Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
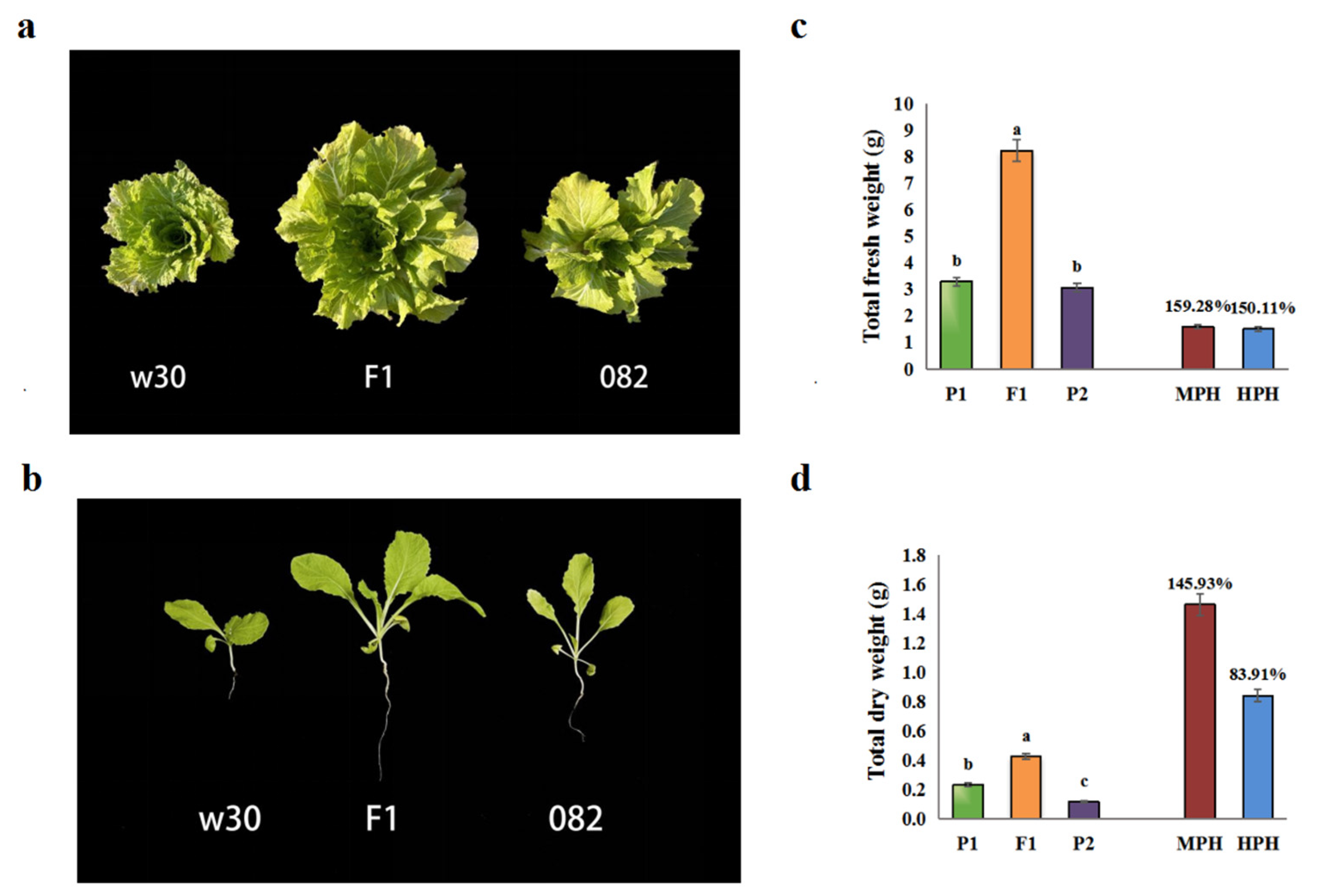
2.1. Heterosis is remarkable in the hybrid F1
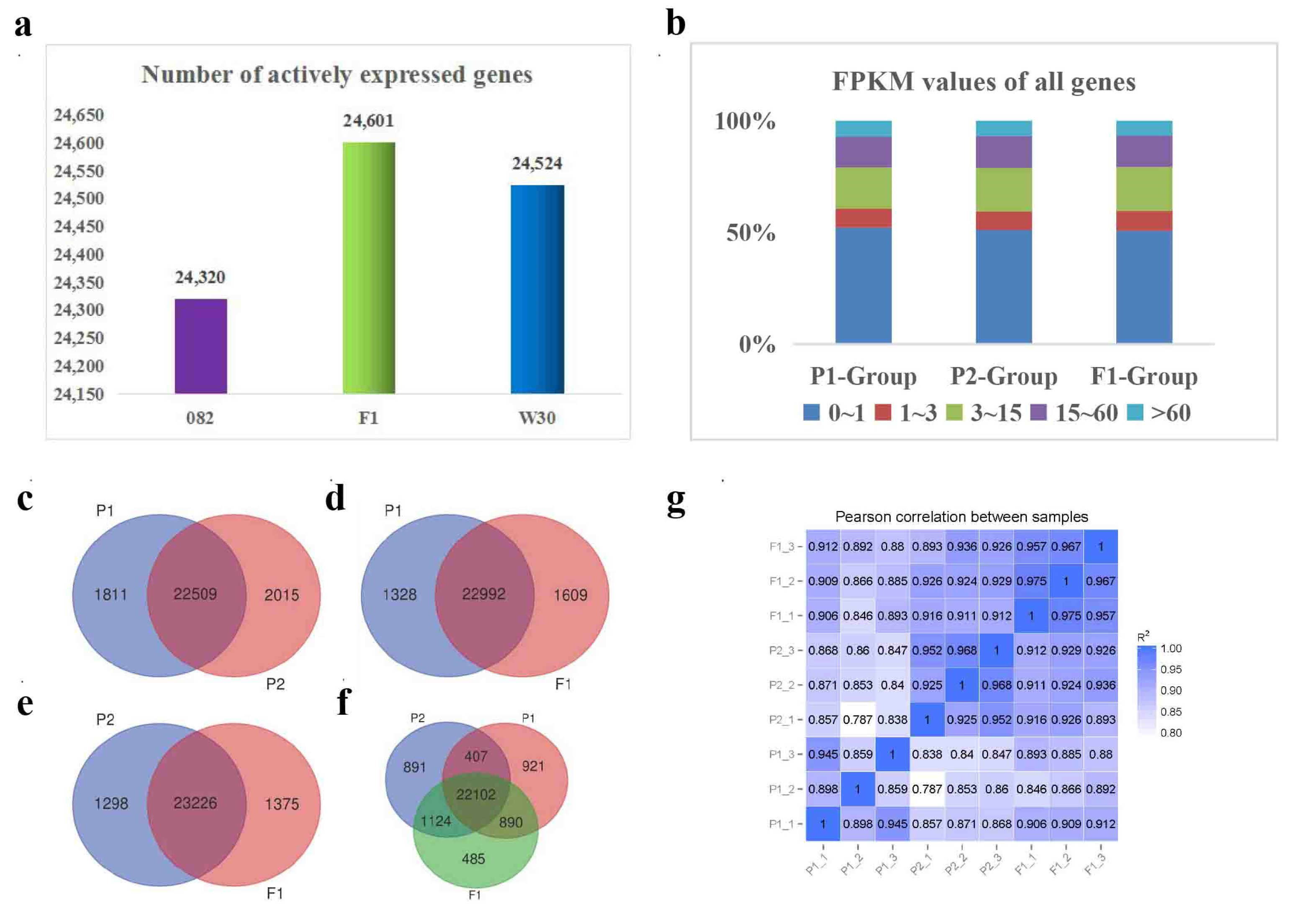
2.2. More genes were actively expressed in the hybrid F1
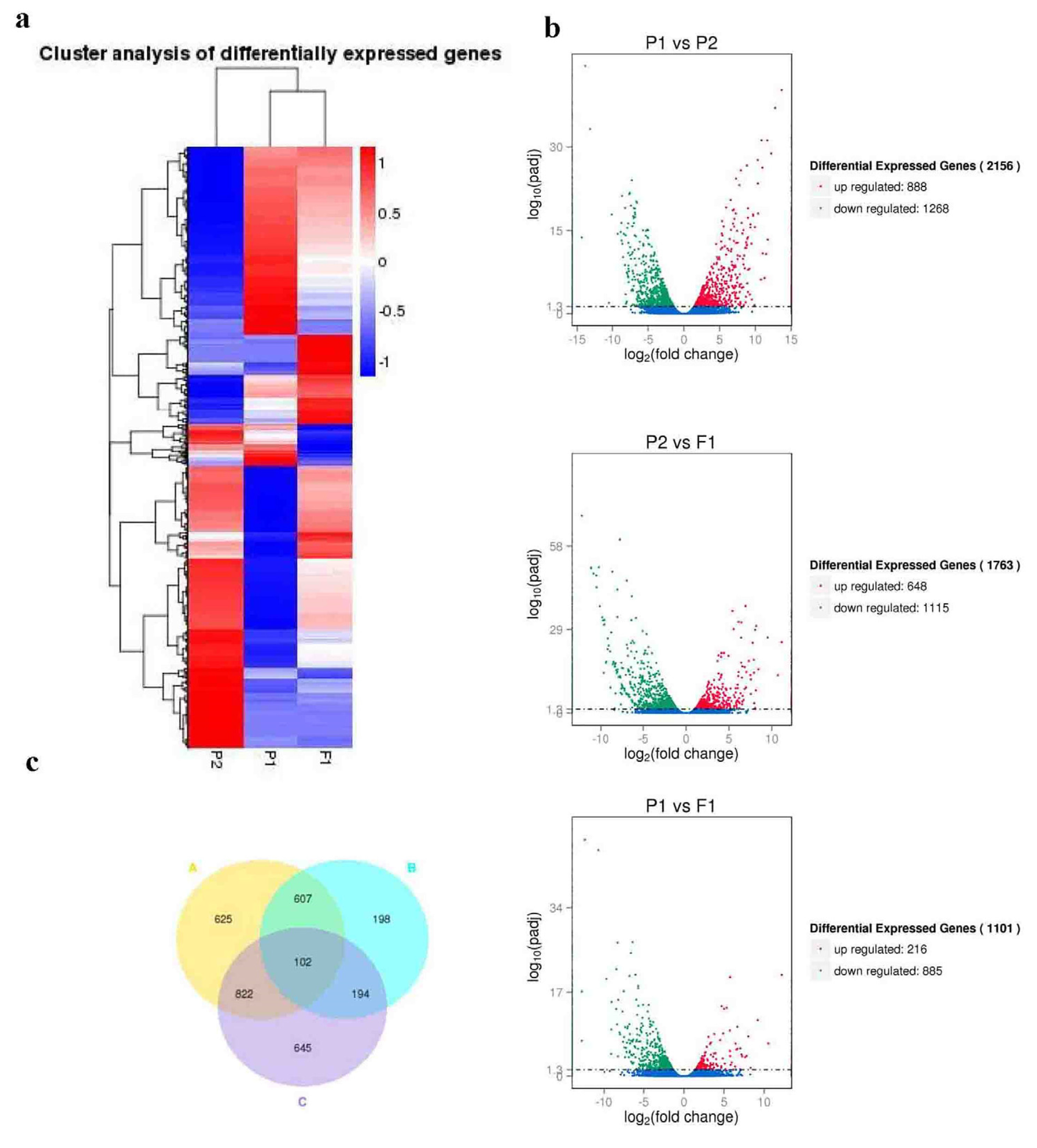
2.3. Transcriptome analysis of DEGs
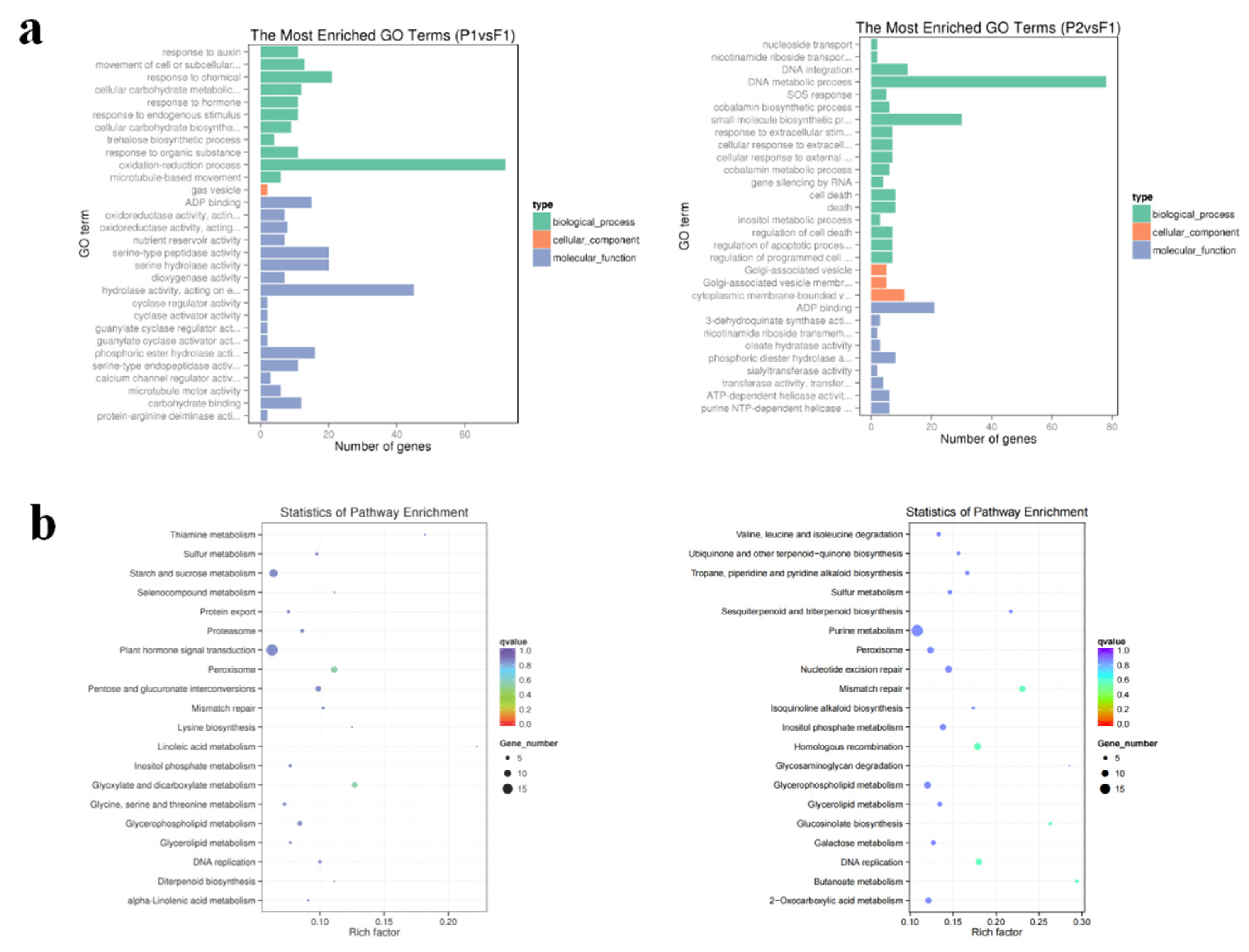
2.4. GO and KEGG analysis of DEGs
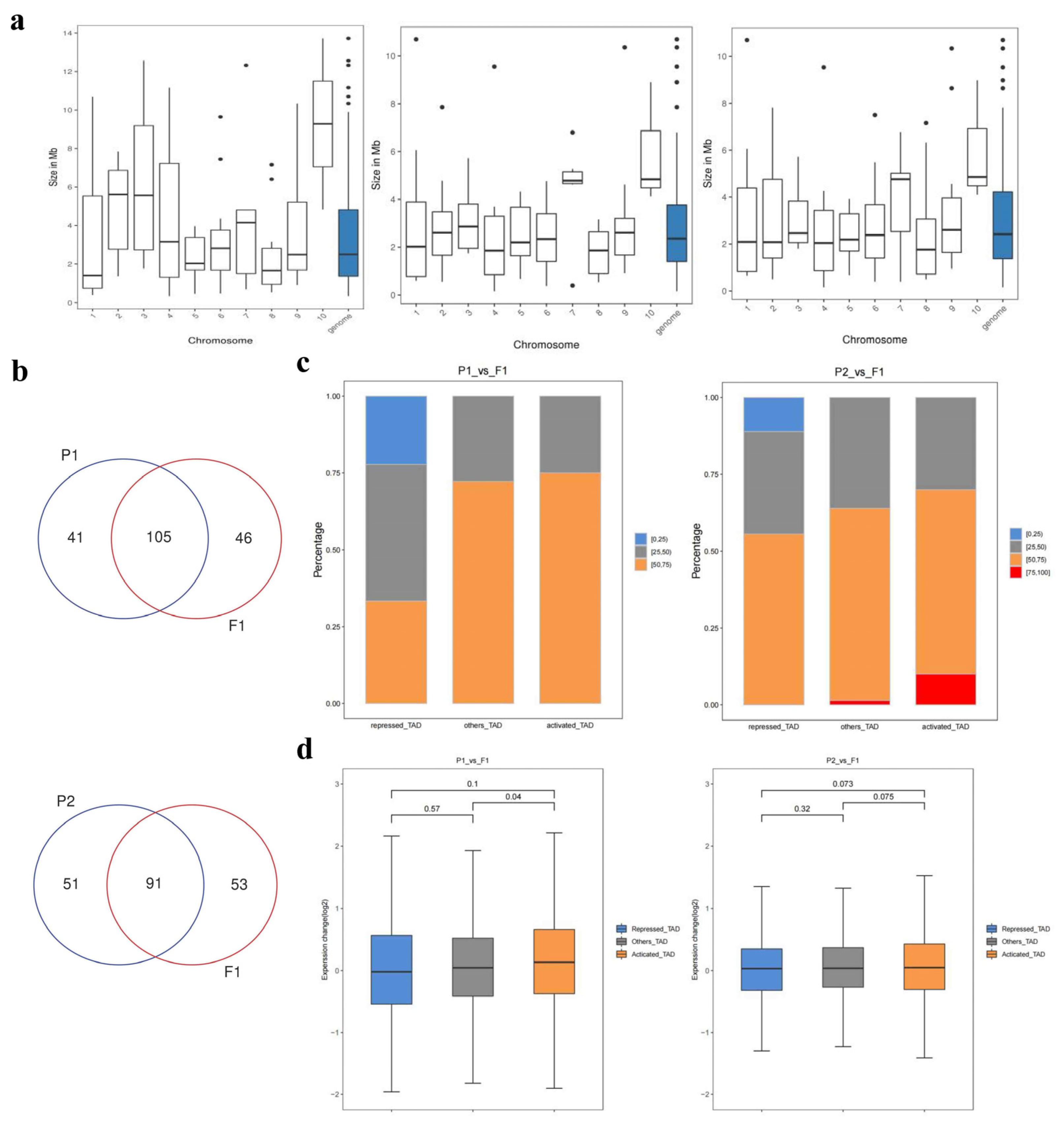
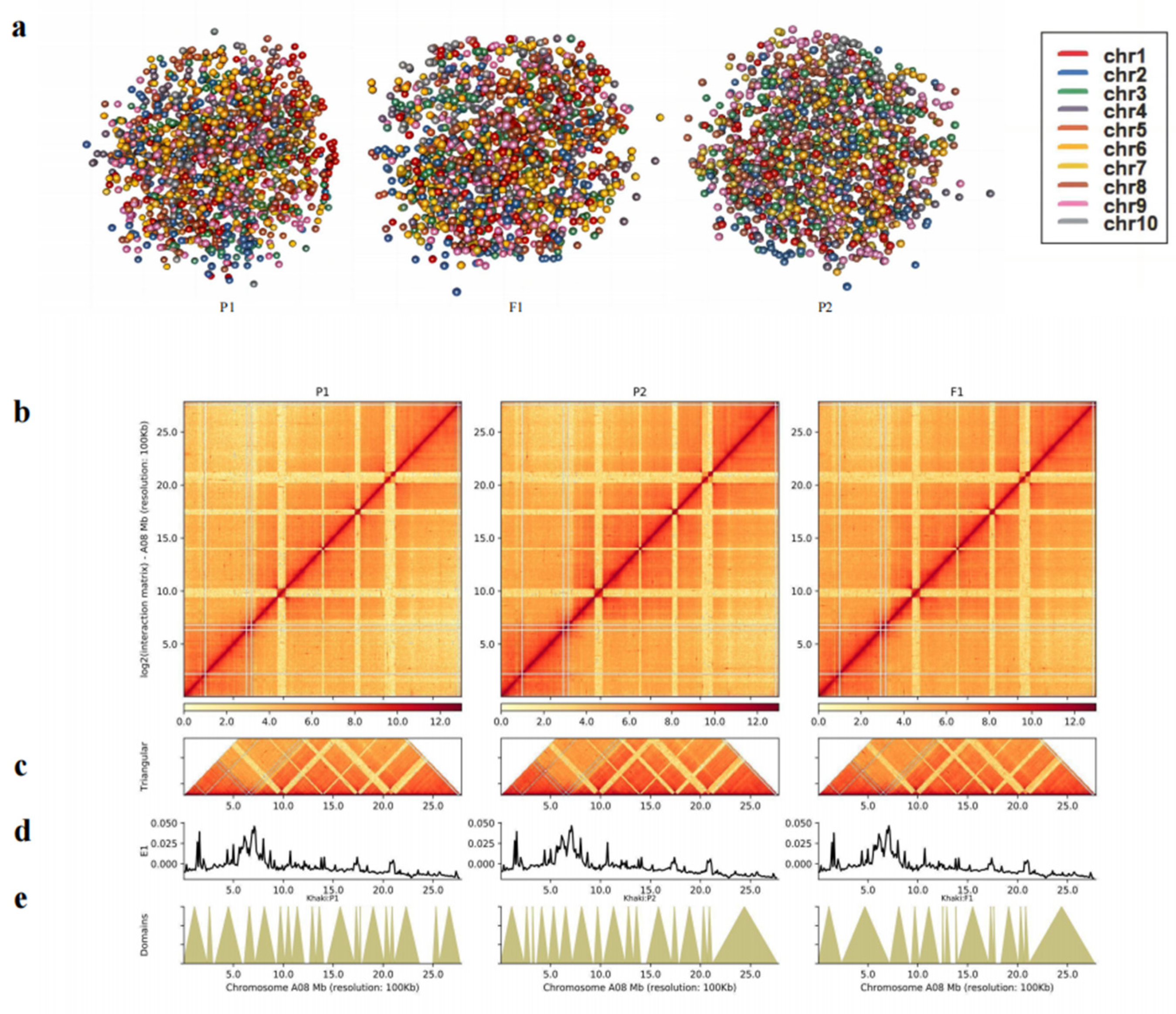
2.5. Genome-wide interaction matrices of parents and F1
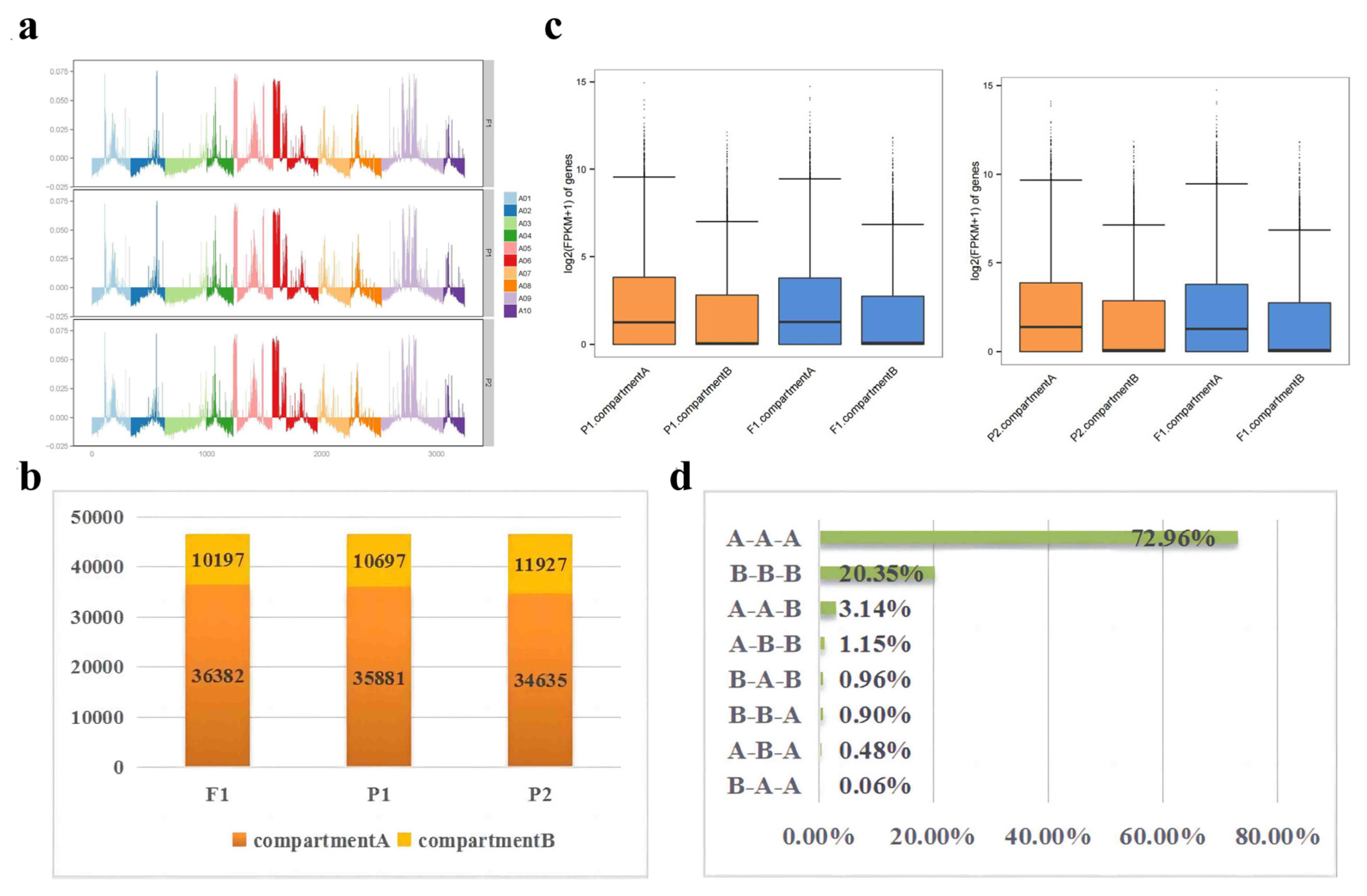
2.6. Identification of A/B Compartment Shifts
2.7. Changes in compartments during hybridization
2.8. Identification of different kinds of topologically associating domains
2.9. Identification of candidate genes for heterosis and verification of qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant materials, growth conditions, and sample collection
4.2. Evaluation of heterosis
4.3. Hi-C libraries preparation and sequencing
4.4. Hi-C read mapping
4.5. RNA-seq experiment and sequencings
4.6. RNA-seq data analysis
4.7. Quantitative reverse-transcription PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEGs | Differentially expressed genes |
