Submitted:
01 April 2025
Posted:
02 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Preparation of the Conditioned Medium
2.4. Insemination and Treatment
2.5. Analysis of LVF
2.6. Statistical Analysis
3. Results
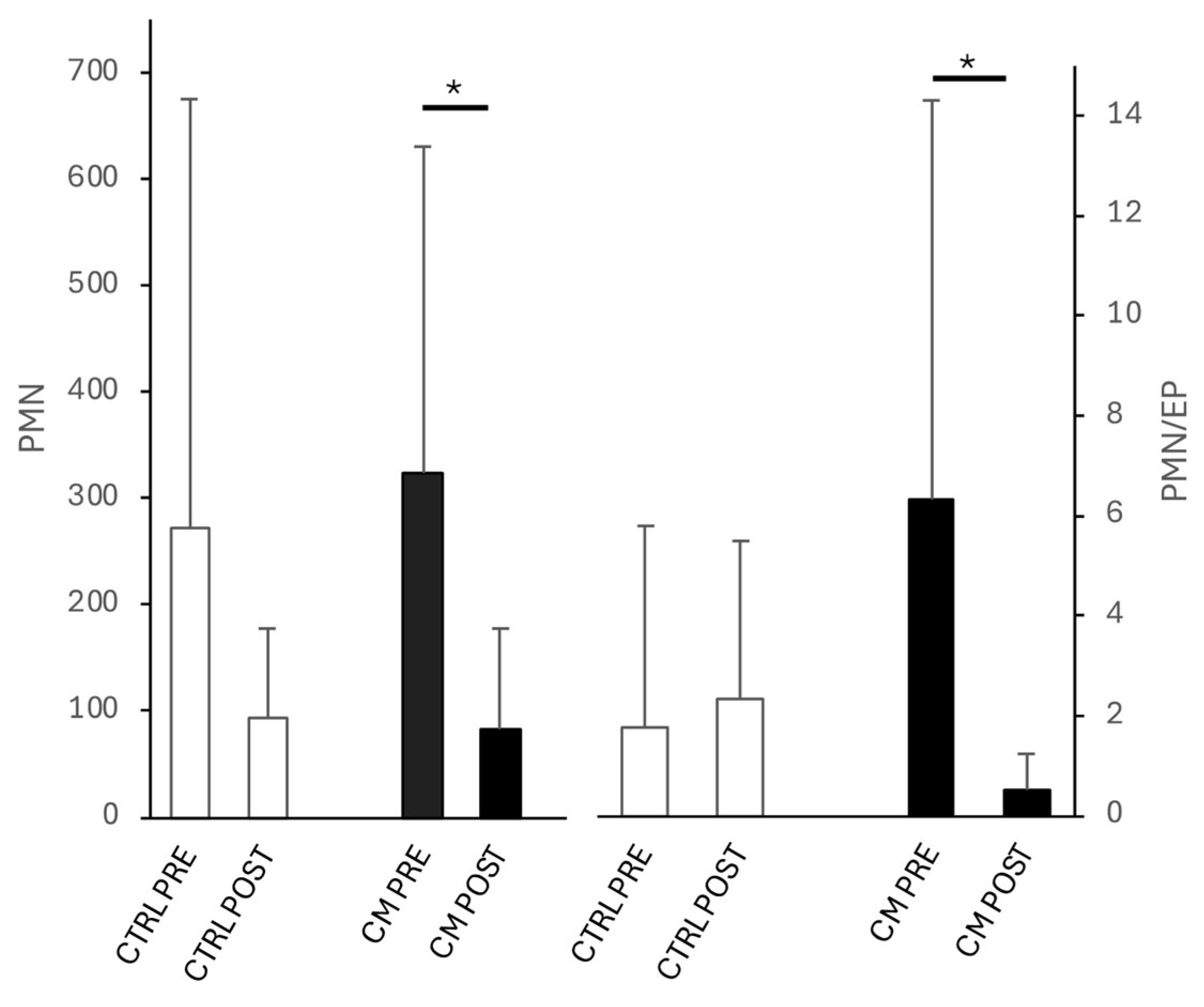
3.1. Clinical Finding
3.2. Bacterial Isolation and Identification
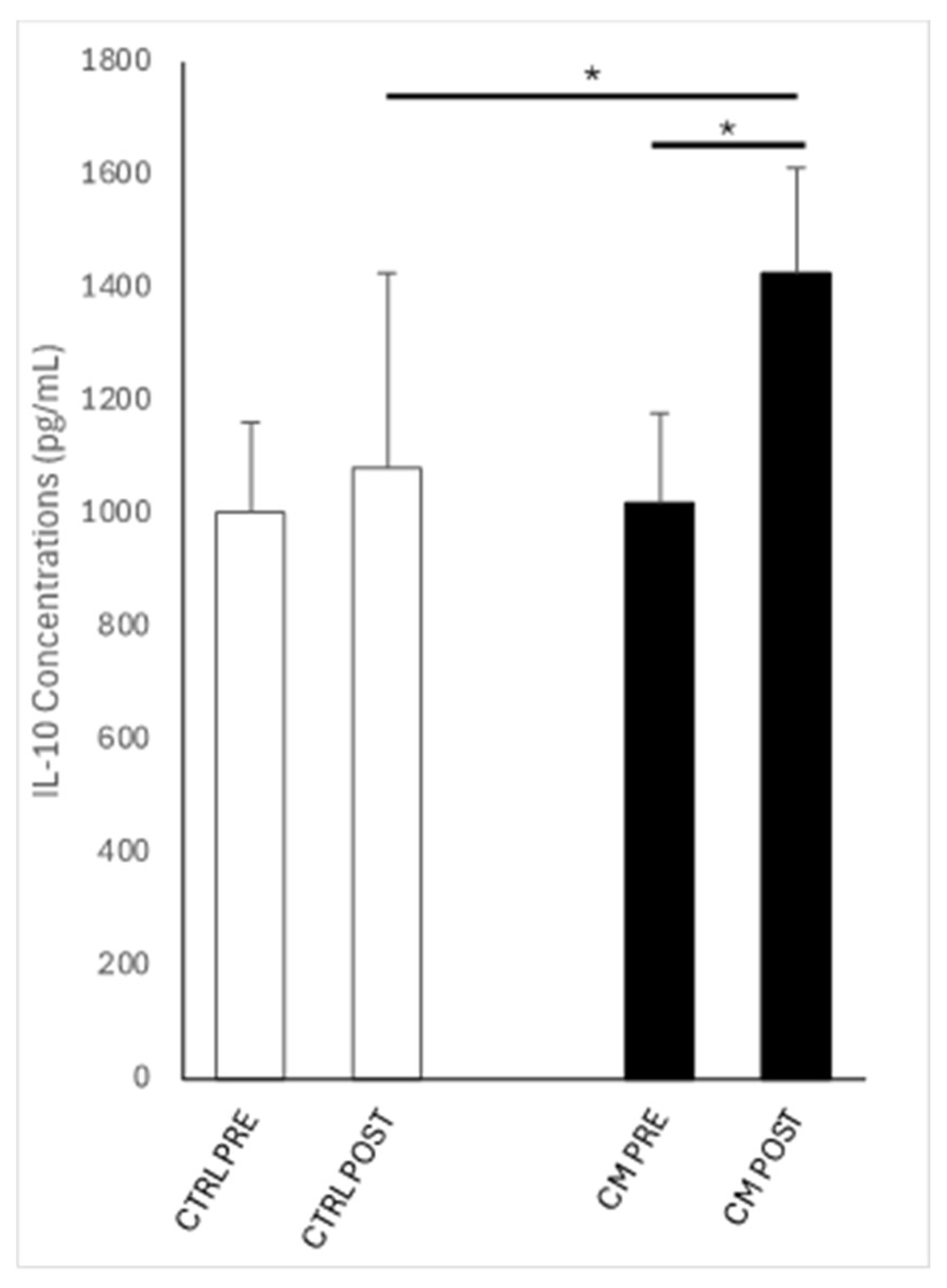
3.3. IL-10 Concentrations
3.4. Pregnancy Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | Conditioned medium |
| CTR | Control group |
| DMEM | Dulbecco’s Minimum Essential Medium |
| DPBS | Dulbecco’s phosphate buffered saline |
| E. coli | Escherichia Coli |
| EP | Epithelial cells |
| HPF | High power field |
| IL-10 | Interleukin-10 |
| PMN | Polimorphonuclear neutrophils |
| POST | After treatment |
| PRE | Before treatment |
| TRT | Treated group |
| UC | Umbilical cord |
| WJ-MSC-CM | Wharton’s jelly mesenchymal stromal/stem cell-derived medium |
References
- Troedsson, M.H.T. Uterine Clearance and Resistance to Persistent Endometritis in the Mare. Theriogenology 1999, 52, 461–471. [CrossRef]
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical Problems of Adult Horses, as Ranked by Equine Practitioners. J Am Vet Med Assoc 1991, 198, 1745–1747. [CrossRef]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares - a Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int J Mol Sci 2020, 21. [CrossRef]
- Alghamdi, A.S.; Foster, D.N.; Carlson, C.S.; Troedsson, M.H.T. Nitric Oxide Levels and Nitric Oxide Synthase Expression in Uterine Samples from Mares Susceptible and Resistant to Persistent Breeding-Induced Endometritis. American Journal of Reproductive Immunology 2005, 53, 230–237. [CrossRef]
- Katila, T. Onset and Duration of Uterine Inflammatory Response of Mares after Insemination with Fresh Semen. Biol Reprod 1995, 52, 515–517. [CrossRef]
- Troedsson, M.H.T.; Liu, I.K.M.; Crabo, B.G. Sperm Transport and Survival in the Mare. Theriogenology 1998, 49, 905–915. [CrossRef]
- Troedsson, M.H.T. Therapeutic Considerations for Mating-Induced Endometritis. Pferdeheilkunde Equine Medicine 1997, 13, 516–520. [CrossRef]
