Submitted:
08 December 2023
Posted:
08 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Preparation and Basic Biological Characteristics of pBD114
2.2. Proinflammatory and Anti-Inflammatory Activity of pBD114 on Mouse Mononuclear Macrophage RAW264.7
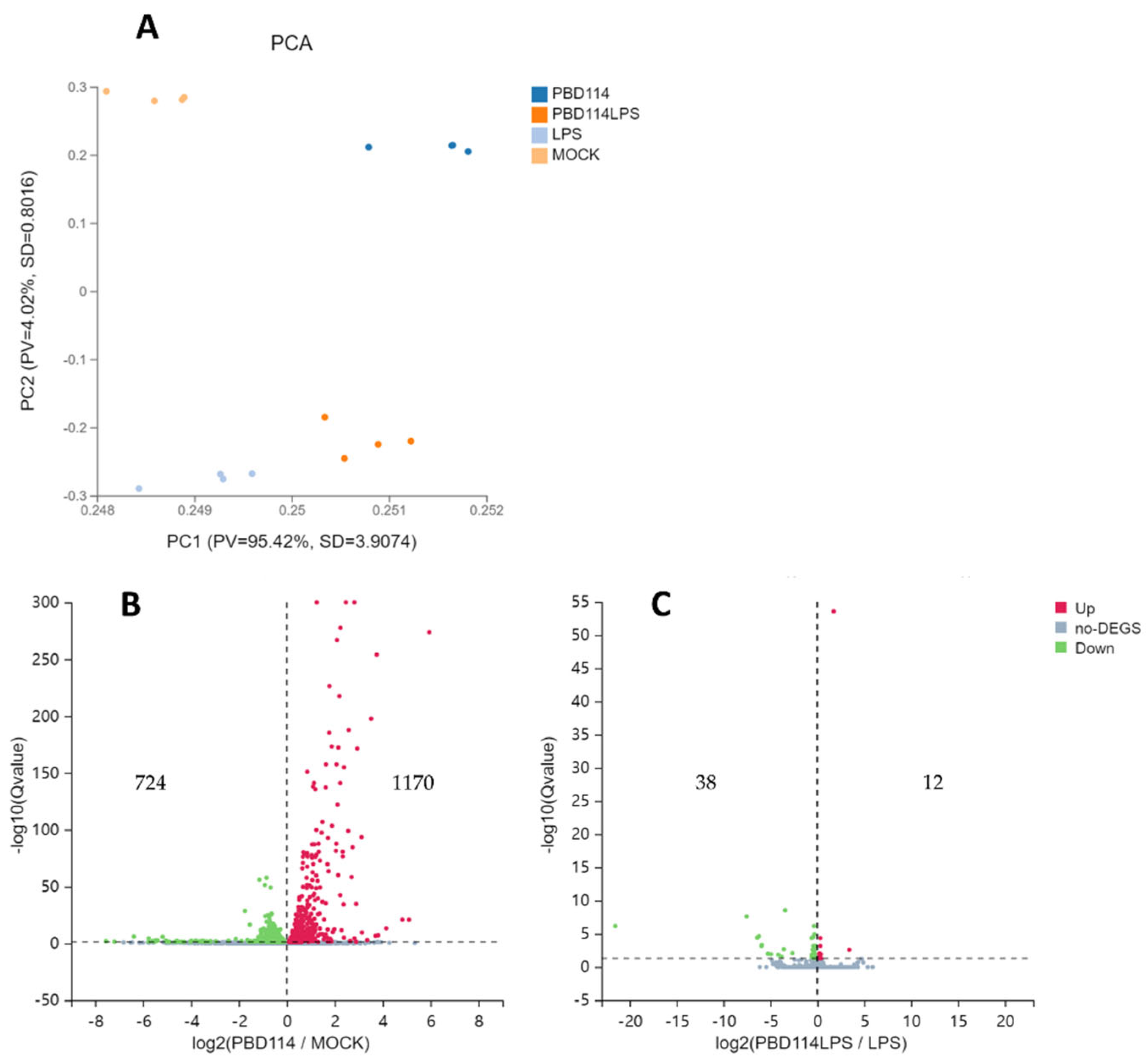
