Submitted:
20 December 2023
Posted:
20 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
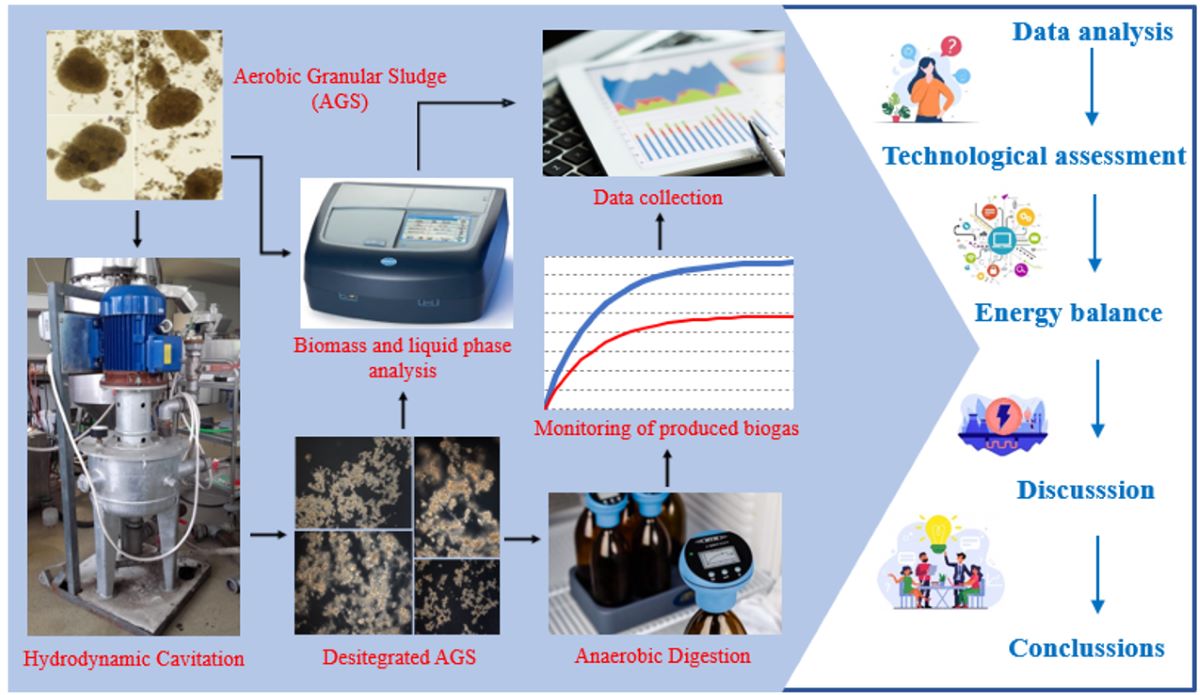
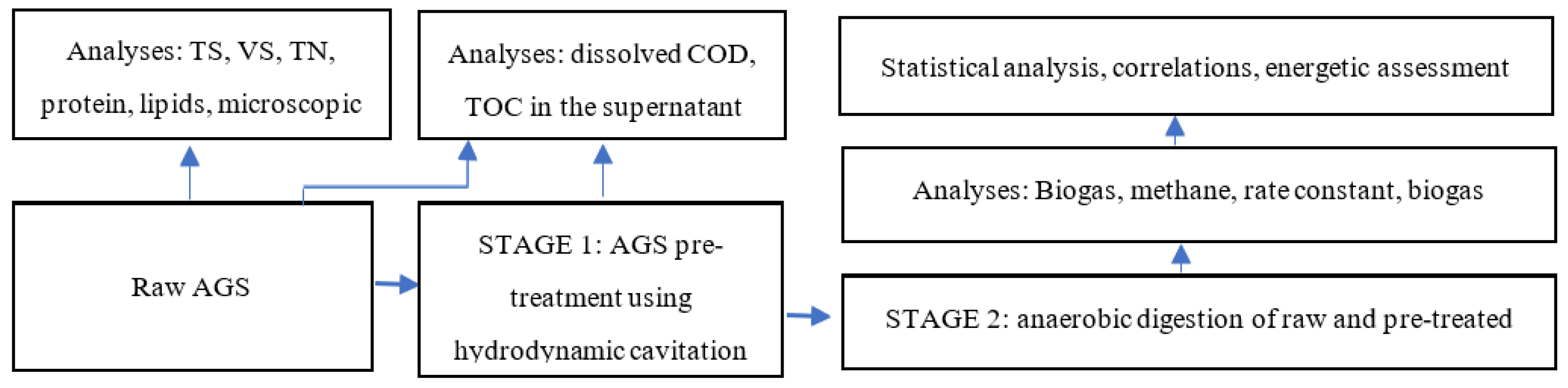
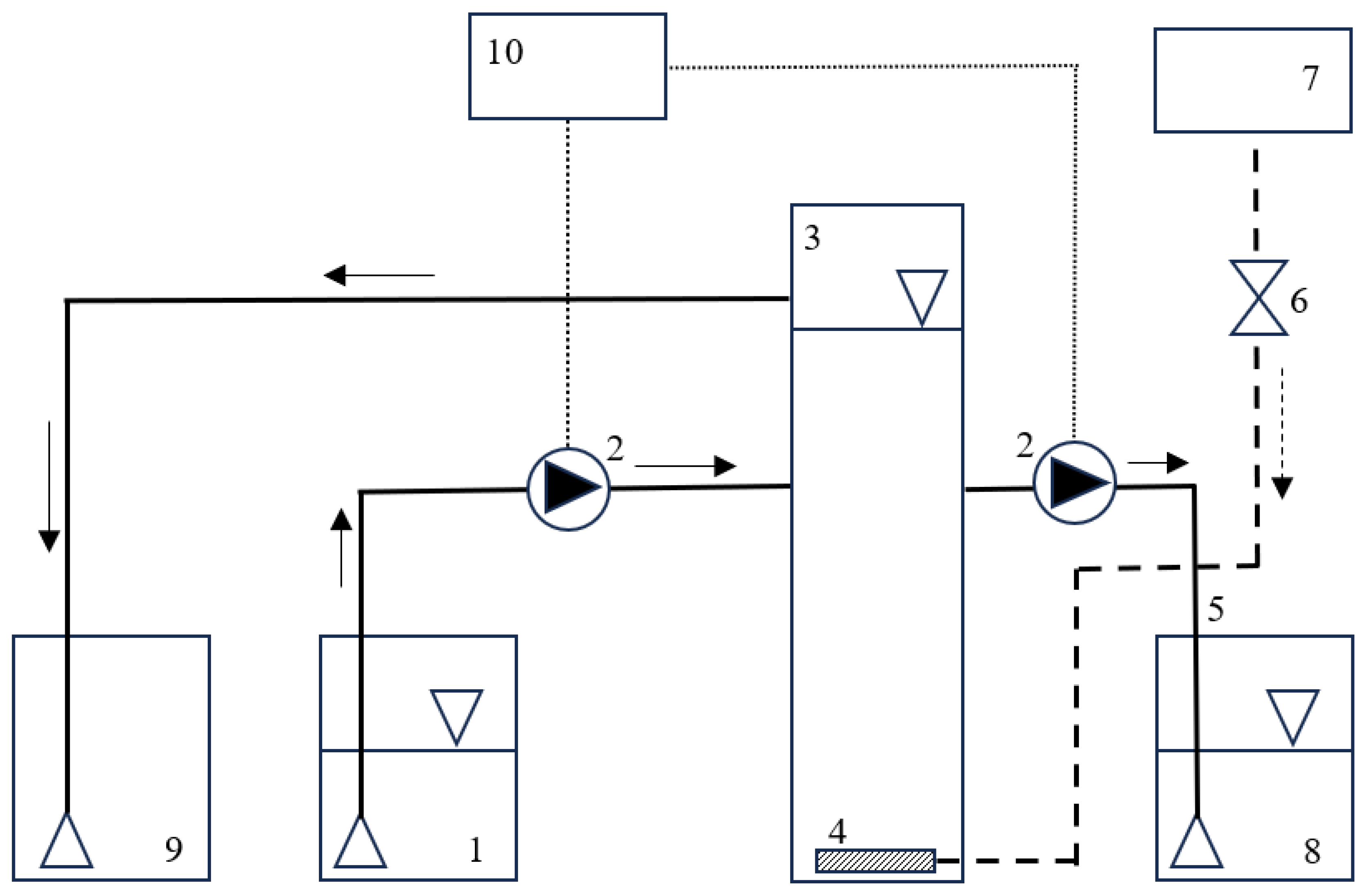
2.1. Organisation of the experiment
2.2. Materials
2.2.1. Aerobic granular sludge (AGS)
2.2.2. Inokulum of the anaerobic sludge (AS)
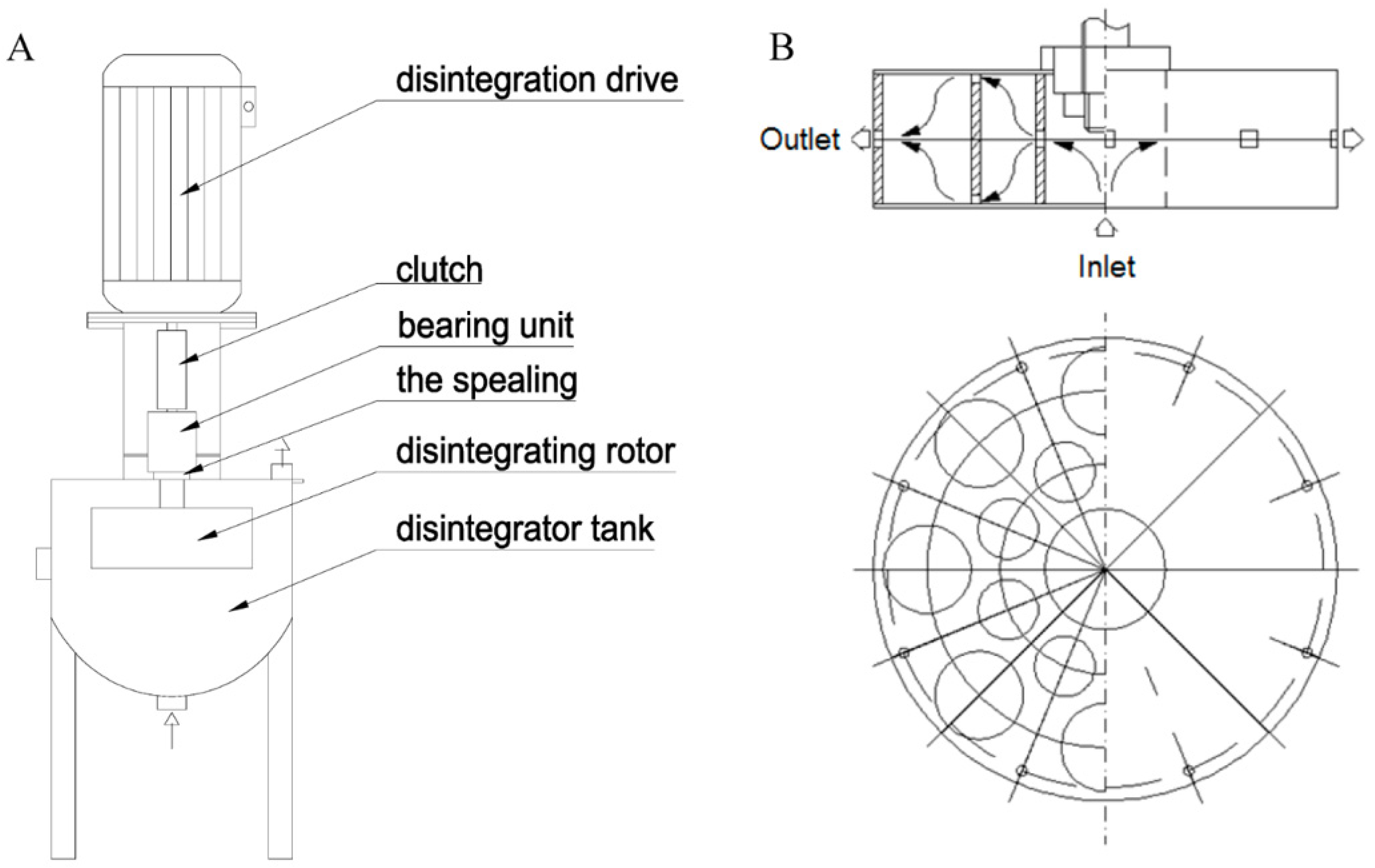
2.2.3. Hydrodynamic cavitation generator
2.2.4. Respirometric measurements
2.3. Calculation Methods
2.4. Analytical procedures
2.5. Statistical methods
3. Results and discussion
3.1. Aerobic granular sludge properties
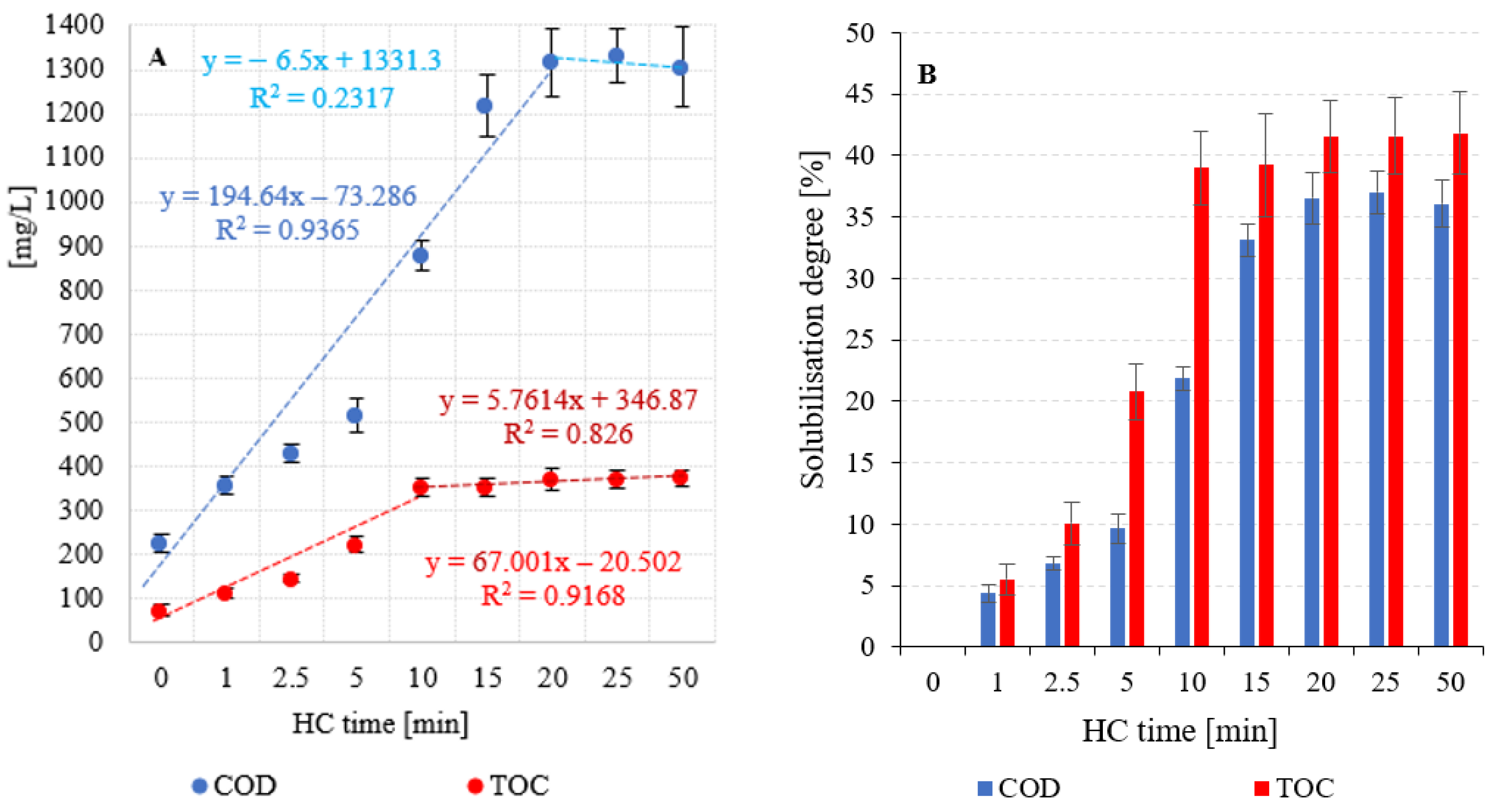
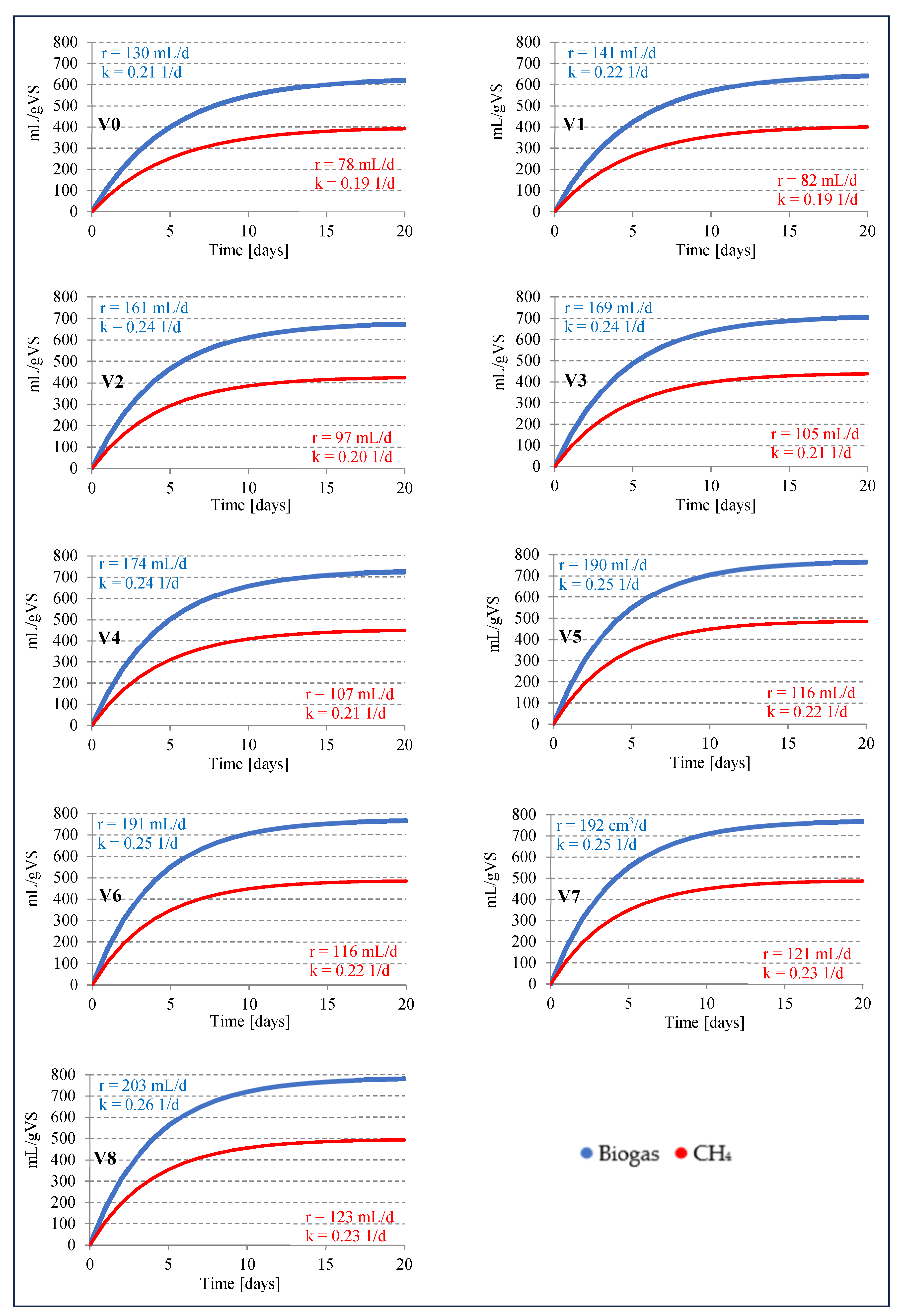
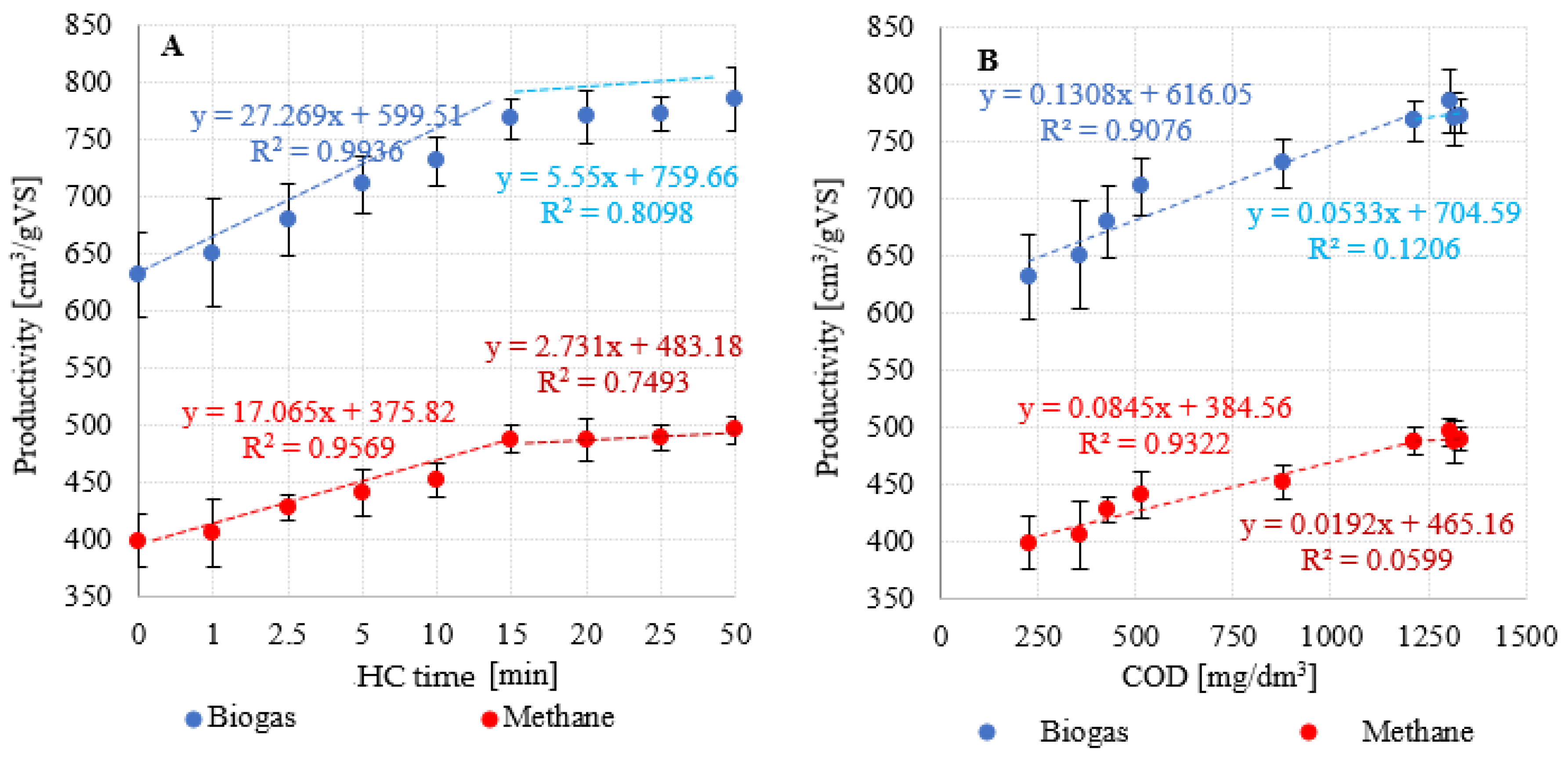
3.2. Efficiency and kinetics of anaerobic digestion
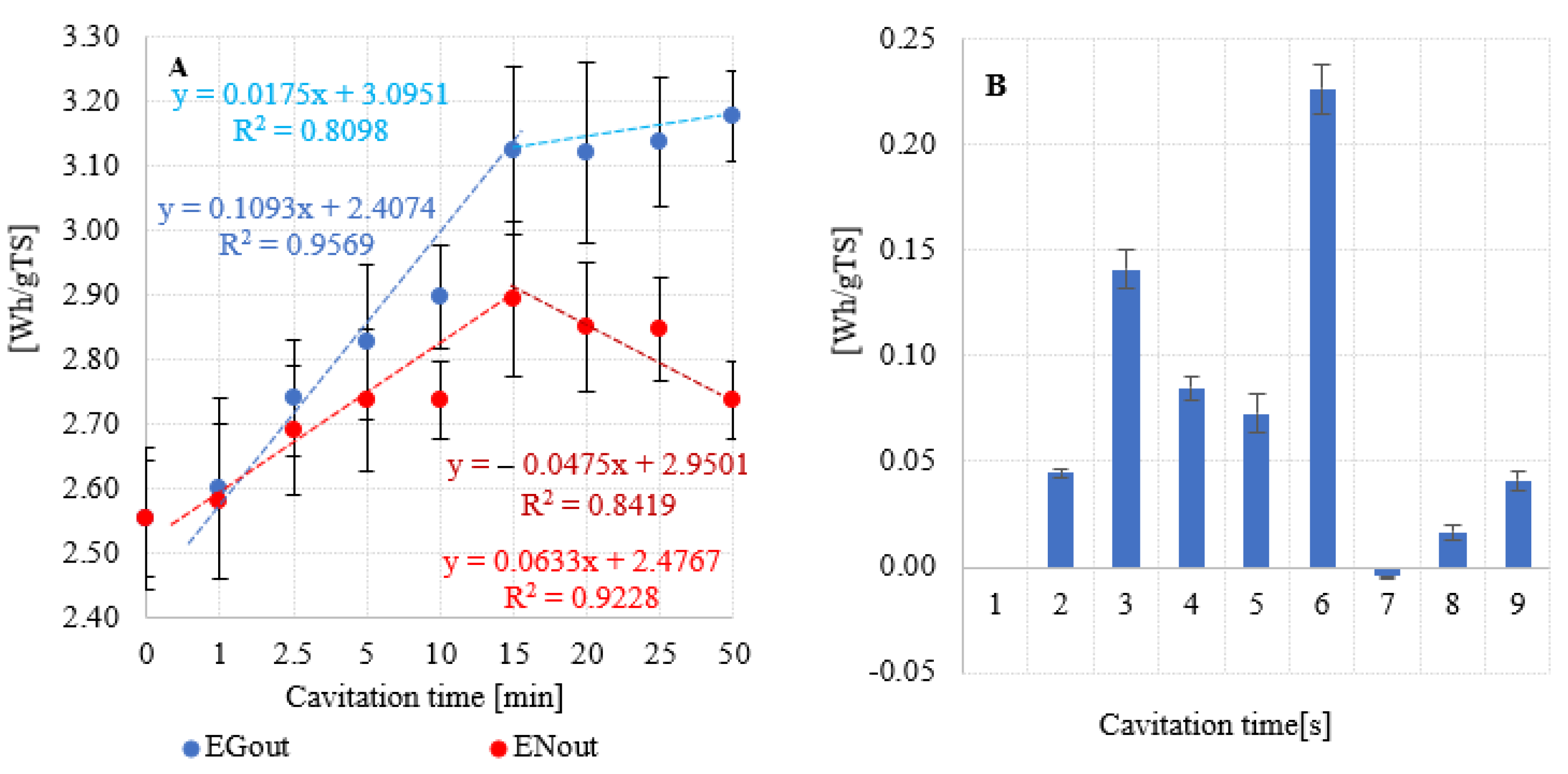
3.3. Energy balance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; van Lier, J. B.; de Kreuk, M. Digestibility of Waste Aerobic Granular Sludge from a Full-Scale Municipal Wastewater Treatment System. Water Res. 2020, 173, 115617. [CrossRef]
- Yeasmin, F.; Rasheduzzaman, M.; Manik, M.; Hasan, M. M. Activated Sludge Process for Wastewater Treatment. Adv. Innov. Approaches Environ. Biotechnol. Ind. Wastewater Treat. 2023, 23–50. [CrossRef]
- Nancharaiah, Y. V.; Sarvajith, M. Granular Stability, Nitrogen and Phosphorus Removal Pathways of Aerobic Granular Sludge Treating Real Municipal Wastewater at Different Temperatures. J. Environ. Chem. Eng. 2023, 11 (5), 110769. [CrossRef]
- Sharma, K. S.; Panchal, K.; Chhimwal, M.; Kumar, D. Integrated Detection and Natural Remediation Technology as a Low-Cost Alternative for Wastewater Treatment. Heal. Sci. Rev. 2023, 8, 100111. [CrossRef]
