Submitted:
19 June 2025
Posted:
23 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
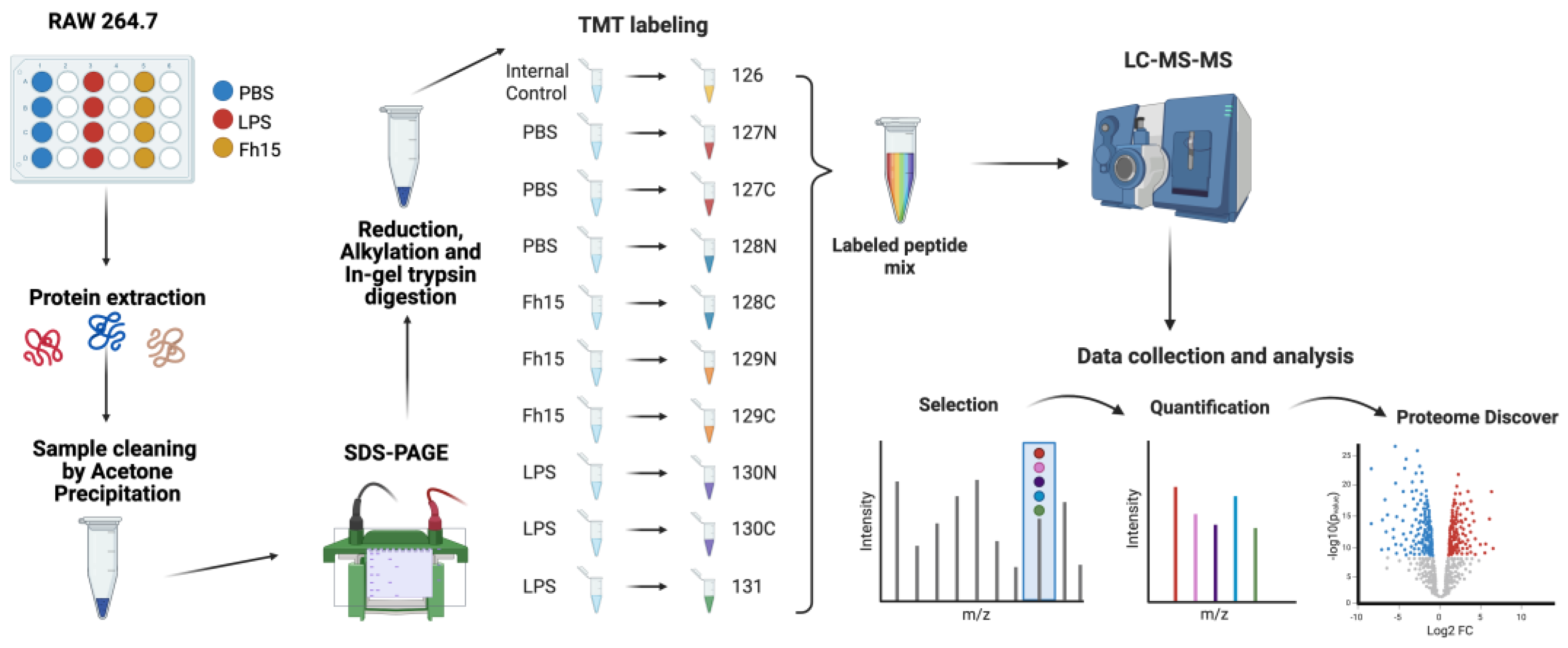
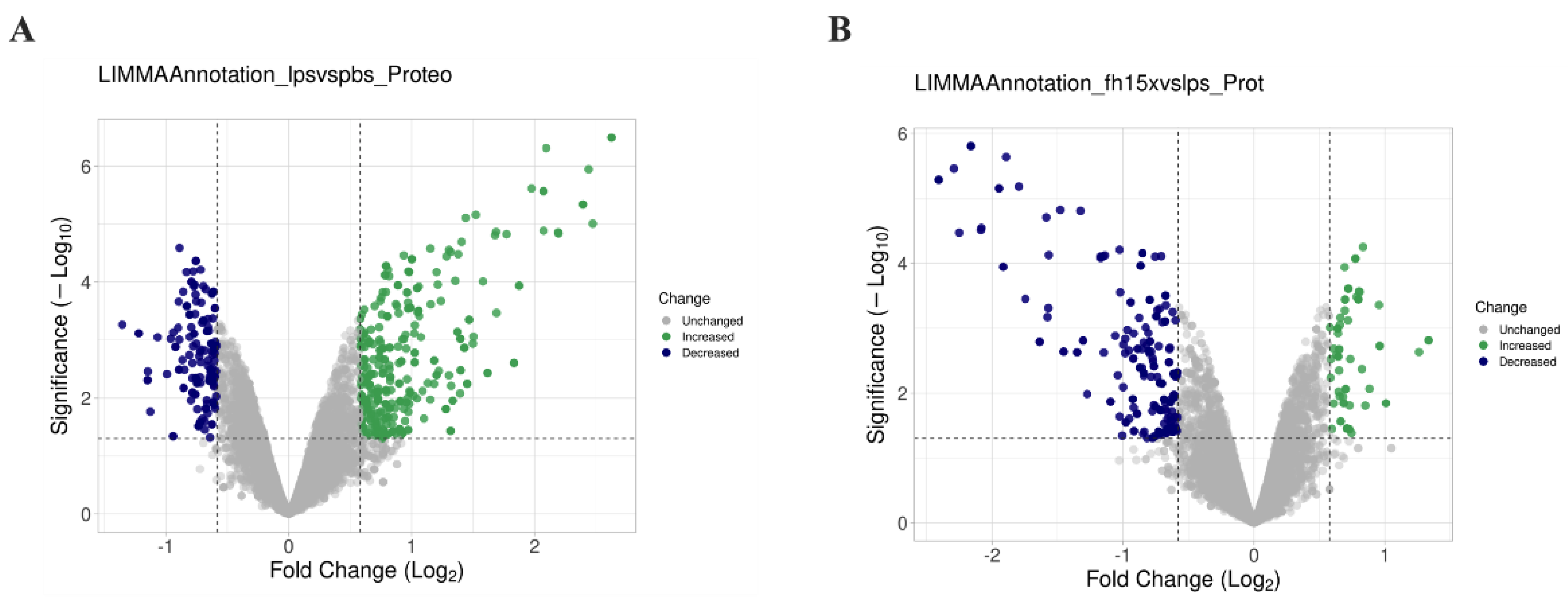
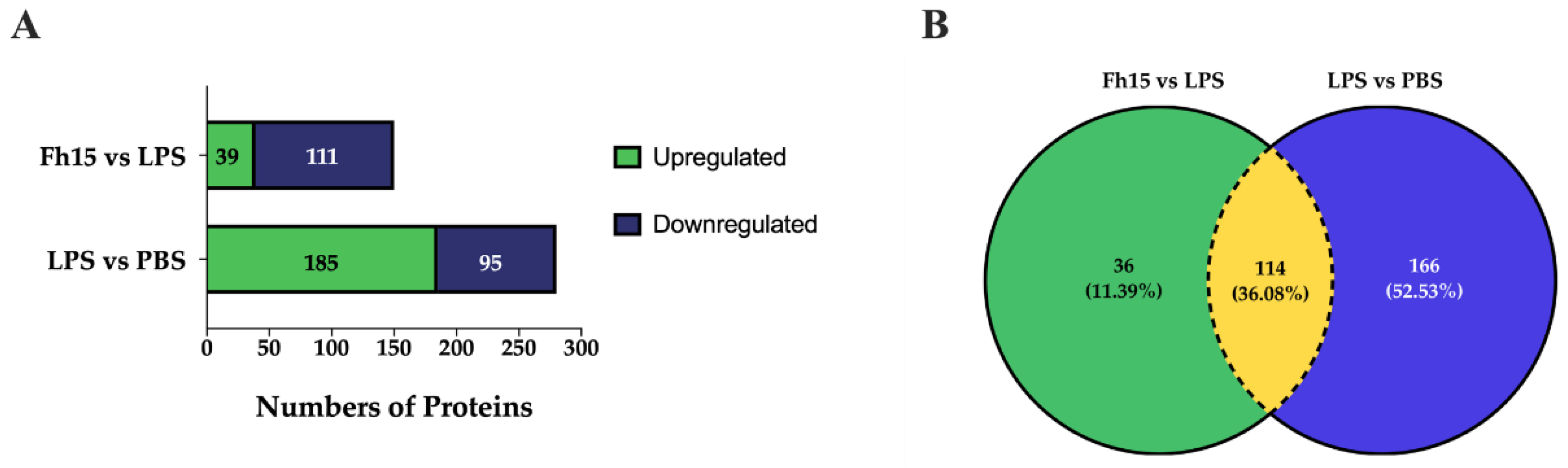
2.1. Quantitative Proteomics Analysis of Macrophages-like Cells Exposed to LPS or Fh15
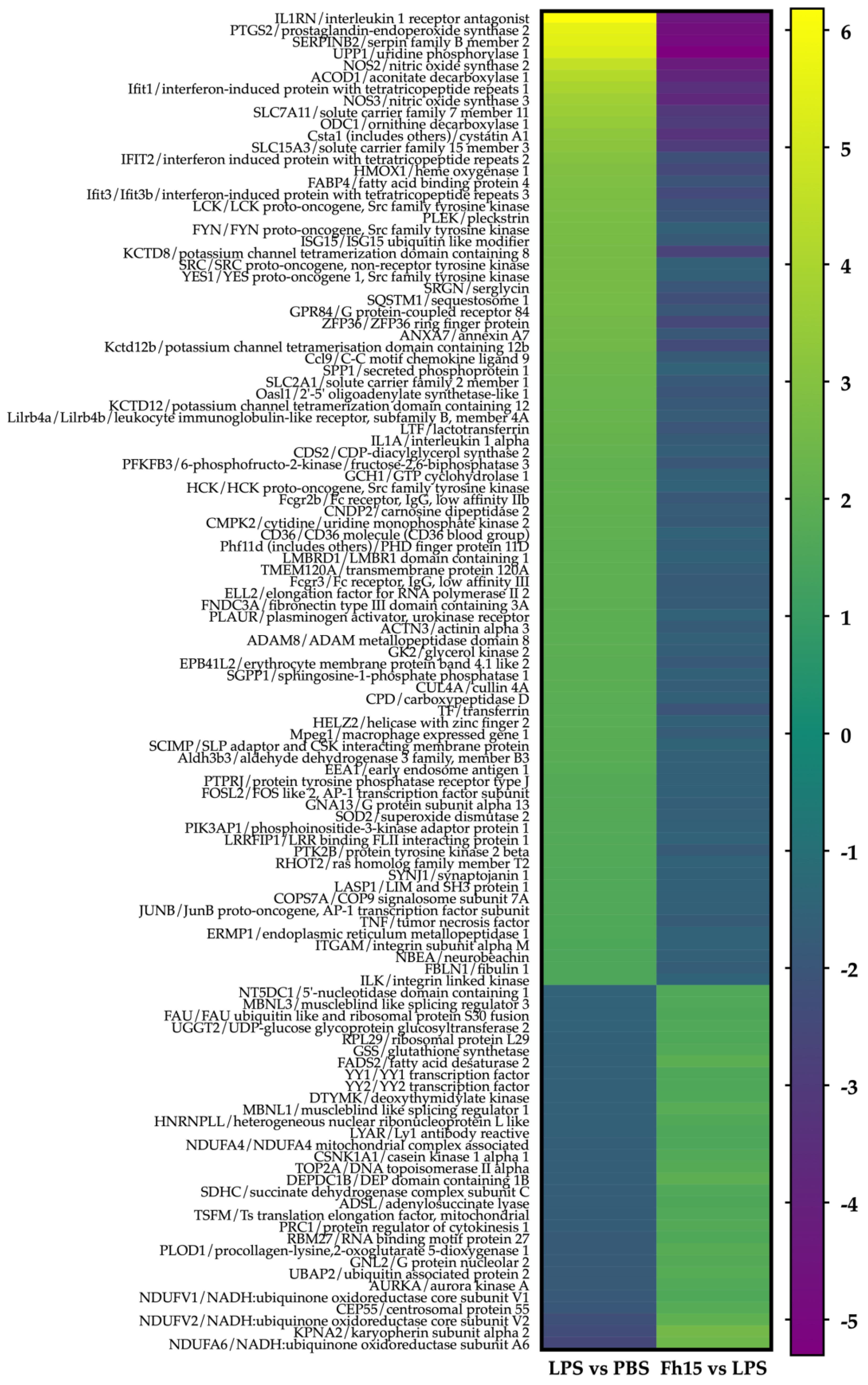
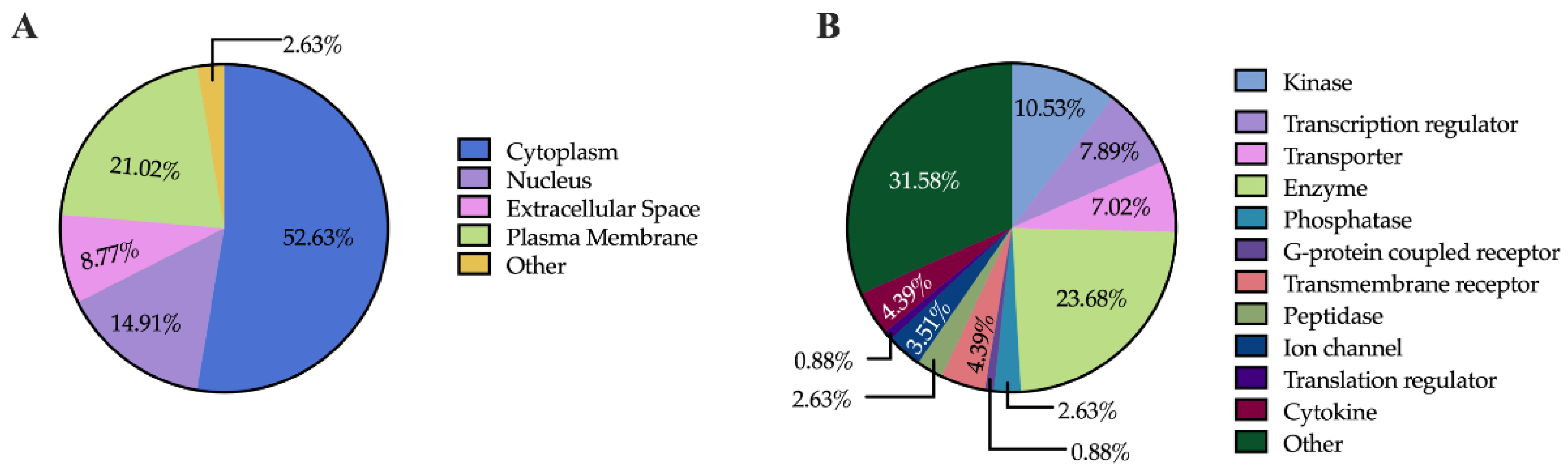
2.2. Subcellular Localization and Function of Dysregulated Proteins
2.3. IPA Results and Functional Enrichment Analysis
| Fh15 vs LPS | LPS vs PBS | ||||||||
| Symbol | Gene Name | ID | Location | Type(s) | Fold Change | p-value | Fold Change | p-value | |
| NOS2 | nitric oxide synthase 2 | P29477 | Cytoplasm | enzyme | -4.242 | 0.0000309 | 4.584 | 0.0000144 | |
| Lck | Lck proto-oncogene, Src family tyrosine kinase | P06240 | Cytoplasm | kinase | -2.137 | 0.0137 | 2.736 | 0.00567 | |
| TNF-α | tumor necrosis factor | P06804 | Extracellular Space | cytokine | -1.79 | 0.00493 | 1.597 | 0.00382 | |
| IL-1α | interleukin 1 alpha | P01582 | Extracellular Space | cytokine | -1.787 | 0.0394 | 2.195 | 0.0055 | |
| CD36 | CD36 molecule (CD36 blood group) | A0A0G2JFB7 | Plasma Membrane | trans- membrane receptor |
-1.538 | 0.000565 | 2.028 | 0.000765 | |
| SOD2 | superoxide dismutase 2 | P09671 | Cytoplasm | enzyme | -1.665 | 0.000527 | 1.722 | 0.0000756 | |
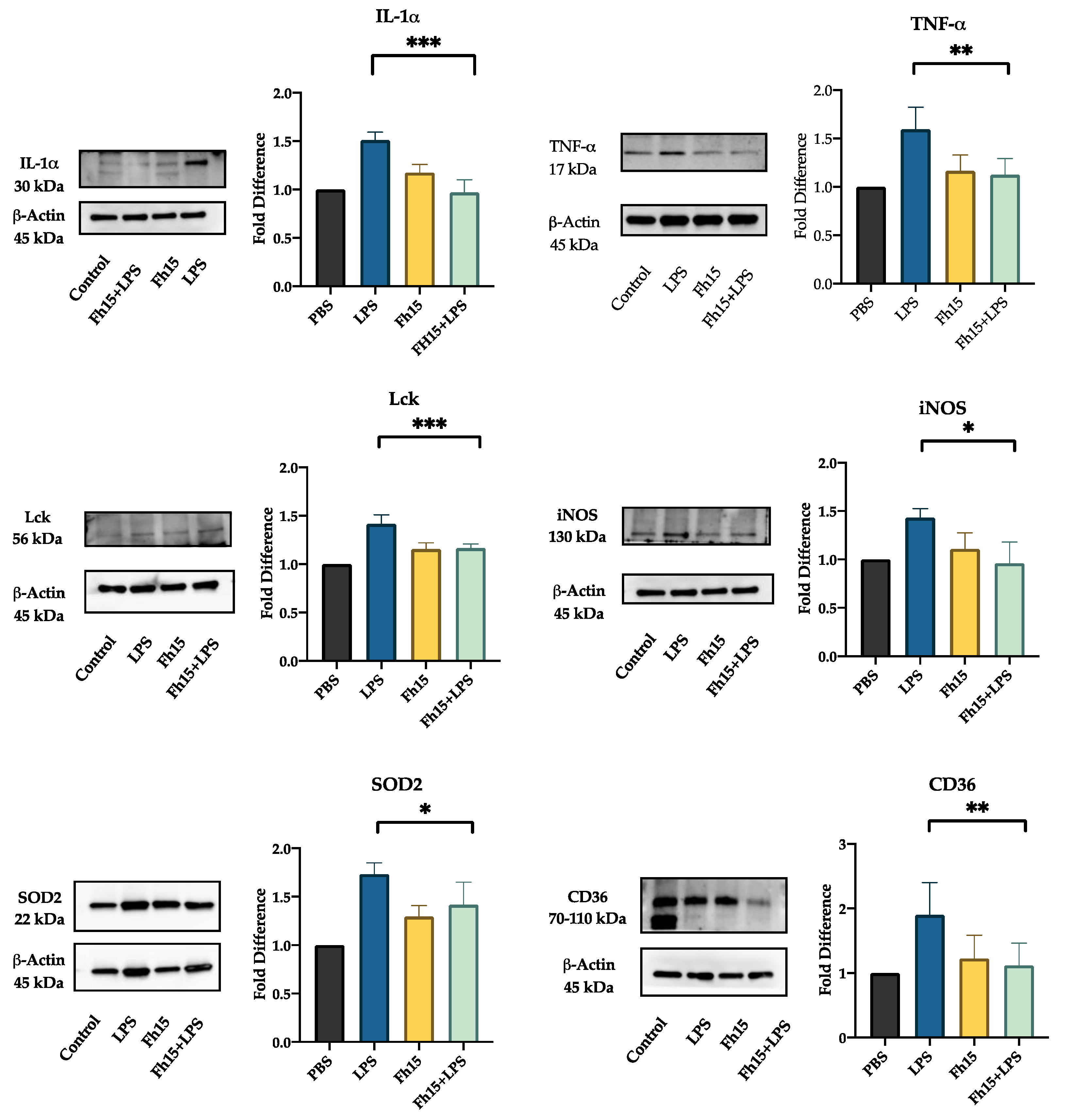
2.4. Validation of Selected Downregulated Proteins by Fh15 Using Western Blot
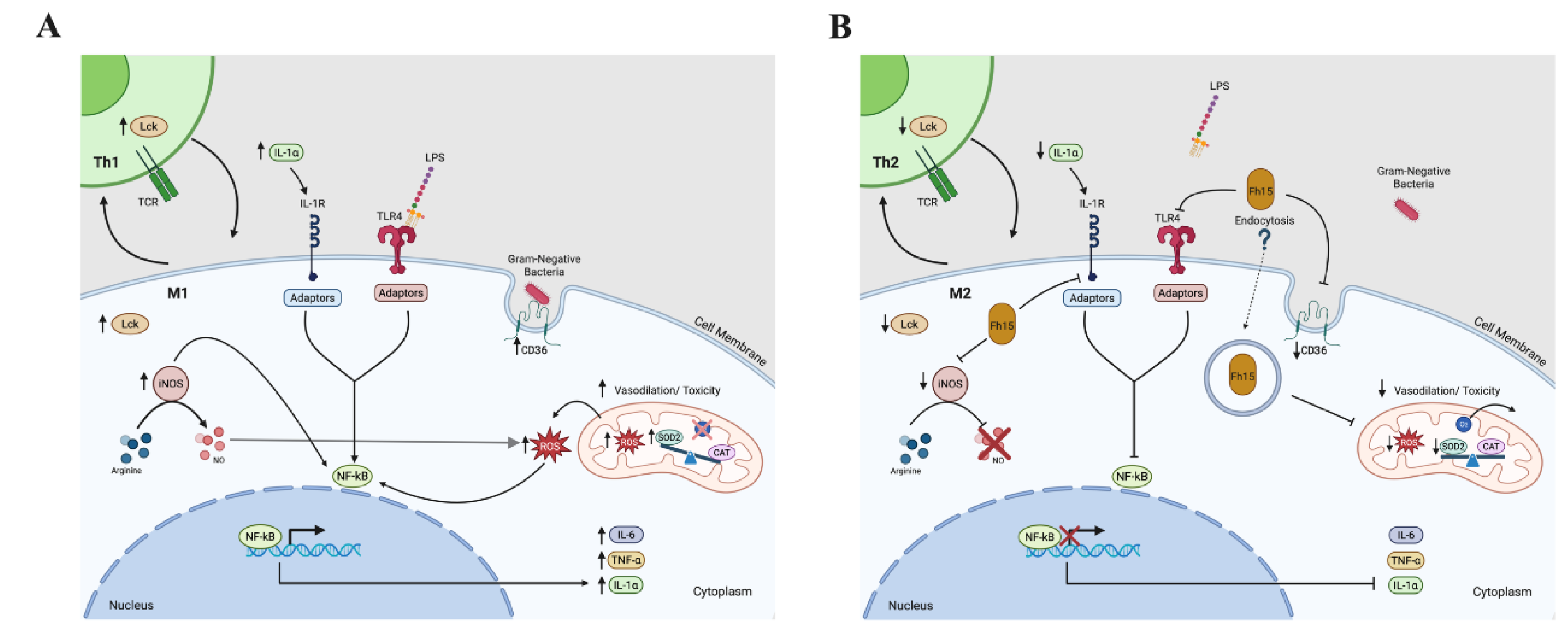
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Recombinant Fh15
4.3. Cell Line and Maintenance
4.4. Mouse Primary Cells Isolation and Differentiation
4.5. Protein Extraction and Quantification
4.6. Preparation of Protein Samples for Tandem Mass Tag (TMT) Labelling
4.7. TMT-Labelling, Fractionation, and Mass Spectrometry Analysis
4.8. Protein Identification, and Bioinformatics Analyses
4.9. Protein Validation by Quantitative Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B | B |
| TLR4 | Toll-like receptor-4 |
| LPS | Lipopolysaccharide |
| FABP | Fatty acid binding protein |
| SIRS | Systemic inflammatory response syndrome |
| CARS | Compensatory anti-inflammatory response syndrome |
| TMT | Tandem mass tag |
| LC/MS-MS | Liquid chromatography /Mass spectrometry |
| HRP | Horseradish peroxidase |
| Lck | Lymphocyte-specific protein tyrosine kinase |
| CD36 | Cluster differentiation-36 |
| IL1 | Interleukin-1 |
| Tumor necrosis factor | |
| SOD2 | Manganese-dependent superoxide dismutase |
| iNOS2 | Inducible nitric oxide synthase-2 |
| BMDM | Bone marrow derived macrophage |
References
- Hubner MP, Layland LE, Hoerauf A. Helminths and their implication in sepsis - a new branch of their immunomodulatory behaviour? Pathog Dis. 2013;69(2):127-41. [CrossRef] [PubMed] [PubMed Central]
- Allen JE, Sutherland TE. Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin Immunol. 2014;26(4):329-40. Epub 20140711. [CrossRef] [PubMed] [PubMed Central]
