1. Introduction
When Branemark introduced osseointegration to North America at the Toronto Conference, in 1982, it was considered a quantum leap in the replacement of missing teeth with an implant that could mimic nature. With the continual optimization of macro- and microgeometry of dental implants and clinical techniques, implant survival rates have reached 96.4% in a10-year follow-up.1 However, the prevalence of peri-implantitis and its intractability to treatment has called into question whether titanium is the “gold standard” for dental implants.2 In fact, there has been a growing confluence of forces, catalyzed by a cosmetic zeitgeist, in support of a metal-free alternative such as zirconia that could potentially address biological, esthetic, and sensitivity concerns with titanium implants.
Accumulations of titanium particles have been detected in regional lymph nodes, bones and distant organs after implant placement in the jaw.3,4 Moreover, when titanium was placed in contact with fluoride in the saliva, a corrosion process has been demonstrated.5 Sridhar et al. also found that bacterial biofilms potentiated oxidation on the surface of titanium implants in an acidic environment.6 The longer implants were in situ, researchers found higher concentrations of corrosion products. There was also a synergistic effect of bacteria biofilm and cyclic occlusal loading (tribocorrosion), negatively affecting the surface of titanium dental implants. It has been reported that early formed biofilms have shown a higher accumulation on titanium implants compared with zirconia implants.7 Furthermore, zirconia has been documented to have a lower surface energy and wettability than titanium.8,9
Another limitation of titanium is its grey color which is difficult to mask in patients with a thin gingival biotype, compromising the cosmetic outcome.10 A 5-year cumulative esthetic complication rate of 7% has been reported with titanium implants.11 Continual craniofacial growth has been shown to lead to labialization of anterior implants over a lifetime.12 Given the growing cosmetic demand by 12% per year, fueled by the media, patients are becoming highly discriminating when there is even a slight discrepancy in the gingival color scalloping an implant restoration in the esthetic zone.13,14 Using the Pink Esthetic Score (PES), the esthetic superiority of zirconia implants has been established, compared to titanium.15 Without soft tissue grafting, zirconia implants led to less discoloration of the mucosa than titanium implants.16
Allergy to metals has also been implicated in autoimmunity and a putative link to neuroendocrinology.17-19 Investigators have postulated that in vivo, metal ions activate T-cells, via cytokines, affect the hypothalamus-pituitary-adrenal axis. While titanium allergy has been estimated at a low prevalence (0.6%), a significantly higher risk of an allergic reaction was found in patients with a predilection for a post-operative allergy compatible response.20 For these patients, an allergy test would be propitious to perform. Hypersensitivity reactions to zirconia, on the other hand are rare.21
Zirconium dioxide (ZrO2), a chemically inert material, is most often applied in dentistry as 3 mol% yittria-stabilized tetragonal zirconia polycrystal (3Y-TZP) to afford a flexural strength similar to titanium alloy, while having comparable osteoconductivity.22,23 This strength is achieved through a mechanism of phase transformation toughening with an opportune 4% volume expansion to counteract crack propagation.24,25 However, a legitimate concern about zirconia is its low temperature degradation (LTD) in the presence of water, resulting in a slow transformation from the tetragonal phase to the monoclinic phase, causing degradation.26
The phenomenon of LTD is influenced by the porosity of the material, inclusion of residual stresses, yittrium segregation, status of cubic phase, and grain size (<1 micron is ideal), stabilizer content of processed material, duration and temperature of sintering, and the use of dental procedures such as sandblasting or grinding.27 The concomitant use of lower-grade powders, higher sintering temperatures and direct exposure to oral fluids has the potential to trigger LTD, but clinical documentation of LTD has yet to be established.27-29 Attempts have been made to enhance the physical characteristics of zirconia ceramics by adding 20% of alumina to the material.30 This new compound has been identified as alumina-toughened zirconia. Initial short-term clinical data have been encouraging.31
Zirconia implants are generally fabricated by a subtractive manufactured technique by grinding in a pre-sintered or fully sintered state.32 This technique, however, requires a post-processing phase such as sandblasting or laser treatment to achieve the microtopography of a roughened surface and quicker bone apposition compared with machine surfaces.33,34 However, reduced survival rate of zirconia implants has been documented using a post-production surface roughening method.35 A promising alternative method of production is ceramic injection molding, allowing the shape of the molds to deliver definitive surface characteristics.36,37 Another workflow for production of zirconia implants without post-processing treatment is of additive manufacturing, or 3D printing.38 In addition to eliminating a source of LTD, these manufacturing techniques are markedly more cost effective, due to the lack of waste and precipitous wear of grinding machinery. However, more studies will be needed to assess dynamic loading and aging of these alternative techniques for the predictable production of zirconia implants.
