Submitted:
22 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
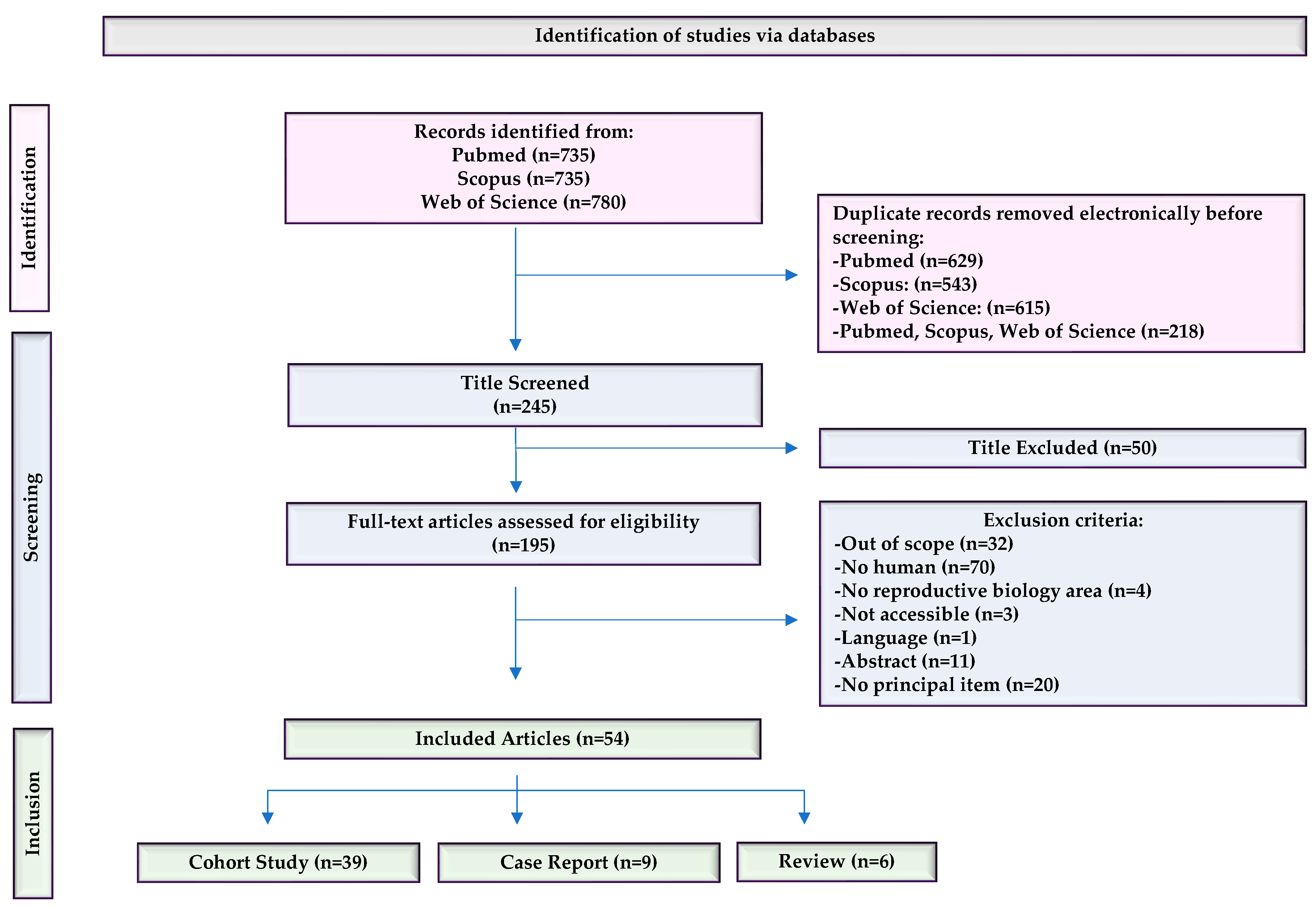
2.1. Compilation of Relevant Bibliographic Source
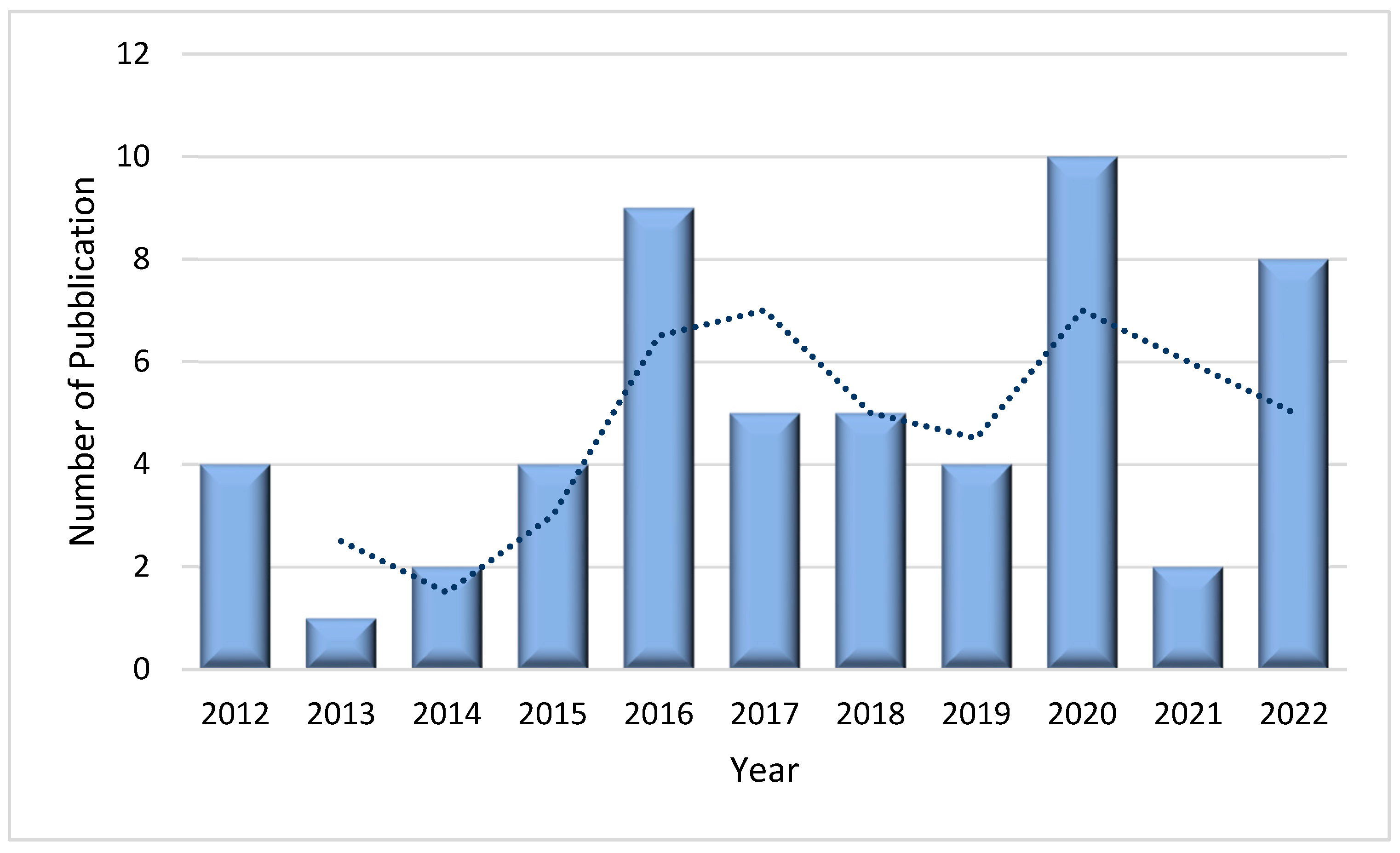
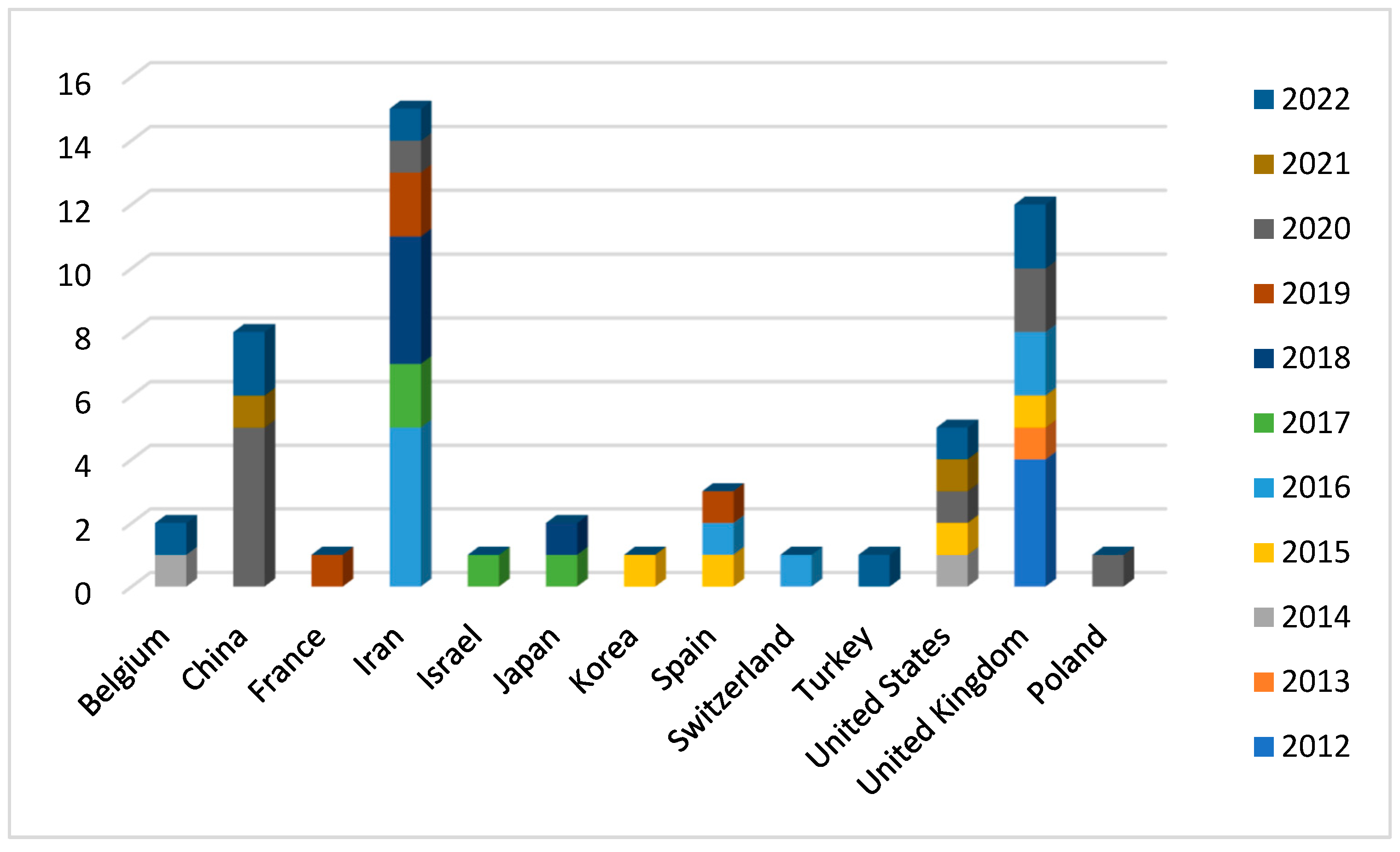
2.2. Bibliometric Analysis
2.3. Bibliographical Analysis
2.4. Analysis of PLCζ
2.4.1. PLCζ mechanism of action
2.4.2. PLCζ level, expression, and localization in relation to semen analysis
2.4.3. Level, expression, localization of PLCζ and fertilization ability
2.4.5. PLCZ1 mutation identified in infertile males
2.4.6. PLCζ in globozoospermic patients
2.4.7. AOA treatment in men with PLCζ dysfunction
2.4.8. Treatment approach in men with PLCζ impairment
3. Discussion
4. Materials and Methods
4.1. Literature search and information processing
4.2. Study selection and eligibility criteria
4.3. Data extraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, C.; Meng, X.; Coward, K. SPERM FACTORS AND EGG ACTIVATION: Phospholipase C zeta (PLCZ1) and the clinical diagnosis of oocyte activation deficiency. Reproduction (Cambridge, England) 2022, 164, F53–F66. [CrossRef]
- Cheung, S.; Parrella, A.; Tavares, D.; Keating, D.; Xie, P.; Rosenwaks, Z.; Palermo, G.D. Single-center thorough evaluation and targeted treatment of globozoospermic men. J Assist Reprod Genet 2021, 38, 2073–2086. [Google Scholar] [CrossRef]
- Cheung, S.; Xie, P.; Parrella, A.; Keating, D.; Rosenwaks, Z.; Palermo, G.D. Identification and treatment of men with phospholipase Cζ–defective spermatozoa. Fertility and sterility 2020, 114, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Jones, C.; Melo, P.; Ross, C.; Mounce, G.; Child, T.; Coward, K. Antigen unmasking does not improve the visualization of phospholipase C zeta in human spermatozoa. Asian journal of andrology 2022, 24, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Rahimizadeh, P.; Topraggaleh, T.R.; Nasr-Esfahani, M.H.; Ziarati, N.; Mirshahvaladi, S.; Esmaeili, V.; Seifi, S.; Eftekhari-Yazdi, P.; Shahverdi, A. The alteration of PLCζ protein expression in unexplained infertile and asthenoteratozoospermic patients: A potential effect on sperm fertilization ability. Molecular reproduction and development 2020, 87, 115–123. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jellerette, T.; Salicioni, A.M.; Hoi, C.L.; Yoo, M.S.; Coward, K.; Parrington, J.; Grow, D.; Cibelli, J.B.; Visconti, P.E.; Mager, J.; Fissore, R.A. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. Journal of Clinical Investigation 2008, 118. [Google Scholar] [CrossRef]
- Amdani, S.N.; Yeste, M.; Jones, C.; Coward, K. Phospholipase C zeta (PLCζ) and male infertility: Clinical update and topical developments. Advances in biological regulation 2016, 61, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J., Ph.D; Jones, C.; Mounce, G., M.Sc; Ramadan, W.M., M.Sc; Lemmon, B., B.M; Heindryckx, B., Ph.D; de Sutter, Petra, M.D., Ph.D; Parrington, J., Ph.D; Turner, K., Ph.D; Child, T., M.D; McVeigh, E., M.D; Coward, K., Ph.D Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertility and sterility 2013, 99, 107–117.e3. [CrossRef]
- Yelumalai, S., Ph.D; Yeste, M., Ph.D; Jones, C.; Amdani, S.N., M.Sc; Kashir, J., Ph.D; Mounce, G., M.Sc; Da Silva, S.J.M., Ph.D; Barratt, C.L., Ph.D; McVeigh, E., M.D; Coward, K., Ph.D Total levels, localization patterns, and proportions of sperm exhibiting phospholipase C zeta are significantly correlated with fertilization rates after intracytoplasmic sperm injection. Fertility and sterility 2015, 104, 561–568.e4. [CrossRef]
