Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Myeloblasts from patients with acute myeloid leukemia exhibit varying levels of mesothelin expression
2.2. Mitochondrial respiration is enhanced in bone marrow of AML patients with mesothelin positivity
2.3. Enhancement of mitochondrial respiration and ATP production in AML cells by mesothelin stimulation
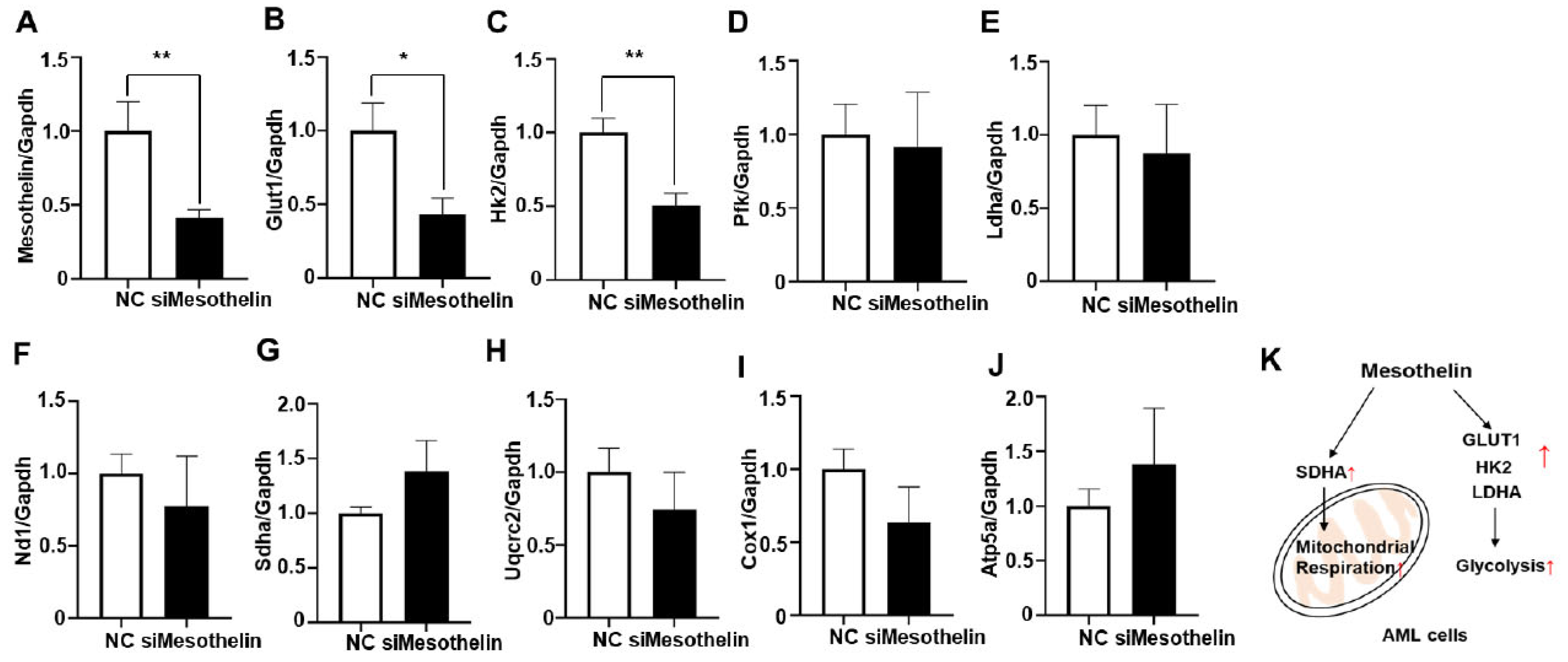
2.4. Induction of glycolytic enzymes and mitochondrial complex II gene expression by mesothelin in AML cells
3. Discussion
4. Materials and Methods
4.1. Cell culture and transfection
4.2. Human bone marrow samples
4.3. Flow cytometry of bone marrow samples
4.4. Measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR)
4.5. RNA isolation and quantitative real- time PCR
4.6. Statistical analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ryu, I.; Ryu, M.J.; Han, J.; Kim, S.J.; Lee, M.J.; Ju, X.; Yoo, B.H.; Lee, Y.L.; Jang, Y.; Song, I.C.; Chung, W.; Oh, E.; Heo, J.Y.; Kweon, G.R. L-Deprenyl exerts cytotoxicity towards acute myeloid leukemia through inhibition of mitochondrial respiration. Oncol Rep 2018, 40, 3869–3878. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y. Human acute myeloid leukemia stem cells: evolution of concept. Blood Res 2022, 57, 67–74. [Google Scholar] [CrossRef] [PubMed]
