Submitted:
02 November 2023
Posted:
02 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Natural occurring compounds - Pyrethrins
2.1. Chrysanthemum cinerariaefolium
2.2. Chemichal structures and clasification
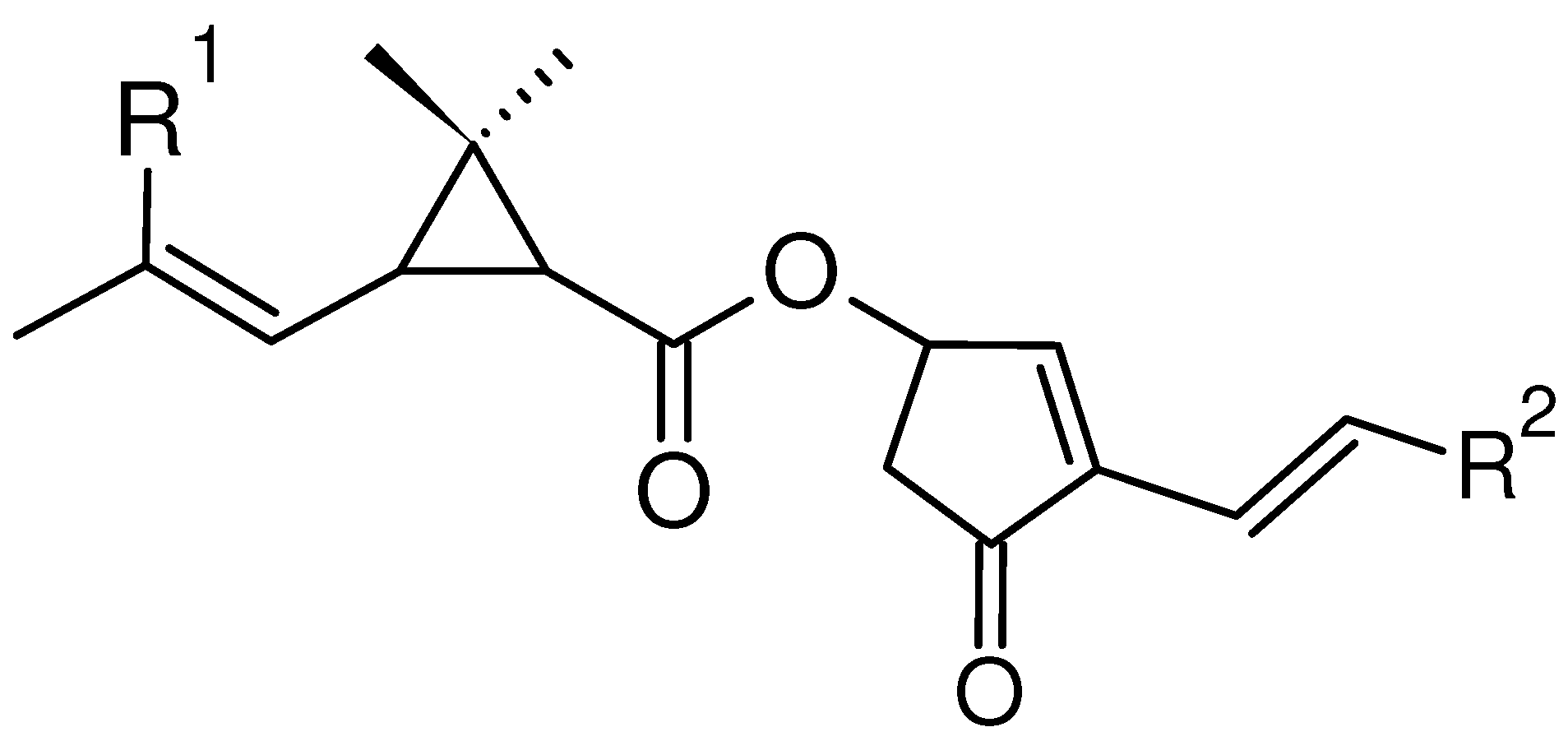
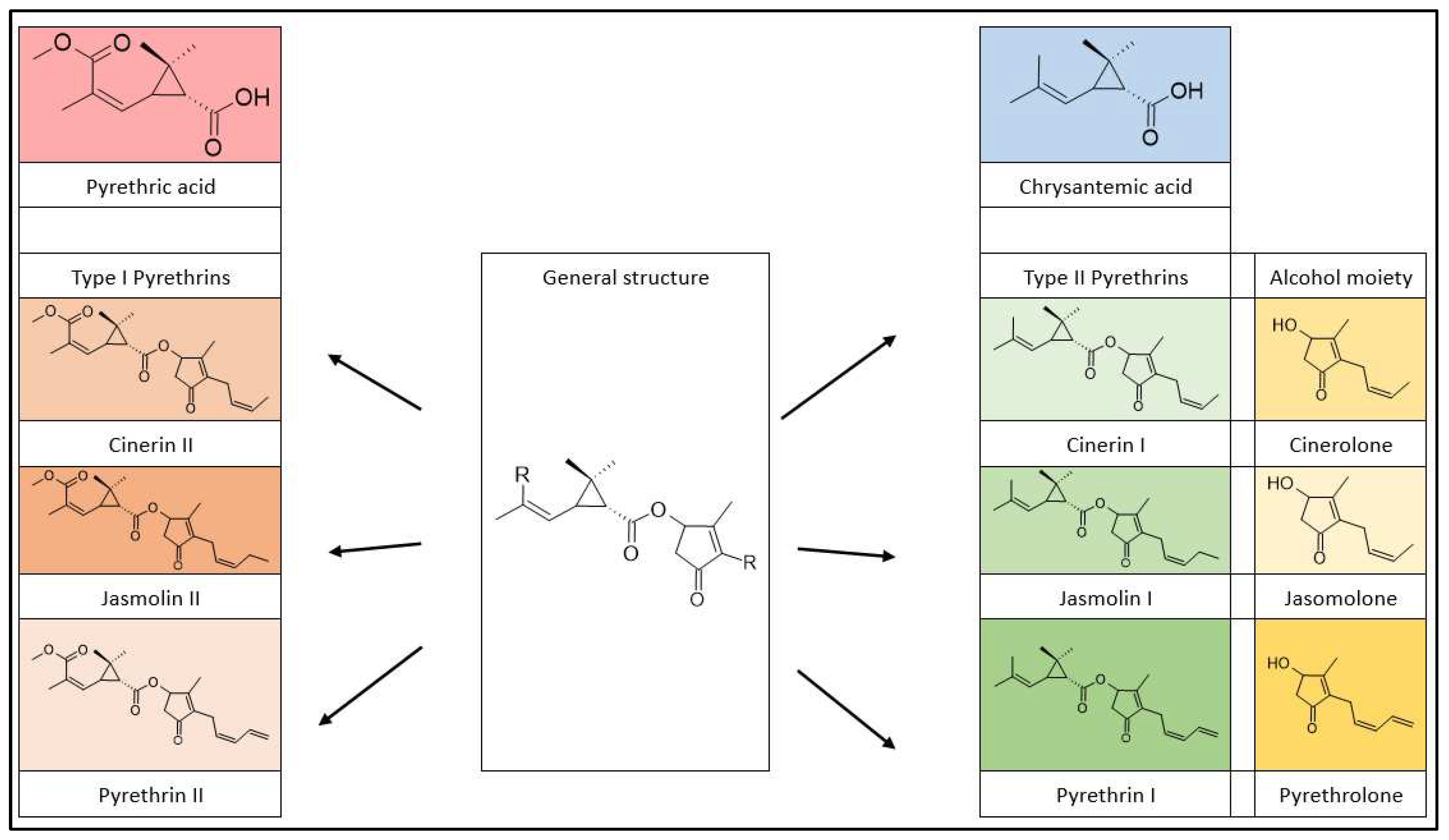
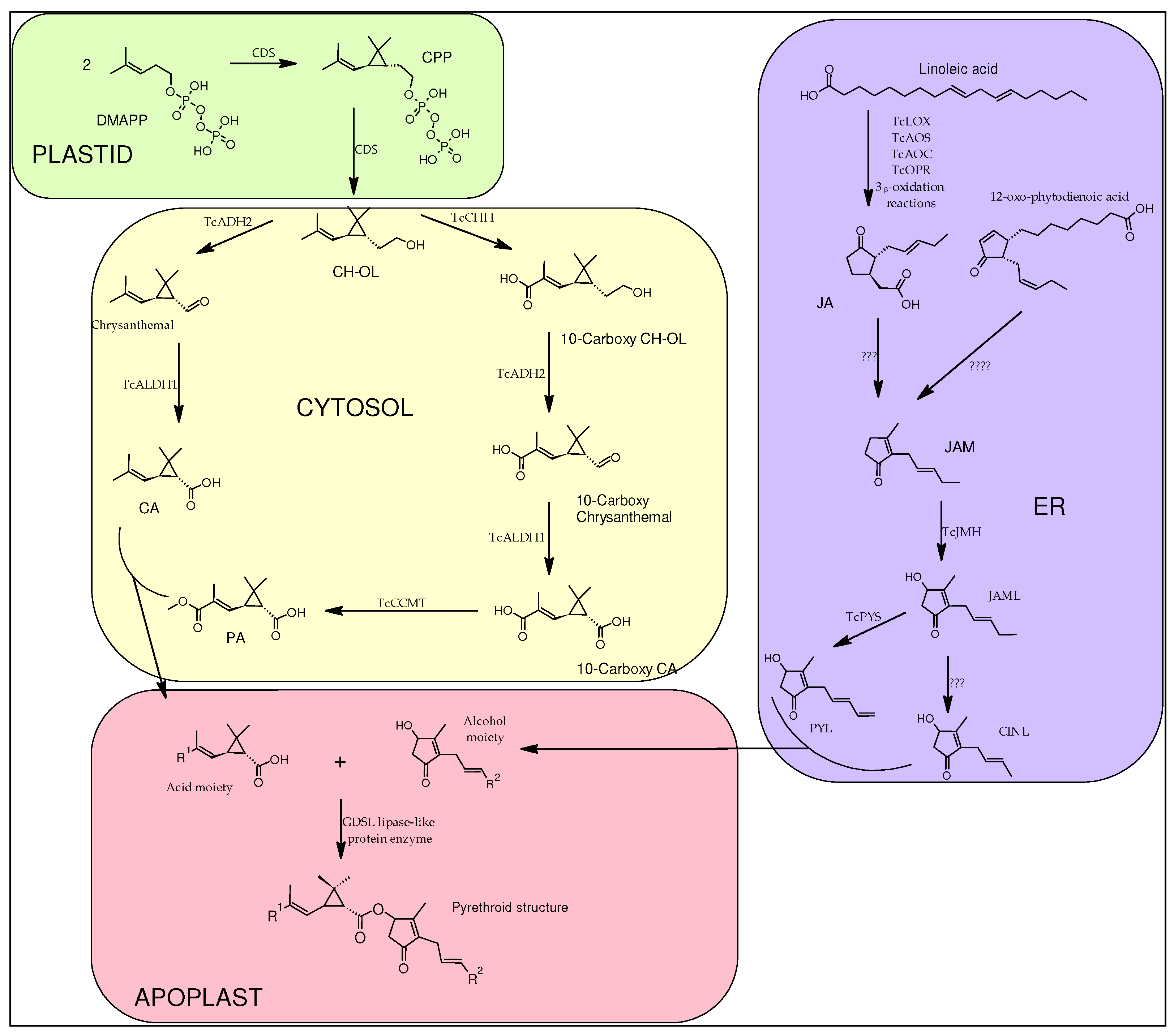
2.3. Biosynthesis
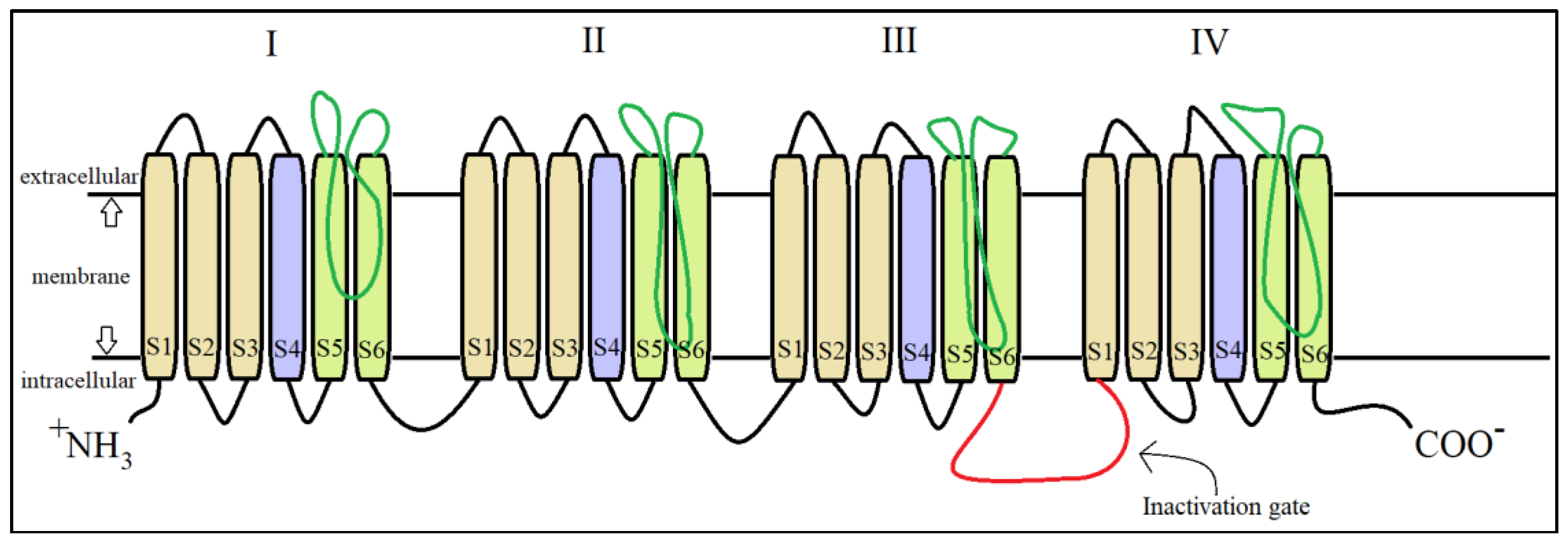
2.3. Mechanism of action
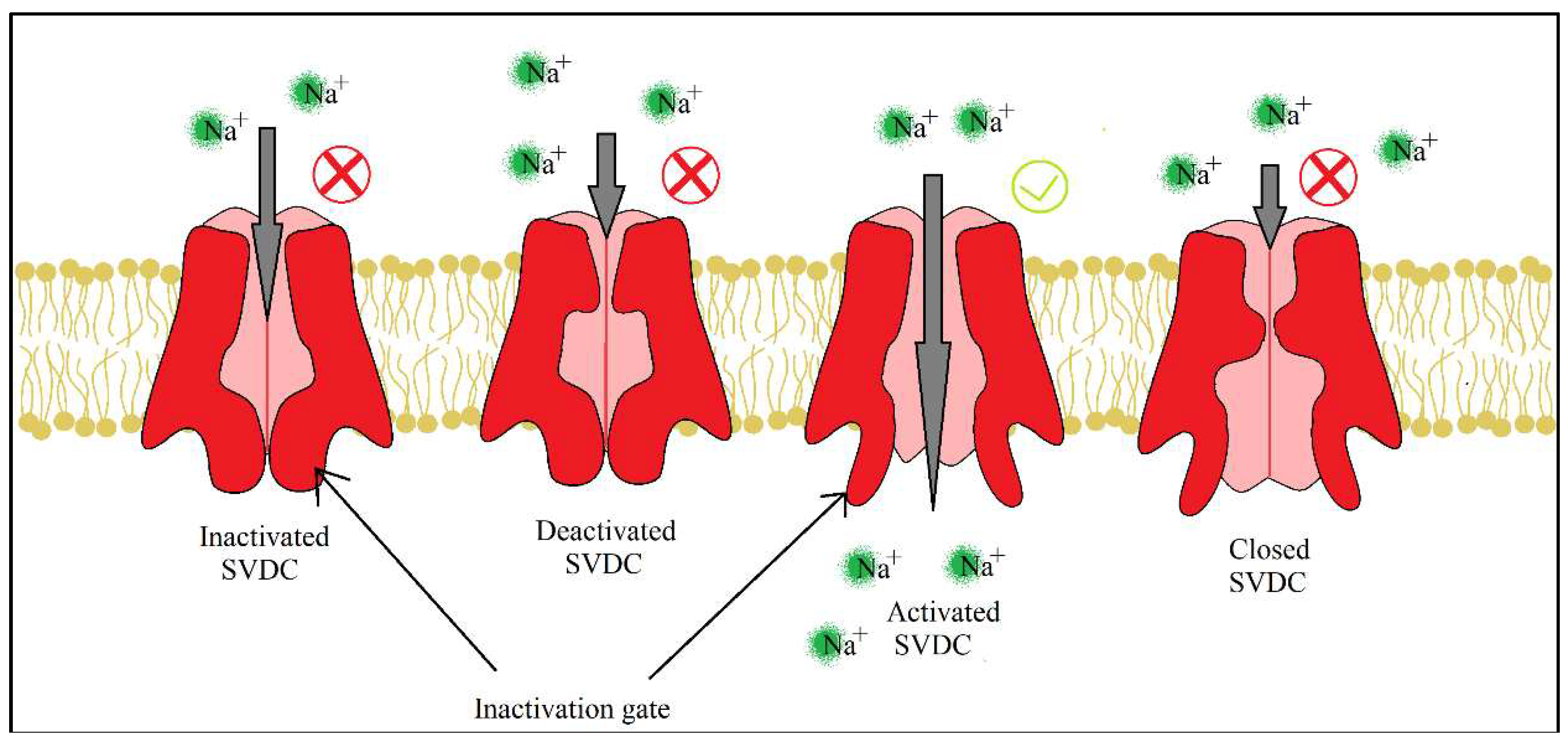
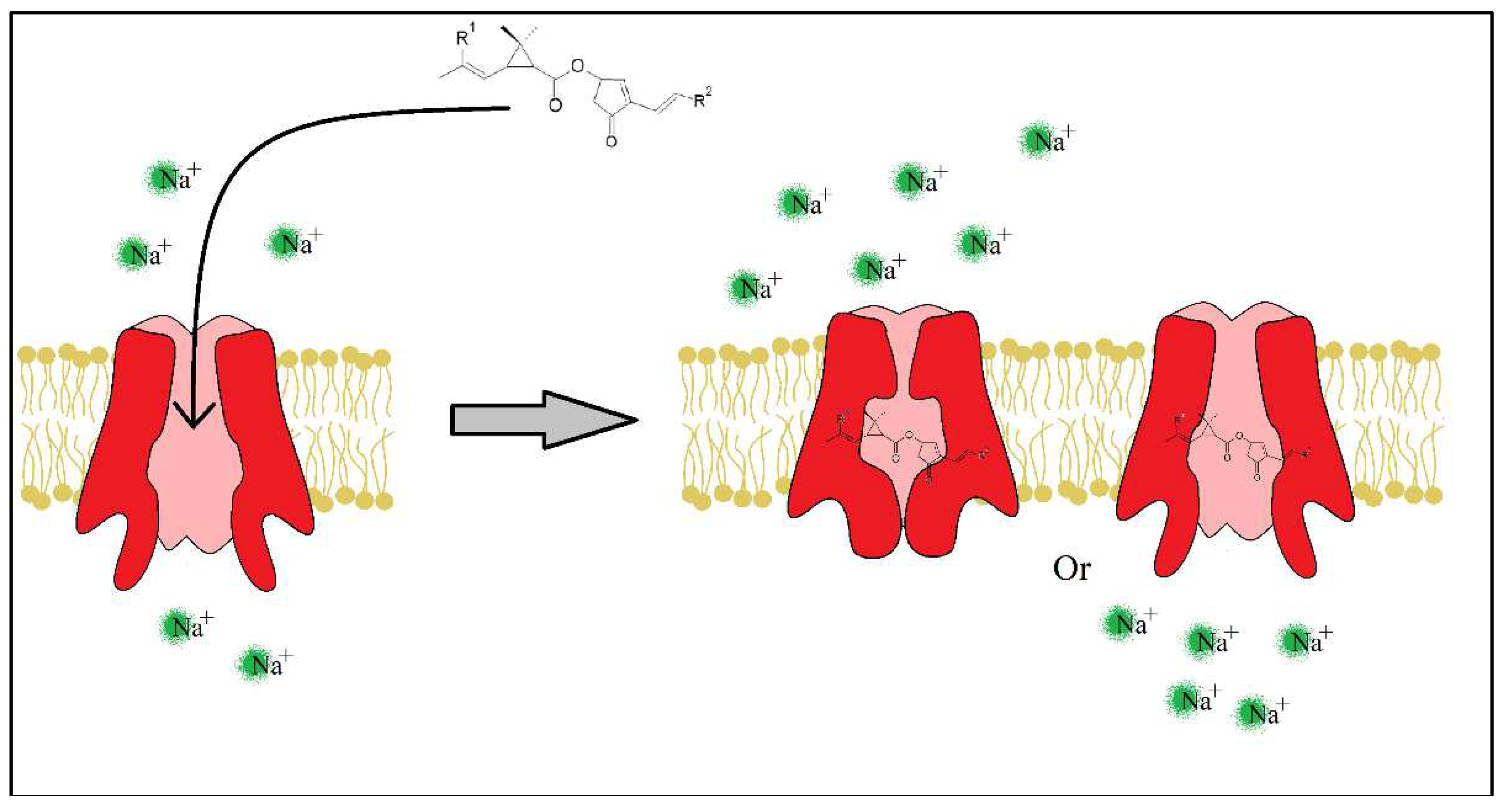
2.4. General and pharmaceutical uses
3. Pyrethrin derivatives – Pyrethroids
3.1. General considerations about pyrethroids
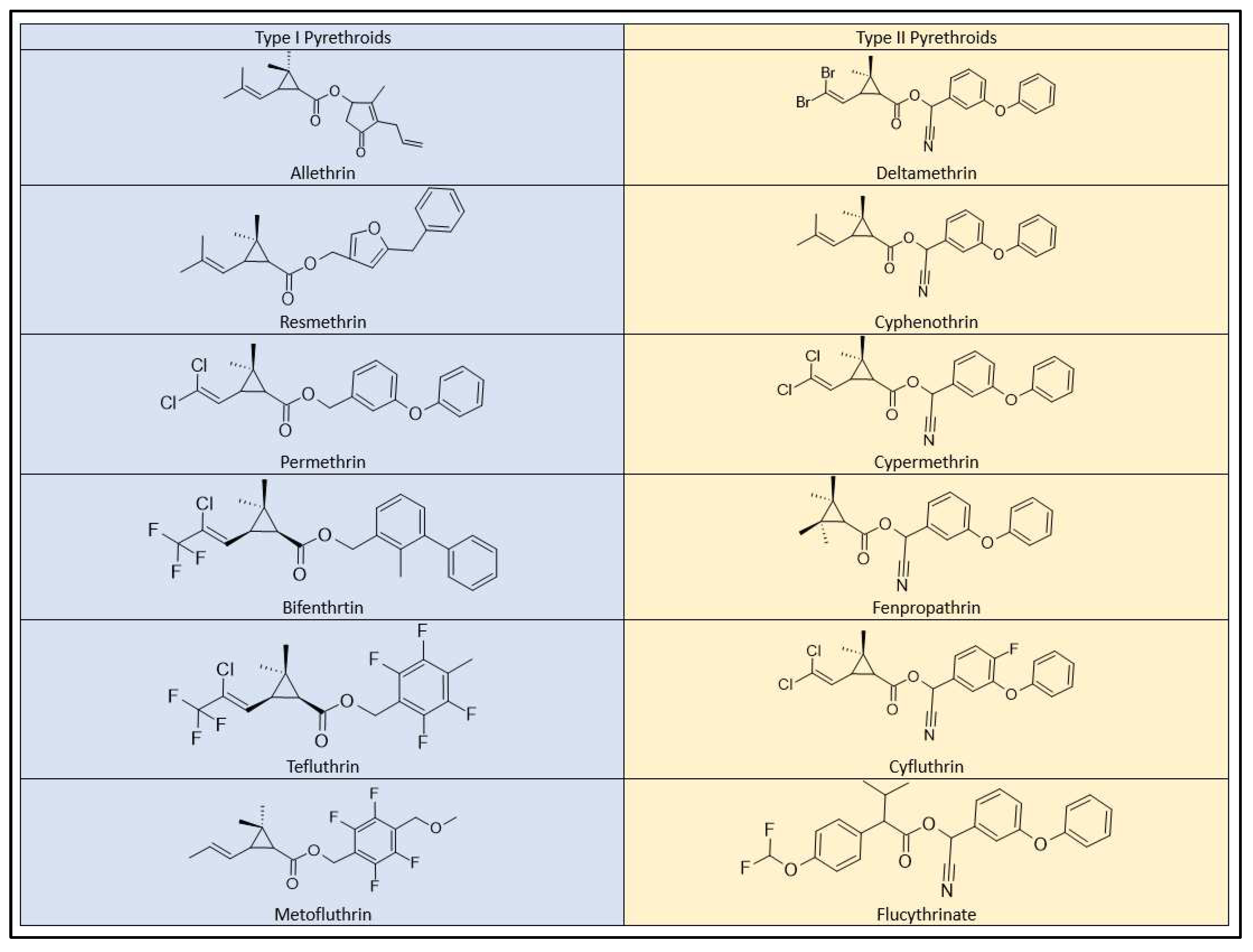
3.2. Classification of pyrethroids
3.3. Synthesis strategies of pyrethroids: approaches and mechanistic considerations
3.4. Toxicological insights into pyrethroids: human and environmental implications
3.4.1. Toxicity in Humans
3.4.2. Biological Mechanisms and Environmental Impact
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Thacker JR. 2002. Chapter 1, A brief history of arthropod control. In:Thacker JR, editor. An introduction to arthropod pest control. London: Cambridge University Press; p. 1–26.
- Isman, M. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden Age of Insecticide Research: Past, Present, or Future? Annu. Rev. Èntomol. 1998, 43, 1–16. [Google Scholar] [CrossRef]
- SCPOP. 2001. Stockholm Convention on Persistent Organic Pollutants. [accessed]. http://chm.pops.int/portals/0/repository/convention_text/unep-pops-cop-convtext-full.english.
- Thatheyus, A.; Selvam, A.G. Synthetic Pyrethroids: Toxicity and Biodegradation. Appl. Ecol. Environ. Sci. 2013, 1, 33–36. [Google Scholar] [CrossRef]
- Chrustek A, Hoły nska-Iwan I, Dziembowska I, Bogusiewicz J, Wroblewski M, Cwynar A, Olszewska-Słonina D. 2018. Current research on thesafety of pyrethroids used as insecticides. Medicina (Kaunas). 54(4):61.
- Peshin R, Bandral RS, Zhang W, Wilson L, Dhawan AK. 2009. Integrated pest management: a global overview of history, programs and adoption. In: Peshin R, Dhawan AK, editors. Integrated Pest Management: Innovation-Development Process: Volume 1. Dordrecht: Springer Netherlands; p. 1–49.
- Mahmood I, Imadi SR, Shazadi K, Gul A, Hakeem KR. 2016. Chapter 13, Effects of Pesticides on Environment. In: Hakeem KR, editor. Plant, Soil and Microbes. Switzerland: Springer International Publishing; p. 253–269.
- G. A. McLAUGHLIN, “History of Pyrethrum,” in Pyrethrum, Elsevier, 1973, pp. 3–15. [CrossRef]
- Demoute, J. A brief review of the environmental fate and metabolism of pyrethroids. Pestic. Sci. 1989, 27, 375–385. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.-W.; Zhou, L.; Xu, Z.-Z.; Li, J.-J.; Hu, H.; Luo, J.; Zheng, R.-R.; Wang, Y.-Y.; Wang, C.-Y. Transcriptional Responses and GCMS Analysis for the Biosynthesis of Pyrethrins and Volatile Terpenes in Tanacetum coccineum. Int. J. Mol. Sci. 2021, 22, 13005. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Classen, B.; Kobold, U.; Vostrowsky, O.; Klingaup, F. Pflanzliche Insektizide IV*. Die insektizide Wirkung des ätherischen Öls aus dem Balsamkraut, Chrysanthemum balsamita L. J. Pest Sci. 1987, 60, 31–34. [Google Scholar] [CrossRef]