- Heytens, E.; Parrington, J.; Coward, K.; Young, C.; Lambrecht, S.; Yoon, S.; Fissore, R.; Hamer, R.; Deane, C.; Ruas, M.; Grasa, P.; Soleimani, R.; Cuvelier, C.; Gerris, J.; Dhont, M.; Deforce, D.; Leybaert, L.; De Sutter, P. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. VO - 24 RT - Journal Article. Human reproduction (Oxford, England) OP - 2417-2428 . [CrossRef]
- Kashir, J.; Konstantinidis, M.; Jones, C.; Lemmon, B.; Chang Lee, H.; Hamer, R.; Heindryckx, B.; Deane, C.M.; De Sutter, P.; Fissore, R.A.; Parrington, J.; Wells, D.; Coward, K. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Human reproduction (Oxford) 2012, 27, 222–231. [Google Scholar] [CrossRef]
- Torra-Massana, M.; Cornet-Bartolomé, D.; Barragán, M.; Durban, M.; Ferrer-Vaquer, A.; Zambelli, F.; Rodriguez, A.; Oliva, R.; Vassena, R. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Human reproduction (Oxford) 2019, 34, 1494–1504. [Google Scholar] [CrossRef]
- Keiji Kuroda; Jan J. Brosens; Siobhan Quenby; Satoru Takeda; editors Treatment Strategy for Unexplained Infertility and Recurrent Miscarriage, Springer Singapore: Singapore, 2018. [CrossRef]
- Kashir, J.; Ganesh, D.; Jones, C.; Coward, K. Oocyte activation deficiency and assisted oocyte activation: mechanisms, obstacles and prospects for clinical application. Human reproduction open 2022, 2022, hoac003. [Google Scholar] [CrossRef]
- Gat, I.; Orvieto, R. "This is where it all started" - the pivotal role of PLCζ within the sophisticated process of mammalian reproduction: a systemic review. Basic and clinical andrology 2017, 27, 9. [Google Scholar] [CrossRef]
- Kashir, J.; Buntwal, L.; Nomikos, M.; Calver, B.L.; Stamatiadis, P.; Ashley, P.; Vassilakopoulou, V.; Sanders, D.; Knaggs, P.; Livaniou, E.; Swann, K.; Lai, F.A. Antigen unmasking enhances visualization efficacy of the oocyte activation factor, phospholipase C zeta, in mammalian sperm. Molecular human reproduction 2017, 23, 54–67. [Google Scholar] [CrossRef]
- Lee, H.C.; Arny, M.; Grow, D.; Dumesic, D.; Fissore, R.A.; Jellerette-Nolan, T. Protein phospholipase C Zeta1 expression in patients with failed ICSI but with normal sperm parameters. J Assist Reprod Genet 2014, 31, 749–756. [Google Scholar] [CrossRef]
- Durban, M.; Barragán, M.; Colodron, M.; Ferrer-Buitrago, M.; De Sutter, P.; Heindryckx, B.; Vernaeve, V.; Vassena, R. PLCζ disruption with complete fertilization failure in normozoospermia. J Assist Reprod Genet 2015, 32, 879–886. [Google Scholar] [CrossRef]
- Ferrer-Vaquer, A.; Barragan, M.; Freour, T.; Vernaeve, V.; Vassena, R. PLCζ sequence, protein levels, and distribution in human sperm do not correlate with semen characteristics and fertilization rates after ICSI. J Assist Reprod Genet 2016, 33, 747–756. [Google Scholar] [CrossRef]
- Chithiwala, Z.H.; Lee, H.C.; Hill, D.L.; Jellerette-Nolan, T.; Fissore, R.; Grow, D.; Dumesic, D.A. Phospholipase C-zeta deficiency as a cause for repetitive oocyte fertilization failure during ovarian stimulation for in vitro fertilization with ICSI: a case report. J Assist Reprod Genet 2015, 32, 1415–1419. [Google Scholar] [CrossRef]
- Azad, N.; Nazarian, H.; Ghaffari Novin, M.; Masteri Farahani, R.; Piryaei, A.; Heidari, M.H.; Abdollahpour Alitappeh, M. Oligoasthenoteratozoospermic (OAT) men display altered phospholipase C ζ (PLCζ) localization and a lower percentage of sperm cells expressing PLCζ and post-acrosomal sheath WW domain-binding protein (PAWP). Bosnian Journal of Basic Medical Sciences 2018, 18, 178–184. [Google Scholar] [CrossRef]
- Tavalaee, M.; Kiani-Esfahani, A.; Nasr-Esfahani, M.H. Relationship between phospholipase C-zeta, semen parameters, and chromatin status. Systems biology in reproductive medicine 2017, 63, 259–268. [Google Scholar] [CrossRef]