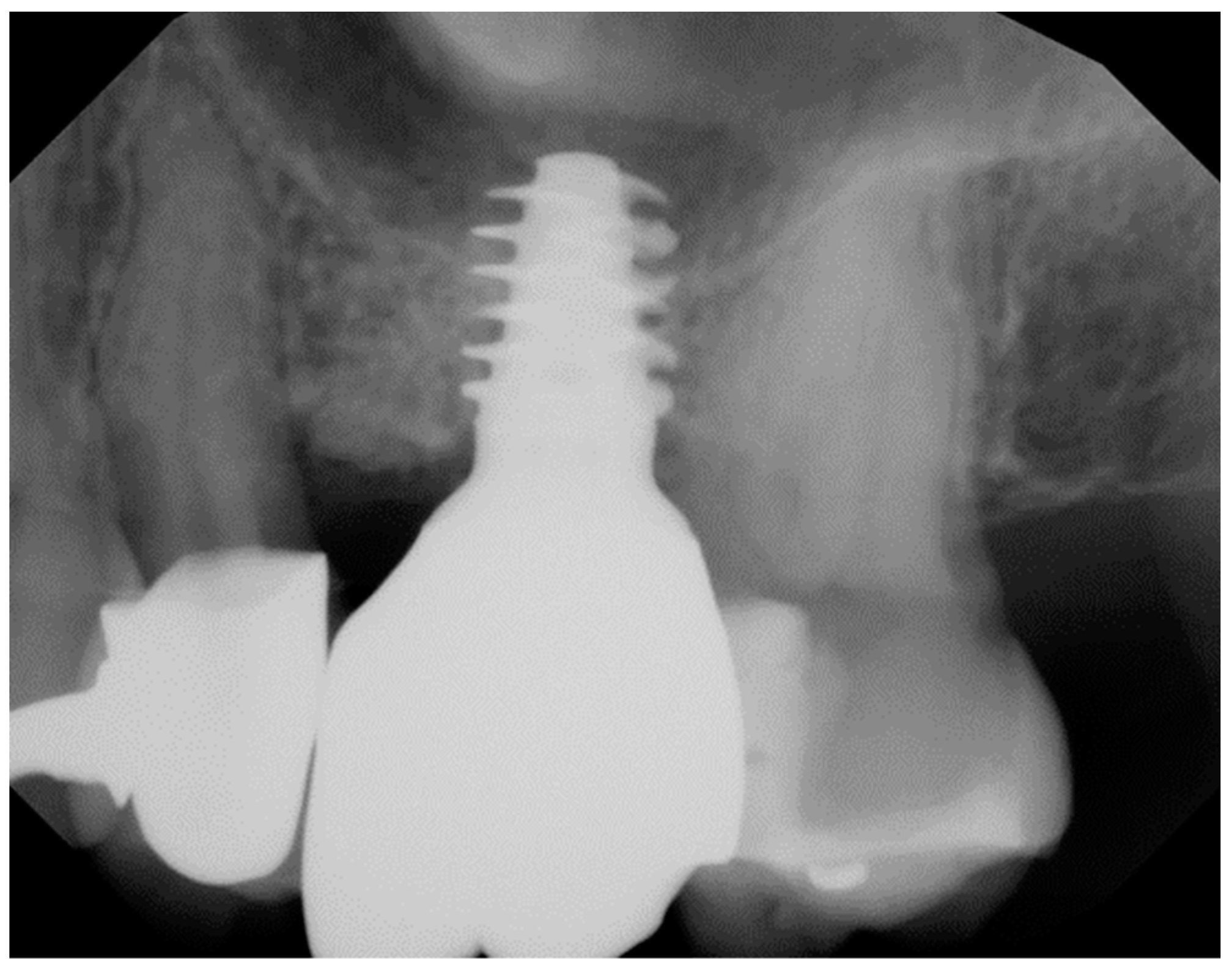
Geometry and diameter also play a significant role in the success of zirconia implants. Excessive implant thread depth is not recommended as the surgical placement might generate high bending forces on the implant body when engaging the bony perimeter of the osteotomy, especially in patients with dense bone (
Figure 1).
39 Additionally, narrow diameter zirconia implants (<3.75 mm) have been reported to have a higher incidence of fracture compared with the regular diameter implants.
40-42
The purpose of this mapping review is to present the breadth of evidence to answer the question, “What do studies reveal about the efficacy of zirconia implants”? This includes primary preclinical and clinical research as well as reviews.
2. Search Strategy
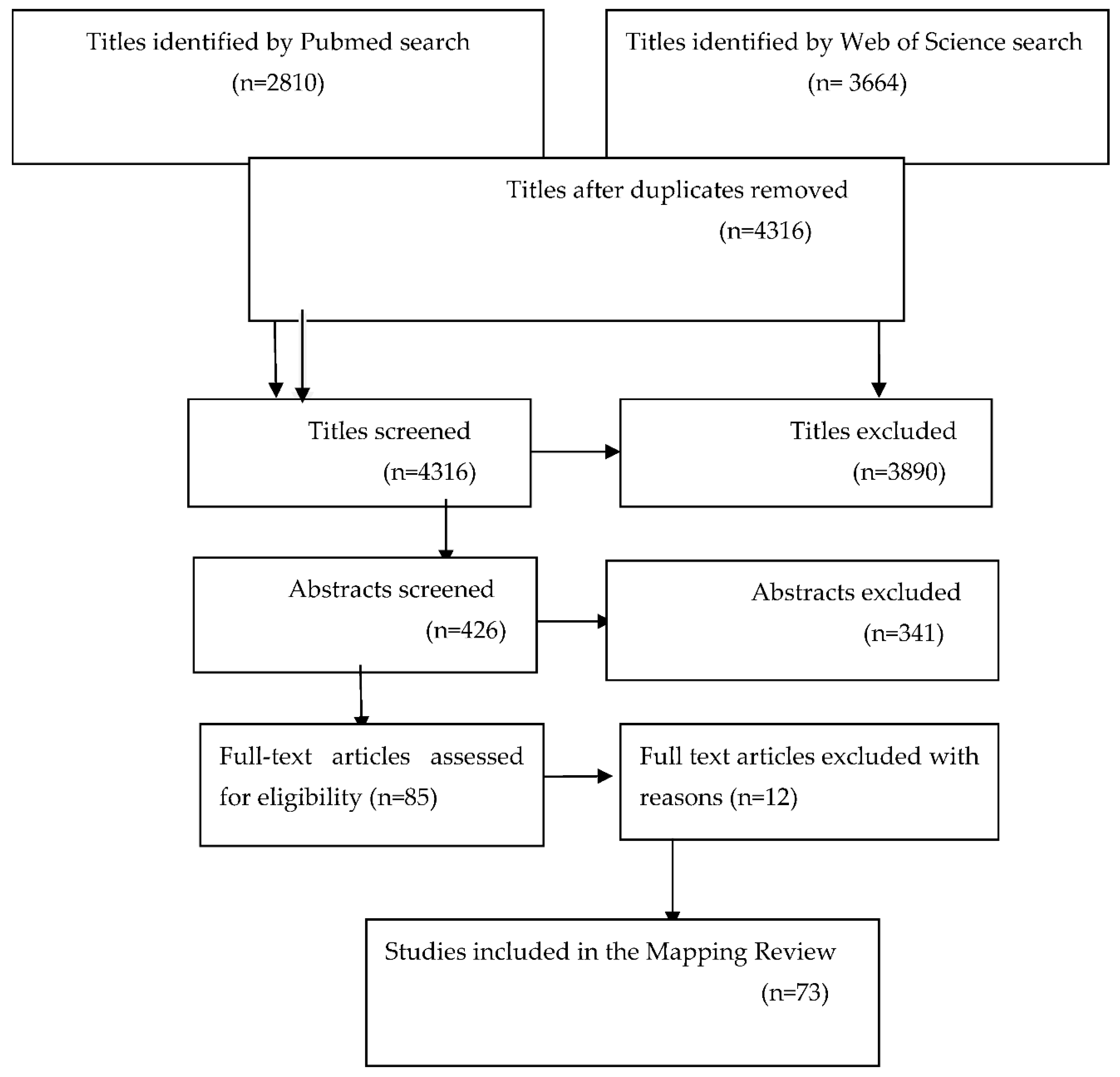
An electronic search of Pubmed and Web of Science databases was performed to select articles from January 1, 2000 to October 1, 2023, using zirconia implants as specific search term. From 6474 titles, 426 abstracts and 85 full-text articles were screened. Additional hand searches were included from the following journals:
International Journal of Oral & Maxillofacial Implants, Journal of Clinical Periodontology, Journal of Periodontology, Clinical Oral Implants Research, Clinical Implant Dentistry and Related Research, and Journal of Prosthodontics. A total of 73 articles were included, from the time period bracketed, that met eligibility criteria of outcome measures with zirconia implants and stipulated the design of the implant system (
Figure 2).