- Sutherland TE, Logan N, Ruckerl D, Humbles AA, Allan SM, Papayannopoulos V, Stockinger, B., Maizels, R.M., Allen, J.E. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol. 2014;15(12):1116-25. Epub 20141019. [CrossRef] [PubMed] [PubMed Central]
- Gondorf F, Berbudi A, Buerfent BC, Ajendra J, Bloemker D, Specht S, Schmidt, D., Neumann, A.L., Layland, L.E., Hoerauf, A., Hubner, M.P. Chronic filarial infection provides protection against bacterial sepsis by functionally reprogramming macrophages. PLoS Pathog. 2015;11(1):e1004616. [CrossRef] [PubMed] [PubMed Central]
- Flajnik MF, Kasahara M. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity. 2001;15(3):351-62. [CrossRef] [PubMed]
- Laird DJ, De Tomaso AW, Cooper MD, Weissman IL. 50 million years of chordate evolution: seeking the origins of adaptive immunity. Proc Natl Acad Sci U S A. 2000;97(13):6924-6. [CrossRef] [PubMed] [PubMed Central]
- Buitrago G, Harnett MM, Harnett W. Conquering rheumatic diseases: are parasitic worms the answer? Trends Parasitol. 2023;39(9):739-48. Epub 20230722. [CrossRef] [PubMed]
- Rook GA, Brunet LR. Old friends for breakfast. Clin Exp Allergy. 2005;35(7):841-2. [CrossRef] [PubMed]
- Cheng Y, Yu Y, Zhuang Q, Wang L, Zhan B, Du S, Liu Y., Huang, J., Hao, J., Zhu, X. Bone erosion in inflammatory arthritis is attenuated by Trichinella spiralis through inhibiting M1 monocyte/macrophage polarization. iScience. 2022;25(3):103979. Epub 20220224. [CrossRef] [PubMed] [PubMed Central]
- Cheng Y, Zhu X, Wang X, Zhuang Q, Huyan X, Sun X, Huang, J., Zhan, B., Zhu, X. Trichinella spiralis Infection Mitigates Collagen-Induced Arthritis via Programmed Death 1-Mediated Immunomodulation. Front Immunol. 2018;9:1566. Epub 20180726. [CrossRef] [PubMed] [PubMed Central]
- Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, Dunne, D.W. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21(4):169-76. [CrossRef] [PubMed]
- Li X, Yang Y, Qin S, Kong F, Yan C, Cheng W, Pan, W., Yu, Q., Hua, H., Zheng, K., Tang, R. The impact of Clonorchis sinensis infection on immune response in mice with type II collagen-induced arthritis. BMC Immunol. 2020;21(1):7. [CrossRef] [PubMed] [PubMed Central]
- Lund ME, O’Brien BA, Hutchinson AT, Robinson MW, Simpson AM, Dalton JP, Donnelly, S. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS One. 2014;9(1):e86289. [CrossRef] [PubMed] [PubMed Central]
- Shayesteh Z, Hosseini H, Nasiri V, Haddadi Z, Moradi N, Beikzadeh L, Sezavar, M., Heidari, A., Zibaei, M. Evaluating the preventive and curative effects of Toxocara canis larva in Freund’s complete adjuvant-induced arthritis. Parasite Immunol. 2020;42(11):e12760. Epub 20200615. [CrossRef] [PubMed]
- Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183(3):1577-86. Epub 20090708. [CrossRef] [PubMed]
- Armina-Rodriguez A, Ocasio-Malave, C, Méndez-Torres LB, Valdés-Fernández B, Espino AM. Fasciola hepatica Fh15 promote survival in a mouse septic shock model and downregulates inflammatory cytokines. J. Immunol. 2023; 210, 82-02.
- Martin I, Caban-Hernandez K, Figueroa-Santiago O, Espino AM. Fasciola hepatica fatty acid binding protein inhibits TLR4 activation and suppresses the inflammatory cytokines induced by lipopolysaccharide in vitro and in vivo. J Immunol. 2015;194(8):3924-36. [CrossRef] [PubMed] [PubMed Central]
