1. Introduction
Humans are physiologically omnivorous and animal meat provides several essential nutrients (vitamin B12, essential amino acids, complete proteins, heme iron, creatine etc.) for maintaining health and promoting organism development, especially for children. Historically, livestock provides us foods of animal origin including meat. Although, during the last two centuries population growth, urbanization, economic growth, and flourishing markets all lead to the rapid increase in demand for meat and animal products on a global scale [
1]. Global meat production has tripled over the last four decades alone and is expected to more than double by the year 2050 to reach a total capacity of 470 million tons [
2]. Thus, the livestock sector can be considered as the fastest growing agricultural subsector as of today, employing 1.3 and supporting about 4 billion people worldwide. Of the total global protein consumption animal proteins now represent 40 % and this number is anticipated to increase with increasing population [
3].
Unfortunately, modern livestock production systems are associated with multiple environmental problems such as soil and water pollution, loss of habitat and biodiversity, increased soil erosion, and greenhouse gas emissions. World meat production at present is contributing between 15 to 24 % of total greenhouse gas emissions (higher than the transport industry) [
2]. Considering that the fossil energy to animal protein ratios as of now are at their lowest in the case of broilers (4:1) and at their highest in the case of lamb (57:1) it can be concluded that traditional meat production systems are also associated with energy inefficiencies, which only aggravate the summarized environmental concerns [
4].
Furthermore, even though animal meat is the most accessible source of essential nutrients, the production and consumption of meat, has been reported to also causes negative effects on human health. For example, there are reports that consumption of red and processed meat increases the risk of developing colon, lung, pancreatic and prostate cancers [
5,
6,
7,
8,
9,
10] and other chronic diseases [
6,
9,
11].
Adding to the stated above, traditional meat production is often unsanitary and relatively inefficient from the point of view of raw material transformation. By removing animals from the meat production process, most of the summarized problems may be alleviated [
12]. Thus, in recent years, cell-based meat (CBM), plant-based meat (PBM) and filamentous fungi-based meat (FBM) have raised great interest as efficient animal meat substitutes.
Cell-based meat (CBM), also known as in vitro meat, lab-grown meat, or cultured meat, refers to a range of products that are produced by cultivating animal cells in laboratory or industrial equipment. The technology behind CBM was developed upon advancements in stem cell biology, such as induced pluripotent stem cells, and tissue engineering techniques that were originally developed for medical purposes. CBM production consists of the isolation and cultivation of muscle and fat cells, the formulation of xeno-free culture mediums, the development of scaffolds, and the cultivation process within a bioreactor [
2].
Although, concerning human health, large-scale in vitro meat manufacturing may require increased artificial hormone supplementation into the cultivation medium. Additionally, so far, no viable methods have been adapted for the production of CBM on large scale without the use of antibiotics to prevent bacterial infections [
13]. Due to slower growth, the contamination risk in mammalian cell cultures is much higher than in microbial or yeast processes [
14]. Additionally, mammalian cells are generally difficult to work with since they are fragile in nature and the conventionally applied cultivation mediums are expensive, which in turn impacts the feasibility of such production [
15]. Furthermore, a notable challenge associated to in vitro meat production is scale-up [
1]. Current CBM production systems can reach production volumes of several dozen m
3 at maximum (per one bioreactor), which is insufficient to satisfy the potential consumer demand [
1]. Thus, current cell-based meat manufacturing at an industrial scale is hampered by high production costs, scale-up difficulties, and gaps in fundamental knowledge about employing cell cultures for food application [
12].
Another key challenge is the perception of in vitro meat by the public. Several limitations of CBM have been outlined previously, e.g., social, economic, and technical constraints including uncertain acceptance by the consumers [
16]. The latter
prima facie seems to be a serious challenge, which renders the perception of in vitro meat as unattractive and ‘unnatural’. Additionally, although cultured meat does not require slaughtering of farm animals for its production, multiple vegetarian sub-classes (such as emotional vegetarians) are considered not likely to include CBM in their diet. The mentioned statement can be explained by the fact that cultured meat is still formally meat and multiple vegetarian groups have already developed a visceral resistance to meat itself, not simply as a fear of health problems or disapproval of farming practices but more as a moral emotion of disgust and revulsion [
17].
Another alternative is fungal-based meat, e.g., mycoproteins, which compared to proteins from plant or animal sources poses multiple characteristic advantages. Products derived from fungi exhibit distinct nutritional profiles, lower production costs, and greater environmental benefits. Notably, filamentous fungi can be cultivated on inexpensive substrates, and even in substrates comprised from food industry by-products, e.g., sugarcane bagasses, and agricultural or forestry waste materials [
18,
19,
20] The meat-like texture of fungal mycelium is suitable to form traditional shaped food, e.g., meatballs, nuggets etc., which benefits the consumer acceptance. However, a major disadvantage is the sensory role, which might delay the filamentous fungi-based meat integration into Western markets, as it occasionally may be perceived as tough to chew and bitter. In eastern countries, fungi-based foods already have taken a niche in everyday human diets, therefore the acceptance of fungal derived products on a global scale could be a matter of time [
21].
Plant-based meat can be described as a meat-like substance, which is vegetarian and/or vegan friendly, as it uses ingredients derived solely from plant sources. Such products aim to recreate the flavor and texture close to that of animal meat using compounds derived from plants, to make it more compelling for consumers. However, recipe development and cooking methods play a crucial role in reaching the mentioned aim. The conventional components used in such recipes include proteins (sourced from soy and potatoes), fats (derived from coconut and sunflower oils), carbohydrates (such as potato starch and corn starch), nutritional additives (like yeast extract and vitamins), and other additives (including beet juice extract, apple extract, and plant hemoglobins), which are usually added to recreate ‘meat-like’ flavors [
22]. Genetically modified yeast strains, for example
Komagataella phaffii, previously known as
Pichia pastoris, were utilized to produce soy leghemoglobin (LegH), which mimics the color and flavor of meat in plant-based meatballs, patties, rissoles, etc. [
23]. Leghemoglobin is usually added to plant-based meat products in 0.5-2 % by mass (like the quantity of myoglobin in animal meat).
It’s worth noting that the LegH protein used in these processes via recombinant microorganism strains has received safety approval from the Food and Drug Administration (FDA) [
24]. As the GMO is not itself an ingredient of plant-based meat, such products are not considered genetically modified (or containing genetically modified ingredients) according to both European and US legislations. Thus, recent trends indicate that LegH meat substitutes will gain more interest in the near future, which will inevitably give rise to the demand for efficient cultivation/fermentation systems of plant-based meat mass production.
Apart from artificially adding LegH to the end product, the development of fermented PBM (using leghemoglobin containing cultures) has been investigated. The utilization of microorganisms or the construction of microbial consortia with specific bioconversion capabilities (e.g., proteolysis, aromatization via amino acid metabolism, or other) can offer a highly efficient means to make PBM more palatable. Presently, this approach is used to develop fermented products based on pea protein, aimed at diminishing the characteristics of the legumes and introducing new flavors (e.g., fruity or dairy-like palate) that are more appealing to consumers [
25,
26].
LegH producing recombinant microorganism strains, which were studied previously include
Candida,
Hansenula,
Komagataella, and
Toruplosis genus representatives [
27]. The mentioned strains are considered methylotrophic yeasts, which utilize alcohols (usually methanol) as substrates, and in their recombinant form can produce LegH via methanol-inducible promoter element. Although,
Komagataella phaffii seems to have promoted the most noticeable interest for LegH production, due to the positive characteristics of this strain, e.g., high growth rates and recombinant protein production capacities, a wide industrial application history in production of both pharma and food products and availability of standardized gene alteration methods [
28].