2.3. RNA-seq Analyzed the Mechanism of pBD114 on Inflammation of Mouse Mononuclear Macrophage RAW264.7
2.3.1. Differentially Expressed Genes
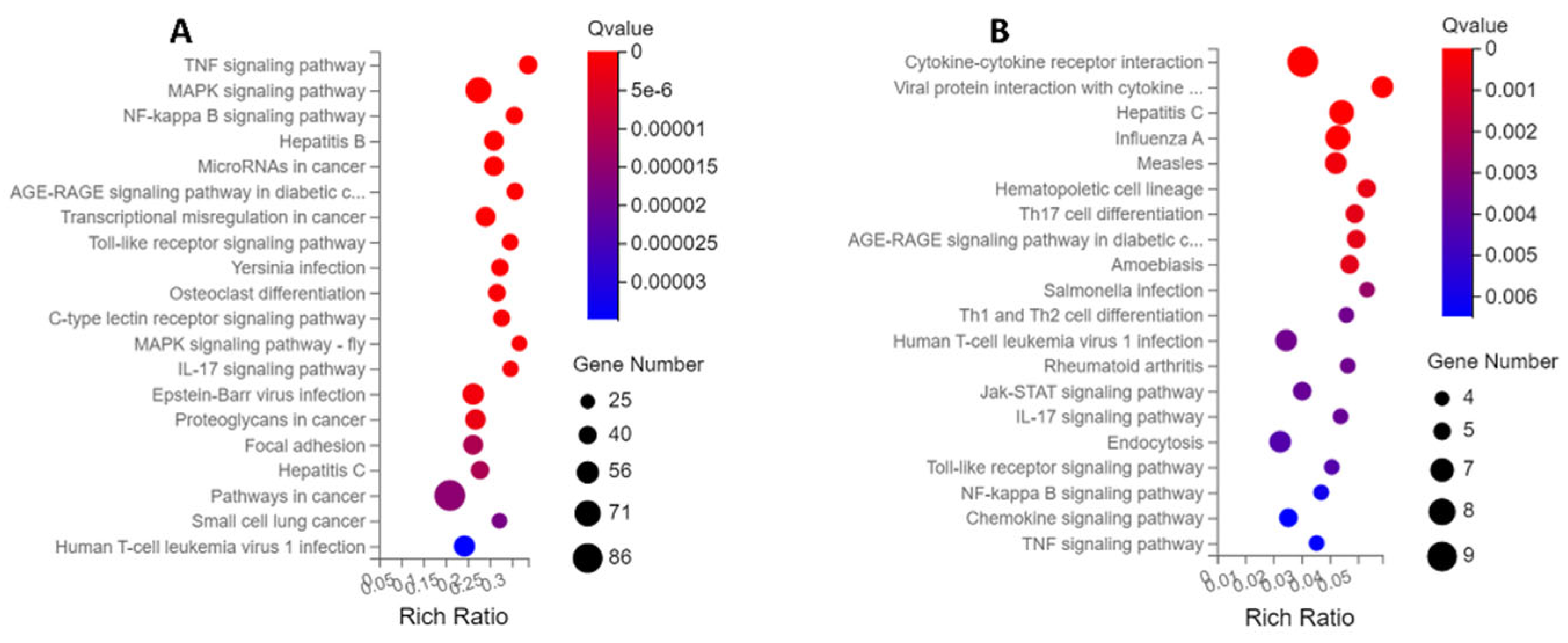
2.3.2. KEGG Enrichment Analysis
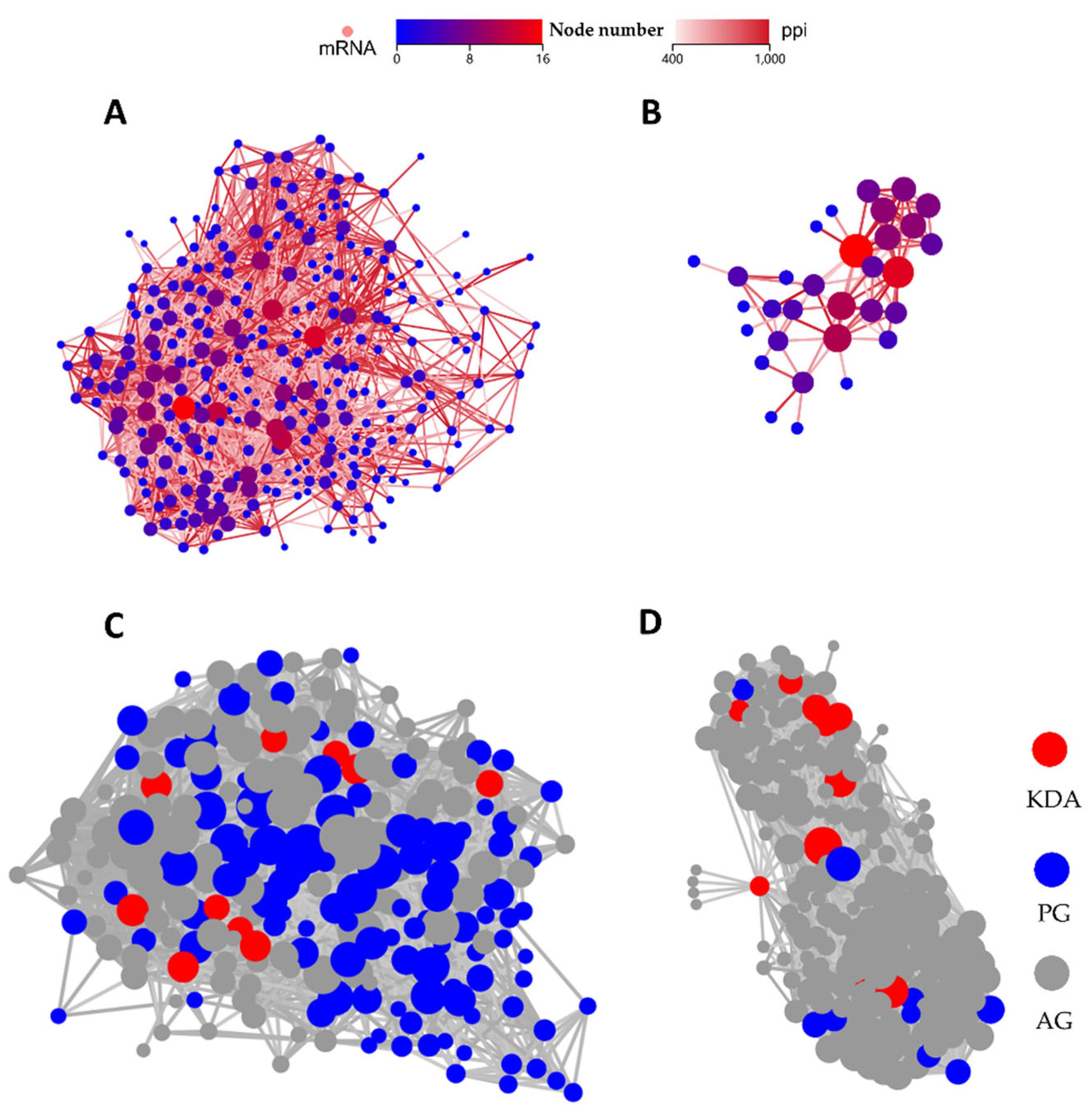
2.3.3. PPI and KDA Analysis
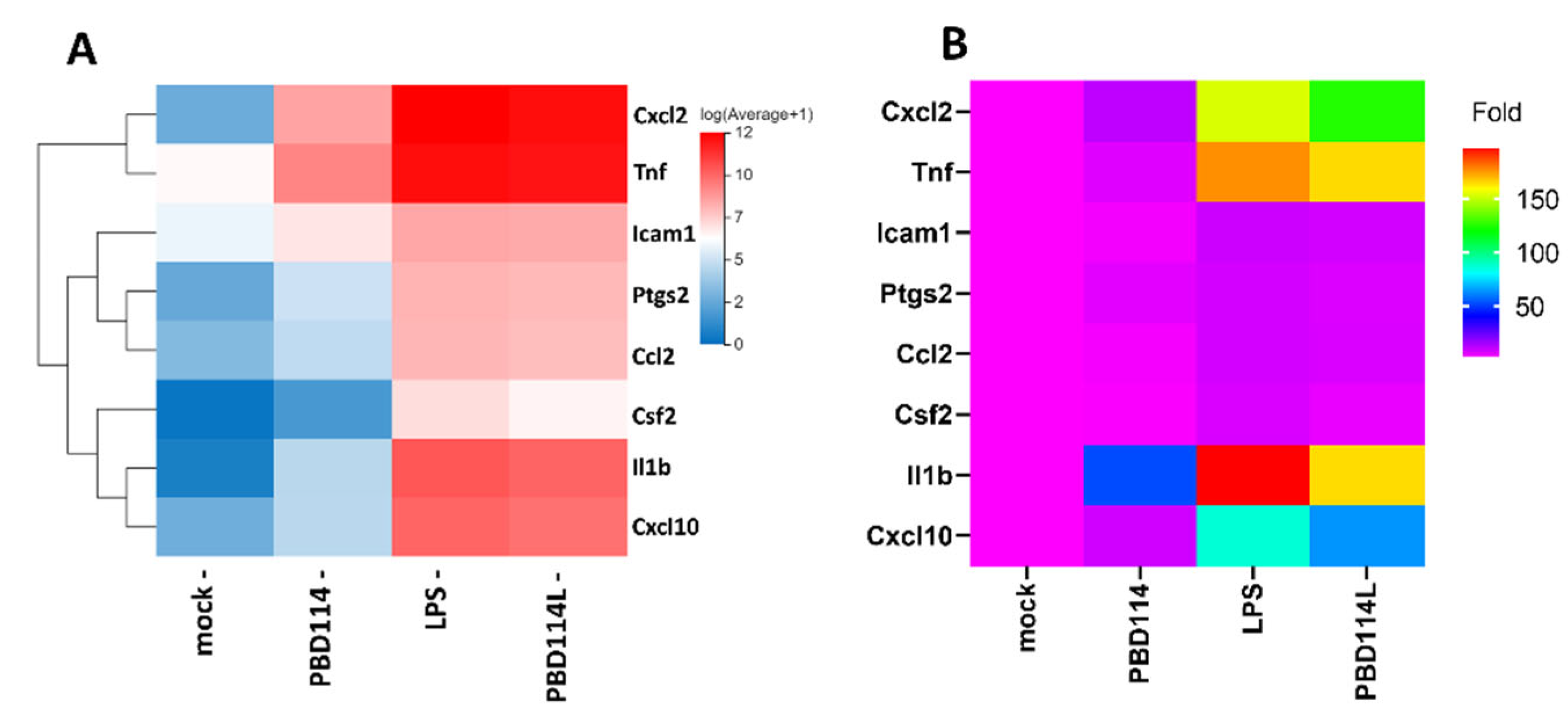
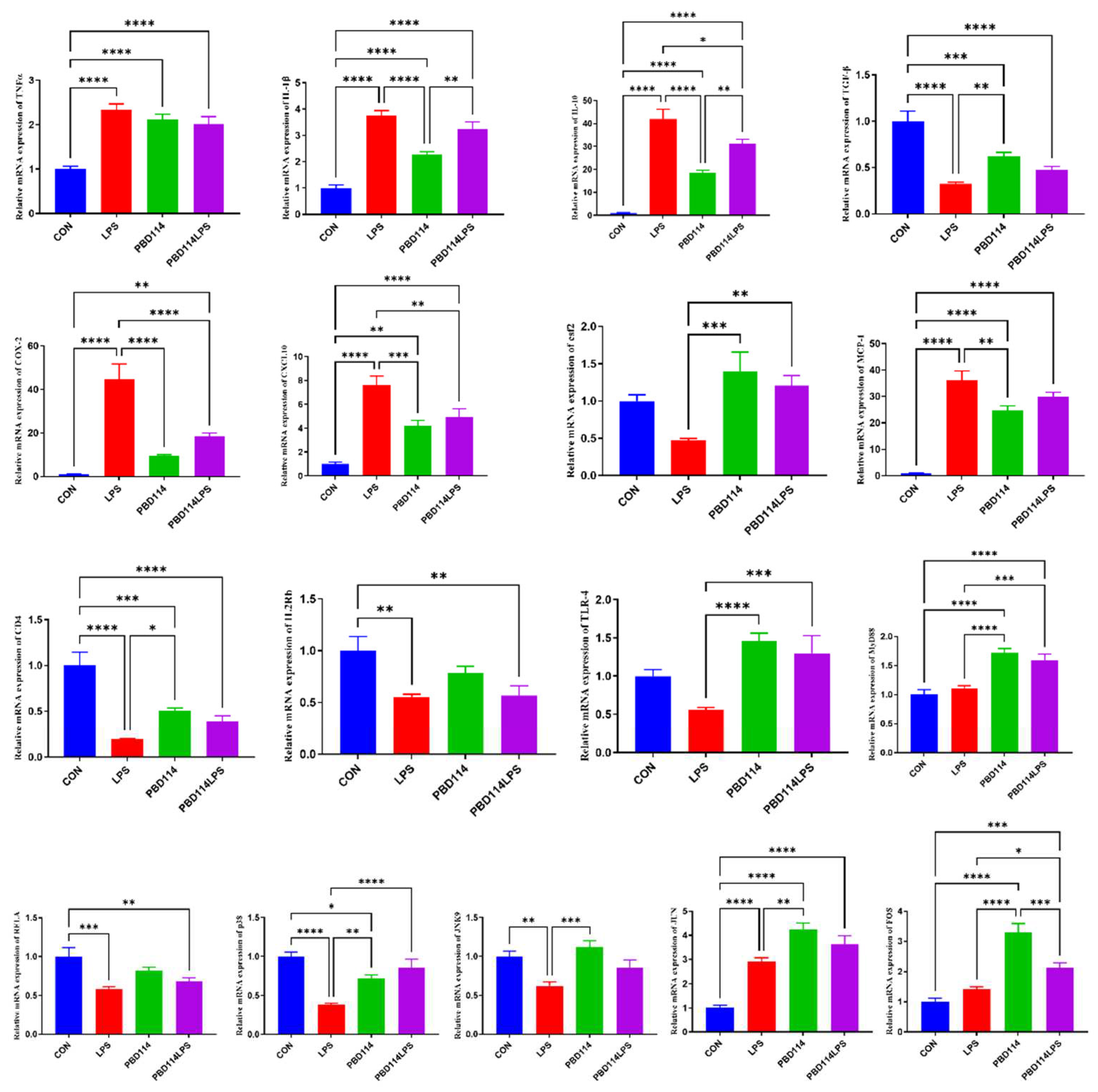
