Submitted:
27 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Native form of hydroxyapatite: description of structure and functions in the body
2. Hydroxyapatite as a bone grafting material
- The range of values of the structural parameter of the bone implant. Bone grafts have different ability to withstand mechanical loading. For unmodified grafts based on cortical bone a relatively high resistance to mechanical loads is shown in comparison with grafts based on cancellous bone. Thus, the mechanical properties of bone grafts without additional modification directly depend on the properties of the donor site [39].
- Spectrum of osteogenic properties. The ability of the graft to initiate neoosteogenesis [40] due to the preserved pool of graft cells including osteoblast precursors [41] is extremely important. Due to the presence of cellular elements, necessary growth factors and matrix framework, such a graft is able to modulate angiogenesis, adequate perfusion [42,43] and activity of progenitor cells [44].
- Osteoconductive properties. Ability to provide an optimal environment for normal metabolism, proliferation and differentiation of cell populations [48].
3. Native substitutes for autologous bone grafting material: allogeneic and xenogeneic bone grafts
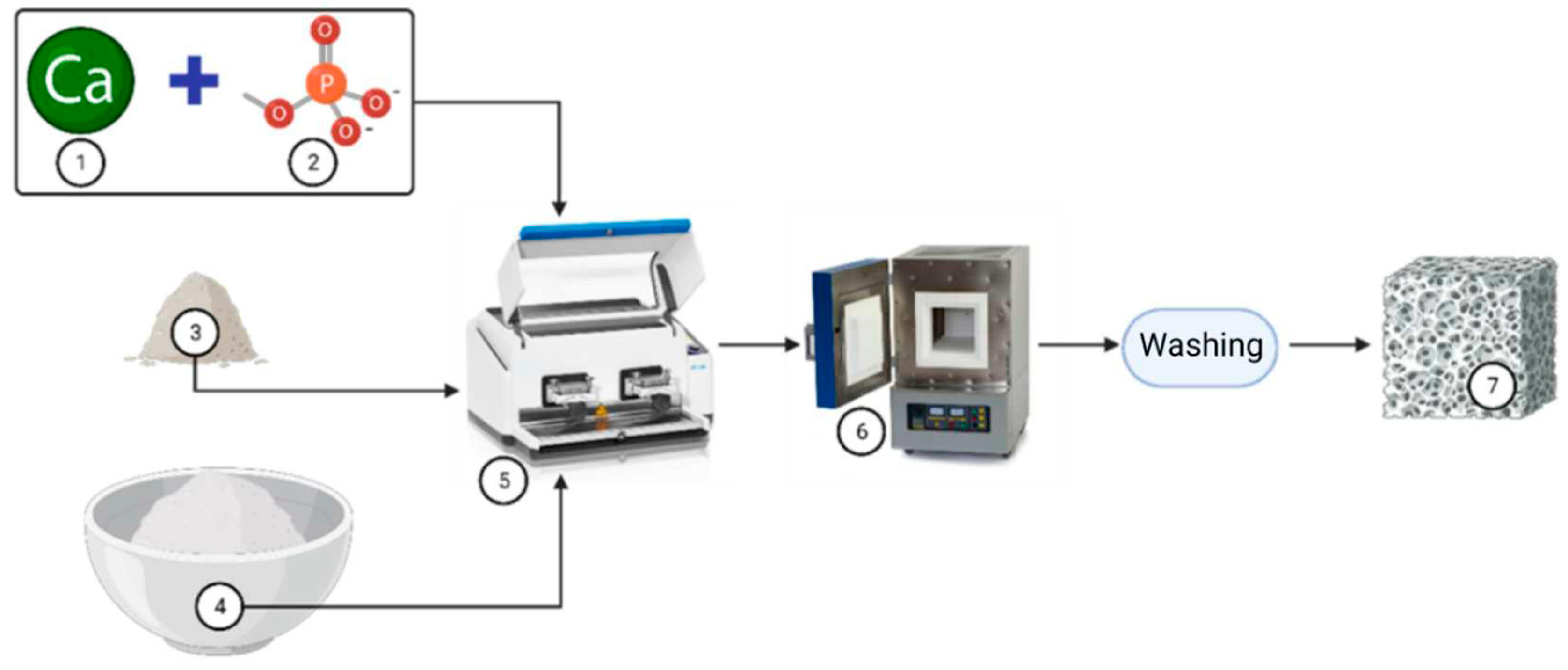
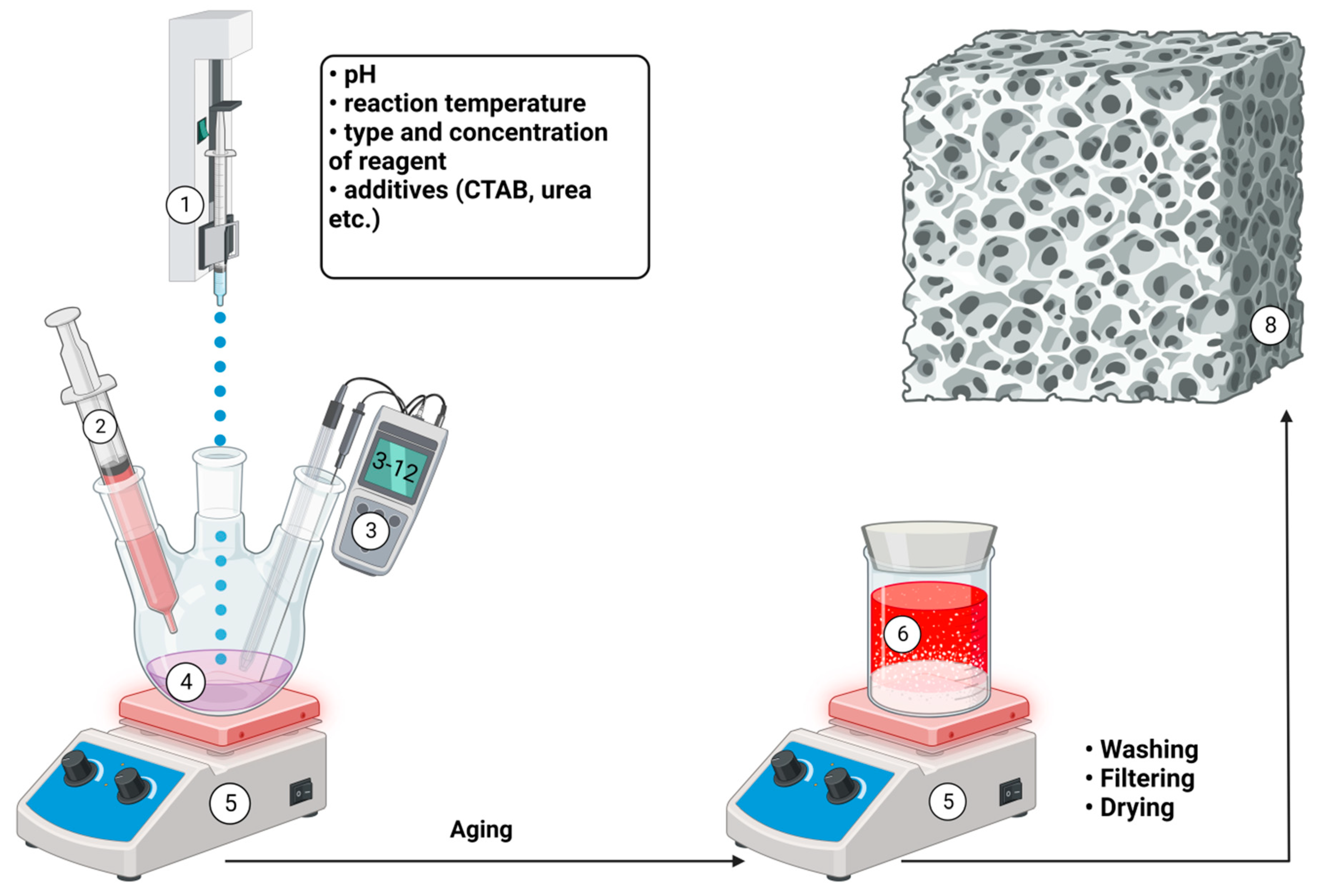
4. Semisynthetic and synthetic bone substitutes
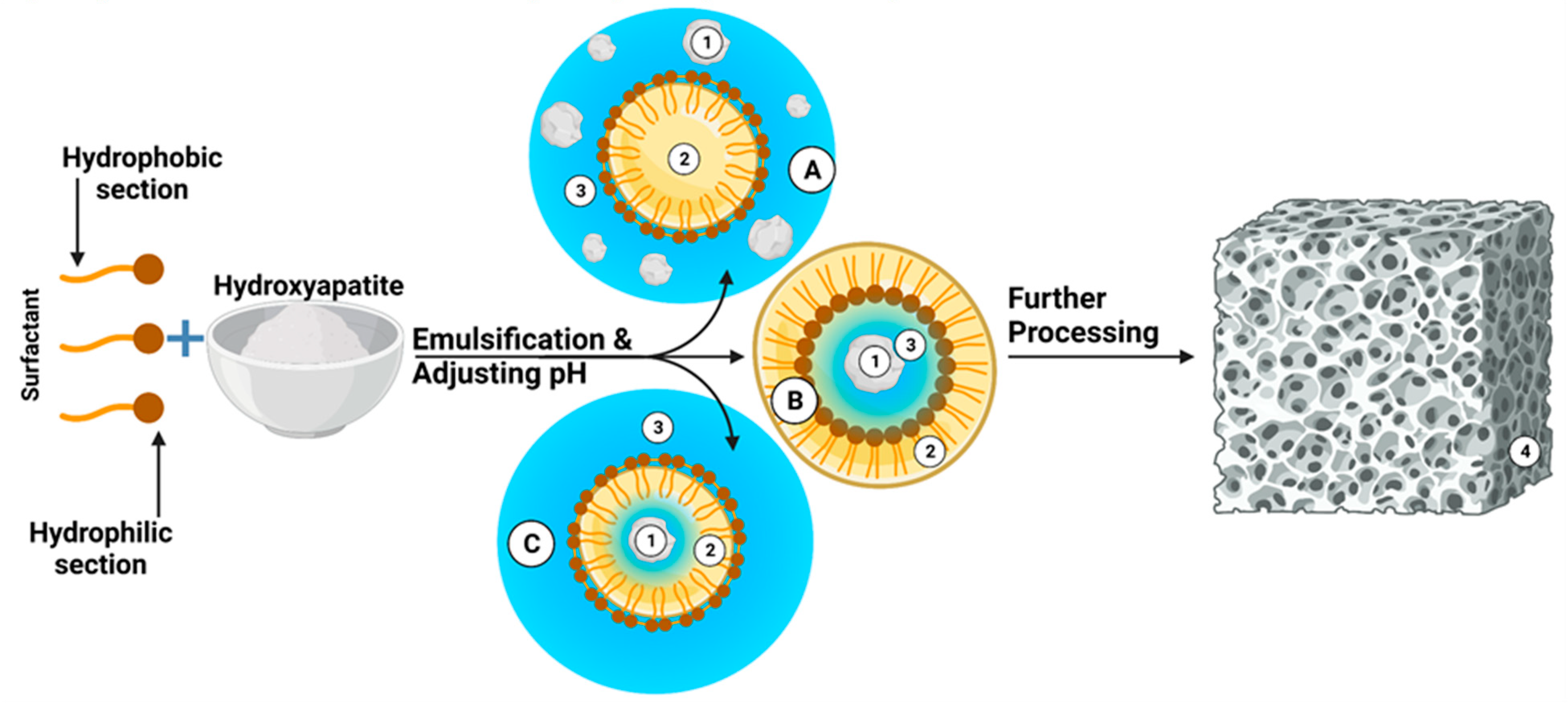
Semi-synthetic and synthetic bone substitutes in research and clinical practice
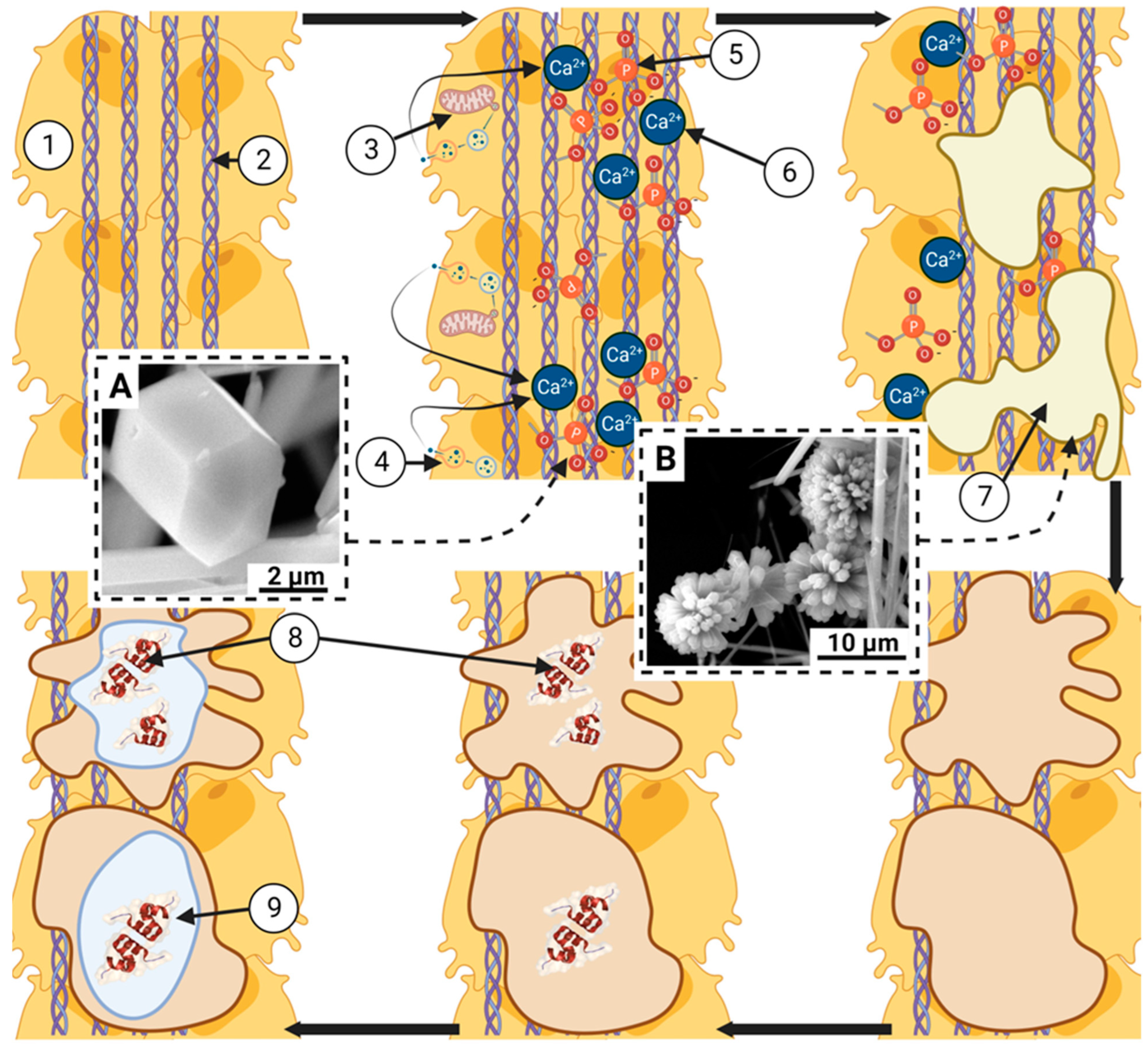
5. Interaction between mononuclear phagocytic cells and hydroxyapatite-based transplant material
6. Conclusion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luedemann, R.E. , Thomas, K.A., Cook, S.D. Particulate aluminum oxide as a bone graft material. Biomater Med Devices Artif Organs 1986, 14, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M. , Son, J.S., Kang, S.S., Kim, G., Choi, S.H. Bone regeneration of hydroxyapatite/alumina bilayered scaffold with 3 mm passage-like medullary canal in canine tibia model. Biomed Res Int 2015, 2015, 235108. [Google Scholar] [CrossRef]
- Pujari, S. , Hoess, A., Shen, J., Thormann, A., Heilmann, A., Tang, L., Karlsson-Ott, M. Effects of nanoporous alumina on inflammatory cell response. J Biomed Mater Res A 2014, 102, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Mahon, O.R. , Browe, D.C., Gonzalez-Fernandez, T., Pitacco, P., Whelan, I.T., Von Euw, S., Hobbs, C., Nicolosi, V., Cunningham, K.T., Mills, K.H.G., Kelly, D.J., Dunne, A. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 2020, 239, 119833. [Google Scholar] [CrossRef]
- Wang, R. , Hua, Y., Wu, H., Wang, J., Xiao, Y.C., Chen, X., Ao, Q., Zeng, Q., Zhu, X., Zhang, X. Hydroxyapatite nanoparticles promote TLR4 agonist-mediated anti-tumor immunity through synergically enhanced macrophage polarization. Acta Biomater 2023, 164, 626–640. [Google Scholar] [CrossRef]
- Burr, D.B. Changes in bone matrix properties with aging. Bone 2019, 120, 85–93. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A. , Drabczyk, A., Florkiewicz, W., Głąb, M., Kudłacik-Kramarczyk, S., Słota, D., Tomala, A., Tyliszczak, B. Review of the Applications of Biomedical Compositions Containing Hydroxyapatite and Collagen Modified by Bioactive Components. Materials (Basel) 2021, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Veremeev, A. , Bolgarin R., Nesterenko V., Andreev-Andrievskiy A., Kutikhin A. Native Bovine Hydroxyapatite Powder, Demineralised Bone Matrix Powder, and Purified Bone Collagen Membranes Are Efficient in Repair of Critical-Sized Rat Calvarial Defects. Materials (Basel) 2020, 13, 3393. [Google Scholar] [CrossRef] [PubMed]
- Indurkar, A. , Choudhary R., Rubenis K., Locs J. Role of carboxylic organic molecules in interfibrillar collagen mineralization. Front Bioeng Biotechnol 2023, 11, 1150037. [Google Scholar] [CrossRef]
- Simon, P. , Grüner D., Worch H., Pompe W., Lichte H., El Khassawna T., Heiss C., Wenisch S., Kniep R. First evidence of octacalcium phosphate@osteocalcin nanocomplex as skeletal bone component directing collagen triple-helix nanofibril mineralization. Sci Rep 2018, 8, 13696. [Google Scholar] [CrossRef]
- Carvalho, M.S. , Cabral J.M.S., da Silva C.L., Vashishth D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers (Basel) 2021, 13, 1095. [Google Scholar] [CrossRef]
- Xu, Z. , Yang C., Wu F., Tan X., Guo Y., Zhang H., Wang H., Sui X., Xu Z., Zhao M., Jiang S., Dai Z., Li Y. Triple-gene deletion for osteocalcin significantly impairs the alignment of hydroxyapatite crystals and collagen in mice. Front Physiol. 2023, 14, 1136561. [Google Scholar] [CrossRef]
- Koblenzer, M. , Weiler M., Fragoulis A., Rütten S., Pufe T., Jahr H. Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model. Cells 2022, 11, 2702. [Google Scholar] [CrossRef]
- Pastero, L. , Bruno M., Aquilano D. Habit Change of Monoclinic Hydroxyapatite Crystals Growing from Aqueous Solution in the Presence of Citrate Ions: The Role of 2D Epitaxy. Crystals 2018, 8, 308. [Google Scholar] [CrossRef]
- Nudelman, F. , Pieterse K., George A., Bomans P.H., Friedrich H., Brylka L.J., Hilbers P.A., de With G., Sommerdijk N.A. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Kawska, A. , Hochrein O., Brickmann J., Kniep R., Zahn D. The nucleation mechanism of fluorapatite-collagen composites: ion association and motif control by collagen proteins. Angew Chem Int Ed Engl 2008, 47, 4982–4985. [Google Scholar] [CrossRef]
- Moriishi, T. , Ozasa R., Ishimoto T., Nakano T., Hasegawa T., Miyazaki T., Liu W., Fukuyama R., Wang Y., Komori H., Qin X., Amizuka N., Komori T. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet 2020, 16, e1008586. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. What is the function of osteocalcin? J Oral Biosci 2020, 62, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. , Zhang Z., Pan H. Bone Apatite Nanocrystal: Crystalline Structure, Chemical Composition, and Architecture. Biomimetics (Basel) 2023, 8, 90. [Google Scholar] [CrossRef]
- Fleet, M.E. The carbonate ion in hydroxyapatite: recent X-ray and infrared results. Front Biosci (Elite Ed) 2013, 5, 643–652. [Google Scholar] [CrossRef]
- Bonar, L.C. , Shimizu M., Roberts J.E., Griffin R.G., Glimcher M.J. Structural and composition studies on the mineral of newly formed dental enamel: a chemical, x-ray diffraction, and 31P and proton nuclear magnetic resonance study. J Bone Miner Res 1991, 6, 1167–1176. [Google Scholar] [CrossRef]
- Bang, L.T. , Long B.D., Othman R. Carbonate hydroxyapatite and silicon-substituted carbonate hydroxyapatite: synthesis, mechanical properties, and solubility evaluations. ScientificWorldJournal 2014, 2014, 969876. [Google Scholar] [CrossRef]
- Saghiri, M.A. , Vakhnovetsky J., Vakhnovetsky A., Ghobrial M., Nath D., Morgano S.M. Functional role of inorganic trace elements in dentin apatite tissue-Part 1: Mg, Sr, Zn, and Fe. J Trace Elem Med Biol 2022, 71, 126932. [Google Scholar] [CrossRef]
- Landis, W.J. , Glimcher M.J. Electron diffraction and electron probe microanalysis of the mineral phase of bone tissue prepared by anhydrous techniques. J Ultrastruct Res 1978, 63, 188–223. [Google Scholar] [CrossRef]
- Bigi, A. , Boanini E., Gazzano M. Ion Substitution in Biological and Synthetic Apatites. 2016, 235–266. [Google Scholar] [CrossRef]
- Bertinetti, L. , Tampieri A., Landi E., Ducati C., Midgley P. A., Coluccia S., Martra G. Surface structure, hydration, and cationic sites of nanohydroxyapatite: UHR-TEM, IR, and microgravimetric studies. Journal of Physical Chemistry C 2007, 111, 4027–4035. [Google Scholar] [CrossRef]