Conventionally,
Komagataella phaffii fermentations for recombinant protein production are operated in fed-batch, which is justified by the ability to provide controlled nutrient feeding, thus ensuring a possibility to optimize cell growth and protein expression [
29]. Furthermore, this approach extends the production phase, resulting in higher biomass and protein titers (in case the protein is endogenous) compared to traditional batch fermentations, which are often limited by nutrient depletion after the growth phase. Although, controlling feeding rates in fed-batch fermentations can be challenging, as it requires precise monitoring and adjustment to avoid issues such as nutrient imbalances (growth limitation) and the formation of byproducts (acetates and ethanol in the case of
K. phaffii), all of which can impact the overall success of the fermentation process [
30].
In
K. phaffii recombinant protein production the regulation of synthetic pAOX1 variants is based on principles of repression/promotion, using glucose or glycerol as the only substrate. At high concentrations of glycerol or glucose, pAOX1 variants are repressed, but at lower substrate availability are promoted during fed-batch cultures. In contrast to pGAP-controlled protein production with glucose as the substrate, the protein production typically increases with specific growth rates close to
μmax. The opposite is often found for pAOX1-controlled high-level recombinant protein formation induced by methanol, where maximum specific secretion rates were observed at a
μ considerably below μ
max.
K. phaffii strains with novel pAOX1 variant promoters, which are cultured with glycerol, are a combination of the two mentioned systems with respect to promoter features and substrate. As a result, product formation is not predictable
a priori and has to be determined empirically for each combination of promoter and heterologous gene [
31].
The most commonly used medium for the high cell density fed-batch fermentation of
K. phaffii is the basal salt medium (BSM) proposed by Invitrogen (USA) [
29,
32,
33]. The two-stage cultivation on glycerol and methanol associated with Invitrogen’s “Pichia Fermentation Process Guidelines” is well documented and present in most pAOX1-promoter-based cultivation strategies. However, recent trends advocate for a move away from standard protocols towards a more conceptual approach, which allows for the development of process-specific strategies tailored both to the specific combination of product/genetic construct and the characteristics of the bioreactor equipment [
34]. Furthermore, during the vast history of BSM application, authors have outlined multiple important problems, e.g., precipitation, unbalanced composition, and high ionic strength, which affect the culture performance. For example, as a result of precipitation, the actual concentration of dissolved minerals remaining in the medium is difficult to determine and also the turbidity caused by the salts complicates the measurement of cell densities (optical densities). Additionally, the BSM medium implies the use of NH
4OH for both pH control and the supply of nitrogen, which is necessary for cell growth. The mentioned fact can pose a significant problem in cases, where the nitrogen amount in the medium must be maintained at specific values and, thus, cannot be interlinked with other control mechanisms [
33]. Hence, the drawbacks of BSM give rise to the necessity to develop other physiologically rational and suitable mediums for efficient recombinant protein production in
Komagataella phaffii [
33].
Considering the rise in interest towards LegH production processes and apparent blank spots regarding the fermentation process specifics, the focus of the present research was aimed at the development and application of a production protocol for recombinant production of LegH protein by Komagataella phaffii through fed-batch fermentations in an alternative medium. The composition of the fermentation media and feeding solution was chosen among the ones published in the literature (with modifications), which in contrast to BSM do not incorporate nitrogen in the form of ammonium hydroxide (allowing independent pH and nitrogen level control) and do not influence medium turbidity.
2. Materials and Methods
2.1. Yeast Strain and Media
The
Komagataella phaffii X-33 strain used a basis for this work was the same as described by Pentjuss et. al. [
35]. Although, for the overexpression of leghemoglobin in the mentioned strain,
Komagataella phaffii genes involved in the heme pathway were chosen to be paired with strong promoters and integrated into the yeast genome. Sequences for HEM1 (XP_002491645.1) and HEM2 (BA75_04933T0) were identified by searching (BLAST)
Saccharomyces cerevisiae homologs in NCBI
Komagataella phaffii GS115 entries. The genes were synthesized by Twist Bioscience (USA). Gene sequences are attached in
Appendix A. Expression construct (pGAP-HEM1-TDH4tt-pAOX1-HEM2-chr4_0883tt) was created by Golden Gate Assembly as described in Prielhoffer et al. 2017 [
36]. The transformation was done by electroporation and positive colonies screened out by colony PCR.
The obtained yeast strain was maintained on YPD agar plates, containing the following reagents (per liter): yeast extract 10 g, bactopeptone 20 g, glucose 20 g, and agar 20 g. The inoculum for bioreactor fermentations was grown in 200 mL Erlenmeyer flask with 100 mL of medium containing (per liter) glucose 20 g, peptone 20 g, and yeast extract 10 g. Incubation was performed for 48 h at 30 °C in orbital shaker-incubator ES-20 (Biosan Ltd., Latvia).
A synthetic growth medium was used during cultivations in the laboratory bioreactor, see
Table 1. Each experiment was split into three stages: (1) batch phase – biomass growth without feeding supply, (2) fed-batch biomass growth with feeding supply and (3) LegH production induction. Leghemoglobin production was induced by activating the methanol feeding supply after an optimal fermentation time obtaining the biomass concentration of around 70 g dry cell weight (DCW) per liter. Determination of biomass DCW and leghemoglobin concentration is described below.
2.2. Fed-batch cultivations
Bioreactor fed-batch fermentation processes were carried out in a 5.4 L total volume EDF-5.4_1 (Bioreactors.net AS, Latvia) bench top system. During the fed-batch fermentations, the temperature was maintained at 30 °C, pH was controlled to be at 6.0 ± 0.2, dissolved oxygen was kept at 40 ± 5 %-sat. The pH was controlled by two peristaltic pumps, which automatically dosed H2SO4 (20 W%) or NaOH (10 W%) solutions into the medium. The foam level was registered via a conductivity sensor, which was mounted on the top lid of the bioreactor’s vessel. Upon reaching the set level the designated peristaltic pump automatically dosed Antifoam A (Sigma, Germany) until the foam level decreased to normal levels. The dissolved oxygen (DO) level was maintained according to a cascade algorithm, which operated with two control principles in a defined succession, e.g., primarily regulating the agitation rate (from 100 to 800 rpm), and secondly by enriching the inlet gas with oxygen. A flow rate of gas was maintained at 2.0 standard liter per minute (slpm) throughout the fermentation process.
The CO2 and O2 concentrations were measured online in the off-gas with the help of BlueInOneCell gas analyzer (BlueSens gas sensor GmbH, Germany). The ethanol concentration estimate was determined with an ethanol/methanol sensor MeOH Sensor (Raven BioTech, Canada).
The gas analysis data, e.g., respiratory quotient (RQ) and oxygen uptake rate (OUR) were calculated automatically by the EDF-5.4_1 system from the off-gas data.
The biomass samples were harvested aseptically for off-line product and metabolite measurements. The biomass concentration was determined through optical density (OD) measurements at 600 nm with a spectrophotometer Jenway UV-Visible Spectrophotometer 7205 (Cole-Parmer Instrument Co. Europe, UK). The glucose concentration in the medium was measured by a blood sugar analyzer AccuCheck ACTIVE (Roche, Switzerland).
The permittivity was measured by the Incyte VCD sensor (Hamilton Company, Switzerland).
