1. Introduction
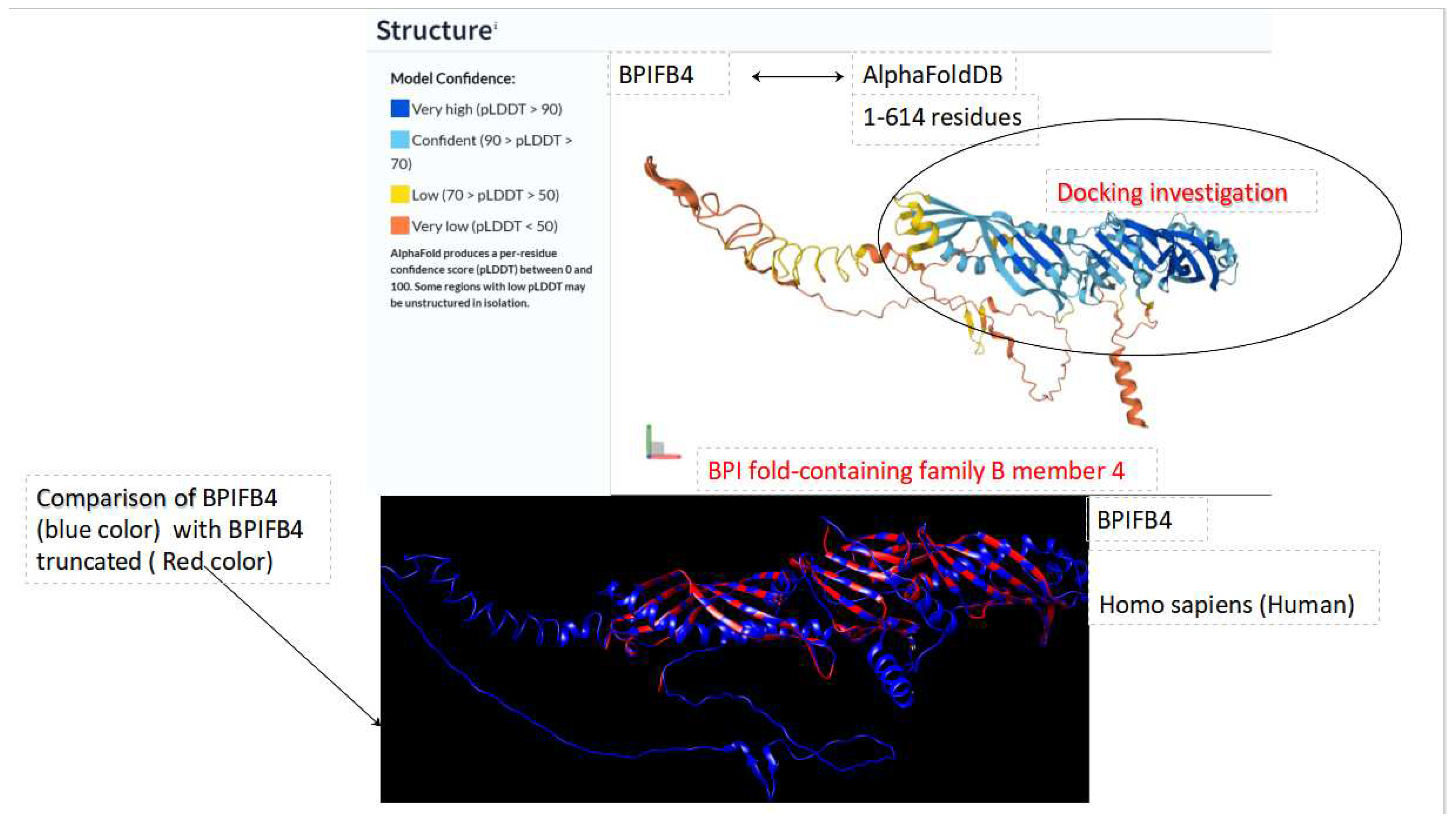
BPI fold-containing family B member 4 (BPIFB4) is a human protein encoded by the BPIFB4 gene, formerly identified as "palate, lung, and nasal epithelial carcinoma-associated protein 4" from the LPLUNC4 gene. The BPIFB4 gene produces four transcripts (splice variants), with three well-characterized isoforms.This protein is prominently expressed in the olfactory epithelium (nasal mucosa) and exhibits elevated levels in the gonads (testis, ovary) and the pituitary gland. Additionally, moderate levels are detected in white blood cells, particularly monocytes. BPIFB4 can be either localized within the cell cytoplasm or secreted, circulating systemically in the blood plasma.