- Bres, E.F. , Steuer P., Voegel J.C., Frank R.M., Cuisinier F.J. Observation of the loss of the hydroxyapatite sixfold symmetry in a human fetal tooth enamel crystal. J. Observation of the loss of the hydroxyapatite sixfold symmetry in a human fetal tooth enamel crystal. J Microsc 1993, 170 (Pt 2), 147–154. [Google Scholar] [CrossRef]
- LeGeros, R.Z. , Ito A., Ishikawa K., Sakae T., LeGeros J.P. Fundamentals of hydroxyapatite and related calcium phosphates. P. Fundamentals of hydroxyapatite and related calcium phosphates. Adv. Biomater. Fundam. Process. Appl. 2009, 19–52. [Google Scholar] [CrossRef]
- Rey, C. , Renugopalakrishnan V., Collins B., Glimcher M.J. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif Tissue Int 1991, 49, 251–258. [Google Scholar] [CrossRef]
- Suzuki, O. , Hamai R., Sakai S. The material design of octacalcium phosphate bone substitute: increased dissolution and osteogenecity. Acta Biomater 2023, 158, 1–11. [Google Scholar] [CrossRef]
- Zhou C., Zhang X., Ai J. et al. Chiral hierarchical structure of bone minerals. Nano Res 2022, 15, 1295–1302. [CrossRef]
- Guarino, V. , Veronesi F., Marrese M., Giavaresi G., Ronca A., Sandri M., Tampieri A., Fini M., Ambrosio L. Needle-like ion-doped hydroxyapatite crystals influence osteogenic properties of PCL composite scaffolds. Biomed Mater 2016, 11, 015018. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. , Hasegawa T., Hongo H., Amizuka N. Alternating lamellar structure in human cellular cementum and rat compact bone: Its structure and formation. J Oral Biosci 2019, 61, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, U.E. , Congiu T., Marchese M., Spagnuolo F., Quacci D. Morphometry and patterns of lamellar bone in human Haversian systems. Anat Rec (Hoboken) 2012, 295, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Locke, M. Structure of long bones in mammals. J Morphol 2004, 262, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury. 2021, 52 Suppl 2, S18–S22. [Google Scholar] [CrossRef]
- Dissaux, C. , Ruffenach L., Bruant-Rodier C., George D., Bodin F., Rémond Y. Cleft Alveolar Bone Graft Materials: Literature Review. Cleft Palate Craniofac J 2022, 59, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V. , Einhorn T.A., Marsh D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M. , Gremse F., Rosenhain S., Bartella A.K., Hölzle F., Kessler P., Lethaus B. Comparison of Bone Grafts From Various Donor Sites in Human Bone Specimens. J Craniofac Surg 2018, 29, 1661–1665. [Google Scholar] [CrossRef]
- Hamada, T. , Matsubara H., Hikichi T., Shimokawa K., Tsuchiya H. Rat model of an autologous cancellous bone graft. Sci Rep 2021, 11, 18001. [Google Scholar] [CrossRef]
- Mehta, D.D. , Dankert J.F., Buchalter D.B., Kirby D.J., Patel K.S., Rocks M., Hacquebord J.H., Leucht P. Iliac Crest and Distal Radius Autografts Exhibit Distinct Cell-Intrinsic Functional Differences. J Hand Surg Am, 0363. [Google Scholar] [CrossRef]
- Stahl, A. , Yang Y.P. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng Part B Rev 2021, 27, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Taqi M., Llewellyn C.M., Estefan M. Fibula Tissue Transfer 2023, In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK563283/.
- Fillingham, Y. , Jacobs J. Bone grafts and their substitutes. Bone Joint J. 2016; 98-B(1 Suppl A):6-9. [CrossRef]
- Street, M. , Gao R., Martis W., Munro J., Musson D., Cornish J., Ferguson J. The Efficacy of Local Autologous Bone Dust: A Systematic Review. Spine Deform 2017, 5, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hazballa, D. , Inchingolo A.D., Inchingolo A.M., Malcangi G., Santacroce L., Minetti E., Di Venere D., Limongelli L., Bordea I.R., Scarano A., Lorusso F., Xhajanka E., Laforgia A., Inchingolo F., Greco Lucchina A., Dipalma G. The effectiveness of autologous demineralized tooth graft for the bone ridge preservation: a systematic review of the literature. J Biol Regul Homeost Agents, 2021; 35, 283–294. [Google Scholar] [CrossRef]
- Peterson, J.R. , Chen F., Nwankwo E., Dekker T.J., Adams S.B. The Use of Bone Grafts, Bone Graft Substitutes, and Orthobiologics for Osseous Healing in Foot and Ankle Surgery. Foot Ankle Orthop 2019, 4, 2473011419849019. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A. , Alkhutari A.S., Abotaleb B., Altairi N.H., Del Fabbro M. Do osteoconductive bone substitutes result in similar bone regeneration for maxillary sinus augmentation when compared to osteogenic and osteoinductive bone grafts? A systematic review and frequentist network meta-analysis. Int J Oral Maxillofac Surg 2020, 49, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Green, J. History and development of suction-irrigation-reaming. Injury 2010, 41 Suppl 2, S24-31. [Google Scholar] [CrossRef]
- van de Wall, B.J.M. , Beeres F.J.P., Rompen I.F., Link B.C., Babst R., Schoeneberg C., Michelitsch C., Nebelung S., Pape H.C., Gueorguiev B., Knobe M. RIA versus iliac crest bone graft harvesting: A meta-analysis and systematic review. Injury 2022, 53, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Laubach M., Weimer L.P., Bläsius F.M., Hildebrand F., Kobbe P., Hutmacher D.W. Complications associated using the reamer-irrigator -aspirator (RIA) system: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2023, 143, 3823–3843. [CrossRef] [PubMed]
- Chen, N.T. , Glowacki J., Bucky L.P., Hong H.Z., Kim W.K., Yaremchuk M.J. The roles of revascularization and resorption on endurance of craniofacial onlay bone grafts in the rabbit. Plast Reconstr Surg 1994, 93, 714–722. [Google Scholar] [CrossRef]
- Burchardt, H. The biology of bone graft repair. Clin Orthop Relat Res. 1983, 28–42. [Google Scholar] [CrossRef]
- Ku, J.K. , Ghim M.S., Park J.H., Leem D.H. A ramus cortical bone harvesting technique without bone marrow invasion. J Korean Assoc Oral Maxillofac Surg 2023, 49, 100–104. [Google Scholar] [CrossRef]
- Vandeputte, T. , Bigorre M., Tramini P., Captier G. Comparison between combined cortical and cancellous bone graft and cancellous bone graft in alveolar cleft: Retrospective study of complications during the first six months post-surgery. J Craniomaxillofac Surg 2020, 48, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.K.E. , Jukic C.C., Zedler S.T. Management of an extensive equine juvenile ossifying fibroma by rostral mandibulectomy and reconstruction of the mandibular symphysis using String of Pearls plates with cortical and cancellous bone autografts. Vet Surg 2019, 48, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kobbe, P. , Laubach M., Hutmacher D.W., Alabdulrahman H., Sellei R.M., Hildebrand F. Convergence of scaffold-guided bone regeneration and RIA bone grafting for the treatment of a critical-sized bone defect of the femoral shaft. Eur J Med Res 2020, 25, 70. [Google Scholar] [CrossRef] [PubMed]
- Ehredt, D.J. Jr,, Rogers B., Takhar J., Payton P., Siesel K. Percutaneous Harvest of Calcaneal Bone Autograft: Quantification of Volume and Definition of Anatomical Safe Zone. J Foot Ankle Surg 2022, 61, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R. , Mataliotakis G.I., Angoules A.G., Kanakaris N.K., Giannoudis P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury, 2011; 42, S3-15. [Google Scholar] [CrossRef]
