1. Introduction
Infertility is a complex and multifactorial condition affecting a significant proportion of couples globally [
1]; often leading to emotional distress and a huge financial burden. Traditional treatments, including hormonal therapies and assisted reproductive technologies, have varying degrees of success which are to an extent influenced by specific indications. The success rates with in-vitro-fertilization (IVF) is about 15-60% [
2,
3,
4] depending on the age of the woman and other factors [
5,
6,
7] and 60-60% with pre-implantation genetic testing and implantation of normal embryos [
8]. For most of the unsuccessful IVF-ETs where the embryo is karyotypically normal, various factors have been implicated including impaired endometrial receptivity. Reduce endometrial receptivity is thought in some cases to be secondary to immune dysfunction or immune abnormalities [
7]. One such immune abnormality is increased natural killer (N) cells, although this remains controversial [9, 10 ]. These cells (peripheral and uterine) have been described as the potential culprit in recurrent pregnancy loss and implantation failure especially in cases with high numbers pre-conception [
11] and with demonstrably sustained markers of cytotoxic activity in both the peripheral [
12] and uterine NK cells [
13]. Increased NK cell cytotoxicity has been associated with recurrent implantation failure and recurrent miscarriages [10, 14,15,16]. Inhibiting the cytotoxic activities of these NK cells could potentially increase implantation rates and reduce the risk of miscarriage [
17]. Various agents have been tried including aspirin, progesterone, low molecular weight heparin, intravenous immunoglobulin (18,19] and corticosteroids [
20], but none of these have been shown to be effective in increasing success pregnancy rates.
An approach that has therefore been proposed to modulate the immune dysfunction associated with high NK cells is the use of intralipids [
21,
22].
Intralipid® is an emulsion of polyunsaturated fatty acids derived from soybean oil, glycerin and egg phospholipid that is commonly used as a component of parenteral nutrition in patients unable to tolerate an oral diet. Although the exact mechanism by which Intralipid® modulates immune modulation remains unclear, its active ingredient, soya oil, has been reported to be capable of inhibiting pro-inflammatory mediators, specifically Th1 cells [
23]
thus favouring successful implantation and pregnancy. It has been reported to have a variety of immune-modulatory and anti-inflammatory actions including decreased production of IL-2, TNFα, IL-1β and suppression of NK cell activity that may last several weeks after a single infusion [
17,
23,
24]. Additionally, it has been shown that intralipid therapy boosted serum granulocyte colony-stimulating factor (G-CSF), a cytokine reported to be associated with successful implantation following embryo transfer in IVF cycles [
25]. Although there have been several studies on the use of intralipids in assisted reproduction, the data have not been consistent. Having said that, two recent systematic reviews and meta-analyses concluded that intralipid administration may improve IVF outcomes, especially in women with recurrent implantation failure or recurrent spontaneous miscarriages [
26,
27]. However, they concluded that due to some limitations, intralipid use in women undergoing IVF should be with caution until more data become available. Such data would ideally be from prospective randomized controlled trials, but it could also come from cases series. We have been using intralipid in women with recurrent implantation failure or recurrent spontaneous miscarriages in our Reproductive Unit for the past few years. The aim of this study was to review the outcomes from these women undergoing assisted reproduction techniques, to add to the accumulating data. Apart from the outcomes of the ART in the group with recurrent implantation failure, we also set out to determine how the outcomes compared to those in women who had not had recurrent implantation failure.
2. Materials and Methods
This was a retrospective study of women undergoing assisted reproductive technology (ART) - specifically IVF-ET at the Feto Maternal Medical Centre, Qatar for the period September 2023 to September 2024. Institutional review board approval was obtained (FMC-IRB-004 12th March 2024) and since this was an anonymized review of the records, no patient informed consent was required.
Intralipid® was administered to women who had had recurrent implantation failure (RIF) defined as three or more unsuccessful IVF/ICSI-ET of at least 3 good quality embryo transfers. To be included in the study, the women would have been thoroughly investigated and obvious causes of infertility (e.g. tubal factor, anatomical, hormonal, haematological and autoimmune).) excluded. Male factor was not an exclusion criterion if good quality sperms were generated after routine preparation.
The electronic records of the women were reviewed and from these, various variables were extracted. These included patient demographics, hormonal (FSH/LH levels, TSH and free T4) investigation results (classified as normal and if abnormal - specified), type of reproductive pathology requiring treatment by ART, other laboratory data (AMH levels, uNK cells - thrombophilia screen,) and ultrasound findings. Other variables collected included past medical and surgical history, co-morbidities (such as diabetes, hypertension etc.) and other associated conditions (such as PCOS, uterine fibroids).
The ART variables collected included - indication for ART, ovulation induction regimen, timing of the intralipid administration (with relation to embryo replacement) and duration of administration and outcomes of the ART where available. We also collected information on additional medications used (e.g. aspirin or steroids during the ART procedure).
The ART outcomes were classified as (1) chemical pregnancy - where there was a urine positive pregnancy test or serum hCG>=5IU/L but with no ultrasound demonstrated intrauterine pregnancy (2) clinical pregnancy - where there was a gestational sac with or without a fetal pole demonstrated on ultrasound (3) miscarriage - where an ultrasound demonstrated loss of a clinical pregnancy <22 weeks after the ET or 24 weeks of gestation and (d) pregnancy progressing beyond 24 weeks (livebirth or otherwise). For each woman who received intralipids a comparative group (the case either before or after the index case) matched for age and who had not had recurrent implantation failure and was selected.