- Ramos-Benitez MJ, Ruiz-Jimenez C, Ramos-Perez, WD, Mendez LB, Osuna A, Espino AM. Fh15 blocks the LPS-induced cytokine storm while modulating peritoneal macrophage migration and CD38 expression within spleen macrophages in a mouse model of septic shock. mSphere 2018;6(3): e00548-18. [CrossRef]
- Rosado-Franco JJ, Armina-Rodriguez A, Marzan-Rivera N, Burgos AG, Spiliopoulos N, Dorta-Estremera SM, Mendez, L.B., Espino, A.M. Recombinant Fasciola hepatica Fatty Acid Binding Protein as a Novel Anti-Inflammatory Biotherapeutic Drug in an Acute Gram-Negative Nonhuman Primate Sepsis Model. Microbiol Spectr. 2021;9(3):e0191021. [CrossRef] [PubMed] [PubMed Central]
- Espino AM, Hillyer GV. Identification of fatty acid molecules in a Fasciola hepatica immunoprophylactic fatty acid-binding protein. J Parasitol. 2001;87(2):426-8. [CrossRef] [PubMed]
- Bell C, English L, Boulais J, Chemali M, Caron-Lizotte O, Desjardins M, Thibault, P. Quantitative proteomics reveals the induction of mitophagy in tumor necrosis factor-alpha-activated (TNFalpha) macrophages. Mol Cell Proteomics. 2013;12(9):2394-407. [CrossRef] [PubMed] [PubMed Central]
- Ricchiuto P, Iwata H, Yabusaki K, Yamada I, Pieper B, Sharma A, Aikawa, M., Singh, S.A. mIMT-visHTS: A novel method for multiplexing isobaric mass tagged datasets with an accompanying visualization high throughput screening tool for protein profiling. J Proteomics. 2015;128:132-40. [CrossRef] [PubMed]
- Rouzer CA, Ivanova PT, Byrne MO, Milne SB, Marnett LJ, Brown HA. Lipid profiling reveals arachidonate deficiency in RAW264.7 cells: Structural and functional implications. Biochemistry. 2006;45(49):14795-808. [CrossRef] [PubMed] [PubMed Central]
- Borges-Velez G, Arroyo JA, Cantres-Rosario YM, Rodriguez de Jesus A, Roche-Lima A, Rosado-Philippi J, Rosario-Rodriguez, L.J., Correa-Rivas, M.S., Campos-Rivera, M., Melendez, L.M. Decreased CSTB, RAGE, and Axl Receptor Are Associated with Zika Infection in the Human Placenta. Cells. 2022;11(22). [CrossRef] [PubMed] [PubMed Central]
- Rosario-Rodriguez LJ, Cantres-Rosario YM, Carrasquillo-Carrion K, Rodriguez-De Jesus AE, Cartagena-Isern LJ, Garcia-Requena LA, Roche-Lima, A., Melendez, L.A. Quantitative Proteomics Reveal That CB2R Agonist JWH-133 Downregulates NF-kappaB Activation, Oxidative Stress, and Lysosomal Exocytosis from HIV-Infected Macrophages. Int J Mol Sci. 2024;25(6). [CrossRef] [PubMed] [PubMed Central]
- Zenon-Melendez CN, Carrasquillo Carrion K, Cantres Rosario Y, Roche Lima A, Melendez LM. Inhibition of Cathepsin B and SAPC Secreted by HIV-Infected Macrophages Reverses Common and Unique Apoptosis Pathways. J Proteome Res. 2022;21(2):301-12. [CrossRef] [PubMed] [PubMed Central]
- Borges-Velez G, Rosado-Philippi J, Cantres-Rosario YM, Carrasquillo-Carrion K, Roche-Lima A, Perez-Vargas J, Gonzalez-Martinez, A., Correa-Rivas, M.S., Melendez, L.M. Zika virus infection of the placenta alters extracellular matrix proteome. J Mol Histol. 2022;53(2):199-214. [CrossRef] [PubMed] [PubMed Central]