2.3. Determination of Biomass Dry Cell Weight
The relationship between the dry cell weight (DCW) of biomass and the absorbance at a wavelength of 600 nm was determined using gravimetry. Initially, a portion of the growth medium containing
K. phaffii cells was harvested from the bioreactor during the exponential growth phase. The absorbance of this solution was then measured at 600 nm. Subsequently, the solution was divided into two equal parts. One part was placed in a convection oven and dried at 60 °C for a minimum of 24 to 48 h, or until the mass reading reached equilibrium. The second part of the solution underwent centrifugation at 3500 rpm for 10 minutes with lab-scale centrifuge Z 167 M (Hermle Labortechnik GmbH, Germany). The resulting supernatant was filtered through a glass filter and then transferred to a convection oven for drying at 60 °C for at least 24 to 48 h, until the mass reading reached equilibrium. Once both the supernatant and growth medium samples reached a constant mass, their masses were measured and used to calculate correlation coefficients. The following mathematical correlation (Equation (1)) was used to calculate the correlation coefficient:
where, X
DCW – biomass concentration in the fermentation medium, g(DCW)·L
−1; OD
600 – absorbance of the solution measured at 600 nm; m
x – mass of dry residue (left from the solution containing
K. phaffii cells), g; ms
n – mass of dry residue (left from the supernatant), g; V
x – volume of the solution containing
K. phaffii cells, L; V
sn – volume of the supernatant, L.
During the present study, the correlation coefficients value was determined as 0.145 g(DCW)·L−1·A.U.−1.
2.4. Determination of leghemoglobin concentration
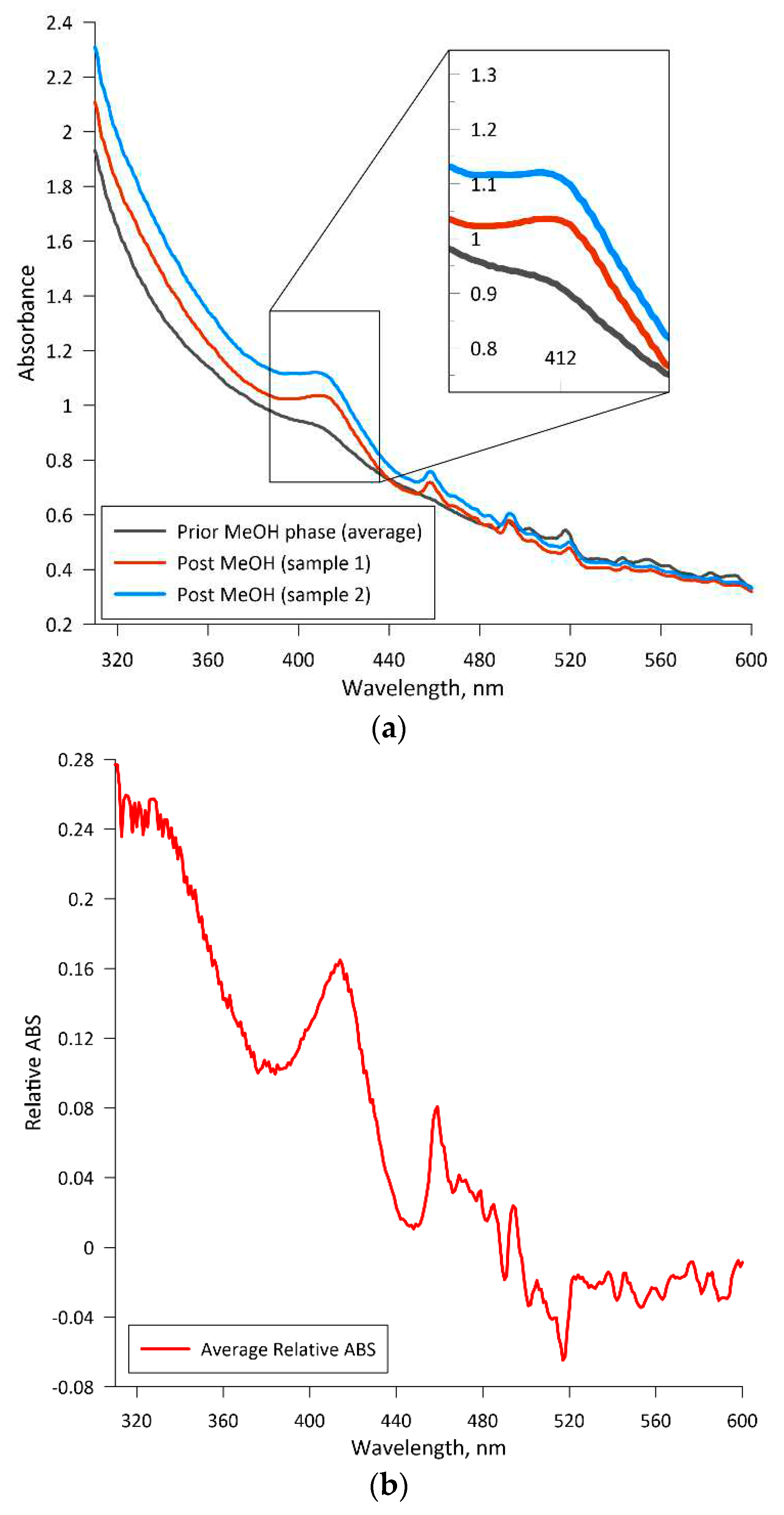
The concentration of leghemoglobin was determined through a developed absorption spectroscopy method, which was developed based on the information published by
Hopp et. al. [
37]. The first phase of this analysis involves washing the yeast biomass. Medium samples were harvested and transferred into four 15 mL vials at particular cultivation time points and then centrifuged. After centrifugation, the supernatant was carefully removed, and a saline solution (9 g·L
-1 NaCl) was added to the remaining precipitate, reaching the 7.5 mL mark. The contents of each two samples were then combined into one vial. This procedure was repeated until a single 15 mL vial with precipitate was obtained.
The second phase of the analysis was the cell disruption. Initially, an anti-coagulant solution, containing per liter: 1.5 g Na-EDTA and 0.2 g NaOH, was added to the 15 mL vial containing the previously obtained biomass precipitate. Suspension was achieved through vigorous agitation using a vortexer LLG-uniTEXER (Lab Logistics Group GmbH, Germany). Next, 1.5 mL of the suspension was transferred into two 2 mL vials. Each vial was then filled with glass beads with a diameter of 0.40-0.60 mm until the liquid level reached the 2.0 mL mark. These vials were then vortexed for at least 15 minutes, which was followed by refrigeration at 4 °C for 10 minutes. The vortexing and refrigeration steps are repeated a total of two times. Finally, the vials were centrifuged at 3500 rpm for 10 minutes. The supernatant is then harvested and diluted as needed with the anti-coagulant solution. The absorption spectra of the solutions were measured within the range of 300 to 600 nm using a quartz cuvette with a light path of 1 cm and a spectrophotometer Jenway UV-Visible Spectrophotometer 7205 (Cole-Parmer Instrument Co. Europe, UK).
