Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Neuroinflammation and AD
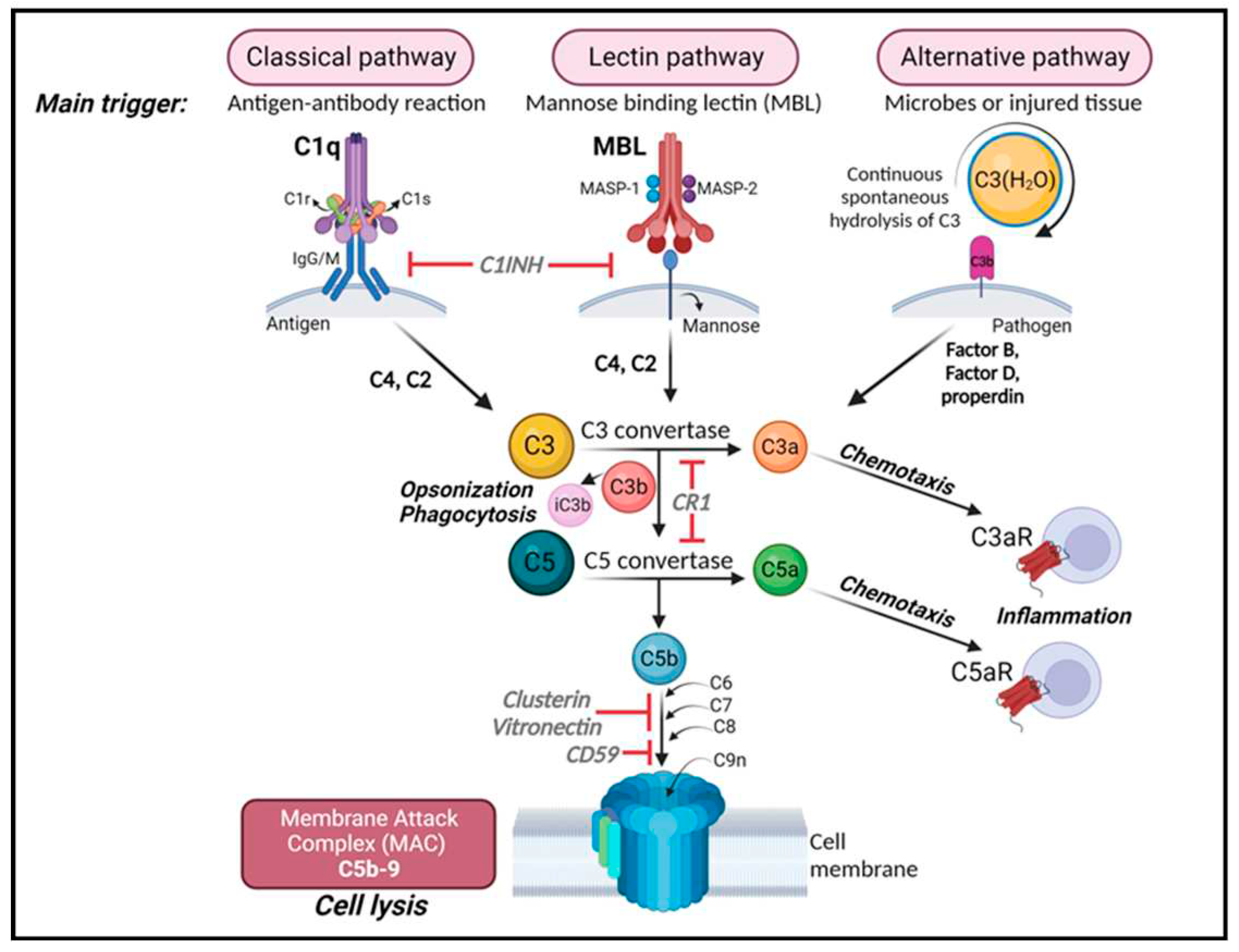
3. Complement system
4. Complement System in the pathogenesis of AD
5. Complement component C3 and AD
6. Driving mechanisms of complement-mediated CNS dysfunction
7. Complement components as therapeutical targets in AD
8. Crosstalk between the complement system and other inflammatory players in AD
9. Complement and Tau pathology
10. Future directions
11. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Selkoe, D.J. and J. Hardy, The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med, 2016. 8(6): p. 595-608. [CrossRef]
- World Alzheimer Report 2018 The State of the art of dementia research: New Frontiers. Alzheimer's Disease International, 2018.
