. Teucrium Polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages. Molecules, 2022, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Hayat, M.Q.; Mumtaz, A.S. A Study on Elemental Contents of Medicinally Important Species of Artemisia L. (Asteraceae) Found in Pakistan. J. Med. Plants Res., 2010, 4, 2256–2263. [Google Scholar]
- Ullah, S.; Dastagir, G.; Ullah, A.; Daud, F.; Ahmad, A.; Naz, M.; Noor, L.; Khan, A. ASSESSMENT OF MEDICINAL FLOWERS WITH SPECIAL EMPHASIS ON ELEMENTAL AND NUTRITIONAL ANALYSIS. J. Xi’an Shiyou Univ. Nat. Sci. Ed., 2023, 19, 1136–1151. [Google Scholar]
- Wang, Z.; Fan, S.; Wu, J.; Zhang, C.; Xu, F.; Yang, X.; Li, J. Application of Long-Wave near Infrared Hyperspectral Imaging for Determination of Moisture Content of Single Maize Seed. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc., 2021, 254, 119666. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Effects of Sample Size, Dry Ashing Temperature and Duration on Determination of Ash Content in Algae and Other Biomass. Algal Res., 2019, 40, 10–18. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, E.; Wilberg, M.J.; Lee, W.C.; Choi, K.S.; Kang, C.K. Physiological Processes and Gross Energy Budget of the Submerged Longline-Cultured Pacific Oyster Crassostrea Gigas in a Temperate Bay of Korea. PLoS One, 2018, 13, 1–24. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res., 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.; Charles, A.L. In Vitro Antioxidant Activity of Kyoho Grape Extracts in DPPH and ABTS Assays: Estimation Methods for EC50 Using Advanced Statistical Programs. Food Chem., 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Mascolo, N.; Izzo, A.A.; Autore, G.; Barbato, F.; Capasso, F. Nitric Oxide and Castor Oil-Induced Diarrhea. J. Pharmacol. Exp. Ther., 1994, 268, 291–295. [Google Scholar]
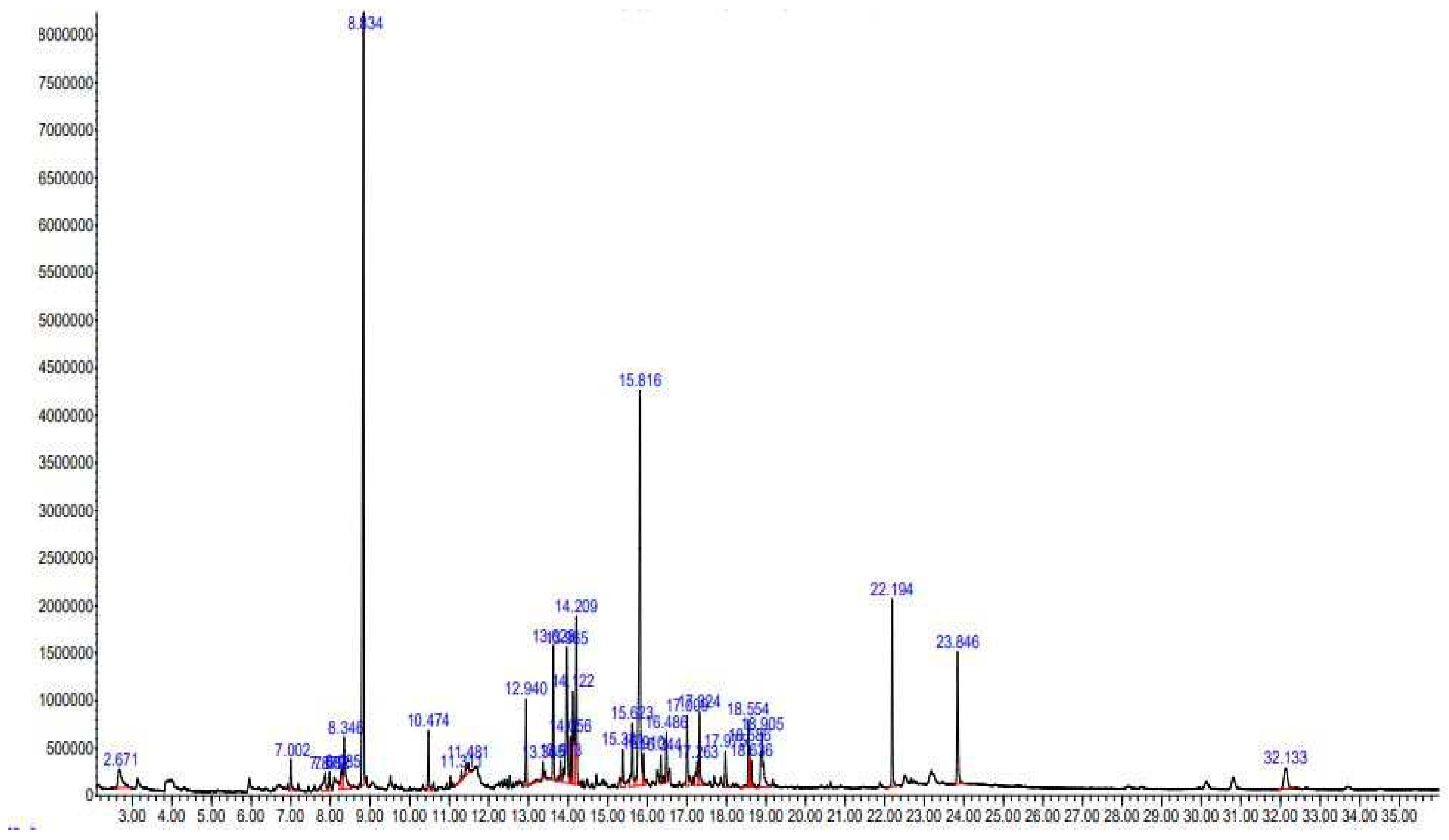
| S. No | RT | %Area | SI | Name of Compound | MF | MW (g/mol) | Prob. % |
|---|---|---|---|---|---|---|---|
| 1 | 2.671 | 1.94 | 55207 | 1,3-Dihydroxyacetone dimer | C3H6O3 | 90.08 | 64 |
| 2 | 7.002 | 0.99 | 23823 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144.13 | 96 |
| 3 | 7.879 | 1.17 | 23777 | 4-Methyl itaconate | C6H8O4 | 144.13 | 43 |
