Submitted:
17 December 2023
Posted:
28 December 2023
You are already at the latest version
Abstract
Keywords:
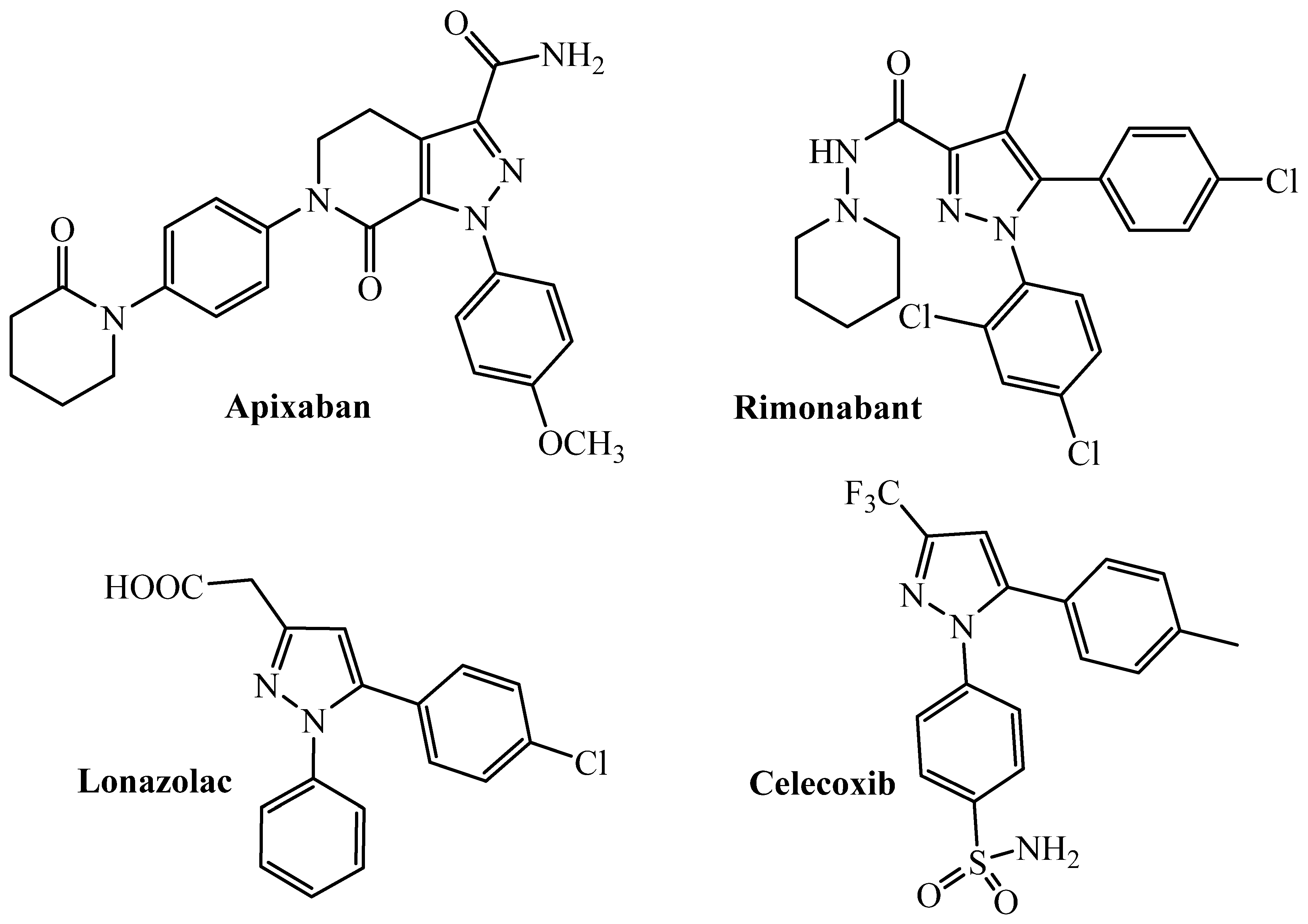
1. Introduction
2. Results and Discussion
3. Materials and methods
3.1. General Information
3.2. Synthesis of Compounds
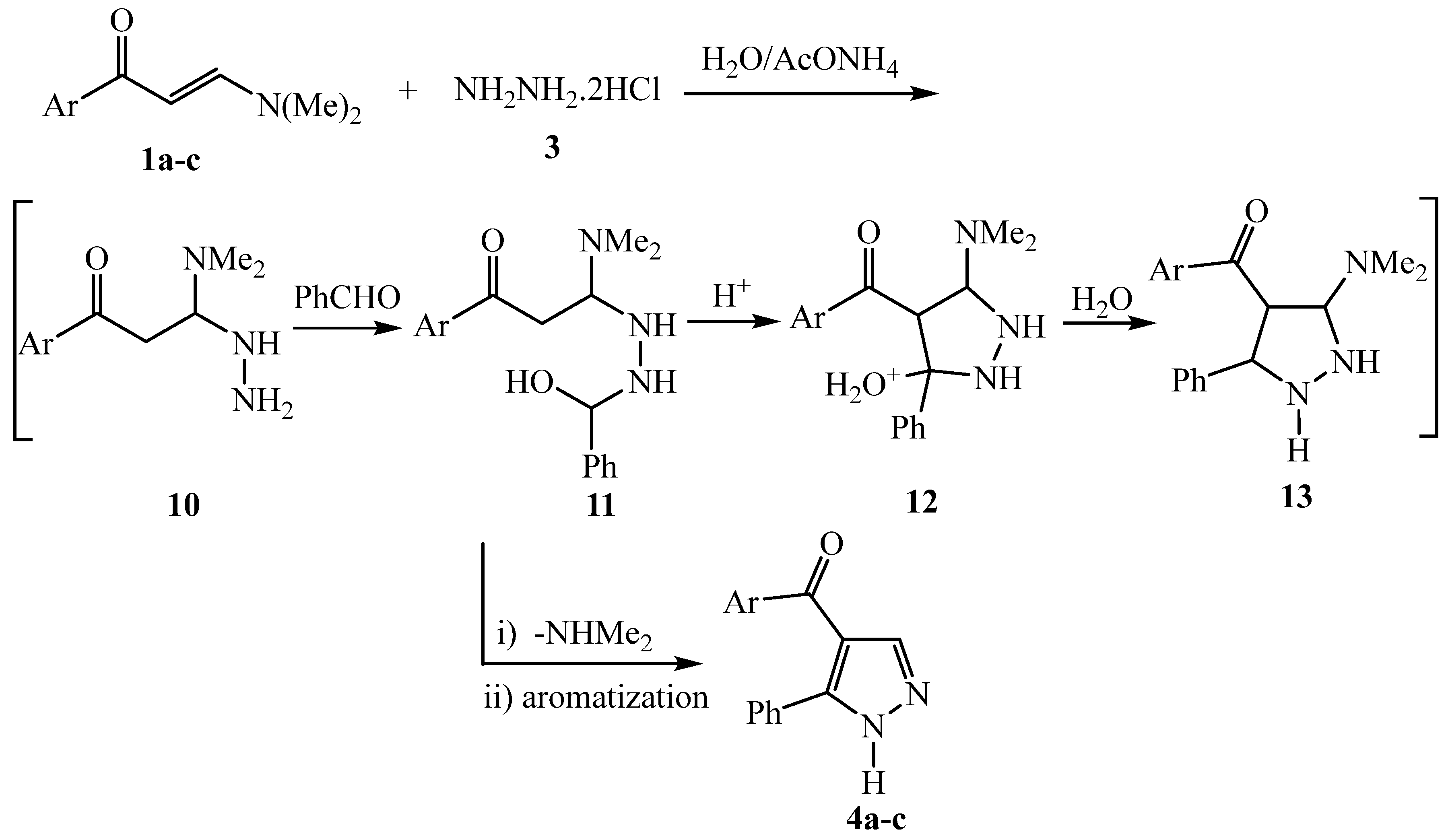
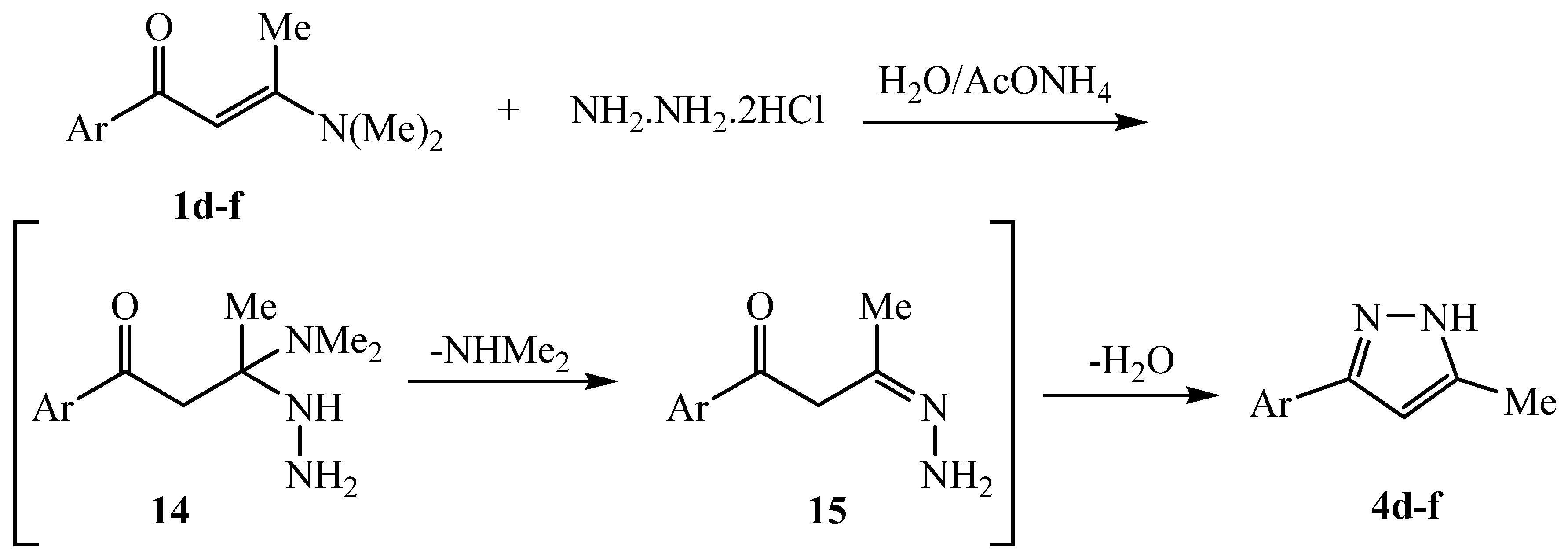
3.2.1. General procedure for compounds 1a-c and 1d-f
3.2.2. 3-Hydroxy-1-phenylbut-2-en-1-one 1d
3.2.3. 3-(Dimethylamino)-1-(thiophen-2-yl)but-2-en-1-one 1e
3.2.4. 3-(Dimethylamino)-1-(furan-2-yl)but-2-en-1-one 1f
3.2.5. General procedure for the preparation of 4a-c and 4d-f
3.2.6. Phenyl(3-phenyl-1H-pyrazol-4-yl)methanone 4a
3.2.7. (3-phenyl-1H-pyrazol-4-yl)(thiophen-2-yl)methanone 4b
