Submitted:
05 February 2025
Posted:
06 February 2025
You are already at the latest version
Abstract
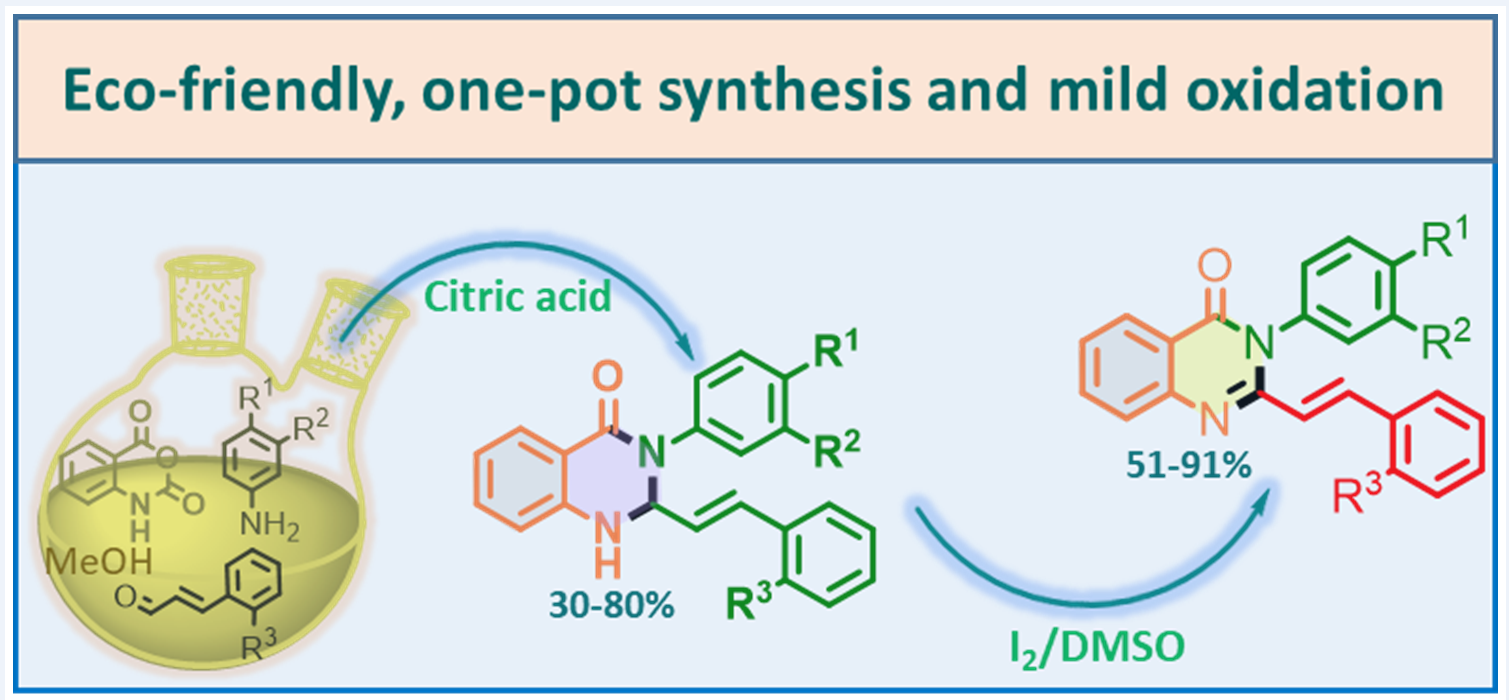
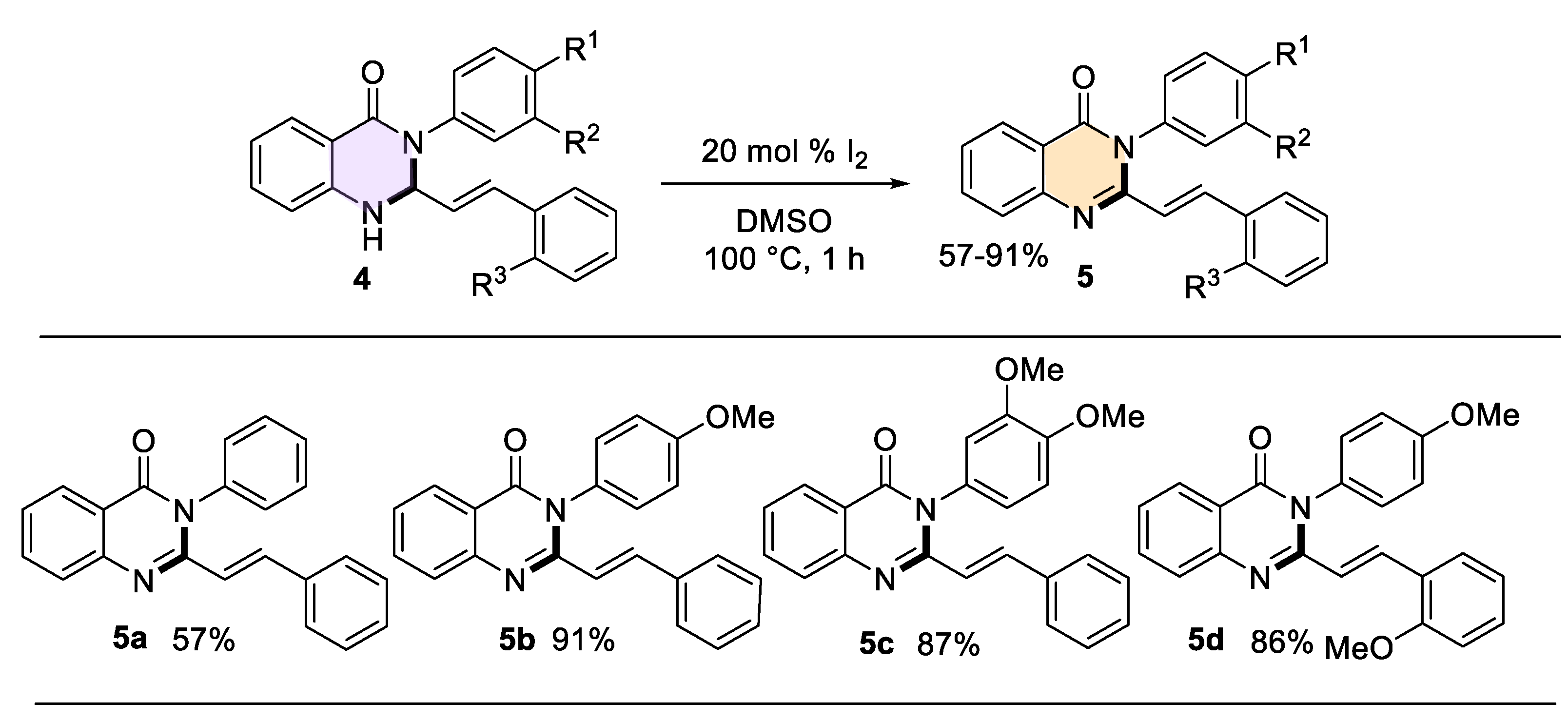
We hereby report a simple and efficient method for the preparation of (E)-3-aryl-2-styryl-2,3-dihydroquinazolin-4-(1H)-ones, from isatoic anhydride, anilines and cin-namaldehydes in the presence of 20 mol% citric acid in methanol at 60 °C for 2 h. The styryl-dihydroquinazolin-4-(1H)-one products were obtained in moderate and good yields (30-80 %) through the three-component condensation reaction, under an environment-friendly protocol. The latter were easily transformed into styrylquinazolin-4-(3H)-one derivatives with 57-91 % yields using a mild oxidation with I2/DMSO system for less than 60 min.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
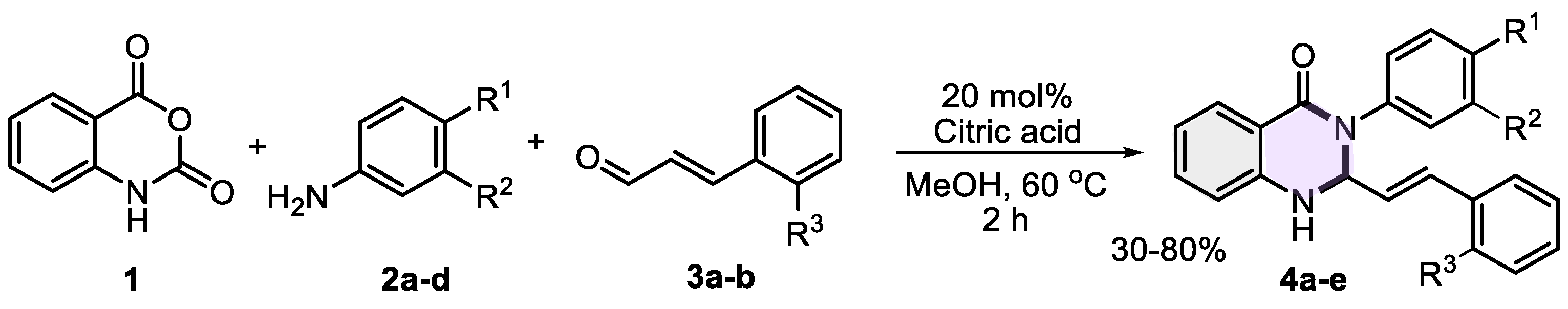
2.2. General Procedure for the Synthesis of (E)-3-aryl-2-styryl-2,3-dihydroquinazolin-4(1H)-one derivatives 4a−e
2.2.1 3-Phenyl-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4a)
2.2.2 3-(4-Methoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4b)
2.2.3 3-(3,4-Dimethoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4c)
2.2.4 3-(4-Methoxyphenyl)-2-(2-methoxystyryl)-2,3-dihydroquinazolin-4(1H)-one (4d)
2.2.5 3-(4-Bromophenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4e)