| 4 | 7.982 | 0.53 | 30998 | .alpha.-Terpineol | C10H18O | 154.24 | 91 |
| 5 | 8.285 | 0.93 | 17229 | 1,2,3-Propanetriol, 1-acetate | C5H10O4 | 134.13 | 37 |
| 6 | 8.346 | 2.52 | 10680 | Benzofuran, 2,3-dihydro- | C8H8O | 120.15 | 95 |
| 7 | 8.834 | 23.69 | 29336 | 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl)- | C10H16O | 152.23 | 97 |
| 8 | 10.474 | 1.59 | 41104 | p-tert.-Butylcatechol | C10H14O2 | 166.22 | 70 |
| 9 | 11.311 | 0.24 | 12718 | 6-Methyl-2,3-dihydropyran-2,4-dione | C6H6O3 | 126.11 | 15 |
| 10 | 11.481 | 0.64 | 18760 | 2-Hydroxy-3-methylbenzaldehyde | C8H8O2 | 136.15 | 46 |
| 11 | 12.94 | 2.06 | 102111 | Cyclohexanemethanol, 4-ethenyl-.alpha.,.alpha.,4-trimethyl-3-(1-methylethenyl)-, [1R-(1.alpha.,3.alpha.,4.beta.)]- | C15H26O | 222.37 | 91 |
| 12 | 13.365 | 0.39 | 99194 | Caryophyllene oxide | C15H24O | 220.35 | 81 |
| 13 | 13.628 | 3.09 | 101977 | .alpha.-epi-7-epi-5-Eudesmol | C15H26O | 222.36 | 99 |
| 14 | 13.813 | 0.54 | 102063 | 2-((2S,4aR)-4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalen-2-yl) propan-2-ol | C15H26O | 222.37 | 99 |
| 15 | 13.965 | 5.62 | 80415 | Cyclohexene, 6-ethenyl-6-methyl-1-(1-methylethyl)-3-(1-methylethylidene)-, (S)- | C15H24 | 204.35 | 64 |
| 16 | 14.056 | 1.1 | 101915 | Hinesol | C15H26O | 222.37 | 91 |
| 17 | 14.122 | 3.09 | 102105 | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- | C15H26O | 222.37 | 99 |
| 18 | 14.209 | 5.29 | 102072 | (1aR,3aS,7S,7aS,7bR)-1,1,3a,7-Tetramethyldecahydro-1H-cyclopropa[a]naphthalen-7-ol | C15H26O | 222.37 | 83 |
| 19 | 15.381 | 1.2 | 123744 | 7-(2-Hydroxypropan-2-yl)-1,4a-dimethyldecahydronaphthalen-1-ol | C15H28O2 | 240.38 | 93 |
| 20 | 15.623 | 2.26 | 39830 | 1H-Indene, 1-ethylideneoctahydro-7a-methyl-, cis- | C12H20 | 164.29 | 89 |
| 21 | 15.816 | 14.92 | 44358 | Acetic acid, (3-fluorophenyl) methyl ester | C9H9FO2 | 168.16 | 25 |
| 22 | 15.91 | 0.92 | 150160 | 4-Methyl-1,2-bis(trimethylsilyloxy)pentane | C12H30O2Si2 | 262.54 | 22 |
| 23 | 16.344 | 0.65 | 99301 | 7R,8R-8-Hydroxy-4-isopropylidene-7-methylbicyclo [5.3.1] undec-1-ene | C15H25O | 220.35 | 86 |
| 24 | 16.486 | 1.31 | 173460 | 6-(2-Hydroxypropan-2-yl)-4,8a-dimethyl-2,3,4,6,7,8-hexahydro-1H-naphthalen-1-ol, 1-acetate | C17H28O3 | 280.40 | 53 |
