Submitted:
07 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
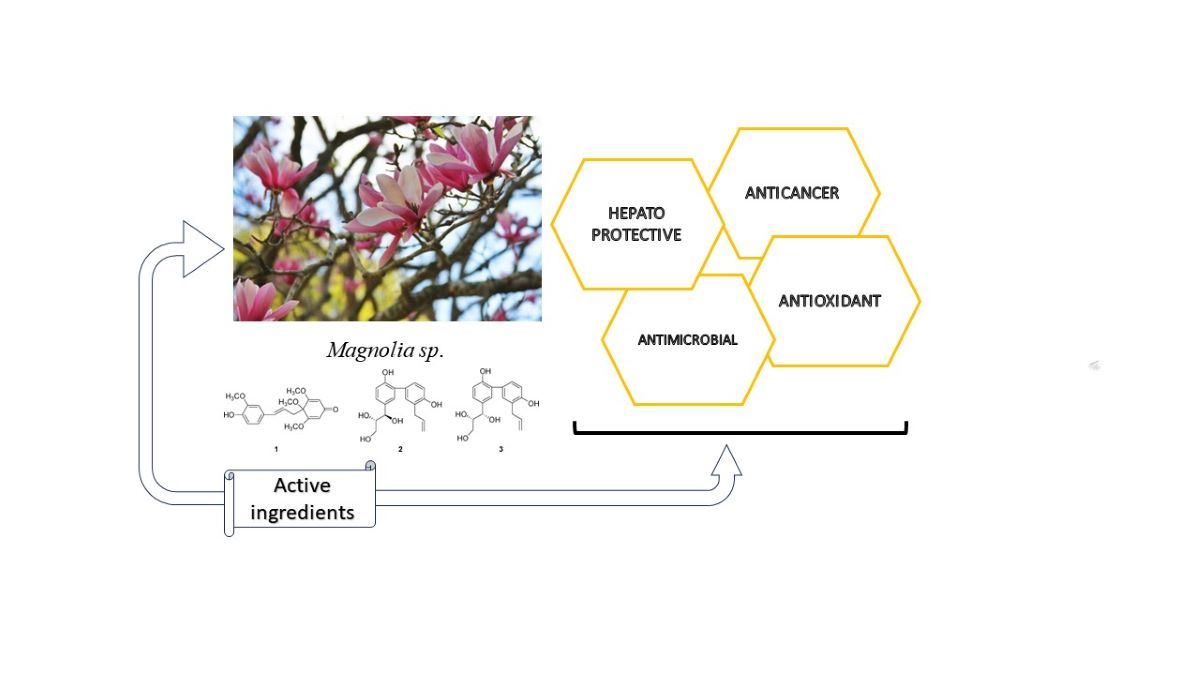
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials. Magnolia Samples, Reagents, Microbial Strains, Culture Media
2.2. Methods
2.2.1. Identification of Total Polyphenols and Determination of Antioxidant Activity
2.2.2. Identification and Quantification of Phenolic Compounds
2.2.3. Identification of Volatile Compounds
2.2.4. Antibacterial Activity/Determination of MIC (Minimum Inhibitory Concentration)
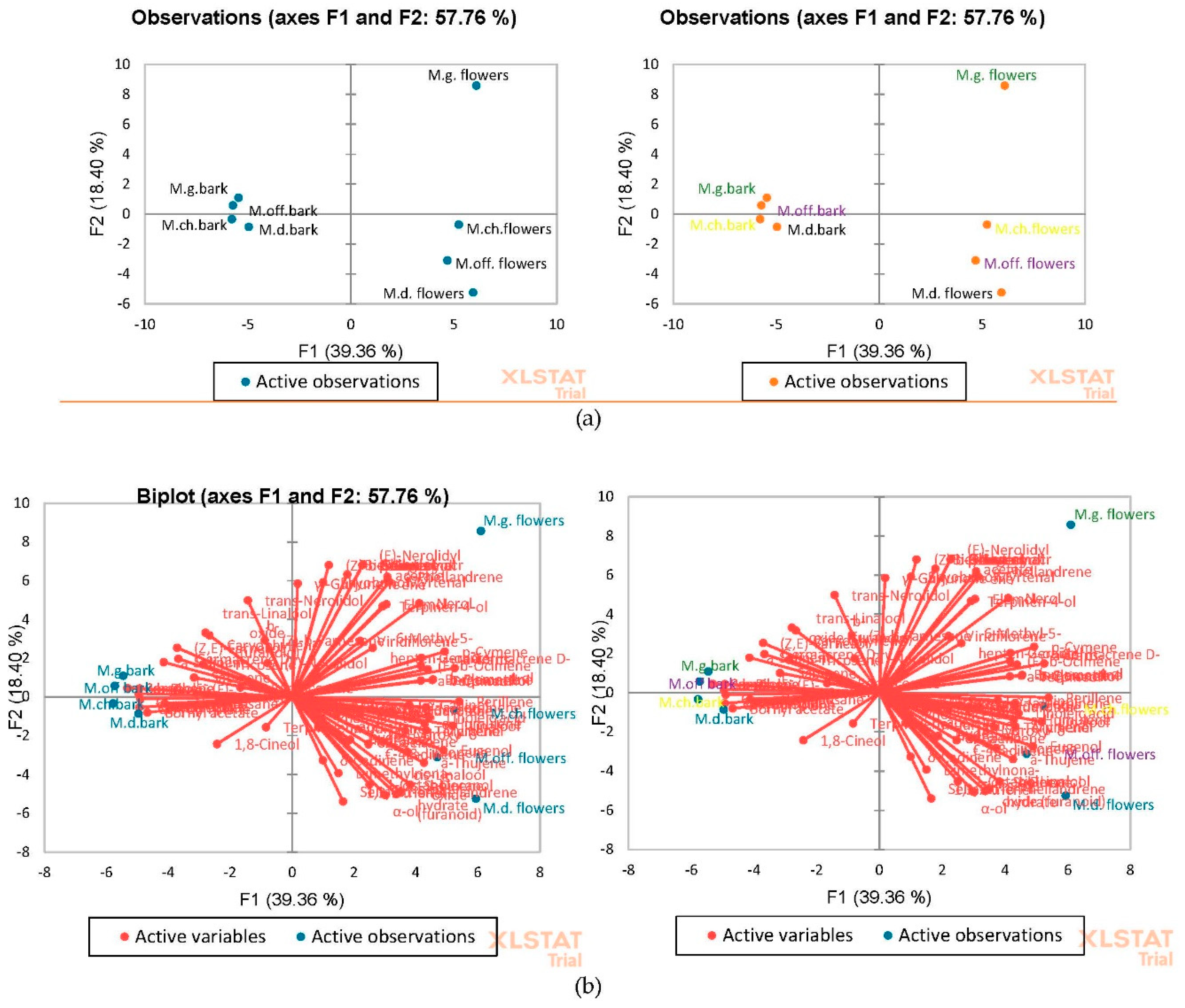
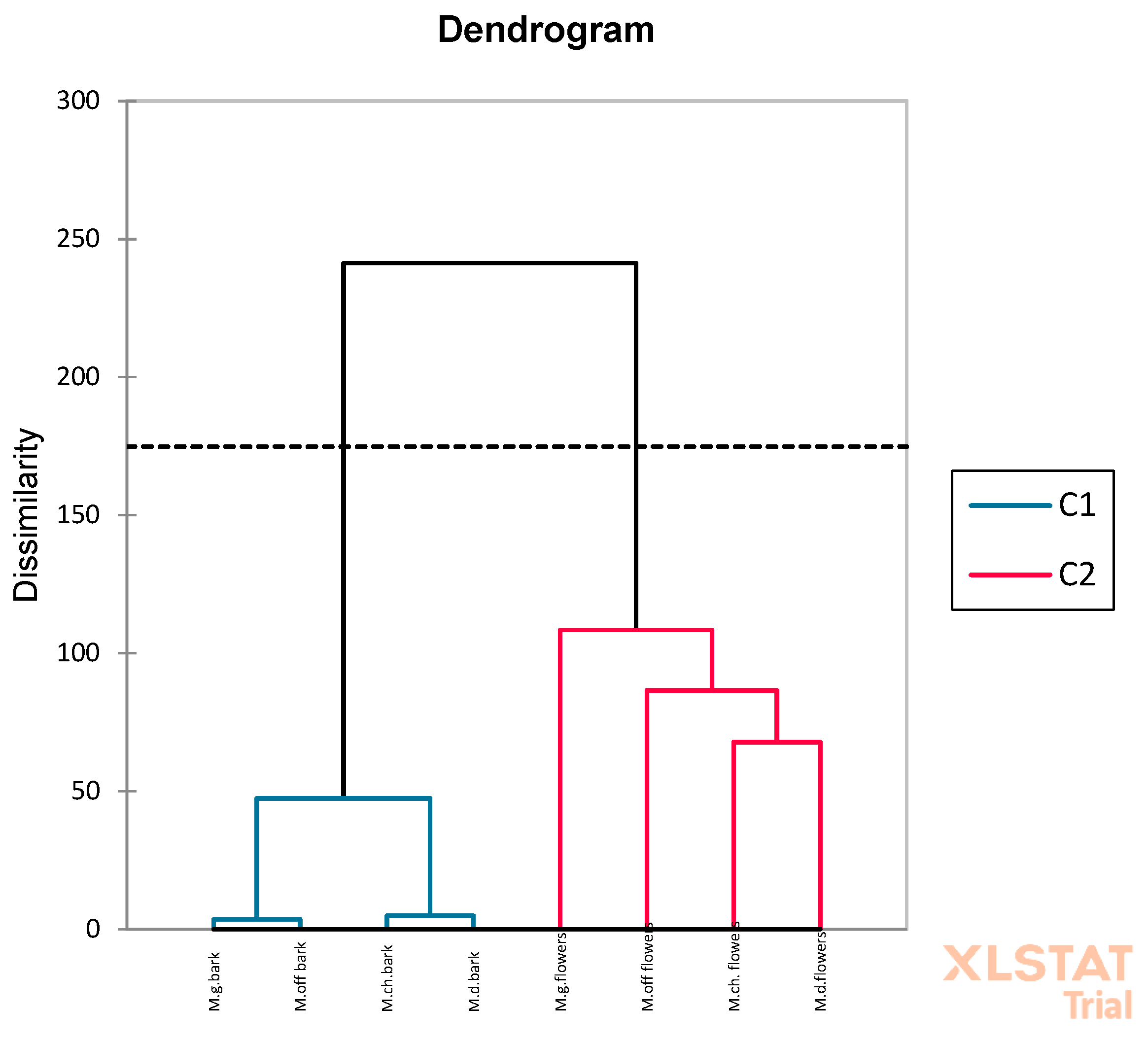
2.2.5. Multivariate Statistical Analyses
3. Results
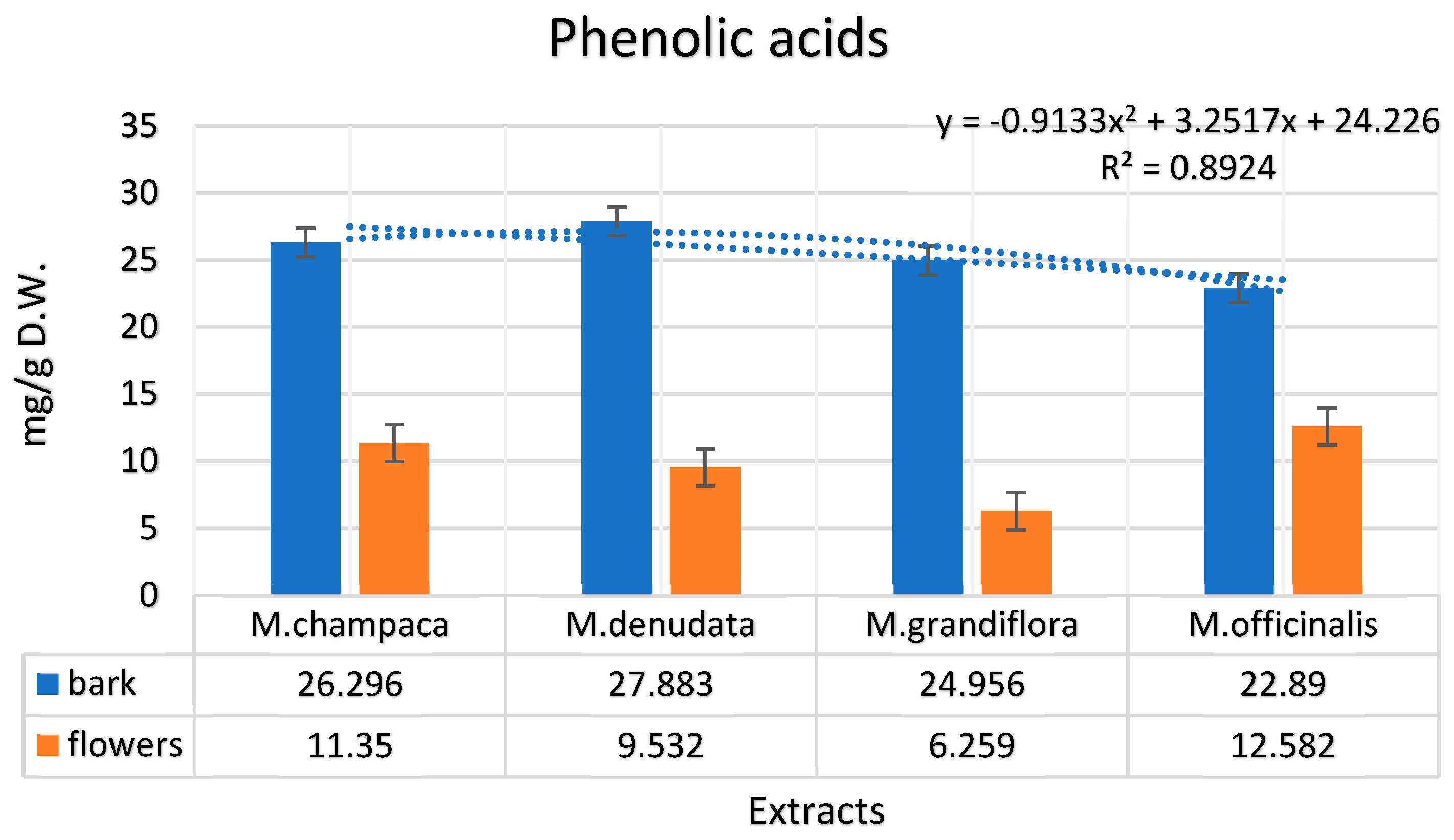
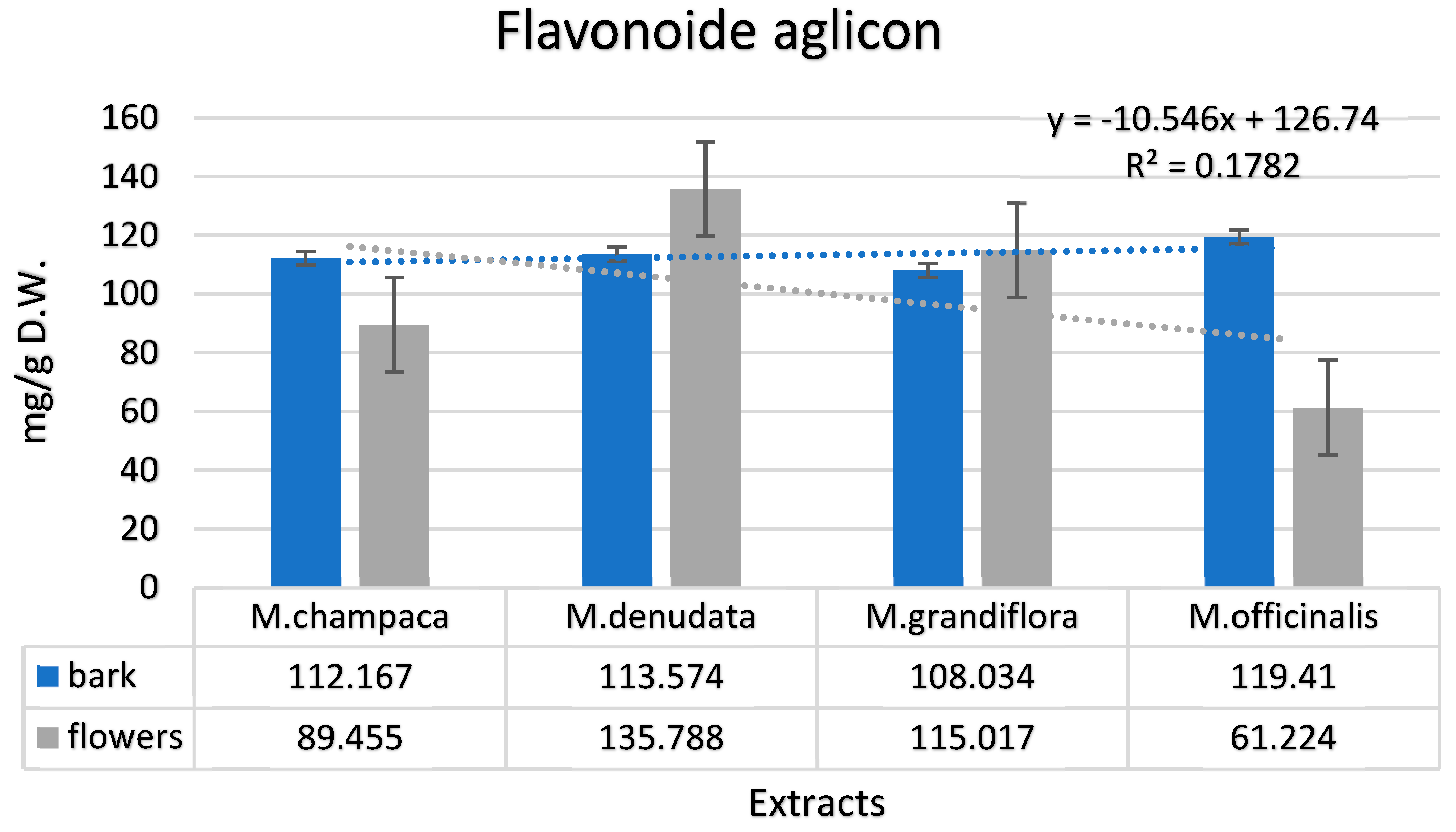
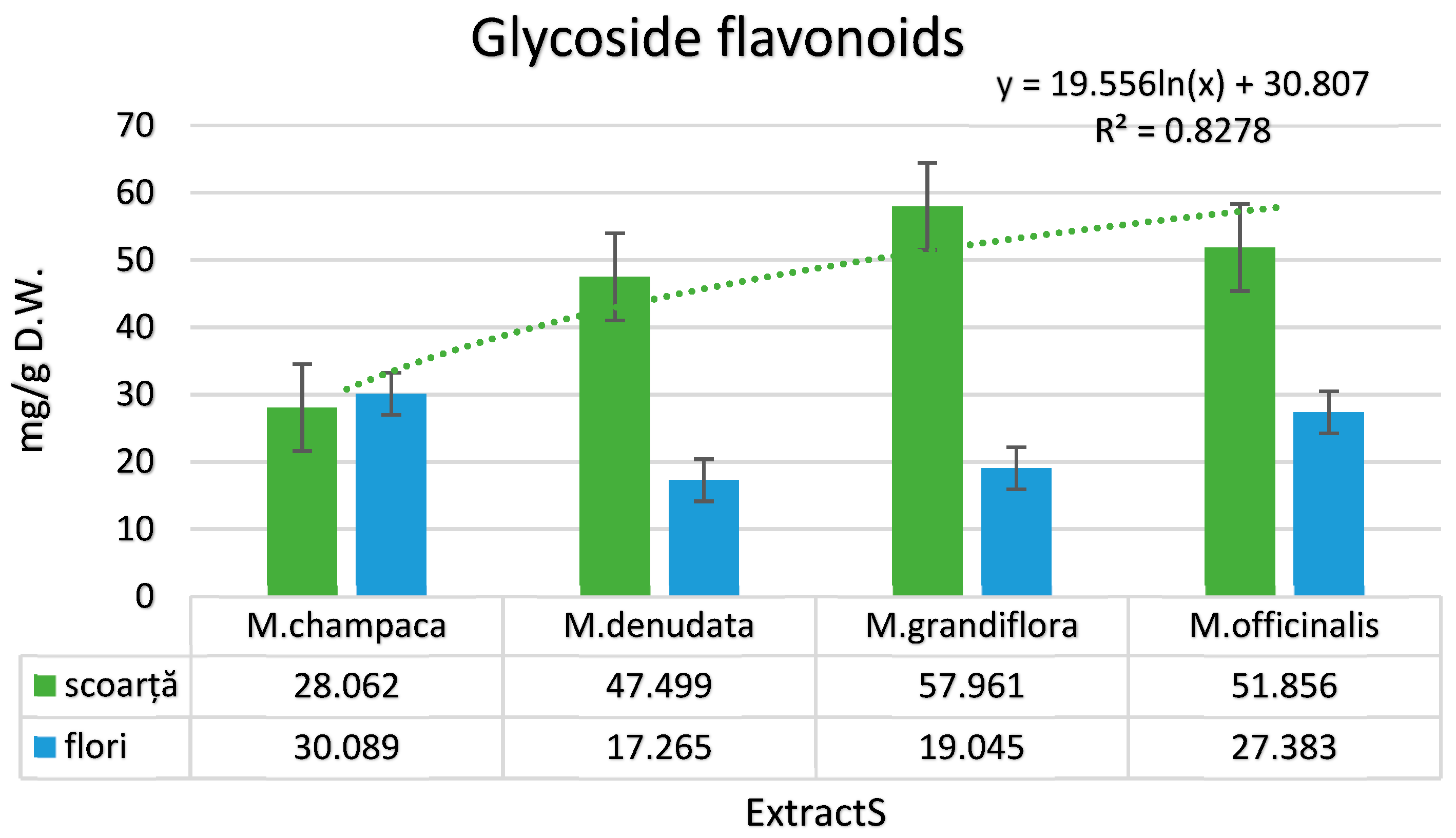
3.1. Identification and Quantification of Phenolic Compounds in Magnolia Extracts
3.2. Identification and Quantification of Volatile Compounds in Magnolia Extracts
3.3. Antibacterial Action of Hydroethanolic Magnolia Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef]
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of Microbial Products in Cosmetic Industry. Nat. Prod. Bioprospect. 2019, 9, 267–278.
- Poivre, M.; Duez, P. Biological Activity and Toxicity of the Chinese Herb Magnolia officinalis Rehder & E. Wilson (Houpo) and Its Constituents. J Zhejiang Univ Sci B 2017, 18, 194–214. [Google Scholar] [PubMed]