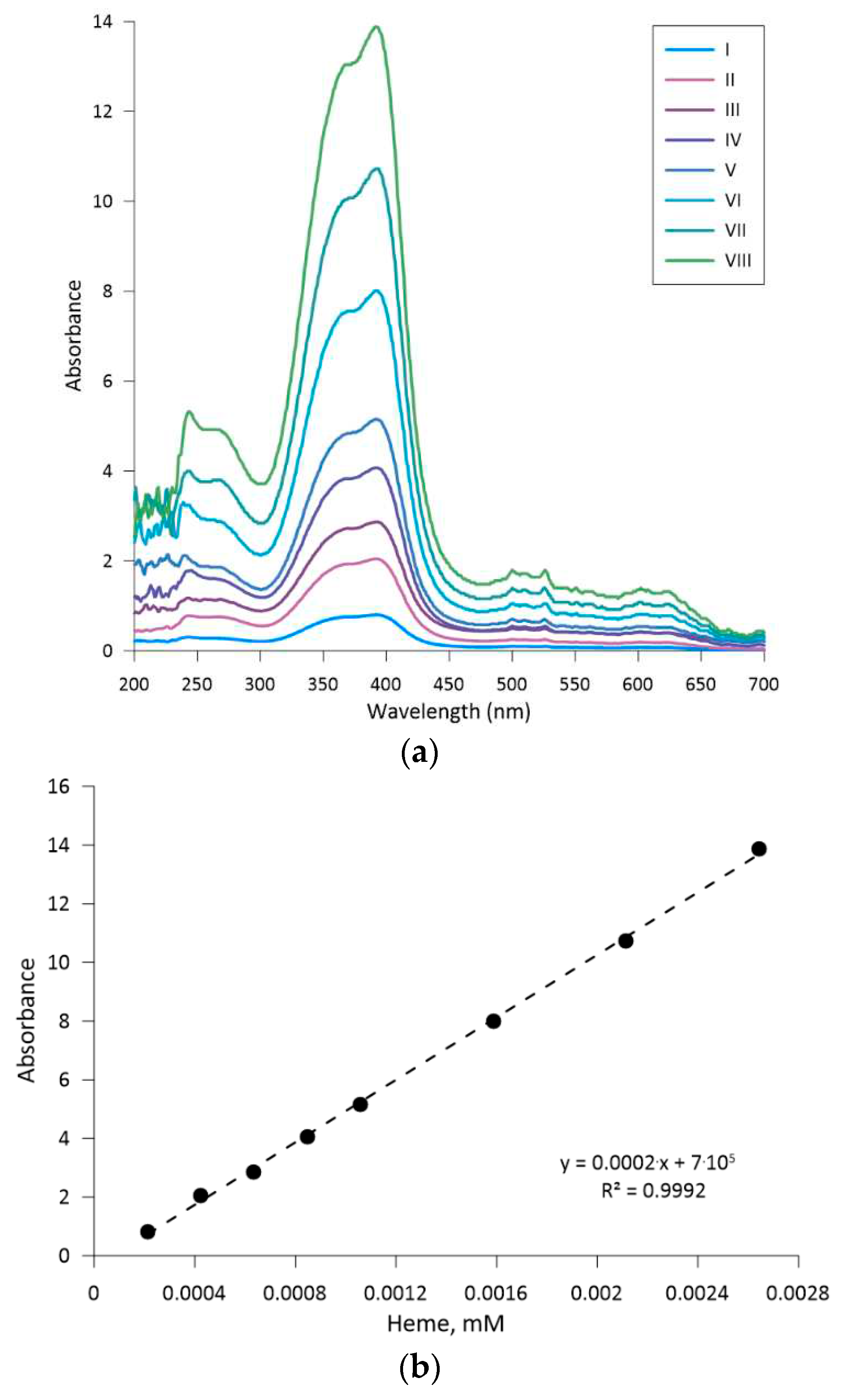
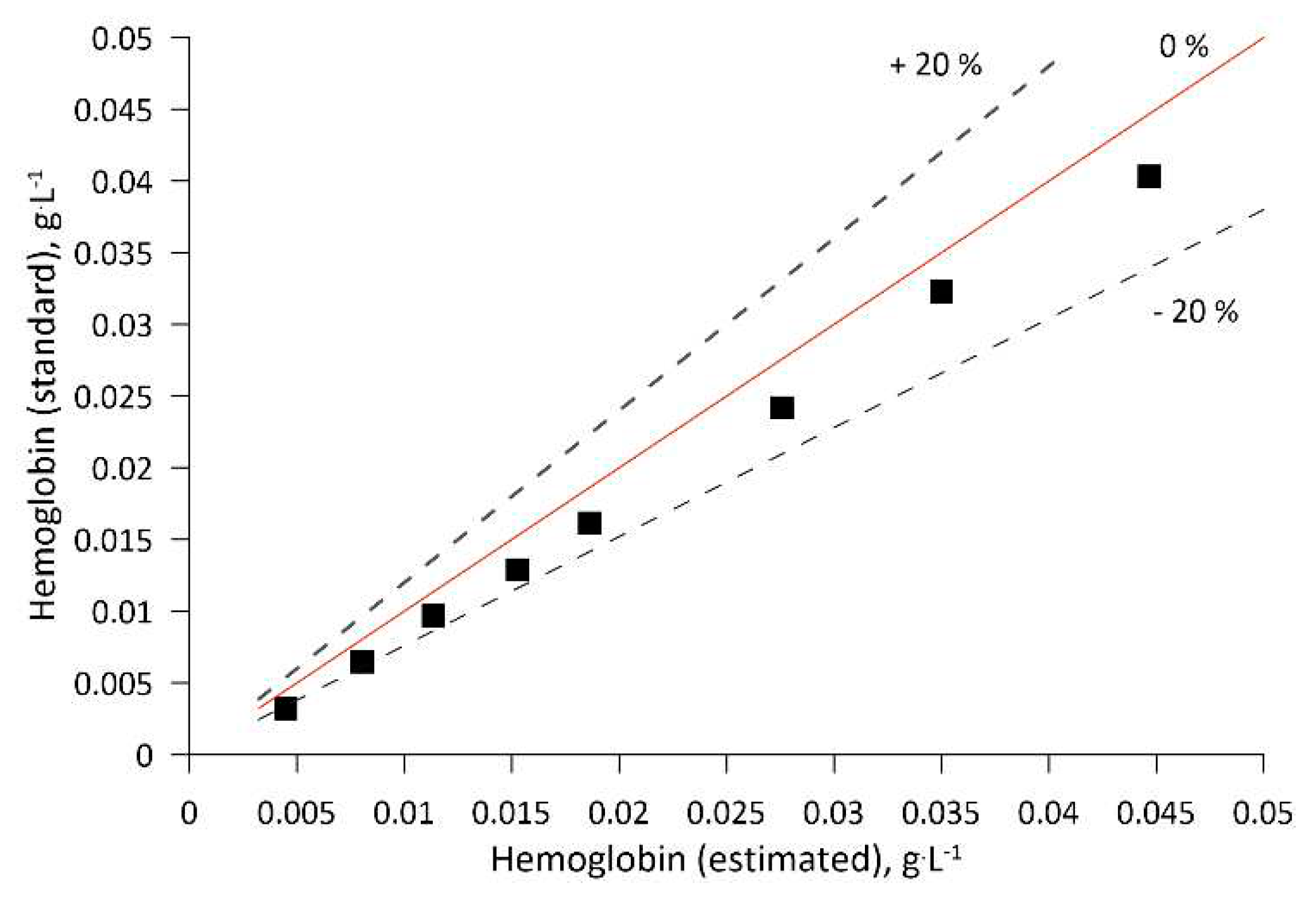
The absorption value at 415 nm was used for the calculation of leghemoglobin concentration in the biomass sample. The correlation between the absorption and LegH concentration was determined through calibration of the described method using analogous hemeproteins and compounds, e.g., hemin (51280, Sigma-Aldrich Co, USA) and hemoglobin (08449, Sigma-Aldrich Co, USA). As the heme molecule in leghemoglobin, hemin, and hemoglobin is responsible for light absorption at the characteristic wavelength, the A.U. vs Heme molecule molar concentration was constructed using hemin and validated for the hemoglobin case (see
Appendix B).