2.1. Intralipid Protocol
Intralipid 20% was administered as an intravenous infusion, prepared by diluting 100 mL of Intralipid in 250 mL of normal saline, and infused over a period of 2-hours in an outpatient setting. The intralipid was administered either 1- 2 days before ET or on the day of embryo transfer and was continued every 2 weeks until 12 weeks provided there was a positive BHCG and/or confirmed intrauterine pregnancy.
2.2. Statistical Analysis
Continuous variables are presented as means and standards deviation (SD) where they are normally distributed while categorical data are presented as frequences (percentages). Where continuous data are not normally distributed, medians and ranges were used. The independent sample t-test was used to compare outcomes between the study and control groups. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software.
3. Results
A total of 113 women who underwent assisted reproductive treatment at our centre and met the inclusion criteria were included in the study - 51 of these had intralipid and constituted the index group while 62 did not and constituted the comparative group.
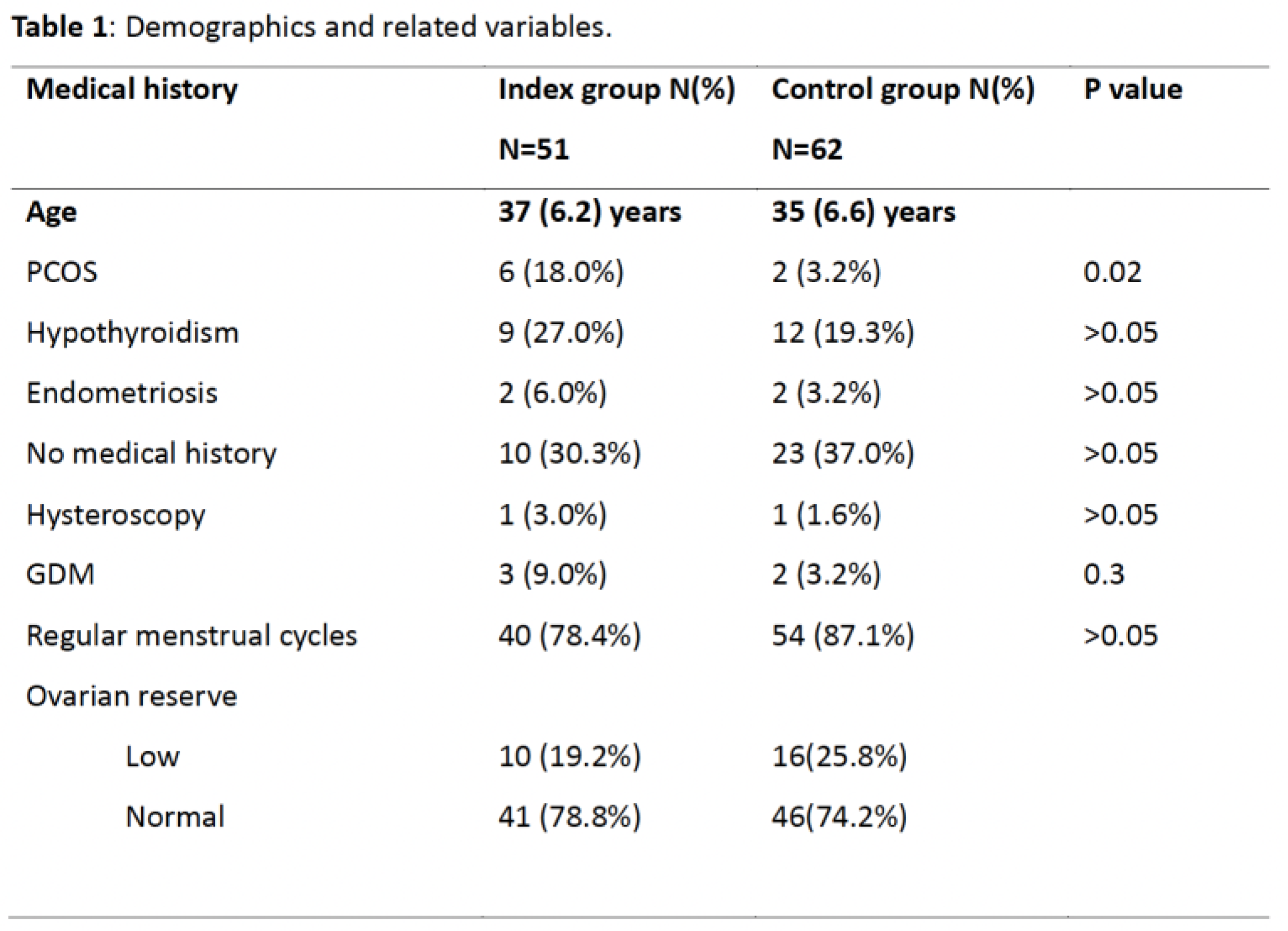
Table 1 shows the demographics, and other variables extracted from the records of the cohort. The mean (SD) age was 37(6.0) years for the index group and 35 (6.6) years for the comparative group (P>0.05). Hypothyroidism and polycystic ovary syndrome (PCOS) were more common in the index than comparative groups but only PCOS was statistically significantly so(P<0.05). Ovarian reserve was not significantly different in the two groups. It was normal in 78.8% (N=41) of the index group and 74.2% (N=46) in the comparative group (P>0.05). Most women (78.4% in the index vs 87.1% in the comparative groups, P>0.05) had regular menstrual cycles. Elevated uNK cells were present in 34 (65.4%) of those in the index group. The prevalence of other immunological factors such as antiphospholipid syndrome was very small in both groups to be of any statistical significance.