- Saqib, F.; Mushtaq, Z.; Janbaz, K.H.; Imran, I.; Deawnjee, S.; Zia-Ul-Haq, M. Pharmacological Basis for the Medicinal Use of Michelia champaca in Gut, Airways and Cardiovascular Disorders. Asian Pac J Trop Med 2018, 11, 292–296. [Google Scholar]
- Sinha, R.; Varma, R. Michelia champaca L. (Swarna Champa): A Review. Int. J. Enhanc. Res. Sci. Technol. Eng. 2016, 5, 78–83. [Google Scholar]
- Taprial, S.; Kashyap, D.; Mehta, V.; Kumar, S.; Kumar, D. Antifertility Effect of Hydroalcoholic Leaves Extract of Michelia champaca L.: An Ethnomedicine Used by Bhatra Women in Chhattisgarh State of India. J. Ethnopharmacol. 2013, 147, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, Y.; Xia, B.; He, F.; Pan. H. Components and Antibacterial Functions of Volatile Organic Compounds from Leaves and Flowers of Michelia champaka. J. Northeast For. Univ. 2012, 40, 71–74. [Google Scholar]
- Al-Sagheer, N.A. Magnolia champaca (L.) Baill. Ex Pierre (Magnoliaceae): A First Report and a New Record in the Arabian Peninsula (Yemen). J. Saudi Soc. Agric. Sci. 2021, 20, 243–247. [Google Scholar] [CrossRef]
- Sahoo, C.; Champati, B.B.; Dash, B.; Jena, S.; Ray, A.; Panda, P.C.; Nayak, S.; Sahoo, A. Volatile Profiling of Magnolia champaca Accessions by Gas Chromatography Mass Spectrometry Coupled with Chemometrics. Molecules. 2022, 27, 7302.
- Yoon, H. Effects of Aging on the Phenolic Content and Antioxidant Activities of Magnolia denudata Flower Extracts. Food Sci Biotechnol. 2014, 23, 1715–1718. [Google Scholar]
- Du, J.; Wang, M.L.; Chen, R.Y.; Yu, D.Q. Chemical Constituents from the Leaves of Magnolia denudata. J Asian Nat Prod Res. 2001, 3, 313–319.