- Suda, A.J. , Schamberger C.T., Viergutz T. Donor site complications following anterior iliac crest bone graft for treatment of distal radius fractures. Arch Orthop Trauma Surg 2019, 139, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Li, G. , Li P., Chen Q., Thu H.E., Hussain Z. Current Updates on Bone Grafting Biomaterials and Recombinant Human Growth Factors Implanted Biotherapy for Spinal Fusion: A Review of Human Clinical Studies. Curr Drug Deliv 2019, 16, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R. , Matthies L., Windisch P., Gosau M., Jung R., Brodala N., Stefanini M., Kleinheinz J., Payer M., Henningsen A., Al-Nawas B., Knipfer C. Horizontal augmentation techniques in the mandible: a systematic review. Int J Implant Dent 2022, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M. , Kheradmandi R., Salehi M., Alizadeh M., Ten Hagen T.L.M., Falahati M.; Criteria, Challenges, and Opportunities for Acellularized Allogeneic/Xenogeneic Bone Grafts in Bone Repairing. ACS Biomater Sci Eng 2022, 8, 3199–3219. [Google Scholar] [CrossRef]
- Salem, D. , Alshihri A., Arguello E., Jung R.E., Mohmed H.A., Friedland B. Volumetric Analysis of Allogenic and Xenogenic Bone Substitutes Used in Maxillary Sinus Augmentations Utilizing Cone Beam CT: A Prospective Randomized Pilot Study. Int J Oral Maxillofac Implants 2019, 34, 920–926. [Google Scholar] [CrossRef]
- Govoni, M. , Vivarelli L., Mazzotta A., Stagni C., Maso A., Dallari D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials (Basel) 2021, 14, 3290. [Google Scholar] [CrossRef]
- Jordana, F. , Le Visage C., Weiss P. Substituts osseux [Bone substitutes]. Med Sci (Paris) 2017, 33, 60–65. [Google Scholar] [CrossRef]
- Lomas, R. , Chandrasekar A., Board T.N. Bone allograft in the U.K.: perceptions and realities. Hip Int 2013, 23, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Scheufler, K.M. , Diesing D. Einsatz von Knochenersatzmaterialien bei Fusionen der Wirbelsäule [Use of bone graft replacement in spinal fusions]. Orthopade 2015, 44, 146–153. [Google Scholar] [CrossRef]
- Brink, O. The choice between allograft or demineralized bone matrix is not unambiguous in trauma surgery. Injury 2021, 52 Suppl 2, S23–S28. [Google Scholar] [CrossRef]
- Windhager, R. , Hobusch G.M., Matzner M. Allogene Transplantate für biologische Rekonstruktionen von Knochendefekten [Allogeneic transplants for biological reconstruction of bone defects]. Orthopade 2017, 46, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L. , Pietrzak W.S., Blanton M.W. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg 2005, 16, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Kotelnikov, G.P. , Kolsanov A.V., Volova L.T., Trunin D.A., Popov N.V., Nikolaenko A.N., Stepanov G.V. Tekhnologiia proizvodstva personifitsirovannogo rekonstruktivnogo allogennogo kostnogo implantata [Technology of manufacturing of personalized reconstructive allogenic bone graft (in Russian only)]. Khirurgiia (Mosk) 2019, 65-72. Russian. [CrossRef]
- Weinraub, G.M. Orthobiologics: a survey of materials and techniques. Clin Podiatr Med Surg 2005, 22, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E. , Doll B.A., Futrell F.W., Schmitz J.P., Hollinger J.O. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- Dinopoulos, H.T. , Giannoudis P.V. Safety and efficacy of use of demineralised bone matrix in orthopaedic and trauma surgery. Expert Opin Drug Saf 2006, 5, 847–866. [Google Scholar] [CrossRef]
- Wu, J. , Liu F., Wang Z., Liu Y., Zhao X., Fang C., Leung F., Yeung K.W.K., Wong T.M. The Development of a Magnesium-Releasing and Long-Term Mechanically Stable Calcium Phosphate Bone Cement Possessing Osteogenic and Immunomodulation Effects for Promoting Bone Fracture Regeneration. Front Bioeng Biotechnol 2022, 9, 803723. [Google Scholar] [CrossRef]
- Würdinger, R. , Donkiewicz P. Allogeneic cortical struts and bone granules for challenging alveolar reconstructions: An innovative approach toward an established technique. J Esthet Restor Dent 2020, 32, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Bieganowski, T. , Buchalter D.B., Singh V., Mercuri J.J., Aggarwal V.K., Rozell J.C., Schwarzkopf R. Bone loss in aseptic revision total knee arthroplasty: management and outcomes. Knee Surg Relat Res 2022, 34, 30. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B. , Kadow-Romacker A., Pruss A., Haas N.P., Schmidmaier G. Quantification of growth factors in allogenic bone grafts extracted with three different methods. Cell Tissue Bank 2007, 8, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, T.J. , Arts J.J. Bioactive and osteoinductive bone graft substitutes: definitions, facts and myths. Injury 2011; 42 Suppl 2:S26-9. [CrossRef]
- Rocha, T. , Cavalcanti A.S., Leal A.C., Dias R.B., da Costa R.S., Ribeiro G.O., Guimarães J.A.M., Duarte M.E.L. PTH1-34 improves devitalized allogenic bone graft healing in a murine femoral critical size defect. Injury 2021, 52 Suppl 3, S3–S12. [Google Scholar] [CrossRef]
- Golubovsky J.L., Ejikeme T., Winkelman R., Steinmetz M.P. Osteobiologics. Oper Neurosurg (Hagerstown) 2021, 21(Suppl 1):S2-S9. [CrossRef]
- Veronesi, F. , Martini L., Giavaresi G., Fini M. Bone regenerative medicine: metatarsus defects in sheep to evaluate new therapeutic strategies for human long bone defect. A systematic review. Injury 2020, 51, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Shih, S. , Askinas C., Caughey S., Vernice N., Berri N., Dong X., Spector J.A. Sourcing and development of tissue for transplantation in reconstructive surgery: A narrative review. J Plast Reconstr Aesthet Surg 2023, 83, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Płomiński, J. , Kwiatkowski K. Przeszczepy kostne [Bone grafts (editorial)]. Pol Merkur Lekarski 2006, 21, 507–510. [Google Scholar]
- Kwiecien, G.J. , Aliotta R., Bassiri Gharb B., Gastman B., Zins J.E. The Timing of Alloplastic Cranioplasty in the Setting of Previous Osteomyelitis. Plast Reconstr Surg 2019, 143, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Egol, K.A. , Nauth A., Lee M., Pape H.C., Watson J.T., Borrelli J. Jr. Bone Grafting: Sourcing, Timing, Strategies, and Alternatives. J Orthop Trauma 2015; 29 Suppl 12:S10-4. [CrossRef]
- Viola, A. 3rd, Appiah J., Donnally C.J. 3rd, Kim Y.H., Shenoy K. Bone Graft Options in Spinal Fusion: A Review of Current Options and the Use of Mesenchymal Cellular Bone Matrices. World Neurosurg 2022, 158, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Abedi, A. , Formanek B., Russell N., Vizesi F., Boden S.D., Wang J.C., Buser Z. Examination of the Role of Cells in Commercially Available Cellular Allografts in Spine Fusion: An in Vivo Animal Study. J Bone Joint Surg Am 2020, 102, e135. [Google Scholar] [CrossRef]
- Kinaia B.M., Daweri O., Gala R., Turows A., Harunani A., Neely A.L. Management of vertical bony defect using novel xenogeneic/allogeneic bone graft: A case report. Clin Adv Periodontics 2023. [CrossRef]
- Bansal, M.R. , Bhagat S.B., Shukla D.D. Bovine cancellous xenograft in the treatment of tibial plateau fractures in elderly patients. Int Orthop 2009, 33, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Naros, A. , Bayazeed B., Schwarz U., Nagursky H., Reinert S., Schmelzeisen R., Sauerbier S. A prospective histomorphometric and cephalometric comparison of bovine bone substitute and autogenous bone grafting in Le Fort I osteotomies. J Craniomaxillofac Surg 2019, 47, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lim, J. , Jun S.H., Tallarico M., Park J.B., Park D.H., Hwang K.G., Park C.J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials (Basel) 2022, 15, 5294. [Google Scholar] [CrossRef]