3.2.8. Furan-2-yl(3-phenyl-1H-pyrazol-4-yl)methanone 4c
3.2.9. 5-methyl-3-phenyl-1H-pyrazole 4d
3.2.10. 5-methyl-3-(thiophen-2-yl)-1H-pyrazole 4e
3.2.11. 3-(Furan-2-yl)-5-methyl-1H-pyrazole 4f
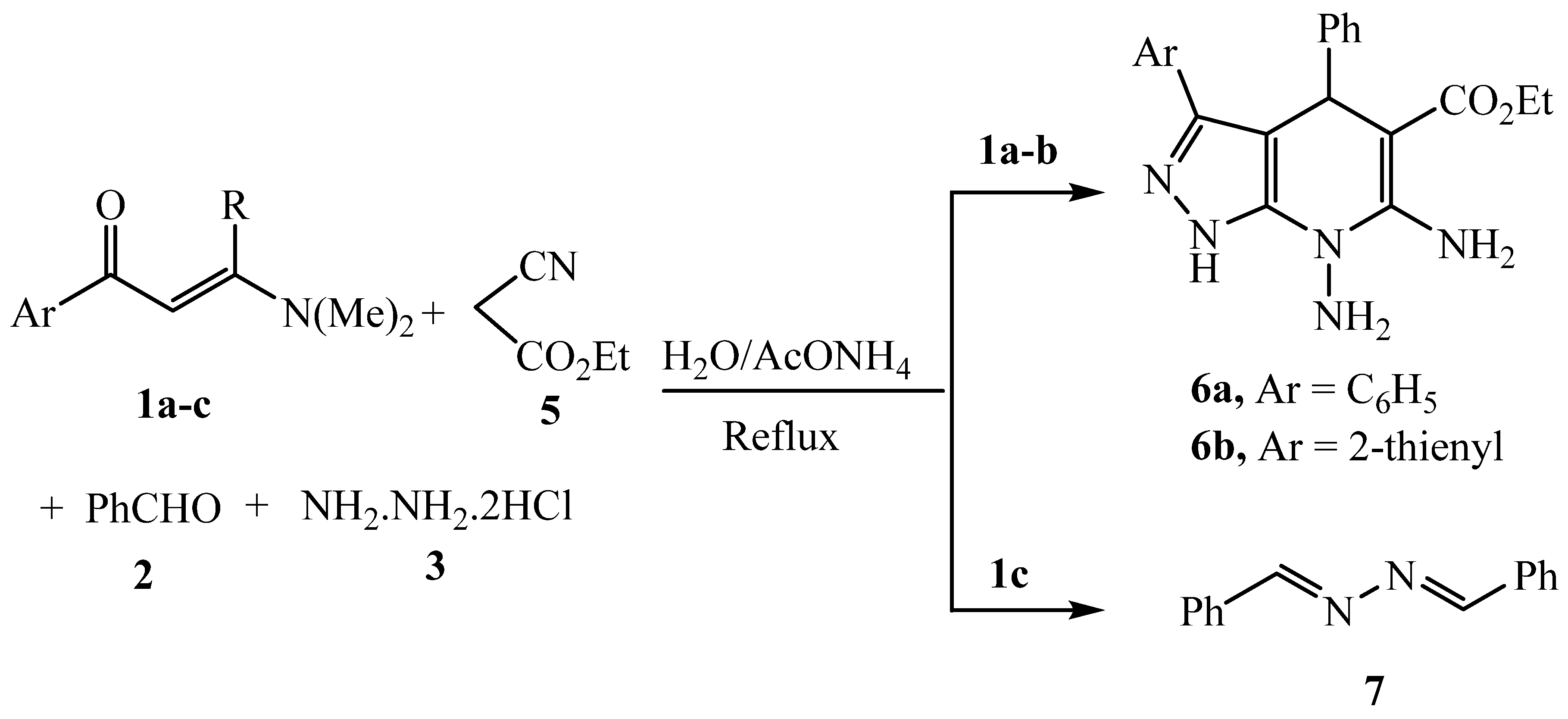
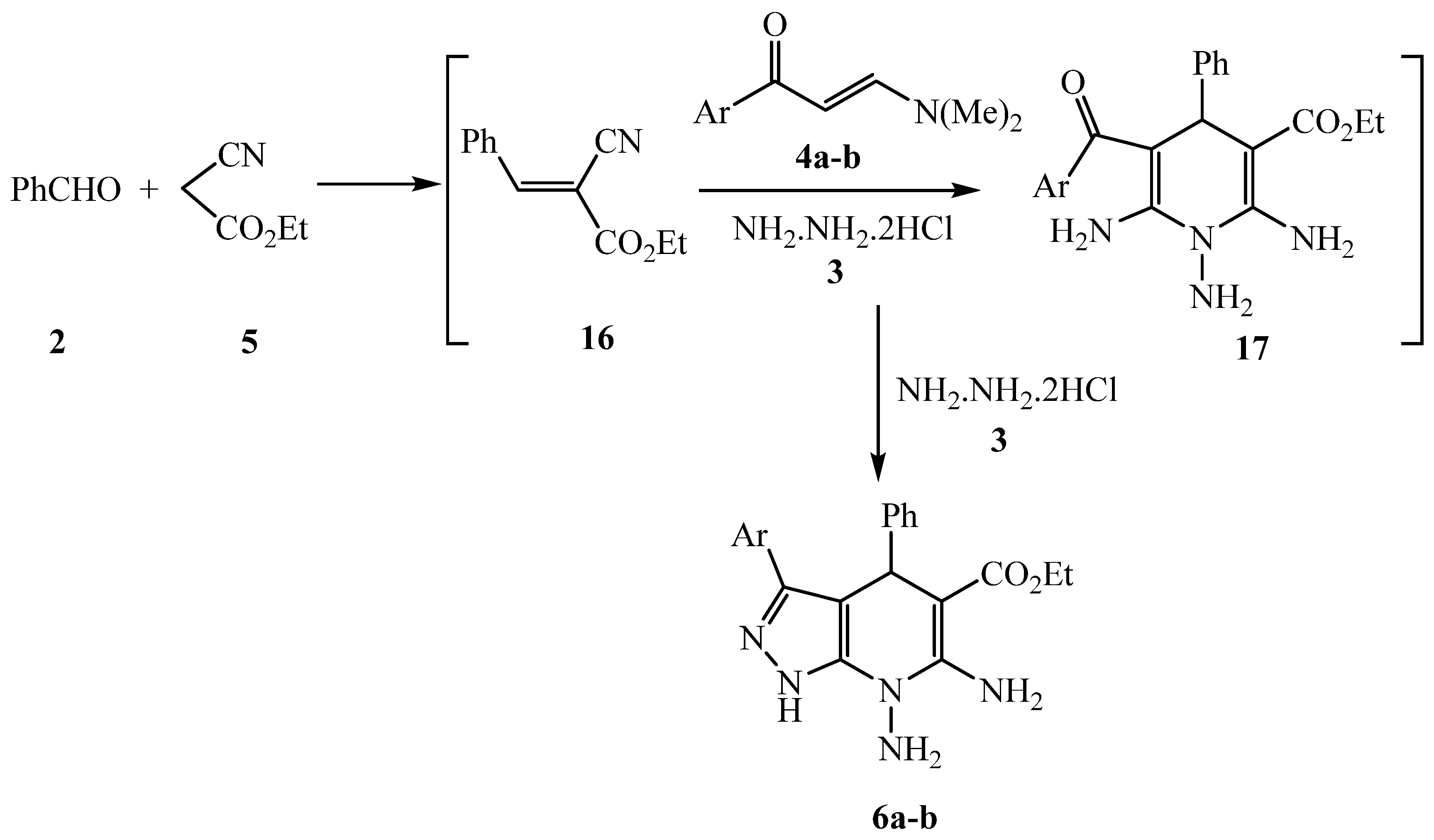
3.2.12. General procedure for the synthesis of 6a-b and 7
3.2.13. Ethyl 6,7-diamino-3,4-diphenyl-4,7-dihydro-1H-pyrazolo[3,4-b]pyridine-5-carboxylate 6a
3.2.14. Ethyl 6,7-diamino-4-phenyl-3-(thiophen-2-yl)-4,7-dihydro-1H-pyrazolo[3,4-b]pyridine-5-carboxylate 6b
3.2.15. 1,2-Dibenzylidenehydrazine 7
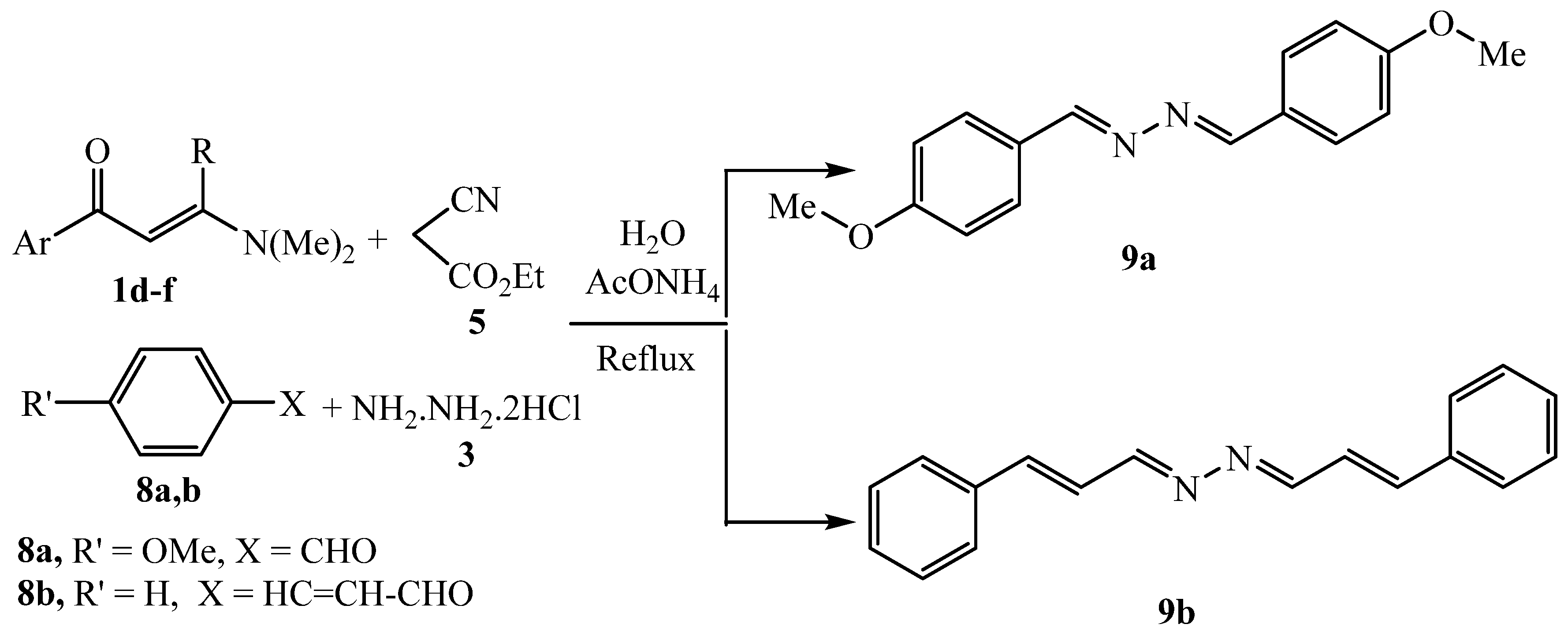
3.2.16. General procedure for the preparation of 9a
3.2.17. 1,2-bis(4-methoxybenzylidene)hydrazine 9a