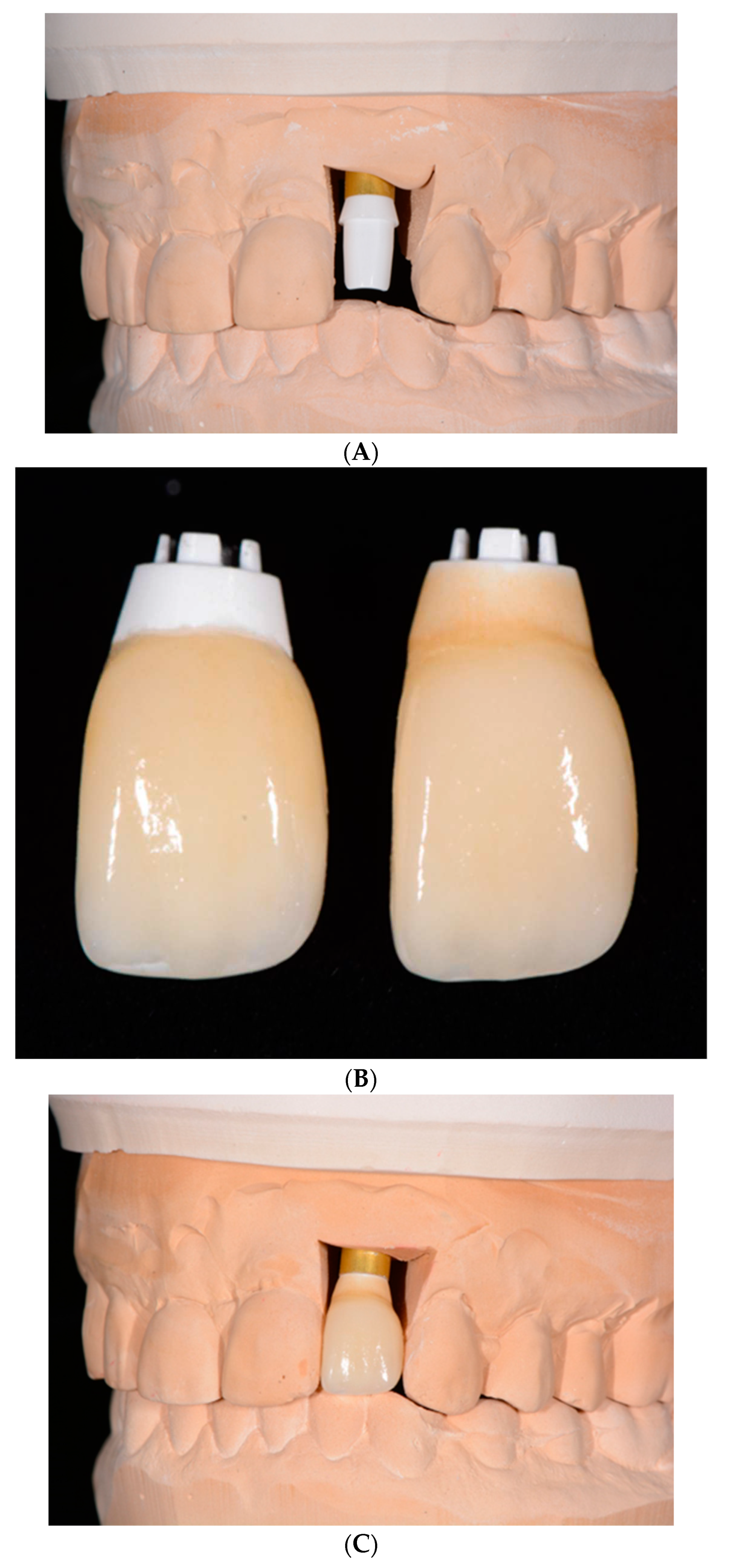
3. One-piece Zirconia Implants
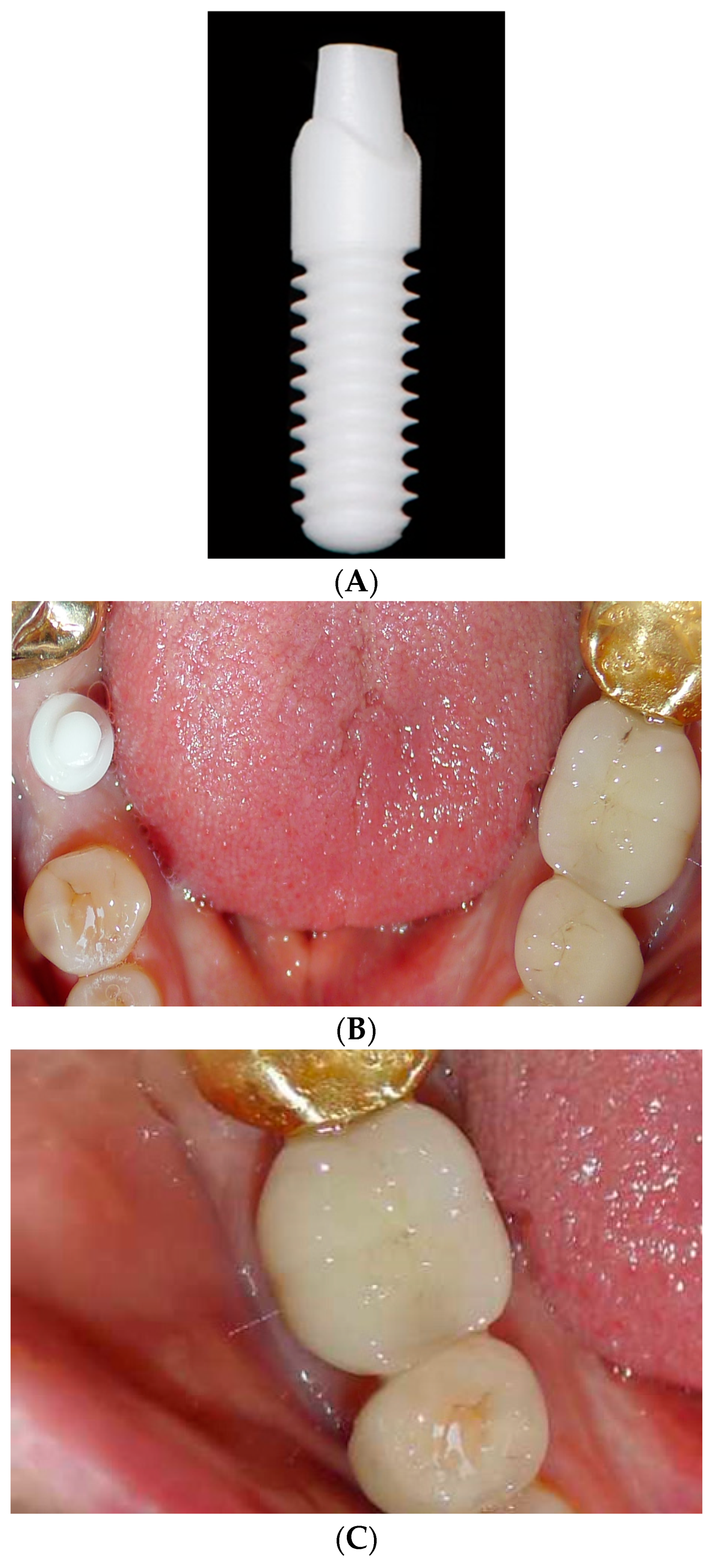
Most of zirconia implants have been produced and clinically researched as one-piece implants (
Figure 3A–C).