2.3. General Procedure for the Synthesis of (E)-3-aryl-2-styrylquinazolin-4(3H)-one derivatives 5a−d
2.3.1 3-Phenyl-2-styrylquinazolin-4(3H)-one (5a)
2.3.2 3-(4-Methoxyphenyl)-2-styrylquinazolin-4(3H)-one (5b)
2.3.3 3-(3,4-Dimethoxyphenyl)-2-styrylquinazolin-4(3H)-one (5c)
2.3.4 3-(4-Methoxyphenyl)-2-(2-methoxystyryl)-quinazolin-4(3H)-one (5d)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
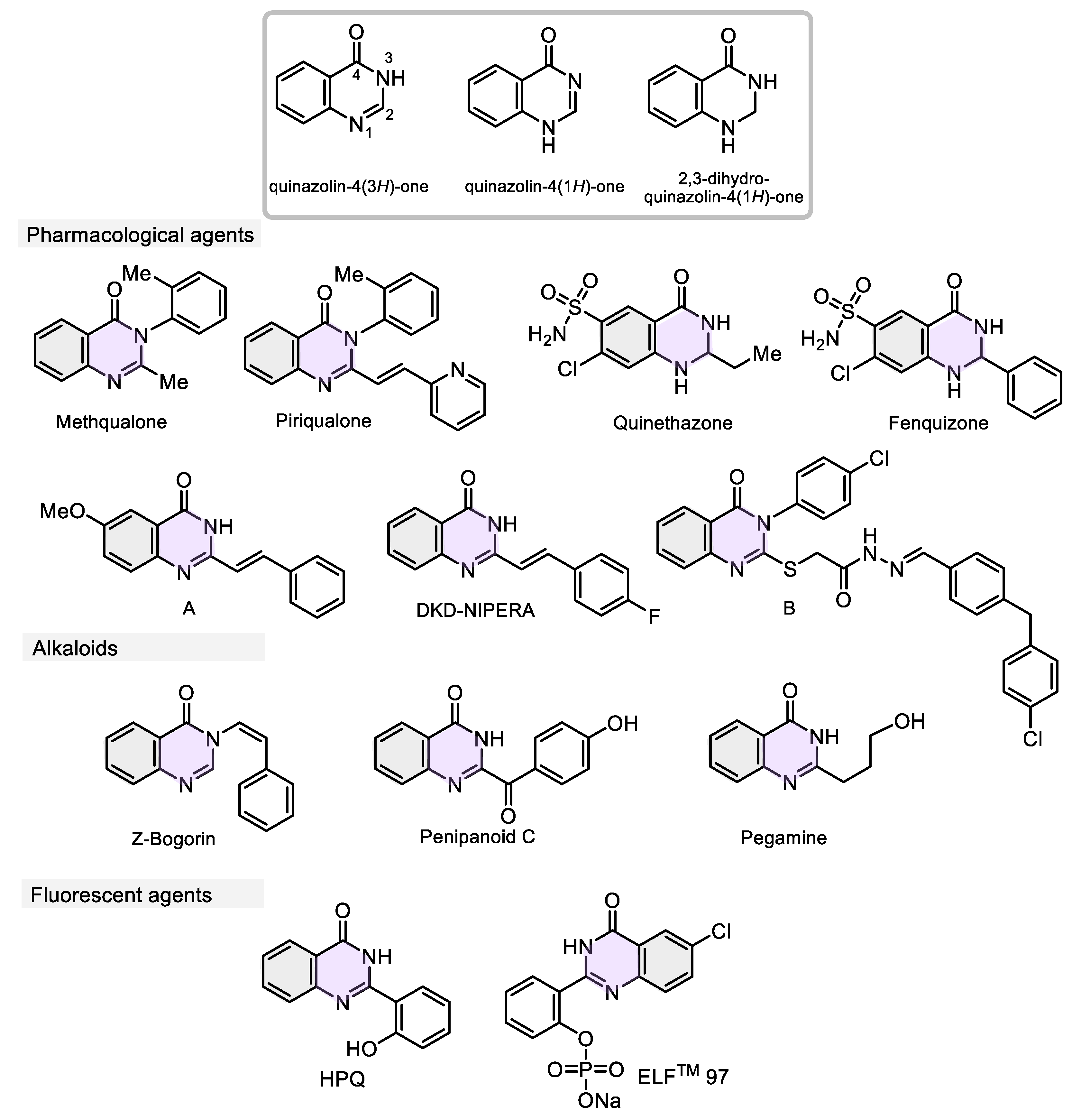
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S. A.; Arshia; Ishtiaq, M. ; Khan, K. M. Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Alsibaee, A. M.; Al-Yousef, H. M.; Al-Salem, H. S. Quinazolinones, the winning horse in drug discovery. Molecules 2023, 28, 978. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, B. K.; Pradhan, K.; Nanda, A. K.; Chakraborty, R. Implication of Quinazoline-4(3H)-Ones in Medicinal Chemistry: A Brief Review. J. Chem. Biol. Ther. 2015, 1, 1000104. [Google Scholar]
- Mahato, A.; Srivastava, B.; Nithya, S. Chemistry Structure Activity Relationship and Biological Activity of Quinazoline-4 (3H)-One Derivatives. Inventi Rapid Med. Chem. 2011, 2, 13–19. [Google Scholar]
- Kaur, J.; Kaur, S.; Muskan; Kaur, N. ; Kumar, V.; Anand, A. Unveiling the Therapeutic Potential of Quinazolinone Derivatives in Cancer Treatment: A Comprehensive Exploration. ChemistrySelect 2024, 9, e202401366. [Google Scholar] [CrossRef]