- Troedsson, M.H.; Liu, I.K. Uterine Clearance of Non-Antigenic Markers (51Cr) in Response to a Bacterial Challenge in Mares Potentially Susceptible and Resistant to Chronic Uterine Infections. J Reprod Fertil Suppl 1991, 44, 283–288.
- Carnevale, E.M.; Ramirez, R.J.; Squires, E.L.; Alvarenga, M.A.; Vanderwall, D.K.; McCue, P.M. Factors Affecting Pregnancy Rates and Early Embryonic Death after Equine Embryo Transfer. Theriogenology 2000, 54, 965–979. [CrossRef]
- Bucca, S.; Carli, A.; Buckley, T.; Dolci, G.; Fogarty, U. The Use of Dexamethasone Administered to Mares at Breeding Time in the Modulation of Persistent Mating Induced Endometritis. Theriogenology 2008, 70, 1093–1100. [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.T. Endometrial Inflammatory Markers of the Early Immune Response in Mares Susceptible or Resistant to Persistent Breeding-Induced Endometritis. Reproduction 2013, 145, 289–296. [CrossRef]
- Rota, A.; Furzi, C.; Panzani, D.; Camillo, F. Studies on Motility and Fertility of Cooled Stallion Spermatozoa. Reproduction in Domestic Animals 2004, 39, 103–109. [CrossRef]
- Riddle, W.T.; LeBlanc, M.M.; Stromberg, A.J. Relationships between Uterine Culture, Cytology and Pregnancy Rates in a Thoroughbred Practice. Theriogenology 2007, 68, 395–402. [CrossRef]
- Kareskoski, M.; Venhoranta, H.; Virtala, A.M.; Katila, T. Analysis of Factors Affecting the Pregnancy Rate of Mares after Inseminations with Cooled Transported Stallion Semen. Theriogenology 2019, 127, 7–14. [CrossRef]
- Freeman, D.A.; Weber, J.A.; Geary, R.T.; Woods, G.L. Time of Embryo Transport through the Mare Oviduct. Theriogenology 1991, 36, 823–830. [CrossRef]
- Christoffersen, M.; Woodward, E.M.; Bojesen, A.M.; Petersen, M.R.; Squires, E.L.; Lehn-Jensen, H.; Troedsson, M.H.T. Effect of Immunomodulatory Therapy on the Endometrial Inflammatory Response to Induced Infectious Endometritis in Susceptible Mares. Theriogenology 2012, 78, 991–1004. [CrossRef]
- Friso, A.M.; Segabinazzi, L.G.T.M.; Cyrino, M.; Correal, S.B.; Freitas-Dell’Aqua, C.P.; Teoro do Carmo, M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Periovulatory Administration of Firocoxib Did Not Alter Ovulation Rates and Mitigated Post-Breeding Inflammatory Response in Mares. Theriogenology 2019, 138, 24–30. [CrossRef]