- Elazzouzi, H.; Fadili, K.; Cherrat, A.; Amalich, S.; Zekri, N.; Zerkani, H.; Tagnaout, I.; Hano, C.; Lorenzo, J.M.; Zair, T. Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants 2022, 11, 2578. [Google Scholar] [CrossRef]
- Gupta, V.; Khan, S.; Verma, R.K.; Shanker, K.; Singh, S.V.; Rahman, L.U. Overexpression of chrysanthemyl diphosphate synthase (CDS) gene in Tagetes erecta leads to the overproduction of pyrethrin. Transgenic Res. 2022, 31, 625–635. [Google Scholar] [CrossRef]
- Zito, S.W.; Zieg, R.G.; Staba, E.J. Distribution of Pyrethrins in Oil Glands and Leaf Tissue of Chrysanthemum cinerariaefolium. Planta Medica 1983, 47, 205–207. [Google Scholar] [CrossRef]
- Grdiša, M.; Babić, S.; Periša, M.; Carović-Stanko, K.; Kolak, I.; Liber, Z.; Jug-Dujaković, M.; Satovic, Z. Chemical Diversity of the Natural Populations of Dalmatian Pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch.Bip.) in Croatia. Chem. Biodivers. 2013, 10, 460–472. [Google Scholar] [CrossRef]
- Matsuda, K.; Kikuta, Y.; Haba, A.; Nakayama, K.; Katsuda, Y.; Hatanaka, A.; Komai, K. Biosynthesis of pyrethrin I in seedlings of Chrysanthemum cinerariaefolium. Phytochemistry 2005, 66, 1529–1535. [Google Scholar] [CrossRef]
- Li, W.; Lybrand, D.B.; Zhou, F.; Last, R.L.; Pichersky, E. Pyrethrin Biosynthesis: The Cytochrome P450 Oxidoreductase CYP82Q3 Converts Jasmolone To Pyrethrolone. Plant Physiol. 2019, 181, 934–944. [Google Scholar] [CrossRef]
- Xu, H.; Li, W.; Schilmiller, A.L.; van Eekelen, H.; de Vos, R.C.H.; Jongsma, M.A.; Pichersky, E. Pyrethric acid of natural pyrethrin insecticide: complete pathway elucidation and reconstitution in Nicotiana benthamiana. New Phytol. 2019, 223, 751–765. [Google Scholar] [CrossRef]
- Xu, H.; Moghe, G.D.; Wiegert-Rininger, K.; Schilmiller, A.L.; Barry, C.S.; Last, R.L.; Pichersky, E. Coexpression Analysis Identifies Two Oxidoreductases Involved in the Biosynthesis of the Monoterpene Acid Moiety of Natural Pyrethrin Insecticides in Tanacetum cinerariifolium. Plant Physiol. 2018, 176, 524–537. [Google Scholar] [CrossRef]
- Matsui, R.; Takiguchi, K.; Kuwata, N.; Oki, K.; Takahashi, K.; Matsuda, K.; Matsuura, H. Jasmonic acid is not a biosynthetic intermediate to produce the pyrethrolone moiety in pyrethrin II. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Li, W.; Zhou, F.; Pichersky, E. Jasmone Hydroxylase, a Key Enzyme in the Synthesis of the Alcohol Moiety of Pyrethrin Insecticides. Plant Physiol. 2018, 177, 1498–1509. [Google Scholar] [CrossRef]