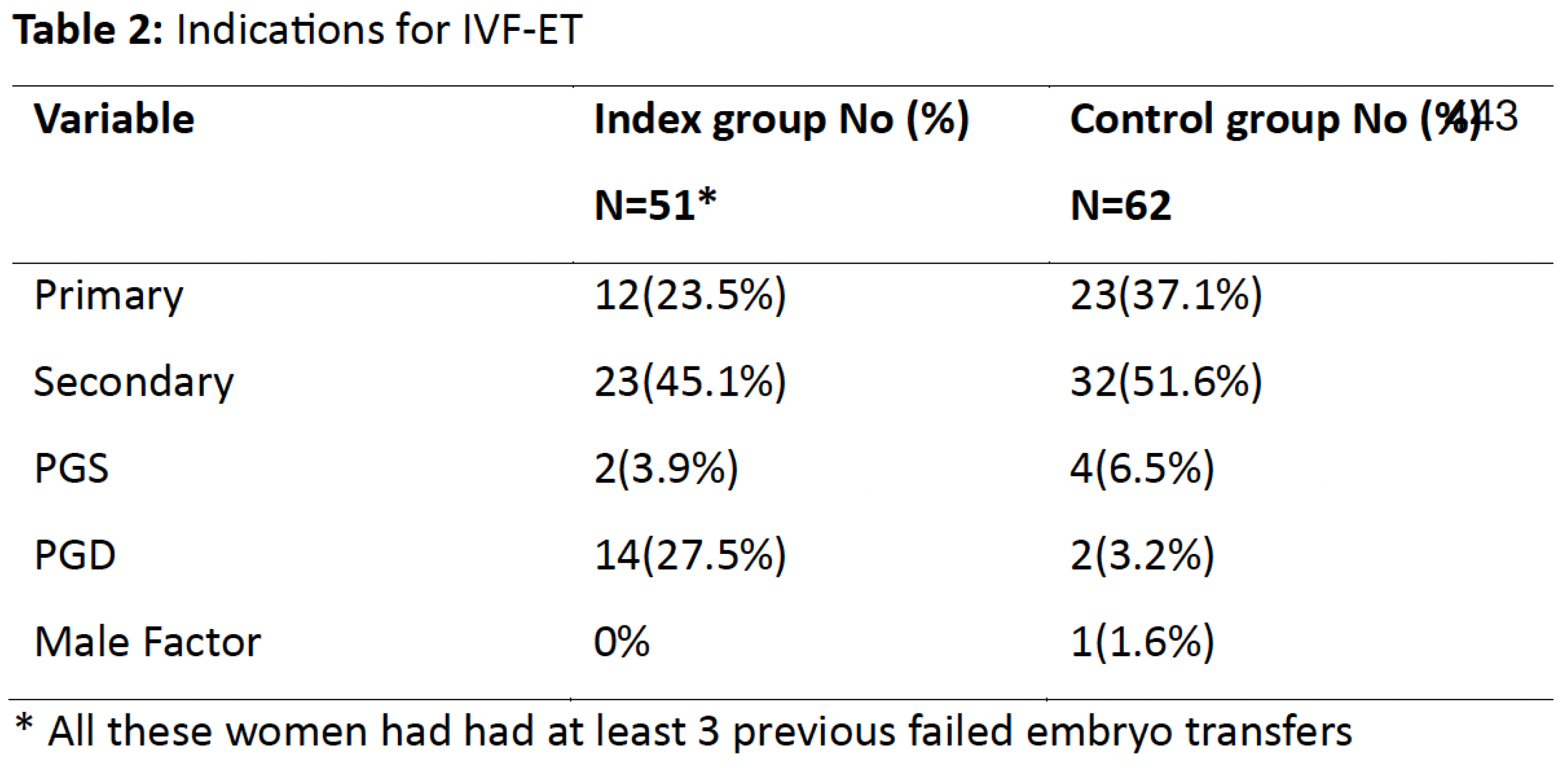
The indications for IVF are shown in Table 2. It was performed for secondary infertility (45.1%), followed by primary infertility (23.5%) in the study group and secondary infertility (51.6%) followed by primary infertility (37.1%) in the comparative group. Other indications included preimplantation genetic diagnosis (PGD, 27.5% vs3.2%), preimplantation genetic screening (PGS, 3.9% vs 6.5%) and male factor infertility (0% vs1.6%).
Ovarian stimulation in the index group was commonly with the antagonist regimen which consisted of (Cetrotide, Orgalutran, Gonal-F and Ovitrelle). A small number of all the cases (5=9.5 %) were offered the long-term agonist protocols (Deacetyl). Ovulation induction in the comparative group was primarily N=(34;54.8%) with the antagonist regimen using Gonal-F, Merional, and Cetrotide.