- Leblanc, M.; Causey, R. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reproduction in Domestic Animals 2009, 44, 10–22. [CrossRef]
- Scoggin, C.F. Endometritis: Nontraditional Therapies. Veterinary Clinics of North America - Equine Practice 2016, 32, 499–511. [CrossRef]
- Troedsson, M.H.T.; Nielsen, J.M. Non-Antibiotic Treatment of Equine Endometritis. Pferdeheilkunde 2018, 34, 17–22. [CrossRef]
- Del Prete, C.; Montano, C.; Cocchia, N.; de Chiara, M.; Gasparrini, B.; Pasolini, M.P. Use of Regenerative Medicine in the Treatment of Endometritis in Mares: A Systematic Review and Meta-Analysis. Theriogenology 2024, 227, 9–20. [CrossRef]
- Caplan, A.I. What’s in a Name? Tissue Eng Part A 2010, 16, 2415–2417. [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [CrossRef]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Influence of Adult Mesenchymal Stem Cells on In Vitro Vascular Formation. https://home.liebertpub.com/tea 2009, 15, 1751–1761. [CrossRef]
- Mambelli, L.I.; Mattos, R.C.; Winter, G.H.Z.; Madeiro, D.S.; Morais, B.P.; Malschitzky, E.; Miglino, M.A.; Kerkis, A.; Kerkis, I. Changes in Expression Pattern of Selected Endometrial Proteins Following Mesenchymal Stem Cells Infusion in Mares with Endometrosis. PLoS One 2014, 9, e97889. [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different Populations and Sources of Human Mesenchymal Stem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Communication and Signaling 2011, 9, 1–14. [CrossRef]
- Marx, C.; Gardner, S.; Harman, R.M.; Van de Walle, G.R. The Mesenchymal Stromal Cell Secretome Impairs Methicillin-Resistant Staphylococcus Aureus Biofilms via Cysteine Protease Activity in the Equine Model. Stem Cells Transl Med 2020, 9, 746–757. [CrossRef]
- Silva-Carvalho, A.É.; Cardoso, M.H.; Alencar-Silva, T.; Bogéa, G.M.R.; Carvalho, J.L.; Franco, O.L.; Saldanha-Araujo, F. Dissecting the Relationship between Antimicrobial Peptides and Mesenchymal Stem Cells. Pharmacol Ther 2022, 233, 108021. [CrossRef]