| FPKM | Fragments per kilobase of transcript sequence per million base pairs |
| GO | Gene ontology |
| MPV | Mid-parent value |
| qRT-PCR | Quantitative real-time PCR |
| QTL | Quantitative trait locus |
References
- Birchler, J.A.; Yao, H.; Chudalayandi, S.; Vaiman, D.; Veitia, R.A. Heterosis. Plant Cell 2010, 22, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Hoecker, N. Towards the molecular basis of heterosis. Trends Plant Sci 2007, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Birchler, J.A.; Auger, D.L.; Riddle, N.C. In search of the molecular basis of heterosis. Plant Cell 2003, 15, 2236–2239. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Baldauf, J.A. Heterosis in plants. Curr Biol 2018, 28, R1089–R1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, M.; Zhang, Q.; Wei, X.; Huang, X. Exploring the molecular basis of heterosis for plant breeding. J Integr Plant Biol 2020, 62, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.F. Dominance of Linked Factors as a Means of Accounting for Heterosis. PNAS 1917, 3, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.B. THE MENDELIAN THEORY OF HEREDITY AND THE AUGMENTATION OF VIGOR. Science (New York, N.Y.) 1910, 32, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Davenport, C.B. DEGENERATION, ALBINISM AND INBREEDING. Science (New York, N.Y.) 1908, 28, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.F. 90 years ago: The beginning of hybrid maize. Genetics 1998, 148, 923–928. [Google Scholar] [CrossRef]
- East, E.M. Heterosis. Genetics 1936, 21, 375–397. [Google Scholar] [CrossRef]
- Williams, W. Heterosis and the genetics of complex characters. Nature 1959, 184, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Richey, F.D. MOCK-DOMINANCE AND HYBRID VIGOR. Science 1942, 96, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, R.; Uezone, K.; Ishikura, S.; Osabe, K.; Peacoc, W.J.; Dennis, E.S. Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breeding Sci 2018, 68, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet 2013, 14, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.R.; Gorkin, D.U.; Ren, B. Chromatin Domains: The Unit of Chromosome Organization. Mol Cell 2016, 62, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.V.; Scheid, O.M. Stress-induced structural changes in plant chromatin. Curr Opin Plant Biol 