43,44 The advantage of this design is that by eliminating the abutment-implant microgap and micromovements, marginal bone loss (MBL) may be diminshed.
45 Nevertheless, the design has several drawbacks. Surgical placement is more challenging to meet both the bony compartment parameters and prosthetic design requirements. Grafting and augmentation options are also more limited. No angled abutments are available and secondary corrections of shape by grinding must be avoided because of the impact on reducing the fracture strength (from 2084 N to 804N).
46,47 Furthermore, one-piece implants cannot be protected from mastication after placement. Patients with parafunction, or those who are not compliant with admonitions of dietary limitations, would not be candidates. One-piece implants also tend to be inserted more deeply in the esthetic zone to hide the crown margin which compromises cement extrusion removal, potentiating peri-implant disease.
48
A prospective multicenter clinical investigation evaluating the 5-year outcome of single-tooth zirconia one-piece implants divulged a similar success and survival rate reported for titanium implant restorations.22 Balmer et al. reported 5-year results of a prospective cohort investigation with a survival rate of 98.4% and the mean marginal bone loss was 0.7 mm + 0.6 mm.49 The authors observed that after the initial mean MBL before loading the implants, no additional significant marginal bone level change was evident. Hassouna et al. compared the restorations of one-piece fusion-sputtered zirconia implants with one-piece titanium implants in a clinical and radiological study, with a 5-year follow-up.50 There was no significant difference between marginal bone loss of the immediately loaded titanium or zirconia implants placed in the maxillary first premolar site. However, when evaluating specific survival and success data for one-piece zirconia implants after an 8-year follow-up, there were differences between immediately placed and delayed placement zirconia implants.51 While the survival was 100% for both groups, the immediately placed implants had a success rate of 94.7% and the delayed implants 87.5%. The PES also revealed a more favorable esthetic score for the immediate zirconia implants, compared with the delayed methodology. The inclusion criteria for immediate placement of zirconia implants in this study included adequate bone volume and strict compliance, while the exclusion criteria included no inflammation, no hypermastication or clenching, and no untreated periodontal disease during a 3-month healing period. It must be noted that the inclusion/exclusion criteria were not as stringent for the delayed placement approach. This may help to explain why other investigations with different criteria for patient candidacy found no significant differences in survival and complications in immediate and delayed placement of titanium or zirconia implants.52,53
Overall, a recent systematic review and meta-analysis performed on 11 studies has established, in the short-term, one-piece ceramic implants achieve osseointegration similar to titanium implants, with a stable marginal bone response.54 For the long-term, the MBL change was on average 1.24 mm + 0.16 mm. The risk of implant fracture is low for current commercially available implants. Immediate loading or temporization of the implants does not appear to interfere with the course of osseointegration. However, it is important to note that significant heterogeneity, limited sample sizes, and lack of long-term randomized clinical studies compromise definitive conclusions regarding single-piece zirconia implant comparisons with titanium implants.55
