1. Introduction
China's lignite is rich in resources and occupies the main position in the coal consumption structure. In the process of mining, lignite is prone to spontaneous ignition when exposed to air, which is an important object of mine fire prevention and control. People have studied the law of spontaneous combustion by constantly exploring the relevant factors of spontaneous combustion of coal. Based on various principles such as isolating the contact between the coal surface and oxygen, reducing the coal temperature below the combustion temperature, improving the activation energy of the coal oxidation reaction from the perspective of chemical technology, and inhibiting the spontaneous combustion reaction process of coal, technical measures such as grouting, filling, pressure equalization, inhibition, inert gas, gel and three-phase foam are proposed to inhibit the spontaneous combustion of coal [
1].Among all kinds of existing fire prevention technologies, the research on the use of inhibitors for fire prevention is still in the process of development [2-5]. Currently, commonly used inhibitors can be divided into: (1)liquid film is formed on the surface of coal seam from the perspective of inhibition mechanism, thereby preventing further contact reaction between coal molecules and oxygen in the air.(2)The blocking agent reacts with heat release, and the surrounding water is heated and vaporized to absorb heat, thereby preventing the heat accumulation inside the coal seam.(3)The inhibitor reacts with the active groups in the macromolecular structure of coal to reduce the number of active groups, thereby preventing the coal-oxygen reaction [
6]. From the point of view of inhibition properties, the existing inhibitors can be divided into physical, chemical and composite inhibitors, physical inhibitors can effectively fix the water in coal, reduce the possibility of coal spontaneous combustion, and prevent the oxygen in coal composite, mainly including chlorine salts, halogen salt inhibitors, gel inhibitors, foam inhibitors, polymer inhibitors and so on. Compared with raw coal, the peak temperature and total heat release of the inorganic salt retarded coal sample decreased significantly, and the apparent activation energy increased significantly, thus effectively preventing the spontaneous combustion reaction of coal [
7]. Predecessors analyzed the flame retardant effect of inorganic salt retardants on different coal types by changing the types of coal and retardants. It is concluded that different inorganic salt inhibitors should be used for different coal types [
8].
Chemical inhibitors can directly interfere with the REDOX reaction in coal and interrupt the chain reaction by forming a stable structure with its active functional groups [
9]. By studying the structural fragments of coal molecules, it was proposed that metal ions can form coordination bonds with atoms in some active groups, thus reducing the activity of reactive groups in coal to react with oxygen. In view of this theory, predecessors have used a variety of test and analysis methods to study the changes of several inorganic salt inhibitors on the structure of coal from the molecular point of view, and evaluated their respective inhibition effectiveness. For example, the mechanism of inhibiting coal spontaneous combustion by CaCl
2 was studied, and the existence of N active groups in coal was systematically studied. The results show that calcium ions react with N-containing active groups in coal structure to form stable complexes, improve the active structure of coal, and greatly improve the stability and antioxidant capacity of coal [
10]. Through thermal analysis, infrared spectrum, oxidation analysis and other experimental tests, it is proved that phosphate and MgCl
2, as synergistic inhibitors to inhibit coal spontaneous combustion, can effectively reduce the heat in the process of coal spontaneous combustion, and reduce the functional groups such as -OH and -CH
2 in treated samples [
11]. According to the temperature program oxidation experiment and Fourier infrared spectrum analysis, it can be seen that the sodium persulfate treatment of coal samples can increase the amount of stable ethers, alkyls and carboxylic acids in the molecular structure, thus significantly improving the flame retardant performance [
12].From the perspective of technical means, in order to effectively solve the limitations and deficiencies of a single type of chemical retarder, predecessors proposed to combine it with different types of retarder to form a composite retarder to inhibit spontaneous combustion of coal. Examples include carbon/oxygen free radical trapping agent and magnesium chloride complex [
14], foam carrier combined with thermoplastic and coal powder [
13], Sodium polyacrylate - anthocyanin complex inhibitor, and the use of temperature-sensitive smart gel coating tea polyphenols [
15]. In order to overcome the problems of easy loss of chemical reagents and short resistance life in the use of traditional chemical inhibitors, the technology of microcapsule encapsulation of chemical reagents can improve the stability of traditional chemical reagents and provide convenience for injection into coal seams. The principle is to inject the microcapsule inhibitor into the coal seam in advance when the coal body is not oxidized [34]. When the oxidation of the coal seam reaches a certain temperature point, the chemical inhibitor wrapped in the microcapsule begins to act for inhibition. At present, the microcapsule coated with tea polyphenol inhibitors and ethylenediamine tetraacetic acid coated with polyethylene glycol 20000 and pentaerythritol stearate have significant chemical inhibition effects and can improve the activation energy of the reaction [
16].