- Li, J.; Tanaka, M.; Kurasawa, K.; Ikeda, T.; Nohara, T. Lignan and Neolignan Derivatives from Magnolia denudata. Chem. Pharm. Bull. 2005, 53, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, S.-Y.; Lee, S.Y.; Kim, J.K.; Park, S.U. Analysis of Metabolites in White Flowers of Magnolia Denudata Desr. and Violet Flowers of Magnolia Liliiflora Desr. Molecules. 2018, 23, 1558. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; Zhang, M.; Chen, X.; Yang, J. Analysis on aroma components of flowers of five Magnolia denudata cultivars. IOP Conf. Ser.: Earth Environ. Sci. 2021, 705.
- Huang, H.C.; Hsieh, W.Y.; Niu, Y.L.; Chang, T.M. Inhibition of Melanogenesis and Antioxidant Properties of Magnolia grandiflora L. Flower Extract. BMC Complement Altern Med. 2012, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Schühly, W.; Khan, I.; Fischer, N.H. The Ethnomedicinal Uses of Magnoliaceae from the Southeastern United States as Leads in Drug Discovery. Pharmaceutical Biology. 2001, 39, 63–69.
- Del Valle-Mondragón, L.; Tenorio-López, F.A.; Torres-Narváez, J.C.; Zarco-Olvera, G.; Pastelín-Hernández, G. Study of Magnolia grandiflora Extracts on Guinea Pig Cardiac Muscle. Archivos de cardiología de México. 2004, 74, 108–117.
- Dave, P.C.; Vogler, B.; Setzer, W.N. Composition of the Floral Essential Oil of Magnolia grandiflora L. (Magnoliaceae): Intraspecific and Floral Maturity Variations. Journal of Essential Oil Bear Plant.