- de Beauchamp, L.; Himonas, E.; Helgason, G.V. Mitochondrial metabolism as a potential therapeutic target in myeloid leukaemia. Leukemia 2022, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fovez, Q.; Laine, W.; Goursaud, L.; Berthon, C.; Germain, N.; Degand, C.; Sarry, J.E.; Quesnel, B.; Marchetti, P.; Kluza, J. Clinically Relevant Oxygraphic Assay to Assess Mitochondrial Energy Metabolism in Acute Myeloid Leukemia Patients. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Basak, N.P.; Banerjee, S. Mitochondrial dependency in progression of acute myeloid leukemia. Mitochondrion 2015, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Panina, S.B.; Pei, J.; Kirienko, N.V. Mitochondrial metabolism as a target for acute myeloid leukemia treatment. Cancer Metab 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.A.; Schiller, G.J.; Kambhampati, S.; Tallman, M.S.; Douer, D.; Minden, M.D.; Yee, K.W.; Gupta, V.; Brandwein, J.; Jitkova, Y.; Gronda, M.; Hurren, R.; Shamas-Din, A.; Schuh, A.C.; Schimmer, A.D. A Phase 1 study of intravenous infusions of tigecycline in patients with acute myeloid leukemia. Cancer Med 2016, 5, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- IDH-Mutated AML: Beyond Enasidenib and Ivosidenib Monotherapy: Highlights From SOHO 2021. J Adv Pract Oncol 2022, 13 (Suppl 1), 12–14. [CrossRef]