2.3.4. Validation of RNA-Seq Results via Quantitative PCR Analysis
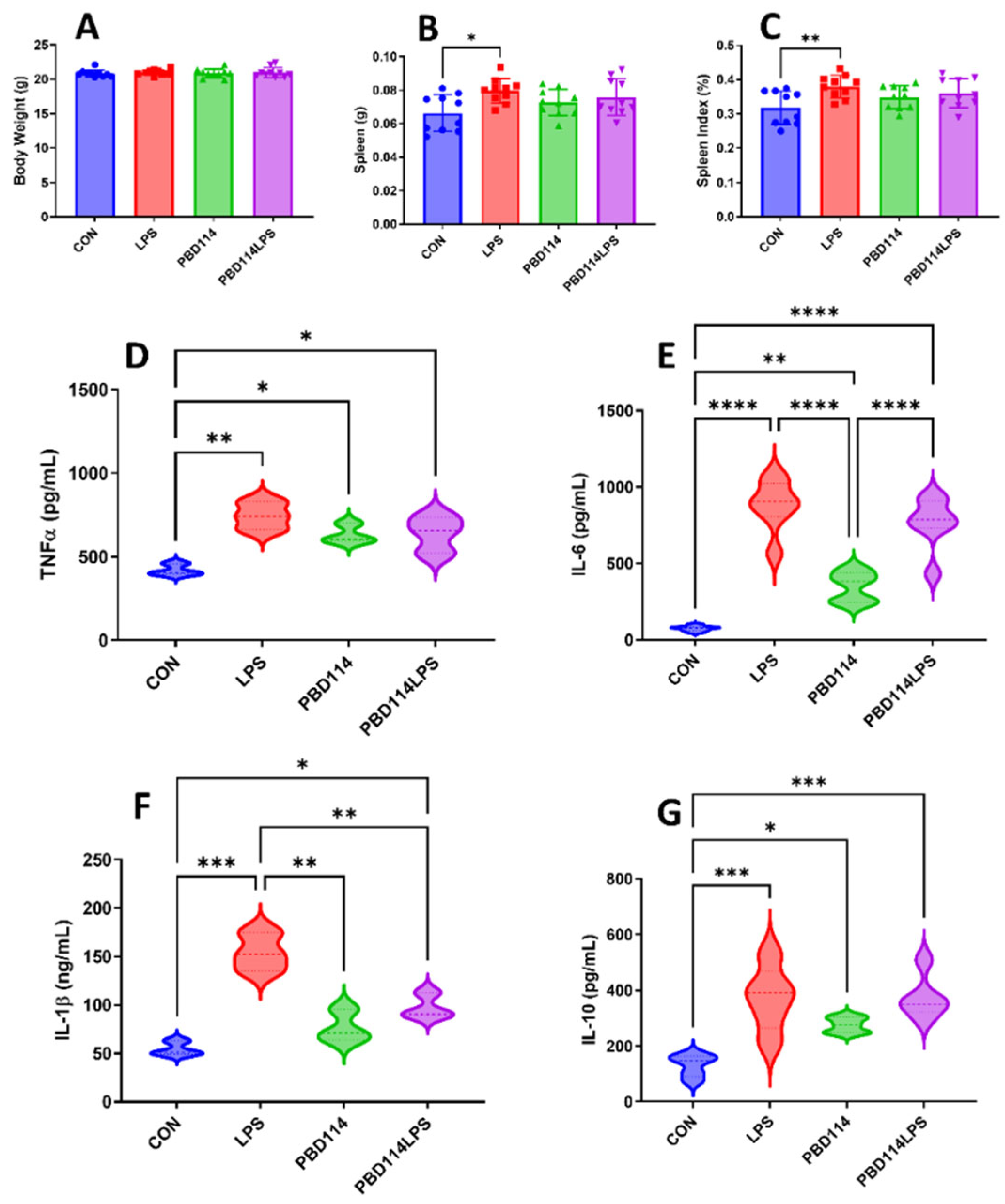
2.4. Modulation of Inflammatory Responses in Mice by pBD114
3. Discussion
4. Materials and Methods
4.1. Chemical Solid Phase Synthesis of pBD114
4.2. Minimal Inhibitory Concentration
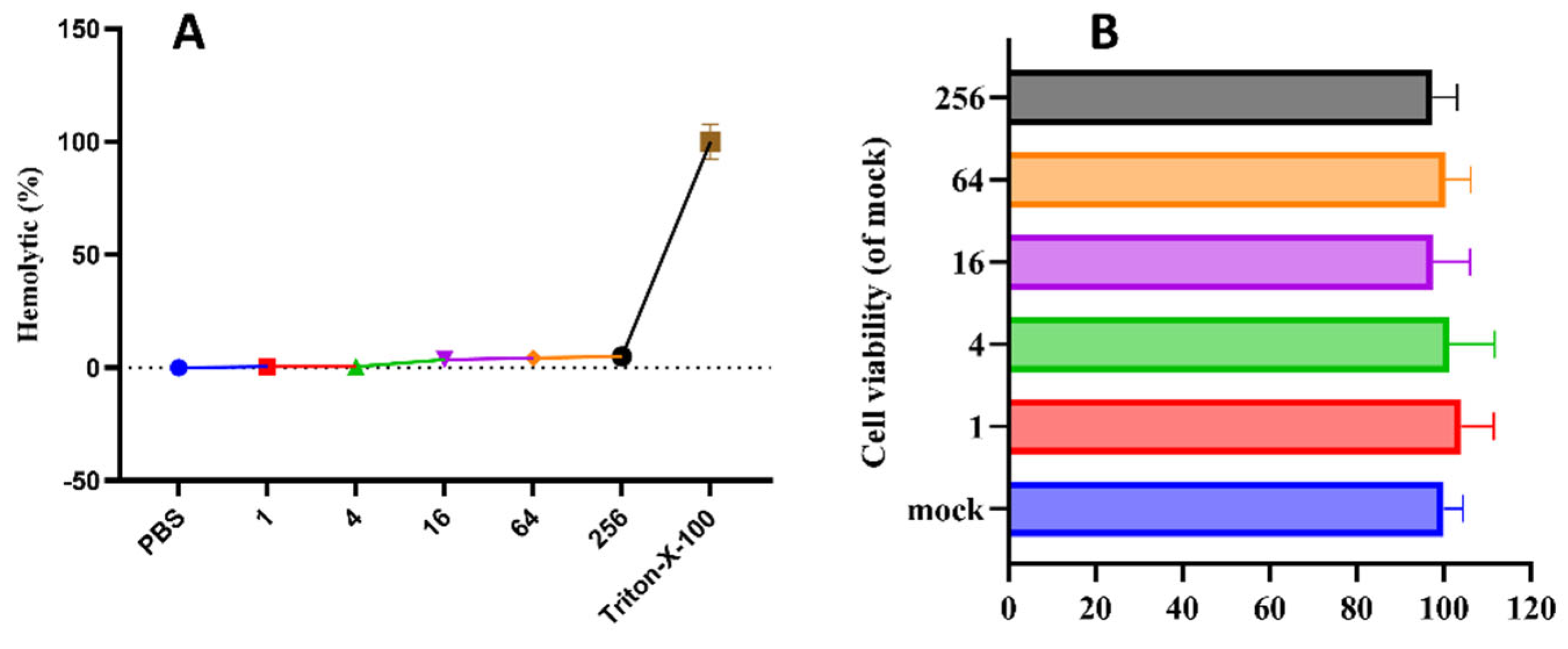
4.3. Hemolytic Activity Assay
4.4. Cell Culture and Treatment
- 1)
- Cytotoxicity of pBD114: The cells were inoculated into 96-well culture plates and cultured until the fusion reached 50%. The cells were treated with pBD114 at the final concentration of 0, 1, 4, 16, 64, and 256 μg/mL for 24 h. CCK-8 detected the cell activity of pBD114.