- Farag, M.A.; Al-Mahdy, D.A. Comparative Study of the Chemical Composition and Biological Activities of Magnolia grandiflora and Magnolia virginiana Flower Essential Oils. Nat. Prod. Res. 2013, 27, 1091–1097. [Google Scholar] [CrossRef]
- Guerra-Boone, L.; Álvarez-Román, R.; Salazar-Aranda, R.; Torres-Cirio, A.; Rivas-Galindo, V. M.; de Torres, N. W.; González, G. M. G.; Pérez-López, L. A. Chemical Compositions and Antimicrobial and Antioxidant Activities of the Essential Oils from Magnolia grandiflora, Chrysactinia mexicana, and Schinus molle Found in Northeast Mexico. Nat. Prod. Commun. 2013, 8, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Kuan, L.Y.; Chen, W.L.; Chen, J.H.; Hsu, F.T.; Liu, T.T.; Chen, W.T.; Wang, K.L.; Chen, W.C.; Liu, Y.C.; Wang, W.S. Magnolol Induces Apoptosis and Inhibits ERK-modulated Metastatic Potential in Hepatocellular Carcinoma Cells. In Vivo 2018, 32, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Garza, B.; Echeverria, A.; Gonzalez, F.; Castillo, O.; Eubanks, T.; Bandyopadhyay, D. Phytochemical Investigation of Magnolia grandiflora Green Seed Cones: Analytical and Phytoceutical Studies. Food Science & Nutrition. 2019, 7, 1761–1767. [Google Scholar]
- Latif, A.; Du, Y.; Dalal, S.R.; Fernández-Murga, M.L.; Merino, E.F.; Cassera, M.B.; Kingston, D.G. Bioactive Neolignans and Other Compounds from Magnolia grandiflora L.: Isolation and Antiplasmodial Activity. Chemistry & Biodiversity. 2017, 14, 1002. [Google Scholar]
- Ma, H.; Bai, X.; Sun, X.; Li, B.; Zhu, M.; Dai, Y.; Wu, C.Z. 2020. Anti-Cancer Effects of Methanol-Ethyl Acetate Partitioned Fraction from Magnolia grandiflora in Human Non-Small Cell Lung Cancer H1975 Cells. Journal of Bioenergetics and Biomembranes. 2020, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Park, E.J.; Park, Y.J.; Ponce-Zea, J.; Doan, T.P.; Ryu, B.; Oh, W.K. Sesquiterpene Lactone and Its Unique Proaporphine Hybrids from Magnolia grandiflora L. and Their Anti-Inflammatory Activity. Phytochemistry. 2022, 200, 1–10. [Google Scholar] [CrossRef]
- Martínez-Báez, A.Z.; Oranday-Cárdenas, M.A.; Verde-Star, M.J.; Arévalo-Niño, K.; González-González, G.M.; Rodríguez-Garza, R.G. Preliminary Study on the Antioxidant and Antibacterial Activity of Methanolic Extracts of Azadirachta indica, Juglans regia, Tecoma stans, and Magnolia grandiflora. Revista Mexicana de Ciencias Farmacéuticas. 2016, 47, 36–44. [Google Scholar]
- Morshedloo, M.R.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Maggi, F. Chemical Composition, Antioxidant Activity and Cytotoxicity on Tumour Cells of the Essential Oil from Flowers of Magnolia grandiflora Cultivated in Iran. Nat Prod Res. 2017, 31, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tao, C.; Huang, X.; Chen, Z.; Wang, L.; Li, X.; Ma, M.; Wu, Z. CT2-3, A Novel Magnolol Analogue Suppresses NSCLC Cells Through Triggering Cell Cycle Arrest and Apoptosis. Bioorganic Med. Chem. 2020, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, T.; Alejandre-Rosas, J. A. ; Ramos-Ligonio, Ángel; Castillo-Morales, M.; Reyes-Pérez, J. J.; Esquivel-Valenzuela, B. Composición Química, Actividad Antioxidante y Citotoxicidad de Extractos de Flores de Magnolia grandiflora L. Encontradas en el Sureste de México. Biotecnia. 2022, 25, 5–13.
- Chen, X.; Hu, Y.; Shan, L.; Yu, X.; Hao, K.; Wang, G. Xue. Magnolol and Honokiol from Magnolia officinalis Enhanced Antiviral Immune Responses Against Grass Carp Reovirus in Ctenopharyngodon idella Kidney Cells. Fish Shellfish Immunol. 2017, 63, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. J.; Choi, D. Y.; Yun, Y. P.; Han, S. B.; Kim, H. M.; Lee, K. Ethanol Extract of Magnolia officinalis Prevents Lipopolysaccharide-Induced Memory Deficiency Via Its Antineuroinflammatory and Antiamyloidogenic Effects. Phyther. Res. 2013, 27, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Szczepanski, M.J.; Lee, Y.J. Magnolol Induces Apoptosis Via Inhibiting the EGFR/PI3K/Akt Signaling Pathway in Human Prostate Cancer Cells. J. Cell Biochem. 2009, 106, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lu, Y.; Fan, Y.; Ning, Q.; Li, W. Enhanced Oral Bioavailability of Magnolol Via Mixed Micelles and Nano-suspensions Based on Soluplus- Poloxamer 188. Drug Deliv. 2020, 27, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. R.; Tan, R.; Qu, W. M.; Wu, Z.; Wang, Y.; Urade, Y. Magnolol, A Major Bioactive Constituent of the Bark of Magnolia officinalis, Exerts Antiepileptic Effects Via the GABA/Benzodiazepine Receptor Complex in Mice. Br. J. Pharmacol. 2011, 164, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Wu, L. Insights on the Multifunctional Activities of Magnolol. BioMed Research International. 2019, 1-15.
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-Breaking Antioxidant Activity of Hydroxylated and Methoxylated Magnolol Derivatives: The Role of H-Bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Cao, Y.; Chen, P.; Zhang, L. Study on the Effect of Magnoliae Officinalis Cortex on Gastrointestinal Motility Disorders. China Med. Her. 2018, 15, 31–34. [Google Scholar]
- Chei, S.; Oh, H.J.; Song, J.H.; Seo, Y.J.; Lee, K.; Lee, B.Y. Magnolol Suppresses TGF-β-Induced Epithelial-to-Mesenchymal Transition in Human Colorectal Cancer Cells. Front. Oncol. 2019, 9, 752. [Google Scholar] [CrossRef]
- Chen, Y. H.; Huang, P. H.; Lin, F. Y.; Chen, W. C.; Chen, Y. L.; Yin, W. H.; et al. Magnolol: A Multifunctional Compound Isolated from the Chinese Medicinal Plant Magnolia officinalis. Eur. J. Integr. Med. 2011, 3, 317–324. [Google Scholar] [CrossRef]
- Chen, C.H.; Hsu, F.T.; Chen, W.L.; Chen, J.H. Induction of Apoptosis, Inhibition of MCL-1, and VEGF-A Expression Are Associated with the Anti-Cancer Efficacy of Magnolol Combined with Regorafenib in Hepatocellular Carcinoma. Cancers. 2021, 13, 2066. [Google Scholar] [CrossRef]
- Jin, W.; Wang, X.; Zeng, Y.; Lan, Y.; Wang, X. Magnolol Suppressed Cell Migration and Invasion and Induced Cell Apoptosis via Inhibition of the NF-κB Signaling Pathway by Upregulating microRNA-129 in Multiple Myeloma. Neoplasma. 2021, 68, 404–415.
- Lin, H.L.; Cheng, W.T.; Chen, L.C.; Ho, H.O.; Lin, S.Y.; Hsieh, C.M. Honokiol/Magnolol-Loaded Self-Assembling Lecithin-Based Mixed Polymeric Micelles (lbMPMs) for Improving Solubility to Enhance Oral Bioavailability. Int. J. Nanomed. 2021, 16, 651–665.
- Sakaue, Y.; Domon, H.; Oda, M.; Takenaka, S.; Kubo, M.; Fukuyama, Y.; et al. Anti-Biofilm and Bactericidal Effects of Magnolia Bark-Derived Magnolol and Honokiol on Streptococcus mutans. Microbiol. Immunol. 2016, 60, 10–16.
- Usach, I.; Alaimo, A.; Fernández, J.; Ambrosini, A.; Mocini, S.; Ochiuz, L.; Peris, J.E. Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics. 2021, 13, 224.
- Behbehani, J.; Shreaz, S.; Irshad, M.; Karched, M. The Natural Compound Magnolol Affects Growth, Biofilm Formation, and Ultrastructure of Oral Candida Isolates. Microb. Pathog. 2017, 113, 209–217.
- Chang, B.; Lee, Y.; Ku, Y.; Bae, K.; Chung, C. Antimicrobial Activity of Magnolol and Honokiol against Periodontopathic Microorganisms. Planta Medica. 1998, 64, 367–369.
- Chang, H.; Chang, C. Y.; Lee, H. J.; Chou, C. Y.; Chou, T. C. Magnolol Ameliorates Pneumonectomy and Monocrotaline-Induced Pulmonary Arterial Hypertension in Rats through Inhibition of Angiotensin II and Endothelin-1 Expression. Phytomedicine. 2018, 51, 205–213.
- Hsieh, S. F.; Chou, C. T.; Liang, W. Z.; Kuo, C. C.; Wang, J. L.; Hao, L. J.; et al. The Effect of Magnolol on Ca2+ Homeostasis and Its Related Physiology in Human Oral Cancer Cells. Arch. Oral Biol. 2018, 89, 49–54.