3.2.18. 1,2-bis-3-phenylallylidene)hydrazine 9b
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Musilek, K.; Dolezal, M.; Gunn-Moore, F.; Kuca, K. Design, evaluation and structure-Activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med. Res. Rev. 2011, 31, 548–75. [Google Scholar] [CrossRef]
- Figueroa-Villar, J.D.; Petronilho, E.C.; Kuca, K.; Franca, T.C.C. Review about Structure and Evaluation of Reactivators of Acetylcholinesterase Inhibited with Neurotoxic Organophosphorus Compounds. Curr. Med. Chem. 2021, 28, 1422–42. [Google Scholar] [CrossRef] [PubMed]
- Demkowicz, S.; Rachon, J.; Daśko, M.; Kozak, W. Selected organophosphorus compounds with biological activity. Applications in medicine RSC Adv. 2016, 6, 7101–12. [Google Scholar] [CrossRef]
- Joly, D.; Bouit, P.A.; Hissler, M. Organophosphorus derivatives for electronic devices. J Mater Chem. C. 2016, 4, 3686–98. [Google Scholar] [CrossRef]
- Elguero, J., et al. (2002) Pyrazoles as Drugs: Facts and Fantasies. Targets in Heterocyclic Systems. 2002, 6, 52–98. ISBN 88-86208-19-7.
- Eicher, T.; Hauptman, S. The chemistry of Heterocycles Structure, Reactions, Synthesis and Application (Translated by Suschitzky H, Suschitzky J.). Georg Thieme: Stuttgart, 1995.
- Huang, Y.R.; Katzenellenbogen, J.A. Regioselective synthesis of 1,3,5-triaryl-4-alkylpyrazoles: novel ligands for the estrogen receptor. Org Lett. 2000, 2, 2833–6. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Wang, M.; Zhang, S.; Voronkov, M.V; Steel, P.J. Regioselective Synthesis of Polysubstituted Pyrazoles and Isoxazoles. J. Org. Chem. 2001, 66, 6787–91. [Google Scholar] [CrossRef]
- Kim, J.; Song, H.; Park, S.B. Orthogonal Regioselective Synthesis of N-Alkyl-3-substituted Tetrahydroindazolones. Eur. J. Org. Chem. 2010, 20, 3815–22. [Google Scholar] [CrossRef]
- Chimichi, S.; Boccalini, M.; Matteucci, H. Regioselective synthesis of 2,5-dihydro-4H-pyrazolo[4,3-c]quinolin-4-ones by the cyclization of 3-acyl-4-methoxy-1-methylquinolinones with hydrazines. Tetrahedron. 2008, 64, 9275–79. [Google Scholar] [CrossRef]
- Padwa, A. “1,3-Dipolar Cycloaddition Chemistry; John Wiley: Vol.1. New York: Ny; 1984.