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement, 2023. 19(4): p. 1598-1695.
- Organization, W.H., World Health Organization (WHO), 2023.
- Terry, R.D., et al., Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol, 1991. 30(4): p. 572-80. [CrossRef]
- Masliah, E., et al., Immunoelectron microscopic study of synaptic pathology in Alzheimer's disease. Acta Neuropathol, 1991. 81(4): p. 428-33. [CrossRef]
- Hammond, T.R., et al., Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity, 2019. 50(1): p. 253-271 e6. [CrossRef]
- Keren-Shaul, H., et al., A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell, 2017. 169(7): p. 1276-1290 e17. [CrossRef]
- Stubbington, M.J.T., et al., Single-cell transcriptomics to explore the immune system in health and disease. Science, 2017. 358(6359): p. 58-63. [CrossRef]
- Felsky, D., et al., Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun, 2019. 10(1): p. 409. [CrossRef]
- Jansen, I.E., et al., Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet, 2019. 51(3): p. 404-413. [CrossRef]
- Ponath, G., et al., Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat Commun, 2018. 9(1): p. 5337. [CrossRef]
- Mathys, H., et al., Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep, 2017. 21(2): p. 366-380. [CrossRef]
- Walport, M.J., Complement. Second of two parts. N Engl J Med, 2001. 344(15): p. 1140-4. [CrossRef]
- Walport, M.J., Complement. First of two parts. N Engl J Med, 2001. 344(14): p. 1058-66. [CrossRef]
- Stephan, A.H., B.A. Barres, and B. Stevens, The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci, 2012. 35: p. 369-89. [CrossRef]
- Schafer, D.P., et al., Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 2012. 74(4): p. 691-705. [CrossRef]
- Stevens, B., et al., The classical complement cascade mediates CNS synapse elimination. Cell, 2007. 131(6): p. 1164-78. [CrossRef]
- Gorelik, A., et al., Developmental activities of the complement pathway in migrating neurons. Nat Commun, 2017. 8: p. 15096. [CrossRef]
- Carpanini, S.M., M. Torvell, and B.P. Morgan, Therapeutic Inhibition of the Complement System in Diseases of the Central Nervous System. Front Immunol, 2019. 10: p. 362. [CrossRef]
- Hammond, T.R., S.E. Marsh, and B. Stevens, Immune Signaling in Neurodegeneration. Immunity, 2019. 50(4): p. 955-974. [CrossRef]
- Hong, S., et al., Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 2016. 352(6286): p. 712-716. [CrossRef]
- Shi, Q., et al., Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med, 2017. 9(392). [CrossRef]
- Shi, Q., et al., Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J Neurosci, 2015. 35(38): p. 13029-42. [CrossRef]
- Krukowski, K., et al., Traumatic Brain Injury in Aged Mice Induces Chronic Microglia Activation, Synapse Loss, and Complement-Dependent Memory Deficits. Int J Mol Sci, 2018. 19(12). [CrossRef]
- Wu, T., et al., Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep, 2019. 28(8): p. 2111-2123 e6. [CrossRef]
- Heneka, M.T., et al., Neuroinflammation in Alzheimer's disease. Lancet Neurol, 2015. 14(4): p. 388-405. [CrossRef]
- Rogers, J., et al., Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci U S A, 1992. 89(21): p. 10016-20. [CrossRef]
- McGeer, P.L., et al., Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett, 1989. 107(1-3): p. 341-6. [CrossRef]
- McGeer, P.L., et al., Occurrence of HLA-DR reactive microglia in Alzheimer's disease. Ann N Y Acad Sci, 1988. 540: p. 319-23. [CrossRef]
- McGeer, P.L., et al., Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett, 1987. 79(1-2): p. 195-200. [CrossRef]
- Zotova, E., et al., Inflammatory components in human Alzheimer's disease and after active amyloid-beta42 immunization. Brain, 2013. 136(Pt 9): p. 2677-96. [CrossRef]
- Eikelenboom, P. and F.C. Stam, Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol, 1982. 57(2-3): p. 239-42. [CrossRef]
- Kamphuis, W., et al., GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS One, 2012. 7(8): p. e42823. [CrossRef]
