Submitted:
19 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
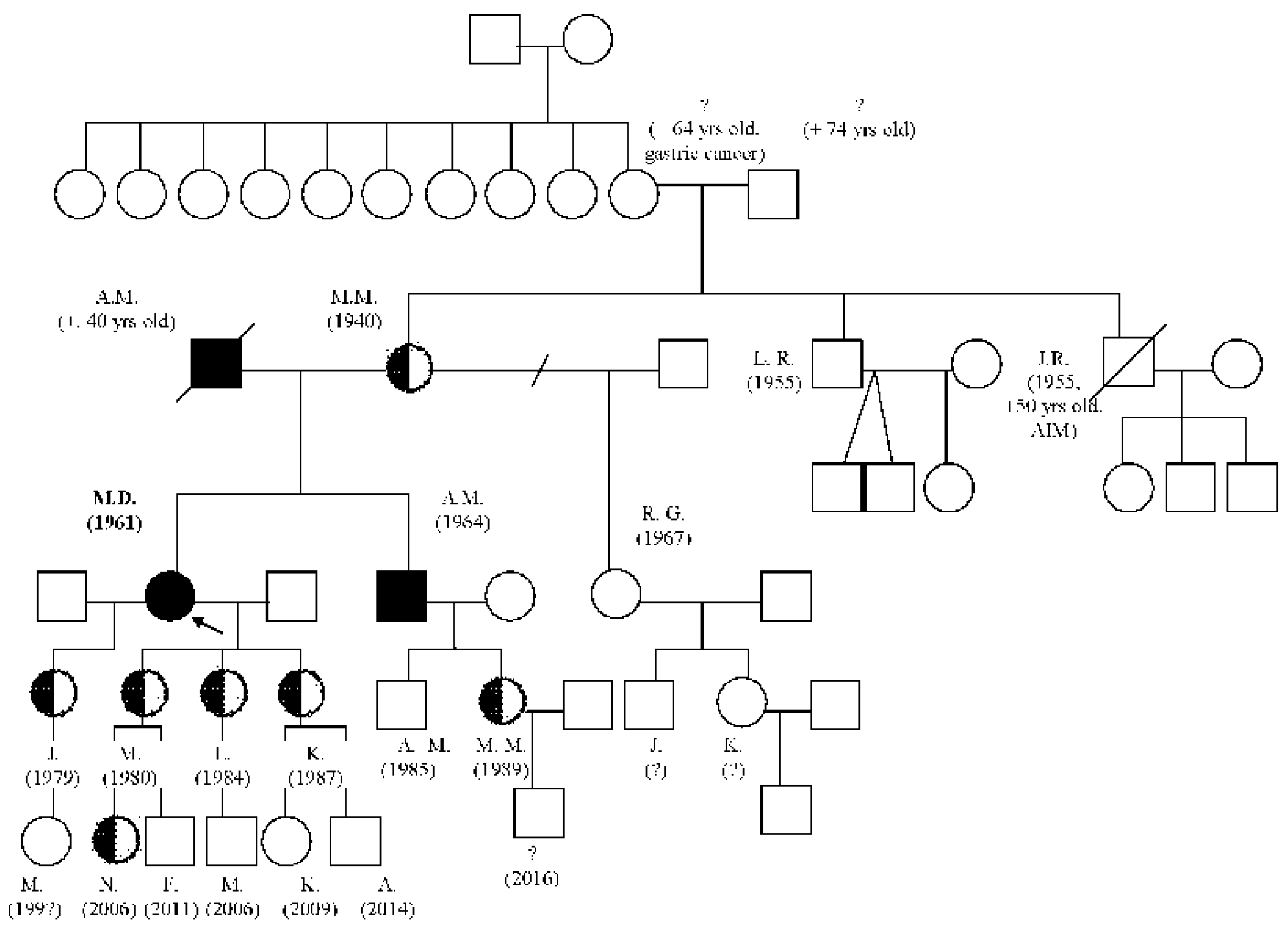
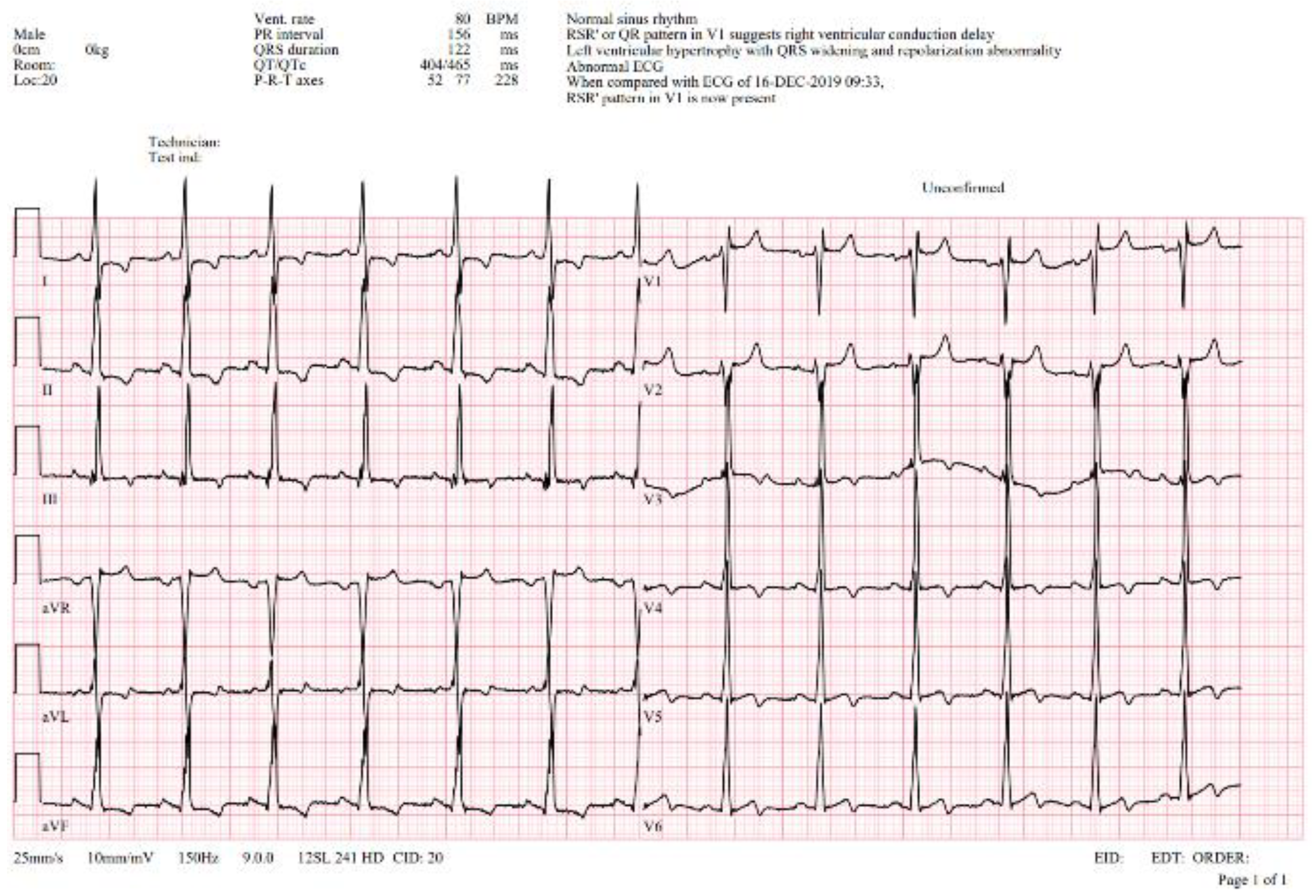
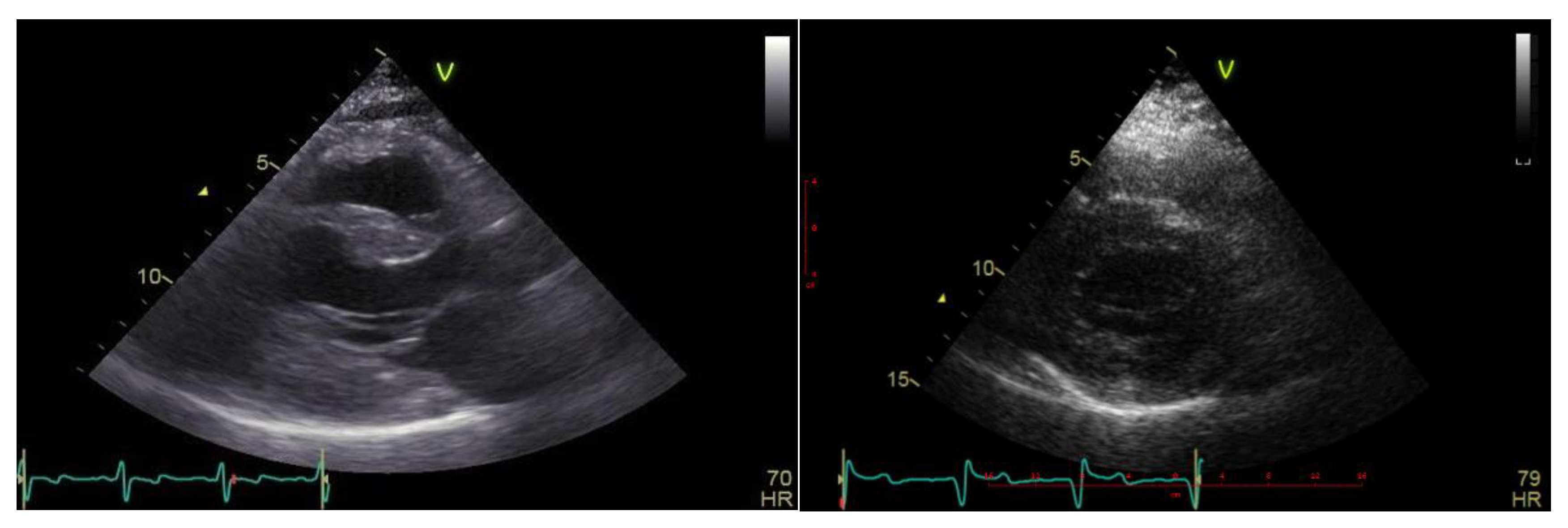
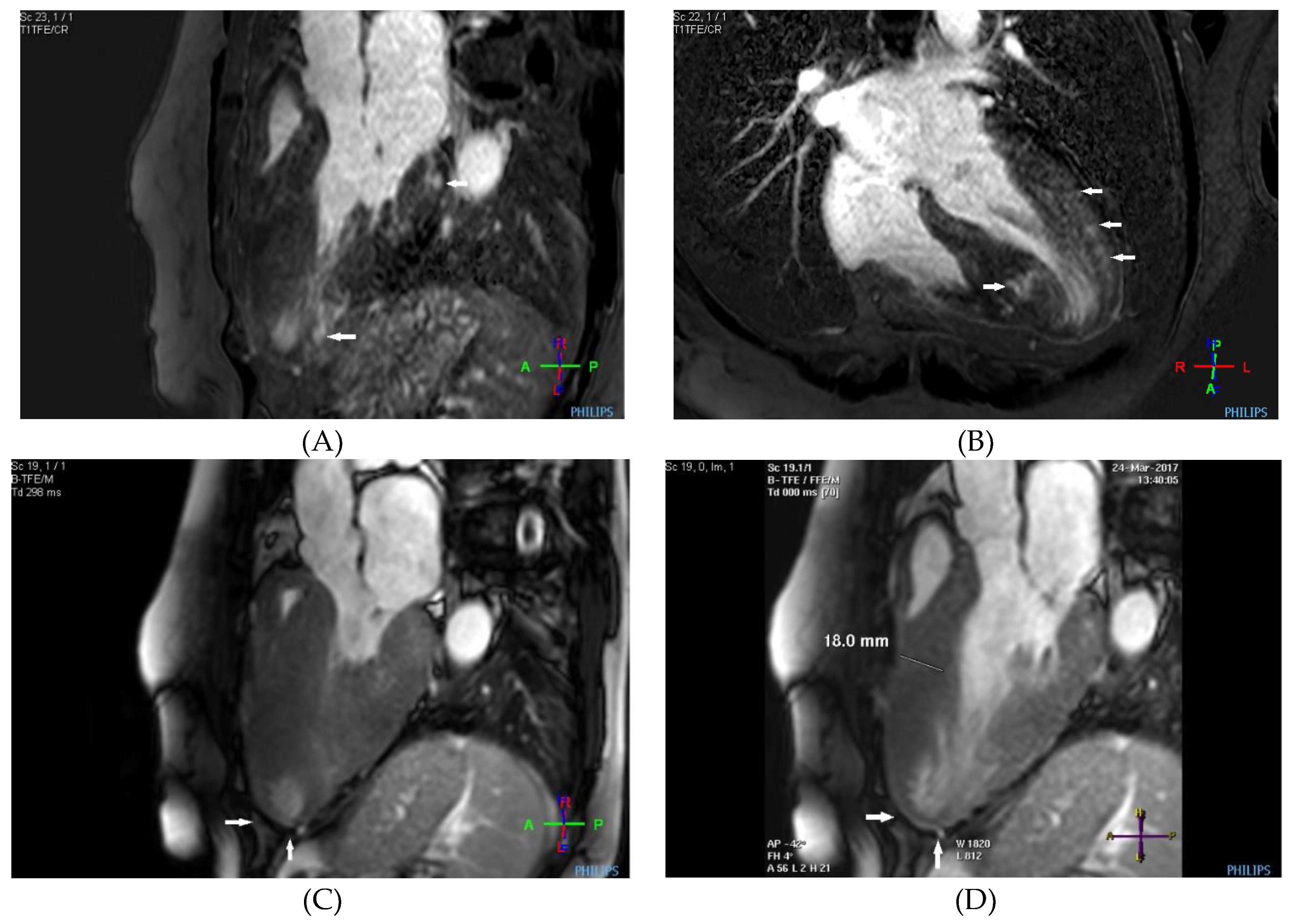
Case description
Discussion
Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- A. Ortiz, D.P. Germain, R.J. Desnick, J. Politei, M. Mauer, A. Burlina, C. Eng, R.J. Hopkin, D. Laney, A. Linhart, S. Waldek, E. Wallace, F. Weidemann, and W.R. Wilcox, Fabry disease revisited: Management and treatment recommendations for adult patients. Molecular genetics and metabolism 123 (2018) 416-427. [CrossRef]
- D.P. Germain, A. Fouilhoux, S. Decramer, M. Tardieu, P. Pillet, M. Fila, S. Rivera, G. Deschênes, and D. Lacombe, Consensus recommendations for diagnosis, management and treatment of Fabry disease in paediatric patients. Clinical genetics 96 (2019) 107-117. [CrossRef]