- Hosseiniyan Khatibi, S.M.; Kheyrolahzadeh, K.; Barzegari, A.; Rahbar Saadat, Y.; Zununi Vahed, S. Medicinal Signaling Cells: A Potential Antimicrobial Drug Store. J Cell Physiol 2020, 235, 7731–7746. [CrossRef]
- Cai, Y.; Li, J.; Jia, C.; He, Y.; Deng, C. Therapeutic Applications of Adipose Cell-Free Derivatives: A Review. Stem Cell Res Ther 2020, 11, 1–16. [CrossRef]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici; Bülle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but Not Murine Multipotent Mesenchymal Stromal Cells Exhibit Broad-Spectrum Antimicrobial Effector Function Mediated by Indoleamine 2,3-Dioxygenase. Leukemia 2011 25:4 2011, 25, 648–654. [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front Immunol 2017, 8, 248157. [CrossRef]
- Cortés-Araya, Y.; Amilon, K.; Rink, B.E.; Black, G.; Lisowski, Z.; Donadeu, F.X.; Esteves, C.L. Comparison of Antibacterial and Immunological Properties of Mesenchymal Stem/Stromal Cells from Equine Bone Marrow, Endometrium, and Adipose Tissue. Stem Cells Dev 2018, 27, 1518–1525. [CrossRef]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res Int 2014, 2014, 965849. [CrossRef]
- Kim, H.O.; Choi, S.M.; Kim, H.S. Mesenchymal Stem Cell-Derived Secretome and Microvesicles as a Cell-Free Therapeutics for Neurodegenerative Disorders. Tissue Eng Regen Med 2013, 10, 93–101. [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular Mechanisms Responsible for Anti-Inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv Exp Med Biol 2019, 1084, 187–206. [CrossRef]
- Lanci, A.; Merlo, B.; Grandis, A.; Mariella, J.; Castagnetti, C.; Iacono, E. Gross and Histological Examination of Wharton’s Jelly in the Equine Umbilical Cord. Theriogenology 2023, 209, 184–192. [CrossRef]
- Iacono, E.; Brunori, L.; Pirrone, A.; Pagliaro, P.P.; Ricci, F.; Tazzari, P.L.; Merlo, B. Isolation, Characterization and Differentiation of Mesenchymal Stem Cells from Amniotic Fluid, Umbilical Cord Blood and Wharton’s Jelly in the Horse. Reproduction 2012, 143, 455–468. [CrossRef]
- Merlo, B.; Pirondi, S.; Iacono, E.; Rossi, B.; Ricci, F.; Mari, G. Viability, in Vitro Differentiation and Molecular Characterization of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Cryopreserved in Serum and Serum-Free Medium.