- Kammers K, Cole RN, Tiengwe C, Ruczinski I. Detecting Significant Changes in Protein Abundance. EuPA Open Proteom. 2015;7:11-9. [CrossRef] [PubMed] [PubMed Central]
- Goedhart J, Luijsterburg MS. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci Rep. 2020;10(1):20560. Epub 20201125. [CrossRef] [PubMed] [PubMed Central]
- Hortova-Kohoutkova M, Tidu F, De Zuani M, Sramek V, Helan M, Fric J. Phagocytosis-Inflammation Crosstalk in Sepsis: New Avenues for Therapeutic Intervention. Shock. 2020;54(5):606-14. [CrossRef] [PubMed] [PubMed Central]
- Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L622-L45. [CrossRef] [PubMed]
- Singh J, Lee Y, Kellum JA. A new perspective on NO pathway in sepsis and ADMA lowering as a potential therapeutic approach. Crit Care. 2022;26(1):246. [CrossRef] [PubMed] [PubMed Central]
- Xu W, Hou H, Yang W, Tang W, Sun L. Immunologic role of macrophages in sepsis-induced acute liver injury. Int Immunopharmacol. 2024;143(Pt 2):113492. [CrossRef] [PubMed]
- Diep S, Maddukuri M, Yamauchi S, Geshow G, Delk NA. Interleukin-1 and Nuclear Factor Kappa B Signaling Promote Breast Cancer Progression and Treatment Resistance. Cells. 2022;11(10). [CrossRef] [PubMed] [PubMed Central]
- Tian B, Nowak DE, Brasier AR. A TNF-induced gene expression program under oscillatory NF-kappaB control. BMC Genomics. 2005;6:137. [CrossRef] [PubMed] [PubMed Central]
- Horkova V, Drobek A, Mueller D, Gubser C, Niederlova V, Wyss L, King, C.G., Zehn, D., Stepanek, O. Dynamics of the Coreceptor-LCK Interactions during T Cell Development Shape the Self-Reactivity of Peripheral CD4 and CD8 T Cells. Cell Rep. 2020;30(5):1504-14 e7. [CrossRef] [PubMed] [PubMed Central]
- Qin Z, Hou P, Lin H, Chen M, Wang R, Xu T. Inhibition of Lck/Fyn kinase activity promotes the differentiation of induced Treg cells through AKT/mTOR pathway. Int Immunopharmacol. 2024;134:112237. [CrossRef] [PubMed]
- Bailey JD, Diotallevi M, Nicol T, McNeill E, Shaw A, Chuaiphichai S, Hale, A., Starr, A., Nandi, M., Stylianou, E., McShane, H., Davis, S., Fischer, R., Kessler, B.M., McCullagh, J., Chanoon, K.M., Crabtree, M.J. Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation. Cell Rep. 2019;28(1):218-30 e7. [CrossRef] [PubMed] [PubMed Central]
- Ishihara Y, Takemoto T, Itoh K, Ishida A, Yamazaki T. Dual role of superoxide dismutase 2 induced in activated microglia: oxidative stress tolerance and convergence of inflammatory responses. J Biol Chem. 2015;290(37):22805-17. [CrossRef] [PubMed] [PubMed Central]
- Grajchen E, Wouters E, van de Haterd B, Haidar M, Hardonniere K, Dierckx T, VanBroeckhoven, J., Erens, C., Hendrix, S., Kerdine-Romer, S., Hendricks, J.J.A., Bogie, J.F.J. CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation. J Neuroinflammation. 2020;17(1):224. [CrossRef] [PubMed] [PubMed Central]
- Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74(4):479-85. Epub 2003/09/10. [CrossRef] [PubMed]
- Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti, N., Muzio, M., Arditi, M. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275(15):11058-63. [CrossRef] [PubMed]
- Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37(5):1043-55. [CrossRef] [PubMed]
- Ramos-Benitez MJ, Ruiz-Jimenez C, Aguayo V, Espino AM. Recombinant Fasciola hepatica fatty acid binding protein suppresses toll-like receptor stimulation in response to multiple bacterial ligands. Sci Rep. 2017;7(1):5455. [CrossRef] [PubMed] [PubMed Central]
- Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. [CrossRef] [PubMed] [PubMed Central]
- Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, Saqid, U, Deng, J., Chatterjee, S., Block, M.L., Vogel, S.M., Malik, A.B., Consolaro, M.E., Christman, J.W., Minshall, R.D., Gantner, B.N., Bonini, M.G. NOS1-derived nitric oxide promotes NF-kappaB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med. 2015;212(10):1725-38. [CrossRef] [PubMed] [PubMed Central]
- Jafarzadeh S, Nemati M, Zandvakili R, Jafarzadeh A. Modulation of M1 and M2 macrophage polarization by metformin: Implications for inflammatory diseases and malignant tumors. Int Immunopharmacol. 2025;151:114345. [CrossRef] [PubMed]
- Parisi L, Gini E, Baci D, Tremolati M, Fanuli M, Bassani B, Farronato, G., Bruno, A., Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J Immunol Res. 2018;2018:8917804. [CrossRef] [PubMed] [PubMed Central]
- Xia T, Fu S, Yang R, Yang K, Lei W, Yang Y, Zhang, Q., Zhao, Y., Yu, J., Yu, L., Zhang, T. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J Inflamm (Lond). 2023;20(1):33. [CrossRef] [PubMed] [PubMed Central]
- Khabipov A, Kading A, Liedtke KR, Freund E, Partecke LI, Bekeschus S. RAW 264.7 Macrophage Polarization by Pancreatic Cancer Cells - A Model for Studying Tumour-promoting Macrophages. Anticancer Res. 2019;39(6):2871-82. [CrossRef] [PubMed]
- Zhang B, Zhang Y, Yao G, Gao J, Yang B, Zhao Y, Rao, Z., Gao, J. M2-polarized macrophages promote metastatic behavior of Lewis lung carcinoma cells by inducing vascular endothelial growth factor-C expression. Clinics (Sao Paulo). 2012;67(8):901-6. [CrossRef] [PubMed] [PubMed Central]
- Zecha J, Satpathy S, Kanashova T, Avanessian SC, Kane MH, Clauser KR, Mertins, P., Carr, S.A., Kuster, B. TMT Labeling for the Masses: A Robust and Cost-efficient, In-solution Labeling Approach. Mol Cell Proteomics. 2019;18(7):1468-78. [CrossRef] [PubMed] [PubMed Central]
- Hussaarts L, Garcia-Tardon N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, Ozir-Fazalalikhan, A., Berbee, J.F., Willems van Dijk, K., van Hamelen, V., Yazdanbakhsh, M., Guigas, B. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29(7):3027-39. [CrossRef] [PubMed]
- Ma Y, Li J, Liu Y, Zhao H, Qi X, Sun Y, Chen, J., Zhou, J., Ma, X., Wang, L. Identification and exploration of a new M2 macrophage marker MTLN in alveolar echinococcosis. Int Immunopharmacol. 2024;131:111808. [CrossRef] [PubMed]
- Wang Z, Hao C, Zhuang Q, Zhan B, Sun X, Huang J, Cheng, Y., Zhu, X. Excretory/Secretory Products from Trichinella spiralis Adult Worms Attenuated DSS-Induced Colitis in Mice by Driving PD-1-Mediated M2 Macrophage Polarization. Front Immunol. 2020;11:563784. [CrossRef] [PubMed] [PubMed Central]