- Martelli, M.P.; Martino, G.; Cardinali, V.; Falini, B.; Martinelli, G.; Cerchione, C. Enasidenib and ivosidenib in AML. Minerva Med 2020, 111, 411–426. [Google Scholar] [CrossRef]
- Moore, J.W.; Pelcovits, A.; Reagan, J.L. Azacitidine and Venetoclax in AML. N Engl J Med 2020, 383, 2088. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D'Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; Hesselberth, J.R.; Abbott, D.; Schatz, D.; Gutman, J.A.; Purev, E.; Smith, C.; Jordan, C.T. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med 2018, 24, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Satta, T.; Li, L.; Chalasani, S.L.; Hu, X.; Nkwocha, J.; Sharma, K.; Kmieciak, M.; Rahmani, M.; Zhou, L.; Grant, S. Dual mTORC1/2 Inhibition Synergistically Enhances AML Cell Death in Combination with the BCL2 Antagonist Venetoclax. Clin Cancer Res 2023, 29, 1332–1343. [Google Scholar] [CrossRef]
- Stahl, M.; Menghrajani, K.; Derkach, A.; Chan, A.; Xiao, W.; Glass, J.; King, A.C.; Daniyan, A.F.; Famulare, C.; Cuello, B.M.; Horvat, T.Z.; Abdel-Wahab, O.; Levine, R.L.; Viny, A.D.; Stein, E.M.; Cai, S.F.; Roshal, M.; Tallman, M.S.; Goldberg, A.D. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv 2021, 5, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Ganguly, S.; Singh, A.; Palanichamy, J.K.; Chopra, A.; Bakhshi, R.; Bakhshi, S. Mitochondrial complex II and V activity is enhanced in pediatric acute myeloid leukemia. Am J Blood Res 2021, 11, 534–543. [Google Scholar] [PubMed]
