Submitted:
14 November 2023
Posted:
15 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
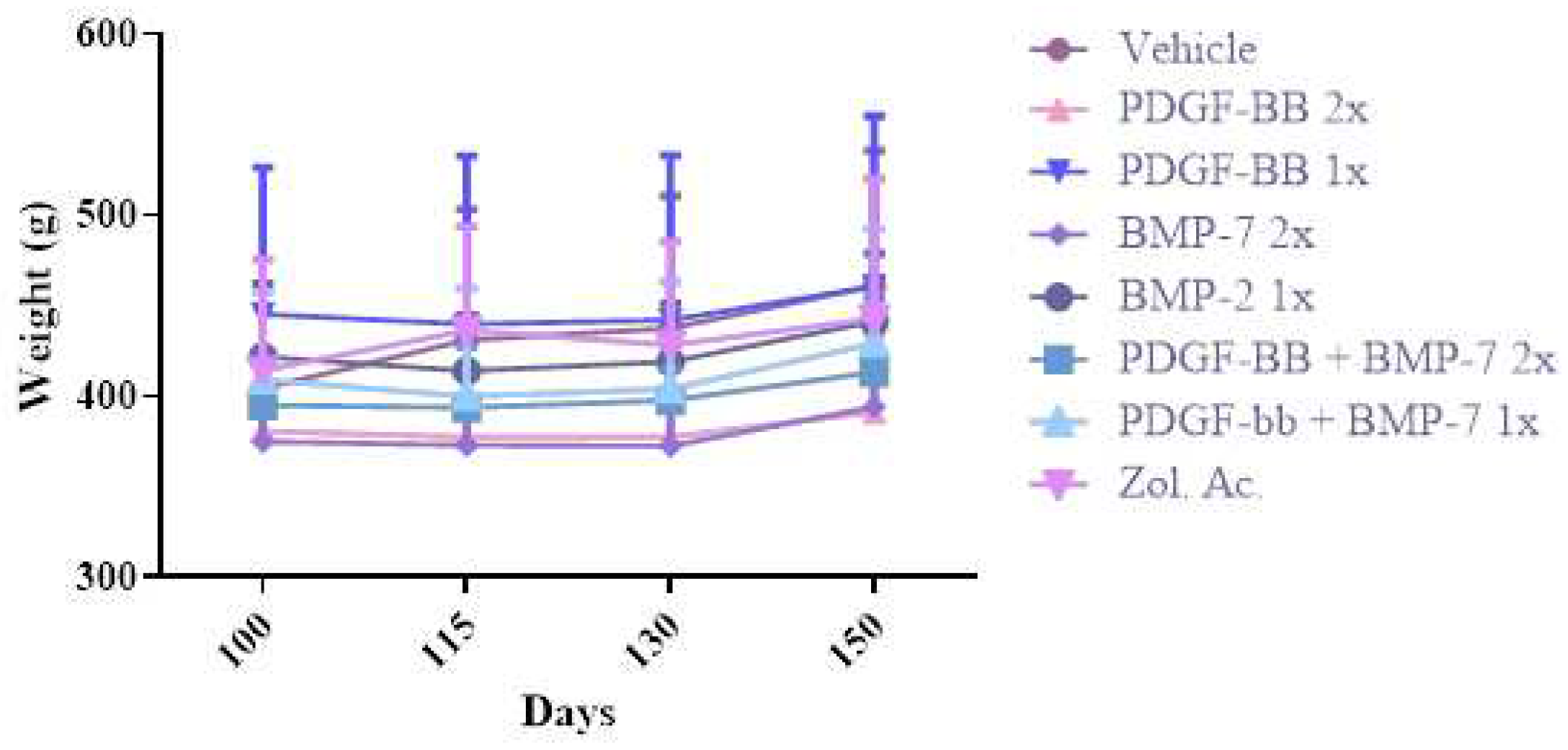
2.1. Growth Factors
2.2. Animals and Treatment
2.3. Blood and serum collection
2.4. Quantification of Alkaline Phosphatase (ALP)
2.5. X-ray
2.6. Micro CT