These findings suggest diverse functions for BPIFB4 in various tissues, emphasizing significant expression in olfactory and gonadal tissues and the potential for circulation in the bloodstream[
1,
2,
3]. BPIFB4 belongs to the BPI fold protein superfamily, characterized by the presence of the bactericidal/permeability-increasing protein fold (BPI fold). This fold is created by two similar domains arranged in a "boomerang" shape. The BPI fold is a structural motif often found in proteins involved in host defense mechanisms and innate immune responses, and it plays a role in interactions with bacteria and other pathogens. The "boomerang" shape refers to the characteristic structure formed by these domains, contributing to the functional properties of proteins within this superfamily[
4].
Analyzing numerous centenarian populations has enabled the identification of the longevity-associated variant (LAV) within the BPIFB4 gene. Individuals exhibiting elevated circulating levels of LAV-BPIFB4 seem to experience cardioprotective effects and display immunomodulatory properties, particularly in terms of anti-inflammatory responses[
5,
6,
7].
This study represents the inaugural exploration of this protein using Molecular Docking [
8] using Autodock Vina [
9] with Pyrx program[
10], aiming to identify the most effective molecule for enhanced binding.
A preliminary Virtual Screening study using the Pyrx program [
10]scrutinized hundreds of drugs and natural molecules. This process identified potential lead compounds with the capability to interact with this protein.
3. Results and Discussion
The BPIFB4 protein is typically present in the olfactory epithelium, mononuclear cells, macrophage-like cells, and various progenitor/stem cell types. In the olfactory mucosa, it is thought to function similarly to other BPIFB proteins by contributing to the innate immune response when exposed to bacteria. When circulating in the bloodstream, BPIFB4 is presumed to engage with endothelial cells that line blood vessels, leading to vasorelaxation in the smooth muscle cells of blood vessels [
5]. The examination of various groups of centenarians has led to the discovery of the longevity-associated variant (LAV) within the BPIFB4 gene. Those with heightened circulating levels of LAV-BPIFB4 demonstrate apparent cardioprotective effects and exhibit immunomodulatory features, specifically with anti-inflammatory characteristics [
5,
6,
7].
In this groundbreaking research, two potential candidates were identified through Docking analysis [
8,
9], demonstrating a high binding energy score (approximately -9.5 kcal/mol) for interaction with BPIFB4. This discovery follows comprehensive preliminaryVirtual Screening studies [
10] that assessed hundreds of drugs and natural molecules.
The outcomes of the docking analysis are presented in
Figure 3, and
Figure 4, elucidating the particular chemical bonds formed by Amentoflavone, a biflavonoid, and Bilobetin, a flavonoid oligomer, in interaction with the BPIFB protein.