4. Two-piece Zirconia Implants
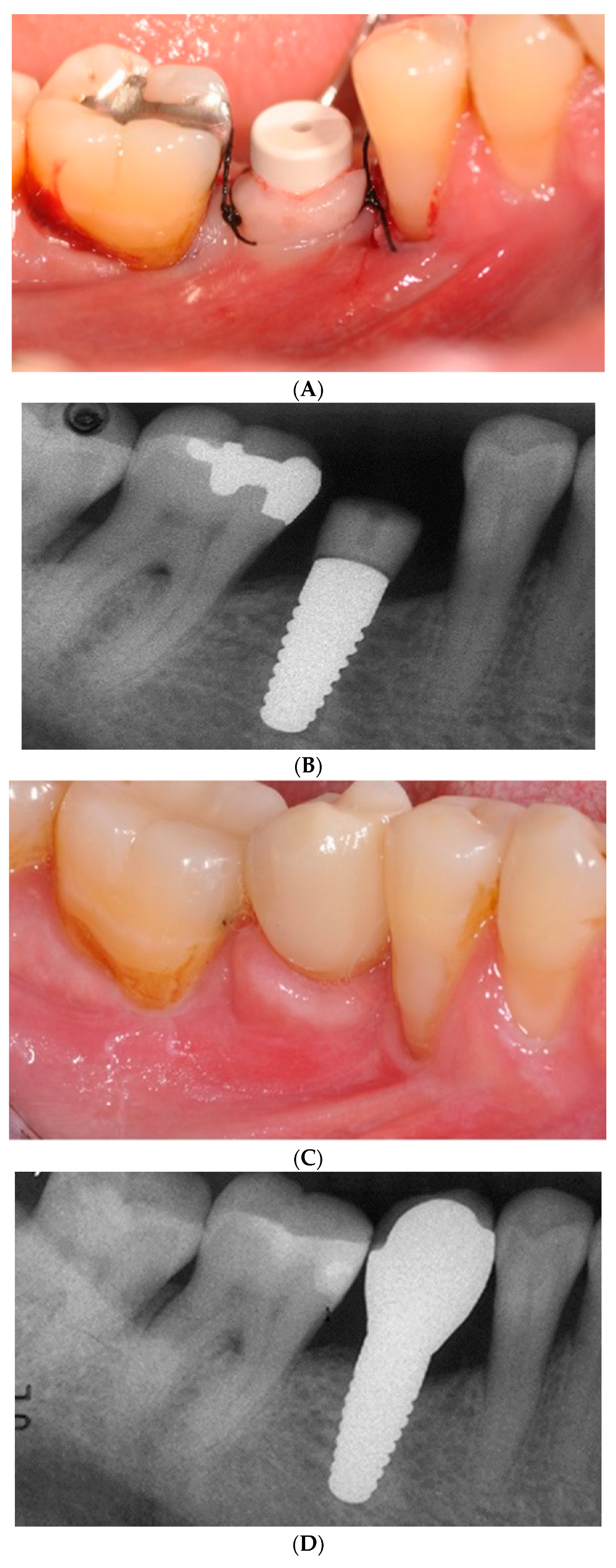
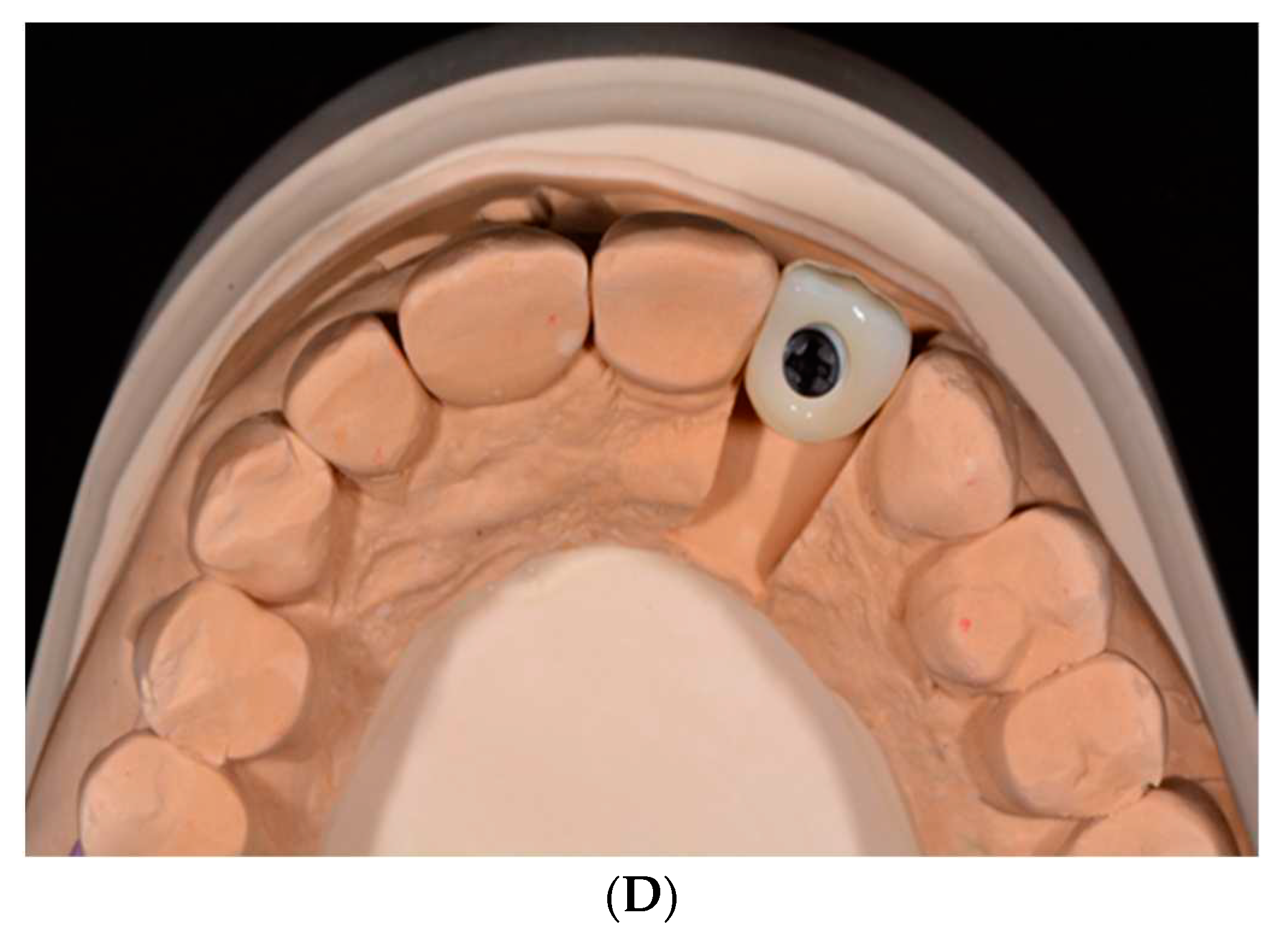
Two-piece implants (
Figure 5A–D) are designed for submerged healing which may minimize the initial bone resorption.
56 A prospective randomized clinical trial (RCT) of two-piece titanium and zirconia implants followed for 80 months, albeit in a small sample (28), divulged no significant differences in peri-implant disease, bleeding on probing, MBL, PES, implant or abutment fractures, or debonding of crowns.
57 Moreover, investigations with shorter observational periods ranging from 18-30 months were in agreement with this RCT and the survival ranged between 93.3% and 96.6%. A cohort study on 46 patients restored with two-piece zirconia implants in the posterior maxilla and, with a mean follow-up time of 9 years, reported a high survival rate. However, this study did report frequent mechanical and technical complications. Furthermore, a contemporaneous prospective cohort study on two-piece zirconia implants, with at least a 6-year follow-up, revealed a troubling 83% cumulative implant survival rate and a 63% success rate.
58 Implant failure was noted with 5 patients due to “aseptic loosening” or a sudden mechanical breakdown of the interface between the bone and the implant. Fracture of a standard diameter implant and a rapid progression of peri-implantitis were responsible for 2 more implant failures. Abutment fracture, using an adhesive material for the design, was the second most common technical complication. Using a two-piece implant design of a lobular connection mated with a PEEK abutment screwed to the implant, a retrospective cross-sectional 5-year study divulged a 73% implant survival rate, 82% success rate (
Figure 4). Machined zirconia components have not been shown to reach the same precision as titanium counterparts.
59 All these studies are limited by their heterogenous methodology applied, lack of randomization, relatively small sample size and lack of blinding of the participants or examiner.
5. Custom-made Root-analogue Zirconia Implants
The concept of replacing a tooth upon removal with an analogue that mimics the contours of its predecessor has many potential benefits. These include uncomplicated immediate implant placement, a minimally invasive surgical approach, decreased number of surgeries, and an absence of a microgap for bacterial adherence.47,60-62 While root analogue implants using titanium were introduced in 1992 by Lundgren,63 zirconia has offered esthetic and biological enhancements, without giving up strength. This includes no metal aura in thin mucosal biotypes, increased corrosion resistance, high biocompatibility, and hypoallergenicity.64,65
The manufacturing of a custom-made root analogue zirconia implant can be accomplished by using computer aided design/computer aided manufacturing as well as additive or subtractive manufacturing combined with cone beam computed technology (CBCT).