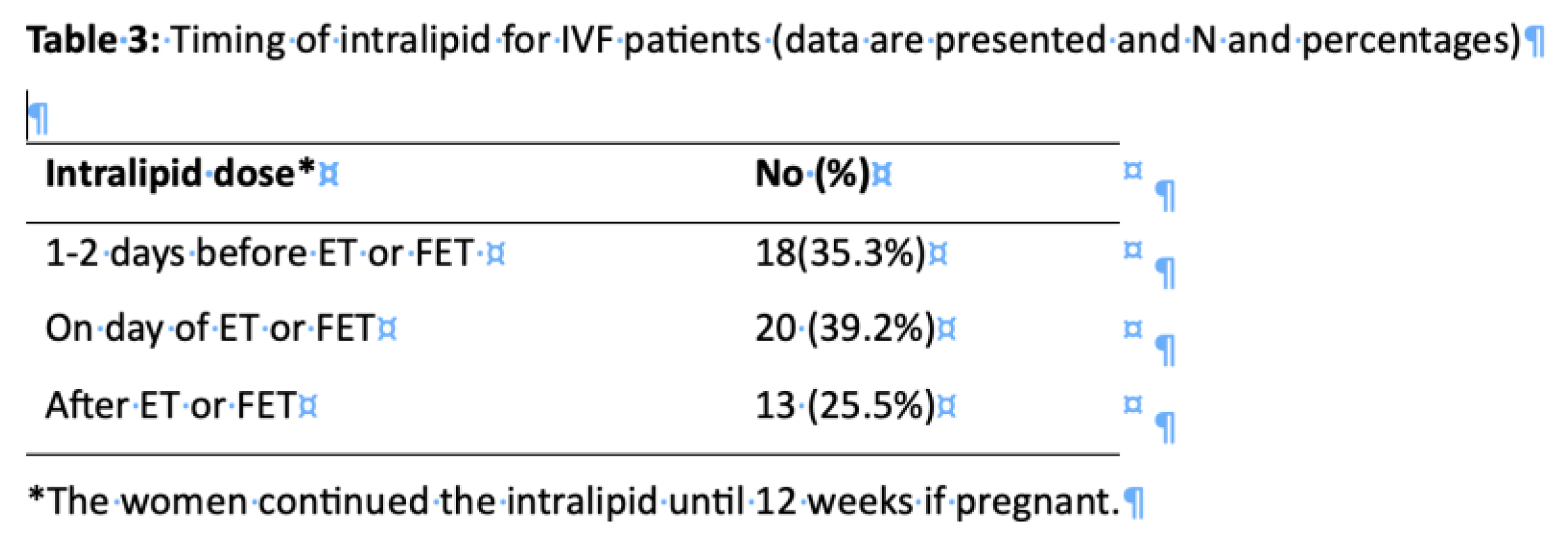
Table 3 shows the intralipid regimen for the patients. There was no consistency in the timing of the administration of intralipid. It was initiated either a day or two before embryo transfer in 18(35.3%) cases, on the day of embryo transfer in 20 (39.2%) cases and after embryo transfer in 13(25.5%).
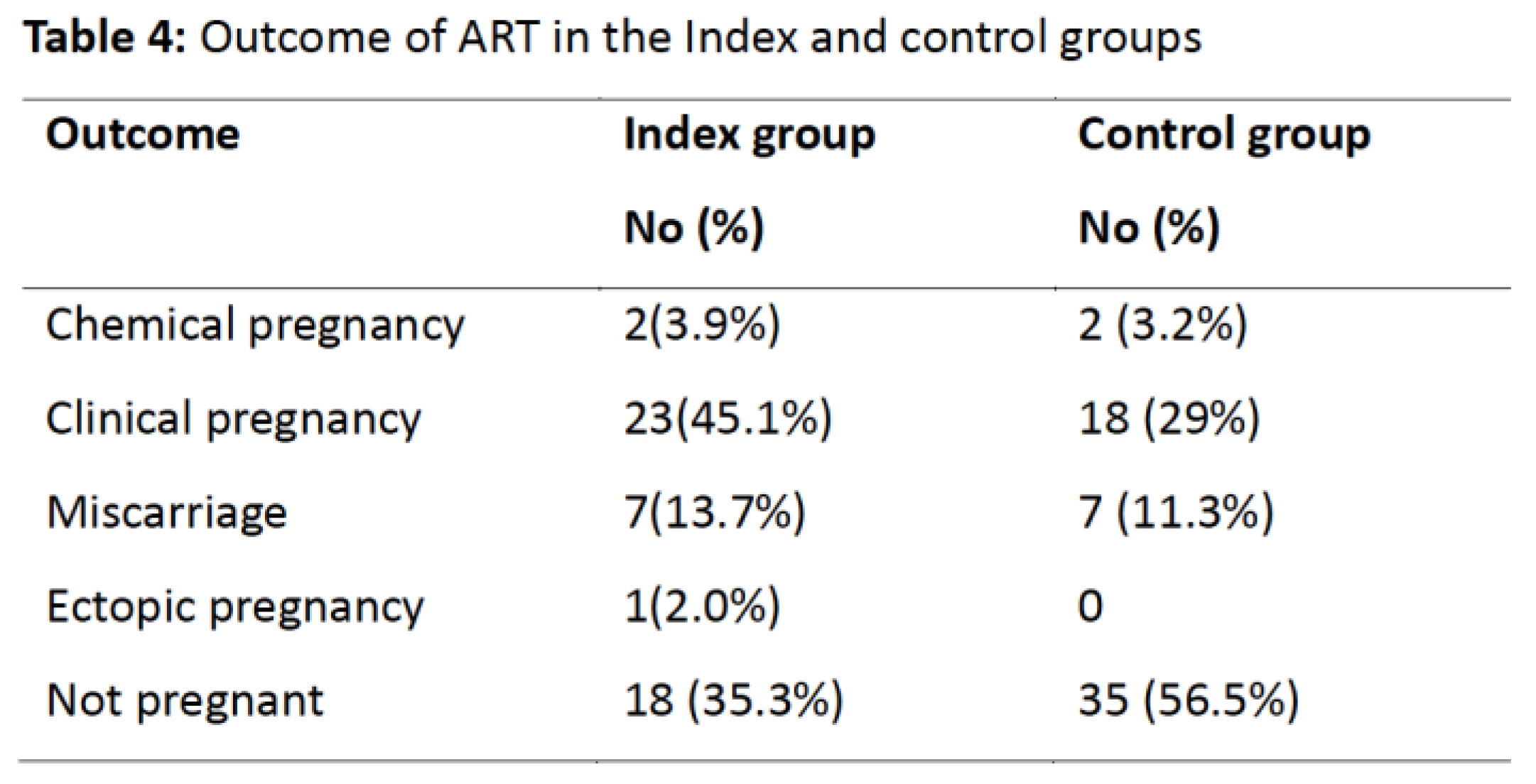
The outcomes of the ART in the cohort are shown in Table 4. There were 23 (45.1%) clinical pregnancies, 7 (13.7%) miscarriages, 2(3.9%) chemical pregnancies; 1(2.0%) was an ectopic pregnancy and 18(35.3%) were not pregnant. In the comparative group there were 18 (29%) clinical pregnancies, 7 (11.3%) miscarriages, 2 (3.2%) chemical pregnancies and 35(56.5%) were not pregnant. The overall pregnancy rate was statistically different in the two groups (P=0.043).