Considering the mentioned procedure, the following equation was used to calculate the amount of produced leghemoglobin per gram of dry cell mass:
where,
YP/DCW – yield of leghemoglobin per gram of yeast biomass, g·g(DCW)
−1;
cLegH – concentration of leghemoglobin in the final sample (measured through light absorption, see
Appendix B).
4. Discussion
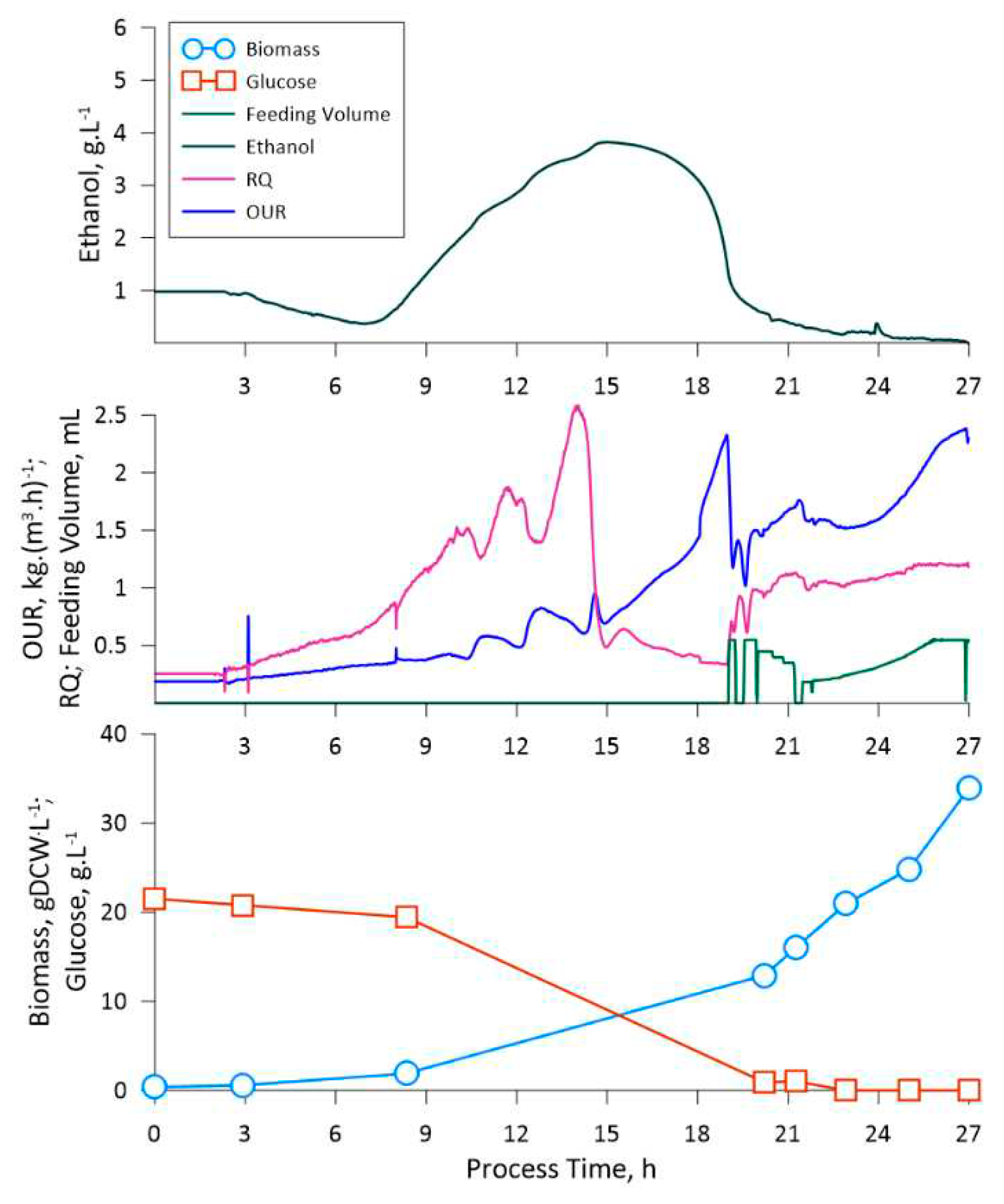
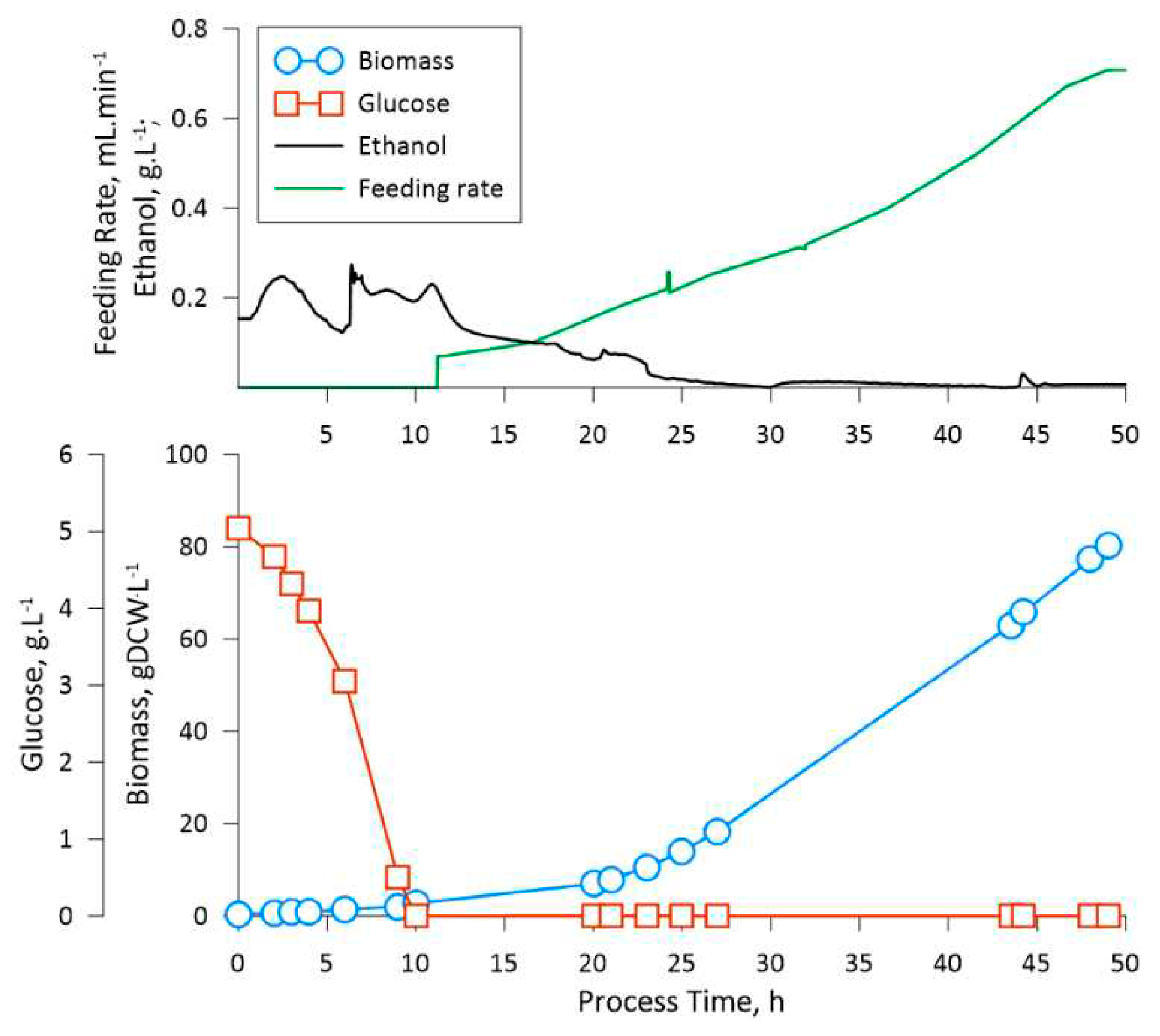
Primarily, the obtained experimental data on ethanol production in K. phaffii cultivations under the conditions of excess substrate, indicate that the even at relatively low initial glucose concentrations (5 g·L -1) ethanol synthesis is stimulated. From cultivations 1 – 3 it can be observed that the ethanol concentration during the batch phase is proportional to the initial glucose concentration, e.g., 0.2, 1.8 and 3.8 g·L -1 of ethanol at initial glucose concentration of 5, 10 and 20 g·L -1 respectively. The latter could be a potential bottleneck in the utilization of glucose-containing fermentation mediums for processes, where K. phaffii is used as the production platform. One aspect is the inefficient utilization of available substrate for biomass growth, e.g., a portion of the available substrate is converted into the secondary metabolite. Even though eventually, when the glucose is depleted, the yeast switches to ethanol consumption, such intermediate processes lower the final biomass yield as a portion of carbon is lost during the conversion from glucose to ethanol. The latter can be observed from the difference in Yxs estimated for the batch phase of the first (higher ethanol production) cultivation and the feeding phase of the third (no ethanol production) cultivation. The second most noticeable aspect is the lag, which is present when the biomass switches from one substrate to another, which could prolong the production process. For example, in the case of the LegH production phase the lag of switching from glucose to methanol amounted to roughly 10 hours.