66 This technique is limited to cases of periodontally sound teeth with a sufficiently deep socket, an atraumatic extraction and absence of periapical disease. The root analogue is manufactured after a crown preparation is completed, the application of micro-retentions limited to the interdental space, and the diameter of the analogue is reduced (
Figure 6).
43,60
A scoping review on custom-made zirconia root analogue implants has concluded that this technique is promising and may prevent a loss of alveolar bone volume with maintenance of the peri-implant soft tissues, while attaining ideal optical and mechanical properties.62 Nonetheless, there is a need for investigations that can assess how zirconia-based surfaces can be enhanced to promote the absorption of proteins preparatory to migration of osteogenic cells. Since the usual acid-etching procedures cannot used with zirconia-based materials without aging, novel micro- and nanotechnologies are warranted to assure the percentage of osseointegration is acceptable along the time of bone healing, especially when subtractive manufacturing is used.67 More long-term, clinically controlled studies should follow.
6. Discussion
While a 2023 systematic review and meta-analysis has concluded that commercially available zirconia implants demonstrated reliable clinical outcomes based on survival rates, pocket depth and MBL values. the preponderance of studies was evaluating one-piece implants.68 Their conclusion was more well-designed clinical studies and randomized clinical studies comparing zirconia and titanium implants are needed. When attempting to compare the survival and success of zirconia compared with titanium implants, a recent meta-analysis has highlighted the poor evidence available as the authors were relegated to only 2 RCTs with 12-month follow-up data.69 A 2021 European Academy of Osseointegration Consensus Conference suggested that titanium implants may present relatively higher survival and success rates than zirconia implants over a short-term follow-up, but longer term controlled studies are lacking to clarify the superiority. Nonetheless, previous systematic reviews have reported a higher 5-year survival rate for titanium implants with single crowns.70 This has likely been the result of combining data between the one-piece and two-piece zirconia implants. Survival of one-piece implants for that follow-up period have been 98.4%,49 but the survival of two-piece zirconia implants, which offer more restorative flexibility, in multiple studies has still averaged below titanium implants at 87%.71,72
While implant survival is homogeneously reported, success criteria has not been well defined for zirconia implants. Short-term investigations have indicated that success for zirconia implants ranges from 93-100%,11,73 but longer follow-up studies have success rates ranging from 57.5% to 93.3%.69 This is likely due to differences in implant position, combining data from one- and two-piece implants and variation in observation time.
Root analogue zirconia implants offer a minimally invasive and simplified technique for replacing a tooth, but the design is limited to patients without a pre-existing periodontal defect and an atraumatic extraction. Moreover, this approach requires a CBCT, scanning and manufacturing armamentaria. Only short-term follow-up on a small number of patients have demonstrated 100% survival and minimal marginal bone loss.74
The three iterations of zirconia implants all offer advantages and disadvantages in their design. Overall, zirconia has outstanding mechanical flexural and compressive strengths, excellent wear resistance and is esthetic. One-piece zirconia implants eliminate a microgap that can be the source for an inflammatory infiltrate. Medium and long-term studies have corroborated the high success rate of the one-piece zirconia implants. On the other hand, they do not offer flexibility in design and require immediate loading protocol for all patients. Two-piece zirconia implants have been designed to offer surgical and restorative latitude in placement. However, the fit of machined components has been problematic and is the source of lower success rates compared to the one-piece implant. Root analogues have the advantage of a simplified surgical operation, but longer-term studies are lacking.
The promise of zirconia as a biocompatible, esthetic and fracture resistant replacement for titanium implants with similar or greater success has not been clearly realized in the literature. Future work on standardizing the zirconia implant and its surface to meet osseointegration needs, two-piece design improvements, more longitudinal comparative studies with titanium implants assessed with definitive success criteria/patient related outcomes, and a careful evaluation the effects of guided bone regeneration and regenerative procedures would be instrumental in promoting enhanced outcomes and a broader acceptance. The use of three-dimensional imaging to evaluate the bony adaptation around zirconia implants would also divulge substantive data for future improvements.
The limitation of this review has been the need to draw on heterogeneous evidence. The lack of data on number of zirconia implants, populations, operators, randomization, type of complications, zirconia material/design, and long-term comparative follow-up with titanium implants have compromised more reliable deductions about the present state of the art of zirconia implants in its various iterations. Future directions should include more robust N-sizes for randomized clinical trials and split mouth design methodology to assess the long-term success of the 3 different iterations of zirconia implants compared to titanium implants.
7. Conclusions
Zirconia implants offer a potential solution to esthetic and biological limitations of titanium implants, but a combination of design and material enhancements, as well as more rigorous comparative long-term studies are needed before validating their more universal application.