- Samper, J.C. A Review of a Practitioner’s Perspective on Endometrial Edema. Pferdeheilkunde 2010, 26, 14–18. [CrossRef]
- Leblanc, M.M.; Act, D. How to Perform and Interpret Findings From a Low-Volume Uterine Flush. 2011.
- Nocera, F.P.; D’eletto, E.; Ambrosio, M.; Fiorito, F.; De Martino, L.; De Martino, L. Occurrence and Antimicrobial Susceptibility Profiles of Streptococcus Equi Subsp. Zooepidemicus Strains Isolated from Mares with Fertility Problems. Antibiotics (Basel) 2021, 11. [CrossRef]
- Cocchia, N.; Paciello, O.; Auletta, L.; Uccello, V.; Silvestro, L.; Mallardo, K.; Paraggio, G.; Pasolini, M.P. Comparison of the Cytobrush, Cottonswab, and Low-Volume Uterine Flush Techniques to Evaluate Endometrial Cytology for Diagnosing Endometritis in Chronically Infertile Mares. Theriogenology 2012, 77, 89–98. [CrossRef]
- Nekanti, U.; Mohanty, L.; Venugopal, P.; Balasubramanian, S.; Totey, S.; Ta, M. Optimization and Scale-up of Wharton’s Jelly-Derived Mesenchymal Stem Cells for Clinical Applications. Stem Cell Res 2010, 5, 244–254. [CrossRef]
- Higuchi, O.; Okabe, M.; Yoshida, T.; Fathy, M.; Saito, S.; Miyawaki, T.; Nikaido, T. Stemness of Human Wharton’s Jelly Mesenchymal Cells Is Maintained by Floating Cultivation. Cell Reprogram 2012, 14, 448–455. [CrossRef]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C. V. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. International Journal of Molecular Sciences 2013, Vol. 14, Pages 11692-11712 2013, 14, 11692–11712. [CrossRef]
- Mareschi, K.; Castiglia, S.; Sanavio, F.; Rustichelli, D.; Muraro, M.; Defedele, D.; Bergallo, M.; Fagioli, F. Immunoregulatory Effects on T Lymphocytes by Human Mesenchymal Stromal Cells Isolated from Bone Marrow, Amniotic Fluid, and Placenta. Exp Hematol 2016, 44, 138-150.e1. [CrossRef]
- Bárcia, R.N.; Santos, J.M.; Filipe, M.; Teixeira, M.; Martins, J.P.; Almeida, J.; Água-Doce, A.; Almeida, S.C.P.; Varela, A.; Pohl, S.; et al. What Makes Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Superior Immunomodulators When Compared to Bone Marrow Derived Mesenchymal Stromal Cells? Stem Cells Int 2015, 2015, 583984. [CrossRef]
- Fong, C.Y.; Gauthaman, K.; Cheyyatraivendran, S.; Lin, H.D.; Biswas, A.; Bongso, A. Human Umbilical Cord Wharton’s Jelly Stem Cells and Its Conditioned Medium Support Hematopoietic Stem Cell Expansion Ex Vivo. J Cell Biochem 2012, 113, 658–668. [CrossRef]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol 2020, 38, 1373–1384. [CrossRef]
- Chronopoulos, A.; Kalluri, R. Emerging Role of Bacterial Extracellular Vesicles in Cancer. Oncogene 2020 39:46 2020, 39, 6951–6960. [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology , Function , and Biomedical Applications of Exosomes. Science 2020, 367. [CrossRef]
- Hollinshead, F.K.; Hanlon, D.W.; Hou, W.; Tasma, Z.; Damani, T.; Bouma, G.J.; Murtazina, D.A.; Chamley, L. Use of Equine Embryo -Derived Mesenchymal Stromal Cells and Their Extracellular Vesicles as a Treatment for Persistent Breeding-Induced Endometritis in Susceptible Mares. J Equine Vet Sci 2024, 139, 105079. [CrossRef]