- Tawil, G. , Barbeck M., Unger R., Tawil P., Witte F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int J Oral Maxillofac Implants 2018, 33, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y. , Wang L., Su K., Gao J., Li X., Cheng G. Horizontal bone augmentation and simultaneous implant placement using xenogeneic bone rings technique: a retrospective clinical study. Sci Rep 2021, 11, 4947. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N. , Holloway B.K., Jupiter D.C. A comparative study of incorporation rates between non-xenograft and bovine-based structural bone graft in foot and ankle surgery. J Foot Ankle Surg 2014, 53, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N. , Jupiter D.C. Bone graft substitute: allograft and xenograft. Clin Podiatr Med Surg 2015, 32, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F. , Ferrari D., Balic E., Buser D., Becker J., Sager M. Lateral ridge augmentation using equine- and bovine-derived cancellous bone blocks: a feasibility study in dogs. Clin Oral Implants Res 2010, 21, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Ledford, C.K. , Nunley J.A. 2nd, Viens N.A., Lark R.K. Bovine xenograft failures in pediatric foot reconstructive surgery. J Pediatr Orthop 2013, 33, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P. , Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J Orthop Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Ruffilli, A. , Barile F., Fiore M., Manzetti M., Viroli G., Mazzotti A., Govoni M., De Franceschi L., Dallari D., Faldini C. Allogenic bone grafts and postoperative surgical site infection: are positive intraoperative swab cultures predictive for a higher infectious risk? Cell Tissue Bank 2023, 24, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. , Verma A., Jain A., Goyal T., Kandwal P., Arora S.S. Infection and utilization rates of bone allografts in a hospital-based musculoskeletal tissue bank in north India. J Clin Orthop Trauma 2021, 23, 101635. [Google Scholar] [CrossRef] [PubMed]
- Van Der Merwe, W. , Lind M., Faunø P., Van Egmond K., Zaffagnini S., Marcacci M., Cugat R., Verdonk R., Ibañez E., Guillen P., Marcheggiani Muccioli G.M. Xenograft for anterior cruciate ligament reconstruction was associated with high graft processing infection. J Exp Orthop 2020, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M. , Leonidou A., Aslam-Pervez N., Hamza A., Panteliadis P., Heliotis M., Mantalaris A., Tsiridis E. Biological therapy of bone defects: the immunology of bone allo-transplantation. Expert Opin Biol Ther 2010, 10, 885–901. [Google Scholar] [CrossRef]
- Hinsenkamp, M. , Muylle L., Eastlund T., Fehily D., Noël L., Strong D.M. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project NOTIFY. Int Orthop 2012, 36, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Wei L., Ma B.J., Shao J.L., Ge S.H. [Advances in the Application of Hydroxyapatite Composite Materials in Bone Tissue Engineering]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021, 52, 357–363. [CrossRef] [PubMed]
- George, S.M. , Nayak C., Singh I., Balani K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater Sci Eng 2022, 8, 3162–3186. [Google Scholar] [CrossRef] [PubMed]
- Zaed, I. , Cardia A., Stefini R. From Reparative Surgery to Regenerative Surgery: State of the Art of Porous Hydroxyapatite in Cranioplasty. Int J Mol Sci 2022, 23, 5434. [Google Scholar] [CrossRef] [PubMed]
- Sobczyk-Guzenda, A. , Boniecka P., Laska-Lesniewicz A., Makowka M., Szymanowski H. Micro- and Nanoparticulate Hydroxyapatite Powders as Fillers in Polyacrylate Bone Cement-A Comparative Study. Materials (Basel) 2020, 13, 2736. [Google Scholar] [CrossRef]
- Gao C., Peng S., Feng P. et al. Bone biomaterials and interactions with stem cells. Bone Res 2017, 5, 17059. [CrossRef]
- Zimmermann, E.A. , Ritchie R.O. Bone as a Structural Material. Adv Healthc Mater 2015, 4, 1287–1304. [Google Scholar] [CrossRef]
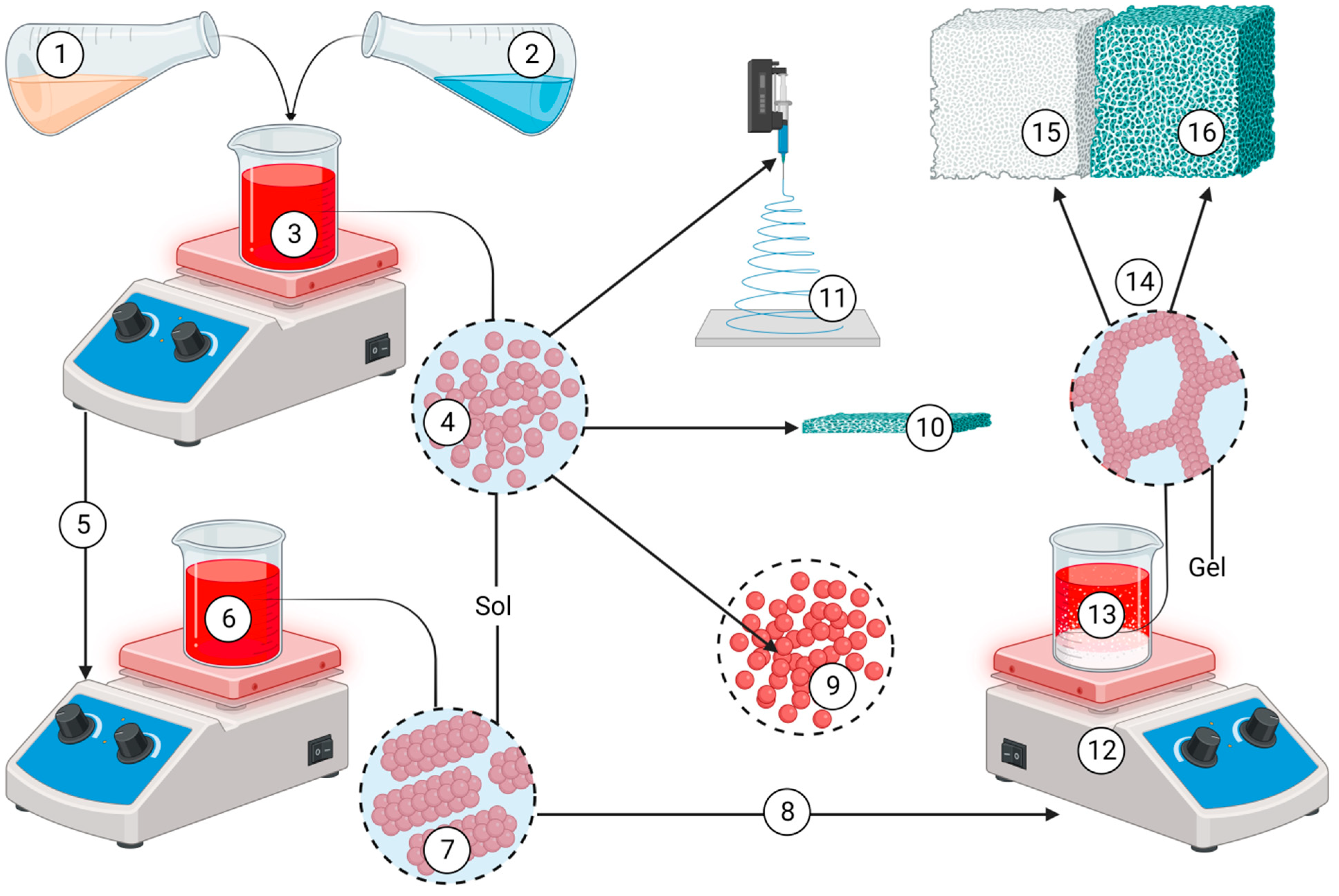
- Kien, P.T. , Phu H.D., Linh N.V.V., Quyen T.N., Hoa N.T. Recent Trends in Hydroxyapatite (HA) Synthesis and the Synthesis Report of Nanostructure HA by Hydrothermal Reaction. Adv Exp Med Biol 2018; 1077:343-354. [CrossRef]
- Fadil, N.A. Synthesis Techniques of Bioceramic Hydroxyapatite for Biomedical Applications. Journal of Biomimetics, Biomaterials and Biomedical Engineering 2023, 59, 59–80. [Google Scholar] [CrossRef]
- Clabel H., J. L., Awan I., Pinto A., Nogueira I., Bezzon V., Leite E. & Balogh D., Mastelaro V., Ferreira S., Marega E. Insights on the mechanism of solid state reaction between TiO 2 and BaCO 3 to produce BaTiO 3 powders: The role of calcination, milling, and mixing solvent. Ceramics International 2019, 46, 2987–3001. [Google Scholar] [CrossRef]
- Sathiyavimal, S. , Vasantharaj S., LewisOscar F., Selvaraj R., Brindhadevi K., Pugazhendhi A. Natural organic and inorganicâ hydroxyapatite biopolymer composite for biomedical applications. Progress in Organic Coatings 2020, 147, 105858. [Google Scholar] [CrossRef]
- Szcześ, A. , Hołysz L., Chibowski E. Synthesis of hydroxyapatite for biomedical applications. Adv Colloid Interface Sci 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Chesley, M. , Kennard R., Roozbahani S., Kim S.M., Kukk K., Mason M. One-step hydrothermal synthesis with in situ milling of biologically relevant hydroxyapatite. Mater Sci Eng C Mater Biol Appl 2020; 113:110962. [CrossRef]
- Fihri, A. , Len C., Varma R., Solhy A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coordination Chemistry Reviews 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Qi, M.L. , He K. , Huang Z.N. et al. Hydroxyapatite Fibers: A Review of Synthesis Methods JOM. 2017, 69, 1354–1360. [Google Scholar] [CrossRef]
- Andrés, N.C. , D'Elía N.L., Ruso J.M., Campelo A.E., Massheimer V.L., Messina P.V. Manipulation of Mg2+-Ca2+ Switch on the Development of Bone Mimetic Hydroxyapatite. ACS Appl Mater Interfaces 2017, 9, 15698–15710. [Google Scholar] [CrossRef]
- Lala, S. , Ghosh M., Das P.K., Kar T., Pradhan S.K. Mechanical preparation of nanocrystalline biocompatible single-phase Mn-doped A-type carbonated hydroxyapatite (A-cHAp): effect of Mn doping on microstructure. Dalton Trans 2015, 44, 20087–20097. [Google Scholar] [CrossRef]
- Wang, M. , Wang L., Shi C., Sun T., Zeng Y., Zhu Y. The crystal structure and chemical state of aluminum-doped hydroxyapatite by experimental and first principles calculation studies. Phys Chem Chem Phys 2016, 18, 21789–21796. [Google Scholar] [CrossRef]
- Kolmas, J. , Kuras M., Oledzka E., Sobczak M. A solid-state NMR study of selenium substitution into nanocrystalline hydroxyapatite. Int J Mol Sci 2015, 16, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J. , Lin H.L., Haung S.M., Liu S.M., Chen W.C. Effect of pH on the In Vitro Biocompatibility of Surfactant-Assisted Synthesis and Hydrothermal Precipitation of Rod-Shaped Nano-Hydroxyapatite. Polymers (Basel) 2021, 13, 2994. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-H. , Lee J.-A., Lee J.-H., Heo Y.-W., Kim J.-J. Effects of pH and reaction temperature on hydroxyapatite powders synthesized by precipitation. Journal of the Korean Ceramic Society 2019, 57, 56–64. [Google Scholar] [CrossRef]
- Wijesinghe, W.P. , Mantilaka M.M., Premalal E.V., Herath H.M., Mahalingam S., Edirisinghe M., Rajapakse R.P., Rajapakse R.M. Facile synthesis of both needle-like and spherical hydroxyapatite nanoparticles: effect of synthetic temperature and calcination on morphology, crystallite size and crystallinity. Mater Sci Eng C Mater Biol Appl 2014, 42, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.R. , Rutledge L., Randolph L.D., Meenan B.J. Strontium-substituted hydroxyapatite coatings deposited via a co-deposition sputter technique. Mater Sci Eng C Mater Biol Appl 2015, 46, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L. , Salma-Ancane K., Stipniece L., Meenan B.J., Boyd A.R. The deposition of strontium and zinc Co-substituted hydroxyapatite coatings. J Mater Sci Mater Med 2017, 28, 51. [Google Scholar] [CrossRef] [PubMed]