- Kaeding, A.J.; Barwe, S.P.; Gopalakrishnapillai, A.; Ries, R.E.; Alonzo, T.A.; Gerbing, R.B.; Correnti, C.; Loken, M.R.; Broderson, L.E.; Pardo, L.; Le, Q.H.; Tang, T.; Leonti, A.R.; Smith, J.L.; Chou, C.K.; Xu, M.; Triche, T.; Kornblau, S.M.; Kolb, E.A.; Tarlock, K.; Meshinchi, S. Mesothelin is a novel cell surface disease marker and potential therapeutic target in acute myeloid leukemia. Blood Adv 2021, 5, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis 2011, 32, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Hung, W.C.; Wang, P.; Paul, C.; Konstantopoulos, K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep 2013, 3, 1870. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Castro, S.; Tang, T.; Loeb, A.M.; Hylkema, T.; McKay, C.N.; Perkins, L.; Srivastava, S.; Call, L.; Smith, J.; Leonti, A.; Ries, R.; Pardo, L.; Loken, M.R.; Correnti, C.; Fiorenza, S.; Turtle, C.J.; Riddell, S.; Tarlock, K.; Meshinchi, S. Therapeutic Targeting of Mesothelin with Chimeric Antigen Receptor T Cells in Acute Myeloid Leukemia. Clin Cancer Res 2021, 27, 5718–5730. [Google Scholar] [CrossRef] [PubMed]