- Kazimierowicz, J.; Dębowski, M. Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives. Sustain. 2022, Vol. 14, Page 10904 2022, 14 (17), 10904. [CrossRef]
- Cheng, L.; Wei, M.; Guo, G.; Hu, Q.; Li, B.; Jiang, Y.; Hu, Z. Effects of Feeding Mode on the Formation and Stability of Aerobic Granular Sludge under Combined Antibiotic Stress. Chem. Eng. J. 2023, 475, 145996. [CrossRef]
- Zielinski, M.; Debowski, M.; Kazimierowicz, J. The Effect of Static Magnetic Field on Methanogenesis in the Anaerobic Digestion of Municipal Sewage Sludge. Energies 2021, Vol. 14, Page 590 2021, 14 (3), 590. [CrossRef]
- Hidaka, T.; Tsushima, I.; Tsumori, J. Comparative Analyses of Microbial Structures and Gene Copy Numbers in the Anaerobic Digestion of Various Types of Sewage Sludge. Bioresour. Technol. 2018, 253, 315–322. [CrossRef]
- Kosar, S.; Isik, O.; Akdag, Y.; Gulhan, H.; Koyuncu, I.; Ozgun, H.; Ersahin, M. E. Impact of Seed Sludge Characteristics on Granulation and Performance of Aerobic Granular Sludge Process. J. Clean. Prod. 2022, 363, 132424. [CrossRef]
- Dababat, S.; Berzio, S.; Wichern, M.; Lübken, M. Anaerobic Digestibility of Aerobic Granular Sludge from Continuous Flow Reactors: The Role of Granule Size Distribution. Water Sci. Technol. 2023, 87 (12), 3047–3058. [CrossRef]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Comparison of Ultrasonic and Hydrothermal Cavitation Pretreatments of Cattle Manure Mixed with Straw Wheat on Fermentative Biogas Production. Waste and Biomass Valorization 2019, 10 (4), 747–754.