2015, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.; De Lucia, F.; Mylne, J.S.; Zhu, D.; Ohmido, N.; Pendle, A.; Kato, N.; Shaw, P.; Dean, C. Physical clustering of FLC alleles during Polycomb-mediated epigenetic silencing in vernalization. Gene Dev 2013, 27, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiong, J.; Shalby, N.; Zhuo, C.; Jia, Y.; Yang, Q.-Y.; Tu, J. Comparison of dynamic 3D chromatin architecture uncovers heterosis for leaf size in Brassica napus. J. Adv. Res 2022, 42, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Sexton, T.; Cavalli, G. The Role of Chromosome Domains in Shaping the Functional Genome. Cell 2015, 160, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Lupianez, D.G.; Kraft, K.; Heinrich, V.; Krawitz, P.; Brancati, F.; Klopocki, E.; Hom, D.; Kayserili, H.; Opitz, J.M.; Laxova, R.; et al. Disruptions of Topological Chromatin Domains Cause Pathogenic Rewiring of Gene-Enhancer Interactions. Cell 2015, 161, 1012–1025. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. NAR 2022. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wang, X.; Liu, B.; Wu, J.; Liang, J.; Cui, Y.; Cheng, F.; Wang, X. Brassica rapa Genome 2.0: A Reference Upgrade through Sequence Re-assembly and Gene Re-annotation. Mol Plant 2017, 10, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Muller, S.; Neusser, M.; von Hase, J.; Calcagno, E.; Cremer, M.; Solovei, I.; Cremer, C.; Cremer, T. Evolutionary conservation of chromosome territory arrangements in cell nuclei from higher primates. PNAS 2002, 99, 4424–4429. [Google Scholar] [CrossRef] [PubMed]
- Ghavi-Helm, Y.; Jankowski, A.; Meiers, S.; Viales, R.R.; Korbel, J.O.; Furlong, E.E.M. Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression. Nat Genet 2019, 51, 1272. [Google Scholar] [CrossRef] [PubMed]
- Grob, S.; Schmid, M.W.; Grossniklaus, U. Hi-C Analysis in Arabidopsis Identifies the KNOT, a Structure with Similarities to the flamenco Locus of Drosophila. Mol Cell 2014, 55, 678–693. [Google Scholar] [CrossRef]
- Gibcus, J.H.; Dekker, J. The Hierarchy of the 3D Genome. Molecular Cell 2013, 49, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Cavalli, G. Organization and function of the 3D genome(vol 17, 661, 2016). Nat Rev Genet 2016, 17. [Google Scholar]