- Kikuta, Y.; Ueda, H.; Takahashi, M.; Mitsumori, T.; Yamada, G.; Sakamori, K.; Takeda, K.; Furutani, S.; Nakayama, K.; Katsuda, Y.; et al. Identification and characterization of a GDSL lipase-like protein that catalyzes the ester-forming reaction for pyrethrin biosynthesis in Tanacetum cinerariifolium– a new target for plant protection. Plant J. 2012, 71, 183–193. [Google Scholar] [CrossRef]
- K. Matsuda, “Pyrethrin Biosynthesis and Its Regulation in Chrysanthemum cinerariaefolium,” 2011, pp. 73–81. [CrossRef]
- Baldwin, I.T.; Karb, M.J.; Callahan, P. Foliar and floral pyrethrins ofChrysanthemum cinerariaefolium are not induced by leaf damage. J. Chem. Ecol. 1993, 19, 2081–2087. [Google Scholar] [CrossRef]
- Kikuta, Y.; Ueda, H.; Nakayama, K.; Katsuda, Y.; Ozawa, R.; Takabayashi, J.; Hatanaka, A.; Matsuda, K. Specific Regulation of Pyrethrin Biosynthesis in Chrysanthemum cinerariaefolium by a Blend of Volatiles Emitted from Artificially Damaged Conspecific Plants. Plant Cell Physiol. 2011, 52, 588–596. [Google Scholar] [CrossRef]
- Ueda, H.; Matsuda, K. VOC-mediated within-plant communications and nonvolatile systemic signals upregulate pyrethrin biosynthesis in wounded seedlings of Chrysanthemum cinerariaefolium. J. Plant Interactions 2011, 6, 89–91. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Knipple, D.C. Knockdown Resistance to DDT and Pyrethroids in the House Fly (Diptera: Muscidae): From Genetic Trait to Molecular Mechanism. Ann. Èntomol. Soc. Am. 1999, 92, 909–915. [Google Scholar] [CrossRef]
- Dong, K. Insect sodium channels and insecticide resistance. Invertebr. Neurosci. 2007, 7, 17–30. [Google Scholar] [CrossRef]
- N., E., P., A., & D., W. (2012). Pyrethroids and Their Effects on Ion Channels. In Pesticides - Advances in Chemical and Botanical Pesticides. InTech. [CrossRef]
- Murayama, K.; Abbott, N.; Narahashi, T.; Shapiro, B.I. Effects of allethrin and Condylactis toxin on the kinetics of sodium conductance of crayfish axon membranes. Comp. Gen. Pharmacol. 1972, 3, 391–400. [Google Scholar] [CrossRef]
- Narahashi, T. Mode of action of pyrethroids. 1971, 44, 337–45. [Google Scholar]
- D. M. Soderlund, “Toxicology and Mode of Action of Pyrethroid Insecticides,” in Hayes’ Handbook of Pesticide Toxicology, Elsevier, 2010, pp. 1665–1686. [CrossRef]
- D. M. Soderlund, “Neurotoxicology of pyrethroid insecticides,” 2020, pp. 113–165. [CrossRef]
- B. P. S. Khambay and P. J. Jewess, “Pyrethroids,” in Comprehensive Molecular Insect Science, Elsevier, 2005, pp. 1–29. [CrossRef]
- Du, Y.; Lee, J.-E.; Nomura, Y.; Zhang, T.; Zhorov, B.S.; Dong, K. Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem. J. 2009, 419, 377–385. [Google Scholar] [CrossRef]
- O’Reilly, A.O.; Khambay, B.P.S.; Williamson, M.S.; Field, L.M.; Wallace, B.A.; Davies, T.G.E. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem. J. 2006, 396, 255–263. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A. Use and abuse of pyrethrins and synthetic pyrethroids in veterinary medicine. Vet. J. 2009, 182, 7–20. [Google Scholar] [CrossRef]