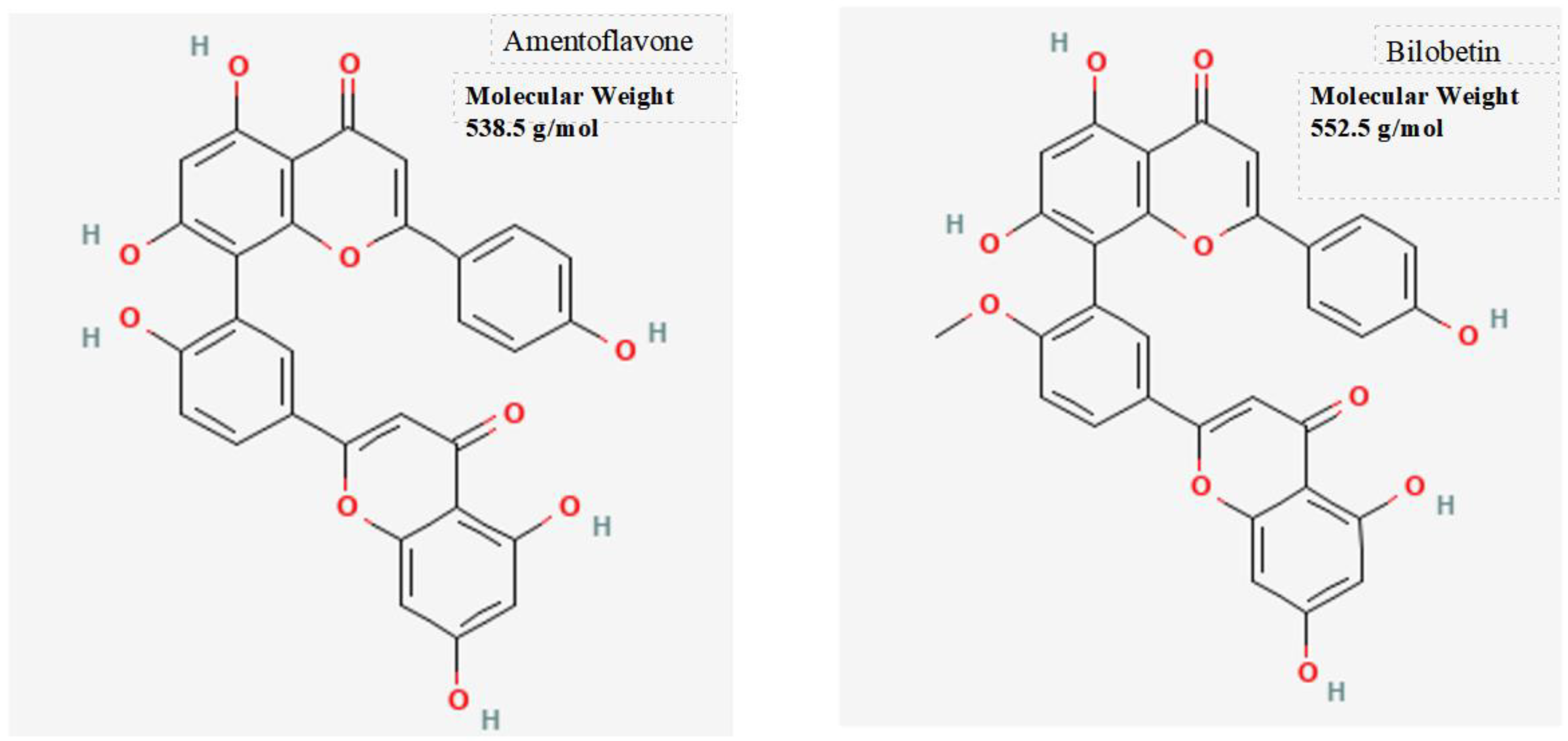
The noteworthy molecules identified in this study are two natural substances: Amentoflavone and Bilobetin. They are two highly similar molecules from a chemical structure perspective.
In summary, the docking analysis revealed that Bilobetin, a distinct flavonoid present in Ginkgo biloba leaves, exhibited a binding energy of approximately -9.4 kcal/mol with the BPIFB protein. Conversely, Amentoflavone, characterized as a biflavonoid consisting of two linked flavonoid units, demonstrated a binding energy of approximately -9.5 kcal/mol with the BPIFB protein.
The observed characteristics of both Bilobetin and Amentoflavone, along with their interactions with the crucial BPIFB4 protein, position them as promising candidates for biological applications. This is especially noteworthy considering the involvement of BPIFB4 in various biological processes, particularly in relation to the longevity-associated variant (LAV) of the BPIFB4 gene.