- Montemagno, C.; Cassim, S.; Pouyssegur, J.; Broisat, A.; Pages, G. From Malignant Progression to Therapeutic Targeting: Current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Le, K.; Wang, J.; Zhang, T.; Guo, Y.; Chang, H.; Wang, S.; Zhu, B. Overexpression of Mesothelin in Pancreatic Ductal Adenocarcinoma (PDAC). Int J Med Sci 2020, 17, 422–427. [Google Scholar] [CrossRef]
- Servais, E.L.; Colovos, C.; Rodriguez, L.; Bograd, A.J.; Nitadori, J.; Sima, C.; Rusch, V.W.; Sadelain, M.; Adusumilli, P.S. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 2012, 18, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, P. Mesothelin as a biomarker for targeted therapy. Biomark Res 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin confers pancreatic cancer cell resistance to TNF-alpha-induced apoptosis through Akt/PI3K/NF-kappaB activation and IL-6/Mcl-1 overexpression. Mol Cancer 2011, 10, 106. [Google Scholar] [CrossRef]
- Goo, C.K.; Lim, H.Y.; Ho, Q.S.; Too, H.P.; Clement, M.V.; Wong, K.P. PTEN/Akt signaling controls mitochondrial respiratory capacity through 4E-BP1. PLoS One 2012, 7, e45806. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.R.; Hamill, D.; Kolb, E.A.; Gopalakrishnapillai, A.; Barwe, S.P. Mesothelin: An Immunotherapeutic Target beyond Solid Tumors. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Mesbahi, Y.; Trahair, T.N.; Lock, R.B.; Connerty, P. Exploring the Metabolic Landscape of AML: From Haematopoietic Stem Cells to Myeloblasts and Leukaemic Stem Cells. Front Oncol 2022, 12, 807266. [Google Scholar] [CrossRef] [PubMed]