- Taplin, D. Pyrethrins and Pyrethroids in Dermatology. Arch. Dermatol. 1990, 126, 213–21. [Google Scholar] [CrossRef]
- Churcher, T.S.; Lissenden, N.; Griffin, J.T.; Worrall, E.; Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 2016, 5, e16090. [Google Scholar] [CrossRef]
- Gleave, K.; Lissenden, N.; Chaplin, M.; Choi, L.; Ranson, H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Emergencias 2021, 2021, CD012776. [Google Scholar] [CrossRef]
- Corcellas, C.; Feo, M.L.; Torres, J.P.; Malm, O.; Ocampo-Duque, W.; Eljarrat, E.; Barceló, D. Pyrethroids in human breast milk: Occurrence and nursing daily intake estimation. Environ. Int. 2012, 47, 17–22. [Google Scholar] [CrossRef]
- Feo, M.L.; Eljarrat, E.; Manaca, M.N.; Dobaño, C.; Barcelo, D.; Sunyer, J.; Alonso, P.L.; Menendez, C.; Grimalt, J.O. Pyrethroid use-malaria control and individual applications by households for other pests and home garden use. Environ. Int. 2012, 38, 67–72. [Google Scholar] [CrossRef]
- Ranson, H.; N’guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Duchon, S.; Bonnet, J.; Marcombe, S.; Zaim, M.; Corbel, V. Pyrethrum: A Mixture of Natural Pyrethrins Has Potential for Malaria Vector Control. J. Med Èntomol. 2009, 46, 516–522. [Google Scholar] [CrossRef]
- LaFORGE, F.B.; Barthel, W.F. CONSTITUENTS OF PYRETHRUM FLOWERS. XVIII. THE STRUCTURE AND ISOMERISM OF PYRETHROLONE AND CINEROLONE. J. Org. Chem. 1945, 10, 114–120. [Google Scholar] [CrossRef]
- Ujihara, K. The history of extensive structural modifications of pyrethroids. J. Pestic. Sci. 2019, 44, 215–224. [Google Scholar] [CrossRef]
- Kim, K.-B.; Anand, S.S.; Kim, H.J.; White, C.A.; Bruckner, J.V. Toxicokinetics and Tissue Distribution of Deltamethrin in Adult Sprague–Dawley Rats. Toxicol. Sci. 2007, 101, 197–205. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden Age of Insecticide Research: Past, Present, or Future? Annu. Rev. Èntomol. 1998, 43, 1–16. [Google Scholar] [CrossRef]
- Bradberry, S.M.; A Cage, S.; Proudfoot, A.T.; Vale, J.A. Poisoning due to Pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Fai, P.B.A.; Kinfack, J.S.T.; Towa, Y.J.T. Acute effects of binary mixtures of Type II pyrethroids and organophosphate insecticides on Oreochromis niloticus. Ecotoxicology 2017, 26, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Kuivila, K.M.; Hladik, M.L.; Ingersoll, C.G.; Kemble, N.E.; Moran, P.W.; Calhoun, D.L.; Nowell, L.H.; Gilliom, R.J. Occurrence and Potential Sources of Pyrethroid Insecticides in Stream Sediments from Seven U.S. Metropolitan Areas. Environ. Sci. Technol. 2012, 46, 4297–4303. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.J.; Pastoor, T.P. Pyrethroid epidemiology: a quality-based review. Crit. Rev. Toxicol. 2018, 48, 297–311. [Google Scholar] [CrossRef]