- Elmaged, A.; Sammour, A. Notes- Action of Hydroxylamine, Hydrazine Hydrate, and Phenylhydrazine on 2-Acetoaceto-1-naphthol J. Org. Chem. 1960, 25, 1458–59. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Vicente, J.d.; Bonnert. R.V. A Novel One-Pot Method for the Preparation of Pyrazoles by 1,3-Dipolar Cycloadditions of Diazo Compounds Generated in Situ. J. Org. Chem. 2003, 68, 5381–83. [CrossRef]
- Lèvai, A.; Silva, A.M.S.; Cavaleiro, J.A.S.; Alkorta, I.; Elguero, J.; Jekö, J. Synthesis of Pyrazoles by Treatment of 3-Benzylchromones, 3-Benzylflavones and Their 4-Thio Analogues with Hydrazine. Eur. J. Org. Chem. 2006, 2006, 2825–32. [Google Scholar] [CrossRef]
- Sviridov, S.I.; Vasil'ev, A.A.; Shorshnev, S.V. Straightforward transformation of isoxazoles into pyrazoles: renewed and improved. Tetrahedron. 2007, 63, 12195–201. [Google Scholar] [CrossRef]
- Chavva, K.; Pillalamarri, S.; Banda, V.; Gautham, S.; Gaddamedi, J.; Yedla, P.; Kumar, C.G.; Banda, N. Synthesis and biological evaluation of novel alkyl amide functionalized trifluoromethyl substituted pyrazolo[3,4-b] pyridine derivatives as potential anticancer agents. Bioorg Med Chem Lett. 2013, 23, 5893–5. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Perumal, S.; Menéndez, J.C.; Mancinelli, M.; Ranieri, S.; Mazzanti, A. Axial Chirality of 4-Arylpyrazolo[3,4-b]pyridines. Conformational Analysis and Absolute Configuration. J. Org. Chem. 2014, 79, 11039–50. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Xu, P.; Dai, Y.; Luo, C.; Sun, Y.; Ai, J.; Geng, M.; Duan, W. Discovery of Substituted 1H-Pyrazolo[3,4-b] pyridine Derivatives as Potent and Selective FGFR Kinase Inhibitors. ACS Med. Chem. Lett. 2016, 7, 629–34. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia, G.; Berteina-Raboin, S.; Guillaumet, G. Synthesis and Functionalization of 1H-Pyrazolo[3,4-b] pyridines Involving Copper and Palladium-Promoted Coupling Reactions. Tetrahedron Lett. 2004, 45, 2389–92. [Google Scholar] [CrossRef]
- El-Emary, T.I.; Chin, J. Synthesis of Newly Substituted Pyrazoles and Substituted Pyrazolo[3,4-b]Pyridines Based on 5-Amino-3-Methyl-1-Phenylpyrazole. Chem Soc. 2007, 54, 507–18. [Google Scholar] [CrossRef]
- Ghaedi, A.; Bradajee, G.R.; Mirshokrayi, A.; Mahdavi, M.; Shafiee, A.; Akbarzadeh, T. Facile, novel and efficient synthesis of new pyrazolo[3,4-b]pyridine products from condensation of pyrazole-5-amine derivatives and activated carbonyl groups. RSC Adv. 2015, 5, 89652–58. [Google Scholar] [CrossRef]
- Kendre, D.B.; Toche, R.B.; Jachak, M.N. Synthesis of pyrazolo[3,4-b]pyridines and attachment of amino acids and carbohydrate as linkers. J. Heterocycl. Chem. 2008, 45, 1281–86. [Google Scholar] [CrossRef]
- Chebanov, V.A.; Saraev, V.E.; Desenko, S.M.; Chernenko, V.N.; Knyazeva, I.V.; Groth, U.; Glasnov, T.N.; Kappe, C.O. Tuning of Chemo- and Regioselectivities in Multicomponent Condensations of 5-Aminopyrazoles, Dimedone, and Aldehydes. J. Org. Chem. 2008, 73, 5110–18. [Google Scholar] [CrossRef]
- Fu, R.G.; You, Q.D.; Yang, L.; Wu, W.T.; Jiang, C.; Xu, X.L. Design, synthesis and bioevaluation of dihydropyrazolo[3,4-b]pyridine and benzo[4,5]imidazo[1,2-a]pyrimidine compounds as dual KSP and Aurora-A kinase inhibitors for anti-cancer agents. Bioorg. Med. Chem. 2010, 18, 8035–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liu, Y.P.; Xu, B.H.; Wang, X.H.; Jiang, B.; Tu, S.J. Microwave-assisted chemoselective reaction: a divergent synthesis of pyrazolopyridine derivatives with different substituted patterns. Tetrahedron. 2011, 67, 9417–25. [Google Scholar] [CrossRef]
- Hao, Y.; Xu, X.P.; Chen, T.; Zhao, L.L.; Ti, S.J. Multicomponent approaches to 8-carboxylnaphthyl-functionalized pyrazolo[3,4-b]pyridine derivatives. Org. Biomol. Chem. 2012, 10, 724–28. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yao, C.; Wei, X. FeCl3-catalyzed multicomponent synthesis of 8-alkoxycarbonylnaphthyl-functionalized pyrazolo[3,4-b]pyridines involving C-C bond cleavage. Monatsh Chem. 2016, 147, 1597–603. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.B. An Efficient One-Step Synthesis of Heterobiaryl Pyrazolo[3,4-b]pyridines via Indole Ring Opening. Org Lett. 2009, 11, 5214–17. [Google Scholar] [CrossRef] [PubMed]
- Nagender, P.; Kumar, R.N.; Reddy, G.M.; Swaroop, D.K.; Poornachandra, Y.; Kumar, C.G.; Narsaiah, B. Synthesis of novel hydrazone and azole functionalized pyrazolo[3,4-b]pyridine derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4427–32. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew Chem Int Ed Engl. 2006, 45, 7134–86. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Brasche, G.; Gericke, K.M. In: Domino Reaction in Organic Synthesis, Wieley-VCH, Weinheim; 2006.