- Azad, N.; Nazarian, H.; Ghaffari Novin, M.; Masteri Farahani, R.; Piryaei, A.; Heidari, M.H. Phospholipase C zeta parameters in sperm from polymorphic teratozoospermic men. Annals of anatomy 2018, 215, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Mistry, B.V.; BuSaleh, L.; Abu-Dawas, R.; Nomikos, M.; Ajlan, A.; Abu-Dawud, R.; AlYacoub, N.; AlHassan, S.; Lai, F.A.; Assiri, A.M.; Coskun, S. Phospholipase C zeta profiles are indicative of optimal sperm parameters and fertilisation success in patients undergoing fertility treatment. Andrology (Oxford) 2020, 8, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Jones, C.; Amdani, S.N.; Yelumalai, S.; Mounce, G.; da Silva, S.J.M.; Child, T.; Coward, K. Does advancing male age influence the expression levels and localisation patterns of phospholipase C zeta (PLCζ) in human sperm? Scientific Reports 2016, 6, 27543. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.K.; Kim, J.; Kim, J.H.; Chang, J.H.; Jee, B.C.; Kim, S.H. Relationship between phospholipase C zeta immunoreactivity and DNA fragmentation and oxidation in human sperm. Obstetrics & Gynecology Science 2015, 58, 232–238. [CrossRef]
- Tavalaee, M.; Kiani-Esfahani, A.; Nasr-Esfahani, M.H. Relationship between Potential Sperm Factors Involved in Oocyte Activation and Sperm DNA Fragmentation with Intra-Cytoplasmic Sperm Injection Clinical Outcomes. Cell Journal 2017, 18, 588–596. [Google Scholar] [CrossRef]
- Janghorban-Laricheh, E.; Ghazavi-Khorasgani, N.; Tavalaee, M.; Zohrabi, D.; Abbasi, H.; Nasr- Esfahani, M.H. An association between sperm PLCζ levels and varicocele? J Assist Reprod Genet 2016, 33, 1649–1655. [Google Scholar] [CrossRef]
- Tavalaee, M.; Parivar, K.; Shahverdi, A.; Ghaedi, K.; Nasr-Esfahani, M.H. Status of sperm-born oocyte activating factors (PAWP, PLCζ) and sperm chromatin in uncapacitated, capacitated and acrosome-reacted conditions. Human fertility (Cambridge, England) 2017, 20, 96–103. [CrossRef]
- Khakpour, S.; Sadeghi, E.; Tavalaee, M.; Bahadorani, M.; Nasr-Esfahani, M.H. Zeta method: A noninvasive method based on membrane charge for selecting spermatozoa expressing high level of phospholipaseCζ. Andrologia 2019, 51, e13249-n/a. [CrossRef]
- Azad, N.; Nazarian, H.; Nazari, L.; Ghaffari Novin, M.; Piryaei, A.; Heidari, M.H.; Masteri Farahani, R.; Sadjadpour, S.S. Evaluation of PAWP and PLC? Expression in Infertile Men with Previous ICSI Fertilization Failure. Urology journal 2018, 15, 116–121. [Google Scholar] [CrossRef]
- Aras-Tosun, D.; Cakar, Z.; Can, A.; Ozkavukcu, S.; Kaplanoglu, I.; Cinar, O. Phospholipase C-zeta levels are not correlated with fertilisation rates in infertile couples. Andrologia 2022, 54, e14269-n/a. [CrossRef]
- Moreau, J.; Fargeon, S.; Gatimel, N.; Parinaud, J.; Léandri, R.D. Expression of phospholipase PLC Zeta in human spermatozoa: impact of cryopreservation. Andrology (Oxford) 2019, 7, 315–318. [Google Scholar] [CrossRef]
- Kashir, J., Ph.D; Konstantinidis, M., B.Sc; Jones, C.; Heindryckx, B., Ph.D; De Sutter, Petra, M.D., Ph.D; Parrington, J., Ph.D; Wells, D., Ph.D; Coward, K., Ph.D Characterization of two heterozygous mutations of the oocyte activation factor phospholipase C zeta (PLCζ) from an infertile man by use of minisequencing of individual sperm and expression in somatic cells. Fertility and sterility 2012, 98, 423–431. [CrossRef]
- Escoffier, J.; Lee, H.C.; Yassine, S.; Zouari, R.; Martinez, G.; Karaouzène, T.; Coutton, C.; Kherraf, Z.; Halouani, L.; Triki, C.; Nef, S.; Thierry-Mieg, N.; Savinov, S.N.; Fissore, R.; Ray, P.F.; Arnoult, C. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Human molecular genetics 2016, 25, 878–891. [Google Scholar] [CrossRef]