- Yuan, Q.; Gong, H.; Xi, H.; Wang, K. Aerobic Granular Sludge Formation Based on Substrate Availability: Effects of Flow Pattern and Fermentation Pretreatment. Front. Environ. Sci. Eng. 2020, 14 (3), 1–10.
- Jeong, S. Y.; Chang, S. W.; Ngo, H. H.; Guo, W.; Nghiem, L. D.; Banu, J. R.; Jeon, B. H.; Nguyen, D. D. Influence of Thermal Hydrolysis Pretreatment on Physicochemical Properties and Anaerobic Biodegradability of Waste Activated Sludge with Different Solids Content. Waste Manag. 2019, 85, 214–221. [CrossRef]
- Cydzik-Kwiatkowska, A.; Bernat, K.; Zielińska, M.; Gusiatin, M. Z.; Wojnowska-Baryła, I.; Kulikowska, D. Valorization of Full-Scale Waste Aerobic Granular Sludge for Biogas Production and the Characteristics of the Digestate. Chemosphere 2022, 303, 135167. [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion. Int. J. Environ. Res. Public Heal. 2023, Vol. 20, Page 4234 2023, 20 (5), 4234. [CrossRef]
- Zou, X.; Yang, R.; Zhou, X.; Cao, G.; Zhu, R.; Ouyang, F. Effects of Mixed Alkali-Thermal Pretreatment on Anaerobic Digestion Performance of Waste Activated Sludge. J. Clean. Prod. 2020, 259, 120940. [CrossRef]
- Dębowski, M.; Kisielewska, M.; Zieliński, M.; Kazimierowicz, J. The Influence of the Ultrasound Disintegration of Microalgal–Bacterial Granular Sludge on Anaerobic Digestion Efficiency. Appl. Sci. 2023, Vol. 13, Page 7387 2023, 13 (13), 7387. [CrossRef]
- Krul, E. S. Calculation of Nitrogen-to-Protein Conversion Factors: A Review with a Focus on Soy Protein. J. Am. Oil Chem. Soc. 2019, 96 (4), 339–364. [CrossRef]
- Das, A.; Mondal, C. Comparative Kinetic Study of Anaerobic Treatment of Thermally Pretreated Source-Sorted Organic Market Refuse. J. Eng. (United Kingdom) 2015, 2015. [CrossRef]