- T.P. Bernardes, R.D. Foresto, and G.M. Kirsztajn, Fabry disease: genetics, pathology, and treatment. Revista da Associacao Medica Brasileira (1992) 66Suppl 1 (2020) s10-s16. [CrossRef]
- Linhart, and F. Cecchi, Common presentation of rare diseases: Left ventricular hypertrophy and diastolic dysfunction. International journal of cardiology 257 (2018) 344-350. [CrossRef]
- T. Palecek, J. Honzikova, H. Poupetova, H. Vlaskova, P. Kuchynka, L. Golan, S. Magage, and A. Linhart, Prevalence of Fabry disease in male patients with unexplained left ventricular hypertrophy in primary cardiology practice: prospective Fabry cardiomyopathy screening study (FACSS). Journal of inherited metabolic disease 37 (2014) 455-60. [CrossRef]
- D.P. Germain, P.M. Elliott, B. Falissard, V.V. Fomin, M.J. Hilz, A. Jovanovic, I. Kantola, A. Linhart, R. Mignani, M. Namdar, A. Nowak, J.P. Oliveira, M. Pieroni, M. Viana-Baptista, C. Wanner, and M. Spada, The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts. Molecular genetics and metabolism reports 19 (2019) 100454. [CrossRef]
- A. Nowak, T. Mechtler, D.C. Kasper, and R.J. Desnick, Correlation of Lyso-Gb3 levels in dried blood spots and sera from patients with classic and Later-Onset Fabry disease. Molecular genetics and metabolism 121 (2017) 320-324. [CrossRef]
- L. Echevarria, K. Benistan, A. Toussaint, O. Dubourg, A.A. Hagege, D. Eladari, F. Jabbour, C. Beldjord, P. De Mazancourt, and D.P. Germain, X-chromosome inactivation in female patients with Fabry disease. Clinical genetics 89 (2016) 44-54. [CrossRef]
- S. Balendran, P. Oliva, S. Sansen, T.P. Mechtler, B. Streubel, P.N. Cobos, Z. Lukacs, and D.C. Kasper, Diagnostic strategy for females suspected of Fabry disease. Clinical genetics 97 (2020) 655-660. [CrossRef]
- D.P. Germain, E. Brand, A. Burlina, F. Cecchi, S.C. Garman, J. Kempf, D.A. Laney, A. Linhart, L. Maródi, K. Nicholls, A. Ortiz, F. Pieruzzi, S.P. Shankar, S. Waldek, C. Wanner, and A. Jovanovic, Phenotypic characteristics of the p.Asn215Ser (p.N215S) GLA mutation in male and female patients with Fabry disease: A multicenter Fabry Registry study. Molecular genetics & genomic medicine 6 (2018) 492-503. [CrossRef]
- Rodríguez-Marí, M.J. Coll, and A. Chabás, Molecular analysis in Fabry disease in Spain: fifteen novel GLA mutations and identification of a homozygous female. Human mutation 22 (2003) 258. [CrossRef]

| Results | Unit | Cut-off value | |
|---|---|---|---|
| α-Galactosidase A enzyme activity | 0.3 | µmol/l | ˃ 1.2 |
| lyso-Gb3 | 10.0 | ng/mL | 0.0. - 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