- Gu, M. , Li W., Jiang L., Li X. Recent progress of rare earth doped hydroxyapatite nanoparticles: Luminescence properties, synthesis and biomedical applications. Acta Biomater 2022, 148, 22–43. [Google Scholar] [CrossRef] [PubMed]
- LaKrat, M. , Jodati H., Mejdoubi E., Evis Z. Synthesis and characterization of pure and Mg, Cu, Ag, and Sr doped calcium-deficient hydroxyapatite from brushite as precursor using the dissolution-precipitation method. Powder Technology 2022, 413, 118026. [Google Scholar] [CrossRef]
- Shah, R.K. , Fahmi M.N., Mat A.H., Zainal A.A. The synthesis of hydroxyapatite through the precipitation method. Med J Malaysia 2004, 59 Suppl B, 75–76. [Google Scholar]
- Chen, W. , Nichols L., Brinkley F., Bohna K., Tian W., Priddy M.W., Priddy L.B. Alkali treatment facilitates functional nano-hydroxyapatite coating of 3D printed polylactic acid scaffolds. Mater Sci Eng C Mater Biol Appl 2021, 120, 111686. [Google Scholar] [CrossRef]
- Enami, H. , Nakahara I., Ando W., Uemura K., Hamada H., Takao M., Sugano N. Osteocompatibility of Si3N4-coated carbon fiber-reinforced polyetheretherketone (CFRP) and hydroxyapatite-coated CFRP with antibiotics and antithrombotic drugs. J Artif Organs 2023, 26, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Yudyanto, Y. , Utomo J., Rosita R. R., Ariyanto L. P., Fathurochman F., Nasikhudin N., Hartatiek H. Porosity, hardness and microstructure of nano-hydroxyapatite/PEG composite synthesized by co-precipitation temperature variations. AIP Conference Proceedings 2023, 2748, 020002. [Google Scholar] [CrossRef]
- Chen, J. , Yang Y., Etim I.P., Tan L., Yang K., Misra R.D.K., Wang J., Su X. Recent Advances on Development of Hydroxyapatite Coating on Biodegradable Magnesium Alloys: A Review. Materials (Basel) 2021, 14, 5550. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, M. , Nagalakshmi R. Nano Hydroxyapatite for Biomedical Applications Derived from Chemical and Natural Sources by Simple Precipitation Method. Appl Biochem Biotechnol 2023, 195, 3994–4010. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierczyk, F. , Fiołek A., Łukaszczyk A., Kopia A., Sitarz M., Zimowski S., Cieniek Ł., Moskalewicz T. Microstructure and Selected Properties of Advanced Biomedical n-HA/ZnS/Sulfonated PEEK Coatings Fabricated on Zirconium Alloy by Duplex Treatment. Int J Mol Sci 2022, 23, 3244. [Google Scholar] [CrossRef] [PubMed]
- Hu, H. , Lin C., Leng Y. [An investigation of HAP/organic polymer composite coating prepared by electrochemical co-deposition technique (II) characterization of XRD, SEM and mechanic properties]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2003; 20(2):202-4, 236. Chinese. [PubMed]
- Zhang, Y. , Dong K., Wang F., Wang H., Wang J., Jiang Z., Diao S. Three dimensional macroporous hydroxyapatite/chitosan foam-supported polymer micelles for enhanced oral delivery of poorly soluble drugs. Colloids Surf B Biointerfaces 2018, 170, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sivasankari, S. , Kalaivizhi R., Gowriboy N., Ganesh M.R., Shazia Anjum M. Hydroxyapatite integrated with cellulose acetate/polyetherimide composite membrane for biomedical applications. Polymer Composites 2021, 42, 5512–5526. [Google Scholar] [CrossRef]
- Akiyama, N. , Patel K.D., Jang E.J., Shannon M.R., Patel R., Patel M., Perriman A.W. Tubular nanomaterials for bone tissue engineering. J Mater Chem B 2023, 11, 6225–6248. [Google Scholar] [CrossRef]
- Adamu D., B. Synthesis and characterization of bismuth-doped hydroxyapatite nanorods for fluoride removal. Environmental Advances 2023, 12, 100360. [Google Scholar] [CrossRef]
- Asri, R.I. , Harun W.S., Hassan M.A., Ghani S.A., Buyong Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J Mech Behav Biomed Mater 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Kuo M., C. , Yen S. K. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature". Materials Science and Engineering C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Kumar, S. , Gupta R.K., Archana K. et al. Development of Ternary Hydroxyapatite-Al2O3-TiO2 Nanocomposite Coating on Mg Alloy by Electrophoretic Deposition Method. J. of Materi Eng and Perform. [CrossRef]
- Baheti, W. , Lv S., Mila, Ma L., Amantai D., Sun H., He H. Graphene/hydroxyapatite coating deposit on titanium alloys for implant application. J Appl Biomater Funct Mater 2023, 21, 22808000221148104. [Google Scholar] [CrossRef] [PubMed]
- Elias, L. , Hegde A.C. Electrodeposited Metallic/Nanocomposite Coatings. Advances in Corrosion Control of Magnesium and its Alloys. CRC Press. С. [CrossRef]
- Li, G. , Song Y., Chen X., Xu W., Tong G., Zhang L., Li J., Zhu X. Preparation, Corrosion Behavior and Biocompatibility of MgFe-Layered Double Hydroxides and Calcium Hydroxyapatite Composite Films on 316 L Stainless Steel. Materials Today Communications 2022, 34, 105195. [Google Scholar] [CrossRef]
- Sundaramali, G. , Aiyasamy J., Karthikeyan S., Kandavel T., Arulmurugan B., Rajkumar S., Sharma S., Li C., Dwivedi S., Kumar A., Singh R., Eldin S. Experimental investigations of electrodeposited Zn–Ni, Zn–Co, and Ni–Cr–Co–based novel coatings on AA7075 substrate to ameliorate the mechanical, abrasion, morphological, and corrosion properties for automotive applications. Reviews on advanced materials science 2023, 62, 20220324. [Google Scholar] [CrossRef]
- Al-Noaman, A. , Rawlinson S.C.F. Polyether ether ketone coated with nanohydroxyapatite/graphene oxide composite promotes bioactivity and antibacterial activity at the surface of the material. European Journal of Oral Sciences, 1294. [Google Scholar] [CrossRef]
- Gao, Q. , Zhang L., Chen Y., Nie H., Zhang B., Li H. Interfacial Design and Construction of Carbon Fiber Composites by Strongly Bound Hydroxyapatite Nanobelt-Carbon Nanotubes for Biological Applications. ACS Appl Bio Mater 2023, 6, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J. , Jang J.M. Electrodeposition of hydroxyapatite nanoparticles onto ultra-fine TiO2 nanotube layer by electrochemical reaction in mixed electrolyte. J Nanosci Nanotechnol 2011, 11, 7167–7171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. , Qiang L., Liu Y., Fan M., Si X., Zheng P. Biomaterial-assisted tumor therapy: A brief review of hydroxyapatite nanoparticles and its composites used in bone tumors therapy. Front Bioeng Biotechnol 2023, 11, 1167474. [Google Scholar] [CrossRef]
- Kargozar, S. , Mollazadeh S., Kermani F., Webster T.J., Nazarnezhad S., Hamzehlou S., Baino F. Hydroxyapatite Nanoparticles for Improved Cancer Theranostics. J Funct Biomater 2022, 13, 100. [Google Scholar] [CrossRef]
- Gómora-Figueroa A., P. Oil emulsions in naturally fractured Porous Media. Petroleum 2019, 5, 215–226. [Google Scholar] [CrossRef]
- Liang, Q. Surfactant-assisted synthesis of photocatalysts: Mechanism, synthesis, recent advances and environmental application. Chemical engineering journal 2019, 372, 429–451. [Google Scholar] [CrossRef]
- Sadat-Shojai, M. , Khorasani M.T., Dinpanah-Khoshdargi E., Jamshidi A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Ioiţescu, A. Synthesis and characterization of hydroxyapatite obtained from different organic precursors by sol–gel method. Journal of thermal analysis and calorimetry 2009, 96, 937–942. [Google Scholar] [CrossRef]