- Li, W.; Li, C.; Zhu, N.; Yuan, H.; Shen, Y. The Extent of Sludge Solubilization Allows to Estimate the Efficacy of Ozonation for Removal of Polycyclic Aromatic Hydrocarbons (PAHs) in Municipal Sewage Sludge. J. Hazard. Mater. 2021, 413, 125404. [CrossRef]
- Ndobeni, A.; Oyekola, O.; Welz, P. J. Organic Removal Rates and Biogas Production of an Upflow Anaerobic Sludge Blanket Reactor Treating Sugarcane Molasses. South African J. Chem. Eng. 2019, 28, 1–7. [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in Biogas Production: Pretreatment and Codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [CrossRef]
- Li, C.; Nges, I. A.; Lu, W.; Wang, H. Assessment of the Degradation Efficiency of Full-Scale Biogas Plants: A Comparative Study of Degradation Indicators. Bioresour. Technol. 2017, 244, 304–312. [CrossRef]
- Kim, M.; Kim, B. C.; Nam, K.; Choi, Y. Effect of Pretreatment Solutions and Conditions on Decomposition and Anaerobic Digestion of Lignocellulosic Biomass in Rice Straw. Biochem. Eng. J. 2018, 140, 108–114. [CrossRef]
- Şaǧban, F. O. T.; Dindar, E.; Cirakoglu, C.; Keskinler, B. Hydrodynamic Cavitation of Waste-Activated Sludge. https://home.liebertpub.com/ees 2018, 35 (8), 775–784. [CrossRef]
- Kim, H.; Sun, X.; Koo, B.; Yoon, J. Y. Experimental Investigation of Sludge Treatment Using a Rotor-Stator Type Hydrodynamic Cavitation Reactor and an Ultrasonic Bath. Process. 2019, Vol. 7, Page 790 2019, 7 (11), 790. [CrossRef]
- Lee, I.; Han, J. I. The Effects of Waste-Activated Sludge Pretreatment Using Hydrodynamic Cavitation for Methane Production. Ultrason. Sonochem. 2013, 20 (6), 1450–1455. [CrossRef]