- Kim, A.; Lee, S. Y.; Seo, C. S.; Chung, S. K. Ethanol Extract of Magnoliae cortex (EEMC) Limits Teratoma Formation of Pluripotent Stem Cells by Selective Elimination of Undifferentiated Cells through the p53-Dependent Mitochondrial Apoptotic Pathway. Phytomedicine. 2020, 69.
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of Tyrosinase Inhibitory, Antioxidant, Antimicrobial, and Antiaging Activities of Magnolia officinalis Extracts After Aspergillus niger Fermentation. BioMed Research International. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Song, L.; Dai, G.; Xu, M.; Zhu, L.; Zhang, W.; et al. Antidepressant Effects of Magnolol in a Mouse Model of Depression Induced by Chronic Corticosterone Injection. Steroids. 2018, 135, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for Cancer Therapeutics: A Traditional Medicine That Can Modulate Multiple Oncogenic Targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Q.; Fu, Y.; Ding, R.-B.; Qi, X.; Zhou, X.; Sun, Z.; Bao, J. Magnolol as a Potential Anticancer Agent: A Proposed Mechanistic Insight. Molecules. 2022, 27, 6441. [Google Scholar] [CrossRef]
- Emran, A.A.; Chinna Chowdary, B.R.; Ahmed, F.; Hammerlindl, H.; Huefner, A.; Haass, N.K.; Schuehly, W.; Schaider, H. Magnolol Induces Cell Death Through PI3K/Akt-Mediated Epigenetic Modifications Boosting Treatment of BRAF- and NRAS-Mutant Melanoma. Cancer Med. 2019, 8, 1186–1196. [Google Scholar] [CrossRef]
- Fried, L. E.; Arbiser, J. L. Honokiol, A Multifunctional Antiangiogenic and Antitumor Agent. Antioxid. Redox Signal 2009, 11, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y. H.; Kim, T.; Kim, R.; Ha, H. Magnolol Inhibits Osteoclast Differentiation via Suppression of RANKL Expression. Molecules. 2018, 23, 1598. [Google Scholar] [CrossRef]
- Lee, W. L.; Tang, Y. Q.; Yap, W. H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers. 2020, 12, 48. [Google Scholar]
- Peng, C.Y.; Yu, C.C.; Huang, C.C.; Liao, Y.W.; Hsieh, P.L.; Chu, P.M.; Yu, C.H.; Lin, S.S. Magnolol Inhibits Cancer Stemness and IL-6/Stat3 Signaling in Oral Carcinomas. J. Formos. Med. Assoc. 2022, 121, 51–57. [Google Scholar] [CrossRef]
- Ranaware, A. M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N. K.; Sethi, G.; et al. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Tsai, J.J.; Chen, J.H.; Chen, C.H.; Chung, J.G.; Hsu, F.T. Apoptosis Induction and ERK/NF-κB Inactivation Are Associated with Magnolol-Inhibited Tumor Progression in Hepatocellular Carcinoma in Vivo. Environ. Toxicol. 2020, 35, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Chen, J.; Huang, X.; Chen, Z.; Li, X.; Li, Y.; Xu, Y.; Ma, M.; Wu, Z. CT1-3, A Novel Magnolol-Sulforaphane Hybrid Suppresses Tumorigenesis Through Inducing Mitochondria-Mediated Apoptosis and Inhibiting Epithelial Mesenchymal Transition. Eur. J. Med. Chem. 2020, 199, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Min, K.J.; Kwon, T.K. Magnolol Enhances the Therapeutic Effects of TRAIL Through DR5 Upregulation and Downregulation of c-FLIP and Mcl-1 Proteins in Cancer Cells. Molecules. 2020, 25, 4591. [Google Scholar] [CrossRef]
- Ho, K.Y.; Tsai, C.C.; Chen, C.P.; et al. Antimicrobial Activity of Honokiol and Magnolol Isolated from Magnolia officinalis. Phytother. Res. 2001, 15, 139–141. [Google Scholar] [CrossRef]
- Bang, K.H.; Kim, Y.K.; Min, B.S.; Na, M.K.; Rhee, Y.H.; Lee, J.P.; Bae, K.H. Antifungal Activity of Magnolol and Honokiol. Archives of Pharmacal Research. 2000, 23, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Tsai, C.C.; Chen, C.P.; Huang, J.S.; Lin, C.C. Antimicrobial Activity of Honokiol and Magnolol Isolated from Magnolia officinalis. Phytoterapy Research. 2004, 15, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Jung, E.; Park, Y.; Kim, K.; Park, B.; Jung, K.; Park, E.; Kim, J.; Park, D. In Vitro Antibacterial and Anti-Inflammatory Effects of Honokiol and Magnolol Against Propionibacterium sp. European Journal of Pharmacology. 2004, 496, 189–195. [Google Scholar] [CrossRef]
- Syu, W.J.; Shen, C.C.; Lu, J.J.; Lee, G.H.; Sun, C.M. Antimicrobial and Cytotoxic Activities of Neolignans from Magnolia officinalis. Chemistry & Biodiversity. 2004, 1, 530–537. [Google Scholar]
- Chiu, K. C.; Shih, Y. H.; Wang, T. H.; Lan, W. C.; Li, P. J.; Jhuang, H. S.; et al. In Vitro Antimicrobial and Anti-Proinflammation Potential of Honokiol and Magnolol Against Oral Pathogens and Macrophages. J. Formos. Med. Assoc. 2020, 120, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Choi, E. J.; Kim, H. I.; Kim, J. A.; Jun, S. Y.; Kang, S. H.; Park, D. J.; et al. The Herbal-Derived Honokiol and Magnolol Enhance Immune Response to Infection with Methicillin-Sensitive Staphylococcus aureus (MSSA) and Methicillin-Resistant S. aureus (MRSA). Appl. Microbiol. Biotechnol. 2015, 99, 4387–4396. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.T.; L. T. Huong; I. A. Ogunwande. Antimicrobial, Larvicidal Activities and Composition of the Leaf Essential Oil of Magnolia coco (Lour.) DC. Rec. Nat. Prod. 2020, 14, 372–377. [Google Scholar] [CrossRef]
- Deng, Y.; Han, X.; Tang, S.; Xiao, W.; Tan, Z.; Zhou, C.; et al. Magnolol and Honokiol Regulate the Calcium-Activated Potassium Channels Signaling Pathway in Enterotoxigenic Escherichia coli-Induced Diarrhea Mice. Eur. J. Pharmacol. 2015, 755, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Dodds, M.W.; Tian, M.; Inui, T. Synergistic Antibacterial Effects of Magnolia Bark Extract and L-Arginine, N-Alpha-Lauroyl Ethyl Ester on Plaque Biofilm. US Patent 2019, 10, 265–367. [Google Scholar]
- Dong, J.; Ding, H.; Liu, Y.; Yang, Q.; Xu, N.; Yang, Y.; et al. Magnolol Protects Channel Catfish from Aeromonas hydrophila Infection via Inhibiting the Expression of Aerolysin. Vet. Microbiol. 2017, 211, 119–123. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Kwag, E.H.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; et al. Magnoliae Cortex and Maize Modulate Porphyromonas gingivalis-Induced Inflammatory Reactions. J. Periodontal Implant Sci. 2018, 48, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.; Jeong, S.Il.; Jahng, K.Y.; Yu, K.Y. Antimicrobial Effects and Resistant Regulation of Magnolol and Honokiol on Methicillin-Resistant Staphylococcus aureus. Biomed. Res. Int. 2015, 2015, 1–10. [Google Scholar]
- Li, T.; Yang, S.R.; Chen, M. Antibacterial Mechanism of Ginger Mix-Fried Magnolia Bark Extract Against Escherichia coli and Staphylococcus aureus. Mod Food Sci Technol 2016, 32, 84–92. [Google Scholar]
- Li, W.L.; Zhao, X.C.; Zhao, Z.W. In Vitro Antimicrobial Activity of Honokiol Against Staphylococcus aureus in Biofilm Mode. J Asian Nat Prod Res 2016, 18, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Oufensou, S.; Scherm, B.; Pani, G.; Balmas, V.; Fabbri, D.; Dettori, M.A.; et al. Honokiol, Magnolol and Its Monoacetyl Derivative Show Strong Antifungal Effect on Fusarium Isolates of Clinical Relevance. PLoS One 2019, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.Y.; Zhang, X.J.; Han, J.; Li, Y.Q.; Wang, G.C. In Vitro Synergism of Magnolol and Honokiol in Combination with Antibacterial Agents Against Clinical Isolates of Methicillin-Resistant Staphylococcus aureus (MRSA). BMC Complement. Altern. Med. 2015, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Stegarus, D.I.; Lengyel, E.; Apostolescu, G.F.; Botoran, O.R.; Tanase, C. Phytochemical Analysis and Biological Activity of Three Stachys Species (Lamiaceae) from Romania. Plants 2021, 10, 2710. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Popescu, D.I.; Lengyel, E.; Apostolescu, F.G.; Soare, L.C.; Botoran, O.R.; Șuțan, N.A. Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania. Horticulturae 2022, 8, 952. [Google Scholar] [CrossRef]
- Ibrahim, N.; Kebede, A. In Vitro Antibacterial Activities of Methanol and Aqueous Leave Extracts of Selected Medicinal Plants Against Human Pathogenic Bacteria. Saudi J Biol Sci. 2020, 27, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Ni, C.L.; Shen, Y.C.; Huang, Y.L.; Kuo, C.H.; Wu, T.S.; Chen, C.C. Phenolic Constituents from the Stem Bark of Magnolia officinalis. J. Nat. Prod. 2009, 72, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xie, Y.; Chen, Z.; Fan, Y.; Liu, Y.; Gao, Q.; Li, J.; Bai, J.; Yang, Y. Antimicrobial Activity and Mechanism of Magnolia officinalis Root Extract Against Methicillin-Resistant Staphylococcus aureus Based on Mannose Transporter. Industrial Crops and Products 2023, 201, 116953. [Google Scholar] [CrossRef]
- Yahaya, A.A.H.; Salleh, W.M.N.H.W.; Ghani, N.A. Magnolia Genus – A Systematic Review on the Composition and Biological Properties of Its Essential Oils. La Rivista Italiana delle Sostanze Grasse 2022, 99, 249-261.