References
- Howe, M.-S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, SJ. Peri-implantitis after 40 years: Evidence, mechanisms, and implications: A mapping review. J Prosthet Dent 2023. [Online ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- del Amo, F.S.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Nelson, K.; Tarnow, D.; Wang, H.-L.; Giannobile, W. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2017, 97, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Fovet, Y.; Gal, J.-Y.; Toumelin-Chemla, F. Influence of pH and fluoride concentration on titanium passivating layer: stability of titanium dioxide. Talanta 2001, 53, 1053–1063. [Google Scholar] [CrossRef]
- Sridhar, S.; Wang, F.; Wilson, T.G.; Palmer, K.; Valderrama, P.; Rodrigues, D.C. The role of bacterial biofilm and mechanical forces in modulating dental implant failures. J. Mech. Behav. Biomed. Mater. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Chiou, L.-L.; Panariello, B.H.D.; Hamada, Y.; Gregory, R.L.; Blanchard, S.; Duarte, S. Comparison of In Vitro Biofilm Formation on Titanium and Zirconia Implants. BioMed Res. Int. 2023, 2023, 1–7. [Google Scholar] [CrossRef]
- Nishihara, H.; Adanez, M.H.; Att, W. Current status of zirconia implants in dentistry: preclinical tests. J. Prosthodont. Res. 2018, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Al-Radha, A.S.D.; Dymock, D.; Younes, C.; O’Sullivan, D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J. Dent. 2012, 40, 146–153. [Google Scholar] [CrossRef]
- van Brakel, R.; Noordmans, H.J.; Frenken, J.; de Roode, R.; de Wit, G.C.; Cune, M.S. The effect of zirconia and titanium implant abutments on light reflection of the supporting soft tissues. Clin. Oral Implant. Res. 2011, 22, 1172–1178. [Google Scholar] [CrossRef]
- Roehling, S.; Woelfler, H.; Hicklin, S.; Kniha, H.; Gahlert, M. A Retrospective Clinical Study with Regard to Survival and Success Rates of Zirconia Implants up to and after 7 Years of Loading. Clin. Implant. Dent. Relat. Res. 2015, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Daftary, F.; Mahallati, R.; Bahat, O.; Sullivan, R.M. Lifelong Craniofacial Growth and the Implications for Osseointegrated Implants. Int. J. Oral Maxillofac. Implant. 2013, 28, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Theobald, A.H.; Wong, B.K.J.; Quick, A.N.; Thomson, W.M. The impact of the popular media on cosmetic dentistry. N Z Dent J 2006, 102, 58–63. [Google Scholar]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Kniha, K.; Kniha, H.; Grunert, I.; Edelhoff, D.; Hölzle, F.; Modabber, A. Esthetic Evaluation of Maxillary Single-Tooth Zirconia Implants in the Esthetic Zone. Int. J. Periodontics Restor. Dent. 2019, 39, e195–e201. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.; Hüsler, J.; Jung, R. Discoloration of the Peri-implant Mucosa Caused by Zirconia and Titanium Implants. Int. J. Periodontics Restor. Dent. 2016, 36, 39–45. [Google Scholar] [CrossRef]
- Stejskal, V.; Hudecek, R.; Stejskal, J.; Sterzl, I. Diagnosis and treatment of metal-induced side-effects. Neuro Endocrinol Lett 2006, 7–16. [Google Scholar]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Payne, A.G.T.; De Silva, R.K.; Duncan, W.J. Titanium allergy: could it affect dental implant integration? Clin Oral Implants Res 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef]
- Dawson-Amoah, K.G.; Waddell, B.S.; Prakash, R.; Alexiades, M.M. Adverse Reaction to Zirconia in a Modern Total Hip Arthroplasty with Ceramic Head. Arthroplast. Today 2020, 6, 612–616. [Google Scholar] [CrossRef]
- Gahlert, M.; Kniha, H.; Laval, S.; Gellrich, N.-C.; Bormann, K.-H. Prospective Clinical Multicenter Study Evaluating the 5-Year Performance of Zirconia Implants in Single-Tooth Gaps. Int. J. Oral Maxillofac. Implant. 2022, 37, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Wolkewitz, M.; Hinze, M.; Han, J.; Bächle, M.; Butz, F. Biomechanical and histological behavior of zirconia implants: an experiment in the rat. Clin. Oral Implant. Res. 2009, 20, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Vagkopoulou, T.; Koutayas, S.O.; Koidis, P.; Strub, J.R. Zirconia in dentistry: Part 1. Discovering the nature of an upcoming bioceramic. Eur. J. Esthet. Dent. 2009, 4, 130–151. [Google Scholar] [PubMed]
- Sanon, C.; Chevalier, J.; Douillard, T.; Cattani-Lorente, M.; Scherrer, S.S.; Gremillard, L. A new testing protocol for zirconia dental implants. Dent. Mater. 2015, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lughi, V.; Sergo, V. Low temperature degradation -aging- of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef]
- Chevalier, J.; Loh, J.; Gremillard, L.; Meille, S.; Adolfson, E. Low-temperature degradation in zirconia with a porous surface. Acta Biomater. 2011, 7, 2986–2993. [Google Scholar] [CrossRef]
- Deville, S.; Chevalier, J.; Gremillard, L. Influence of surface finish and residual stresses on the ageing sensitivity of biomedical grade zirconia. Biomaterials 2006, 27, 2186–2192. [Google Scholar] [CrossRef]
- Kohal, R.; Wolkewitz, M.; Mueller, C. Alumina-reinforced zirconia implants: survival rate and fracture strength in a masticatory simulation trial. Clin. Oral Implants Res. 2010, 21, 1345–1352. [Google Scholar] [CrossRef]
- Spies, B.C.; Sperlich, M.; Fleiner, J.; Stampf, S.; Kohal, R. Alumina reinforced zirconia implants: 1-year results from a prospective cohort investigation. Clin. Oral Implant. Res. 2015, 27, 481–490. [Google Scholar] [CrossRef]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implant. Res. 2018, 29, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Rohr, N.; Hoda, B.; Fischer, J. Surface Structure of Zirconia Implants: An Integrative Review Comparing Clinical Results with Preclinical and In Vitro Data. Materials 2022, 15, 3664. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2021, 13, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.-J.; Burkhardt, F.; Chevalier, J.; Patzelt, S.B.M.; Butz, F. One-Piece Zirconia Oral Implants for Single Tooth Replacement: Five-Year Results from a Prospective Cohort Study. J. Funct. Biomater. 2023, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Spies BC, Maass ME, Adolfsson E, Sergo V, Kiemle T, Berthold C, et al. Long-term stability of an injection-molded zirconia bone-level implant: A testing protocol considering aging kinetics and dynamic fatigue. Dent Mater 2017;33:954-965. [CrossRef] [PubMed]
- Kohal, R.; von Schierholz, C.; Nold, J.; Spies, B.C.; Adolfsson, E.; Vach, K.; Burkhardt, F. Influence of loading and aging on the fracture strength of an injection-molded two-piece zirconia implant restored with a zirconia abutment. Clin. Oral Implant. Res. 2022, 34, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Haseeb SA, K CV, Vijaykumar N, Sree Durga BA, Kumar AS, M KS. Finite element evaluation to compare stress pattern in bone surrounding implant with carbon fiber-reinforced poly-ether-ether-ketone and commercially pure titanium implants. Natl J Maxillofac Surg 2022;13:243-247. [CrossRef] [PubMed]
- Virdee, P.; Bishop, K. A review of the aetiology and management of fractured dental implants and a case report. Br. Dent. J. 2007, 203, 461–466. [Google Scholar] [CrossRef]