- Mancuso, G.; Langone, M.; Andreottola, G.; Bruni, L. Effects of Hydrodynamic Cavitation, Low-Level Thermal and Low-Level Alkaline Pre-Treatments on Sludge Solubilisation. Ultrason. Sonochem. 2019, 59, 104750. [CrossRef]
- Petkovšek, M.; Mlakar, M.; Levstek, M.; Stražar, M.; Širok, B.; Dular, M. A Novel Rotation Generator of Hydrodynamic Cavitation for Waste-Activated Sludge Disintegration. Ultrason. Sonochem. 2015, 26, 408–414. [CrossRef]
- Mancuso, G.; Langone, M.; Di Maggio, R.; Toscano, A.; Andreottola, G. Effect of Hydrodynamic Cavitation on Flocs Structure in Sewage Sludge to Increase Stabilization for Efficient and Safe Reuse in Agriculture. Bioremediat. J. 2022, 26 (1), 41–52. [CrossRef]
- Ferrentino, R.; Andreottola, G. Investigation of Sludge Solubilization and Phosphorous Release in Anaerobic Side-Stream Reactor with a Low Pressure Swirling Jet Hydrodynamic Cavitation Treatment. J. Environ. Chem. Eng. 2020, 8 (5), 104389. [CrossRef]
- Lee, G.; Lee, I.; Han, J. I. A Combined Method of Hydrodynamic Cavitation and Alkaline Treatment for Waste-Activated Sludge Solubilization; N/P Recovery from Anaerobic Granular Sludge. J. Environ. Chem. Eng. 2019, 7 (5), 103329. [CrossRef]
- Zupanc, M.; Humar, B. B.; Dular, M.; Gostiša, J.; Hočevar, M.; Repinc, S. K.; Krzyk, M.; Novak, L.; Ortar, J.; Pandur, Ž.; Stres, B.; Petkovšek, M. The Use of Hydrodynamic Cavitation for Waste-to-Energy Approach to Enhance Methane Production from Waste Activated Sludge. J. Environ. Manage. 2023, 347, 119074. [CrossRef]
- Garlicka, A.; Zubrowska-Sudol, M.; Umiejewska, K.; Roubinek, O.; Palige, J.; Chmielewski, A. Effects of Thickened Excess Sludge Pre-Treatment Using Hydrodynamic Cavitation for Anaerobic Digestion. Energies 2020, Vol. 13, Page 2483 2020, 13 (10), 2483. [CrossRef]