- Hanzel, D.K., et al., High-throughput quantitative histological analysis of Alzheimer's disease pathology using a confocal digital microscanner. Nat Biotechnol, 1999. 17(1): p. 53-7. [CrossRef]
- Stephenson, J., et al., Inflammation in CNS neurodegenerative diseases. Immunology, 2018. 154(2): p. 204-219. [CrossRef]
- Brosseron, F., et al., Body fluid cytokine levels in mild cognitive impairment and Alzheimer's disease: a comparative overview. Mol Neurobiol, 2014. 50(2): p. 534-44. [CrossRef]
- Brosseron, F., et al., Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer's disease. Alzheimers Res Ther, 2018. 10(1): p. 25. [CrossRef]
- Tian, Z., X. Ji, and J. Liu, Neuroinflammation in Vascular Cognitive Impairment and Dementia: Current Evidence, Advances, and Prospects. Int J Mol Sci, 2022. 23(11). [CrossRef]
- Nimmerjahn, A., F. Kirchhoff, and F. Helmchen, Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 2005. 308(5726): p. 1314-8. [CrossRef]
- Sheng, J., C. Ruedl, and K. Karjalainen, Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity, 2015. 43(2): p. 382-93. [CrossRef]
- Lawson, L.J., et al., Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 1990. 39(1): p. 151-70. [CrossRef]
- Hong, S., L. Dissing-Olesen, and B. Stevens, New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol, 2016. 36: p. 128-34. [CrossRef]
- Sarlus, H. and M.T. Heneka, Microglia in Alzheimer's disease. J Clin Invest, 2017. 127(9): p. 3240-3249. [CrossRef]
- Lian, H., et al., Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer's Disease. J Neurosci, 2016. 36(2): p. 577-89. [CrossRef]
- Roy, E.R., et al., Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J Clin Invest, 2020. 130(4): p. 1912-1930. [CrossRef]
- Butovsky, O. and H.L. Weiner, Microglial signatures and their role in health and disease. Nat Rev Neurosci, 2018. 19(10): p. 622-635. [CrossRef]
- Krasemann, S., et al., The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity, 2017. 47(3): p. 566-581 e9. [CrossRef]
- Hansen, D.V., J.E. Hanson, and M. Sheng, Microglia in Alzheimer's disease. J Cell Biol, 2018. 217(2): p. 459-472. [CrossRef]
- Lambert, J.C., et al., Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet, 2009. 41(10): p. 1094-9. [CrossRef]
- Schafer, D.P., E.K. Lehrman, and B. Stevens, The "quad-partite" synapse: microglia-synapse interactions in the developing and mature CNS. Glia, 2013. 61(1): p. 24-36. [CrossRef]
- Paolicelli, R.C., et al., Synaptic pruning by microglia is necessary for normal brain development. Science, 2011. 333(6048): p. 1456-8. [CrossRef]
- Filipello, F., et al., The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity, 2018. 48(5): p. 979-991 e8. [CrossRef]
- Paolicelli, R.C. and C.T. Gross, Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol, 2011. 7(1): p. 77-83. [CrossRef]
- Rogers, J.T., et al., CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci, 2011. 31(45): p. 16241-50. [CrossRef]
- Bailey, C.H., E.R. Kandel, and K.M. Harris, Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb Perspect Biol, 2015. 7(7): p. a021758. [CrossRef]
- Venegas, C., et al., Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer's disease. Nature, 2017. 552(7685): p. 355-361. [CrossRef]
- Arranz, A.M. and B. De Strooper, The role of astroglia in Alzheimer's disease: pathophysiology and clinical implications. Lancet Neurol, 2019. 18(4): p. 406-414. [CrossRef]
- Liddelow, S.A., et al., Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 2017. 541(7638): p. 481-487. [CrossRef]
- Brambilla, R., et al., Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem, 2009. 110(2): p. 765-78. [CrossRef]
- Diniz, L.P., et al., Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Abeta Oligomers in Alzheimer's Disease Model. J Neurosci, 2017. 37(28): p. 6797-6809. [CrossRef]