- Upadhyay, R.; Tandel, P.; Patel, A. B. Halogen-based quinazolin-4 (3H)-one derivatives as MCF-7 breast cancer inhibitors: Current developments and structure–activity relationship. Arch. Pharm. 2024, e2400740. [Google Scholar] [CrossRef]
- Ugale, V. G.; Bari, S. B. Quinazolines: new horizons in anticonvulsant therapy. Eur. J. Med. Chem. 2014, 80, 447−501. [Google Scholar] [CrossRef] [PubMed]
- Utreja, D.; Salotra, R.; Kaur, G.; Sharma, S.; Kaushal, S. Chemistry of quinolines and their agrochemical potential. Curr. Org. Chem. 2022, 26, 1895–1913. [Google Scholar] [CrossRef]
- An, L.; Yang, L.; Yan, T.; Yi, M.; Liu, S.; Li, H.; Bao, X. Synthesis and agricultural antimicrobial evaluation of new quinazoline derivatives containing both a piperazine linker and the N-acetyl moiety. Pest Manag. Sci. 2024, 80, 5307–5321. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Li, X.; Shi, Q.; Wan, Z.; Hu, D.; Jin, L.; Song, B. Synthesis and antiviral bioactivity of novel 3-((2-((1E,4E)-3-oxo-5-arylpenta-1,4-dien-1-yl) phenoxy) methyl)-4(3H)-quinazolinone derivatives. J. Agric. Food Chem. 2014, 62, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wu, W.; Miao, Y.; Tang, Y.; Zhou, Y.; Zheng, L.; Fu, Y.; Song, Z.; Peng, Y. Recent advances in quinazolinones as an emerging molecular platform for luminescent materials and bioimaging. Org. Chem. Front. 2021, 8, 1867–1889. [Google Scholar] [CrossRef]