- 2)
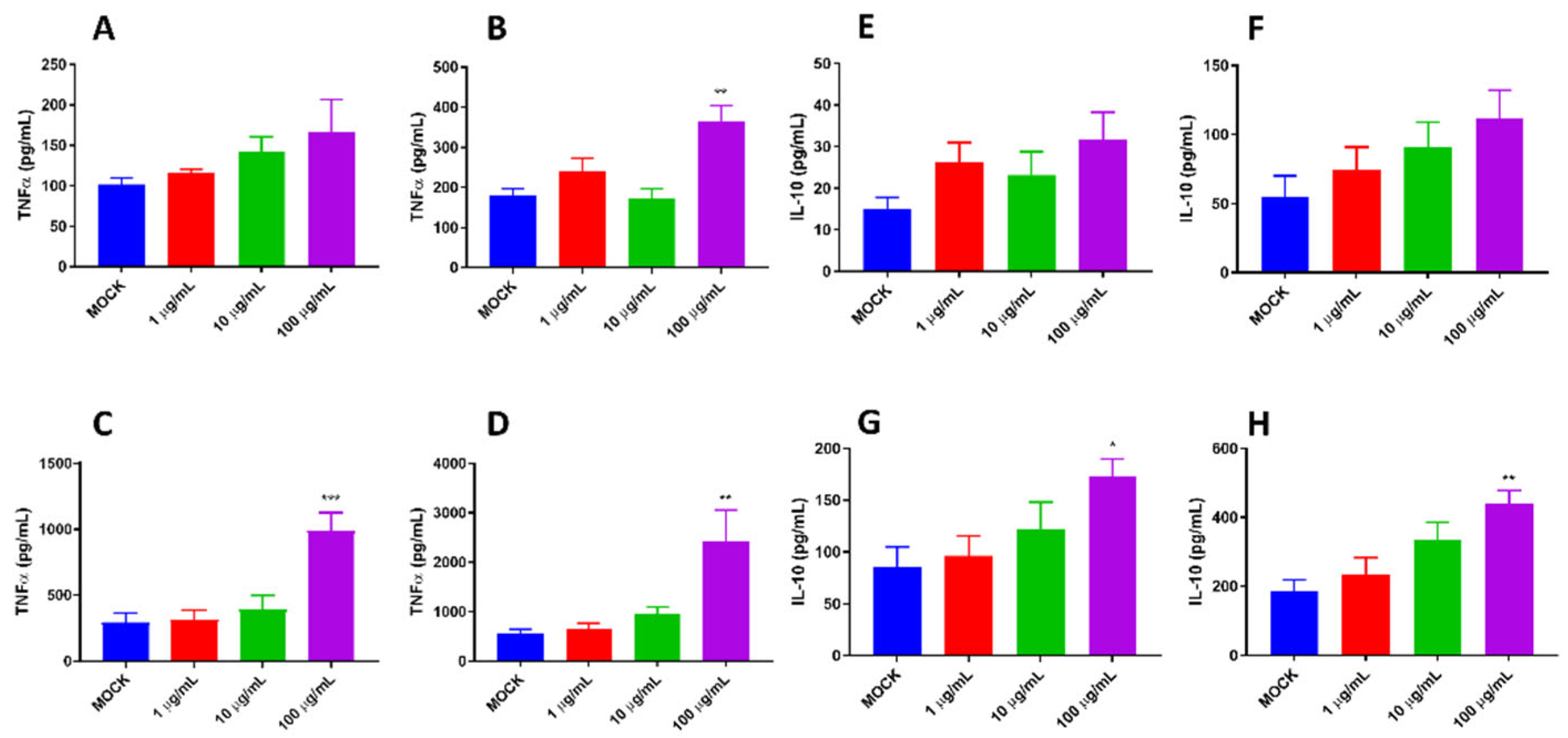
- The effects of pBD114 on inflammatory response in macrophages under normal physiological conditions: Cells were inoculated into 24-well culture plates and cultured until the fusion reached 80%, then treated with 10 and 100 μg/mL pBD114 for 1, 3, 6 and 12 h. Cell culture medium was collected to detect TNF-α (R&D Systems,DY410) and IL-10 (R&D Systems,M1000B) protein concentration by ELISA.
- 3)
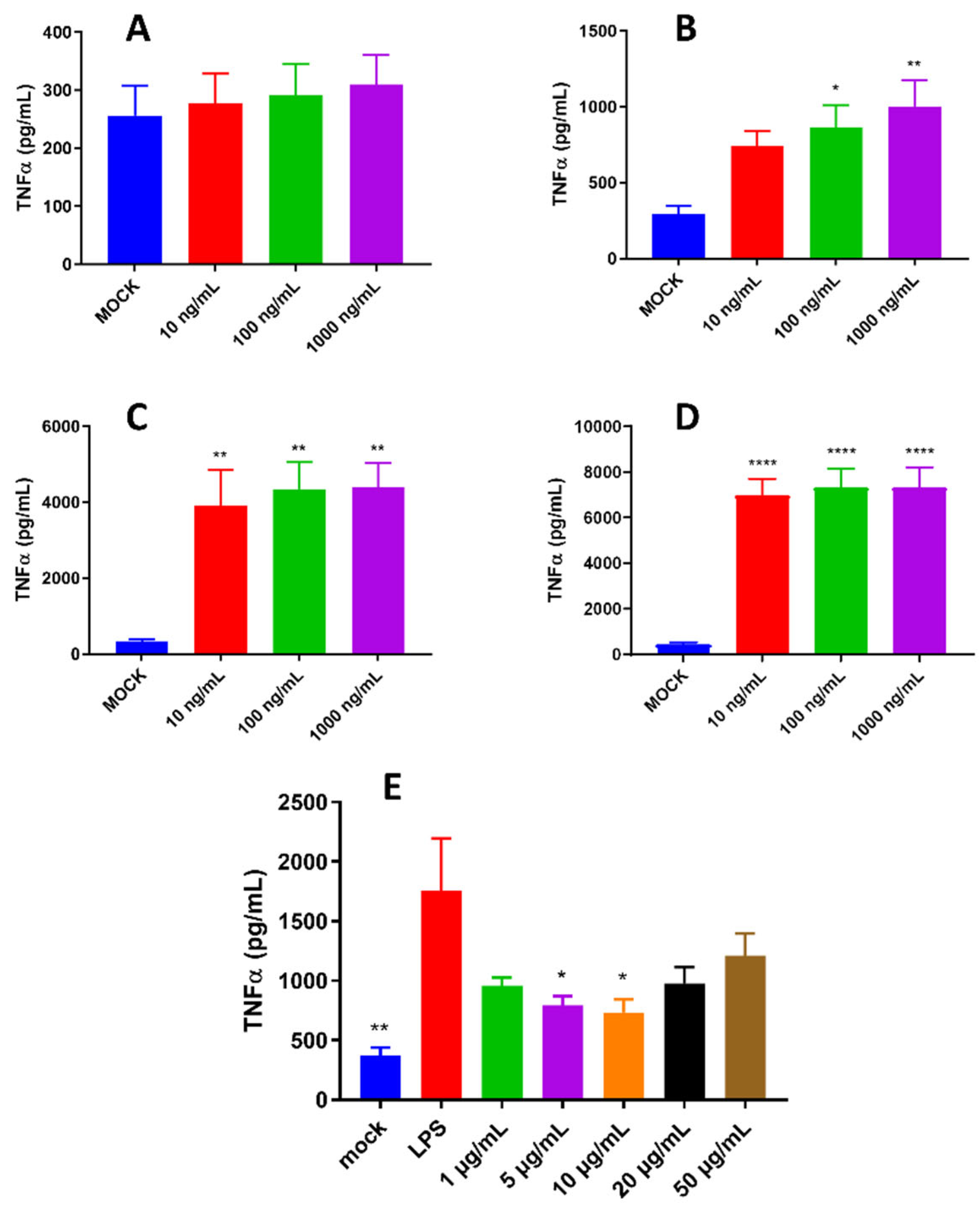
- Optimization of LPS treatment time and concentration: Cells were inoculated into a 24-well culture plate and cultured until the fusion reached 80%, then treated with 1, 5, 10, 20 μg/mL LPS and 50 μg/mL pBD114f for 0.5, 1, 2 and 4 h, cell culture medium was collected, and TNF-α protein concentration was detected by ELISA.