- Lovecká, P.; Svobodová, A.; Macůrková, A.; Vrchotová, B.; Demnerová, K.; Wimmer, Z. Decorative Magnolia Plants: A Comparison of the Content of Their Biologically Active Components Showing Antimicrobial Effects. Plants (Basel). 2020, 9, 879. [Google Scholar] [CrossRef]
| Magnolia species | Source of Extracts | Total Polyphenols mg GAE/g | DPPH IC50 mg/mL |
|---|---|---|---|
| M. champaca | Bark | 73.12±0.25 | 19.50±0.25 |
| Flowers | 68.62±0.21 | 18.66±0.21 | |
| M. denudata | Bark | 65.18±0.28 | 17.01±0.24 |
| Flowers | 55.23±0.34 | 9.99±0.25 | |
| M. grandiflora | Bark | 72.52±0.45 | 19.07±0.32 |
| Flowers | 55.18±0.38 | 10.55±0.18 | |
| M. officinalis | Bark | 98.44±0.49 | 23.23±0.48 |
| Flowers | 66.02±0.28 | 18.78±0.27 |
| Compounds | M. champaca | M. denudata | M. grandiflora | M. officinalis | ||||
|---|---|---|---|---|---|---|---|---|
| Bark | Flowers | Bark | Flowers | Bark | Flowers | Bark | Flowers | |
| Phenolic Acids (mg/g dry matter) | ||||||||
| Gallic | 5.021 | 1.191 | 1.278 | 1.782 | 2.371 | 0.984 | 1.999 | 2.035 |
| 4-hydroxybenzoic | 9.289 | 4.133 | 11.097 | 3.191 | 5.667 | 1.224 | 10.012 | 6.127 |
| p-coumaric | 0.111 | 2.671 | 3.108 | 0.154 | n.d. | 1.793 | 0.010 | 1.119 |
| Salicylic | 7.789 | n.d. | 4.578 | n.d. | 9.123 | n.d. | 2.333 | n.d. |
| Caffeic | 0.019 | 0.662 | 0.027 | 0.276 | 0.113 | 1.044 | 0.022 | 0.993 |
| Caftaric | n.d. | 0.011 | 0.001 | n.d | n.d. | 0.015 | 0.017 | 0.024 |
| Cinnamic | 0.092 | 0.023 | 0.002 | 0.064 | n.d. | 0.048 | 0.076 | n.d |
| Chlorogenic | 1.357 | 2.543 | 4.504 | 3.934 | 4.441 | 1.147 | 5.619 | 2.281 |
| Ellagic | 0.487 | n.d. | 0.983 | 0.001 | 0.578 | 0.001 | 0.568 | n.d. |
| Ferulic | 1.129 | 0.111 | 1.222 | 0.127 | 1.341 | n.d. | 0.991 | n.d. |
| Syringic | 1.002 | n.d. | 1.083 | n.d. | 1.321 | 0.001 | 1.242 | 0.001 |
| Vanillic | n.d. | 0.005 | n.d. | 0.003 | 0.001 | 0.002 | 0.001 | 0.002 |
| Total | 26.296 | 11.35 | 27.883 | 9.532 | 24.956 | 6.259 | 22.89 | 12.582 |
| Aglycone Flavonoids (mg/g dry matter) | ||||||||
| Catechin | 85.333 | 52.744 | 91.227 | 78.936 | 71.772 | 66.033 | 81.034 | 32.333 |
| Myricetin | 1.024 | 0.221 | 0.023 | 2.003 | 0.992 | n.d. | 0.835 | n.d. |
| Luteolin | 2.001 | 0.001 | 0.779 | 0.287 | 1.429 | n.d. | 1.002 | 0.110 |
| Taxifolin | 0.004 | 1.983 | n.d. | 5.661 | n.d. | 1.111 | 0.002 | 2.003 |
| Eriodictyol | n.d. | 0.001 | n.d. | n.d. | 0.001 | 0.021 | 0.001 | n.d. |
| Apigenin | 0.772 | 4.229 | 0.189 | 12.002 | 0.991 | 7.456 | 1.283 | 2.342 |
| Quercetin | 0.615 | 0.357 | 0.995 | 1.911 | 0.937 | 0.999 | 0.691 | 1.001 |
| Epicatechin | 22.418 | 29.919 | 20.361 | 34.988 | 31.912 | 39.397 | 34.562 | 23.435 |
| Total | 112.167 | 89.455 | 113.574 | 135.788 | 108.034 | 115.017 | 119.41 | 61.224 |
| Glycosidic flavonoids (mg/g dry matter) | ||||||||
| Rutin | 27.035 | 23.923 | 45.327 | 15.075 | 56.782 | 17.249 | 51.103 | 20.200 |
| Luteolin-7-O-glucoside | 1.002 | 5.552 | 2.033 | 2.003 | 1.023 | 1.279 | 0.435 | 6.771 |
| Isoquercetin | 0.004 | 0.213 | n.d. | 0.016 | n.d. | 0.111 | n.d. | 0.098 |
| Hyperoside | n.d. | 0.111 | n.d. | 0.032 | n.d. | n.d. | n.d. | 0.011 |
| Kaempferol-3-O-rutinoside | 0.012 | 0.241 | 0.023 | 0.104 | 0.122 | 0.286 | 0.192 | 0.197 |
| Apigenin-7-O-glucoside | 0.003 | 0.044 | 0.115 | 0.011 | 0.012 | 0.101 | 0.011 | 0.099 |
| Isorhamnetin-3-O-glucoside | 0.002 | n.d. | 0.001 | n.d. | 0.001 | 0.001 | n.d. | n.d. |
| Naringenin-7-O-glucoside | 0.001 | n.d. | n.d. | 0.002 | n.d. | n.d. | n.d. | n.d. |
| Astragalin | n.d. | n.d. | n.d. | 0.001 | n.d. | n.d. | n.d. | n.d. |
| Quercitrin | n.d. | 0.001 | n.d. | n.d. | n.d. | n.d. | 0.001 | n.d. |
| Isorhamnetin-3-O-rutinoside | 0.003 | 0.004 | n.d. | 0.021 | 0.021 | 0.018 | 0.114 | 0.007 |
| Total | 28.062 | 30.089 | 47.499 | 17.265 | 57.961 | 19.045 | 51.856 | 27.383 |
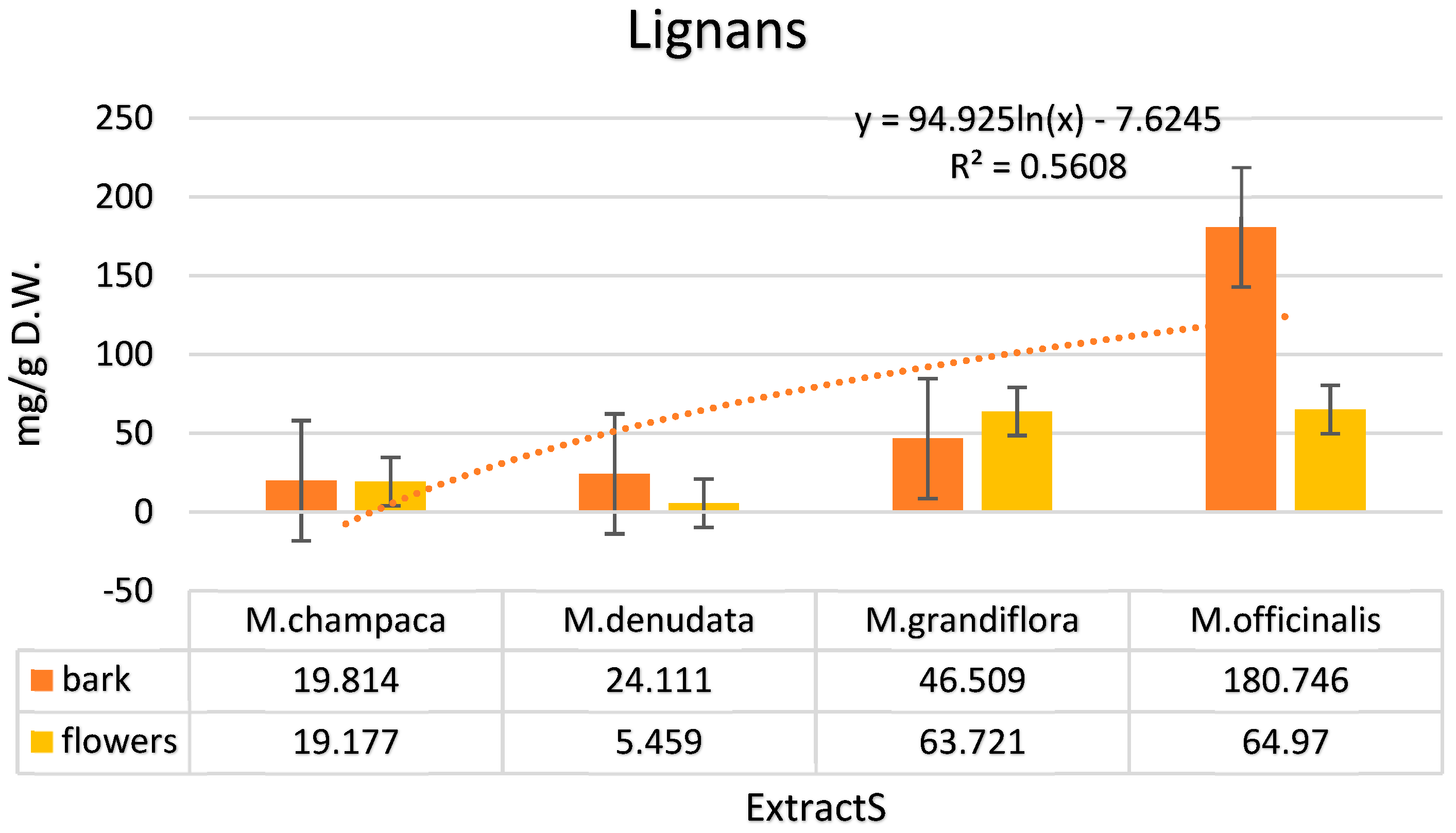
| Lignans | ||||||||
| 4’-O-methylhonokiol | 2.011 | 7.012 | 5.561 | 1.021 | 19.092 | 18.098 | 21.021 | 16.546 |
| Magnolol | 9.319 | 5.666 | 9.999 | 2.001 | 7.021 | 19.021 | 97.093 | 23.021 |
| Honokiol | 5.092 | 4.090 | 5.892 | 2.098 | 6.607 | 18.917 | 56.785 | 13.195 |
| 3-methoxymagnolol | 2.367 | 2.311 | 1.285 | 0.227 | 7.000 | 5.662 | 2.368 | 4.432 |