- Adams PN, Aldridge A, Vukman KV, Donnelly S, O’Neill SM. Fasciola hepatica tegumental antigens indirectly induce an M2 macrophage-like phenotype in vivo. Parasite Immunol. 2014;36(10):531-9. Epub 2014/07/22. [CrossRef] [PubMed]
- Ruiz-Campillo MT, Molina-Hernandez V, Perez J, Pacheco IL, Perez R, Escamilla A, Martinez-Moreno, F.J., Martinez-Moreno, A., Zafra, R. Study of peritoneal macrophage immunophenotype in sheep experimentally infected with Fasciola hepatica. Vet Parasitol. 2018;257:34-9. [CrossRef] [PubMed]
- Zhang Y, Mei X, Liang Y, Zhu B, Sheng Z, Shi W, Wang, D., Huang, W. Newly excysted juveniles (NEJs) of Fasciola gigantica induce mice liver fibrosis and M2 macrophage-like phenotype in vivo. Microb Pathog. 2020;139:103909. [CrossRef] [PubMed]
- Hacariz O, Sayers G, Baykal AT. A proteomic approach to investigate the distribution and abundance of surface and internal Fasciola hepatica proteins during the chronic stage of natural liver fluke infection in cattle. Journal of proteome research. 2012;11(7):3592-604. [CrossRef] [PubMed]
- Wilson RA, Wright JM, de Castro-Borges W, Parker-Manuel SJ, Dowle AA, Ashton PD, Young, N.D., Gasser, R.B., Spithill, T.W. Exploring the Fasciola hepatica tegument proteome. Int J Parasitol. 2011;41(13-14):1347-59. [CrossRef] [PubMed]
- Flynn RJ, Irwin JA, Olivier M, Sekiya M, Dalton JP, Mulcahy G. Alternative activation of ruminant macrophages by Fasciola hepatica. Vet Immunol Immunopathol. 2007;120(1-2):31-40. [CrossRef] [PubMed]
- Quinteros SL, O’Brien B, Donnelly S. Exploring the role of macrophages in determining the pathogenesis of liver fluke infection. Parasitology. 2022;149(10):1364-73. [CrossRef] [PubMed] [PubMed Central]
- Figueroa-Santiago O, Espino A.M. 2014. Fasciola hepatica Fatty Acid Binding Protein Induces the Alternative Activation of Human Macrophages. Inf. Immu. 2014;82(12):5005-12. [CrossRef]
- Lingappan, K. NF-kappaB in Oxidative Stress. Curr Opin Toxicol. 2018;7:81-6. [CrossRef] [PubMed] [PubMed Central]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527-605. [CrossRef] [PubMed]
- Flohe L, Gunzler WA, Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32(1):132-4. [CrossRef] [PubMed]
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito, F., Altavilla, D., Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. [CrossRef] [PubMed] [PubMed Central]
- Pooja G, Shweta, S., Patel, P. Oxidative stress and free radicals in disease pathogenesis: a review Discovery Medicine. 2025;2:104. [CrossRef]
- Alqarni SA, Bineid A, Ahmad SF, Al-Harbi NO, Alqahtani F, Ibrahim KE, Ali, N, Nadeem, A. Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma. Metabolites. 2022;12(9). [CrossRef] [PubMed] [PubMed Central]
- Hauck F, Randriamampita C, Martin E, Gerart S, Lambert N, Lim A, Soulier, J., Maciorowski, Z., Touzot, F., Moshous, D., Quartier, P., Heritier, S., Blanche, S., Rieux-Laucat, F., Brousse, N., Callebaut, I., Veillette, A., Hivroz, C., Fischer, A., Latour, S., Picard, C. Primary T-cell immunodeficiency with immunodysregulation caused by autosomal recessive LCK deficiency. J Allergy Clin Immunol. 2012;130(5):1144-52 e11. [CrossRef] [PubMed]
- Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B. The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol Rev. 2003;191:107-18. [CrossRef] [PubMed]
- Matache C, Onu A, Stefanescu M, Tanaseanu S, Dragomir C, Dolganiuc A, Szegli, G. Dysregulation of p56lck kinase in patients with systemic lupus erythematosus. Autoimmunity. 2001;34(1):27-38. [CrossRef] [PubMed]
- Romagnoli P, Strahan D, Pelosi M, Cantagrel A, van Meerwijk JP. A potential role for protein tyrosine kinase p56(lck) in rheumatoid arthritis synovial fluid T lymphocyte hyporesponsiveness. Int Immunol. 2001;13(3):305-12. [CrossRef] [PubMed]
- Han X, Zhang W, Yang X, Wheeler CG, Langford CP, Wu L, Filippova, N., Friedman, G.K., Ding, Q., Fathaliah-Shaykh, H.M., Gillespie, G.Y., Nabors, L.B. The role of Src family kinases in growth and migration of glioma stem cells. Int J Oncol. 2014;45(1):302-10. [CrossRef] [PubMed] [PubMed Central]
- Conboy CB, Yonkus JA, Buckarma EH, Mun DG, Werneburg NW, Watkins RD, Alva-Ruiz, R., Tomlinson, J.L., Guo, Y., Wang, J., O’Brien, D., McCabe, C.E., Jessen, E., GRaham, R.P., Bujisman, R.C., Vu, D., de Man J., Llyas, S.I., Truty, M.J., Borad, M., Pandey A., Gores, G.J., Smoot, R.L. LCK inhibition downregulates YAP activity and is therapeutic in patient-derived models of cholangiocarcinoma. J Hepatol. 2023;78(1):142-52. [CrossRef] [PubMed] [PubMed Central]
- Gong FC, Ji R, Wang YM, Yang ZT, Chen Y, Mao EQ, Chen, E.Z. Identification of Potential Biomarkers and Immune Features of Sepsis Using Bioinformatics Analysis. Mediators Inflamm. 2020;2020:3432587. [CrossRef] [PubMed] [PubMed Central]
- Kong F, Zhu Y, Xu J, Ling B, Wang C, Ji J, Yang, Q., Liu, X., Shao, L., Zhou, X., Chen, K., Yang, M., Tang, L. The novel role of LCK and other PcDEGs in the diagnosis and prognosis of sepsis: Insights from bioinformatic identification and experimental validation. Int Immunopharmacol. 2025;149:114194. [CrossRef] [PubMed]
- Chakraborty P, Aravindhan V, Mukherjee S. Helminth-derived biomacromolecules as therapeutic agents for treating inflammatory and infectious diseases: What lessons do we get from recent findings? Int J Biol Macromol. 2023;241:124649. [CrossRef] [PubMed]
- Thorne RF, Law EG, Elith CA, Ralston KJ, Bates RC, Burns GF. The association between CD36 and Lyn protein tyrosine kinase is mediated by lipid. Biochem Biophys Res Commun. 2006;351(1):51-6. [CrossRef] [PubMed]
- Muniz-Santos R, Lucieri-Costa G, de Almeida MAP, Moraes-de-Souza I, Brito M, Silva AR, Goncalves-de-Alburquerque, C.F. Lipid oxidation dysregulation: an emerging player in the pathophysiology of sepsis. Front Immunol. 2023;14:1224335. [CrossRef] [PubMed] [PubMed Central]
- Baranova IN, Vishnyakova TG, Bocharov AV, Leelahavanichkul A, Kurlander R, Chen Z, Souza, A.C., Yuen, P.S., Star, R.A., Csako, G., Patterson, A.P., Eggerman, T.L. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J Immunol. 2012;188(3):1371-80. [CrossRef] [PubMed] [PubMed Central]