- Javadian-Elyaderani, S.; Ghaedi, K.; Tavalaee, M.; Rabiee, F.; Deemeh, M.R.; Nasr-Esfahani, M.H. Diagnosis of genetic defects through parallel assessment of PLCζ and CAPZA3 in infertile men with history of failed oocyte activation. Iranian Journal of Basic Medical Sciences 2016, 19, 281–289. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27114798. [PubMed]
- Mu, J.; Zhang, Z.; Wu, L.; Fu, J.; Chen, B.; Yan, Z.; Li, B.; Zhou, Z.; Wang, W.; Zhao, L.; Dong, J.; Kuang, Y.; Sun, X.; He, L.; Wang, L.; Sang, Q. The identification of novel mutations in PLCZ1 responsible for human fertilization failure and a therapeutic intervention by artificial oocyte activation. Molecular human reproduction 2020, 26, 80–87. [Google Scholar] [CrossRef]
- Yuan, P.; Zheng, L.; Liang, H.; Lin, Q.; Ou, S.; Zhu, Y.; Lai, L.; Zhang, Q.; He, Z.; Wang, W. Novel mutations in the PLCZ1 gene associated with human low or failed fertilization. Molecular genetics & genomic medicine 2020, 8, e1470-n/a. [CrossRef]
- Yuan, P.; Yang, C.; Ren, Y.; Yan, J.; Nie, Y.; Yan, L.; Qiao, J. A novel homozygous mutation of phospholipase C zeta leading to defective human oocyte activation and fertilization failure. Human reproduction (Oxford) 2020, 35, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Dai, C.; Guo, J.; Zheng, W.; Zhang, T.; Li, Y.; Lu, C.; Gong, F.; Lu, G.; Lin, G. Novel homozygous variations in PLCZ1 lead to poor or failed fertilization characterized by abnormal localization patterns of PLCζ in sperm. Clinical genetics 2020, 97, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Fan, Y.; Wang, F.; Yan, Z.; Li, M.; Ouyang, J.; Wu, L.; Yin, M.; Zhao, J.; Kuang, Y.; Li, B.; Lyu, Q. Novel mutations in PLCZ1 cause male infertility due to fertilization failure or poor fertilization. Human reproduction (Oxford) 2020, 35, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Kong, S.; Li, C.; Zhang, Z.; He, X.; Wu, H.; Tang, D.; Zha, X.; Tan, Q.; Duan, Z.; Cao, Y.; Zhu, F. A homozygous nonsense mutation of PLCZ1 cause male infertility with oocyte activation deficiency. J Assist Reprod Genet 2020, 37, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sadakierska-Chudy, A.; Patrylak, J.; Janeczko, J.; Chudy, J. Downregulation of gene expression and the outcome of ICSI in severe oligozoospermic patients: A preliminary study. Molecular reproduction and development 2020, 87, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liu, C.; Ma, C.; Yu, H.; Shao, Z.; Gao, Y.; Liu, Y.; Wu, H.; Tang, D.; Tan, Q.; Zhang, J.; Li, K.; Xu, C.; Geng, H.; Zhang, J.; Li, H.; Mao, X.; Ge, L.; Fu, F.; Zhong, K.; Xu, Y.; Tao, F.; Zhou, P.; Wei, Z.; He, X.; Zhang, F.; Cao, Y. Homozygous mutation in SLO3 leads to severe asthenoteratozoospermia due to acrosome hypoplasia and mitochondrial sheath malformations. Reproductive biology and endocrinology 2022, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, X.; Yao, G.; Huang, L.; Wu, S.; Li, X.; Guo, J.; Wen, Y.; Wang, Y.; Shang, L.; Li, N.; Xu, W. A loss-of-function variant in SSFA2 causes male infertility with globozoospermia and failed oocyte activation. Reproductive biology and endocrinology 2022, 20, 1–103. [Google Scholar] [CrossRef] [PubMed]
- Tavalaee, M.; Nasr-Esfahani, M.H. Expression profile of PLCζ, PAWP, and TR-KIT in association with fertilization potential, embryo development, and pregnancy outcomes in globozoospermic candidates for intra-cytoplasmic sperm injection and artificial oocyte activation. Andrology (Oxford) 2016, 4, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kamali-Dolat Abadi, M.; Tavalaee, M.; Shahverdi, A.; Nasr-Esfahani, M.H. Evaluation of PLCζ and PAWP Expression in Globozoospermic Individuals. Cell Journal 2016, 18, 438–445. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27602326.