| Isomagnolol | 1.025 | 0.098 | 1.374 | 0.112 | 6.789 | 2.023 | 3.479 | 7.776 |
| Total | 19.814 | 19.177 | 24.111 | 5.459 | 46.509 | 63.721 | 180.746 | 64.97 |
| Total Phenolic Compounds | 186.339 | 150.071 | 213.067 | 168.044 | 237.46 | 204.042 | 374.902 | 166.159 |
| Compound | RIa | RIb | M. champaca % | M. denudata % | M. grandiflora % | M. officinalis % | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| bark | flowers | bark | flowers | bark | flowers | bark | flowers | |||
| Hexanal | 801 | 800 | - | 0.1 | - | - | - | - | - | - |
| Heptanal | 902 | 902 | - | 0.2 | - | 0.1 | 0.1 | 0.1 | 0.3 | 0.9 |
| α-Thujene | 930 | 929 | 0.1 | 1.2 | 0.1 | 1.5 | 0.4 | 0.7 | 0.5 | 2.1 |
| α-Pinene | 939 | 932 | 0.4 | 0.6 | 1.1 | 0.9 | 0.2 | 0.8 | 0.3 | 1.5 |
| Camphene | 954 | 946 | 1.1 | - | 1.1 | 0.1 | 1.1 | 0.1 | 1.3 | - |
| Sabinene | 975 | 972 | - | tr | - | tr | 0.1 | tr | 0.1 | tr |
| β-Pinene | 979 | 980 | 3.6 | 0.1 | 2.9 | - | 5.2 | tr | 2.3 | tr |
| 6-Methyl-5-hepten-2-one | 986 | 985 | - | 1.1 | 0.1 | - | 0.1 | 0.5 | - | - |
| α-Phellandrene | 1002 | 1001 | - | 0.1 | - | - | - | 0.2 | - | - |
| α-Terpinene | 1016 | 1014 | 0.1 | 0.5 | 0.2 | 0.5 | 0.1 | 0.7 | 0.1 | 1.2 |
| p-Cymene | 1026 | 1024 | - | 0.2 | - | 0.1 | - | 0.2 | - | 0.1 |
| Limonene | 1029 | 1030 | 0.9 | 4.4 | 2.2 | 7.1 | 0.5 | 2.9 | 2.1 | 1.8 |
| 1,8-Cineol | 1031 | 1031 | 0.5 | 0.9 | 2.1 | 1.1 | 0.5 | 0.1 | 1.7 | 0.1 |
| β-Phellandrene | 1034 | 1032 | - | 0.2 | - | 0.4 | - | - | - | 0.1 |
| (Z)-β-Ocimene | 1038 | 1044 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | - |
| Phenylacetaldehyde | 1042 | 1043 | 1.1 | 1.8 | 1.4 | 3.9 | 2.2 | 1.6 | 1.7 | - |
| (E)-β-Ocimene | 1050 | 1052 | 0.1 | 5.7 | 0.1 | 1.9 | 0.1 | 3.4 | 0.1 | 1.1 |
| γ-Terpinene | 1060 | 1058 | 0.2 | 3.2 | 0.1 | 2.1 | 0.1 | 0.8 | 0.2 | 0.3 |
| 1-Octanol | 1068 | 1066 | 0.4 | 2.8 | 2.0 | 4.6 | 2.4 | 1.2 | 1.6 | 1.9 |
| cis-Sabinene hydrate | 1070 | 1070 | - | tr | - | 0.1 | tr | - | - | 0.1 |
| cis-Linalool oxide (furanoid) | 1073 | 1074 | 0.2 | 0.4 | 0.1 | 0.6 | 0.2 | 0.1 | - | 0.3 |
| trans-Linalool oxide (furanoid) | 1087 | 1085 | 0.1 | 0.1 | 0.1 | - | 0.2 | 0.1 | 0.1 | 0.1 |
| Terpinolene | 1092 | 1093 | 0.5 | 0.2 | 0.4 | 0.3 | 0.7 | 0.3 | 0.2 | 0.8 |
| Linalool | 1095 | 1100 | 1.4 | 25.1 | 2.7 | 20.4 | 1.8 | 15.8 | 2.0 | 21.2 |
| €-4,8-Dimethylnona-1,3,7-triene | 1118 | 1120 | 0.1 | 1.2 | 1.0 | 4.4 | 1.1 | 0.4 | 1.3 | - |
| Perillene | 1122 | 1123 | - | 0.1 | - | 0.1 | - | 0.1 | - | 0.1 |
| Camphor | 1141 | 1140 | 2.5 | 1.1 | 3.1 | 1.3 | 6.3 | 0.8 | 6.6 | 3.4 |
| Borneol | 1163 | 1165 | 1.1 | - | 1.2 | 0.1 | 1.3 | 0.1 | 1.1 | - |
| Terpinen-4-ol | 1186 | 1185 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 |
| α-Terpineol | 1189 | 1190 | 0.2 | 2.2 | 0.5 | 0.8 | 0.8 | 1.6 | 0.7 | 2.1 |
| Myrtenol | 1195 | 1193 | 0.1 | 1.1 | 0.1 | 2.9 | 0.1 | 1.4 | 0.1 | 0.7 |
| Myrtenal | 1196 | 1197 | - | 0.3 | tr | - | tr | 0.5 | tr | - |
| β-Citronellol | 1226 | 1223 | 0.4 | 1.8 | 0.9 | 1.3 | 0.7 | 2.3 | 0.5 | 2.9 |
| Nerol | 1230 | 1229 | 0.1 | 1.6 | 0.1 | 1.1 | 0.2 | 4.9 | 0.2 | 2.2 |
| Neral | 1238 | 1237 | 0.1 | 0.1 | - | - | 0.1 | - | - | - |
| Geraniol | 1253 | 1255 | 1.1 | 5.2 | 0.5 | 1.9 | 0.9 | 7.7 | 0.8 | 9.1 |
| Geranial | 1257 | 1258 | 0.1 | 0.3 | 0.2 | 0.1 | 0.3 | 0.3 | 0.1 | 0.9 |
| 1-Decanol | 1270 | 1273 | 0.2 | 0.3 | 0.1 | 0.6 | 0.1 | 0.2 | 0.1 | 0.7 |
| Bornyl acetate | 1289 | 1290 | 25.6 | 2.1 | 14.9 | 5.3 | 14.2 | 4.3 | 17.8 | 11.1 |
| Myrtenyl acetate | 1327 | 1325 | 0.5 | 2.1 | 1.0 | 1.5 | 1.1 | 0.9 | 1.3 | 0.4 |
| Eugenol | 1359 | 1360 | 0.1 | 0.9 | 0.2 | 1.4 | 0.2 | 0.8 | 0.1 | 1.5 |
| α-Copaene | 1388 | 1387 | 0.1 | 0.4 | - | - | 0.1 | - | 0.1 | 0.4 |
| β-Elemene | 1404 | 1405 | 0.8 | 1.2 | 0.7 | 2.0 | 0.7 | 1.9 | 0.3 | 1.0 |
| Ε-Caryophyllene | 1417 | 1419 | 11.1 | 1.2 | 22.4 | 0.9 | 21.1 | 0.7 | 15.6 | 1.2 |
| α-trans-Bergamotene | 1435 | 1435 | - | 0.1 | - | 0.1 | tr | 0.1 | - | - |
| β-Caryophyllene | 1437 | 1432 | 1.2 | 0.2 | 1.8 | 1.3 | 3.4 | 2.9 | 5.6 | 1.1 |
| (Z)-β-Farnesene | 1453 | 1447 | - | 0.1 | - | 0.1 | 0.1 | 0.3 | 0.7 | - |
| α-Humulene | 1455 | 1455 | 0.1 | 0.4 | 0.3 | 1.1 | 0.2 | 0.8 | 0.5 | 0.9 |