- Islam, M. S.; Ranade, V. Enhancing Bmp and Digestibility of Daf Sludge Via Hydrodynamic Cavitation. SSRN 2023, 1–33. [CrossRef]
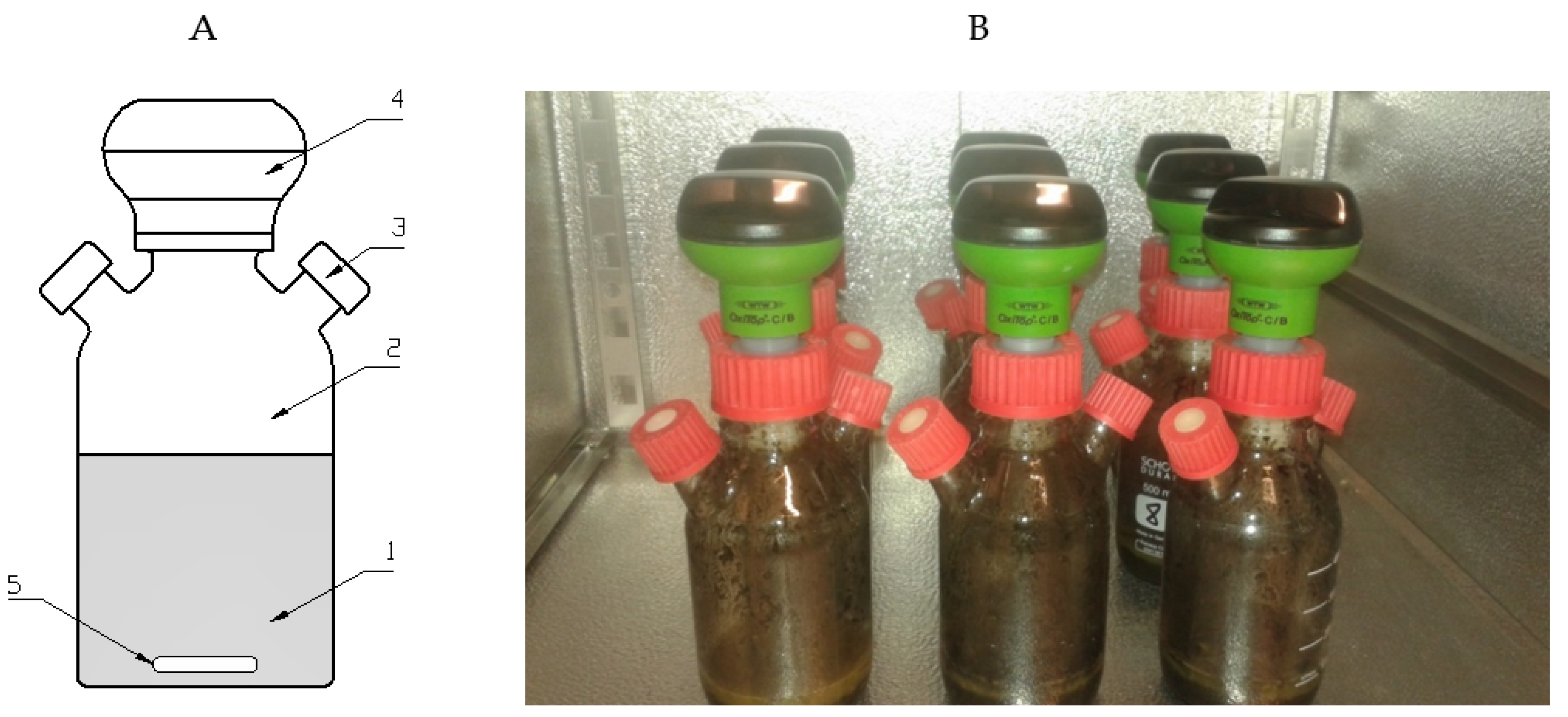
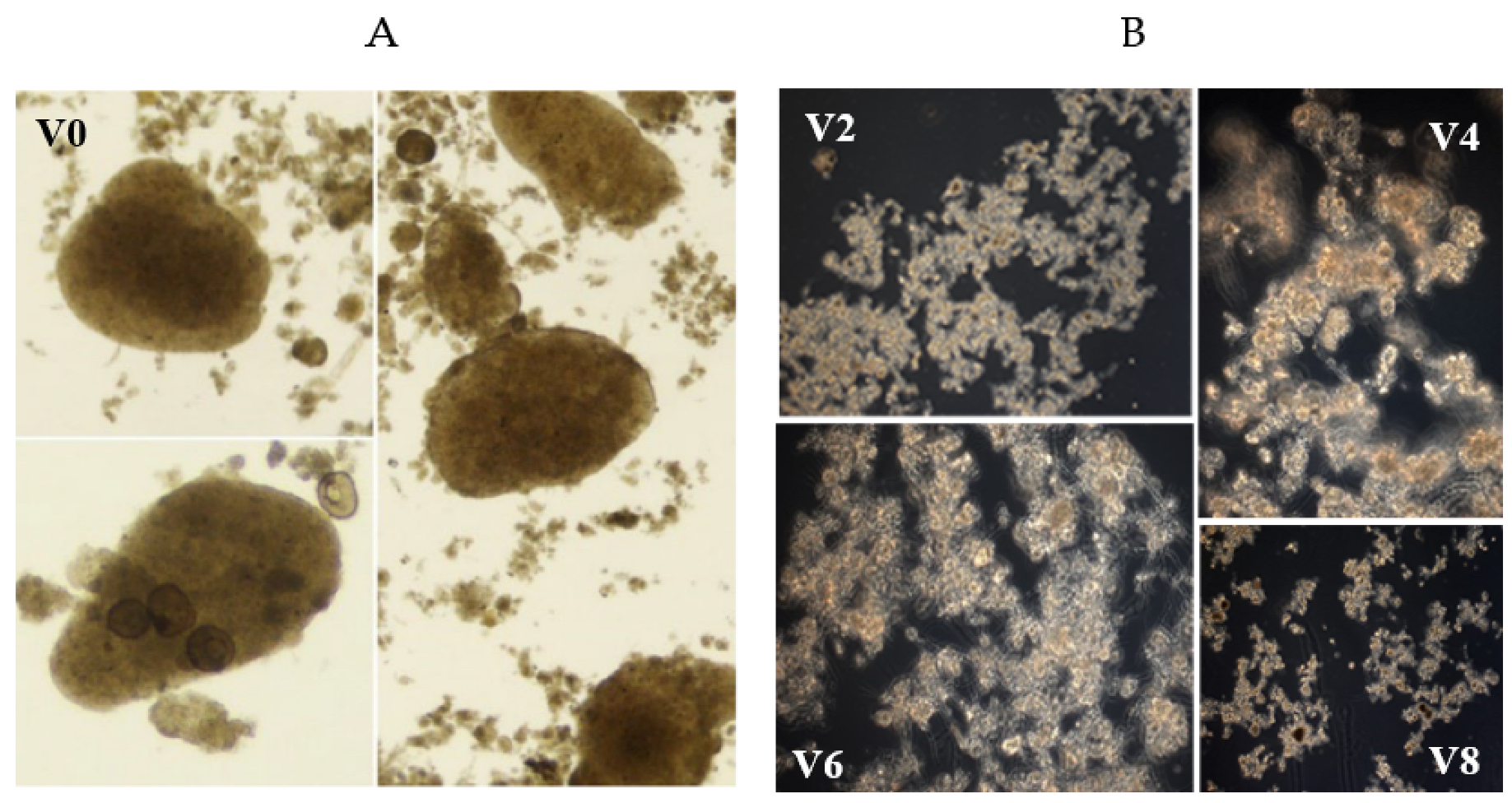
| Stage 1 | ||||
| Variant | HC time [min] | Energy consumption | Temperature of AGS after HC [°C] | |
| [Wh/L] | [Wh/gTS] | |||
| V0 | 0 | - | - | 21,2 |
| V1 | 1 | 1,2 | 0,02 | 21,6 |
| V2 | 2,5 | 2,8 | 0,05 | 21,9 |
| V3 | 5 | 5,2 | 0,09 | 22,4 |
| V4 | 10 | 9,6 | 0,16 | 23,8 |
| V5 | 15 | 13,6 | 0,23 | 27,0 |
| V6 | 20 | 15,6 | 0,27 | 27,5 |
| V7 | 25 | 17,2 | 0,29 | 28,2 |
| V8 | 50 | 26,0 | 0,44 | 28,4 |
| Stage 2 | ||||
| Respirometric measurement of anaerobic digestion: Temerature - 38°C Initial organic load rate – 5,0 gVS/L Retention time – 20 days Frequency of partial pressure measurement – 2h | ||||
| Parameter | Unit | Value | |
|---|---|---|---|
| AGS | AS | ||
| Total solids (TS) | [mg/gFM.] | 58,6±3,1 | 21,0±2,2 |
| Mineral solids (MS) | [mg/gFM] | 16,0±1,8 | 6,4±01,5 |
| Volatile solids (VS) | [mg/gFM] | 42,5±1,8 | 14,6±1,5 |
| Total nitrogen (TN) | [mgN/gTS] | 32,4±2,3 | 30,5±1,9 |
| COD | [mgO2/L] | 225,0 ±19,8 | 412,0±67,2 |
| TOC | [mg/L] | 72,2±13,7 | 290±11,4 |
| TC | [mg/L] | 175,5±13,5 | 352±10,9 |
| IC | [mg/L] | 31,0±9,1 | 62±7,2 |
| Lipids | [mg/gTS] | 0,42±0,1 | 0,34±0,1 |
| Proteins | [mg/gTS] | 202,5±14,4 | 190,6±11,9 |
| Parametr | Unit | HC time [min] (Variant) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (V0) | 1 (V1) | 2,5 (V2) | 5 (V3) | 10 (V4) | 15 (V5) | 20 (V6) | 25 (V7) | 50 (V8) | ||
| Total solids (TS) | [mg/gFM.] | 58,6±3,1 | 53,4±1,7 | 54,5±2,1 | 55,0±1,3 | 56,2±2,6 | 56,7±2,9 | 56,0±2,3 | 55,8±1,4 | 55,7±3,2 |