4. Discussion
This study demonstrated a statistically significant higher clinical pregnancy rate in women undergoing assisted reproductive treatment (ART) who received intralipid
(particularly those with elevated uterine natural killer (uNK) cell activity) and a history of repeated implantation failure (RIF). The overall clinical pregnancy rate in the intralipid group (45.1%) was significantly higher than in the comparative group (29%,
P=0.043). Coulam and Acacio [
18] reported improved pregnancy outcomes in women with immunological abnormalities, including elevated NK cell activity, following intralipid therapy (1). Similarly, Roussev et al. [
17] documented that intralipid therapy significantly reduced NK cell activity and improved live birth rates in patients with prior ART failures [
28]. In two systematic reviews by Asif et al. [
29] and Han et al. [
26] of 2RTCs and 5RTCs composing of 303 and 800 participants respectively, intralipid improved livebirth rate compared to controls by a RR of 2.13 (95%CI 1.35-3.36) and 1.85 (95%CI 1.44-2.38) respectively [
17]. Our findings of higher pregnancy rates are consistent with the conclusions from these systematic reviews. This is not an ideal comparator as the denominator was different, however our findings indicate that intralipid may not only increase the pregnancy rates in women with recurrent implantation failure (RIF) but may even result in higher pregnancy rates in this sub-population when compared to those who had not experienced recurrent implantation failure.
In our series the intralipid was started at three different time points and continued until 12 weeks of gestation where the pregnancy was ongoing. There is no consistency in the timing of intralipids in published studies. In the five randomised studies Al-Zebeidi et al. [
30] administered it on the day of ET and again on the day of pregnancy test; Singh et al. (2019) on the day of oocyte retrieval and again on the day of ET; Gameleldin et al. [
31] 6-7 days before ET and again on the day of a positive pregnancy test; Dakhly et al [
32] on the day of oocyte retrieval and repeated within a week of a positive pregnancy test and then every 2 weeks until the end of the first trimester and El-Khayat et al. [
33] between day 4 and 9 of ovulation stimulation and within a week of a positive pregnancy test. This timing of intralipid in these studies and ours did not have any bearing on the successful pregnancy rates suggesting perhaps that there may be no need to administer more than two doses (the minimum in all the studies) of intralipids. This is more so that Roussev et al. [
17] showed that the effect of intralipids by way of decreasing NK cell cytotoxicity lasted for about 2 weeks post administration. A detailed review of the timing of intralipid in all these studies would indicate that most administered it either on the day of ET or within 7 days of a positive pregnancy test. This would suggest that this is a likely to be the crucial period of the immunological modulation by intralipid. Whether a single dose given during this window will result in similar results is unknown.
Results from our findings and those of most others differed from a few others that showed no difference. In the study by Martin et al [
34] in which the intralipid was administered 7-10 days before embryo transfer or insemination, and again at 6 weeks and at 10 weeks, there were no differences in pregnancy rates when compared to a historical control. Han et al. [
26] suggested that this might have been because of the use of a historical for control. Another study that failed to show any difference was in women aged 40-42 years in whom intralipid was administered in the mid-follicular phase [
35] but the numbers were very small. A randomized controlled trial by Al-Zebeidi et al. [
30] found no significant difference in pregnancy rates with intralipid administration in women without specific immune dysfunctions. These discrepancies emphasize the importance of patient selection and the need for standardized protocols in both diagnostic immunological profiling and timing of intervention.
Table 5 shows most of the studies that have explored the benefit of intralipids in recurrent unexplained implantation failure.