As for the improvement of the inhibition efficiency of the inhibitor, it is still under research and development. It has been found that some microorganisms in nature can react with calcium ions in the environment by their own metabolic activities to generate calcium carbonate precipitation, which can be cemented and condensed among the particles after combining with soil particles. This feature is often applied to soil structure modification in construction to increase soil strength. Reduce dust [
17]. In view of this characteristic, this paper proposes a new method and principle of inhibiting spontaneous combustion of lignite by inducing calcium deposition by microorganisms. A series of controlled experiments are carried out to determine the optimal culture environment for microorganisms, and four coal samples of raw coal, bio-inhibited coal, chemically inhibited coal and water-immersed coal are prepared. SEM, pore size analysis and FT-IR tests were carried out to study the changes of surface morphology, pore structure, active functional groups and other microscopic characteristics of four kinds of lignite samples before and after inhibition, analyze the inhibition mechanism and change rule, and reveal the inhibition mechanism of microbial inhibitor on lignite spontaneous combustion from the perspective of microstructure.
2. Experimental Material
2.1. Preparation of Biological Inhibitors
The Bacillus pasteurelli used in the experiment was purchased from Beijing Beina Biotechnology Co, LTD. Strain number ATCC11859. The urease produced by this strain can decompose urea in two stages to obtain ammonia and carbonic acid. With the addition of calcium source, carbonate ions in the solution combine with calcium ions to form calcium carbonate with strong cementing ability [
18]. The mechanism of applying microorganisms to inhibit the spontaneous combustion of coal is mainly to use the calcium carbonate produced to block the pores of coal and significantly reduce the specific surface area of coal. Reduce oxygen contact with the coal surface. The growth of Bacillus pasteurii is mainly affected by temperature and pH, and it has strong adaptability to the environment and can still survive in the environment of high acid, high alkali and high salt. Studies have shown that the optimal growth temperature of this bacterium is 30℃ in a more alkaline environment [
28], and the optimal cultivation conditions such as pH and cultivation time are determined through further controlled experiments.
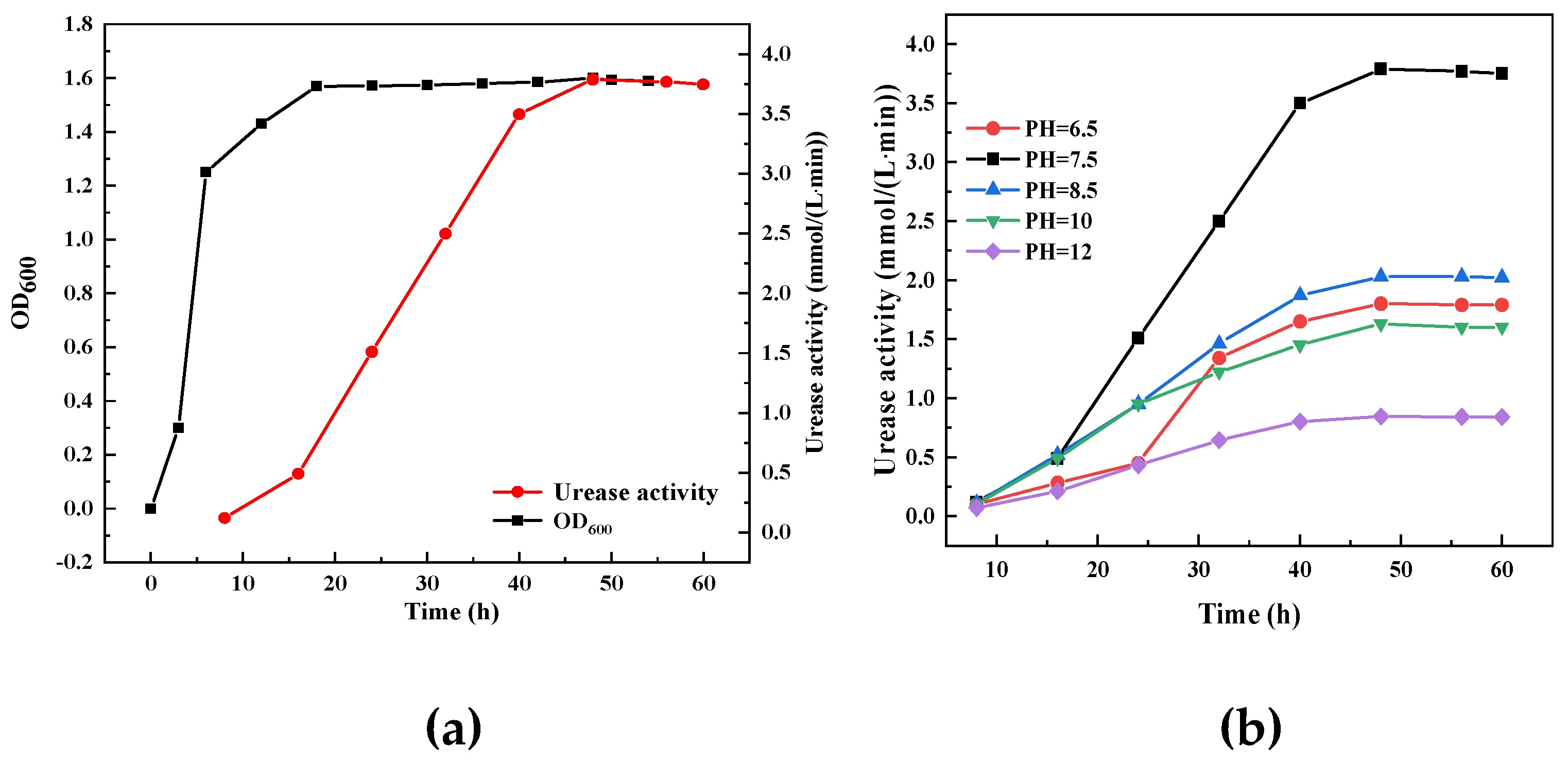
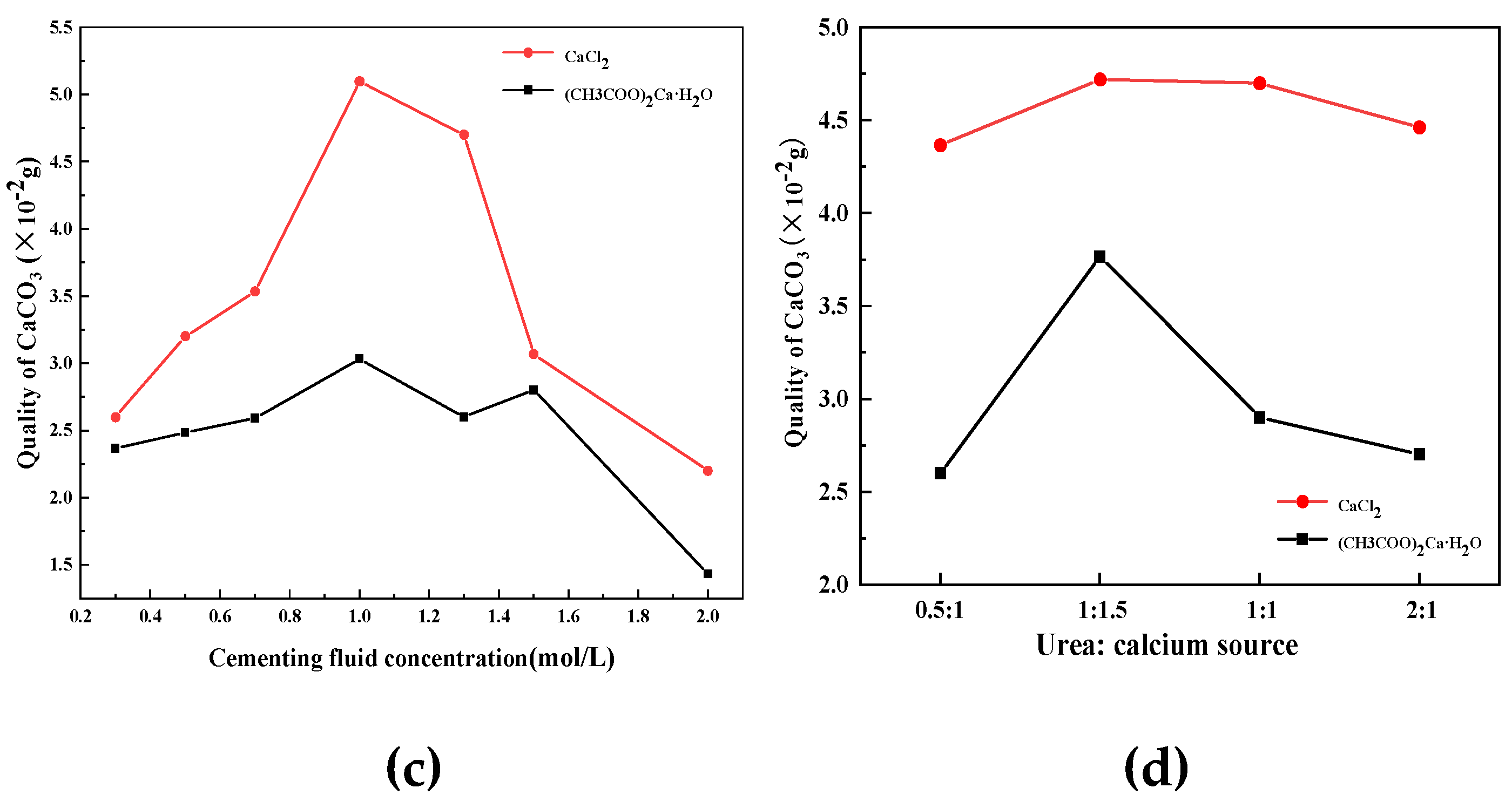
According to the results of the control experiment, the strains were cultured in a weak alkaline environment with a PH of 7.5 for 48h, and the number of microorganisms and urease activity in the culture medium reached the highest. When calcium chloride is selected as calcium source, the concentration of cementing fluid