- Bellaver, B., et al., Astrocyte reactivity influences amyloid-beta effects on tau pathology in preclinical Alzheimer's disease. Nat Med, 2023. 29(7): p. 1775-1781. [CrossRef]
- Lian, H. and H. Zheng, Signaling pathways regulating neuron-glia interaction and their implications in Alzheimer's disease. J Neurochem, 2016. 136(3): p. 475-91. [CrossRef]
- Eikelenboom, P., et al., Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Arch B Cell Pathol Incl Mol Pathol, 1989. 56(4): p. 259-62. [CrossRef]
- Ishii, T. and S. Haga, Immuno-electron-microscopic localization of complements in amyloid fibrils of senile plaques. Acta Neuropathol, 1984. 63(4): p. 296-300. [CrossRef]
- Afagh, A., et al., Localization and cell association of C1q in Alzheimer's disease brain. Exp Neurol, 1996. 138(1): p. 22-32. [CrossRef]
- Stoltzner, S.E., et al., Temporal accrual of complement proteins in amyloid plaques in Down's syndrome with Alzheimer's disease. Am J Pathol, 2000. 156(2): p. 489-99. [CrossRef]
- Reichwald, J., et al., Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J Neuroinflammation, 2009. 6: p. 35. [CrossRef]
- Matsuoka, Y., et al., Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer's disease. Am J Pathol, 2001. 158(4): p. 1345-54. [CrossRef]
- Nonaka, M. and A. Kimura, Genomic view of the evolution of the complement system. Immunogenetics, 2006. 58(9): p. 701-13. [CrossRef]
- Bordet, J., Les leukocytes et les proprietes actives du serum chez les vaccines. Ann. Inst. Pasteur, 1895. 462–506.
- Hadding, U. and H.J. Muller-Eberhard, The ninth component of human complement: isolation, description and mode of action. Immunology, 1969. 16(6): p. 719-35.
- Nilsson, U., Separation and partial purification of the sixth, seventh and eighth components of human haemolytic complement. Acta Pathol Microbiol Scand, 1967. 70(3): p. 469-80. [CrossRef]
- Nilsson, U.R. and H.J. Mueller-Eberhard, Isolation of Beta If-Globulin from Human Serum and Its Characterization as the Fifth Component of Complement. J Exp Med, 1965. 122: p. 277-98. [CrossRef]
- Mueller-Eberhard, H.J. and C.E. Biro, Isolation and Description of the Fourth Component of Human Complement. J Exp Med, 1963. 118: p. 447-66. [CrossRef]
- Pillemer, L. and E.E. Ecker, The Terminology of the Components of Complement. Science, 1941. 94(2445): p. 437. [CrossRef]
- Merle, N.S., et al., Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol, 2015. 6: p. 262. [CrossRef]
- Merle, N.S., et al., Complement System Part II: Role in Immunity. Front Immunol, 2015. 6: p. 257. [CrossRef]
- Coulthard, L.G., O.A. Hawksworth, and T.M. Woodruff, Complement: The Emerging Architect of the Developing Brain. Trends Neurosci, 2018. 41(6): p. 373-384. [CrossRef]
- Bialas, A.R. and B. Stevens, TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci, 2013. 16(12): p. 1773-82. [CrossRef]
- Perez-Alcazar, M., et al., Altered cognitive performance and synaptic function in the hippocampus of mice lacking C3. Exp Neurol, 2014. 253: p. 154-64. [CrossRef]
- Fitzgerald, K.C., et al., Early complement genes are associated with visual system degeneration in multiple sclerosis. Brain, 2019. 142(9): p. 2722-2736. [CrossRef]
- Werneburg, S., et al., Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity, 2020. 52(1): p. 167-182 e7. [CrossRef]
- Sun, Y., et al., Disrupted functional brain connectivity and its association to structural connectivity in amnestic mild cognitive impairment and Alzheimer's disease. PLoS One, 2014. 9(5): p. e96505. [CrossRef]
- Siegel, J.S., et al., Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci U S A, 2016. 113(30): p. E4367-76. [CrossRef]
- Brown, A.S., Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol, 2012. 72(10): p. 1272-6. [CrossRef]
- Sekar, A., et al., Schizophrenia risk from complex variation of complement component 4. Nature, 2016. 530(7589): p. 177-83. [CrossRef]
- Yilmaz, M., et al., Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci, 2021. 24(2): p. 214-224. [CrossRef]