- Yun, Y.H. , Lee J.K. Sol-Gel Coating of Hydroxyapatite on Zirconia Substrate. J Nanosci Nanotechnol 2021, 21, 4169–4173. [Google Scholar] [CrossRef]
- Jaafar, A. , Hecker C., Árki P., Joseph Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering (Basel) 2020, 7, 127. [Google Scholar] [CrossRef]
- Ishikawa, K. , Garskaite E., Kareiva A. Sol–gel synthesis of calcium phosphate-based biomaterials—A review of environmentally benign, simple, and effective synthesis routes. Journal of Sol-Gel Science and Technology 2020, 94, 551–572. [Google Scholar] [CrossRef]
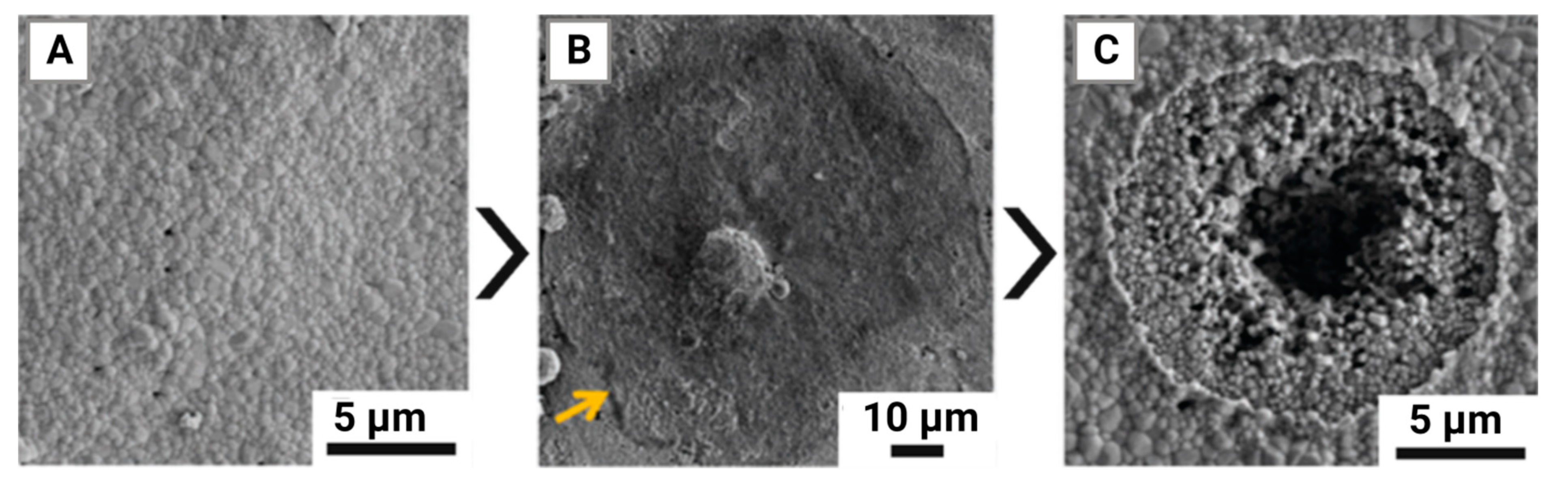
- Molino, G. Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application: A short review. Biomedical Materials 2020, 15, 022001. [Google Scholar] [CrossRef]
- Hernández-Barreto, D.F. , Hernández-Cocoletzi H., Moreno-Piraján J.C. Biogenic Hydroxyapatite Obtained from Bone Wastes Using CO2-Assisted Pyrolysis and Its Interaction with Glyphosate: A Computational and Experimental Study. ACS Omega 2022, 7, 23265–23275. [Google Scholar] [CrossRef]
- Amna, T. Valorization of Bone Waste of Saudi Arabia by Synthesizing Hydroxyapatite. Appl Biochem Biotechnol 2018, 186, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C. , Hsu H.C., Wang H.F., Liou S.P., Ho W.F. Synthesis and Characterization of Nano-Hydroxyapatite Obtained from Eggshell via the Hydrothermal Process and the Precipitation Method. Molecules 2023, 28, 4926. [Google Scholar] [CrossRef]
- Patel, D.K. , Jin B., Dutta S.D., Lim K.T. Osteogenic potential of human mesenchymal stem cells on eggshells-derived hydroxyapatite nanoparticles for tissue engineering. J Biomed Mater Res B Appl Biomater 2020, 108, 1953–1960. [Google Scholar] [CrossRef]
- Prado, J.P.D.S. , Yamamura H., Magri A.M.P., Ruiz P.L.M., Prado J.L.D.S., Rennó A.C.M., Ribeiro D.A., Granito R.N. In vitro and in vivo biological performance of hydroxyapatite from fish waste. J Mater Sci Mater Med 2021, 32, 109. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.Y. , Safwat N., Shehata R., Althubaiti E.H., Kareem S., Atef A., Qari S.H., Aljahani A.H., Al-Meshal A.S., Youssef M., Sami R. Synthesis of Natural Nano-Hydroxyapatite from Snail Shells and Its Biological Activity: Antimicrobial, Antibiofilm, and Biocompatibility. Membranes (Basel) 2022, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Granito, R.N. , Muniz Renno A.C., Yamamura H., de Almeida M.C., Menin Ruiz P.L., Ribeiro D.A. Hydroxyapatite from Fish for Bone Tissue Engineering: A Promising Approach. Int J Mol Cell Med 2018, 7, 80–90. [Google Scholar] [CrossRef]
- Kodali, D. , Hembrick-Holloman V., Gunturu D.R., Samuel T., Jeelani S., Rangari V.K. Influence of Fish Scale-Based Hydroxyapatite on Forcespun Polycaprolactone Fiber Scaffolds. ACS Omega 2022, 7, 8323–8335. [Google Scholar] [CrossRef]
- Shaltout, A.A. , Allam M.A., Moharram M.A. FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim Acta A Mol Biomol Spectrosc 2011, 83, 56–60. [Google Scholar] [CrossRef]
- Pradeep, S. , Jain A.S., Dharmashekara C., Prasad S.K., Akshatha N., Pruthvish R., Amachawadi R.G., Srinivasa C., Syed A., Elgorban A.M., Al Kheraif A.A., Ortega-Castro J., Frau J., Flores-Holguín N., Shivamallu C., Kollur S.P., Glossman-Mitnik D. Synthesis, Computational Pharmacokinetics Report, Conceptual DFT-Based Calculations and Anti-Acetylcholinesterase Activity of Hydroxyapatite Nanoparticles Derived From Acorus Calamus Plant Extract. Front Chem. 2021, 9, 741037. [Google Scholar] [CrossRef]
- Ghate, P. , Prabhu S.D., Murugesan G., Goveas L.C., Varadavenkatesan T., Vinayagam R., Lan Chi N.T., Pugazhendhi A., Selvaraj R. Synthesis of hydroxyapatite nanoparticles using Acacia falcata leaf extract and study of their anti-cancerous activity against cancerous mammalian cell lines. Environ Res 2022, 214 Pt 2. [Google Scholar] [CrossRef] [PubMed]
- Susanto, H. The Characterization of Green Materials of Moringa oleifera Leaf Powder (MOLP) from Madura Island with Different Preparation Methods. IOP Conference Series: Earth and Environmental Science. IOP Publishing 2019, 276, 012005. [Google Scholar] [CrossRef]
- Domene-López, D. , Delgado-Marín J.J., Martin-Gullon I., García-Quesada J.C., Montalbán M.G. Comparative study on properties of starch films obtained from potato, corn and wheat using 1-ethyl-3-methylimidazolium acetate as plasticizer. Int J Biol Macromol 2019, 135, 845–854. [Google Scholar] [CrossRef]
- Khlestkin, V.K. , Rozanova I.V., Efimov V.M., Khlestkina E.K. Starch phosphorylation associated SNPs found by genome-wide association studies in the potato (Solanum tuberosum L.). BMC Genet. [CrossRef]
- Manoj, M. A plant-mediated synthesis of nanostructured hydroxyapatite for biomedical applications: a review. RSC advances 2020, 10, 40923–40939. [Google Scholar] [CrossRef] [PubMed]
- Luo, M. , Li Z., Su M., Gadd G.M., Yin Z., Benton M.J., Pan Y., Zheng D., Zhao T., Li Z., Chen Y. Fungal-induced fossil biomineralization. Curr Biol 2023, 33, 2417–2424. [Google Scholar] [CrossRef] [PubMed]
- Shi, D. , Tong H., Lv M., Luo D., Wang P., Xu X., Han Z. Optimization of hydrothermal synthesis of hydroxyapatite from chicken eggshell waste for effective adsorption of aqueous Pb(II). Environ Sci Pollut Res Int 2021, 28, 58189–58205. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M. Physiochemical characterizations of hydroxyapatite extracted from bovine bones by three different methods: extraction of biologically desirable HAp. Materials Science and Engineering: C 2008, 28, 1381–1387. [Google Scholar] [CrossRef]
- Boudreau, S. Isolation of hydroxyapatite from Atlantic salmon processing waste using a protease and lipase mixture. RSC Sustainability 2023. [CrossRef]
- Mohd Pu'ad, N.A.S. , Koshy P., Abdullah H.Z., Idris M.I., Lee T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed]
- Forero-Sossa, P.A. , Salazar-Martínez J.D., Giraldo-Betancur A.L., Segura-Giraldo B., Restrepo-Parra E. Temperature effect in physicochemical and bioactive behavior of biogenic hydroxyapatite obtained from porcine bones. Sci Rep 2021, 11, 11069. [Google Scholar] [CrossRef]
- Horta M. K., S. Hydroxyapatite from biowaste for biomedical applications: obtainment, characterization and in vitro assays. Materials Research 2023, 26, e20220466. [Google Scholar] [CrossRef]
- Joschek, S. , Nies B., Krotz R., Göferich A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Cao, X. , Zhu J., Zhang C., Xian J., Li M., Nath Varma S., Qin Z., Deng Q., Zhang X., Yang W., Liu C. Magnesium-Rich Calcium Phosphate Derived from Tilapia Bone Has Superior Osteogenic Potential. J Funct Biomater 2023, 14, 390. [Google Scholar] [CrossRef]
- Gani, M.A. , Budiatin A.S., Lestari M.L.A.D., Rantam F.A., Ardianto C., Khotib J. Fabrication and Characterization of Submicron-Scale Bovine Hydroxyapatite: A Top-Down Approach for a Natural Biomaterial. Materials (Basel) 2022, 15, 2324. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C. , Seonwoo H., Jang K.J., Pandey S., Lim J., Park S., Kim J.E., Choung Y.H., Garg P., Chung J.H. Development of novel gene carrier using modified nano hydroxyapatite derived from equine bone for osteogenic differentiation of dental pulp stem cells. Bioact Mater 2021, 6, 2742–2751. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H. , da Silva V.H.P., Ruiz P.L.M., Ussui V., Lazar D.R.R., Renno A.C.M., Ribeiro D.A. Physico-chemical characterization and biocompatibility of hydroxyapatite derived from fish waste. J Mech Behav Biomed Mater 2018, 80, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P. , Kupendra M., Fasim A., Anantharaju K.S., Kottam N., Murthy V.K., More S.S. Synthesis of nano hydroxyapatite from Hypopthalmichthys molitrix (silver carp) bone waste by two different methods: a comparative biophysical and in vitro evaluation on osteoblast MG63 cell lines. Biotechnol Lett 2022, 44, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Mathirat, A. , Dalavi P.A., Prabhu A., Yashaswini Devi G.V., Anil S., Senthilkumar K., Seong G.H., Sargod S.S., Bhat S.S., Venkatesan J. Remineralizing Potential of Natural Nano-Hydroxyapatite Obtained from Epinephelus chlorostigma in Artificially Induced Early Enamel Lesion: An In Vitro Study. Nanomaterials (Basel) 2022, 12, 3993. [Google Scholar] [CrossRef] [PubMed]