- Inger, J. A.; Mihan, E. R.; Kolli, J. U.; Lindsley, C. W.; Bender, A. M. DARK classics in chemical neuroscience: methaqualone. ACS Chem. Neurosci. 2023, 14, 340–350. [Google Scholar] [CrossRef]
- Welch, W. M.; Ewing, F. E.; Huang, J.; Menniti, F. S.; Pagnozzi, M. J.; Kelly, K.; Seymour, P. A.; Guanowsky, V.; Guhan, S.; Guinn, M. R.; Critchett, D.; Lazzaro, J.; Ganong, A. H.; DeVries, K. M.; Staigers, T. L.; Chenard, B. L. Atropisomeric quinazolin-4-one derivatives are potent noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists. Bioorg. Med. Chem. Lett. 2001, 11, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Selvam, T. P.; Kumar, P. V. Quinazoline marketed drugs. Res. Pharm. 2015, 1, https. [Google Scholar]
- Jiang, J. B.; Hesson, D. P.; Dusak, B. A.; Dexter, D. L.; Kang, G. J.; Hamel, E. Synthesis and biological evaluation of 2-styrylquinazolin-4 (3H)-ones, a new class of antimitotic anticancer agents which inhibit tubulin polymerization. J. Med. Chem. 1990, 33, 1721–1728. [Google Scholar] [CrossRef]
- Satpute, D. P.; Shirwadkar, U.; Tharalla, A. K.; Shinde, S. D.; Vaidya, G. N.; Joshi, S.; Vatsa, P. P.; Jain, A., Singh, A. A., Garg, R., Mandoli, A., Kumar, D. Discovery of fluorinated 2-Styryl-4(3H)-quinazolinone as potential therapeutic hit for oral cancer. Bioorg. Med. Chem. 2023, 81, 117193.