- Tavalaee, M.; Nomikos, M.; Lai, F.A.; Nasr-Esfahani, M.H. Expression of sperm PLCζ and clinical outcomes of ICSI-AOA in men affected by globozoospermia due to DPY19L2 deletion. Reproductive biomedicine online 2018, 36, 348–355. [Google Scholar] [CrossRef]
- Kashir, J.; Sermondade, N.; Sifer, C.; Oo, S.L.; Jones, C.; Mounce, G.; Turner, K.; Child, T.; McVeigh, E.; Coward, K. Motile sperm organelle morphology evaluation-selected globozoospermic human sperm with an acrosomal bud exhibits novel patterns and higher levels of phospholipase C zeta. Human reproduction (Oxford) 2012, 27, 3150–3160. [Google Scholar] [CrossRef]
- Nazarian, H.; Azad, N.; Nazari, L.; Piryaei, A.; Heidari, M.H.; Masteri-Farahani, R.; Karimi, M.; Ghaffari-Novin, M. Effect of Artificial Oocyte Activation on Intra-Cytoplasmic Sperm Injection Outcomes in Patients with Lower Percentage of Sperm Containing Phospholipase Cζ: A Randomized Clinical Trial. Journal of Reproduction & Infertility 2019, 20, 3–9. [Google Scholar]
- Meng, X.; Melo, P.; Jones, C.; Ross, C.; Mounce, G.; Turner, K.; Child, T.; Coward, K. Use of phospholipase C zeta analysis to identify candidates for artificial oocyte activation: a case series of clinical pregnancies and a proposed algorithm for patient management. Fertility and sterility 2020, 114, 163–174. [Google Scholar] [CrossRef]
- Nikiforaki, D., M.Sc; Vanden Meerschaut, Frauke, M.D., Ph.D; De Gheselle, S., M.Sc; Qian, C., M.D; Van den Abbeel, E., Ph.D; De Vos, W.H., Ph.D; Deroo, T., Ph.D; De Sutter, Petra, M.D., Ph.D; Heindryckx, B., Ph.D Sperm involved in recurrent partial hydatidiform moles cannot induce the normal pattern of calcium oscillations. Fertility and sterility 2014, 102, 581–588.e1. [CrossRef]
- Cardona Barberán, A.; Boel, A.; Vanden Meerschaut, F.; Stoop, D.; Heindryckx, B. SPERM FACTORS AND EGG ACTIVATION: Fertilization failure after human ICSI and the clinical potential of PLCZ1. Reproduction (Cambridge, England) 2022, 164, F39–F51. [CrossRef]
- Swann, K., Ph.D; Windsor, S., Ph.D; Campbell, K., Ph.D; Elgmati, K., M.Sc; Nomikos, M., Ph.D; Zernicka-Goetz, M., Ph.D; Amso, N., Ph.D; Lai, F.A., Ph.D; Thomas, A., Ph.D; Graham, C., D. Phil Phospholipase C-ζ-induced Ca2+ oscillations cause coincident cytoplasmic movements in human oocytes that failed to fertilize after intracytoplasmic sperm injection. Fertility and Sterility 2012, 97, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Ito, M.; Kuroda, K.; Takeda, S.; Tanaka, A. The establishment of appropriate methods for egg-activation by human PLCZ1 RNA injection into human oocyte. Cell calcium (Edinburgh) 2017, 65, 22–30. [Google Scholar] [CrossRef]
- Mirsanei, J.S.; Sheibak, N.; Zandieh, Z.; Mehdizadeh, M.; Aflatoonian, R.; Tabatabaei, M.; Mousavi, A.S.; Amjadi, F. Microfluidic chips as a method for sperm selection improve fertilization rate in couples with fertilization failure. Arch Gynecol Obstet 2022, 306, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; Glanville, J.; Grimshaw, J.M.; Hróbjartsson, A.; Lalu, M.M.; Li, T.; Loder, E.W.; Mayo-Wilson, E.; McDonald, S.; McGuinness, L.A.; Stewart, L.A.; Thomas, J.; Tricco, A.C.; Welch, V.A.; Whiting, P.; Moher, D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Ref. | PLCζ Features | Population | Sample | PLCζ Identification | Main Findings |
|---|---|---|---|---|---|
| [1] | Localization patterns, expression profile, genetic and identification of PLCZ1 deficit | Infertile patients (N=NA) | NA | NA | The expression and localization of PLCZ1 in human sperm are associated with male infertility. Infertile males showed lower PLCZ1 levels in their sperm than fertile males. PLCZ1 was found to be predominantly expressed in the equatorial region of the sperm but sometimes in combination with other regions. |
| [4] | Method to identify PLCζ | Recurrent FF, low fertilization, TFF, abnormal sperm head morphology (N=12) | Fresh | Immunofluorescence | Specificity testing of antibody-antigen binding indicated that the house method showed more specific binding than spermatozoa treated by the antigen unmasking method (AUM), resulting in the highest relative fluorescence intensity. |
| [5] | Expression profile, localization patterns | Asthenoteratozoospermic (N=40) and unexplained infertile patients (N=40) | Fresh | Immunofluorescence, Western blot | PLCζ expression was significantly reduced in unexplained infertile and asthenoteratozoospermic patients compared to fertile men. No significant differences were observed among the experimental groups in terms of PLCζ localization patterns. |
| [8] | Level, localization patterns | OAD patients with recurrent ICSI failure (N=5) | Frozen | Immunofluorescence | Control subjects presented a significantly higher proportion of sperm exhibiting PLCζ immunofluorescence compared with OAD men. Total levels of PLCζ in sperm from control and OAD patients exhibited significant variance. Predominant PLCζ localization patterns varied between control and OAD samples with no predictable or consistent pattern. |
| [9] | Expression profile, localization patterns | Infertile patients (N=44) | Fresh | Immunofluorescence | Total levels, localization patterns, and the proportion of sperm exhibiting PLCζ are correlated with fertilization rates for ICSI, but not for IVF. |
| [16] | Method to identify PLCζ | Infertile patients (N=NA) | Fresh | Immunofluorescence | All methods of the antigen unmasking method (AUM) enhanced PLCζ visualization efficacy compared to those without AUM. Examination of sperm from individual donors revealed that AUM differentially affects observable PLCζ fluorescence, and the proportion of sperm exhibiting detectable PLCζ fluorescence in sperm from different males. |
| [17] | Expression profile, localization patterns | Normozoospermic with failed or low fertilization (N=4) | Fresh | Western blot and immunofluorescence | Male partners of couples with failed or low success ICSI fertilization but with normal semen analysis parameters showed low expression levels of PLCZ1. |
| [18] | Expression profile, localization patterns | Normozoospermic with TFF (N=1) | Fresh/Frozen | Gene sequencing, immunoblotting, immunofluorescence, MOAT, MOCA, AOA | PLCζ expression level and distribution were significantly disrupted despite the normal sperm activation potential in functional testing using mouse oocytes. |
| [19] | Expression profile, localization patterns, genetics | Low fertilization and TFF(N=15) | Fresh | Gene sequence, Western blot, and immunofluorescence | The distribution pattern of PLCζ did not vary significantly between donor and patient samples. Levels of PLCζ protein in sperm cells showed an interindividual variability both in patient and donor samples. Several SNPs previously reported in infertile patients were also present in fertile men. |