Considering the stated above, 5 g·L-1 of initial glucose in the cultivation mediums could be suitable for K. phaffii based processing using the proposed medium composition as an alternative to BSM. Furthermore, the amount of biomass produced at the end of the pre-feeding phase is enough to implement continuous feeding techniques, e.g., without posing significant technical constraints on the liquid supply equipment (peristaltic pump productivity).
In comparison to medium based on glycerol as the main carbon source, glucose in the applied medium composition seemed to promote similar biomass growth rates [
33]. Although, a decrease in biomass yield from substrate, in relation to previously published data, has been noticed [
38]. The latter confirms the fact that glycerol as a substrate, delivers more ATP than glucose under aerobic conditions, leading to higher biomass and product yields compared to the use of glucose [
39]. Nevertheless, the properties of glucose, which make it highly easier to quantify at-line during the cultivation can justify the use of this substrate especially in process development, where determination of mass fluxes and calculation of growth kinetics is highly important.
In the context of biomass switching to different substrates, the RQ data exhibited high potential as a means of identifying the current state of the cultivation. In respect to the data available in multiple publications, the RQ value denotes the currently utilized substrate type, e.g., RQ values for fat, protein, and carbohydrate are 0.7, 0.8, and 1.0, respectively. RQ of > 1.0 might suggest excessive substrate provision that can result in the production of metabolites linked to the particular overflow metabolism state. A RQ of < 0.7 might suggest underfeeding and/or utilization of ketones and alcohols as substrates [
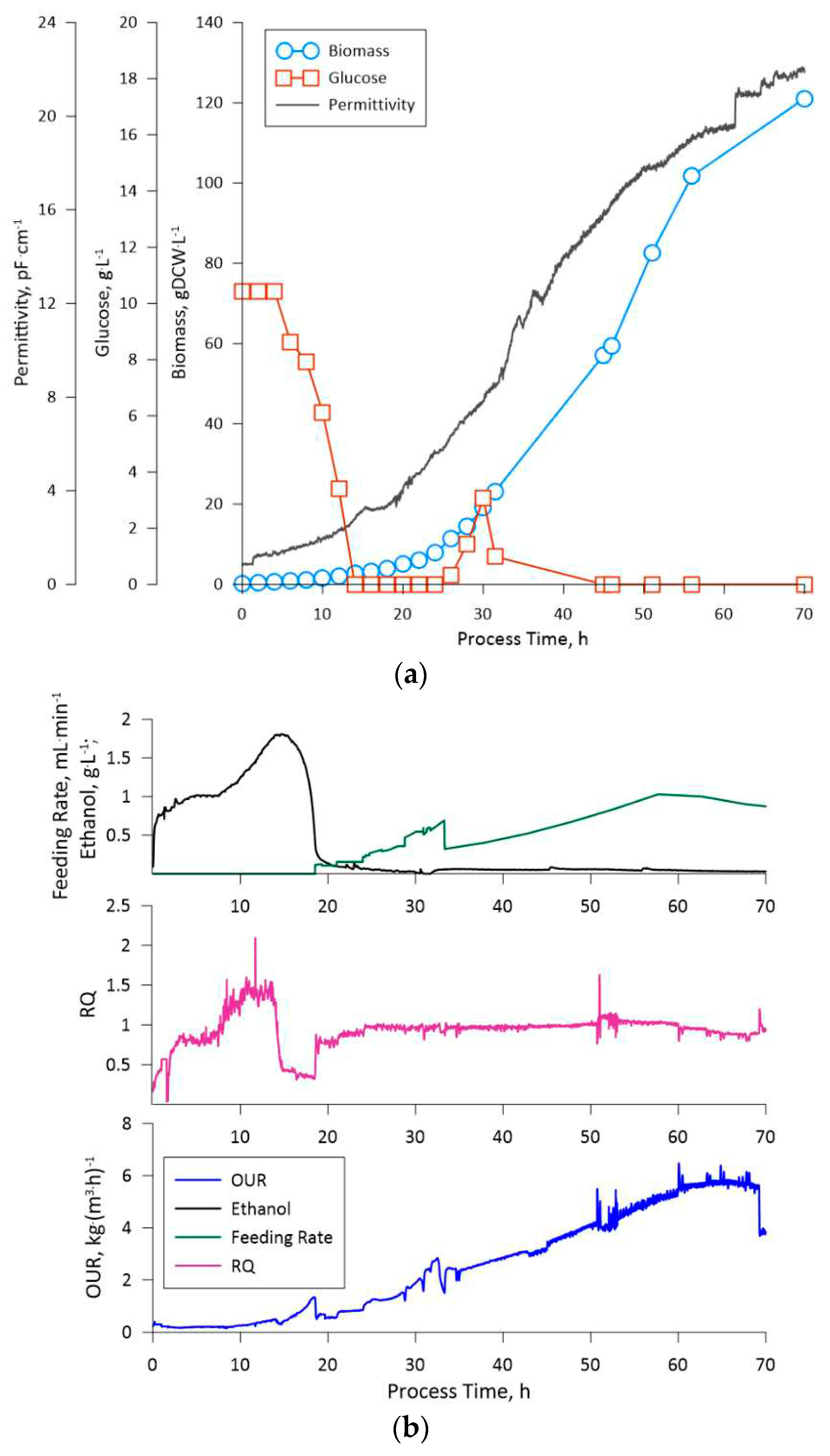
31]. From the presented data of the third cultivation experiment and the LegH production fermentation, the RQ indicates, that during the feeding phase the supplied glucose was utilized solely for biomass growth (RQ = 1.0 ± 0.1) and the methanol was utilized by the cells during production (RQ = 0.6 ± 0.5). Thus, the applied medium and feeding solution compositions did not induce shifts to metabolite synthesis or limited the biomass growth/recombinant protein production.