| 9-epi-(E)-Caryophyllene | 1470 | 1470 | 3.7 | 0.1 | 2.9 | 1.8 | 3.8 | 0.9 | 3.1 | 0.3 |
| γ-Gurjunene | 1477 | 1476 | - | - | - | 0.1 | 0.1 | - | - | |
| Germacrene D | 1485 | 1481 | 11.2 | 2.4 | 5.3 | 1.5 | 4.6 | 5.2 | 7.7 | 4.8 |
| α-Selinene | 1488 | 1489 | 1.2 | 0.1 | 0.8 | 0.5 | 1.3 | 0.7 | 2.3 | 0.1 |
| γ-Muurolene | 1490 | 1494 | 0.1 | - | 0.1 | 0.8 | 0.2 | 0.5 | 0.1 | 0.3 |
| Viridiflorene | 1496 | 1497 | 1.8 | 2.1 | 1.5 | 1.8 | 2.2 | 2.6 | - | 1.2 |
| Bicyclogermacrene | 1500 | 1502 | 0.1 | 0.4 | 0.2 | 0.4 | 0.4 | 1.1 | 0.5 | - |
| δ-Cadinene | 1523 | 1522 | 0.9 | 2.0 | 0.5 | 0.8 | 1.5 | 0.3 | 1.7 | 3.5 |
| Hedycaryol | 1548 | 1545 | - | - | - | - | - | 0.1 | - | - |
| Elemol | 1550 | 1552 | - | - | - | - | - | 0.8 | - | 0.5 |
| trans-Nerolidol | 1556 | 1555 | 0.1 | 0.1 | - | - | 0.1 | 0.1 | 0.1 | - |
| (E)-Nerolidol | 1563 | 1563 | 0.2 | 0.5 | - | 0.1 | 0.6 | 0.2 | 0.3 | 0.1 |
| Palustrol | 1567 | 1569 | - | - | - | - | - | 0.1 | - | - |
| Germacrene D-4-ol | 1576 | 1577 | 0.1 | 1.4 | 0.1 | 1.1 | 0.2 | 1.5 | 0.2 | 1.0 |
| Spathulenol | 1578 | 1579 | 3.6 | 0.1 | 2.8 | 0.5 | 1.7 | 0.1 | 2.2 | 0.1 |
| Globulol | 1585 | 1588 | 0.1 | 0.1 | - | - | - | 0.8 | - | - |
| β-Eudesmol | 1650 | 1651 | - | - | - | - | - | 0.1 | - | - |
| α-Cadinol | 1654 | 1653 | 0.1 | 0.1 | 0.1 | 1.0 | 0.3 | 1.1 | 0.1 | 1.7 |
| Selin-11-en-4-α-ol | 1659 | 1657 | 0.2 | 0.1 | 0.5 | 1.9 | 0.1 | - | 0.4 | 0.5 |
| Shyobunol | 1688 | 1688 | - | - | - | - | 0.1 | 0.2 | 0.1 | 0.1 |
| (E)-Nerolidyl acetate | 1717 | 1713 | - | - | - | - | - | 0.1 | - | - |
| (Z,E)-Farnesol | 1725 | 1722 | 0.3 | 0.4 | 0.5 | - | 0.9 | 0.3 | 0.6 | 0.2 |
| 14-Hydroxy-α-muurolene | 1780 | 1777 | 0.2 | 0.5 | 0.2 | 0.6 | 0.5 | 0.3 | 0.4 | 0.1 |
| 14-Hydroxy-δ-cadinene | 1802 | 1800 | 0.5 | 0.5 | - | 0.4 | 0.1 | 0.2 | 0.1 | 0.3 |
| n-Hexadecanol | 1875 | 1872 | 3.3 | 7.4 | 2.3 | 2.2 | 1.7 | 2.7 | 1.1 | 3.3 |
| n-Heneicosane | 2100 | 2100 | 2.1 | 0.2 | 2.7 | 0.2 | 1.6 | 0.3 | 0.5 | 0.5 |
| Linoleic acid | 2133 | 2130 | 0.1 | 0.6 | 0.1 | 2.2 | 0.1 | 1.4 | 0.4 | 1.0 |
| n-Tricosene | 2300 | 2300 | 7.7 | 1.9 | 5.5 | 1.8 | 1.7 | 4.1 | 4.2 | 2.8 |
| n-Pentacosane | 2500 | 2500 | 1.4 | 1.9 | 4.9 | 1.3 | 1.1 | 2.1 | 2.9 | 1.9 |
| Total | 95.3 | 96.0 | 96.9 | 95.1 | 94.1 | 91.1 | 97.5 | 97.7 | ||
| Other compounds | 4.7 | 4.0 | 3.1 | 4.9 | 5.9 | 8.9 | 2.5 | 2.3 | ||
| Bacterial strains | MIC (µg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampi-cillin | Genta-micin | Tetracy-cline | M. champaca | M. denudata | M. grandiflora | M. officinalis | |||||
| bark | flo-wer | bark | flo-wer | bark | flo-wer | bark | flo-wer | ||||
| Staphylococcus aureus ATCC 43300(MRSA) | n.a. | 30 | n.a. | n.a | n.a | 30 | n.a. | n.a. | 30 | 30 | 30 |
| Staphylococcus epidermidis ATCC 12228 | 10 | 5 | 5 | 15 | 30 | 15 | 30 | 7.5 | 7.5 | 5 | 5 |
| Streptococcus faecalis ATCC 19443 | 2.5 | 2.5 | 2.5 | 15 | 2.5 | 7.5 | 15 | 7.5 | 7.5 | 5 | 2.5 |
| Streptococcus pyogenes ATCC 12347 | 1.25 | 1.25 | 2.5 | 30 | 30 | n.a. | 30 | 15 | n.a. | 15 | n.a. |
| Streptococcus sanguinis ATCC 10556 | 2.5 | 2.5 | n.a. | n.a | n.a. | n.a. | n.a. | n.a. | 30 | 30 | 30 |
| Actinomyces israelii ATCC 12102 | 0.625 | 0.625 | 0.625 | 15 | 30 | 12.5 | 15 | 10 | 10 | 7.5 | 7.5 |
| Propionebacterium acnes ATCC 6921/4311 | 1.25 | 1.25 | 0.625 | 7.5 | 2.5 | 10 | 12.5 | 5 | 7.5 | 5 | 2.5 |
| Enterobacter aerogenes ATCC 13048 | 0.625 | 0.625 | 30 | 15 | 10 | 30 | 30 | 10 | 12.5 | 7.5 | 10 |
| Escherichia coli ATCC 35218 | 0.625 | 0.625 | 30 | n.a. | n.a. | 15 | 30 | 15 | 15 | 10 | 12.5 |
| Klebsiella pneumoniae ATCC 13883 | 0.625 | 0.625 | 30 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 30 | n.a. |
| Prevotella intermedia ATCC 25611 | 0.625 | 0.625 | 0.625 | 5 | 15 | n.a. | n.a. | 10 | 2.5 | 15 | 15 |
| Porphyromonas gingivalis ATCC 33277 | 0.625 | 30 | 15 | 0.625 | 5 | 7.5 | 10 | 5 | 2.5 | 0.625 | 1.25 |
| Proteus vulgaris ATCC 13315 | 0.625 | 0.625 | 10 | 10 | 10 | 15 | 15 | 7.5 | 12.5 | 5 | 10 |
| Pseudomonas aeruginosa ATCC 27853 | n.a. | 0.625 | n.a. | 5 | 12.5 | 12.5 | 15 | 7.5 | 10 | 2.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