- Bordoni, L.; Nasuti, C.; Fedeli, D.; Galeazzi, R.; Laudadio, E.; Massaccesi, L.; López-Rodas, G.; Gabbianelli, R. Early impairment of epigenetic pattern in neurodegeneration: Additional mechanisms behind pyrethroid toxicity. Exp. Gerontol. 2019, 124, 110629. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gan, J.; Cui, X.; Delgado-Moreno, L.; Lin, K. Understanding the bioavailability of pyrethroids in the aquatic environment using chemical approaches. Environ. Int. 2019, 129, 194–207. [Google Scholar] [CrossRef]
- Ullah, S.; Li, Z.; Zuberi, A.; Arifeen, M.Z.U.; Baig, M.M.F.A. Biomarkers of pyrethroid toxicity in fish. Environ. Chem. Lett. 2019, 17, 945–973. [Google Scholar] [CrossRef]
- Deng, F.; Sun, J.; Dou, R.; Yu, X.; Wei, Z.; Yang, C.; Zeng, X.; Zhu, L. Contamination of pyrethroids in agricultural soils from the Yangtze River Delta, China. Sci. Total. Environ. 2020, 731, 139181. [Google Scholar] [CrossRef] [PubMed]
- Cryder, Z.; Wolf, D.; Carlan, C.; Gan, J. Removal of urban-use insecticides in a large-scale constructed wetland. Environ. Pollut. 2021, 268, 115586–115586. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B 2019, 95, 378–400. [Google Scholar] [CrossRef]
- Ravula, A.R.; Yenugu, S. Pyrethroid based pesticides – chemical and biological aspects. Crit. Rev. Toxicol. 2021, 51, 1–24. [Google Scholar] [CrossRef]
- Ray, D.E.; Forshaw, P.J. Pyrethroid Insecticides: Poisoning Syndromes, Synergies, and Therapy. J. Toxicol. Clin. Toxicol. 2000, 38, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch. Toxicol. 2011, 86, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.-M.; Ndiaye, D.; Sabaté, J.-P. Pyrethroids: Exposure and health effects – An update. Int. J. Hyg. Environ. Heal. 2015, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, M.; Guo, X.; Lin, P. Urinary Metabolites of Organophosphate and Pyrethroid Pesticides and Neurobehavioral Effects in Chinese Children. Environ. Sci. Technol. 2016, 50, 9627–9635. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; David, A.; Freire, C.; Fernández, M.F.; D’Cruz, S.C.; Reina-Pérez, I.; Fini, J.-B.; Blaha, L. Pyrethroids and developmental neurotoxicity - A critical review of epidemiological studies and supporting mechanistic evidence. Environ. Res. 2022, 214, 113935. [Google Scholar] [CrossRef]
- Laskowski DA. Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol. 2002;174:49-170. [CrossRef]
- Ii, D.M.S.; Bradham, K.D.; Egeghy, P.P.; Jones, P.A.; Croghan, C.W.; Ashley, P.A.; Pinzer, E.; Friedman, W.; Brinkman, M.C.; Nishioka, M.G.; et al. American Healthy Homes Survey: A National Study of Residential Pesticides Measured from Floor Wipes. Environ. Sci. Technol. 2009, 43, 4294–4300. [Google Scholar] [CrossRef]
- Chapuis, H., Strazewski, P., 2006. Shorter puromycin analog synthesis by means of an efficient StaudingereVilarrasa coupling. Tetrahedron 62, 12108e12115.
- Menn, J.J., 1980. Contemporary frontiers in chemical pesticide research. J. Agric. Food Chem. 28, 2e8.