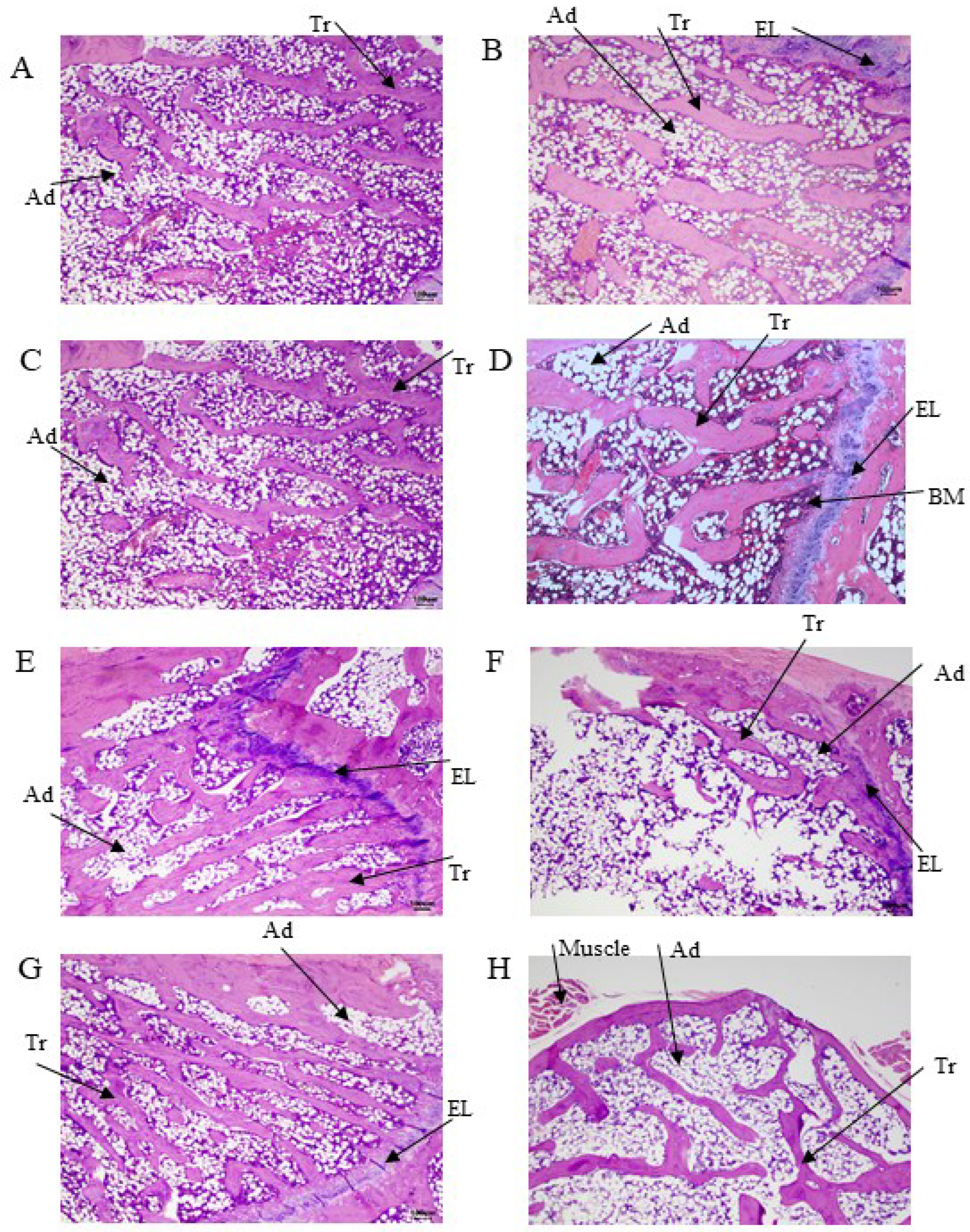
2.7. Histology
3. Results
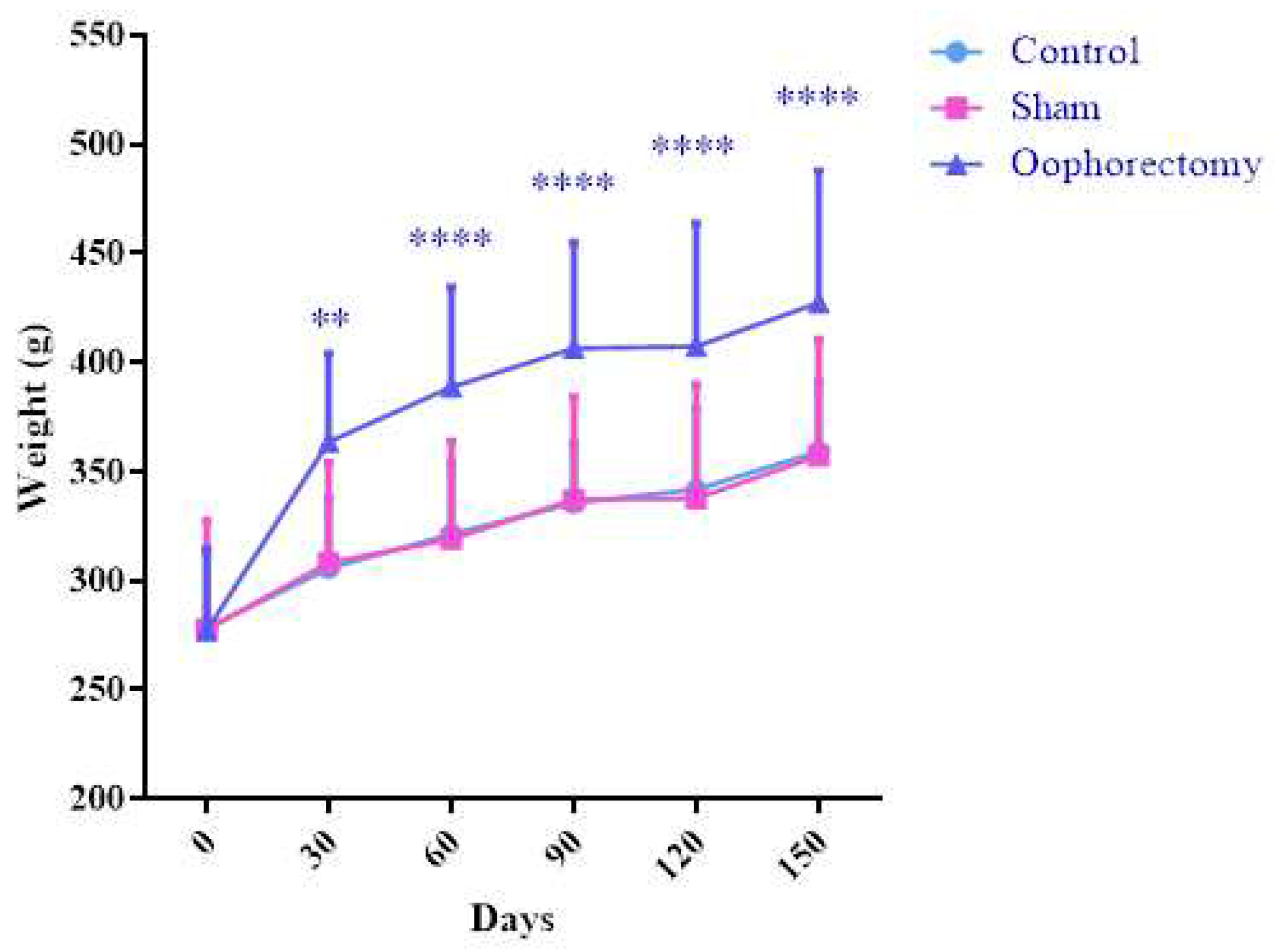
3.1. Oophorectomy causes body mass gain
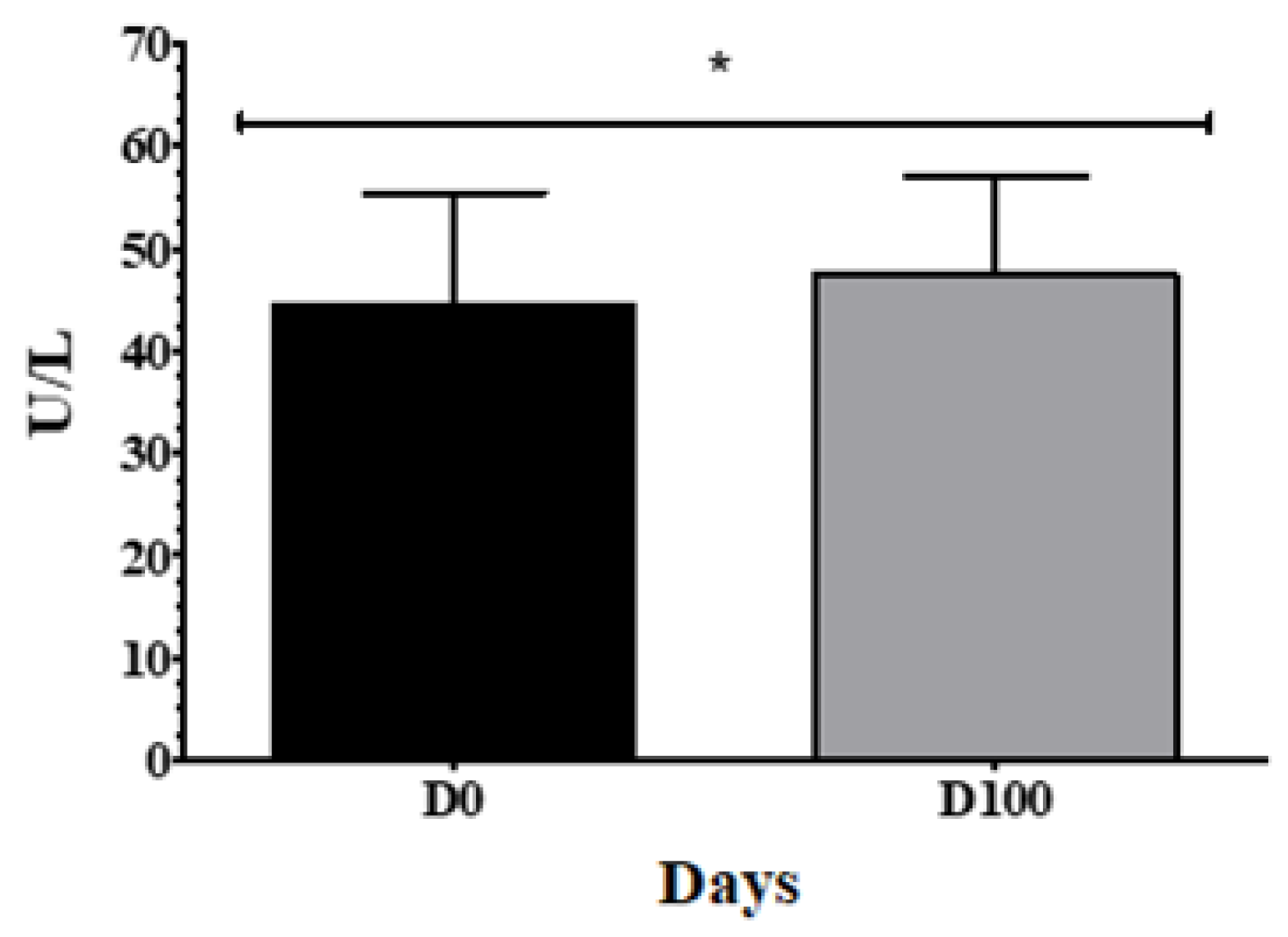
3.2. Oophorectomized animals show increased serum ALP concentration
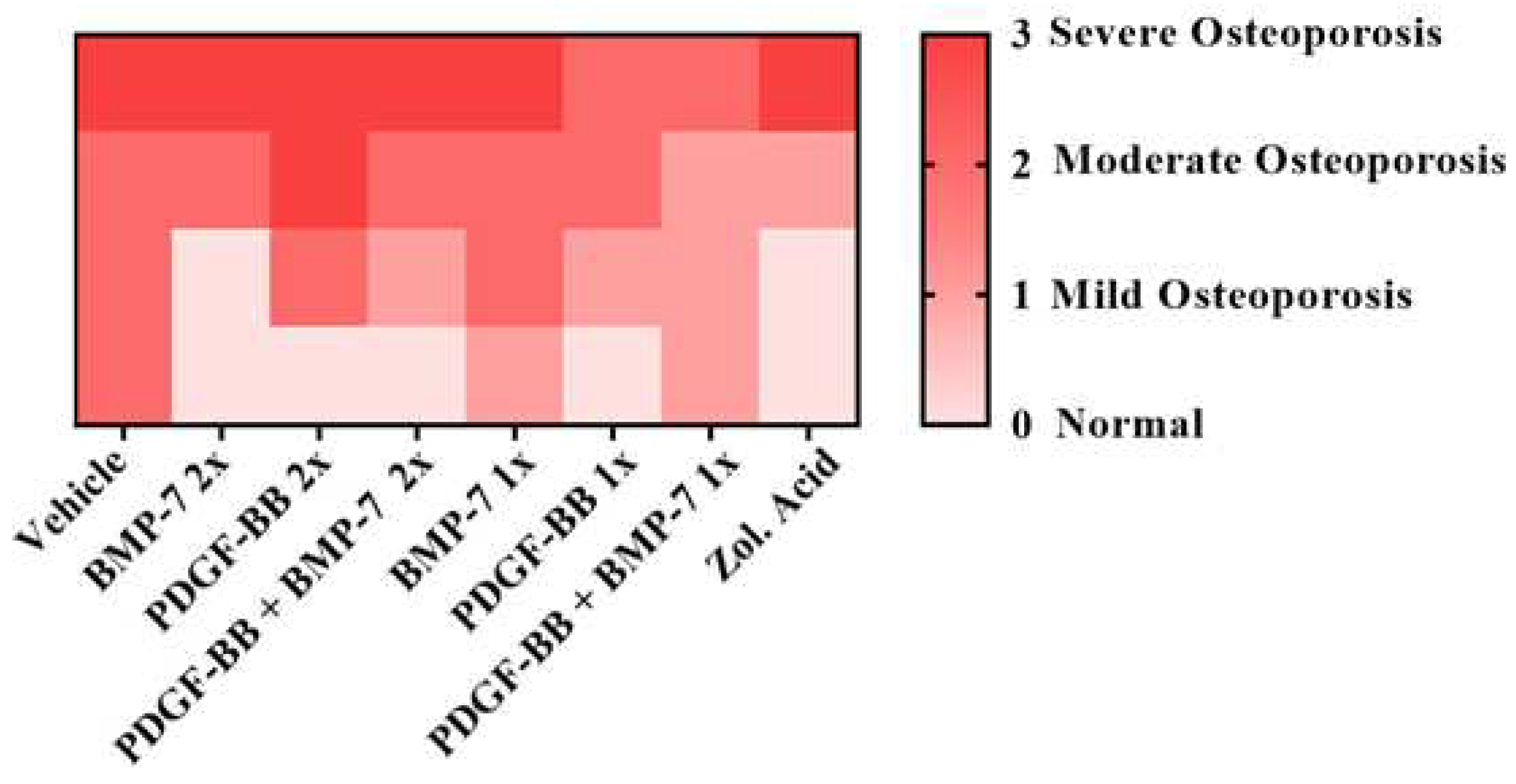