- Athinarayanan, J. , Periasamy V.S., Alshatwi A.A. Simultaneous fabrication of carbon nanodots and hydroxyapatite nanoparticles from fish scale for biomedical applications. Mater Sci Eng C Mater Biol Appl 2020, 117, 111313. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.W. , Kim K.S., Park H., Kim B.S. Marine plankton exoskeletone-derived hydroxyapatite/polycaprolactone composite 3D scaffold for bone tissue engineering. Biomater Sci 2022, 10, 7055–7066. [Google Scholar] [CrossRef] [PubMed]
- Palaveniene, A. , Tamburaci S., Kimna C., Glambaite K., Baniukaitiene O., Tihminlioğlu F., Liesiene J. Osteoconductive 3D porous composite scaffold from regenerated cellulose and cuttlebone-derived hydroxyapatite. J Biomater Appl 2019, 33, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I. , Hidayat H., Purwiandono G., Khoirunisa K., Zahra H.A., Audita R., Sagadevan S. Green Synthesis of Antibacterial Nanocomposite of Silver Nanoparticle-Doped Hydroxyapatite Utilizing Curcuma longa Leaf Extract and Land Snail (Achatina fulica) Shell Waste. J Funct Biomater 2022, 13, 84. [Google Scholar] [CrossRef]
- Mebarki, M. , Coquelin L., Layrolle P., Battaglia S., Tossou M., Hernigou P., Rouard H., Chevallier N. Enhanced human bone marrow mesenchymal stromal cell adhesion on scaffolds promotes cell survival and bone formation. Acta Biomater 2017, 59, 94–107. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials (Basel) 2023, 16, 4117. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.R. , Bonfield W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J Biomed Mater Res 2002, 59, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, S.A. 6 - Carbonate Substituted Hydroxyapatite. Elsevier Ltd 2020; 149-173. [CrossRef]
- Ishikawa, K. , Miyamoto Y., Tsuchiya A., Hayashi K., Tsuru K., Ohe G. Physical and Histological Comparison of Hydroxyapatite, Carbonate Apatite, and β-Tricalcium Phosphate Bone Substitutes. Materials (Basel) 2018, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Everts, V. , Delaissé J.M., Korper W., Jansen D.C., Tigchelaar-Gutter W., Saftig P., Beertsen W. The bone lining cell: its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res 2002, 17, 77–90. [Google Scholar] [CrossRef]
- Carrodeguas, R.G. , De Aza S. α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater 2011, 7, 3536–3546. [Google Scholar] [CrossRef]
- Crespi, R. , Capparè P., Gherlone E. Magnesium-enriched hydroxyapatite compared to calcium sulfate in the healing of human extraction sockets: radiographic and histomorphometric evaluation at 3 months. J Periodontol 2009, 80, 210–218. [Google Scholar] [CrossRef]
- Atilgan, S. An experimental comparison of the effects of calcium sulfate particles and β-tricalcium phosphate/hydroxyapatite granules on osteogenesis in internal bone cavities. Biotechnology & Biotechnological Equipment 2007, 21, 205–210. [Google Scholar] [CrossRef]
- Chen, F. , Wang M., Wang J., Chen X., Li X., Xiao Y., Zhang X. Effects of hydroxyapatite surface nano/micro-structure on osteoclast formation and activity. J Mater Chem B 2019, 7, 7574–7587. [Google Scholar] [CrossRef]
- Matsumoto, M.A. , Caviquioli G., Biguetti C.C., Holgado Lde A., Saraiva P.P., Rennó A.C., Kawakami R.Y. A novel bioactive vitroceramic presents similar biological responses as autogenous bone grafts. J Mater Sci Mater Med 2012, 23, 1447–1456. [Google Scholar] [CrossRef]
- Bellucci, D. , Anesi A., Salvatori R., Chiarini L., Cannillo V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater Sci Eng C Mater Biol Appl 2017, 79, 286–295. [Google Scholar] [CrossRef]
- Bellucci, D. , Braccini S., Chiellini F., Balasubramanian P., Boccaccini A.R., Cannillo V. Bioactive glasses and glass-ceramics versus hydroxyapatite: Comparison of angiogenic potential and biological responsiveness. J Biomed Mater Res A 2019, 107, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.Q.M. , Costa Klaus A.E., Espósito Santos B.F., Carvalho da Costa M., Ervolino E., Coelho de Lima D., Fernandes L.A. Evaluations of hydroxyapatite and bioactive glass in the repair of critical size bone defects in rat calvaria. J Oral Biol Craniofac Res 2020, 10, 422–429. [Google Scholar] [CrossRef]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, D. , Davison N., Habibović P. Human osteoclast formation and resorptive function on biomineralized collagen. Bioact Mater 2021, 8, 241–252. [Google Scholar] [CrossRef]
- Tayton, E. , Purcell M., Aarvold A., Smith J.O., Briscoe A., Kanczler J.M., Shakesheff K.M., Howdle S.M., Dunlop D.G., Oreffo R.O. A comparison of polymer and polymer-hydroxyapatite composite tissue engineered scaffolds for use in bone regeneration. An in vitro and in vivo study. J Biomed Mater Res A 2014, 102, 2613–2624. [Google Scholar] [CrossRef]
- Yang, W. , Both S.K., Zuo Y., Birgani Z.T., Habibovic P., Li Y., Jansen J.A., Yang F. Biological evaluation of porous aliphatic polyurethane/hydroxyapatite composite scaffolds for bone tissue engineering. J Biomed Mater Res A 2015, 103, 2251–2259. [Google Scholar] [CrossRef]
- Deng, X. , Huang B., Hu R., Chen L., Tang Y., Lu C., Chen Z., Zhang W., Zhang X. 3D printing of robust and biocompatible poly(ethylene glycol)diacrylate/nano-hydroxyapatite composites via continuous liquid interface production. J Mater Chem B 2021, 9, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Ielo, I. , Calabrese G., De Luca G., Conoci S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int J Mol Sci 2022, 23, 9721. [Google Scholar] [CrossRef]
- Sun, J.S. , Lin F.H., Hung T.Y., Tsuang Y.H., Chang W.H., Liu H.C. The influence of hydroxyapatite particles on osteoclast cell activities. J Biomed Mater Res 1999, 45, 311–321. [Google Scholar] [CrossRef]
- Han, Y. , Li S., Cao X., Yuan L., Wang Y., Yin Y., Qiu T., Dai H., Wang X. Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo. Sci Rep 2014, 4, 7134. [Google Scholar] [CrossRef] [PubMed]
- Narducci, P. , Nicolin V. Differentiation of activated monocytes into osteoclast-like cells on a hydroxyapatite substrate: an in vitro study. Ann Anat 2009, 191, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Veillat, V. , Spuul P., Daubon T., Egaña I., Kramer I., Génot E. Podosomes: Multipurpose organelles? Int J Biochem Cell Biol 2015, 65, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. , Zhou Y., Xiao C., Zhao W., Wu H., Tang J., Li Z., Yu S., Li X., Min L., Yu Z., Wang G., Wang L., Zhang K., Yang X., Zhu X., Tu C., Zhang X. Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect. Sci Adv. 2019, 5, eaax6946. [Google Scholar] [CrossRef]
- Chen, Z. , Deng J., Cao J., Wu H., Feng G., Zhang R., Ran B., Hu K., Cao H., Zhu X., Zhang X. Nano-hydroxyapatite-evoked immune response synchronized with controllable immune adjuvant release for strengthening melanoma-specific growth inhibition. Acta Biomater 2022, 145, 159–171. [Google Scholar] [CrossRef]