- Song, G.S.; Zhai, H.-L.; Peng, Y.G.; Zhang, L.; Wei, G.; Chen, X.-Y.; Xiao, Y.-G.; Wang, L.; Chen, Y.J.; Wu, B.; et al. Comparative Transcriptional Profiling and Preliminary Study on Heterosis Mechanism of Super-Hybrid Rice. Mol Plant 2010, 3, 1012–1025. [Google Scholar] [CrossRef]
- Wei, G.; Tao, Y.; Liu, G.; Chen, C.; Luo, R.; Xia, H.; Gan, Q.; Zeng, H.; Lu, Z.; Han, Y.; et al. A transcriptomic analysis of superhybrid rice LYP9 and its parents. PNAS 2009, 106, 7695–7701. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, H.; Diao, X.; Liu, Z.; Li, K.; Wu, Y.; Liang, Q.; Wang, H.; Huang, C. Transcriptome profiling and comparison of maize ear heterosis during the spikelet and floret differentiation stages. Bmc Genomics 2016, 17. [Google Scholar] [CrossRef]
- Gaertner, T.; Steinfath, M.; Andorf, S.; Lisec, J.; Meyer, R.C.; Altmann, T.; Willmitzer, L.; Selbig, J. Improved Heterosis Prediction by Combining Information on DNA- and Metabolic Markers. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Lisec, J.; Steinfath, M.; Meyer, R.C.; Selbig, J.; Melchinger, A.E.; Willmitzer, L.; Altmann, T. Identification of heterotic metabolite QTL in Arabidopsis thaliana RIL and IL populations. Plant J 2009, 59, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, B.; Guo, W.; Qin, Y.; Wang, L.; Zhang, Y.; Zhang, T. Quantitative