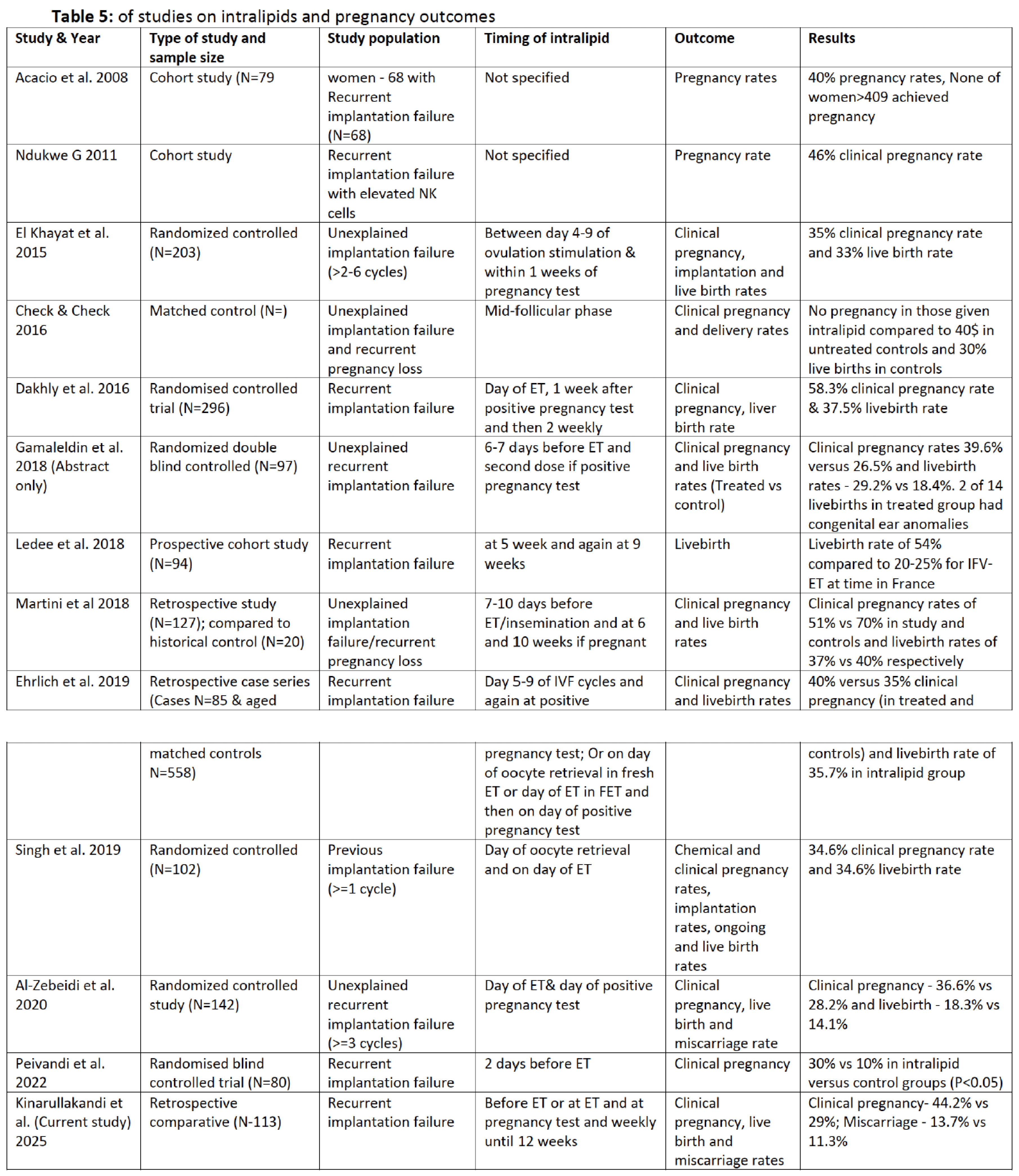
One major weakness in the published studies and ours is failure to exclude other possible contributing immunological factors to the recurrent implantation failure or quantify peripheral and uterine NK cells. We would therefore state that while the evidence for intralipids making a difference in pregnancy rates is reasonably strong, the precise mechanism of action is yet to be elucidated and may be variable including immunological modulation. Some studies that have investigated NK cell activities following infusion of intralipids have shown changes in toxicity perhaps demonstrating modulation. Since the role of peripheral NK cells (which have been shown to be different in origin, function and phenotype from uterine NK cells) in repeated miscarriages and implantation failure remains controversial [
36,
37,
38,
39] we would suggest that there may be other yet to be explored contributing modalities by which this intervention improves pregnancy outcomes.
There were no serious recorded side effects in our cases and no reported congenital abnormalities. Minor adverse effects that have been reported include flushing, headaches, drowsiness, vomiting, sweating, dizziness [
40]. This has been a consistent observation in the various studies although the study by Gamaleldin et al. [
31] reported 2 cases of congenital ear abnormalities. These findings have not been reported in any other study. While it may be safe to conclude from the reported cases that intralipid is not teratogenic and that the reported ear abnormalities may be incidental there is a need to continue to monitor this aspect of intralipids.
5. Conclusions
In this retrospective study we have shown that intralipids administration is associated with an increase in pregnancy rates in women who have had recurrent implantation failure, consistent with most other studies. There is still the need to undertake randomised controlled trials with large numbers to provide more definite evidence and furthermore try to unravel the precise mechanism by which intralipids improve IVF-ET outcomes.
Strengths and Limitations
The study’s retrospective nature limits causal inference and introduces potential bias in data interpretation. The small sample size (113 women) reduces statistical power and generalizability of the findings. The variation in intralipid dosing and timing, lack of standardized NK cell testing, and absence of long-term follow-up on live birth outcomes constrain definitive conclusions.
Nevertheless, our findings support existing evidence suggesting that intralipid may be beneficial in selected patient populations, particularly those with immune-related infertility challenges. Larger, well-powered, randomized controlled trials with standardized protocols are essential to validate these results and to determine optimal patient selection criteria and treatment timing.
The timing of initiation of intralipids was variable - from pre-ET to post ET. Furthermore, we did not have a control group. An ideal case control study would have generated more robust evidence. An ideal control group would have been one in which there had been recurrent implantation failure - matched for age and other factors who did not receive intralipids.
Author Contributions
JCK and OOS conceived the study; SKA collected the data; SKA and JCK analyzed the data; SKA wrote the initial draft which was then reviewed by JCK and then by OOS, AO, MA and BA. All the author approved the final version.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Feto Maternal Centre Institutional Review Board FMC-IRB-004 of 12th March 2024.
Informed Consent Statement
Patient consent was waived due to this being a retrospective study the involved reviewing patient records anonymously.