- Rahpeymai, Y., et al., Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J, 2006. 25(6): p. 1364-74. [CrossRef]
- Westacott, L.J., et al., Dissociable effects of complement C3 and C3aR on survival and morphology of adult born hippocampal neurons, pattern separation, and cognitive flexibility in male mice. Brain Behav Immun, 2021. 98: p. 136-150. [CrossRef]
- Figueiredo, C.P., et al., Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat Commun, 2019. 10(1): p. 3890. [CrossRef]
- Jaffry M, F.I., Jaffry K, Neurological Manifestations of SARS-CoV-2 Infection and the Role of Complement Activation. touchREVIEWS in Neurology, 2022. 18(2): p. 86-92. [CrossRef]
- Lee, M.H., et al., Neurovascular injury with complement activation and inflammation in COVID-19. Brain, 2022. 145(7): p. 2555-2568. [CrossRef]
- Zhou, J., et al., Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer's disease. J Neurochem, 2008. 106(5): p. 2080-92. [CrossRef]
- Maier, M., et al., Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci, 2008. 28(25): p. 6333-41. [CrossRef]
- Thambisetty, M., et al., Effect of complement CR1 on brain amyloid burden during aging and its modification by APOE genotype. Biol Psychiatry, 2013. 73(5): p. 422-8. [CrossRef]
- Hernandez, M.X., et al., Prevention of C5aR1 signaling delays microglial inflammatory polarization, favors clearance pathways and suppresses cognitive loss. Mol Neurodegener, 2017. 12(1): p. 66. [CrossRef]
- Shen, Y., et al., Complement activation by neurofibrillary tangles in Alzheimer's disease. Neurosci Lett, 2001. 305(3): p. 165-8. [CrossRef]
- Daborg, J., et al., Cerebrospinal fluid levels of complement proteins C3, C4 and CR1 in Alzheimer's disease. J Neural Transm (Vienna), 2012. 119(7): p. 789-97. [CrossRef]
- Strohmeyer, R., Y. Shen, and J. Rogers, Detection of complement alternative pathway mRNA and proteins in the Alzheimer's disease brain. Brain Res Mol Brain Res, 2000. 81(1-2): p. 7-18. [CrossRef]
- Goetzl, E.J., et al., High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol, 2018. 83(3): p. 544-552. [CrossRef]
- Cribbs, D.H., et al., Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation, 2012. 9: p. 179. [CrossRef]
- Harold, D., et al., Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet, 2009. 41(10): p. 1088-93. [CrossRef]
- Bellenguez, C., et al., New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet, 2022. 54(4): p. 412-436. [CrossRef]
- Gatz, M., et al., Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry, 2006. 63(2): p. 168-74. [CrossRef]
- Crehan, H., et al., Complement receptor 1 (CR1) and Alzheimer's disease. Immunobiology, 2012. 217(2): p. 244-50. [CrossRef]
- Kucukkilic, E., et al., Complement receptor 1 gene (CR1) intragenic duplication and risk of Alzheimer's disease. Hum Genet, 2018. 137(4): p. 305-314. [CrossRef]
- Lian, H., et al., NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease. Neuron, 2015. 85(1): p. 101-115. [CrossRef]
- El Gaamouch, F., et al., VGF-derived peptide TLQP-21 modulates microglial function through C3aR1 signaling pathways and reduces neuropathology in 5xFAD mice. Mol Neurodegener, 2020. 15(1): p. 4. [CrossRef]
- Xie, J., et al., Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3-C3aR signalling. J Extracell Vesicles, 2023. 12(2): p. e12306. [CrossRef]
- Fu, H., et al., Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Abeta by microglia. Glia, 2012. 60(6): p. 993-1003. [CrossRef]
- Tooyama, I., et al., Correlation of the expression level of C1q mRNA and the number of C1q-positive plaques in the Alzheimer Disease temporal cortex. analysis of C1q mrna and its protein using adjacent or nearby sections. Dement Geriatr Cogn Disord, 2001. 12(4): p. 237-42. [CrossRef]
- Guttikonda, S.R., et al., Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer's disease. Nat Neurosci, 2021. 24(3): p. 343-354. [CrossRef]