3.3. In vivo X-Ray Analysis
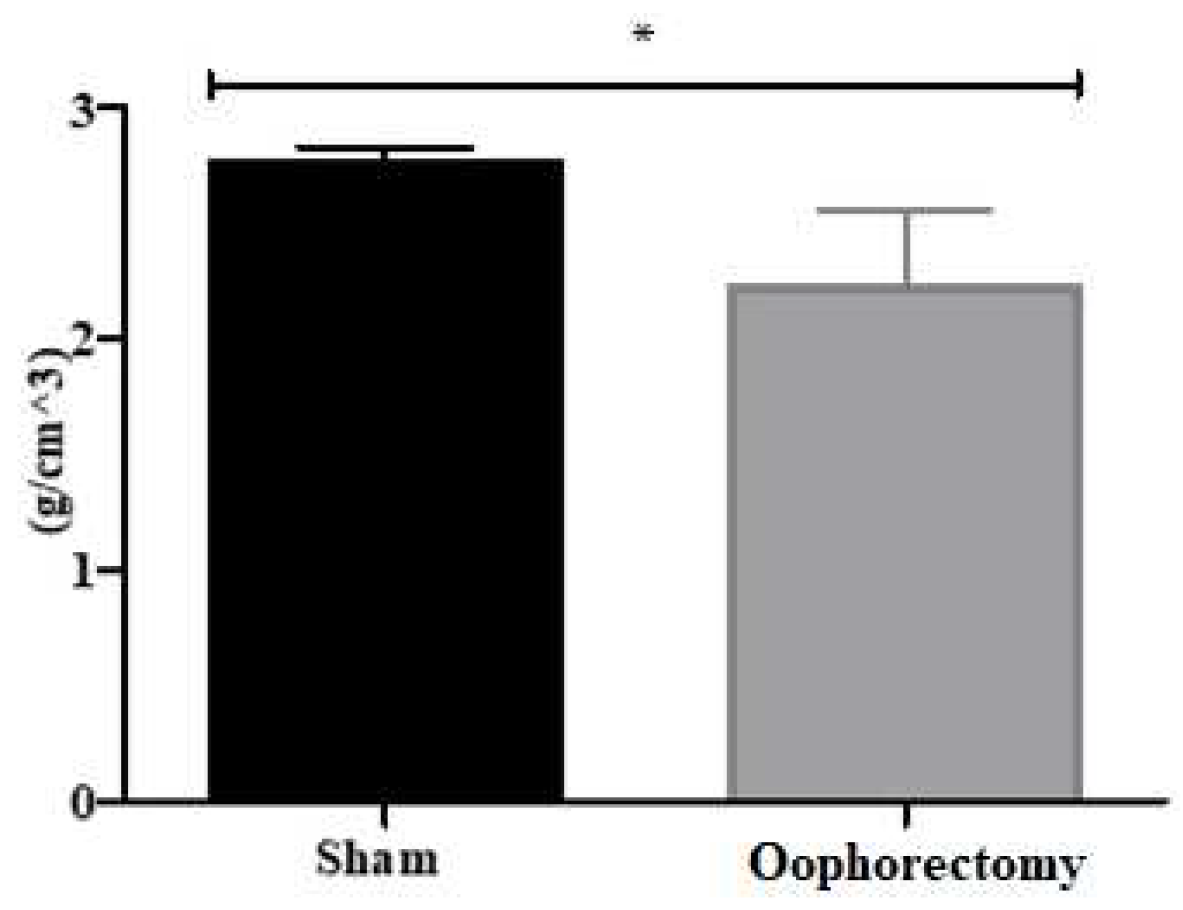
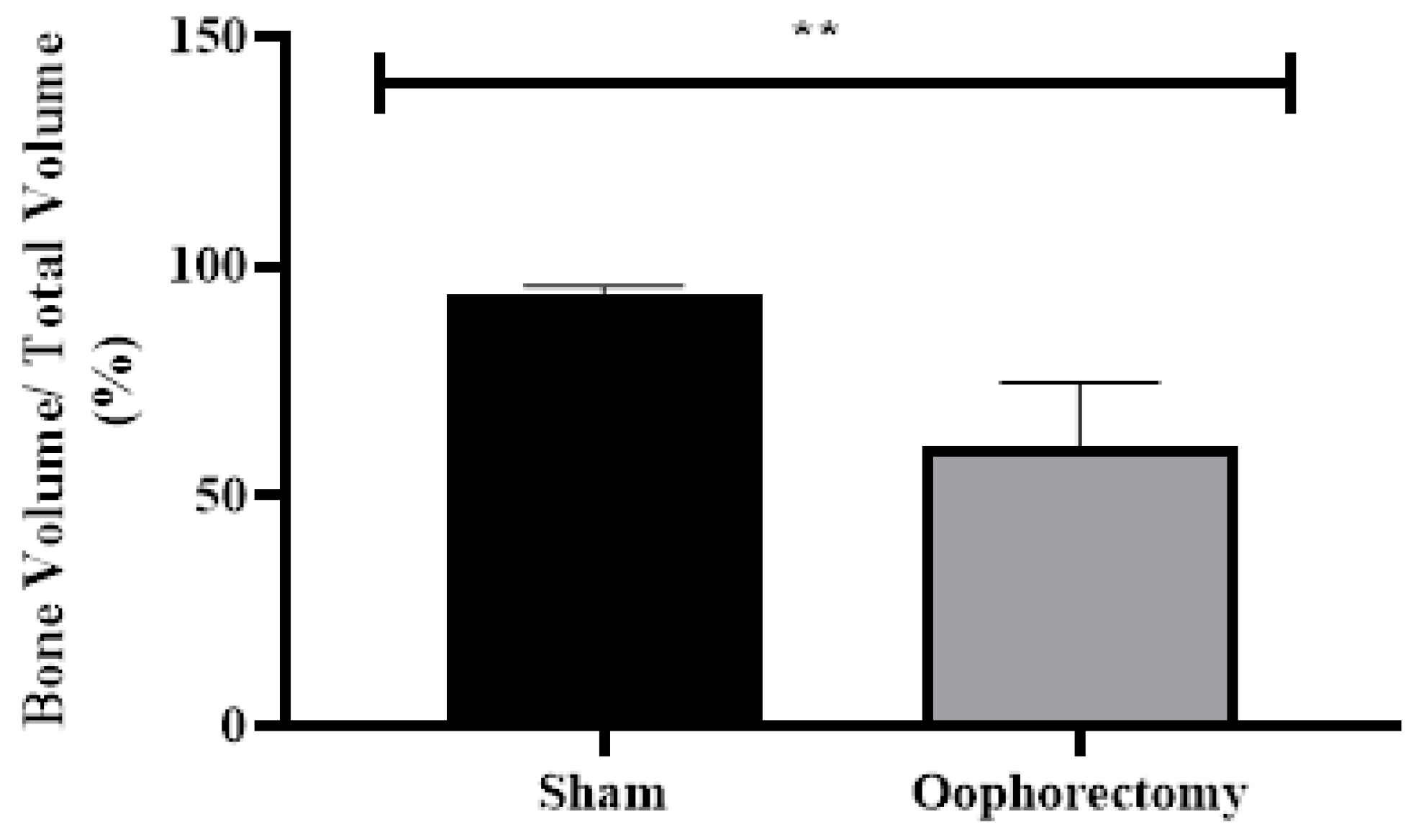
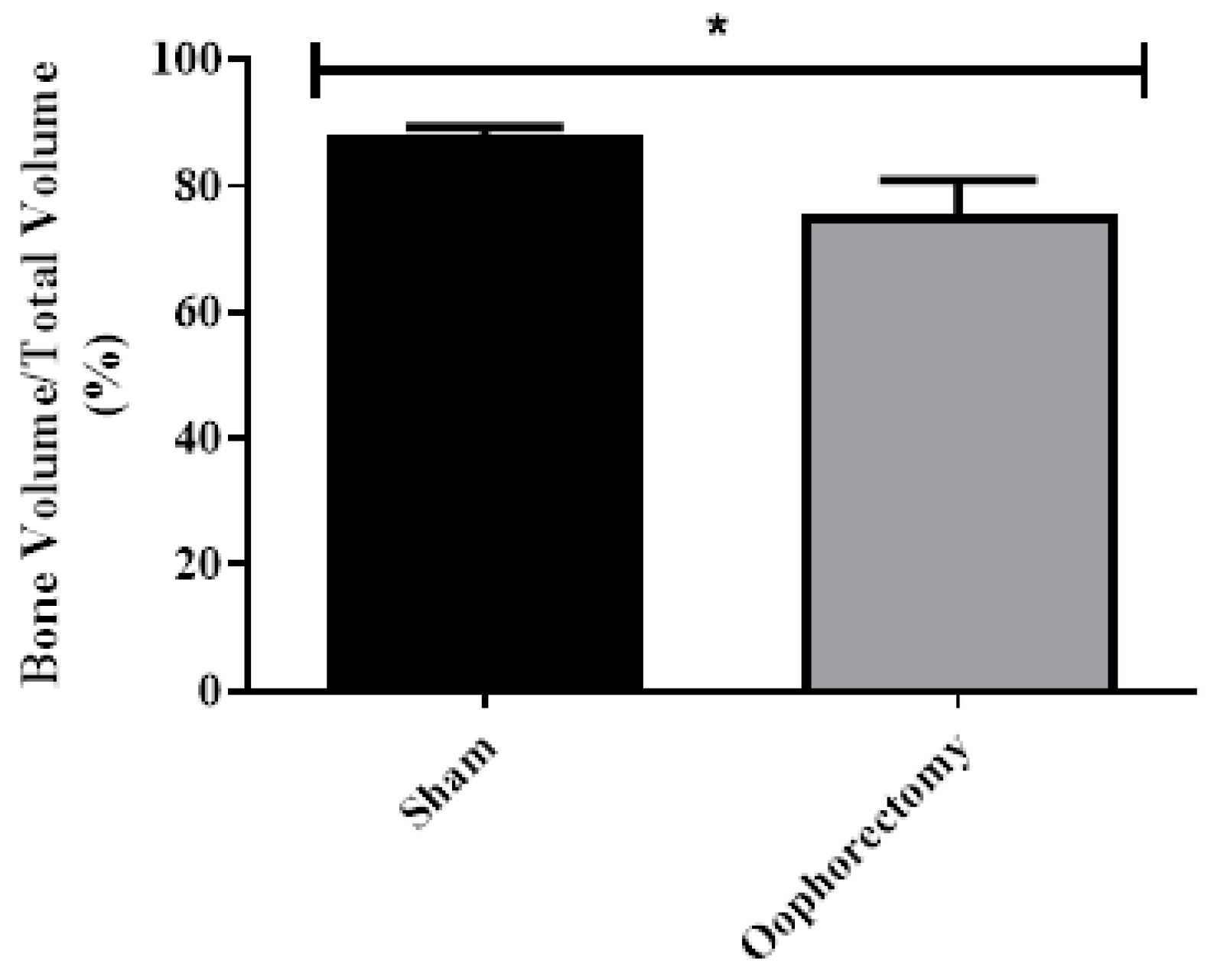
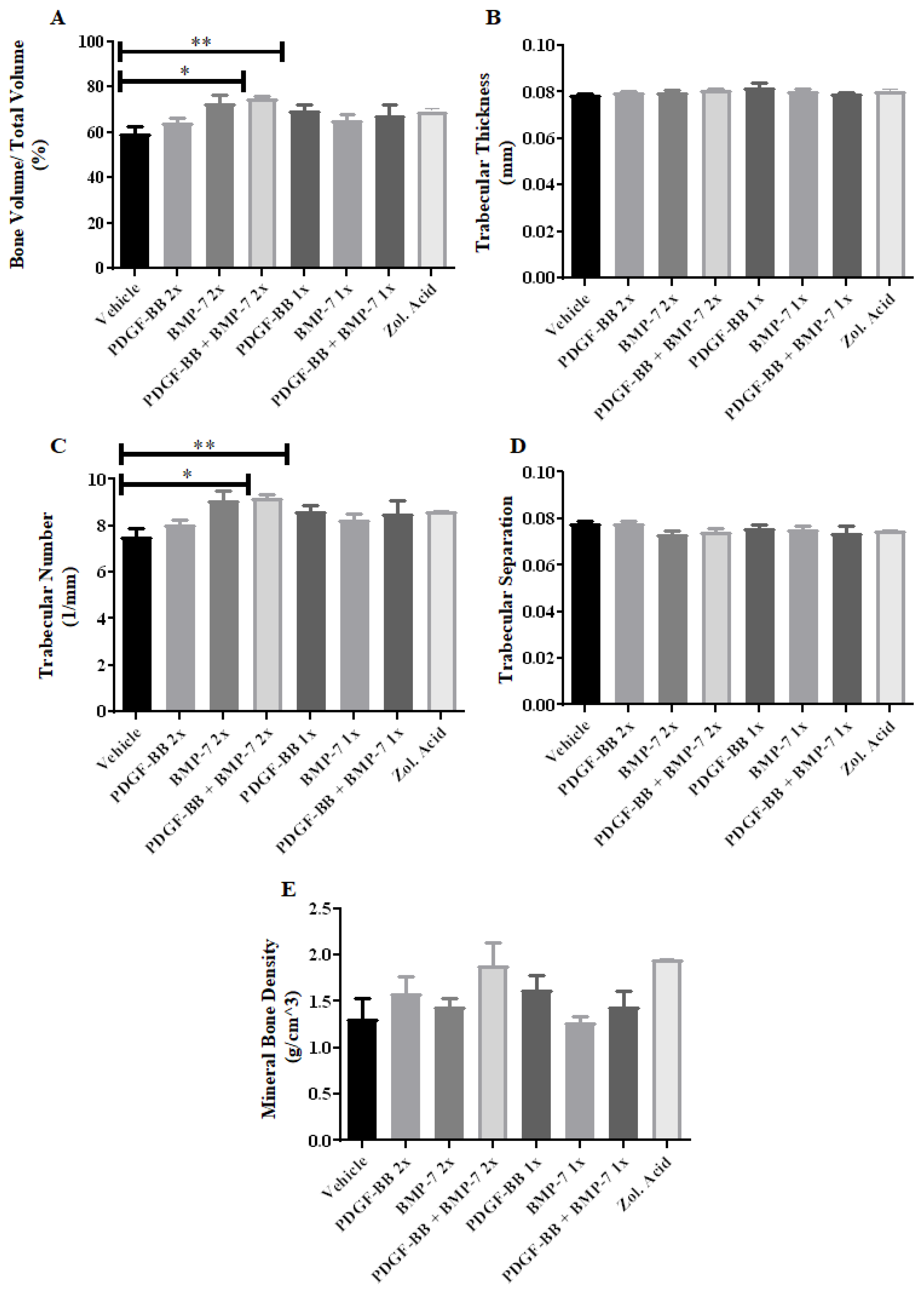
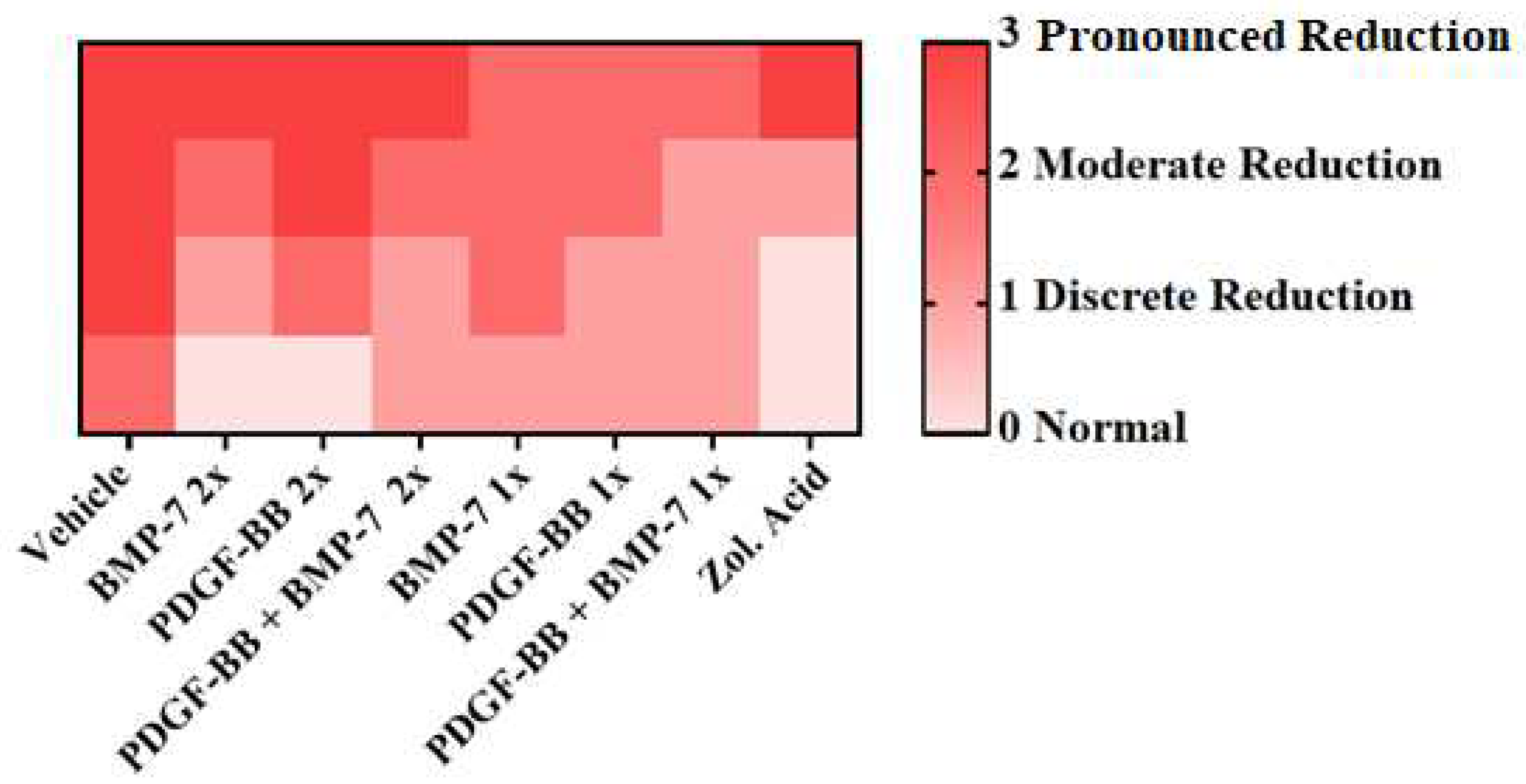
3.4. Micro-CT Analysis
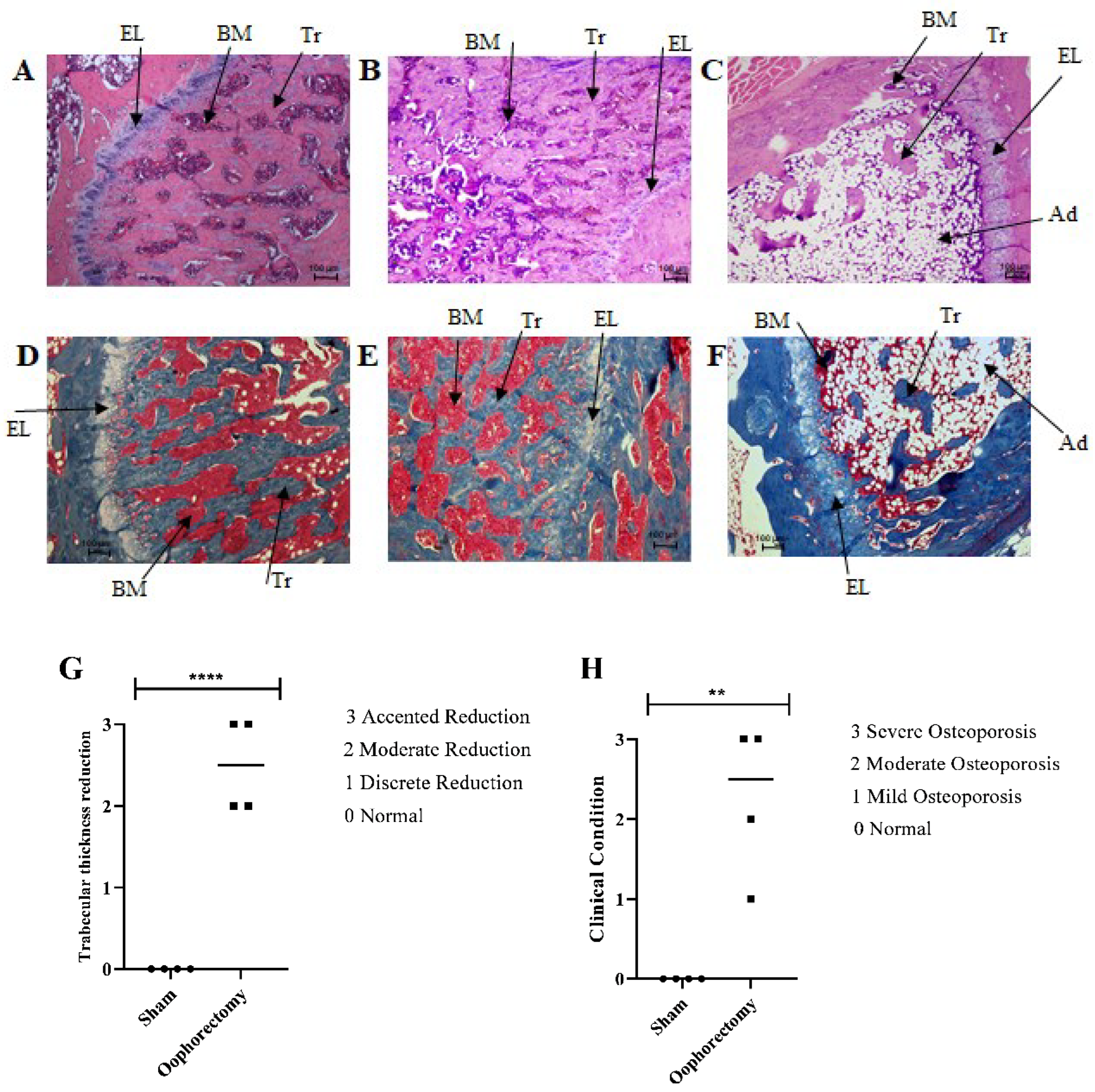
3.5. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Increased Mortality in Patients with a Hip Fracture-Effect of Pre-Morbid Conditions and Post-Fracture Complications. Osteoporosis International 2007, 18, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Forciea, M.A.; McLean, R.M.; Denberg, T.D. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update from the American College of Physicians. Ann Intern Med 2017, 166, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An Estimate of the Worldwide Prevalence and Disability Associated with Osteoporotic Fractures. Osteoporos Int 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Zofkova, I.; Blahos, J. New Molecules Modulating Bone Metabolism – New Perspectives in the Treatment of Osteoporosis. Physiol. Res 2017, 66, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, K.; Mitchell, P. Capture a Fratura 2012, 1–28.