- Sonousi, A.; Hassan, R. A.; Osman, E. O.; Abdou, A. M.; Emam, S. H. Design and synthesis of novel quinazolinone-based derivatives as EGFR inhibitors with antitumor activity. J. Enzyme Inhib. Med. Chem. 2022, 37, 2644–2659. [Google Scholar] [CrossRef]
- Seger, C.; Vajrodaya, S.; Greger, H.; Hofer, O. Structure elucidation and synthesis of a new bioactive quinazolone derivative obtained from Glycosmis Cf. Chlorosperma. Chem. Pharm. Bull. 1998, 46, 1926–1928. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-hydroxypyridones, phenylhydrazones, and a quinazolinone from Isaria farinosa. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef]
- Ma, Z. Z.; Hano, Y.; Nomura, T.; Chen, Y. J. Two new quinazoline-quinoline alkaloids from Peganum nigellastrum. Heterocycles 1999, 8, 1883–1889. [Google Scholar]
- Zhang, X. B.; Waibel, M.; Hasserodt, J. An Autoimmolative Spacer Allows First-Time Incorporation of a Unique Solid-State Fluorophore into a Detection Probe for Acyl Hydrolases. Chem. Eur. J. 3, 792−795.
- Zi-Jun, C. A. I.; Kuang, Y. Q.; Dan, P. A. N.; Wei, L. I. U.; Jiang, J. H. Synthesis and characterization of a novel ELF-97-based fluorescent probe for hydrogen peroxide detection. Chin. J. Anal. Chem. 2015, 43, 1671–1675. [Google Scholar]
- Connolly, D. J.; Cusack, D.; O'Sullivan, T. P.; Guiry, P. J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef]
- Maiden, T. M. M.; Harrity, J. P. A. Recent developments in transition metal catalysis for quinazolinone synthesis. Org. Biomol. Chem. 2016, 14, 8014–8025. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M. M.; Sivaramakrishna, A. Remarkably flexible quinazolinones—synthesis and biological applications. J. Heterocyclic Chem. 2020, 57, 942–954. [Google Scholar] [CrossRef]
- Kumar, P.; Tomar, V.; Joshi, R. K.; Nemiwal, M. Nanocatalyzed synthetic approach for quinazoline and quinazolinone derivatives: A review (2015–present). Synth. Commun. 2022, 52, 795–826. [Google Scholar] [CrossRef]
- Lodhi, A.; Maheria, K. C. Solid acid catalysed synthesis of biologically potent quinazolinones: Environmentally benign approaches. Sustain. Chem. Pharm. 2023, 36, 101265. [Google Scholar] [CrossRef]
- Borah, B.; Swain, S.; Patat, M.; Chowhan, L. R. Recent advances and prospects in the organocatalytic synthesis of quinazolinones. Front. Chem. 2022, 10, 991026. [Google Scholar] [CrossRef]
- Peng, J.-B.; Geng, H.-Q.; Wang, W.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-catalyzed four-component carbonylative synthesis of 2,3-disubstituted quinazolin-4(3H)-ones: Convenient methaqualone preparation. J. Catal. 2018, 365, 10–13. [Google Scholar] [CrossRef]
- Wang, L. C.; Du, S.; Chen, Z.; Wu, X. F. FeCl3-Mediated Synthesis of 2-(Trifluoromethyl) quinazolin-4(3H)-ones from Isatins and Trifluoroacetimidoyl Chlorides. Org. Lett. 2020, 22, 5567–5571. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, L. C.; Zhang, J.; Wu, X. F. Palladium-catalyzed three-component carbonylative synthesis of 2-(trifluoromethyl) quinazolin-4(3H)-ones from trifluoroacetimidoyl chlorides and amines. Org. Chem. Front. 2020, 7, 2499–2504. [Google Scholar] [CrossRef]
- Wang, L. C.; Zhang, Y.; Chen, Z.; Wu, X. F. Palladium-Catalyzed Carbonylative Synthesis of 2-(Trifluoromethyl) quinazolin-4(3H)-ones from Trifluoroacetimidoyl Chlorides and Nitro Compounds. Adv. Synth. Catal. 2021, 363, 1417–1426. [Google Scholar] [CrossRef]
- Abbas, S. Y.; El-Bayouki, K. A.; Basyouni, W. M. Utilization of isatoic anhydride in the syntheses of various types of quinazoline and quinazolinone derivatives. Synth. Commun. 2016, 46, 993–1035. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Salehi, P.; Dabiri, M.; Kozehgary, G. Water-accelerated synthesis of novel bis-2, 3-dihydroquinazolin-4(1H)-one derivatives. Synthesis, 2006; 2006, 344–348. [Google Scholar]