The identification of LAV through the study of centenarian populations suggests that individuals with elevated circulating levels of LAV-BPIFB4 may experience cardioprotective effects [
6,
7] and an immunodulatory response, characterized by anti-inflammatory properties[
6,
7].
It cound be interestring conducting in-depth studies on substances like Amentoflavone and Bilobetin is crucial to understanding their therapeutic effects and potential activation or stimulation of BPIFB4 protein. Some aspects that researchers may explore in such studies:
-
-
Mechanism of Interaction:
Investigate the molecular mechanisms by which Amentoflavone and Bilobetin interact with BPIFB4.
-
-
Functional Effects:
Examine the downstream effects of BPIFB4 activation or stimulation induced by Amentoflavone and Bilobetin.
Assess the impact on cellular and physiological processes related to longevity and cardioprotectiveness.
-
-
Therapeutic Potential:
Evaluate the therapeutic potential of Amentoflavone and Bilobetin in conditions related to cardiovascular health, considering BPIFB4's suggested cardioprotective effects.
Explore their potential in anti-inflammatory and immunomodulatory contexts.
-
-
Bioavailability and Pharmacokinetics:
Investigate the bioavailability and pharmacokinetics of Amentoflavone and Bilobetin to understand how efficiently these substances can reach and interact with BPIFB4 in different tissues.
-
-