- 4)
- The effects of pBD114 on inflammatory response in macrophages under inflammatory activation: Cells were inoculated into a 24-well culture plate and cultured until the fusion reached 80%, then treated with 1, 5, 10, 20, and 50 μg/mL pBD114 and 10 ng/mL LPS for 2 h, cell culture medium was collected, and TNF-α protein concentration was detected by ELISA.
- 5)
- Samples of RNA-seq: Mouse mononuclear macrophage RAW264.7 were inoculated into a 6-well plate and cultured until the fusion reached 80%. The cells were treated with or without pBD114 for 12 h, and 10 ng/mL LPS-induced cells were simultaneously treated with or without 10 μg/mL pBD114 for 2 h. The concentration of TNF-α and IL-10 protein in the culture solution was detected by ELISA. The cells were collected for RNA-seq.
4.5. RNA-Seq and Data Analysis
4.6. Animal Trials
4.7. Quantitative Real-Time PCR Analysis
4.8 Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 2017, 23, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat Immunol 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M. Understanding inflammation, its regulation, and relevance for health: A top scientific and public priority. Brain Behav Immun 2015, 45, 13–14. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front Med 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kotas, Maya E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [CrossRef]
- Straub, R.H. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat Rev Rheumatol 2017, 13, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat Rev Drug Discov 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Cutolo, M.; Pacifici, R. Evolutionary medicine and bone loss in chronic inflammatory diseases—A theory of inflammation-related osteopenia. Semin Arthritis Rheum 2015, 45, 220–228. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. Inflammation: Friend or foe for animal production? Poult Sci 2018, 97, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Chastant, S.; Saint-Dizier, M. Inflammation: Friend or foe of bovine reproduction? Anim Reprod 2019, 16, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.R.G.; Mehat, J.; La Ragione, R.; Behboudi, S. Preventing bacterial disease in poultry in the post-antibiotic era: A case for innate immunity modulation as an alternative to antibiotic use. Front Immunol 2023, 14, 1205869. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ding, J.; Liao, C.; Xu, J.; Liu, X.; Lu, W. Defensins: The natural peptide antibiotic. Adv Drug Deliv Rev 2021, 179, 114008. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front Immunol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Solanki, S.S.; Singh, P.; Kashyap, P.; Sansi, M.S.; Ali, S.A. Promising role of defensins peptides as therapeutics to combat against viral infection. Microb Pathog 2021, 155, 104930. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and regulation of defensins in host defense. Signal Transduct Target Ther 2023, 8, 300. [Google Scholar] [CrossRef]
- Contreras, G.; Shirdel, I.; Braun, M.S.; Wink, M. Defensins: Transcriptional regulation and function beyond antimicrobial activity. Dev Comp Immunol 2020, 104, 103556. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat Rev Microbiol 2021, 19, 786–797. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 2013, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Lu, W. Antimicrobial peptides. Semin Cell Dev Biol 2019, 88, 105–106. [Google Scholar] [CrossRef]
- Brook, M.; Tomlinson, G.H.; Miles, K.; Smith, R.W.P.; Rossi, A.G.; Hiemstra, P.S.; van ’t Wout, E.F.A.; Dean, J.L.E.; Gray, N.K.; Lu, W.; et al. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci 2016, 113, 4350–4355. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat Rev Immunol 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Y.; Hou, J.; Shu, Q.; Yin, Y.; Fu, W.; Han, F.; Hou, T.; Zeng, C.; Nemeth, E.; et al. Increased gene copy number of DEFA1/DEFA3 worsens sepsis by inducing endothelial pyroptosis. Proc Natl Acad Sci 2019, 116, 3161–3170. [Google Scholar] [CrossRef]
- Higazi, M.; Abdeen, S.; Abu-Fanne, R.; Heyman, S.N.; Masarwy, A.; Bdeir, K.; Maraga, E.; Cines, D.B.; Higazi, A.A.-R. Opposing effects of HNP1 (α-defensin-1) on plasma cholesterol and atherogenesis. PLoS ONE 2020, 15, e0231582. [Google Scholar] [CrossRef]
- Abu-Fanne, R.; Stepanova, V.; Litvinov, R.I.; Abdeen, S.; Bdeir, K.; Higazi, M.; Maraga, E.; Nagaswami, C.; Mukhitov, A.R.; Weisel, J.W.; et al. Neutrophil α-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood 2019, 133, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Antikainen, A.A.V.; Sandholm, N.; Trégouët, D.-A.; Charmet, R.; McKnight, A.J.; Ahluwalia, T.S.; Syreeni, A.; Valo, E.; Forsblom, C.; Gordin, D.; et al. Genome-wide association study on coronary artery disease in type 1 diabetes suggests beta-defensin 127 as a risk locus. Cardiovasc Res 2020, 117, 600–612. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Pan, Y.; Shen, L.; Zhang, Y.; Zheng, F.; Shu, Q.; Fang, X. Human Neutrophil Defensins Disrupt Liver Interendothelial Junctions and Aggravate Sepsis. Mediators Inflamm 2022, 2022, 7659282. [Google Scholar] [CrossRef]
- Miani, M.; Le Naour, J.; Waeckel-Enée, E.; Verma, S.c.; Straube, M.; Emond, P.; Ryffel, B.; van Endert, P.; Sokol, H.; Diana, J. Gut Microbiota-Stimulated Innate Lymphoid Cells Support β-Defensin 14 Expression in Pancreatic Endocrine Cells, Preventing Autoimmune Diabetes. Cell Metab 2018, 28, 557–572.e556. [Google Scholar] [CrossRef]
- Kolbinger, F.; Loesche, C.; Valentin, M.-A.; Jiang, X.; Cheng, Y.; Jarvis, P.; Peters, T.; Calonder, C.; Bruin, G.; Polus, F.; et al. β-Defensin 2 is a responsive biomarker of IL-17A–driven skin pathology in patients with psoriasis. J Allergy Clin Immunol 2017, 139, 923–932.e928. [Google Scholar] [CrossRef]