- Murzin, D.Y.; Leino, R. Design. Sustainable chemical technology through catalytic multistep reactions. Chem. Eng. Res. 2008, 86, 1002–10. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int J Mol Sci. 2015, 16, 17101–59. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and solvent effects in Organic chemistry. 4th ed. Wiely-VCH Verlag Gmbll & Co. KGan: Weinheim Germany; 2011.
- Grieco, P.A. organic synthesis in water. Blackie A&P: London; 1998.
- Siskin, M.; Katritzky, A.R. Reactivity of Organic Compounds in Superheated Water: General Background. Chem. Rev. 2001, 101, 825–36. [Google Scholar] [CrossRef]
- Poliakoff, M.; Fitzpatrick, J.M.; Farren, T.R.; Anastas, P.T. Green chemistry: science and politics of change. Science. 2002, 297, 807–10. [Google Scholar] [CrossRef] [PubMed]
- Ghanda, A.; Fokin, V.V. Organic synthesis “on water”. Chem. Rev. 2009, 109, 725–48. [Google Scholar]
- Minakata, S.; Komatsu, M. Organic Reactions on Silica in Water. Chem. Rev. 2009, 109, 711–24. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, J.M. Practical Approaches to Green Solvents. Science. 2002, 297, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Santosh, V.N.; Ajay, P.N.; Mohan, B.K.; Vijay, S.P.; Umesh, D.P.; Kamlesh, R.D.; Shamkant, L.P.; Sidhanath, V.B. One-Pot Four Component Synthesis of 4, 6-Disubstituted 3-Cyano-2- Pyridones in Polyethylene Glycol. Lett. in Org. Chem. 2010, 7, 406–10. [Google Scholar]
- Moustafa, M.S.; Mekheimer, R.A.; Al-Mousawi, S.M.; Abd-Elmonem, M.; El-Zorba, H.; Abdel Hameed, A.M.; Mohamed, T.M.; Sadek, K.U. Microwave-assisted efficient one-pot synthesis of N2-(tetrazol-5-yl)-6-aryl/heteroaryl-5,6-dihydro-1,3,5-triazine-2,4-diamines. Beilstein J. Org. Chem. 2020, 16, 1706–12. [Google Scholar] [CrossRef] [PubMed]
- Alnajjar, A.; Abdelkhalik, M.M.; Riad, H.M.; Sayed, S.M.; Sadek, K.U. Regioselectivity in the Reaction of 2-Aminobenzothiazoles and 2-Aminobenzimidazoles with Enaminonitriles and Enaminones: Synthesis of Functionally Substituted Pyrimido[2,1-b][1,3]benzothiazole and Pyrimido[1,2-a]benzimidazole Derivatives. J. Heterocycl. Chem. 2018, 55, 2760–65. [Google Scholar] [CrossRef]
- Sadek, K.U.; Al-Qalaf, F.; Abdelkhalik, M.M.; Elnagdi, M.H. Cerium (IV) ammonium nitrate as an efficient Lewis acid for the one-pot synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones and their corresponding 2-(1H) thiones. J. Heterocycl. Chem. 2010, 47, 284–87. [Google Scholar] [CrossRef]
- Nazmy, M.H.; Mekheimer, R.A.; Shoman, M.E.; Abo-Elsebaa, M.; Abel-Elmonem, M.; Sadek, K.U. Densely functionalized cinnolines: Controlled microwave-assisted facile one-pot multi-component synthesis and in vitro anticancer activity via apoptosis induction. Bioorg. Chem. 2020, 101, 103932. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Allam, S.M.R.; Al-Sheikh, M.A.; Moustafa, M.S.; Almousawi, S.M.; Mostafa, Y.A.; Youssif, B.G.M.; Gomaa, H.A.M.; Hayallah, A.M.; Abdelaziz, M.; Sadek, K.U. Discovery of new pyrimido [5, 4-c] quinolines as potential antiproliferative agents with multitarget actions: Rapid synthesis, docking, and ADME studies. Bioorg. Chem. 2022, 121, 105693. [Google Scholar] [CrossRef]
- Stephan, M.; Panther, J.; Wilbert, F.; Ozog, P.; Müller, T.J.J. Heck Reactions of Acrolein or Enones and Aryl Bromides – Synthesis of 3-Aryl Propenals or Propenones and Consecutive Application in Multicomponent Pyrazole Syntheses. Eur. J. Org. Chem. 2020, 2086–92. [Google Scholar] [CrossRef]
- Al-Omran, F.; Al-Awadhi, N.; Abdel Khalik, M.M.; Kaul, K.; Abu EL-Khair, A.; Elnagdi, M.H. 1-Substituted 3-Dimethylaminoprop-2-en-1-ones as Building Blocks in Heterocyclic Synthesis: Routes to 6-Aryl- and 6-Heteroaryl-2H-pyran-2-ones and 6- and 4-Arylpyridin-2(1H)-ones. J Chem. Res. (S). 1997, 84–85. [Google Scholar] [CrossRef]
- Chemical Book (E,E)-bis(phenylmethylidene)hydrazine CAS No.588-68-1 Chemical Name: Benzaldehyde Azine.