Cellular and Animal Studies:
Conduct cellular and animal studies to validate the findings obtained from molecular docking studies.
-
-
Clinical Trials:
If preclinical studies are promising, consider progressing to clinical trials to evaluate the safety and efficacy of Amentoflavone and Bilobetin in humans, particularly in the context of longevity and cardiovascular health.
Given the potential implications for health and longevity, continued research on these substances and their interactions with BPIFB4 could contribute valuable insights to both basic science and clinical applications.
Figure 2.
comparison 3D structure of amentoflavone with 3D structure bilobetin respectively.
Figure 2.
comparison 3D structure of amentoflavone with 3D structure bilobetin respectively.
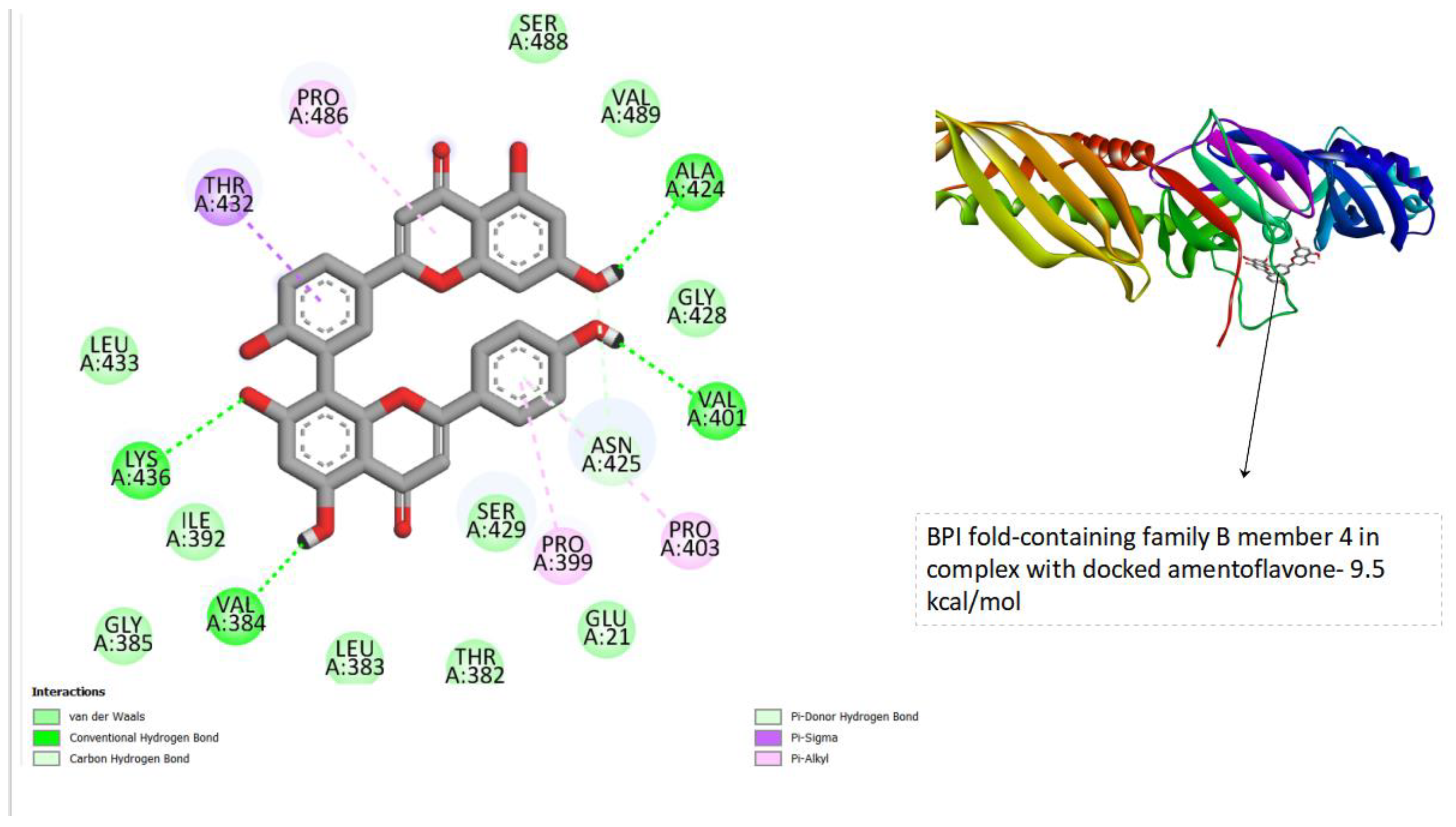
Figure 3.
displays the docking outcomes of BPI fold-containing family B member 4 in conjunction with Amentoflavone within as analyzed by Autodock Vina through Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Amentoflavone. Meanwhile, the right side exhibits the potential Ligand Binding Site of the protein, highlighting the specific location of Amentoflavone.
Figure 3.
displays the docking outcomes of BPI fold-containing family B member 4 in conjunction with Amentoflavone within as analyzed by Autodock Vina through Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Amentoflavone. Meanwhile, the right side exhibits the potential Ligand Binding Site of the protein, highlighting the specific location of Amentoflavone.