- Lu, W.; de Leeuw, E. Pro-inflammatory and pro-apoptotic properties of Human Defensin 5. Biochem Biophys Res Commun 2013, 436, 557–562. [Google Scholar] [CrossRef]
- Biragyn, A.; Ruffini, P.A.; Leifer, C.A.; Klyushnenkova, E.; Shakhov, A.; Chertov, O.; Shirakawa, A.K.; Farber, J.M.; Segal, D.M.; Oppenheim, J.J.; et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 2002, 298, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.V.; Fiedoruk, K.; Daniluk, T.; Piktel, E.; Bucki, R. Expression and Function of Host Defense Peptides at Inflammation Sites. Int J Mol Sci 2019, 21. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.R.; Davidson, D.J.; Dorin, J.R. The Dichotomous Responses Driven by β-Defensins. Front Immunol 2020, 11. [Google Scholar] [CrossRef]
- Koeninger, L.; Armbruster, N.S.; Brinch, K.S.; Kjaerulf, S.; Andersen, B.; Langnau, C.; Autenrieth, S.E.; Schneidawind, D.; Stange, E.F.; Malek, N.P.; et al. Human β-Defensin 2 Mediated Immune Modulation as Treatment for Experimental Colitis. Front Immunol 2020, 11. [Google Scholar] [CrossRef]
- Su, G.; Luo, Y.; Chen, D.; Yu, B.; He, J. NF-κB-dependent induction of porcine β-defensin 114 regulates intestinal epithelium homeostasis. Int J Biol Macromol 2021, 192, 241–249. [Google Scholar] [CrossRef]
- Xie, K.; Su, G.; Chen, D.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Luo, Y.; Yan, H.; Li, H.; et al. The immunomodulatory function of the porcine β-defensin 129: Alleviate inflammatory response induced by LPS in IPEC-J2 cells. Int J Biol Macromol 2021, 188, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, Q.-h.; Sun, J.; Zhang, M.; Xiang, Y.-q. Porcine β-defensin-2 alleviates AFB1-induced intestinal mucosal injury by inhibiting oxidative stress and apoptosis. Ecotoxicol Environ Saf 2023, 262, 115161. [Google Scholar] [CrossRef]
- Zhang, K.; Lian, S.; Shen, X.; Zhao, X.; Zhao, W.; Li, C. Recombinant porcine beta defensin 2 alleviates inflammatory responses induced by Escherichia coli in IPEC-J2 cells. Int J Biol Macromol 2022, 208, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, Q.H.; Sun, J.; Zhang, M.; Xiang, Y.Q. Porcine β-defensin-2 alleviates AFB1-induced intestinal mucosal injury by inhibiting oxidative stress and apoptosis. Ecotoxicol Environ Saf 2023, 262, 115161. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, M.; Sun, J.; Li, Y.; Zu, S.; Xiang, Y.; Jin, X. Porcine β-defensin-2 alleviates aflatoxin B1 induced intestinal mucosal damage via ROS-Erk(1/2) signaling pathway. Sci Total Environ 2023, 905, 167201. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Sun, Y.; Huang, C.; Wang, A.; Xu, J.; Zhou, H.; Li, L.; Zhou, R. Porcine β-defensin 2 confers enhanced resistance to swine flu infection in transgenic pigs and alleviates swine influenza virus-induced apoptosis possibly through interacting with host SLC25A4. Antiviral Res 2022, 201, 105292. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.; Huang, J.; Liu, X.; Yang, X.; Jin, H.; Huang, Q.; Li, L.; Zhou, R. Porcine Beta-Defensin 2 Provides Protection Against Bacterial Infection by a Direct Bactericidal Activity and Alleviates Inflammation via Interference With the TLR4/NF-κB Pathway. Front Immunol 2019, 10, 1673. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.; Rijnders, M.; Claassen, E.A.; van Dijk, A.; Haagsman, H.P. Porcine beta-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol Immunol 2008, 45, 386–394. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat Rev Drug Discov 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Xie, K.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; et al. Differential expression, molecular cloning, and characterization of porcine beta defensin 114. J Anim Sci Biotechnol 2019, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-t.; Xiao, Y.-P.; Li, J.-J.; Ran, J.-S.; Yin, L.-Q.; Liu, Y.-P.; Zhang, L. Molecular characterization of a novel ovodefensin gene in chickens. Gene 2018, 678, 233–240. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Chertov, O.; Oppenheim, J.J. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol 2000, 68, 9–14. [Google Scholar] [CrossRef]
- Corrales-García, L.L.; Serrano-Carreón, L.; Corzo, G. Improving the heterologous expression of human β-defensin 2 (HBD2) using an experimental design. Protein Expr Purif 2020, 167, 105539. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; An, Y.; Wang, Z.; Yin, W.; Sun, N.; Sun, Y.; Khan, A.; Li, H. Antibacterial Activity of Recombinant Porcine β-Defensin 2. Pak Vet J 2019, 39, 2074–7764. [Google Scholar] [CrossRef]
- Lian, S.; Lin, X.; Zhan, F.; Shen, X.; Liang, Y.; Li, C. Transcriptome Analysis Reveals the Multiple Functions of pBD2 in IPEC-J2 Cells against E. coli. Int J Mol Sci 2022, 23, 9754. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Sun, Y.; Qiu, X.; Huang, J.; Wang, A.; Zhang, Q.; Pang, S.; Huang, Q.; Zhou, R.; Li, L. The Intracellular Interaction of Porcine beta-Defensin 2 with VASH1 Alleviates Inflammation via Akt Signaling Pathway. J Immunol 2022, 208, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int Immunol 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Merly, L.; Smith, S.L. Murine RAW 264.7 cell line as an immune target: Are we missing something? Immunopharmacol Immunotoxicol 2017, 39, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Pae, H.B.; Lam, V.; Cederberg, R.; Hofs, E.; Krystal, G. Comparison of RAW264.7, human whole blood and PBMC assays to screen for immunomodulators. J Immunol Methods 2018, 452, 26–31. [Google Scholar] [CrossRef]