- Kim, H.K.; Lee, J.S.; Kim, J.H.; Seon, J.K.; Park, K.S.; Jeong, M.H.; Yoon, T.R. Bone-Forming Peptide-2 Derived from BMP-7 Enhances Osteoblast Differentiation from Multipotent Bone Marrow Stromal Cells and Bone Formation. Exp Mol Med 2017, 49, e328-7. [Google Scholar] [CrossRef] [PubMed]
- Segredo-Morales, E.; García-García, P.; Évora, C.; Delgado, A. BMP Delivery Systems for Bone Regeneration: Healthy vs Osteoporotic Population. Review. J Drug Deliv Sci Technol 2017, 42, 107–118. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Chen, X.; Hu, Y.; Wu, J.; Chen, S.; Chang, J.; Wang, G.; Gao, Y. Emodin Promotes the Osteogenesis of MC3T3-E1 Cells via BMP-9/Smad Pathway and Exerts a Preventive Effect in Ovariectomized Rats. Acta Biochim Biophys Sin (Shanghai) 2017, 49, 867–878. [Google Scholar] [CrossRef]
- Bartelt, A.; Behler-Janbeck, F.; Beil, F.T.; Koehne, T.; Müller, B.; Schmidt, T.; Heine, M.; Ochs, L.; Yilmaz, T.; Dietrich, M.; et al. Lrp1 in Osteoblasts Controls Osteoclast Activity and Protects against Osteoporosis by Limiting PDGF-RANKL Signaling. Bone Res 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Lee, Z.H.; Kim, H.-J.; Ryoo, H.M. A Novel Osteogenic Activity of Suberoylanilide Hydroxamic Acid Is Synergized by BMP-2. J Bone Metab 2015, 22, 51–56. [Google Scholar] [CrossRef]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Structure, Biological Function and Therapeutic Applications. Arch Biochem Biophys 2014, 561, 64–73. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The Family of Bone Morphogenetic Proteins. Kidney Int 2000, 57, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Leboy, P.; Grasso-Knight, G.; D’Angelo, M.; Volk, S.W.; Lian, J. v; Drissi, H.; Stein, G.S.; Adams, S.L. Smad-Runx Interactions during Chondrocyte Maturation. J Bone Joint Surg Am 2001, 83-A Suppl, S15-22. [CrossRef]
- Caplan, A.I.; Correa, D. PDGF in Bone Formation and Regeneration: New Insights into a Novel Mechanism Involving MSCs. Journal of Orthopaedic Research 2011, 29, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of Platelet-Derived Growth Factors in Physiology and Medicine. Genes Dev 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Onikepe, A.O.; MacKrell, J.; Einhorn, T.; Bradica, G.; Lynch, S.; Hart, C.E. Accelerated Fracture Healing in the Geriatric, Osteoporotic Rat with Recombinant Human Platelet-Derived Growth Factor-BB and an Injectable Beta-Tricalcium Phosphate/Collagen Matrix. J Orthop Res 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; et al. PDGF-BB Secreted by Preosteoclasts Induces Angiogenesis during Coupling with Osteogenesis. Nat Med 2014, 20, 1–12. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Henshaw, K.; Bird, H.; Jones, E.; Giannoudis, P. v. The Effect of Bone Morphogenetic Protein-2, Bone Morphogenetic Protein-7, Parathyroid Hormone, and Platelet-Derived Growth Factor on the Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Osteoporotic Bone. J Orthop Trauma 2010, 24, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Valenzuela, J.C.; Halcsik, E.; Bassi, E.J.; Demasi, M.A.; Granjeiro, J.M.; Sogayar, M.C. Expression, Purification, Bioactivity, and Partial Characterization of a Recombinant Human Bone Morphogenetic Protein-7 Produced in Human 293T Cells. Mol Biotechnol 2010, 46, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lasota, A.; Danowska-Klonowska, D. Experimental Osteoporosis--Different Methods of Ovariectomy in Female White Rats. Rocz Akad Med Bialymst 2004, 49 Suppl 1, 129–131. [Google Scholar]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Description of a New Method of Ovariectomy in Female Rats. Rev Bras Reumatol 2012, 52, 462–470. [Google Scholar]
- Azzi, C.M.G.; Nishiyama, A.C.O.C. Desenvolvimento de Um Modelo Animal Para Osteoporose Induzido Por Ooforectomia Em Rato Fêmea Nude (Rowett). São Paulo University (USP), 2017.
- Marcu, F.; Bogdan, F.; Muţiu, G.; Lazăr, L. The Histopathological Study of Osteoporosis. Rom J Morphol Embryol 2011, 52, 321–325. [Google Scholar]
- Carlsson, C.; Weisbrod, S. Ossos, Articulações, Tendões e Ligamentos. In Bases da Patologia em Veterinária; Zachary, J.F., McGavin, M.D., Eds.; Elsevier Ltd: Rio de Janeiro, 2013; pp. 923–974. [Google Scholar]
- Xu, H.; Liu, T.; Hu, L.; Li, J.; Gan, C.; Xu, J.; Chen, F.; Xiang, Z.; Wang, X.; Sheng, J. Effect of Caffeine on Ovariectomy-Induced Osteoporosis in Rats. Biomed Pharmacother 2019, 112, 108650. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. The Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Thibodaux, R.J.; Neira, L.F.V.; Messina, O.D. Osteoporosis: A Clinical and Pharmacological Update. Clin Rheumatol 2019, 38, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Heiman, M.L. Increased Weight Gain after Ovariectomy Is Not a Consequence of Leptin Resistance. Am J Physiol Endocrinol Metab 2001, 280, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.Y.; Hong, Y.S.; Park, S.-H.; Ju, J.H. Increased Serum Alkaline Phosphatase Levels Correlate with High Disease Activity and Low Bone Mineral Density in Patients with Axial Spondyloarthritis. Semin Arthritis Rheum 2015, 45, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Mukaiyama, K.; Kamimura, M.; Uchiyama, S.; Ikegami, S.; Nakamura, Y.; Kato, H. Elevation of Serum Alkaline Phosphatase (ALP) Level in Postmenopausal Women Is Caused by High Bone Turnover. Aging Clin Exp Res 2015, 27, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, A. V.; Dimitrova, A.A.; Kostov, K.G.; Russeva, A.L.; Atanasova, M.A.; Blagev, A.B.; Betova, T.M.; Trifonov, R.G. Changes of Serum Concentrations of Alkaline Phosphatase and Metalloproteinase-9 in an Ovariectomized Wistar Rat Model of Osteoporosis. Journal of Biomedical and Clinical Research 2017, 10, 32–36. [Google Scholar] [CrossRef]
- Lang, J.; Zhao, Q.; He, Y.; Yu, X. Bone Turnover Markers and Novel Biomarkers in Lung Cancer Bone Metastases. Biomarkers 2018, 23, 518–526. [Google Scholar] [CrossRef]