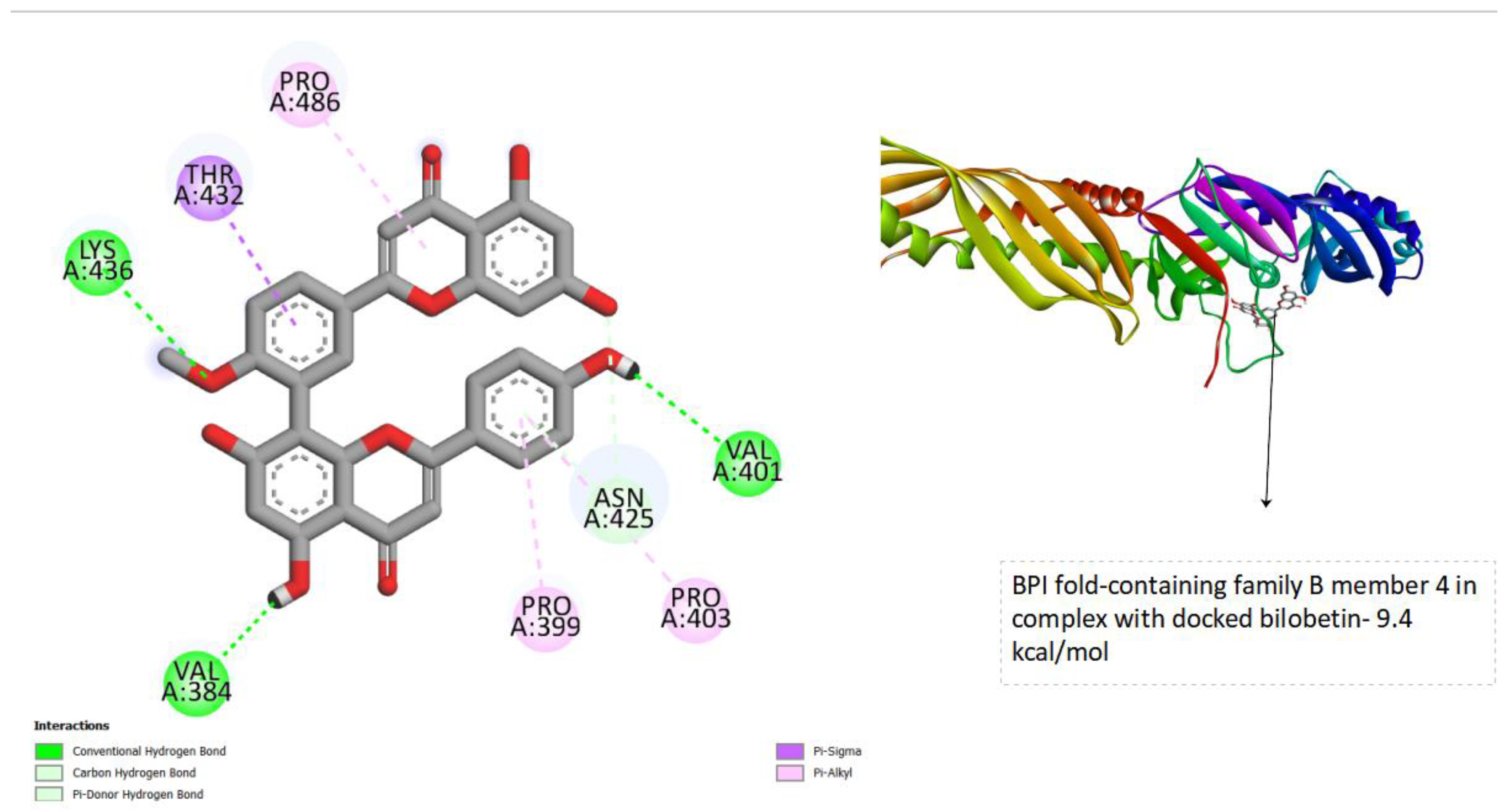
Figure 4.
displays the docking outcomes of BPI fold-containing family B member 4 in conjunction with Bilobetin within, as analyzed by Autodock Vina through Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Bilobetin. Meanwhile, the right side exhibits the potential Ligand Binding Site of the protein, highlighting the specific location of Bilobetin.
Figure 4.
displays the docking outcomes of BPI fold-containing family B member 4 in conjunction with Bilobetin within, as analyzed by Autodock Vina through Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Bilobetin. Meanwhile, the right side exhibits the potential Ligand Binding Site of the protein, highlighting the specific location of Bilobetin.
Table 1.
shows the comparison of predicted toxicity parameters with Amentoflavone and Bilobetin through pKCSM Server.
Table 1.
shows the comparison of predicted toxicity parameters with Amentoflavone and Bilobetin through pKCSM Server.
| Compounds |
AMES
toxicity |
Max.
tolerated dose(human) (logmg/kg/day) |
hERG I inhibitor |
hERG II inhibitor |
Oral Rat Acute Toxicity (LD50) (mol/kg) |
Oral Rat Chronic Toxicity (LOAEL)
(log mg/kg_bw/day) |
Hepatotoxicity |
| Amentoflavone |
no |
0.438 |
no |
yes |
2.527 |
3.572 |
no |
| Bilobetin |
no |
0.437 |
no |
yes |
2.56 |
2.217 |
no |