trait loci mapping for yield and its components by using two immortalized populations of a heterotic hybrid in Gossypium hirsutum L. Mol Breeding 2012, 29, 297–311. [Google Scholar] [CrossRef]
- Zhang, H.Y.; He, H.; Chen, L.-B.; Li, L.; Liang, M.-Z.; Wang, X.-F.; Liu, X.-G.; He, G.-M.; Chen, R.-S.; Ma, L.-G.; et al. A genome-wide transcription analysis reveals a close correlation of promoter INDEL polymorphism and heterotic gene expression in rice hybrids. Mol Plant 2008, 1, 720–731. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Du, Z.; Wei, L.; Fang, H.; Dong, Q.; Niu, J.; Li, Y.; Gao, J.; Zhang, M.Q.; et al. The loss of heterochromatin is associated with multiscale three-dimensional genome reorganization and aberrant transcription during cellular senescence. Genome Res 2021, 31, 1121. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y. Deciphering Hi-C: From 3D genome to function. Cell Biol Toxico 2019, 35, 15–32. [Google Scholar] [CrossRef] [PubMed]
- van Berkum, N.L.; Lieberman-Aiden, E.; Williams, L.; Imakaev, M.; Gnirke, A.; Mirny, L.A.; Dekker, J.; Lander, E.S. Hi-C: A method to study the three-dimensional architecture of genomes. JoVE 2010. [Google Scholar] [CrossRef]
- Wingett, S.; Ewels, P.; Furlan-Magaril, M.; Nagano, T.; Schoenfelder, S.; Fraser, P.; Andrews, S. HiCUP: Pipeline for mapping and processing Hi-C data. F1000Research 2015, 4, 1310–1310. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012, 9, 357–U354. [Google Scholar] [CrossRef] [PubMed]
- Szalaj, P.; Tang, Z.; Michalski, P.; Pietal, M.J.; Luo, O.J.; Sadowski, M.; Li, X.; Radew, K.; Ruan, Y.; Plewczynski, D. An integrated 3-Dimensional Genome Modeling Engine for data-driven simulation of spatial genome organization. Genome Res 2016, 26, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat Methods 2015, 12, 357–U121. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 2004, 37, 112. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat Protoc 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
| Hybrid set samples |
Up | Down | B2P | Total |
|---|---|---|---|---|
| 082 vs w30 | 888 (41.19%) | 1268(58.81%) | 2156 | |
| F1 vs 082 | 885(80.38%) | 216(19.62%) | 1101 | |
| F1 vs w30 | 1115(63.24%) | 648(36.76%) | 1763 | |
| F1 vs (082 and w30) | 173 (58.45%) | 32(10.81%) | 91(30.74%) | 296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