- Narasimhulu, M.; Lee, Y. R. Ethylenediamine diacetate-catalyzed three-component reaction for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones and their spirooxindole derivatives. Tetrahedron 2011, 67, 9627–9634. [Google Scholar] [CrossRef]
- Chen, B. H.; Li, J. T.; Chen, G. F. Efficient synthesis of 2, 3-disubstituted-2, 3-dihydroquinazolin-4(1H)-ones catalyzed by dodecylbenzenesulfonic acid in aqueous media under ultrasound irradiation. Ultrason. Sonochem. 2015, 23, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H. B.; Dixit, B. C.; Dixit, R. B. L-Proline catalyzed one-pot multi-component synthesis of 2-(1, 3-diphenyl-1H-pyrazol-4-yl) quinazolin-4(3H)-one derivatives and their biological studies. Chin.Chem. Lett. 2014, 25, 741–744. [Google Scholar] [CrossRef]
- Ramesh, K.; Karnakar, K. G. K. H. V.; Satish, G.; Reddy, K. H. V.; Nageswar, Y. V. D. Tandem supramolecular synthesis of substituted 2-aryl-2, 3-dihydroquinazolin-4(1H)-ones in the presence of β-cyclodextrin in water. Tetrahedron Lett. 2012, 53, 6095–6099. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Salehi, P.; Dabiri, M.; Kozehgary, G. Water-accelerated synthesis of novel bis-2, 3-dihydroquinazolin-4 (1H)-one derivatives. Synthesis 2006, 2006, 344−348. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Arjmandi, R. Acetic acid-promoted, efficient, one-pot synthesis of 2, 3-dihydroquinazolin-4(1H)-ones. Monatsh. Chem. 2011, 142, 631–635. [Google Scholar] [CrossRef]
- Mane, R.; Yaraguppi, D. A.; Ashok, A. K.; Gangadharappa, B.; Chandrakala, K. B.; Kamanna, K. Glutamic acid-catalyzed synthesis of dihydroquinazolinone: anticancer activity, electrochemical behavior, molecular docking, dynamics, simulations and drug-likeness studies. Res. Chem. Intermed, 2024; 1–33. [Google Scholar]
- Fahimi, N.; Sardarian, A. R. Citric acid: A green bioorganic catalyst for one-pot three-component synthesis of 2, 3-dihydroquinazoline-4 (1H)-ones. Curr. Organocatal. 2016, 3, 39–44. [Google Scholar] [CrossRef]
- Rosado-Solano, D. N.; Barón-Rodríguez, M. A.; Sanabria-Florez, P. L.; Luna-Parada, L. K.; Puerto-Galvis, C. E.; Zorro-González, A. F.; Kouznetsov, V. V.; Vargas-Méndez, L. Y. Synthesis, biological evaluation and in silico computational studies of 7-chloro-4-(1H-1,2,3-triazol-1-yl)quinoline derivatives. Search for new controlling agents against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. J. Agric. Food Chem. 2019, 67, 9210–9219. [Google Scholar] [CrossRef]
- Villamizar-Mogotocoro, A. F.; Bonilla-Castañeda, S. M.; Kouznetsov, V. V. Green conditions for the efficient two-step synthesis of new 6-arylphenanthridines from 2-bromoacetoanilides based on microwave-assisted Suzuki-Miyaura cross-coupling and modified Pictet-Spengler dehydrogenative cyclization in a zinc chloride/[Bmim]BF4 mixture. Green Chem. 2022, 24, 7996–8004. [Google Scholar]
- Becerra-Anaya, S. J.; Merchán Arenas, D. R.; Kouznetsov, V. V. A simple and effective protocol for the Pechmann reaction to obtain 4-methylcoumarin derivatives using a high-speed mixer ball mill process. Chemistry 2023, 5, 1077–1088. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J. P. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1, 3, 4-thiadiazole-2-yl]-2-styryl quinazoline-4 (3H)-ones. Eur. J. Med. Chem. 2008, 43, 135–141. [Google Scholar] [CrossRef]