Apart from RQ, the gas analysis data (OUR), which was gathered for the third cultivation gave valuable insight into the changes in the culture state, especially at higher biomass concentrations. At RQ = 1.0 ± 0.1 (glucose feeding stage) the oxygen uptake rate grew proportionally to the biomass concentration, thus indicating that the culture remained in a steady state, e.g., the specific oxygen consumption rate (qox) was constant. The latter indicates on the potential of linking the gas analysis data with the biomass concentration, thus creating an indirect estimator (soft-sensor) for measuring cell densities online for the particular process configuration. Furthermore, the permittivity (viable cell density) data also exhibited good correlation both with OUR and biomass density, thus indicating that natural cell lysis and growth remained proportional throughout the cultivation.
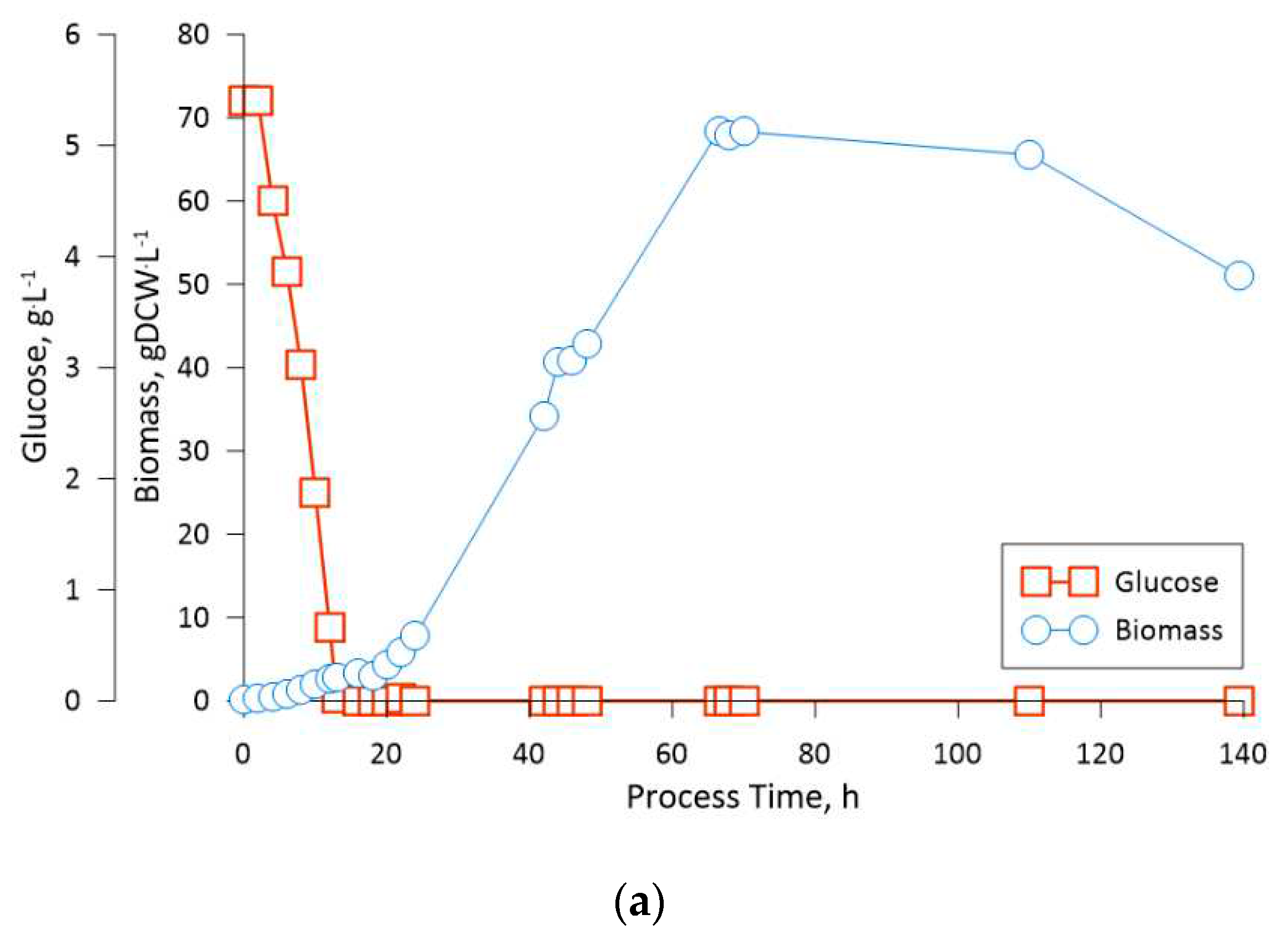
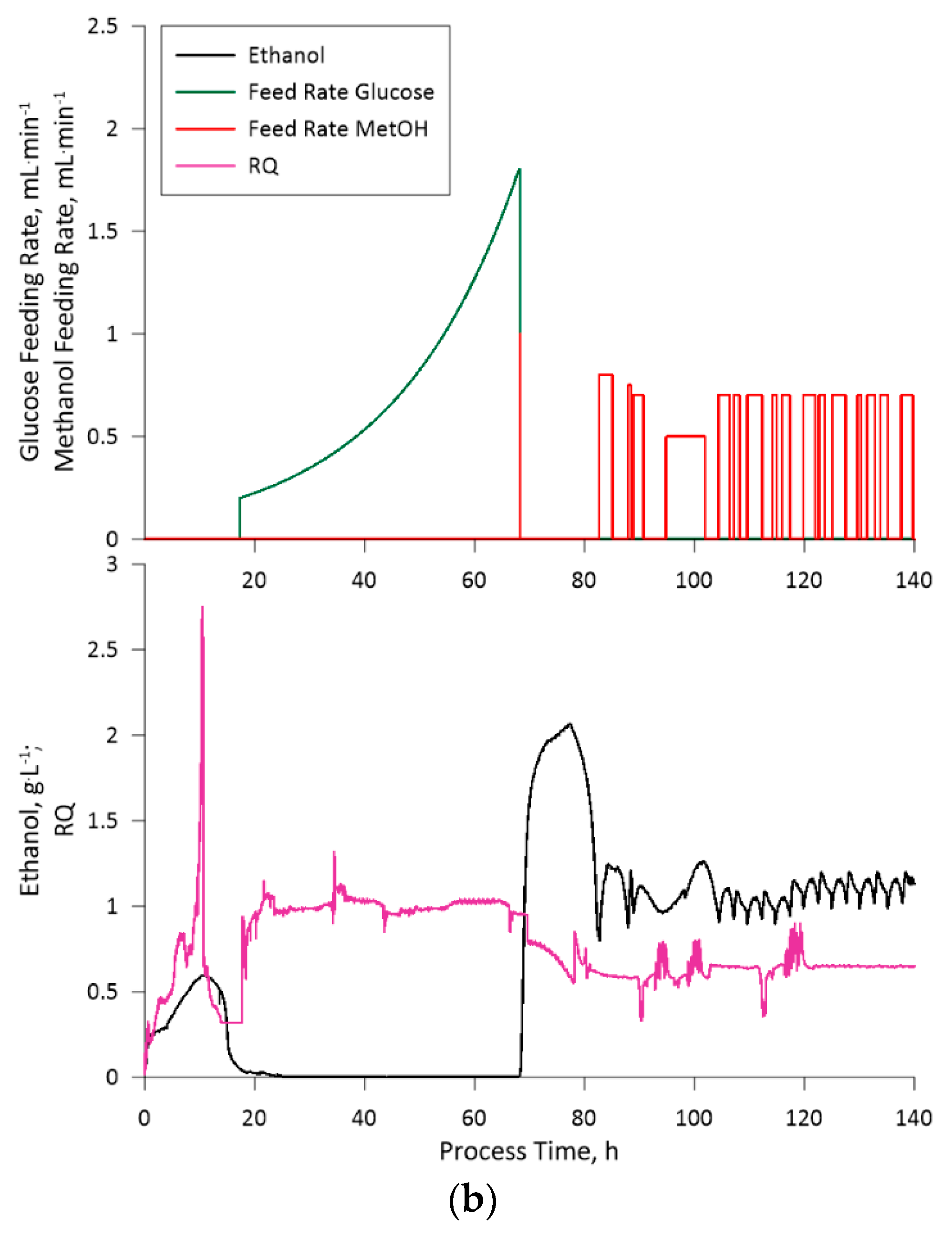
The fed-batch fermentation process for the engineered K. phaffii strain, aimed at leghemoglobin production, was managed, taking into consideration the observation from the previously performed cultivations. This fermentation, as was stated previously, was divided into two phases: glucose feeding phase and the methanol feeding phase. The transition to the methanol feeding phase was strategically set at a biomass concentration of 70 gDCW·L-1. The mentioned cell density was selected due to the slight change in cell size, which was observed on the third cultivations 51st hour through optical microscopy (data not shown). The latter could indicate on an increase in osmatic pressure of the medium due to the accumulation of salts. Thus, even though the applied medium and feeding solution composition showed optimistic results in the context of this study, the composition of said solutes should be optimized as part of future research. Nevertheless, to circumvent this issue a lower biomass concentration was selected for the start of the methanol feeding phase.