- Salamanna, F.; Borsari, V.; Contartese, D.; Nicoli Aldini, N.; Fini, M. Link between Estrogen Deficiency Osteoporosis and Susceptibility to Bone Metastases: A Way towards Precision Medicine in Cancer Patients. Breast 2018, 41, 42–50. [Google Scholar] [CrossRef]
- Rahman, M.M.; Matsuoka, K.; Takeshita, S.; Ikeda, K. Secretion of PDGF Isoforms during Osteoclastogenesis and Its Modulation by Anti-Osteoclast Drugs. Biochem Biophys Res Commun 2015, 462, 159–164. [Google Scholar] [CrossRef]
- Li, D.; Wan, Q.; Pathak, J.L.; Li, Z. Platelet-Derived Growth Factor BB Enhances Osteoclast Formation and Osteoclast Precursor Cell Chemotaxis. J Bone Miner Metab 2017, 35, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J.; Jin, D. Platelet-Derived Growth Factor (PDGF)-BB Stimulates Osteoclastic Bone Resorption Directly: The Role of Receptor Beta. Biochem Biophys Res Commun 1998, 251, 190–194. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP Signalling in Skeletal Development, Disease and Repair. Nat Rev Endocrinol 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Desmyter, S.; Goubau, Y.; Benahmed, N.; de Wever, A.; Verdonk, R. The Role of Bone Morphogenetic Protein-7 (Osteogenic Protein-1) in the Treatment of Tibial Fracture Non-Unions. An Overview of the Use in Belgium. Acta Orthop Belg 2008, 74, 534–537. [Google Scholar] [PubMed]
- Cecchi, S.; Bennet, S.J.; Arora, M. Bone Morphogenetic Protein-7: Review of Signalling and Efficacy in Fracture Healing. J Orthop Translat 2016, 4, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Helbig, L.; Omlor, G.W.; Ivanova, A.; Guehring, T.; Sonntag, R.; Kretzer, J.P.; Minkwitz, S.; Wildemann, B.; Schmidmaier, G. Bone Morphogenetic Proteins - 7 and - 2 in the Treatment of Delayed Osseous Union Secondary to Bacterial Osteitis in a Rat Model. BMC Musculoskelet Disord 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Hilyard, A.C.; Wu, C.; Davis, B.N.; Hill, N.S.; Lal, A.; Lieberman, J.; Lagna, G.; Hata, A.; Rahman, M.M.; et al. Molecular Basis for Antagonism between PDGF and the TGFΒ Family of Signalling Pathways by Control of MiR-24 Expression. EMBO Journal 2010, 29, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Fedorchak, M. v.; Little, S.R. The Influence of Platelet-Derived Growth Factor and Bone Morphogenetic Protein Presentation on Tubule Organization by Human Umbilical Vascular Endothelial Cells and Human Mesenchymal Stem Cells in Coculture. Tissue Eng Part A 2016, 22, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Osteoblast Differentiation by Transcription Factors. J Cell Biochem 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, X.; Zhu, C.; Tang, X.; Yu, F.; Shang, G.W.; Cai, X. Molecular Mechanisms of PPAR-γ Governing MSC Osteogenic and Adipogenic Differentiation. Curr Stem Cell Res Ther 2016, 11, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.A.; Israel, D.I.; Kelly, S.; Luxenberg, D.P. Bone Morphogenetic Protein-2 Causes Commitment and Differentiation in C3H10T1/2 and 3T3 Cells. Growth Factors 1993, 9, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Nishimura, R.; Ikeda, F.; Yamashita, K.; Matsubara, T.; Nokubi, T.; Yoneda, T. Differential Roles of Smad1 and P38 Kinase in Regulation of Peroxisome Proliferator-Activating Receptor Gamma during Bone Morphogenetic Protein 2-Induced Adipogenesis. Mol Biol Cell 2003, 14, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, P.; Ríos, S.; Pastenes, L.; Pino, A.M.; Rodríguez, J.P. Increased Adipogenesis of Osteoporotic Human-Mesenchymal Stem Cells (MSCs) Characterizes by Impaired Leptin Action. J Cell Biochem 2008, 103, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.C.; Berry, R.; Holtrup, B.; Sebo, Z.; Nelson, T.; Fretz, J.A.; Lindskog, D.; Kaplan, J.L.; Ables, G.; Rodeheffer, M.S.; et al. Bone Marrow Adipocytes. Adipocyte 2017, 6, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, X.; Zhi, X.; Cong, W.; Huang, B.; Chen, H.; Wang, Y.; Li, Y.; Wang, L.; Fang, C.; et al. RANKL from Bone Marrow Adipose Lineage Cells Promotes Osteoclast Formation and Bone Loss. EMBO Rep 2021, e52481. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhong, L.; Yao, L.; Wei, Y.; Gui, T.; Li, Z.; Kim, H.; Holdreith, N.; Jiang, X.; Tong, W.; et al. Bone Marrow Adipogenic Lineage Precursors Promote Osteoclastogenesis in Bone Remodeling and Pathologic Bone Loss. Journal of Clinical Investigation 2021, 131. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N Engl J Med 2007, 356, 1809–1822. [Google Scholar] [CrossRef]

| Group | Surgery | Treatment | Number of injections | Dose/Injection | Euthanasia |
|---|---|---|---|---|---|
| Control | Not performed | None | Not applicable | Not applicable | 150 days |
| Sham | Surgery without ovaries remotion | None | Not applicable | Not applicable | 150 days |
|
Zoledronic Acid |
Oophorectomy | Zoledronic acid | Two injections with 22 days interval | 100 µg/kg | 150 days |
| Vehicle | Oophorectomy | None | 2x/week | 20mM Tris-HCl pH 7.2 + 300 mM NaCl | 150 days |
| PDGF-BB 2x | Oophorectomy | PDGF-BB | 2x/week | 20 µg/kg | 150 days |
| BMP-7 2x | Oophorectomy | BMP-7 | 2x/week | 30 µg/kg | 150 days |
| PDGF-BB + BMP-7 2x | Oophorectomy | PDGF-BB + BMP-7 | 2x/week | 20 µg/kg of PDGF-BB + 30 µg/kg of BMP-7 | 150 days |
| PDGF-BB 1x | Oophorectomy | PDGF-BB | 1x/week | 20 µg/kg | 150 days |
| BMP-7 2x | Oophorectomy | BMP-7 | 1x/week | 30 µg/kg | 150 days |
| PDGF-BB + BMP-7 1x | Oophorectomy | PDGF-BB + BMP-7 | 1x/week | 20 µg/kg of PDGF-BB + 30 µg/kg of BMP-7 | 150 days |
| Oophorectomy | Oophorectomy | None | Not applicable | Not applicable | 100 days |
| Score | Trabecular Thickness |
|---|---|
| 0 | Normal |
| 1 | Discrete reduction |
| 2 | Moderate reduction |
| 3 | Pronounced reduction |
| Score | Clinical condition |
|---|---|
| 0 | Normal |
| 1 | Mild osteoporosis |
| 2 | Moderate osteoporosis |
| 3 | Severe osteoporosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