- Zheng, Z.; Wang, J.; Zhang, D.; Guan, X.; Gao, S.; Chen, Z.; Zou, X. Design, Synthesis, and Insecticidal Activities of Novel Monohalovinylated Pyrethroids. J. Agric. Food Chem. 2011, 59, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Zhao, X.-F.; Liu, P.; Meng, X.-H.; Yu, T.; Ji, Y.-L.; Zhang, H.; Zhang, C.; Zhang, Y.; et al. Cypermethrin exposure during puberty disrupts testosterone synthesis via downregulating StAR in mouse testes. Arch. Toxicol. 2009, 84, 53–61. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Wu, W.-Y.; Chen, F.-L.; Guan, X.-L.; Fu, X.-H.; Jiang, P.; Wan, R. Design, synthesis, crystal structure and insecticidal evaluation of novel arylpyrazole derivatives containing cyhalothroyl thiourea moiety. Phosphorus, Sulfur, Silicon Relat. Elements 2017, 192, 911–918. [Google Scholar] [CrossRef]
- Chang, F.; Dutta, S.; Becnel, J.J.; Estep, A.S.; Mascal, M. Synthesis of the Insecticide Prothrin and Its Analogues from Biomass-Derived 5-(Chloromethyl)furfural. J. Agric. Food Chem. 2013, 62, 476–480. [Google Scholar] [CrossRef]
- Kiessling, L. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000, 4, 696–703. [Google Scholar] [CrossRef]
- Thatheyus, A.; Selvam, A.G. Synthetic Pyrethroids: Toxicity and Biodegradation. Appl. Ecol. Environ. Sci. 2013, 1, 33–36. [Google Scholar] [CrossRef]
- Mikata K, Isobe N, Kaneko H. Biotransformation, and enzymatic reactions of synthetic pyrethroids in mammals. Top Curr Chem. 2012; 314:113-35. [CrossRef]
- Bradberry, S.M.; A Cage, S.; Proudfoot, A.T.; Vale, J.A. Poisoning due to Pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Ramchandra, A.M.; Victor, P.J. Pyrethroid Poisoning. Indian J. Crit. Care Med. 2019, 23, S267–S271. [Google Scholar] [CrossRef]
- Matsunaga, T.; Makita, M.; Higo, A.; Nishibe, I.; Dohara, K.; Shinjo, G. Studies on prallethrin, a new synthetic pyrethroid, for indoor applications: I. The insecticidal activities of prallethrin isomers. Med Èntomol. Zoöl. 1987, 38, 219–223. [Google Scholar] [CrossRef]
- Narendra, M.; Kavitha, G.; Kiranmai, A.H.; Rao, N.R.; Varadacharyulu, N. Chronic exposure to pyrethroid-based allethrin and prallethrin mosquito repellents alters plasma biochemical profile. Chemosphere 2008, 73, 360–364. [Google Scholar] [CrossRef]
- Na, H.G.; Kim, Y.-D.; Choi, Y.S.; Bae, C.H.; Song, S.-Y. Allethrin and prallethrin stimulates MUC5AC expression through oxidative stress in human airway epithelial cells. Biochem. Biophys. Res. Commun. 2018, 503, 316–322. [Google Scholar] [CrossRef]
- Bhaskar, E.M.; Moorthy, S.; Ganeshwala, G.; Abraham, G. Cardiac Conduction Disturbance Due To Prallethrin (Pyrethroid) Poisoning. J. Med Toxicol. 2010, 6, 27–30. [Google Scholar] [CrossRef]
- Parlato, F.; Palacios, D.C.B.; Serrano, M.A.; Gonçalves, F.; Carreiro, C.; Gouveia, J. A Suicide Attempt: Deltamethrin Intoxication. Eur. J. Case Rep. Intern. Med. 2022, 9, 003573. [Google Scholar] [CrossRef]
- Osweiler, G.D., 1996. Toxicology. Williams and Wilkins, Philadelphia, PA.