| [20] | Expression profile, localization patterns, AOA | Normozoospermic men with fertilization failure (N=1) | Fresh | Western blot, immunofluorescence, PLCζ bioactivity by an in vitro model of Ca2+ release. | Deficiency of PLCζ in normal-appearing sperm was associated with impaired Ca2+ -dependent oocyte activation during ICSI. Use of calcium ionophore following ICSI improved embryo developmental competence. |
| [21] | Expression profile, localization patterns | Oligoasthenoteratozoospermic patients(N=25) | Fresh | Immunofluorescence | The mean percentage of sperm cells expressing PLCζ was significantly lower in OAT than the control. The localization patten was altered with a predominance in the post-acrosomal region. |
| [22] | Level | Patient with normal (N=32) and abnormal (N=23) semen parameters | Fresh | Flow cytometry | PLCζ was significantly lower in men with abnormal semen parameters compared to those with normal parameters. Significant correlations were observed between sperm concentration, motility, and abnormal morphology with the percentage of PLCζ positive spermatozoa. Negative relationship was observed between the percentage of PLCζ positive spermatozoa and sperm DNA fragmentation. |
| [23] | Expression profile, localization patterns, Mean level | Polymorphic teratozoospermic patients (N=23) | Fresh | Immunofluorescence, Western blot | Relative PLCζ expression was significantly lower in polymorphic teratozoospermic men, as compared with control men; there was no significant difference in localization patterns and proportion of PLCζ expressing sperm between polymorphic teratozoospermic patients and control men. |
| [24] | Expression profile, localization patterns | Infertile patients (N=65) | Fresh | Immunofluorescence and Immunoblotting | Acrosomal and equatorial PLCζ correlated most to sperm health, while dispersed PLCζ correlated to decreased sperm viability. Total levels of PLCζ exhibited significant correlations with sperm parameters. Significantly higher levels of PLCζ were exhibited by cases of fertilization success, alongside higher proportions of Acrosomal + Equatorial patterns, and lower levels of dispersed PLCζ. |
| [25] | Expression profile, localization patterns | Infertile patients (N=27) | Fresh | Immunofluorescence | While the progressive motility parameter was negatively correlated with male age, the proportion, total level, and localization patterns of PLCζ were not associated with male age. |
| [26] | Expression profile, DNA fragmentation, oxidation status | Infertile patients (N=44) | Fresh | Immunofluorescence | The duplicate PLCζ tests on two sperm samples from each patients showed similar results. Immunoreactivity of PLCζ was not associated with donor’s age, sperm concentration, motility, and the percentage of normal form as well as DNA fragmentation index. However, lower expression of PLCζ in sperm may be associated with higher sperm DNA oxidation status. |
| [27] | Expression profile | Infertile patients (N=35) | Fresh | Flow cytometry | PLCζ expression showed a positive correlation with fertilization rate and a negative correlation with the percentage of DNA fragmentation, while no significant relationship was found between PLCζ and embryo quality or pregnancy rate. |
| [28] | Expression profile | Patients with varicocele (N=35) | Fresh | Real-time PCR and Western blot | The mean relative expression of PLCζ was significantly lower in individuals with varicocele compared to fertile men at both transcription and translation levels. The percentage of DNA fragmentation was significantly higher in infertile men with varicocele compared to fertile men. |
| [29] | Expression profile | Normozoospermic men (N=10) | Fresh | Flow cytometry | The proportion of sperm-expressing weak or zero levels of PLCζ were significantly reduced after DGC processing. There were no significant differences among processed DGC, capacitated and acrosome-reacted samples. |
| [30] | Expression profile, Localization patterns | Normozoospermic (N=13) | Fresh | Flow cytometry | The number of PLCζ-positive spermatozoa was significantly low in the Zeta method, but the intensity of PLCζ protein in such spermatozoa was significantly higher than DGC procedure. |
| [31] | Expression profile, protein level | Fertilization Failure (N=15) | Fresh | Immunofluorescence | The mean percentage of PLCζ positive sperm and level of PLCζ protein were significantly lower in FF group compared to the control group. |
| [32] | Expression profile, protein level | Infertile Patients (N=43) | Fresh | Flow cytometry | Quantitative analyses showed no significant difference among the low and high fertilization groups when PLCζ ratio or mean fluorescent intensity was considered. No correlation was found between pregnancy rates and PLCζ quantity. None of the total fertilization failure cases were lack of PLCζ. |
| [33] | Expression profile, Localization patterns | Normozoospermic (N=32) | Fresh/Frozen | Immunofluorescence | The presence of PLCζ on spermatozoa was decreased after freezing–thawing procedures. The percentage of spermatozoa exhibiting PLCζ at post-acrosomal position was significantly greater before freezing while no significant difference was seen for the percentage of spermatozoa exhibiting PLCζ at equatorial position. |
| Ref. | PLCζ Features | Population | PLCZ Identification | Gene Mutation | Main Findings |
|---|---|---|---|---|---|
| [11] | Mutation identification | Recurrent ICSI failure or abnormal sperm morphology (N=9) | Direct sequencing, mini sequencing | PLCζ H233L and PLCζ H398P | PLCζ H233L is predicted to disrupt local protein interaction. Both PLCζ H233Land PLCζ H398P mutations exist on distinct parental chromosomes, the former inherited from the patient’s mother and the latter from his father. |
| [12] | Mutation identification, expression profile | Total or partial FF (N=37) | Immunofluorescence, Western blot, sanger sequencing | Five single-nucleotide missense mutations: p.I120M, p.R197H, p.L224P, p.H233L, and p.S500 L. A frameshift variant p.V326K fs∗25 | Six different mutations were identified in FF patients, and all were found in heterozygosis. PLCZ1 mutations were found in high frequency in patients presenting OAF (54.55%) compared to of no-OAF group (6.67%). None of the controls presented mutation in the PLCZ1 coding sequence. |
| [34] | Localization pattern in HEK293T cells | Recurrent ICSI failure (N=1) | Confocal microscopy, microsequencing | PLCζ H233L and PLCζ H398P | Both PLCζ H233L and PLCζ H398P mutations are present on different alleles and do not alter PLCζ localization in HEK293T cells. |