- Chatterjee, M., et al., C1q is increased in cerebrospinal fluid-derived extracellular vesicles in Alzheimer's disease: A multi-cohort proteomics and immuno-assay validation study. Alzheimers Dement, 2023. [CrossRef]
- Stephan, A.H., et al., A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci, 2013. 33(33): p. 13460-74. [CrossRef]
- Fonseca, M.I., et al., Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J Neurosci, 2004. 24(29): p. 6457-65. [CrossRef]
- Benoit, M.E., et al., C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-beta neurotoxicity. J Biol Chem, 2013. 288(1): p. 654-65. [CrossRef]
- Carpanini, S.M., et al., Terminal complement pathway activation drives synaptic loss in Alzheimer's disease models. Acta Neuropathol Commun, 2022. 10(1): p. 99. [CrossRef]
- Xin, Y.R., et al., The Immune System Drives Synapse Loss During Lipopolysaccharide-Induced Learning and Memory Impairment in Mice. Front Aging Neurosci, 2019. 11: p. 279. [CrossRef]
- Propson, N.E., et al., Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J Clin Invest, 2021. 131(1). [CrossRef]
- Zhang, J., et al., Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron, 2014. 82(1): p. 195-207. [CrossRef]
- Gedam, M., et al., Complement C3aR depletion reverses HIF-1alpha-induced metabolic impairment and enhances microglial response to Abeta pathology. J Clin Invest, 2023. 133(12). [CrossRef]
- Wyss-Coray, T., et al., Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc Natl Acad Sci U S A, 2002. 99(16): p. 10837-42. [CrossRef]
- Choucair-Jaafar, N., et al., Complement receptor 3 (CD11b/CD18) is implicated in the elimination of beta-amyloid peptides. Fundam Clin Pharmacol, 2011. 25(1): p. 115-22. [CrossRef]
- Czirr, E., et al., Microglial complement receptor 3 regulates brain Abeta levels through secreted proteolytic activity. J Exp Med, 2017. 214(4): p. 1081-1092. [CrossRef]
- Fonseca, M.I., et al., Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J Immunol, 2009. 183(2): p. 1375-83. [CrossRef]
- Gomez-Arboledas, A., et al., C5aR1 antagonism alters microglial polarization and mitigates disease progression in a mouse model of Alzheimer's disease. Acta Neuropathol Commun, 2022. 10(1): p. 116. [CrossRef]
- Panayiotou, E., et al., C5aR agonist enhances phagocytosis of fibrillar and non-fibrillar Abeta amyloid and preserves memory in a mouse model of familial Alzheimer's disease. PLoS One, 2019. 14(12): p. e0225417. [CrossRef]
- Carvalho, K., et al., Modulation of C5a-C5aR1 signaling alters the dynamics of AD progression. J Neuroinflammation, 2022. 19(1): p. 178. [CrossRef]
- Wang, C., et al., Microglia mediate forgetting via complement-dependent synaptic elimination. Science, 2020. 367(6478): p. 688-694. [CrossRef]
- Kim, J.H., et al., Gamma subunit of complement component 8 is a neuroinflammation inhibitor. Brain, 2021. 144(2): p. 528-552. [CrossRef]
- McNab, F., et al., Type I interferons in infectious disease. Nat Rev Immunol, 2015. 15(2): p. 87-103. [CrossRef]
- Minter, M.R., et al., Deletion of the type-1 interferon receptor in APPSWE/PS1DeltaE9 mice preserves cognitive function and alters glial phenotype. Acta Neuropathol Commun, 2016. 4(1): p. 72. [CrossRef]
- Roy, E.R., et al., Concerted type I interferon signaling in microglia and neural cells promotes memory impairment associated with amyloid beta plaques. Immunity, 2022. 55(5): p. 879-894 e6. [CrossRef]
- Holtzman, D.M., J. Herz, and G. Bu, Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med, 2012. 2(3): p. a006312. [CrossRef]
- Liu, C.C., et al., Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol, 2013. 9(2): p. 106-18. [CrossRef]
- Yin, C., et al., ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med, 2019. 25(3): p. 496-506. [CrossRef]
- Qin, Q., et al., The Specific Mechanism of TREM2 Regulation of Synaptic Clearance in Alzheimer's Disease. Front Immunol, 2022. 13: p. 845897. [CrossRef]
- Vandendriessche, C., et al., Importance of extracellular vesicle secretion at the blood-cerebrospinal fluid interface in the pathogenesis of Alzheimer's disease. Acta Neuropathol Commun, 2021. 9(1): p. 143. [CrossRef]