Upon enabling methanol feeding, we observed a gradual decrease in cell density over time, which appeared to be linked to fermentation medium dilution. This observation has led us to contemplate the possibility that the methanol concentration in the feeding solution should be increased to maintain an equilibrium between the dilution effects and substrate consumption.
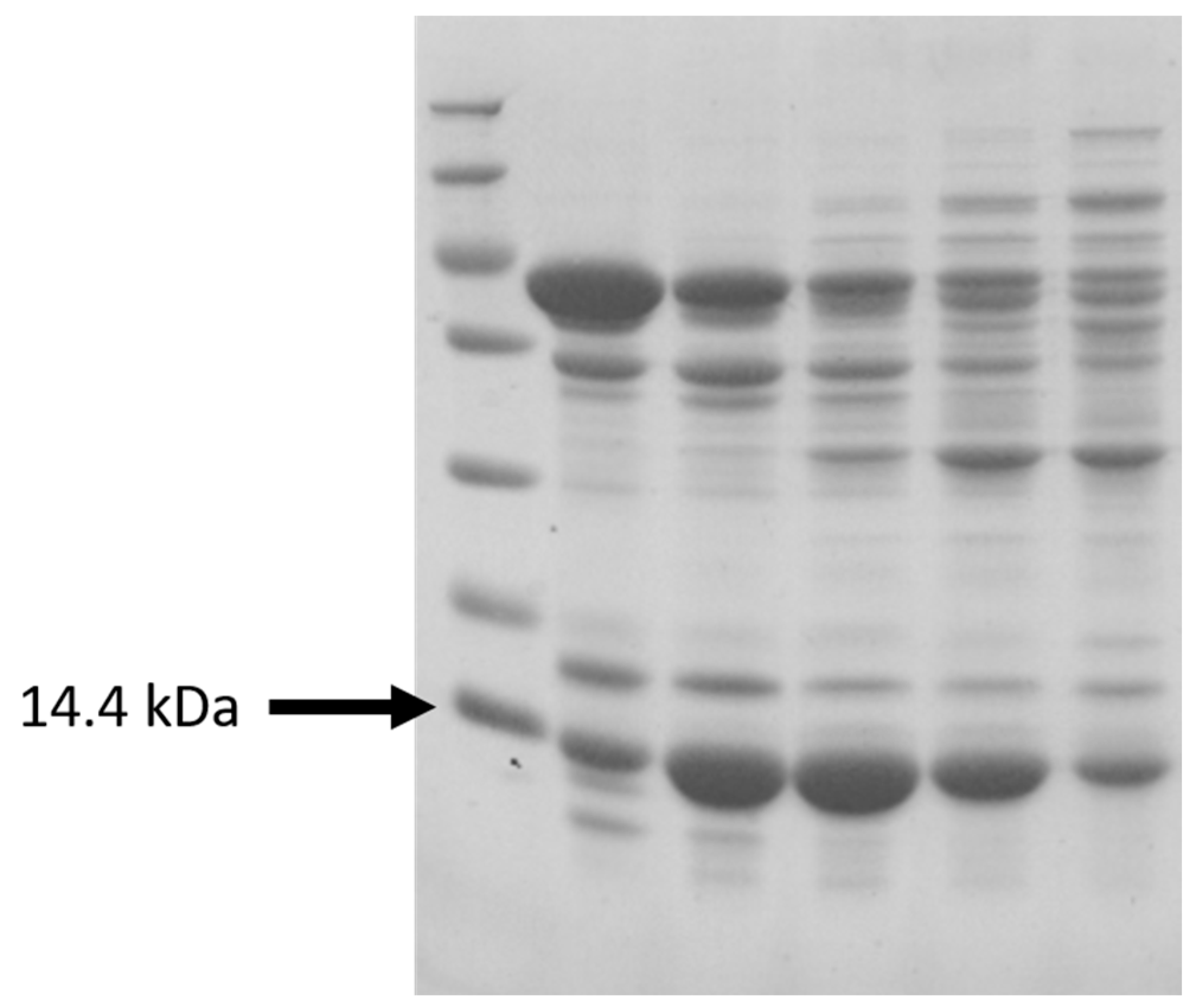
The obtained LegH yield per gram of biomass, as was stated previously, amounted to 0.513 mg·gDCW
-1 (additionally confirmed through gel electrophoresis, see
Appendix C Figure A3). In comparison to the results published by other authors for similar yeast strains, the mentioned yield is comparable, thus indicating that the particular medium composition effect on protein synthesis is negligible and can be attributed to the peculiarities of the specific
K. phaffii modification [
3,
1,
28]. Although, considering the increased LegH yields for recombinant strains holding a higher count of gene alterations as in the results demonstrated by Shao et. al. [
28], the current strain should be improved as part of future studies, with the aim of increasing its efficiency and, as a result, commercial potential as a food-grade LegH production platform.
Considering the main goal set for the current article, e.g., production of leghemoglobin in K. phaffii fermentations, the data gathered from the three fed-batch cultivations enabled the development of a protocol (feeding profiles, medium/feeding solution compositions) suitable for this particular strain. Although, as part of future research multiple crucial points have to be addressed, in order to improve and/or optimize the developed methods, e.g., medium/feeding solution optimization and certain improvements of the engineered K. phaffii strain.