| [35] | Mutation identification | TTF (N=2) | Whole exomic sequencing | Missense homozygote mutation in PLCZ1, c.1465A>T; p.Ile489Phe | The mutation is deleterious, leading to the absence of the protein in sperm, mislocalization of the protein when injected in GV and MII oocytes, highly abnormal Ca2+ transients and early embryonic arrest. |
| [36] | Expression profile | Patients with total, low and high fertilization rates and globozoospermic patients (N=59) | Real time PCR, Western blot | CAPZA3 and PLCζ genes | The results revealed a significant correlation between the expression of CAPZA3 and PLCζ genes. Individuals with low expression of both genes presented low fertilization rates. |
| [37] | Mutation identification, AOA | Recurrent fertilization failure (N=4) | Whole-exome sequencing, Sanger sequencing, Western blot | Homozygous nonsense mutation c.588C>A (p.Cys196∗), homozygous missense mutation c.590G>A (p.Arg197His); a heterozygous mutation c.588C>A (p.Cys196∗) and the missense heterozygous mutation c.1259C>T (p.Pro420Leu), compound heterozygous frameshift mutations c.972_973delAG (p.Thr324fs) and c.1234delA (p.Arg412fs) | Western blot showed that missense mutations decreased the level of PLCZ1, and that nonsense or frameshift mutations resulted in undetectable or truncated proteins. The oocyte activation ability was significantly reduced by these mutations. AOA help to achieve a pregnancy. |
| [38] | Mutation identification | Recurrent partial/total fertilization failure (N=2) | Sanger Sequencing | Compound heterozygous mutations c.1259C>T (p.P420L) and c.1733T>C (p.M578T) in the PLCZ1 gene; homozygous mutation c.1727T>C (p.L576P) | The compound heterozygous mutations c.1259C>T (p.P420L) and c.1733T>C (p.M578T) in the PLCZ1 gene were identified in a patient with TFF while the homozygous mutation c.1727T>C (p.L576P) in a man with partial fertilization failure. These mutations were absent in the control cohort and in the databases.All mutant amino acids are located in key domains and are predicted to impair hydrolytic activity and lead to PLCZ1 dysfunction. |
| [39] | Mutation identification | Recurrent ICSI failure (N=1) | Single-cell trio-seq sequencing, Whole genome sequencing, Sanger sequencing, Western blot | Missense homozygous mutation in PLCζ, c.1658 G>C; p. R553P | PLCζ, c.1658 G>C mutation does not affect the production of the corresponding protein in sperm. However, microinjection of the mRNA transcribed from the PLCζ R553P mutation gene failed to trigger oocyte activation and the subsequent embryo development. |
| [40] | Mutation identification, localization patterns, AOA | TFF and poor ICSI fertilization (N=10) | Whole-exome sequencing, immunofluorescence | A nonsense variation, c.C588A (p.C196X), two missense variations, c.T1048C (p.S350P) and c.C736T (p.L246F). | The three novel homozygous variations in the PLCZ1 gene are predicted to modify its secondary structure impairing its hydrolytic activity. These variations in PLCZ1 led to poor or failed fertilization that could be overcame by ICSI-AOA. |
| [41] | Mutation identification, AOA | FF or poor fertilization (N=14) | Sanger sequencing, Western blot | Nonsense mutation c.588C>A (p. C196∗) and missense mutation c.830T>C (p.L277P), 3 bp in-frame deletion c.1129_1131delAAT (p.N377del), missense mutation c.1733T>C (p.M578T), splicing mutation c.570+1G>T and missense mutation c.1344A>T (p.K448N) | In five families, six new PLCZ1 mutations and one reported mutation were found. Western blot analysis showed no PLCZ1 protein in affected patients' semen. The injection of wild-type PLCZ1 cRNA induced pronuclear formation, while the microinjection of mutant PLCZ1 cRNA did not activate oocytes to induce nuclear formation. AOA treatment successfully rescued fertilization failure in four patients. |
| [42] | Mutation identification, AOA | FF (N=4) | Sanger sequencing, Western blot, immunofluorescence | c.588C>A (p.Cys196Ter) | We identified a novel homozygous PLCZ1 nonsense mutation, c.588C>A (p.Cys196Ter) in an infertile man from a consanguineous family. This results in the non-production of the protein; The use of AOA resulted in an increased rate of normal fertilization. |
| [43] | Expression level | Severe oligozoospermic patients (N=8) | TaqMan low density array | 44 genes | The transcript levels of 21 genes important for spermatogenesis and early preimplantation development, were significantly decreased in the severe oligozoospermic group. Among them, mRNA of AKAP4 and PTK7 was greatly reduced and the transcripts of PLCζ and POU5F1 were not detected in patients with severe oligozoospermia. |
| [44] | Mutation identification, expression level | Asthenoteratozoospermic patients (N=105) | Quantitative RT-PCR, Western blot, immunofluorescence | c.1237A>T: p.Ile413Phe in SLO3 | A homozygous missense variant (c.1237A>T: p.Ile413Phe) in the sperm-specific SLO3 in one Chinese patient was identified. The levels of SLO3 mRNA and protein in spermatozoa were reduced. Sperm of this individual exhibited acrosome hypoplasia, disruption of the mitochondrial sheath, coiled tails, and motility defects. Furthermore, the acrosome reaction, mitochondrial membrane potential, and membrane potential during capacitation were also afflicted. The levels of PLCζ1 were significantly reduced. |
| [45] | Expression profile, AOA | Globozoospermic patient (N=1) | Immunofluorescence, Western blot, Liquid chromatography–mass spectrometry/mass spectrometry, coimmunoprecipitation analyses | Homozygous missense variant (NM_001130445.3: c.3671G > A; p.R1224Q) in the SSFA2 | This study revealed that SSFA2 plays an important role in acrosome formation, and the homozygous c.3671G > A loss-of-function variant in SSFA2 caused globozoospermia. AOA rescued the oocyte activation failure for the patient with the SSFA2 variant. |
| Ref. | PLCζ Features | Population | Sample | PLCζ identification | Main findings |
|---|---|---|---|---|---|
| [2] | Expression profile, genetics, AOA | Globozoospermic patients (N=14) | Fresh | Immunofluorescence | PLCζ was detected in men with the partial form of globozoospermia, whereas it was not detected in those with the complete form.The PLCZ1 gene was mutated and underexpressed in the complete form of globozoospermia. |
| [46] | Expression profile, protein levels | Globozoospermic patients (N=12) | Fresh | Quantitative real-time PCR analysis and Western blot | Levels of PLCζ mRNA in the spermatozoa of fertile men were significantly higher than globozoospermic men. At the protein level, expressions of these factors were low in globozoospermic individuals. High fertilization and pregnancy rates were achieved following ICSI-AOA. |