- Sang, Y.; Patil, A.A.; Zhang, G.; Ross, C.R.; Blecha, F. Bioinformatic and expression analysis of novel porcine β-defensins. Mamm Genome 2006, 17, 332–339. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.; Huang, J.; Liu, X.; Yang, X.; Jin, H.; Huang, Q.; Li, L.; Zhou, R. Porcine Beta-Defensin 2 Provides Protection Against Bacterial Infection by a Direct Bactericidal Activity and Alleviates Inflammation via Interference With the TLR4/NF-κB Pathway. Front Immunol 2019, 10. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Xia, X.; Xiong, H.; Song, D.; Zong, X.; Wang, Y. Porcine β-Defensin 2 Attenuates Inflammation and Mucosal Lesions in Dextran Sodium Sulfate–Induced Colitis. J Immunol Methods 2015, 194, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Choi, K.G.; Mookherjee, N. Host Defense Peptide LL-37-Mediated Chemoattractant Properties, but Not Anti-Inflammatory Cytokine IL-1RA Production, Is Selectively Controlled by Cdc42 Rho GTPase via G Protein-Coupled Receptors and JNK Mitogen-Activated Protein Kinase. Front Immunol 2018, 9, 1871. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat Rev Genet 2019, 20, 631–656. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat Rev Immunol 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target Ther 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, H.; Ha, H.L.; Tassi, I.; Bhardwaj, R.; Claudio, E.; Siebenlist, U. The B-cell tumor promoter Bcl-3 suppresses inflammation-associated colon tumorigenesis in epithelial cells. Oncogene 2016, 35, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Anto Michel, N.; Colberg, C.; Buscher, K.; Sommer, B.; Pramod, A.B.; Ehinger, E.; Dufner, B.; Hoppe, N.; Pfeiffer, K.; Marchini, T.; et al. Inflammatory Pathways Regulated by Tumor Necrosis Receptor-Associated Factor 1 Protect From Metabolic Consequences in Diet-Induced Obesity. Circ Res 2018, 122, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A.; Sousa, C.M.; Zhang, X.; Kim, E.; Akthar, R.; Caumanns, J.J.; Yao, Y.; Mikolajewicz, N.; Ross, C.; Brown, K.R.; et al. Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature 2020, 586, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Markó, L.; Park, J.K.; Henke, N.; Rong, S.; Balogh, A.; Klamer, S.; Bartolomaeus, H.; Wilck, N.; Ruland, J.; Forslund, S.K.; et al. B-cell lymphoma/leukaemia 10 and angiotensin II-induced kidney injury. Cardiovasc Res 2020, 116, 1059–1070. [Google Scholar] [CrossRef]
- Lao, Y.; Yang, K.; Wang, Z.; Sun, X.; Zou, Q.; Yu, X.; Cheng, J.; Tong, X.; Yeh, E.T.H.; Yang, J.; et al. DeSUMOylation of MKK7 kinase by the SUMO2/3 protease SENP3 potentiates lipopolysaccharide-induced inflammatory signaling in macrophages. J Biol Chem 2018, 293, 3965–3980. [Google Scholar] [CrossRef]
- Gong, J.; Fang, C.; Zhang, P.; Wang, P.X.; Qiu, Y.; Shen, L.J.; Zhang, L.; Zhu, X.Y.; Tian, S.; Li, F.; et al. Tumor Progression Locus 2 in Hepatocytes Potentiates Both Liver and Systemic Metabolic Disorders in Mice. Hepatology 2019, 69, 524–544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, S. LncRNA GAS5 suppresses inflammatory responses and apoptosis of alveolar epithelial cells by targeting miR-429/DUSP1. Exp Mol Pathol 2020, 113, 104357. [Google Scholar] [CrossRef] [PubMed]
- Dagvadorj, J.; Mikulska-Ruminska, K.; Tumurkhuu, G.; Ratsimandresy, R.A.; Carriere, J.; Andres, A.M.; Marek-Iannucci, S.; Song, Y.; Chen, S.; Lane, M.; et al. Recruitment of pro-IL-1alpha to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Corbin, A.L.; Gomez-Vazquez, M.; Berthold, D.L.; Attar, M.; Arnold, I.C.; Powrie, F.M.; Sansom, S.N.; Udalova, I.A. IRF5 guides monocytes toward an inflammatory CD11c(+) macrophage phenotype and promotes intestinal inflammation. Sci Immunol 2020, 5. [Google Scholar] [CrossRef]
- Gerbino, V.; Kaunga, E.; Ye, J.; Canzio, D.; O’Keeffe, S.; Rudnick, N.D.; Guarnieri, P.; Lutz, C.M.; Maniatis, T. The Loss of TBK1 Kinase Activity in Motor Neurons or in All Cell Types Differentially Impacts ALS Disease Progression in SOD1 Mice. Neuron 2020, 106, 789–805.e785. [Google Scholar] [CrossRef] [PubMed]
- Semple, F.; MacPherson, H.; Webb, S.; Cox, S.L.; Mallin, L.J.; Tyrrell, C.; Grimes, G.R.; Semple, C.A.; Nix, M.A.; Millhauser, G.L.; et al. Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur J Immunol 2011, 41, 3291–3300. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xie, H.; Su, G.; Chen, D.; Yu, B.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; Zheng, P.; et al. β-Defensin 129 Attenuates Bacterial Endotoxin-Induced Inflammation and Intestinal Epithelial Cell Apoptosis. Front Immunol 2019, 10, 2333. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Wen, P.; He, J.; Zhang, Q.; Qi, H.; Zhang, A.; Liu, D.; Sun, Q.; Wang, Y.; Li, Q.; Wang, W.; et al. SET Domain Group 703 Regulates Planthopper Resistance by Suppressing the Expression of Defense-Related Genes. Int J Mol Sci 2023, 24, 13003. [Google Scholar] [CrossRef]
| Sample | Total Raw Reads, M | Total Clean Reads, M | Total Clean Bases, Gb | Clean Reads Q20, % | Clean Reads Q30, % |
|---|---|---|---|---|---|
| LPS-1 | 23.92 | 23.83 | 1.19 | 97.82 | 93.66 |
| LPS-2 | 23.92 | 23.83 | 1.19 | 97.86 | 93.73 |
| LPS-3 | 23.92 | 23.82 | 1.19 | 97.87 | 93.78 |
| LPS-4 | 22.24 | 22.15 | 1.11 | 97.76 | 93.47 |
| MOCK-1 | 23.92 | 23.83 | 1.19 | 97.77 | 93.53 |
| MOCK-2 | 23.92 | 23.83 | 1.19 | 97.83 | 93.72 |
| MOCK-3 | 23.92 | 23.81 | 1.19 | 97.81 | 93.60 |
| MOCK-4 | 23.92 | 23.83 | 1.19 | 97.84 | 93.75 |
| PBD114-1 | 23.92 | 23.70 | 1.18 | 97.70 | 93.28 |
| PBD114-2 | 23.92 | 23.67 | 1.18 | 97.47 | 92.69 |
| PBD114-3 | 23.92 | 23.66 | 1.18 | 97.77 | 93.52 |
| PBD114-4 | 23.92 | 23.69 | 1.18 | 97.47 | 92.73 |
| PBD114LPS-1 | 23.92 | 23.86 | 1.19 | 97.40 | 92.22 |
| PBD114LPS-2 | 23.92 | 23.85 | 1.19 | 97.46 | 92.40 |
| PBD114LPS-3 | 22.91 | 22.84 | 1.14 | 97.59 | 92.69 |
| PBD114LPS-4 | 21.26 | 21.20 | 1.06 | 98.08 | 93.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