- Sriskanthadevan, S.; Jeyaraju, D.V.; Chung, T.E.; Prabha, S.; Xu, W.; Skrtic, M.; Jhas, B.; Hurren, R.; Gronda, M.; Wang, X.; Jitkova, Y.; Sukhai, M.A.; Lin, F.H.; Maclean, N.; Laister, R.; Goard, C.A.; Mullen, P.J.; Xie, S.; Penn, L.Z.; Rogers, I.M.; Dick, J.E.; Minden, M.D.; Schimmer, A.D. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood 2015, 125, 2120–2130. [Google Scholar] [CrossRef]
- Wang, L.; Cybula, M.; Rostworowska, M.; Wang, L.; Mucha, P.; Bulicz, M.; Bieniasz, M. Upregulation of Succinate Dehydrogenase (SDHA) Contributes to Enhanced Bioenergetics of Ovarian Cancer Cells and Higher Sensitivity to Anti-Metabolic Agent Shikonin. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, X.; Chen, Q.; Kantawong, F.; Wan, S.; Liu, J.; Li, H.; Zhou, J.; Lu, B.; Wu, J. Identification of a Mitochondria-Related Gene Signature to Predict the Prognosis in AML. Front Oncol 2022, 12, 823831. [Google Scholar] [CrossRef]
- Kaneko, O.; Gong, L.; Zhang, J.; Hansen, J.K.; Hassan, R.; Lee, B.; Ho, M. A binding domain on mesothelin for CA125/MUC16. J Biol Chem 2009, 284, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, X.; Sun, X.; Wang, L.; Chen, S. The Glycolytic Switch in Tumors: How Many Players Are Involved? J Cancer 2017, 8, 