- Reiman, E.M., et al., Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun, 2020. 11(1): p. 667. [CrossRef]
- Farrer, L.A., et al., Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA, 1997. 278(16): p. 1349-56. [CrossRef]
- Panitch, R., et al., Integrative brain transcriptome analysis links complement component 4 and HSPA2 to the APOE epsilon2 protective effect in Alzheimer disease. Mol Psychiatry, 2021. 26(10): p. 6054-6064. [CrossRef]
- Ulland, T.K., et al., TREM2 Maintains Microglial Metabolic Fitness in Alzheimer's Disease. Cell, 2017. 170(4): p. 649-663 e13. [CrossRef]
- Pottier, C., et al., TREM2 R47H variant as a risk factor for early-onset Alzheimer's disease. J Alzheimers Dis, 2013. 35(1): p. 45-9. [CrossRef]
- Guerreiro, R., et al., TREM2 variants in Alzheimer's disease. N Engl J Med, 2013. 368(2): p. 117-27. [CrossRef]
- Konishi, H. and H. Kiyama, Microglial TREM2/DAP12 Signaling: A Double-Edged Sword in Neural Diseases. Front Cell Neurosci, 2018. 12: p. 206. [CrossRef]
- Qu, W. and L. Li, Loss of TREM2 Confers Resilience to Synaptic and Cognitive Impairment in Aged Mice. J Neurosci, 2020. 40(50): p. 9552-9563. [CrossRef]
- Scott-Hewitt, N., et al., Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J, 2020. 39(16): p. e105380. [CrossRef]
- Fraser, D.A., K. Pisalyaput, and A.J. Tenner, C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J Neurochem, 2010. 112(3): p. 733-43. [CrossRef]
- Zhang, B., et al., Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell, 2013. 153(3): p. 707-20. [CrossRef]
- Readhead, B., et al., Molecular systems evaluation of oligomerogenic APP(E693Q) and fibrillogenic APP(KM670/671NL)/PSEN1(Deltaexon9) mouse models identifies shared features with human Alzheimer's brain molecular pathology. Mol Psychiatry, 2016. 21(8): p. 1099-111. [CrossRef]
- Haure-Mirande, J.V., et al., Deficiency of TYROBP, an adapter protein for TREM2 and CR3 receptors, is neuroprotective in a mouse model of early Alzheimer's pathology. Acta Neuropathol, 2017. 134(5): p. 769-788. [CrossRef]
- Haure-Mirande, J.V., et al., Integrative approach to sporadic Alzheimer's disease: deficiency of TYROBP in cerebral Abeta amyloidosis mouse normalizes clinical phenotype and complement subnetwork molecular pathology without reducing Abeta burden. Mol Psychiatry, 2019. 24(3): p. 431-446. [CrossRef]
- Audrain, M., et al., Integrative approach to sporadic Alzheimer's disease: deficiency of TYROBP in a tauopathy mouse model reduces C1q and normalizes clinical phenotype while increasing spread and state of phosphorylation of tau. Mol Psychiatry, 2019. 24(9): p. 1383-1397. [CrossRef]
- DeVos, S.L., et al., Synaptic Tau Seeding Precedes Tau Pathology in Human Alzheimer's Disease Brain. Front Neurosci, 2018. 12: p. 267. [CrossRef]
- Bejanin, A., et al., Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain, 2017. 140(12): p. 3286-3300. [CrossRef]
- Britschgi, M., et al., Deficiency of terminal complement pathway inhibitor promotes neuronal tau pathology and degeneration in mice. J Neuroinflammation, 2012. 9: p. 220. [CrossRef]
- Dejanovic, B., et al., Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron, 2018. 100(6): p. 1322-1336 e7. [CrossRef]
- Litvinchuk, A., et al., Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer's Disease. Neuron, 2018. 100(6): p. 1337-1353 e5. [CrossRef]
- Bonham, L.W., et al., The relationship between complement factor C3, APOE epsilon4, amyloid and tau in Alzheimer's disease. Acta Neuropathol Commun, 2016. 4(1): p. 65. [CrossRef]
- Shi, Y., et al., ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature, 2017. 549(7673): p. 523-527. [CrossRef]
- Dejanovic, B., et al., Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer's disease mouse models. Nat Aging, 2022. 2(9): p. 837-850. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