- Gammon, D.W., Chandrasekaran, A., AlNaggar, S.F., 2012. Comparative metabolism and toxicology of pyrethroids in mammals. In: Marrs, T.C. (Ed.), Mammalian Toxicology of Insecticides. RSC Publ, Cambridge, pp. 137-183.
- Anadon, A., Are’s, I., Martı´inez, M.A., et al., 2013. Pyrethrins and synthetic pyrethroids:use in veterinary medicine. In: Ramawat, K.G., Me´rillon, J.M. (Eds.), Natural Products. Springer-Verlag, Berlin, pp. 4061-4086.
- Xu, Q.; Zhu, B.; Dong, X.; Li, S.; Song, X.; Xiao, X.; Zhang, C.; Lv, Y.; Zhang, X.; Li, Y. Pyrethroid pesticide exposure during early pregnancy and birth outcomes in southwest China: a birth cohort study. J. Toxicol. Sci. 2020, 45, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Asensio, M.J.; Hernandez, A.F.; Romero-Molina, D.; Gonzalez-Alzaga, B.; Luzardo, O.P.; Henríquez-Hernández, L.A.; Boada, L.D.; García-Cortés, H.; Lopez-Flores, I.; Sanchez-Piedra, M.D.; et al. Effect of prenatal exposure to organophosphates and pyrethroid pesticides on neonatal anthropometric measures and gestational age. Environ. Res. 2023, 232, 116410. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Lim, Y.-H.; Lee, Y.A.; Shin, C.H.; Kim, B.-N.; Hong, Y.-C.; Kim, J.I. The association of prenatal and childhood pyrethroid pesticide exposure with school-age ADHD traits. Environ. Int. 2022, 161, 107124. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mao, Y.; Xu, B. Association between pyrethroid pesticide exposure and hearing loss in adolescents. Environ. Res. 2020, 187, 109640. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Min, J.-Y.; Min, K.-B. Association between pyrethroids and prostate endpoints; stratified according to renal function. Environ. Int. 2021, 153, 106489. [Google Scholar] [CrossRef]
- Jia, C.; Qiu, G.; Wang, H.; Zhang, S.; An, J.; Cheng, X.; Li, P.; Li, W.; Zhang, X.; Yang, H.; et al. Lipid metabolic links between serum pyrethroid levels and the risk of incident type 2 diabetes: A mediation study in the prospective design. J. Hazard. Mater. 2023, 459, 132082. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.C.; Efthymiou, N.; Konstantinou, C.; Anastasi, E.; Schoeters, G.; Kolossa-Gehring, M.; Katsonouri, A. Oxidative stress of glyphosate, AMPA and metabolites of pyrethroids and chlorpyrifos pesticides among primary school children in Cyprus. Environ. Res. 2022, 212, 113316. [Google Scholar] [CrossRef]
- An, S.; Rauch, S.A.; Maphula, A.; Obida, M.; Kogut, K.; Bornman, R.; Chevrier, J.; Eskenazi, B. In-utero exposure to DDT and pyrethroids and child behavioral and emotional problems at 2 years of age in the VHEMBE cohort, South Africa. Chemosphere 2022, 306, 135569–135569. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Tang, Y. Urinary pyrethroid metabolite and hearing threshold shifts of adults in the United States: A cross-sectional study. PLOS ONE 2022, 17, e0275775. [Google Scholar] [CrossRef]
- Mora, A.M.; Baker, J.M.; Hyland, C.; Rodríguez-Zamora, M.G.; Rojas-Valverde, D.; Winkler, M.S.; Staudacher, P.; Palzes, V.A.; Gutiérrez-Vargas, R.; Lindh, C.; et al. Pesticide exposure and cortical brain activation among farmworkers in Costa Rica. NeuroToxicology 2022, 93, 200–210. [Google Scholar] [CrossRef]
- Bao, W.; Liu, B.; Simonsen, D.W.; Lehmler, H.-J. Association Between Exposure to Pyrethroid Insecticides and Risk of All-Cause and Cause-Specific Mortality in the General US Adult Population. JAMA Intern. Med. 2020, 180, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xiao, X.; Qi, Z.; Chen, L.; Chen, Y.; Xu, L.; Zhang, L.; Song, X.; Li, Y. Effects of prenatal and infant daily exposure to pyrethroid pesticides on the language development of 2-year-old toddlers: A prospective cohort study in rural Yunnan, China. NeuroToxicology 2022, 92, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bo, Y. Associations between pyrethroid exposure and serum sex steroid hormones in adults: Findings from a nationally representative sample. Chemosphere 2022, 300, 134591. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L.; Nemec, M.; Sheets, L.; Sargent, D.; Breckenridge, C. Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. NeuroToxicology 2009, 30, S1–S16. [Google Scholar] [CrossRef]
- Wolansky, M.; Harrill, J. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: A critical review. Neurotoxicology Teratol. 2008, 30, 55–78. [Google Scholar] [CrossRef]
- Bradbury, S.P., Coats, J.R., 1986. Toxicokinetics of fenvalerate in rain-bow trout (Salmo gairdneri). Environ. Toxicol. Chem. 5, 567-576.
- Bradbury, S.P., Coats, J.R., 1989. Comparative toxicology of pyrethroid insecticides. Reviews of Environmental Contamination and Toxicology, US Environmental Res. LabVol. 108. Springer-Verlag, New York, pp. 133-177.