| Mineral solids (MS) | [mg/gFM] | 16,0±1,8 | 15,0±1,2 | 15,1±0,9 | 15,1±1,5 | 15,7±2,1 | 16,1±0,7 | 15,9±1,3 | 15,8±1,1 | 15,4±2,4 |
| Volatile solids (VS) | [mg/gFM] | 42,5±1,8 | 38,4±1,2 | 39,3±0,9 | 39,9±1,5 | 40,6±2,1 | 40,6±0,7 | 40,1±1,3 | 40,1±1,1 | 40,4±2,4 |
| Total nitrogen (TN) | [mgN/gTS] | 32,4±2,3 | 32,2±1,9 | 31,5±1,4 | 32,2±1,6 | 34,5±1,6 | 36,4±2,1 | 38,8±1,2 | 38,5±2,0 | 37,5±0,9 |
| ChZT | [mgO2/L] | 225,0 ±19,8 | 356,0±21,3 | 429,0±19,8 | 514,0±39,2 | 878,0±32,6 | 1217,0±70,8 | 1318,0±77,2 | 1332,0±62,6 | 1305,0±91,1 |
| TOC | [mg/L] | 72,2±13,7 | 111,9±12,9 | 144,5±9,1 | 221,5±17,9 | 352,4±21,2 | 354,1±20,6 | 370,6±23,8 | 370,9±19,9 | 372,8±16,2 |
| TC | [mg/L] | 175,5±13,5 | 138,4±19,1 | 163,1±14,3 | 271,3±17,7 | 410,4±29,2 | 405,0±20,7 | 426,2±31,2 | 427,2±17,4 | 118,7±14,6 |
| IC | [mg/L] | 31,0±9,1 | 47,0±7,2 | 51,2±12,9 | 49,8±17,9 | 58,0±21,2 | 50,9±20,6 | 55,6±23,8 | 56,3±19,9 | 46,5±13,7 |
| Lipids | [mg/gTS] | 0,42±0,1 | 0,6±0,11 | 0,4±0,1 | 0,4±0,1 | 0,4±0,1 | 0,5±0,2 | 0,6±0,1 | 0,6±0,1 | 0,6±0,2 |
| Protein | [mg/gTS] | 202,5±14,4 | 201,3±11,9 | 196,9±8,7 | 201,3±10,0 | 215,6±10,0 | 227,5±13,1 | 242,5±7,5 | 240,6±12,5 | 234,4±5,6 |
| Cavitation time [min] (Variant) | Biogas | Methane | Biogas composition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mL/gFM | mL/gTS | mL/gVS | mL/gFM | mL/gTS | mL/gVS | CH4 [%] | CO2 [%] | O2 [%] | |
| 0 (V0) | 25,6±1,5 | 457,2±26,8 | 631,0±37,0 | 16,2±0,9 | 289,0±16,9 | 398,8±23,4 | 63,2±1,2 | 36,3±1,1 | 0,4±0,1 |
| 1 (V1) | 26,2±1,9 | 461,7±33,5 | 650,3±47,2 | 16,3±1,2 | 288,1±20,9 | 405,8±29,2 | 62,4±1,1 | 37,1±1,0 | 0,4±0,1 |