- Ding, X. , Takahata M., Akazawa T., Iwasaki N., Abe Y., Komatsu M., Murata M., Ito M., Abumi K., Minami A. Improved bioabsorbability of synthetic hydroxyapatite through partial dissolution-precipitation of its surface. J Mater Sci Mater Med 2011, 22, 1247–1255. [Google Scholar] [CrossRef]
- Mestres, G. , Espanol M., Xia W., Persson C., Ginebra M.P., Ott M.K. Inflammatory response to nano- and microstructured hydroxyapatite. PLoS One 2015, 10, e0120381. [Google Scholar] [CrossRef] [PubMed]
- Lebre, F. , Sridharan R., Sawkins M.J., Kelly D.J., O'Brien F.J., Lavelle E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci Rep 2017, 7, 2922. [Google Scholar] [CrossRef]
- Ghanaati, S. , Udeabor S.E., Barbeck M., Willershausen I., Kuenzel O., Sader R.A., Kirkpatrick C.J. Implantation of silicon dioxide-based nanocrystalline hydroxyapatite and pure phase beta-tricalciumphosphate bone substitute granules in caprine muscle tissue does not induce new bone formation. Head Face Med 2013, 9, 1. [Google Scholar] [CrossRef]
- Li, C. , Yang L., Ren X., Lin M., Jiang X., Shen D., Xu T., Ren J., Huang L., Qing W., Zheng J., Mu Y. Groove structure of porous hydroxyapatite scaffolds (HAS) modulates immune environment via regulating macrophages and subsequently enhances osteogenesis. J Biol Inorg Chem 2019, 24, 733–745. [Google Scholar] [CrossRef]
- Zeng, Q. , Wang R., Hua Y., Wu H., Chen X., Xiao Y.C., Ao Q., Zhu X., Zhang X. Hydroxyapatite nanoparticles drive the potency of Toll-like receptor 9 agonist for amplified innate and adaptive immune response. Nano Res 2022, 15, 9286–9297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. , Liang Z., Chen C., Yang X., Fu D., Bao H., Li M., Shi S., Yu G., Zhang Y., Zhang C., Zhang W., Xue C., Sun B. Engineered Hydroxyapatite Nanoadjuvants with Controlled Shape and Aspect Ratios Reveal Their Immunomodulatory Potentials. ACS Appl Mater Interfaces 2021, 13, 59662–59672. [Google Scholar] [CrossRef] [PubMed]
- Shang, L. , Shao J., Ge S. Immunomodulatory Properties: The Accelerant of Hydroxyapatite-Based Materials for Bone Regeneration. Tissue Eng Part C Methods 2022, 28, 377–392. [Google Scholar] [CrossRef] [PubMed]
| Hierarchy | Morphology | Size | Step length and chirality | |
|---|---|---|---|---|
| Needle crystal | 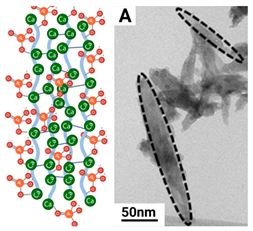 |
Thin, twisted long ribbon | Thickness ~ 5 nm Width 5 to 10 nm Length 50-100 nm | ~ 1.5 µm right-handed |
| Subplate | 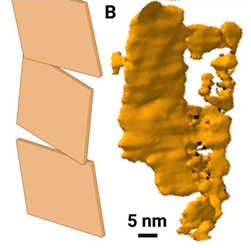 |
Needle-shaped crystal merging laterally at an angle | Thickness 5 nm Width 20 to 60 nm Length 50-100 nm | ~ 4.7 µm left-handed |
| Plate | 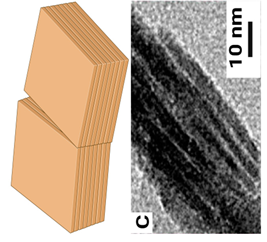 |
Twisted mineral laminae composed of subplates | Thickness 20-40 nm Width 60-150 nm Length 50-100 nm | ~ 4.6 µm left-handed |
| Mineral fibrils |  |
Mineral plates spirally merge at an angle | Width 60-150 nm Length: Micron level |
~ 2.9 µm right-hand side |
| Bundles of mineral fibrils | 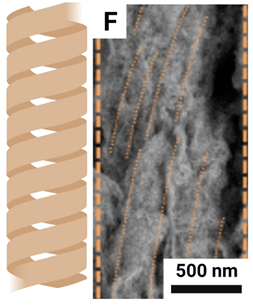 |
Mineral fibrils spirally merge at an angle | Diameter 1-2 microns Length: Micron level |
Coils: 4.2-6.5 µm right-handed |
| Lamellar units | 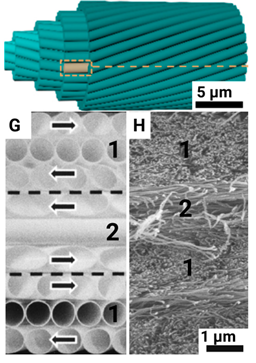 |
The fibril bundles are rotationally stacked layer by layer | Diameter 6-12 microns |
Coils: 48-653 µm, left/right; Stacking: ~ 47.6 µm, right-handed |
| Haver's canals | 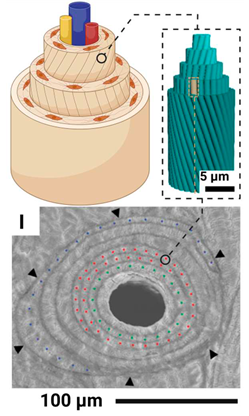 |
Lamellar units are spirally twisted around the blood vessel in the form of coaxial cylinders | Diameter 100-300 microns |
Coils: 617-5,167 µm, right/left-handed; Stacking: ~184 µm, right-handed |
| Compact bone | 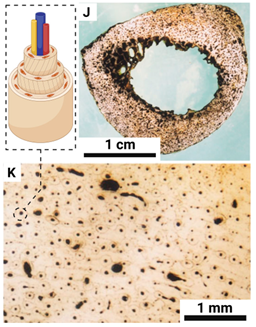 |
The haversack channels are laid in layers in a circular pattern | Thickness ~ 3 mm |
Coils: 13.5-119.4 cm, right/left-handed; Stacking: ~9.7 mm, left-handed |
| Solid bone | 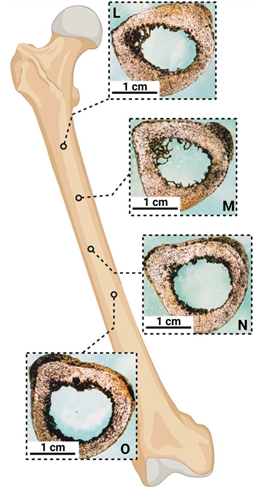 |
Anatomical shape with curve | Diameter ~ 3 cm |
Left-handed |
| Source | Extraction method | Final product | Results of in vitro and in vivo studies | Ref. |
| Cattle bone waste | Calcination without chemical agents. | Hydroxyapatite nanoparticles with good crystallization are hexagonal in size 300-500 nm. | In vitro cytocompatibility studies have shown high rates of cell viability and proliferation when co-cultured together | [164] [187] |
| Horse bone waste | Sintering and processing in ball mill | Hydroxyapatite nanoparticles of two size groups: ≤ 500 and 200 nm. | High ability to induce osteogenic differentiation of dental stem cells | [188] |
| Fish waste M. furnieri | Treatment with NaOH and H2O2 and calcination at 800⁰C | Hydroxyapatite powder with well-crystallized structure. Hydroxyapatite particles with pore size ~8 µm. | Collagen fiber formation, fibroblasts were present and angiogenesis was observed. Tissue proliferation into the graft was observed. | [167] [189] |
| Bone waste of Hypopthalmichthys molitrix | Treatment with NaOH and acetone | Hydroxyapatite powder with an average crystallite size of 58.3 nm. | Cell viability of osteoblast-like MG63 cells is 91%. Positive effect on differentiation and proliferation. | [190] |
| Tempering at 900⁰C | Hydroxyapatite powder with an average crystallite size of 64.3 nm. | MG63 cell viability is 86%. Low indicators of proliferative capacity. | ||
| Tilapia bone waste | Tempering in the temperature range from 600°C to 800°C | Hydroxyapatite grains with a distinct porous structure and a relatively high degree of Mg2+ substitution. | High degree of biocompatibility. Promotes cell proliferation and differentiation compared to commercial pure hydroxyapatite. | [186] |
| Bone waste from E. chlorostigma | Alkaline hydrolysis and calcination at 600°C | Nanoscale hydroxyapatite measuring 29.5 nm for alkaline treatment and 82.12 nm for calcination | High biocompatibility of normal adipose fibroblast L929 cells. High proliferation L929. High remineralizing potential. | [191] |
| L. catla and N. japonicus scales. | Tempering at 800°C and grinding in a bead mill | Hydroxyapatite in the form of nanopowder of porous nanoparticles of size 30-60 nm with crystallites of size 10 nm. | When used in conjunction with the polycaprolactone scaffold, proliferation and excellent adhesion rates were observed | [170] |
| L. lentjan scales | Hydrothermal treatment at 280°C | Hydroxyapatite in the form of rods 50-100 nm long, 8-12 nm in diameter, and spheroids with a diameter of 15-50 nm | Biocompatibility and osteogenic potential for human mesenchymal stem cells. | [192] |
| Plankton | Leaching of solid particles | Porous nanohydroxyapatite | Adhesion, proliferation and viability. Increased expression of bone morphogenetic protein 2, collagen-1, osteocalcin and sialopotein. | [193] |
| Atactodea glabrata shells | Tempering and chemical precipitation | Hydroxyapatite powder in the form of nanoscale rods 15.22 nm | Absence of cytotoxicity. Inhibitory effect against some pathogenic bacteria and fungi. | [168] |
| Sepia cuttlefish skeleton | Hydrothermal treatment with NH4H2PO4 | Hydroxyapatite microspheres 1-2 microns in size with a trace element content close to human bone | Absence of cytotoxicity of MG63 cells. MG63 proliferation. High alkaline phosphatase activity and osteocalcin expression. | [194] |
| A. fulica shell | Tempering. Sintering with (NH4)2HPO4. | Nanoparticles of hydroxyapatite with the size of 87.7-88.9 nm | Distinct antibacterial activity | [195] |
| Eggshell | Hydrothermal acid treatment with H3PO4. | Single-phase crystalline hydroxyapatite (26.5, 40.8 and 25.5 nm) with high Mg and Sr content. | High adhesion of MG63 cells. The cells had distinct filopodia. | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