3430–3440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Duan, Z.; Li, Z.; Ge, F.; Wei, R.; Kong, L. The significance of glycolysis in tumor progression and its relationship with the tumor microenvironment. Front Pharmacol 2022, 13, 1091779. [Google Scholar] [CrossRef]
- Schuringa, J.J.; Bonifer, C. Dissecting Clonal Heterogeneity in AML. Cancer Cell 2020, 38, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Mondet, J.; Lo Presti, C.; Chevalier, S.; Bertrand, A.; Tondeur, S.; Blanchet, S.; Mc Leer, A.; Pernet-Gallay, K.; Mossuz, P. Mitochondria in human acute myeloid leukemia cell lines have ultrastructural alterations linked to deregulation of their respiratory profiles. Exp Hematol 2021, 98, 53–62. [Google Scholar] [CrossRef]
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H.; Andreeff, M. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest 2010, 120, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Mao, L.; Wu, M.; Liu, J.; Yu, S. Challenges of Anti-Mesothelin CAR-T-Cell Therapy. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Kelly, R.J.; Sharon, E.; Pastan, I.; Hassan, R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther 2012, 11, 517–525. [Google Scholar] [CrossRef]
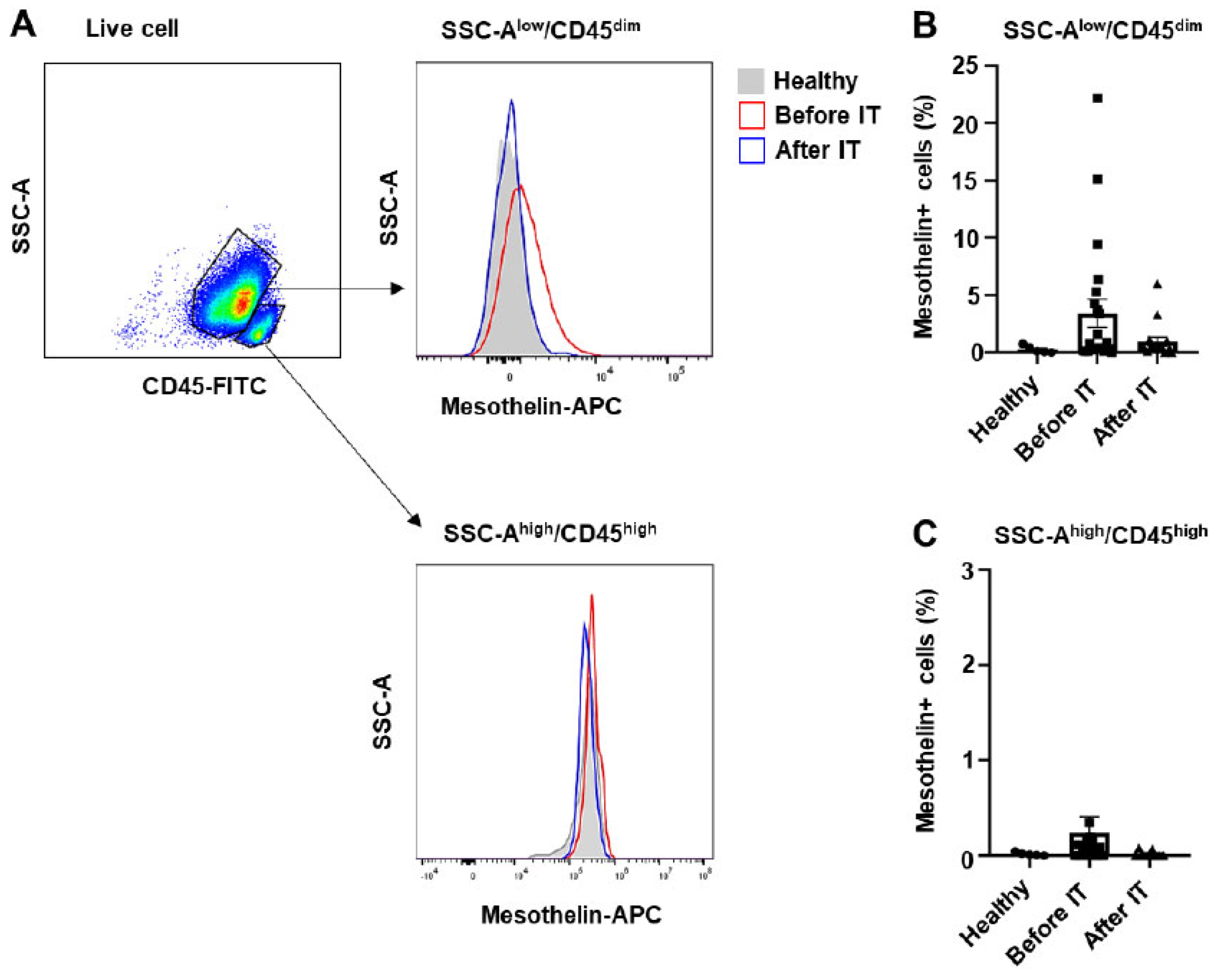
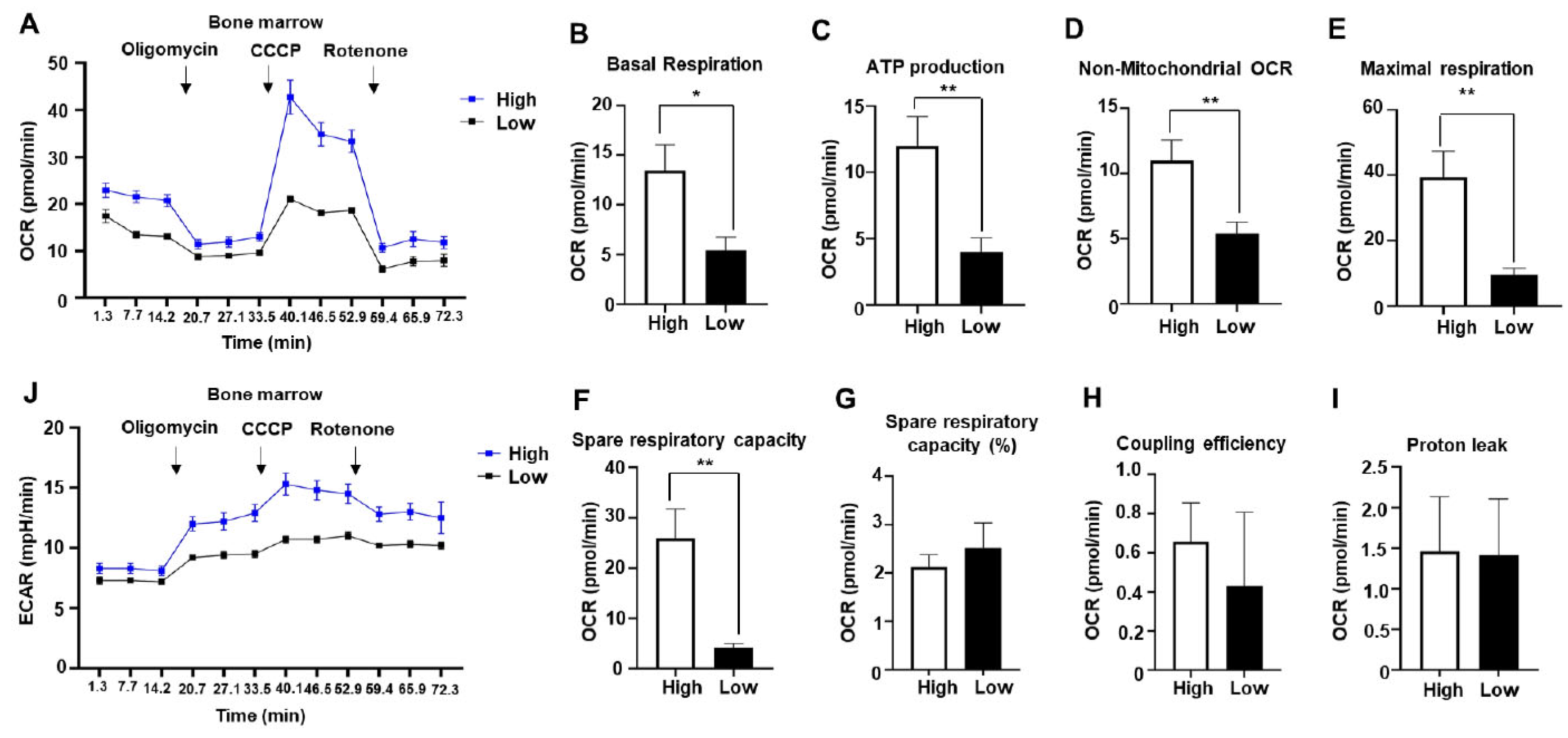
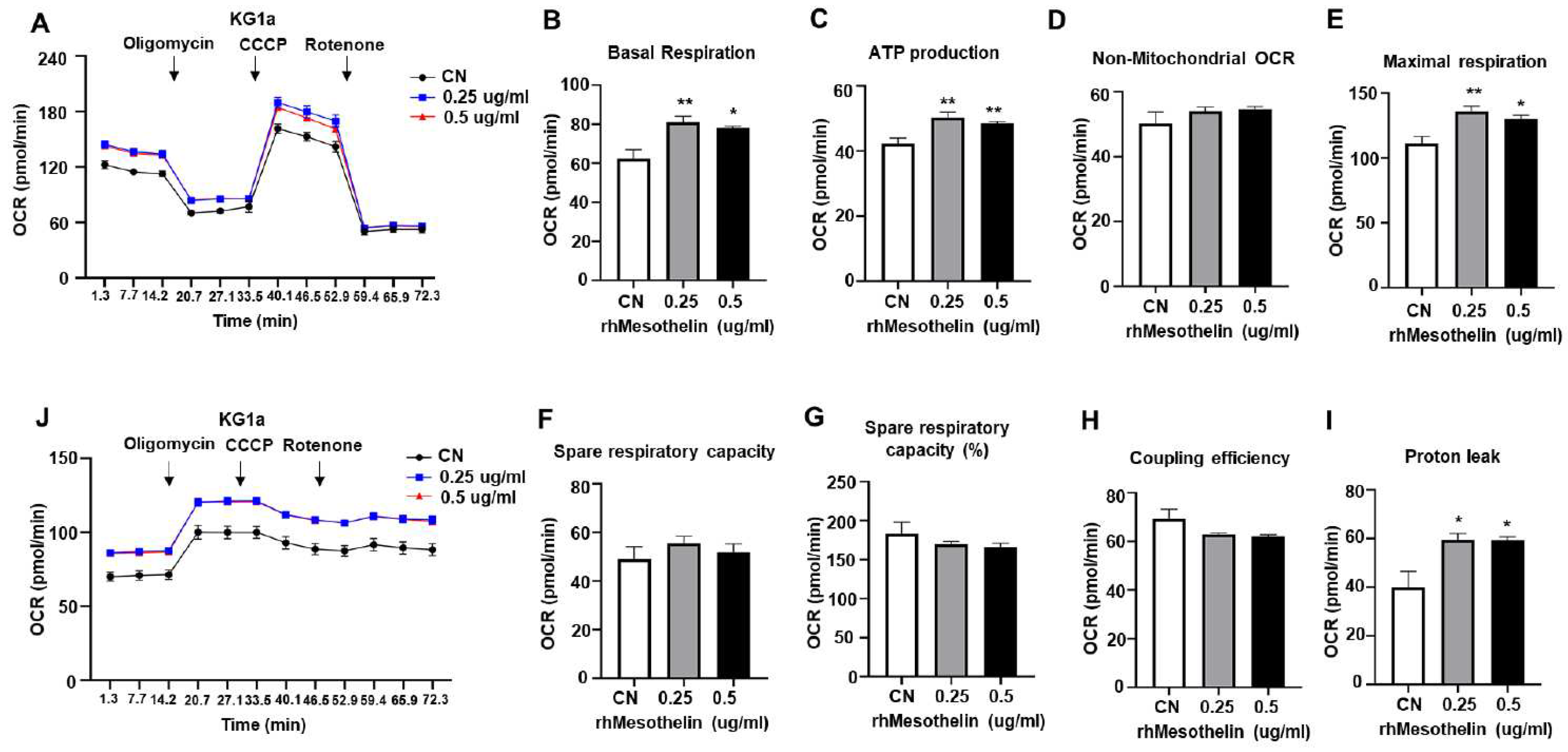
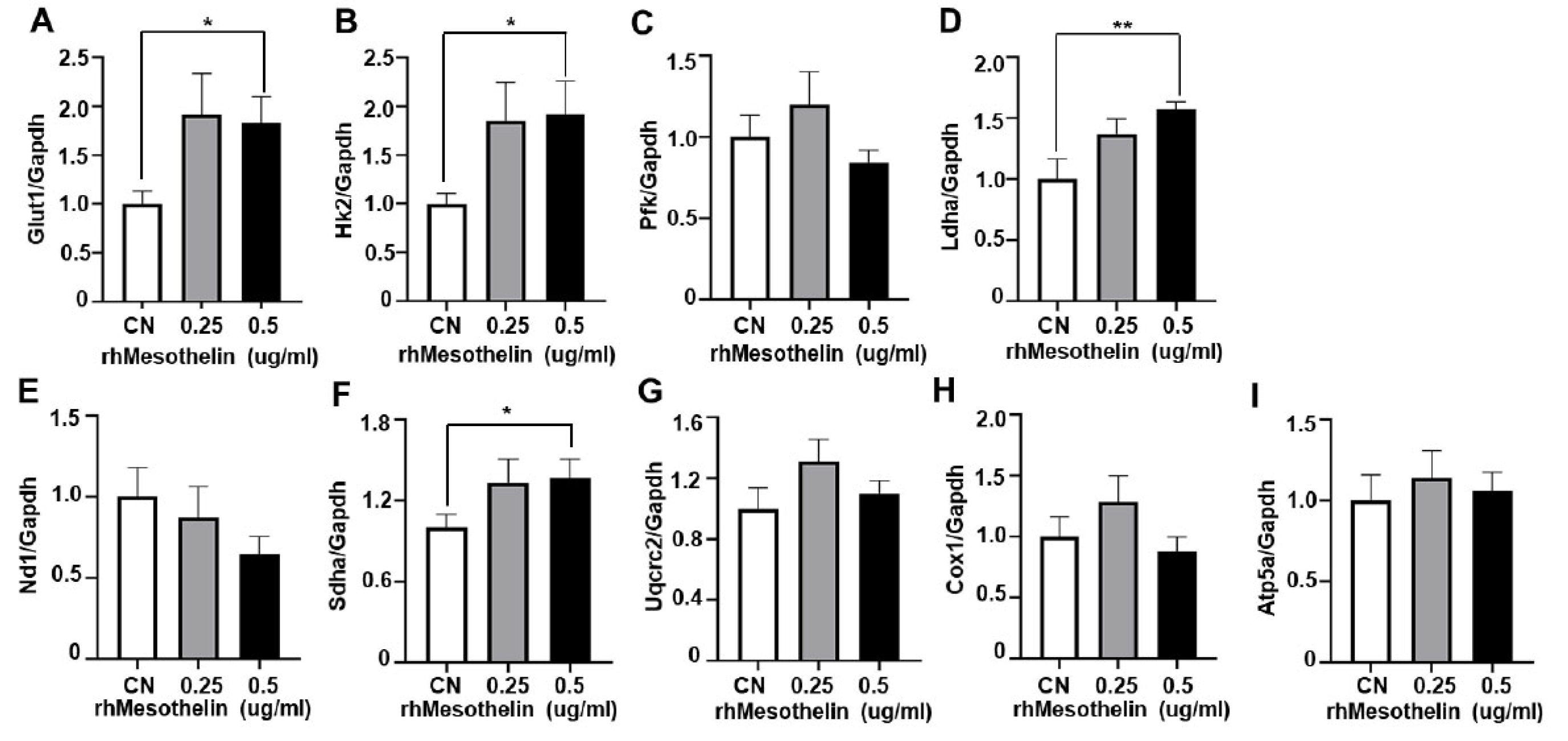