- Raffa, D.; Edler, M. C.; Daidone, G.; Maggio, B.; Merickech, M.; Plescia, S.; Schillaci, D.; Bai, R.; Hamel, E. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur. J. Med. Chem. 2004, 39, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, I. G.; Kim, G. A.; Matochkina, E. G.; Kodess, M. I.; Barykin, N. V.; El´ tsov, O. S.; Nosova, E. V.; Rusinov, G. L.; Charushin, V. N. Synthesis, photochemical and luminescent properties of (E)-2-(2-hydroxyarylethylene)-3-phenylquinazolin-4 (3H)-ones. Russ. Chem. Bull. 2014, 63, 2467–2477. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Molnar, M.; Damm, M.; Reidlinger, C.; Dabiri, M.; Kappe, C. O. Parallel microwave synthesis of 2-styrylquinazolin-4 (3H)-ones in a high-throughput platform using HPLC/GC vials as reaction vessels. J. Comb. Chem. 2009, 11, 676–684. [Google Scholar] [CrossRef]
- Srinivasa Reddy, B.; Naidu, A.; Dubey, P. K. PEG-600-mediated, green and efficient, tandem syntheses of N-subtituted-2-styrylquinazolin-4-ones. Green Chem. Lett. Rev. 2013, 6, 254–261. [Google Scholar] [CrossRef]
- Trashakhova, T. V.; Nosova, E. V.; Valova, M. S.; Slepukhin, P. A.; Lipunova, G. N.; Charushin, V. N. Synthesis and photophysical properties of 2-styrylquinazolin-4-ones. Russ. J. Org. Chem. 2011, 47, 753–761. [Google Scholar] [CrossRef]
- Kumar, D.; Jadhavar, P. S.; Nautiyal, M.; Sharma, H.; Meena, P. K.; Adane, L.; Pancholia, S.; Chakraborti, A. K. Convenient synthesis of 2, 3-disubstituted quinazolin-4 (3H)-ones and 2-styryl-3-substituted quinazolin-4 (3H)-ones: Applications towards the synthesis of drugs. RSC Adv. 2015, 5, 30819–30825. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Delbari, A. S. Novel and efficient one-Pot tandem synthesis of 2-Styryl-Substituted 4 (3H)-Quinazolinones. J. Comb. Chem. 2008, 10, 700–703. [Google Scholar] [CrossRef]
- Gupta, A. D.; Sepay, N.; Mallik, A. K. An efficient microwave-assisted synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones by a three component reaction under catalyst-and solvent-free conditions. Eur. Chem. Bull. 2016, 5, 185–188. [Google Scholar]
- Rupnar, B. D.; Kachave, T. R.; Jawale, P. D.; Shisodia, S. U.; Pawar, R. P. Green and efficient synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones in aqueous medium using ZnFe2O4 catalyst under microwave irradiation. J. Iran. Chem. Soc. 2017, 14, 1853–1858. [Google Scholar] [CrossRef]
- Dutta, A.; Sarma, D. Base promoted metal-free approach towards synthesis of quinazolin-4 (3H)-ones and 2, 3-dihydroquinazolin-4 (1H)-ones under microwave irradiation. Sustain. Chem. Pharm. 2021, 20, 100402. [Google Scholar] [CrossRef]
- Peña-Solórzano, D.; Guilombo, C. E. G.; Ochoa-Puentes, C. Rapid and eco-friendly high yield synthesis of dihydroquinazolinones mediated by urea/zinc chloride eutectic mixture. Sustain. Chem. Pharm. 2019, 14, 100167. [Google Scholar] [CrossRef]
- Mahdavi, M.; Pedrood, K.; Safavi, M.; Saeedi, M.; Pordeli, M.; Ardestani, S. K.; Emami, S.; Adib, M.; Foroumadi, A.; Shafiee, A. Synthesis and anticancer activity of N-substituted 2-arylquinazolinones bearing trans-stilbene scaffold. Eur. J. Med. Chem. 2015, 95, 492–499. [Google Scholar] [CrossRef]
- Mehta, H. B.; Dixit, B. C.; Dixit, R. B. L-Proline catalyzed one-pot multi-component synthesis of 2-(1, 3-diphenyl-1H-pyrazol-4-yl) quinazolin-4 (3H)-one derivatives and their biological studies. Chin. Chem. Lett. 2014, 25, 741–744. [Google Scholar] [CrossRef]
- Wang, J. Q.; Zuo, Z. Y.; He, W. Recent advances of green catalytic system I2/DMSO in C–C and C–Heteroatom bonds formation. Catalysts 2022, 12, 821. [Google Scholar] [CrossRef]
- Singhal, R.; Choudhary, S. P.; Malik, B.; Pilania, M. I2/DMSO-mediated oxidative C–C and C–heteroatom bond formation: a sustainable approach to chemical synthesis. RSC Adv. 2024, 14, 5817–5845. [Google Scholar] [CrossRef]
- Peñaranda Gómez, A.; Puerto Galvis, C.E.; Macías, M.A.; Ochoa-Puentes, C.; Kouznetsov, V.V. I2/DMSO-Promoted the synthesis of chromeno[4,3-b]quinolines through an imine formation/aza-Diels-Alder/aromatization tandem reaction under metal-catalyst and photosensitizer-free conditions Synthesis 2022, 54, 1857–1869.