Data Availability Statement
The data are not publicly available due to the confidentiality of ART procedures but will be shared on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liang, Y.; Huang, J.; Zhao, Q.; Mo, H.; Su, Z.; Feng, S.; Li, S.; Ruan, X. Global, regional, and national prevalence and trends of infertility among individuals of reproductive age (15–49 years) from 1990 to 2021, with projections to 2040. Hum. Reprod. 2025, 40, 529–544. [Google Scholar] [CrossRef] [PubMed]
- IVF Success rates. February 2025. Available online: https://www.cdc.gov/art/success-rates/index.html (accessed on 26 August 2025).
- The current status of IVF: are we putting the needs of the individual first? eClinicalMedicine, Volume 65, 102343.
- Donoso, P.; Staessen, C.; Fauser, B.; Devroey, P. Current value of preimplantation genetic aneuploidy screening in IVF. Hum. Reprod. Updat. 2006, 13, 15–25. [Google Scholar] [CrossRef]
- Yazdani, N.; Khaniani, M.S.; Bastami, M.; Ghasemnejad, T.; Afkhami, F.; Derakhshan, S.M. HLA-G regulatory variants and haplotypes with susceptibility to recurrent pregnancy loss. Int. J. Immunogenetics 2018, 45, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, H.; Popovic-Todorovic, B.; Papanikolaou, E.; Donoso, P.; Devroey, P. An update of luteal phase support in stimulated IVF cycles. Hum. Reprod. Updat. 2007, 13, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Boroujerdnia, M.G.; Nikbakht, R.; Khodadadi, A. Enhancement of peripheral blood CD56dim cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J. Reprod. Immunol. 2012, 95, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30:473-83.
- Zhu, L.; Chen, X.; Xu, Z.; Xu, L.; Mao, T.; Zhang, H. Changes and clinical significance of peripheral blood helper T lymphocyte and natural killer (NK) cells in unexplained recurrent spontaneous abortion (URSA) patients after abortion and successful pregnancy. Clin. Exp. Obstet. Gynecol. 2015, 42, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lédée, N.; Vasseur, C.; Petitbarat, M.; Chevrier, L.; Vezmar, K.; Dray, G.; Chenière, S.; Lobersztajn, A.; Vitoux, D.; Cassuto, G.N.; et al. Intralipid® may represent a new hope for patients with reproductive failures and simultaneously an over-immune endometrial activation. J. Reprod. Immunol. 2018, 130, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Yamaya, A.; Fukui, A.; Kawai, K.; Yano, M.; Honda, H.; Nakagawa, K.; Kamei, H.; Omote, M.; Wakimoto, Y.; Mabuchi, S. A Comparative Study of Intravenous Immunoglobulin and Lipid Emulsion in Patients With Reproductive Failures Associated With NK Cell Abnormalities. Reprod. Med. Biol. 2025, 24, e12662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santillán, I.; Lozano, I.; Illán, J.; Verdú, V.; Coca, S.; Bajo-Arenas, J.M.; Martinez, F. Where and when should natural killer cells be tested in women with repeated implantation failure? J. Reprod. Immunol. 2015, 108, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.W.; Alfirevic, Z.; Quenby, S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum. Reprod. 2011, 26, 1971–1980. [Google Scholar] [CrossRef]
- Meng, L.; Lin, J.; Chen, L.; Wang, Z.; Liu, M.; Liu, Y.; Chen, X.; Zhu, L.; Chen, H.; Zhang, J. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2015, 294, 29–39. [Google Scholar] [CrossRef]
- Kolanska, K.; Suner, L.; Cohen, J.; Ben Kraiem, Y.; Placais, L.; Fain, O.; Bornes, M.; Selleret, L.; Delhommeau, F.; Feger, F.; et al. Proportion of Cytotoxic Peripheral Blood Natural Killer Cells and T-Cell Large Granular Lymphocytes in Recurrent Miscarriage and Repeated Implantation Failure: Case–Control Study and Meta-analysis. Arch. Immunol. et Ther. Exp. 2019, 67, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Roussev RG, Acacio B, Kaider BD, Jackson E, Coulam CB. Duration of intralipid’s suppressive effect on NK cell functional activity. Am J Reprod Immunol. 2008, 60, 258–263. [CrossRef]
- Coulam, C.B.; Acacio, B. Does Immunotherapy for Treatment of Reproductive Failure Enhance Live Births? Am. J. Reprod. Immunol. 2012, 67, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Moraru, M.; Carbone, J.; Alecsandru, D.; Castillo-Rama, M.; García-Segovia, A.; Gil, J.; Alonso, B.; Aguarón, A.; Ramos-Medina, R.; de María, J.M.; et al. Intravenous Immunoglobulin Treatment Increased Live Birth Rate in a Spanish Cohort of Women with Recurrent Reproductive Failure and Expanded CD56+ Cells. Am. J. Reprod. Immunol. 2012, 68, 75–84. [Google Scholar] [CrossRef]
- Robertson, S.A.; Jin, M.; Yu, D.; Moldenhauer, L.M.; Davies, M.J.; Hull, M.L.; Norman, R.J. Corticosteroid therapy in assisted reproduction – immune suppression is a faulty premise. Hum. Reprod. 2016, 31, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Acacio, B.; Coulam, C.; Rinehart, J.; Rinehart, L.; Ng, S.; Roussev, R.; Parrett, S. Pregnancy Outcome After Intralipid Infusion Among Women Experiencing Recurrent Pregnancy Loss. Fertil. Steril. 2008, 89, S11–S11. [Google Scholar] [CrossRef]
- Ndukwe, G. Recurrent embryo implantation failure after in-vitro fertilisation : improved outcome following intralipid infusion in women with elevated T Helper r response. Hum Fertil (Camb);2-11 14(2)1-88 (Abstract).