| Age, median (range) | 59 (19-78) |
|---|---|
| Gender, M:F (%) | 13:10 |
| ELN classification AML with recurrent genetic abnormalities AML with mutated TP53 AML with MR gene mutations AML with MR cytogenetic abnormalities AML, NOS |
22 (95.7%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1 (4.3%) |
| NCCN risk stratification Favorable Intermediate Poor |
8 (34.8%) 11 (47.8%) 4 (17.4%) |
| Peripheral blood tests, median (range) | |
| White blood cell count, x103/uL | 5.49 (0.0095-163.9) |
| Hemoglobin (g/dL) | 8.9 (5.3-12.8) |
| Platelet count, x103/uL | 52 (9.0-266) |
| Absolute neutrophil count, x103/uL | 0.61 (0.1-7.6) |
| LDH (IU/L) | 1023 (389-11000) |
| BM blast, median (range), % | 68.6 (20.2-95.2) |
| Cytogenetics | |
| inv(16)/t(16;16) | 6 (26.0%) |
| t(8;21) | 10 (43.5%) |
| 11q23/KMT2A rearrangements | 4 (17.4%) |
| Somatic mutations FLT3-ITD FLT3-TKD NPM1 DNMT3A IDH2 TET2 NRAS WT1 PTPN11 KIT U2AF1 KRAS ASXL1 SMC3 SRSF2 CBL KMT2C NOTCH2 |
4 (17.4%) 2 (8.7%) 2 (8.7%) 1 (4.3%) 2 (8.7%) 2 (8.7%) 5 (21.7%) 1 (4.3%) 1 (4.3%) 9 (39.1%) 1 (4.3%) 1 (4.3%) 1 (4.3%) 2 (8.7%) 1 (4.3%) 2 (8.7%) 2 (8.7%) 3 (13.0%) |
| Disease status at end of induction (evaluable) | |
| 1st CR | 17 (73.9%) |
| 2nd CR | 0 (0.0%) |
| 3rd CR | 0 (0.0%) |
| Persistent | 2 (8.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