- Ansari, B.A., Kumar, K., 1988. Cypermethrin toxicity: effect on the carbohydrate metabolism of the Indian catfish, Heteropneustes fossilis. Sci. Total Environ. 72, 161-166. [CrossRef]
- Bradbury, S.P.; Coats, J.R. Toxicity of fenvalerate to bobwhite quail (colinus virginianus) including brain and liver residues associated with mortality. J. Toxicol. Environ. Heal. 1982, 10, 307–319. [Google Scholar] [CrossRef]
| Refference | Subjects | Sampling | Exposure assessment1 | Health effects related to pyrethroid exposure |
|---|---|---|---|---|
| [88] | 512 pregnant woman | urine samples | urinary levels of cis-dibromodimethylvinylcyclopropane carboxylic acid (DBCA), 3-phenoxybenzoic acid (3-PBA) and total PYR metabolites | Exposure to PYRs during pregnancy was linked to higher birth weight, increased length at birth, and longer gestational duration, as well as a reduced likelihood of small for gestational age (SGA) or premature birth. |
| [89] | 537 mother-child pairs | urine samples | dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), diethyl phosphate (DEP), diethyl thiophosphate (DETP), diethyl dithiophosphate (DEDTP), 3,5,6-trichloro-2-pyridinol (TCPy; a metabolite of chlorpyrifos, chlorpyrifos-methyl and triclopyr), 3-PBA in urine | The findings of this research suggest that exposure to organophosphate and pyrethroid insecticides during pregnancy could potentially impact the normal growth of the fetus and lead to changes in birth anthropometric measures and gestational age, potentially signaling early disruptions in childhood developmental processes. Such effects might have long-term implications for individual health. Furthermore, our study emphasizes the differing susceptibility between genders to prenatal stressors. Given the limited evidence available at the time of this analysis and the discrepancies in the existing data, further investigation is strongly recommended to validate the reported outcomes and provide additional insights into the observed distinctions. |
| [90] | 524 mother–child pairs | maternal urine samples | 3-PBA in urine | Exposure to 3-PBA both during pregnancy and at age 2 was linked to heightened ADHD symptoms at age 6, whereas exposure during ages 4 and 6 was connected to ADHD symptoms at age 8. These results suggest the presence of various sensitive phases in early life that could influence the development of ADHD symptoms during the school-age period. |
| [91] | 720 adolescents | urine samples | 3-PBA in urine | Exposure to pyrethroid pesticides was found to be correlated with hearing loss in American teenagers. This research offers new insights into the connection between pyrethroid exposure and the sense of hearing. |
| [92] | 1305 subjects | urine samples | 2,2,3,3-tetramethylcyclopropanecarboxylic acid (TMCA), TFBA, 3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2- dimethylcyclopropanecarboxylic acid (CTFCA), 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylic acid (MPCA), DCCA, FPBA, 3-PBA, MTFBL, and MMTFBL. |
The findings of this study indicated that pyrethroid exposure was linked to either heightened levels of total prostate-specific antigen (PSA) or changes in the PSA ratio. Our findings propose that long-term exposure to pyrethroids could potentially harm male reproductive organs, particularly the prostate gland. Additionally, the effects of pyrethroid exposure on the body may vary depending on renal function status. The unstable statistical outcomes and the uncertainty surrounding the carcinogenicity of pyrethroids could be attributed to the limited sample size. Further comprehensive studies are required to establish the reproductive toxicity associated with pyrethroids. |
| [93] | 2012 participants (1006 diabetic cases and 1006 matched controls) | serum semples | Serum pyrethroids determined by gas chromatography-mass spectrometry according to a standardized protocol - deltamethrin and fenvalerate |
The comprehensive analysis of metabolites revealed that serum pyrethroid insecticides, especially deltamethrin, were linked to various plasma lipid metabolites, many of which participated in the glycerophospholipid metabolism pathway, predominantly characterized by PCs and LPCs. Additionally, the study indicated that four plasma metabolites (namely, PC 32:0, PC 34:4, CE 20:0, and TAG 52:5 [18:2]) and the pathway involving glycerophosphoethanolamine might represent the potential mechanism underlying the relationship between serum pyrethroids and the onset of type 2 diabetes (T2D). |
| [94] | 177 children | urine samples | glyphosate (GLY); aminomethylphosphonic acid (AMPA); 3,5,6-trichloro-2-pyridinol (TCPy), the main chlorpyrifos pesticide metabolite; cis-(2,2-dibromovinyl)-2,2-dime thylcyclopropanecarboxylic acid (cis-DBCA); cis-3-(2,2-dichlorovinyl)- 2,2-dimethylcyclopropane-1-carboxylic acid (cis-DCCA); trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (transDCCA); 3-phenoxybenzoic acid (3-PBA); 4-fluoro-3-phenoxybenzoic acid (4-F-3-PBA); and cis-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylic acid (CIF3CA or CFMP) | This study focused on monitoring children’s exposure to pesticides in Cyprus, following the methodology and tools used in the HBM4EU project. While a notable link was found between aminomethylphosphonic acid (AMPA) and the DNA oxidative stress marker in this children’s population, it is essential to replicate these findings in a more extensive study. The absence of significant associations between AMPA/GLY and lipid damage may suggest a biological DNA damage mechanism for AMPA. |
| [95] | 683 mothers | maternal urine samples | cis-DBCA, cis-DCCA, trans-DCCA, and 3-PBA | Exposure during pregnancy to DDT and pyrethroid insecticides might be linked to concerning behaviors in children residing in a malaria-endemic rural area of South Africa. |
| [96] | 726 adults | urine samples | 3-PBA in urine | A direct correlation was noted between 3-PBA and shifts in both low-frequency and high-frequency hearing thresholds in individuals aged 20 to 39 in the United States, suggesting the susceptibility of young adults to the harmful effects of pyrethroid insecticides. |
| [97] | 48 farmworkers | urine samples | five insecticide metabolites [3,5,6-trichloro-2- pyridinol (TCPy; a metabolite of the OP chlorpyrifos) and four metabolites of pyrethroid insecticides: 3-phenoxybenzoic acid (3PBA), 4-fluoro-3-phenoxybenzoic acid (4F3PBA), sum of cis/trans 3-(2,2-dichlorovinyl)− 2,2-dimethylcyclopropanecarboxylic acid (DCCA), and chloro-3,3,3-trifluoro-1-propene-1-yl (CFCA)] | The study showed a connection between exposure to OP and pyrethroid insecticides and a decrease in cortical brain activity in the prefrontal cortex, which might explain previously reported links to cognitive and behavioral function. |
| [98] | 2116 adults | urine samples | 3-PBA in urine | The results of this forward-looking group study revealed that the environmental exposure to pyrethroid insecticides was notably linked to a heightened risk of mortality from all causes within the general adult population in the United States. The observed correlation is probably connected to the detrimental impact of pyrethroids on the cardiovascular system. |
| [99] | 384 pregnant women | urine samples | PYRs metabolites: 3-phenoxybenzoic acid (3PBA), 4-fluoro-3-phenoxybenzoic acid (4F3PBA), and cis-2,2dibromovinyl-2,2-dimethylcyclopropane-1-carboxylic acid (DBCA) in urine samples | The results suggested that women in the final trimester of pregnancy and their infants, aged 6-8 months, were widely exposed to PYRs at low doses. The total concentration of PYRs metabolites in infancy exceeded that during pregnancy. While daily PYRs exposure during the third trimester of pregnancy did not impact toddlers’ language development, exposure to PYRs containing 4F3PBA and DBCA during the 6-8 months age range might delay toddlers’ language development. This demonstrates that the phase between 6-8 months old could be a sensitive window for PYRs exposure that affects toddlers’ language development. The likelihood of language development delay in 2-year-old toddlers could be anticipated by PYRs metabolites with 4F3PBA and DBCA during infancy, at 6-8 months. |
| [100] | 1235 adults | urine samples | 3-PBA in urine | The outcomes of our study revealed that exposure to pyrethroids was linked positively with testosterone (TT) and sex hormone-binding globulin (SHBG) in adults and negatively with circulating free testosterone in males. The current study provides backing to the idea that pyrethroids might have disrupted endocrine function on sex hormones at the observed exposure levels of pyrethroids in U.S. adults. Prolonged and chronic exposure to pesticides could potentially lead to changes in serum sex hormones. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