- Cao D, Luo J, Chen D, Xu H, Shi H, Jing X, Zang, W. CD36 regulates lipopolysaccharide-induced signaling pathways and mediates the internalization of Escherichia coli in cooperation with TLR4 in goat mammary gland epithelial cells. Sci Rep. 2016;6:23132. [CrossRef] [PubMed] [PubMed Central]
- Zamora C, Canto E, Nieto JC, Angels Ortiz M, Juarez C, Vidal S. Functional consequences of CD36 downregulation by TLR signals. Cytokine. 2012;60(1):257-65. [CrossRef] [PubMed]
- Xie Y, Lv H, Chen D, Huang P, Zhou Z, Wang R. A CD36-based prediction model for sepsis-induced myocardial injury. Int J Cardiol Heart Vasc. 2025;57:101615. [CrossRef] [PubMed] [PubMed Central]
- Kim MH, Lim H, Kim OH, Oh BC, Jung Y, Ryu KH, Park, J.W., Park, W.J. CD36 deficiency protects lipopolysaccharide-induced sepsis via inhibiting CerS6-mediated endoplasmic reticulum stress. Int Immunopharmacol. 2024;143(Pt 2):113441. [CrossRef] [PubMed]
- Li Y, Zhang L, Jiao J, Ding Q, Li Y, Zhao Z, Luo, J., Chen, Y., Ruan, X., Zhao, L. Hepatocyte CD36 protects mice from NASH diet-induced liver injury and fibrosis via blocking N1ICD production. Biochim Biophys Acta Mol Basis Dis. 2023;1869(7):166800. [CrossRef] [PubMed]
- Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333(1):19-39. [CrossRef] [PubMed]
- Li L, Li Y, Timothy Sembiring Meliala I, Kasim V, Wu S. Biological roles of Yin Yang 2: Its implications in physiological and pathological events. J Cell Mol Med. 2020;24(22):12886-99. [CrossRef] [PubMed] [PubMed Central]
- Verheul TCJ, van Hijfte L, Perenthaler E, Barakat TS. The Why of YY1: Mechanisms of Transcriptional Regulation by Yin Yang 1. Front Cell Dev Biol. 2020;8:592164. [CrossRef] [PubMed] [PubMed Central]
- Li YL, Tian H, Jiang J, Zhang Y, Qi XW. Multifaceted regulation and functions of fatty acid desaturase 2 in human cancers. Am J Cancer Res. 2020;10(12):4098-111. [PubMed] [PubMed Central]
- Cao K, Lv W, Wang X, Dong S, Liu X, Yang T, Xu, J., Zeng, M., Zou, X., Zhao, D., Ma, Q., Lin, M., Long J., Zang W., Gao F., Feng Z., Liu J. Hypermethylation of Hepatic Mitochondrial ND6 Provokes Systemic Insulin Resistance. Adv Sci (Weinh). 2021;8(11):2004507. [CrossRef] [PubMed] [PubMed Central]
- Wang Q, Li M, Zeng N, Zhou Y, Yan J. Succinate dehydrogenase complex subunit C: Role in cellular physiology and disease. Exp Biol Med (Maywood). 2023;248(3):263-70. [CrossRef] [PubMed] [PubMed Central]
- Na K, Oh BC, Jung Y. Multifaceted role of CD14 in innate immunity and tissue homeostasis. Cytokine Growth Factor Rev. 2023;74:100-7. [CrossRef] [PubMed]
| Antibody Type | Antibody Name | Company | Dilution |
| Primary | Anti-GAPDH | Cell Signaling Technology | 1:1000 |
| Primary | Anti-β-actin | Cell Signaling Technology | 1:1000 |
| Primary | Anti- iNOS | Cell Signaling Technology | 1:1000 |
| Primary | Anti- IL-1α | Cell Signaling Technology | 1:1000 |
| Primary | Anti- TNF- | Cell Signaling Technology | 1:1000 |
| Primary | Anti- Lck | Cell Signaling Technology | 1:1000 |
| Primary | Anti-CD36 | Cell Signaling Technology | 1:1000 |
| Secondary (HRP-conjugated) | Anti-rabbit IgG | Cell Signaling Technology | 1:10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