| 25 | 17.009 | 1.87 | 161203 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.45 | 99 |
| 26 | 17.263 | 1.12 | 37996 | 2,7:3,6-Dimethanonaphthalene, decahydro- | C12H18 | 162.27 | 25 |
| 27 | 17.324 | 2.24 | 143511 | n-Hexadecanoic acid | C16H32O2 | 256.42 | 99 |
| 28 | 17.975 | 1.16 | 54896 | trans-8-Methyl-1.beta.-acetyl-hydrindane | C12H20O | 180.29 | 38 |
| 29 | 18.554 | 1.64 | 191917 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | C19H34O2 | 294.47 | 99 |
| 30 | 18.586 | 0.87 | 189451 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | C19H32O2 | 292.46 | 99 |
| 31 | 18.636 | 0.55 | 194438 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 296.49 | 99 |
| 32 | 18.905 | 3.39 | 171210 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | C18H30O2 | 278.43 | 99 |
| 33 | 22.194 | 4.42 | 295608 | Bis(2-ethylhexyl) phthalate | C24H38O4 | 390.55 | 91 |
| 34 | 23.846 | 4.11 | 295779 | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | C24H38O4 | 390.56 | 95 |
| 35 | 32.133 | 1.97 | 310599 | .gamma.-Sitosterol | C29H50O | 414.70 | 98 |
| P.Parts | Macro nutrients (mg/L) | Micro nutrients (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | K | Na | Cu | Pb | Zn | Mn | Fe | Co | Cd | |
| Leaves | 37.80±0.12 | 5.645±0.29 | 18.65±0.19 | 13.16±1.87 | 0.059±0.09 | 0.08±0.06 | 0.737±0.02 | 0.975±0.03 | 3.350±0.06 | 0.00±0.00 | 0.034±0.02 |
| Stem | 33.75±0.58 | 6.727±0.49 | 19.02±0.26 | 32.51±1.23 | 0.055±0.05 | 0.13±0.11 | 1.185±0.01 | 0.498±0.05 | 0.377±0.09 | 0.00±0.00 | 0.013±0.01 |
| Roots | 10.56±0.20 | 2.952±0.73 | 6.506±0.27 | 18.32±1.73 | 0.013±0.03 | 0.15±0.03 | 0.153±0.01 | 0.281±0.02 | 0.295±0.04 | 0.00±0.00 | 0.00±0.00 |
| P. Parts | Moisture (%) | Ash (%) | Fats (%) | Fibers (%) | Proteins (%) | Carbohydrates (%) | Gross Energy(Kcal/100g) |
|---|---|---|---|---|---|---|---|
| Leaves | 7.70 | 5.00 | 5.38 | 39.00 | 3.21 | 39.71 | 220.10 |
| Stem | 8.09 | 6.03 | 3.39 | 45.03 | 2.92 | 34.54 | 180.34 |
| Roots | 8.57 | 9.77 | 5.96 | 41.77 | 3.62 | 30.31 | 189.40 |
| Mean | 8.12 | 6.93 | 4.91 | 41.93 | 3.25 | 34.85 | 196.61 |
| Classes | Negative Control (Only Lymphocytes) | Positive Control (Lymphocytes + H2O2) | 50mg/100ml | 75mg100ml | 100mg/100ml |
|---|---|---|---|---|---|