- Gahlert, M.; Burtscher, D.; Grunert, I.; Kniha, H.; Steinhauser, E. Failure analysis of fractured dental zirconia implants. Clin. Oral Implant. Res. 2011, 23, 287–293. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Al-Nawas, B. Narrow-diameter implants: A systematic review and meta-analysis. Clin Oral Implants Res 2018, 29 Suppl 16, 21–40. [Google Scholar] [CrossRef]
- Atalay, P.; Öztaş, D.D. Fatigue resistance and fracture strength of narrow-diameter one-piece zirconia implants with angled abutments. J. Esthet. Restor. Dent. 2022, 34, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, S.J. Has zirconia made a material difference in implant prosthodontics? A review. Dent. Mater. 2019, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Grohmann, P.; Sailer, I.; Steinhart, Y.; Fehér, A.; Hämmerle, C.; Strub, J.R.; Kohal, R. Evaluation of a one-piece ceramic implant used for single-tooth replacement and three-unit fixed partial dentures: a prospective cohort clinical trial. Clin. Oral Implant. Res. 2015, 27, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Hermann JS, Buser D, Schenk RK, Schoolfield JD, Cochran DL. Biologic Width around one- and two-piece titanium implants. Clin Oral Implants Res 2001;12:559-571. [CrossRef] [PubMed]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implant. Res. 2009, 20, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000 2017;73:241-258. [CrossRef]
- Staubli N, Walter C, Schmidt JC, Weiger R, Zitzmann NU. Excess cement and the risk of peri-implant disease - a systematic review. Clin Oral Implants Res 2017;28:1278-1290. [CrossRef]
- Balmer, M.; Spies, B.C.; Kohal, R.-J.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral Implants Res. 2020, 31, 452–462. [Google Scholar] [CrossRef]
- Hassouna, M.; Al-Zordk, W.; Aboshilib, M.; Ghazy, M. Clinical and radiographic prospective study of customized one-piece titanium and one-piece fusion-sputtered zirconia implants: five-year mean follow-up. BMC Oral Heal. 2022, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, S.; Liebermann, A.; Mast, G.; Heitzer, M.; Möhlhenrich, S.C.; Hölzle, F.; Kniha, H.; Kniha, K. Evaluation of one-piece zirconia dental implants: An 8-year follow-up study. Clin. Oral Investig. 2023, 27, 3415–3421. [Google Scholar] [CrossRef]
- Patel, R.; Ucer, C.; Wright, S.; Khan, R.S. Differences in Dental Implant Survival between Immediate vs. Delayed Placement: A Systematic Review and Meta-Analysis. Dent. J. 2023, 11, 218. [Google Scholar] [CrossRef]
- Grassi, F.; Capogreco, M.; Consonni, D.; Bilardi, G.; Buti, J.; Kalemaj, Z. Immediate occlusal loading of one-piece zirconia implants: five-year radiographic and clinical evaluation. Int. J. Oral Maxillofac. Implant. 2015, 30, 671–680. [Google Scholar] [CrossRef]
- Neugebauer, J.; Schoenbaum, T.; Pi-Anfruns, J.; Yang, M.; Lander, B.; Blatz, M.; Fiorellini, J. Ceramic Dental Implants: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2023, 38, 30–36. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef]
- Gamper, F.B.; Benic, G.I.; Sanz-Martin, I.; Asgeirsson, A.G.; Hämmerle, C.H.F.; Thoma, D.S. Randomized controlled clinical trial comparing one-piece and two-piece dental implants supporting fixed and removable dental prostheses: 4- to 6-year observations. Clin. Oral Implant. Res. 2017, 28, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Steyer, E.; Theisen, K.; Stagnell, S.; Jakse, N.; Payer, M. Two-piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clin. Oral Implant. Res. 2020, 31, 388–396. [Google Scholar] [CrossRef]
- Cionca N, Hashim D, Mombelli A. Two-piece zirconia implants supporting all-ceramic crowns: Six-year results of a prospective cohort study. Clin Oral Implants Res 2021;32:695-701. [CrossRef]
- Preis, V.; Kammermeier, A.; Handel, G.; Rosentritt, M. In vitro performance of two-piece zirconia implant systems for anterior application. Dent. Mater. 2016, 32, 765–774. [Google Scholar] [CrossRef]
- Pirker, W.; Kocher, A. Immediate, non-submerged, root-analogue zirconia implant in single tooth replacement. Int. J. Oral Maxillofac. Surg. 2008, 37, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Pirker, W.; Kocher, A. Immediate, non-submerged, root-analogue zirconia implants placed into single-rooted extraction sockets: 2-year follow-up of a clinical study. Int. J. Oral Maxillofac. Surg. 2009, 38, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Pessanha-Andrade, M.; Sordi, M.B.; Henriques, B.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Custom-made root-analogue zirconia implants: A scoping review on mechanical and biological benefits. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2018, 106, 2888–2900. [Google Scholar] [CrossRef]
- Lundgren D, Rylander H, Andersson M, Johansson C, Albrektsson T. Healing-in of root analogue titanium implants placed in extraction sockets. An experimental study in the beagle dog. Clin Oral Implants Res 1992;3:136-143. [CrossRef]
- Pirker, W.; Wiedemann, D.; Lidauer, A.; Kocher, A. Immediate, single stage, truly anatomic zirconia implant in lower molar replacement: A case report with 2.5 years follow-up. Int. J. Oral Maxillofac. Surg. 2011, 40, 212–216. [Google Scholar] [CrossRef]
- Van Dooren, E.; Calamita, M.; Calgaro, M.; Coachman, C.; Ferencz, J.L.; Pinho, C.; Silva, N.R. Mechanical, biological and clinical aspects of zirconia implants. Eur J Esthet Dent 2012, 7, 396–417. [Google Scholar] [PubMed]
- Regish, K.M.; Sharma, D.; Prithviraj, D.R. An Overview of Immediate Root Analogue Zirconia Implants. J. Oral Implant. 2013, 39, 225–233. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Gahlert, M.; Bacevic, M.; Woelfler, H.; Laleman, I. Clinical and radiographic outcomes of zirconia dental implants—A systematic review and meta-analysis. Clin. Oral Implant. Res. 2023, 34, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Padhye, N.M.; Calciolari, E.; Zuercher, A.N.; Tagliaferri, S.; Donos, N. Survival and success of zirconia compared with titanium implants: a systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 6279–6290. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implant. Res. 2007, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Müller, N.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: A prospective clinical study. Clin. Oral Implant. Res. 2014, 26, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, G.; Torchio, C.; Felice, P.; Leone, M.; Esposito, M. Immediate occlusal versus non-occlusal loading of single zirconia implants. A multicentre pragmatic randomised clinical trial. Eur J Oral Implantol 2010, 3, 111–20. [Google Scholar]
- Jank S, Hochgatterer G. Success rate of two-piece zirconia implants: a retrospective statistical analysis. Implant Dent 2016, 25, 193–198. [Google Scholar] [CrossRef]
- Mangano, F.G.; De Franco, M.; Caprioglio, A.; Macchi, A.; Piattelli, A.; Mangano, C. Immediate, non-submerged, root-analogue direct laser metal sintering (DLMS) implants: a 1-year prospective study on 15 patients. Lasers Med Sci. 2013, 29, 1321–1328. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).