- Laroye, C.; Boufenzer, A.; Jolly, L.; Cunat, L.; Alauzet, C.; Merlin, J.L.; Yguel, C.; Bensoussan, D.; Reppel, L.; Gibot, S. Bone Marrow vs Wharton’s Jelly Mesenchymal Stem Cells in Experimental Sepsis: A Comparative Study. Stem Cell Res Ther 2019, 10. [CrossRef]
- Lange-Consiglio, A.; Funghi, F.; Cantile, C.; Idda, A.; Cremonesi, F.; Riccaboni, P. Case Report: Use of Amniotic Microvesicles for Regenerative Medicine Treatment of a Mare With Chronic Endometritis. Front Vet Sci 2020, 7, 529611. [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 2018, 156, 16–27. [CrossRef]
- Abdelnaby, E.A.; Abdallah, A.N.; Anwar, I.M.; El-Tookhy, O.S.; Shamaa, A.A. The Therapeutic Effect of Stem Cell-Derived Exosomes in the Treatment of Chronic Endometritis as Assessed by Histopathological, Doppler and Hormonal Expression in Arabian Mares. Equine Vet Educ 2024, 36, 347–356. [CrossRef]
- Cyktor, J.C.; Turner, J. Interleukin-10 and Immunity against Prokaryotic and Eukaryotic Intracellular Pathogens. Infect Immun 2011, 79, 2964–2973. [CrossRef]
- Ferris, R.A.; Frisbie, D.D.; McCue, P.M. Use of Mesenchymal Stem Cells or Autologous Conditioned Serum to Modulate the Inflammatory Response to Spermatozoa in Mares. Theriogenology 2014, 82, 36–42. [CrossRef]
- Lange-Consiglio, A.; Gaspari, G.; Funghi, F.; Capra, E.; Cretich, M.; Frigerio, R.; Bosi, G.; Cremonesi, F. Amniotic Mesenchymal-Derived Extracellular Vesicles and Their Role in the Prevention of Persistent Post-Breeding Induced Endometritis. Int J Mol Sci 2023, 24, 5166. [CrossRef]
- Tongu, E.A. de O.; Segabinazzi, L.G.T.M.; Alvarenga, M.L.; Monteiro, A.; Papa, F.O.; Alvarenga, M.A. Allogenic Mesenchymal Stem Cell-Conditioned Medium Does Not Affect Sperm Parameters and Mitigates Early Endometrial Inflammatory Responses in Mares. Theriogenology 2021, 169, 1–8. [CrossRef]
- Canisso, I.F.; Stewart, J.; Coutinho da Silva, M.A. Endometritis: Managing Persistent Post-Breeding Endometritis. Vet Clin North Am Equine Pract 2016, 32, 465–480. [CrossRef]
- Walter, J.; Neuberg, K.P.; Failing, K.; Wehrend, A. Cytological Diagnosis of Endometritis in the Mare: Investigations of Sampling Techniques and Relation to Bacteriological Results. Anim Reprod Sci 2012, 132, 178–186. [CrossRef]
- Beltaire, K.A.; Cheong, S.H.; Coutinho da Silva, M.A. Retrospective Study on Equine Uterine Fungal Isolates and Antifungal Susceptibility Patterns (1999-2011). Equine Vet J Suppl 2012, 44, 84–87. [CrossRef]
- Del Prete, C.; Nocera, F.P.; Piegari, G.; Palumbo, V.; De Martino, L.; Cocchia, N.; Paciello, O.; Montano, C.; Pasolini, M.P. Use of Cytobrush for Bacteriological and Cytological Diagnosis of Endometritis in Mares. Vet World 2024, 17, 398. [CrossRef]
- Albihn, A.; Båverud, V.; Magnusson, U. Uterine Microbiology and Antimicrobial Susceptibility in Isolated Bacteria from Mares with Fertility Problems. Acta Vet Scand 2003, 44, 121–129. [CrossRef]