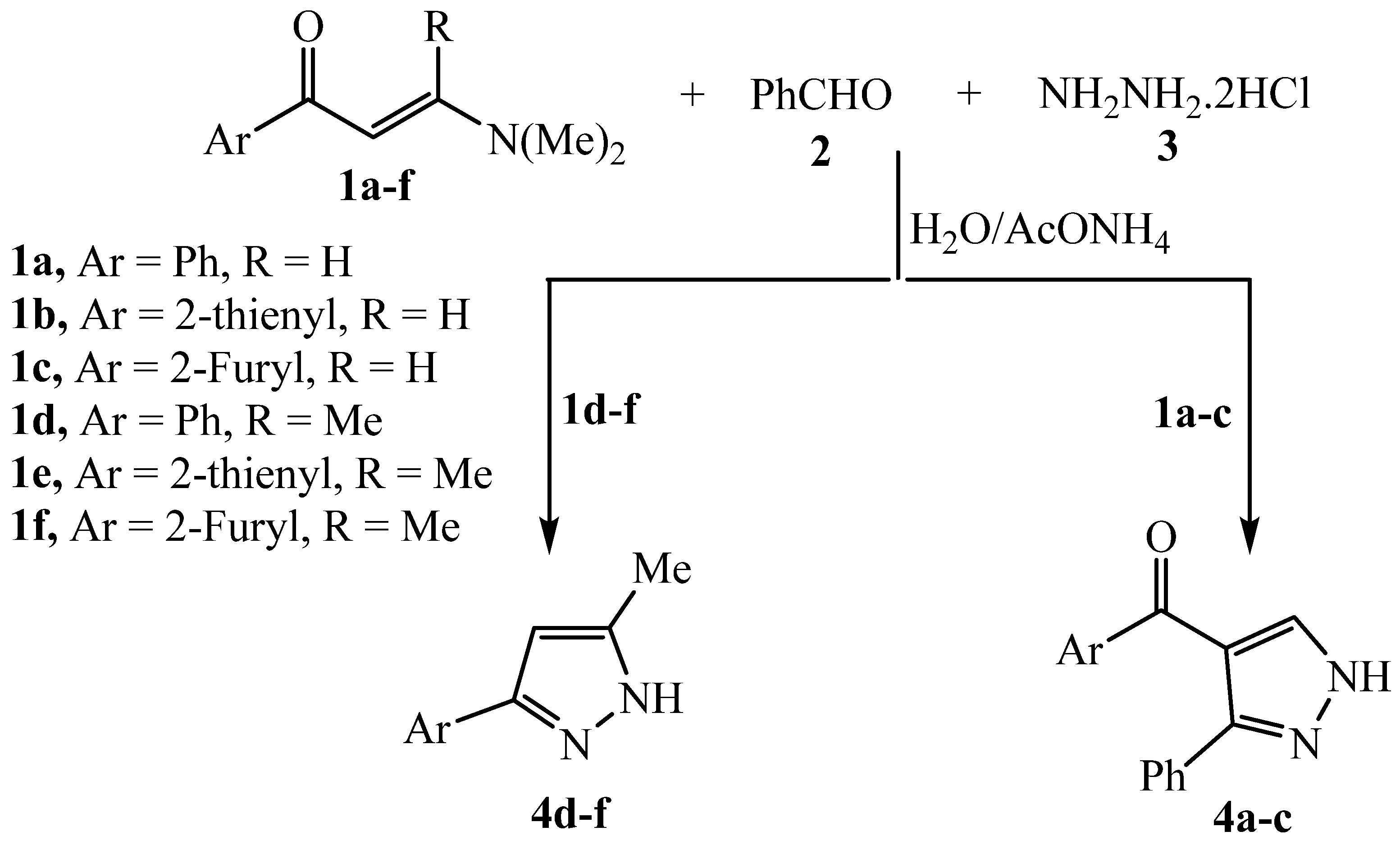
| Product | R | Ar | Yield % | m.p [oC] |
|---|---|---|---|---|
| 4a | H | Phenyl | 75 | 160-162 |
| 4b | H | 2-Thienyl | 77 | 156-158 |
| 4c | H | 2-Furyl | 73 | 170-172 |
| 4d | Methyl | Phenyl | 60 | 124-126 |
| 4e | Methyl | 2-Thienyl | 66 | 173-175 |
| 4f | Methyl | 2-Furyl | 63 | 162-164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