- Granato, D.; Blum, S.; Rössle, C.; Le Boucher, J.; Malnoë, A.; Dutot, G. Effects of Parenteral Lipid Emulsions With Different Fatty Acid Composition on Immune Cell Functions In Vitro. J. Parenter. Enter. Nutr. 2000, 24, 113–118. [Google Scholar] [CrossRef]
- Singh, N.; Davis, A.A.; Kumar, S.; Kriplani, A. The effect of administration of intravenous intralipid on pregnancy outcomes in women with implantation failure after IVF/ICSI with non-donor oocytes: A randomised controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 45–51. [Google Scholar] [CrossRef]
- Henshaw, J.; Tremellen, K. Intralipid infusion therapy as an adjunct treatment in women experiencing adenomyosis-related infertility. SAGE Journals 2023. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, H.N.; Kim, M.K.; Lyu, S.W.; Lee, W.S. Efficacy of intralipid administration to improve in vitro fertilization outcomes: A systematic review and meta-analysis. Clin. Exp. Reprod. Med. 2021, 48, 203–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marchand, G.J.; Masoud, A.T.; Ulibarri, H.; Arroyo, A.; Coriell, C.; Goetz, S.; Moir, C.; Moberly, A.; Gonzalez, D.; Blanco, M.; et al. Effect of a 20% intravenous fat emulsion therapy on pregnancy outcomes in women with RPL or RIF undergoing IVF/ICSI: a systematic review and meta-analysis. . 2023, 9, 236–245. [Google Scholar] [PubMed] [PubMed Central]
- Roussev, R.G.; Ng, S.C.; Coulam, C.B. Natural Killer Cell Functional Activity Suppression By Intravenous Immunoglobulin, Intralipid and Soluble Human Leukocyte Antigen-G. Am. J. Reprod. Immunol. 2007, 57, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Asif S, Al-Ahwany H, Chittawar PB, Nigdelis MP, Toulis KA, Goulis DG, et al. Immune therapies for women with history of unsuccessful implantation undergoing IVF/ICSI treatment: a Cochrane collaboration systematic review. Hum Reprod. 2018, 33, 85.
- Al-Zebeidi, J.; Agdi, M.; Lary, S.; Al-Obaid, S.; Salim, G.; Al-Jaroudi, D. Effect of empiric intravenous intralipid therapy on pregnancy outcome in women with unexplained recurrent implantation failure undergoing intracytoplasmic sperm injection-embryo transfer cycle: a randomized controlled trial. Gynecol. Endocrinol. 2019, 36, 131–134. [Google Scholar] [CrossRef]
- Gamaleldin I, Gomaa MF, Shafik A, Akande V. Intralipid infusion does not improve live birth rates in women with unexplained recurrent implantation failure and may increase the risk of congenital malformations, a double-blinded randomised controlled trial. BJOG 2018, 125, 31–32.
- Dakhly, D.M.; Bayoumi, Y.A.; Sharkawy, M.; Allah, S.H.G.; Hassan, M.A.; Gouda, H.M.; Hashem, A.T.; Hatem, D.L.; Ahmed, M.F.; El-Khayat, W. Intralipid supplementation in women with recurrent spontaneous abortion and elevated levels of natural killer cells. Int. J. Gynecol. Obstet. 2016, 135, 324–327. [Google Scholar] [CrossRef] [PubMed]
- El-Khayat, W.; El Sadek, M. Intralipid for repeated implantation failure (RIF): a randomized controlled trial. Fertil. Steril. 2015, 104, e26. [Google Scholar] [CrossRef]
- Martini, A.; Jasulaitis, S.; Fogg, L.F.; Uhler, M.L.; Hirshfeld-Cytron, J. Evaluating the utility of intralipid infusion to improve live birth rates in patients with recurrent pregnancy loss or recurrent implantation failure. J. Hum. Reprod. Sci. 2018, 11, 261–268. [Google Scholar] [CrossRef]
- Check, J.; Check, D. Intravenous intralipid therapy is not beneficial in having a live delivery in women aged 40-42 years with a previous history of miscarriage or failure to conceive despite embryo transfer undergoing in vitro fertilization-embryo transfer. Clin. Exp. Obstet. Gynecol. 2016, 43, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Manaster, I.; Mandelboim, O. The Unique Properties of Uterine NK Cells. Am. J. Reprod. Immunol. 2010, 63, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Manaster, I.; Mandelboim, O. The Unique Properties of Human NK Cells in the Uterine Mucosa. Placenta 2008, 29, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human Circulating and Tissue-Resident CD56bright Natural Killer Cell Populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feyaerts, D.; Kuret, T.; van Cranenbroek, B.; van der Zeeuw-Hingrez, S.; van der Heijden, O.W.H.; van der Meer, A.; Joosten, I.; van der Molen, R.G. Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire. Hum. Reprod. 2018, 33, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, R.; Hull, M.L.; Walkley, J.; Sacks, G. Intralipid Immunotherapy for Repeated IVF Failure. Fertil. Reprod. 2019, 01, 154–160. [Google Scholar] [CrossRef]
- Peivandi S, Mortazavi L, Gordani N, Zamaniyan M, Asgarian-Omran H, Ajami A et al. Effect of Intralipid Infusion on Pregnancy Outcome in Infertile Women with History of Implantation Failure: A Single Blind Randomized Clinical Trial. J Mazandaran Univ Med Sci 2022, 32, 16–26. Available online: http://jmums.mazums.ac.ir/article-1-17611-en.html.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).