- Frontoso, R.; De Carlo, E.; Pasolini, M.P.; van der Meulen, K.; Pagnini, U.; Iovane, G.; De Martino, L. Retrospective Study of Bacterial Isolates and Their Antimicrobial Susceptibilities in Equine Uteri during Fertility Problems. Res Vet Sci 2008, 84, 1–6. [CrossRef]
- Nocera, F.P.; Capozzi, L.; Simone, D.; Pizzano, F.; Iovane, V.; Bianco, A.; Parisi, A.; De Martino, L. Multi-Locus Sequence Typing and in Vitro Antimicrobial Resistance of Equine Streptococcus Equi Subspecies Zooepidemicus Strains. Vet Res Commun 2024, 48, 215–224. [CrossRef]
- Nocera, F.P.; Ambrosio, M.; Conte, A.; Di Palma, T.; Castaldo, S.; Pasolini, M.P.; Fiorito, F.; De Martino, L. Importance of Broth-Enrichment Culture in Equine Endometritis Diagnosis. New Microbiol 2021, 44, 19–23.
- Däubener, W.; Schmidt, S.K.; Heseler, K.; Spekker, K.H.; MacKenzie, C.R. Antimicrobial and Immunoregulatory Effector Mechanisms in Human Endothelial Cells Indoleamine 2,3-Dioxygenase versus Inducible Nitric Oxide Synthase. Thromb Haemost 2009, 102, 1110–1116. [CrossRef]
- Marx, C.; Gardner, S.; Harman, R.M.; Wagner, B.; Van de Walle, G.R. Mesenchymal Stromal Cell-Secreted CCL2 Promotes Antibacterial Defense Mechanisms through Increased Antimicrobial Peptide Expression in Keratinocytes. Stem Cells Transl Med 2021, 10, 1666–1679. [CrossRef]
| ID | Cycle | PRE | POST | Pregnancy |
|---|---|---|---|---|
| 1 | CTR |
Streptococcus equi subsp. zooepidemicus; Escherichia coli |
Streptococcus equi subsp. zooepidemicus; Escherichia coli | Y |
| 2 | Escherichia coli | Escherichia coli | N | |
| 3 | Streptococcus equinus; Escherichia coli | Streptococcus equinus; Escherichia coli | N | |
| 4 | Pseudomonas putida | Pseudomonas putida | N | |
| 5 | Staphylococcus aureus | Staphylococcus aureus | N | |
| 6 | Escherichia coli | Escherichia coli | N | |
| 7 | Enterococcus faecalis; Escherichia coli | Enterococcus faecalis; Escherichia coli | N | |
| 8 | Deftia tsurunatensis | Escherichia coli | N | |
| 1 | TRT |
Escherichia coli; Streptococcus equi subsp. zooepidemicus |
Escherichia coli; Streptococcus equi subsp. zooepidemicus |
N |
| 2 | Streptococcus equinus | Streptococcus equinus | Y | |
| 3 | Escherichia coli; Staphylococcus schleiferi | Escherichia coli; Staphylococcus schleiferi | Y | |
| 4 |
Escherichia coli; Enterococcus faecalis; Staphylococcus aureus |
Escherichia coli; Enterococcus faecalis; Staphylococcus aureus |
Y | |
| 5 | Staphylococcus aureus; Streptococcus dysgalactiae; Escherichia coli |
Staphylococcus aureus; Streptococcus dysgalactiae; Escherichia coli |
Y | |
| 6 |
Streptococcus dysgalactiae; Klebsiella pneumoniae |
Streptococcus dysgalactiae | N | |
| 7 | Pseudomonas spp. | Escherichia coli | Y | |
| 8 | - |
Escherichia coli; Streptococcus equi subsp zooepidemicus |
N | |
| 9 | - | Escherichia coli; | N | |
| 10 | - | - | Y | |
| 11 | - | - | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