| 2,5 (V2) | 27,8±1,3 | 480,1±22,4 | 680,0±31,8 | 18,4±0,8 | 324,6±14,1 | 427,8±11,5 | 62,9±0,9 | 36,0±0,6 | 1,1±0,3 |
| 5 (V3) | 29,2±1,7 | 505,2±29,4 | 710,3±41,4 | 18,1±1,1 | 331,9±18,3 | 441,0±20,1 | 62,1±2,3 | 36,7±2,1 | 1,2±0,2 |
| 10 (V4) | 30,6±1,6 | 527,9±27,6 | 730,6±38,3 | 19,2±1,0 | 342,6±17,4 | 452,3±14,7 | 61,9±1,6 | 37,0±1,5 | 1,1±0,1 |
| 15 (V5) | 30,8±1,4 | 548,3±24,9 | 767,6±34,9 | 19,3±0,9 | 342,8±15,6 | 487,6±12,1 | 63,5±1,2 | 36,1±1,1 | 0,3±0,1 |
| 20 (V6) | 29,6±1,3 | 557,5±24,5 | 769,3±33,8 | 18,7±0,8 | 352,9±15,5 | 487,0±18,2 | 63,3±0,6 | 35,8±0,4 | 0,9±0,2 |
| 25 (V7) | 29,9±1,6 | 539,4±28,9 | 772,1±41,3 | 18,7±1,0 | 336,6±18,0 | 489,5±10,7 | 63,4±0,8 | 36,0±0,6 | 0,5±0,2 |
| 50 (V8) | 30,5±1,2 | 541,0±21,3 | 785,2±30,9 | 21,4±0,7 | 393,4±13,2 | 495,9±12,3 | 63,2±1,4 | 35,9±1,0 | 0,9±0,4 |
| Variant | HK time [min] |
Energy consumption [kWh] | Specific energy input (Es) [Wh/gTS] | CH4 yeld mL/gTS |
CH4 energy value (YCH4) [Wh/L] |
Gross energy output (EGout) Wh/gTS |
Net energy output (ENout) Wh/g TS |
Net energy gain (Enet) Wh/g TS |
|---|---|---|---|---|---|---|---|---|
| V0 | 0 | 0 | 0 | 278,6 | 9,17 | 2,55 | 2,55 | 0,000 |
| V1 | 1 | 0,03 | 0,02 | 283,4 | 2,60 | 2,58 | 0,045 | |
| V2 | 2,5 | 0,07 | 0,05 | 298,8 | 2,74 | 2,69 | 0,141 | |
| V3 | 5 | 0,13 | 0,09 | 308,1 | 2,82 | 2,73 | 0,085 | |
| V4 | 10 | 0,24 | 0,16 | 316,0 | 2,90 | 2,74 | 0,073 | |
| V5 | 15 | 0,34 | 0,23 | 340,6 | 3,12 | 2,89 | 0,226 | |
| V6 | 20 | 0,39 | 0,27 | 340,2 | 3,12 | 2,85 | -0,004 | |
| V7 | 25 | 0,43 | 0,29 | 342,0 | 3,14 | 2,85 | 0,016 | |
| V8 | 50 | 0,65 | 0,44 | 346,4 | 3,18 | 2,74 | 0,041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