| Class 0 | 91.43 ± 1.52 | 38.00 ± 2.30 | 88.42 ± 1.37 | 85.47 ± 2.37 | 75.67 ± 1.53 |
| Class 1 | 11.30 ± 0.60 | 55.43 ± 1.07 | 13.53 ± 2.27 | 18.27 ± 1.57 | 15.66 ± 0.50 |
| Class 2 | 2.56 ± 0.47 | 13.37 ± 0.48 | 3.43 ± 0.63 | 1.56 ± 0.27 | 26.44 ± 1.02 |
| Class 3 | 1.30 ± 0.00 | 5.53 ± 1.07 | 4.38 ± 1.15 | 3.26 ± 0.37 | 5.53 ± 1.06 |
| TCS | 20.32±0.65 | 98.76±2.18 | 33.53±1.52* | 31.17±0.76* | 85.13±1.23* |
| Classes | Negative Control (Only Lymphocytes) | Positive Control (Lymphocytes + H2O2) | 50mg/100ml | 75mg100ml | 100mg/100ml |
|---|---|---|---|---|---|
| Class 0 | 88.0 ± 2.30 | 35.63 ± 2.5 | 84.34 ± 2.5 | 66.52 ± 3.2 | 56.31 ± 2.13 |
| Class 1 | 8.54 ± 1.43 | 40.50 ± 3.25 | 35.53 ± 1.41 | 23.40 ± 2.0 | 20.42 ± 3.6 |
| Class 2 | 6.35 ± 1.5 | 27.26 ± 2.17 | 30.43 ± 2.5 | 18.63 ± 2.5 | 15.47 ± 2.1 |
| Class 3 | 4.43 ± 2.0 | 16.30 ± 1.20 | 10.33 ± 2.5 | 7.54 ± 1.1 | 4.38 ± 1.0 |
| TCS | 34.53±2.32 | 143.8±2.06 | 127.38±1.16 | 83.28±0.65 | 44.08±1.40 |
| Plant Extract | Conc. (µg/ml) | (%) DPPH radical scavenging activity | |||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | IC50 (µg/ml) | ||
| Ascorbic acid | 100 | 49.47±1.58 | 55.52±1.23 | 60.2±1.34 | 65.84 |
| 200 | 56.09±1.53 | 61.13±1.11 | 71.3±2.32 | ||
| 300 | 72.47±1.88 | 83.38±1.03 | 91.5±2.34 | ||
| Methanol | 100 | 35.66±1.29 | 45.14±1.20 | 52.5±1.07 | 150.78 |
| 200 | 45.77±1.62 | 57.07±0.94 | 66.3±2.32 | ||
| 300 | 55.71±0.57 | 65.92±1.19 | 79.5±2.54 | ||
| Aqueous | 100 | 43.53±0.74 | 51.66±0.69 | 56.2±1.51 | 81.62 |
| 200 | 53.57±2.10 | 62.57±1.47 | 67.5±2.35 | ||
| 300 | 61.30±2.29 | 72.30±1.13 | 76.5±1.36 | ||
| Treatment | Antidiarrheal activity of crude methanolic extract of F. africana. | ||||
|---|---|---|---|---|---|
| Dose (mg/kg) | Mean length of intestine | Mean distance travelled by charcoal | Transit of intestine Percent (%) | Percent inhibition % | |
| Normal saline | 10ml/kg | 54.8 | 49.2740±4.25 | 89.90% | 10.09% |
| Atropine sulphate | 10mg/kg | 55.42 | 18.0820±3.93 | 32.62% | 67.37% |
| Methanolic Extract |
100 mg/kg | 51.83 | 20.9240±2.54 | 40.36% | 59.63% |
| 200 mg/kg | 51.35 | 13.7300±9.72 | 26.73% | 73.26% | |
| 300 mg/kg | 51.68 | 8.3788±3.16 | 16.21% | 83.78% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