| [47] | Expression profile, protein levels | Globozoospermic patients (N=21) | Fresh | Real time polymerase chain reaction (qPCR) and Western blot | Expression of PLCζ was significantly reduced at RNA and protein levels in globozoospermic patients compared to fertile men. |
| [48] | Expression profile, protein levels | Globozoospermic patients with DPY19L2 deletion (N=32) | Fresh | Quantitative real-time PCR analysis and Western blot | The relative expression of PLCζ at RNA and protein levels in globozoospermic men with DPY19L2 deletion was significantly lower compared with fertile men. The fertilization rate in globozoospermic couples following ICSI-AOA was significantly lower. Implantation and pregnancy rates were not jeopardized by DPY19L2 deletion in these couples. |
| [49] | Expression profile, localization patterns, level | Globozoospermic patients (N=3) | Fresh | Quantitative immunofluorescence | Completely round-headed globozoospermic sperm were either devoid of PLCz immunofluorescence or exhibited an abnormal, punctate, pattern of PLCz localization. Most sperm with an acrosomal bud exhibited punctate patterns of PLCz localization within the sperm head. MSOME select spermatozoa with higher total level and proportion of spermatozoa exhibiting PLCζ. |
| Ref. | PLCζ Features | Population | Sample | PLCζ identification | Main findings |
|---|---|---|---|---|---|
| [3] | AOA and clinical outcomes | Poor Fertilization/ FF (N=76) | Fresh | Immunofluorescence, MOAT | For couples with oocyte-related OAD, successful fertilization, term pregnancies and deliveries were achieved with an adjusted superovulation protocol. In couples with a sperm-related OAD, as determined by PLCζ assay, assisted gamete treatment was successful. |
| [50] | AOA and clinical outcomes | Infertile patients with PLCζ deficient (N=220) | Fresh | Immunofluorescence | The fertilization rate was significantly lower in the group without AOA compared to the control group. Cleavage and embryo quality scores were not substantially different between groups of control, with and without AOA treatment. |
| [51] | Expression profile, localization patterns, AOA, and clinical outcomes | Teratozoospermic men, TFF, low fertilization rate (N=43) | Fresh | Immunofluorescence | Compared with fertile controls, infertile males had significantly lower levels of PLCζ and/or a significantly lower proportion of sperm exhibiting PLCζ. In men with a significant PLCζ reduction in both parameters, the fertilization and clinical pregnancies rate improved significantly after AOA treatment. |
| [52] | Measurement of fertilizing and calcium-releasing ability | Recurrent partial hydatidiform mole pregnancy(N=1) | Fresh | Immunofluorescence, MOAT | Sperm that previously provoked recurrent partial hydatidiform mole pregnancies due to dispermic fertilization is not able to trigger the normal pattern of Ca2+ oscillations in oocytes. In subsequent ICSI cycles, fertilization failure was overcome with AOA treatment which led to normal deliveries. |
| Ref. | PLCζ Features | PLCζ identification | Treatment | Main findings/function |
|---|---|---|---|---|
| [7] | Review on the application of PLCζ in diagnostic and therapeutic medicine | NGS | hrPLCζ | NGS represents a more sensitive and less time-consuming method for screening PLCζ genetic variants and carefully designed promoters. The most outstanding priority is to synthesize a pure hrPLCZ1 with which to generate a monoclonal antibody and protein crystals. |
| [13] | Review on the effectiveness of the oocyte’s activation methods | NA | AOA, hrPLCζ | The use of calcium ionophore directly increases [Ca2+] but it can have a potential cytotoxic or mutagenic adverse effect on eggs and embryos. The PLCZ1 RNA injection can induce an optimal pattern of Ca2+ oscillations as that at fertilization, leading to the parthenogenetic development of blastocysts. Nevertheless, the adverse effect of RNA injection on human oocytes is still unclear. |
| [14] | Review of the safety and efficiency of AOA | NA | AOA | Contradictory studies on the safety and efficacy of AOA do not yet allow for the establishment of AOA as standard practice in the clinic. The main scientific concern is the non-physiological method of Ca2+ release mediated by most AOA agents, coupled with a lack of holistic understanding regarding the physiological mechanism(s) underlying Ca2+ release at oocyte activation. |
| [15] | Review on the function of PLCζ and new approach focusing on its clinical applicability | NA | hrPLCζ | ICSI failure may be related to impaired PLCζ activity. Microinjection of recombinant human PLCζ to human oocytes after ICSI fertilization failure may trigger Ca2+ oscillations and achieve successful fertilization. |
| [53] | Review of genetic causes of FF, the use of PLCZ1 as a diagnostic marker or therapeutic molecule treatments | MOAT, MOCA, HOCA | hrPLCζ | Heterologous (MOAT and MOCA) and homologous ICSI tests (HOCA) have a high predictive potential for identifying sperm-related deficiencies, but they are technically difficult and require animal facilities and specialized equipment. The injection of human rPLCZ1 protein results in multiple Ca+2 oscillations resembling the physiological calcium. However, it is not yet commercially available. In the meantime, AOA with calcium ionophores remains the best strategy. |
| [54] | Calcium oscillation mechanism | Particle image velocimetry (PIV) and Ca2+ sensitive fluorescent dye | hrPLCζ | Microinjection of PLCζ cRNA into human oocytes that had failed to fertilize after ICSI resulted in the appearance of prolonged Ca2+ oscillations. Ca2+ concentration change was accompanied by a small, coordinated movement of the cytoplasm that could be detected using particle image velocimetry (PIV) analysis. |
| [55] | Pattern of Ca2+ oscillation with different activation methods | PCR technique | hrPLCζ | The pattern of Ca2+ oscillations after PLCZ1 RNA injection exhibited similar characteristics to that after ICSI treatment. Human PLCZ1 RNA is a better therapeutic agent to rescue human oocytes from failed activation, leading to normal and efficient development. |
| [56] | Expression levels of PLCZ1 with microfluidics sperm selection | Reverse transcription-polymerase chain reaction (RT-PCR) | Sperm selection thought DGC and microfluidics | The RT-PCR results showed that there was a significant increase in the expression of PLCZ1 and TNP1 genes in the sperm of groups that were selected using microfluidics sperm selection compared to the group that underwent the density gradient centrifugation method. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