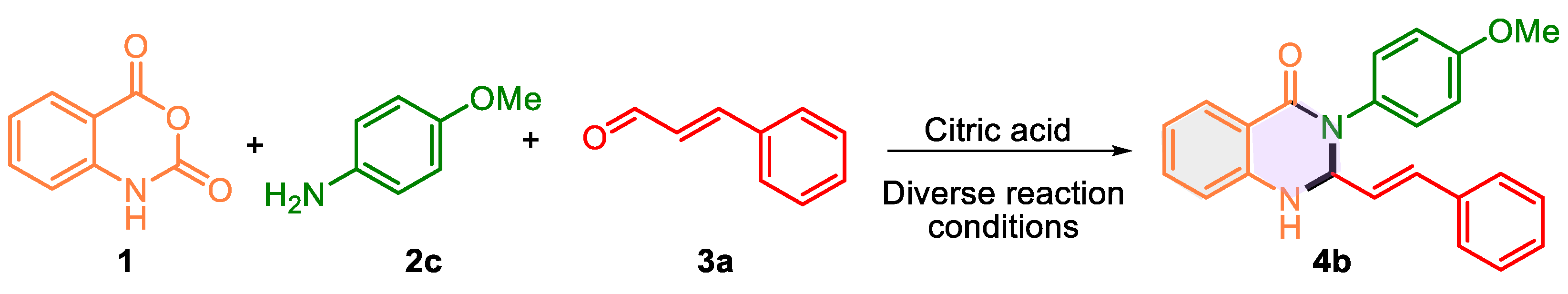
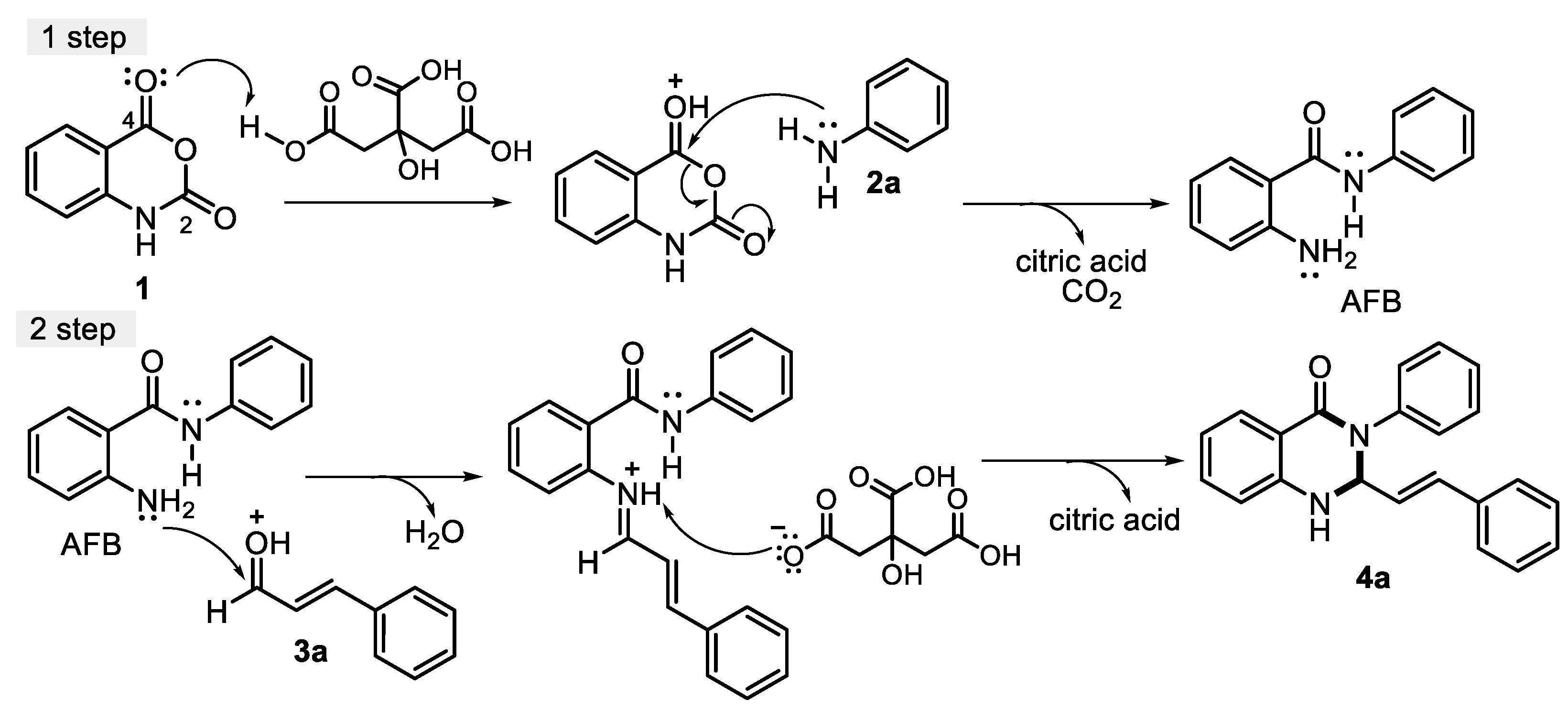
| Entry | Citric acid (mol %) | Dissolvent | t, °C | T (h) | Yield, % |
|---|---|---|---|---|---|
| 1 | 40 | Methanol | 60 | 2 | 76 |
| 2 | 40 | Methanol | 100b | 10 min | 40 |
| 3 | 40 | Methanol | 160b | 15 min | 20 |
| 4 | 20 | Methanol | 60 | 2 | 80 |
| 5 | -- | Urea/ZnCl2 | 110 | 1 | 20c |
| Comp. | R1 | R2 | R3 | Mp., °C | Yield, % |
|---|---|---|---|---|---|
| 4a | H | H | H | 192−194 | 54 |
| 4b | OMe | H | H | 234−236 | 80 |
| 4c | OMe | OMe | H | 227−228 | 70 |
| 4d | OMe | H | OMe | 162−164 | 61 |
| 4e | Br | H | H | 183−185 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