is 1M, the ratio of cementing fluid to bacterial fluid is 1:1, and the ratio of urea to calcium chloride is 1:1.5, the quality of calcium carbonate is the highest. The influence of culture conditions on biological bacteria solution is shown in
Figure 1.
2.2. Preparation of Experimental Coal Sample
The object of this experiment was lignite, which was accurately tested and analyzed according to the standard method of experimental coal sample [
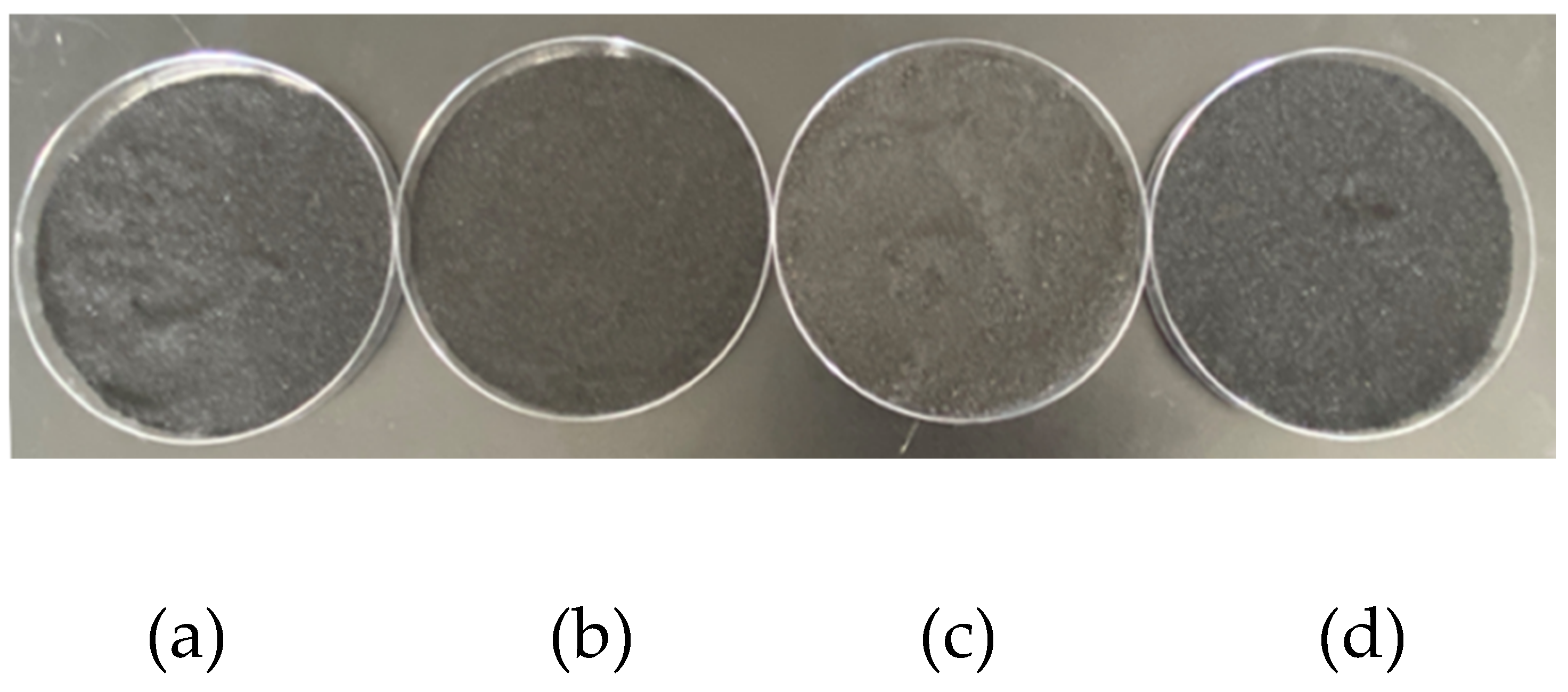
19]. Fresh coal samples were taken from the site and sealed and stored. After crushing treatment in the laboratory, samples with particle sizes of 40~80 mesh and 200~300 mesh were selected and dried for 48 hours under vacuum environment for subsequent use. Four groups of lignite coal powder with a mass of 5g were weighed, and one group of coal samples was set as raw coal samples without treatment and placed in a 40°drying oven for 48 hours under vacuum drying. The remaining three groups are: Add 10 mL of soaked coal sample after 24 hours of distillation and drying, add 1 mol/L calcium chloride solution and 5 mL of 1 mol/L sodium bicarbonate solution respectively, and react 24 h after drying chemically inhibited coal sample. In addition, 5 mL calcium chloride solution with a concentration of 1 mol/L and 5 mL microbial culture medium cultured for 48 h (bacterial activity OD600=1.6) were added to the bioinhibited coal samples, which were dried after reaction for 24 h. The drying method of each sample was the same as that of raw coal. After sealing, four groups of experimental samples were obtained after different treatments, and the subsequent four groups of coal samples were respectively carried out. The prepared experimental coal samples are shown in
Figure 2.
3. Experimental Methods
3.1. Electron Microscope Experiment of Retarded Lignite
The four groups of coal samples were dehydrated and gold sprayed to ensure their good electrical characteristics. QuantaTM250 scanning electron microscope was used to observe and analyze their surface structural characteristics respectively.
3.2. Low Temperature Nitrogen Adsorption Experiment
Weigh about 2.5g coal sample and put it into the sample tube for 10h degassing treatment. Before and after degassing, weigh the empty tube mass M1 of the sample tube and the sample tube mass M2 of the coal sample with degassing and removing water in the pores respectively. After that, the NOVA touch rapid automatic specific surface area and aperture analyzer was used to perform low-temperature nitrogen adsorption experiments on coal samples at ambient temperature above 77K and relative pressure range of 0.008~0.98. The measurement principle is to use nitrogen as the measuring medium and take advantage of the adsorption characteristics of coal to measure the specific surface area, total pore volume and average pore size of the sample [
31]. After measurement, BET equation was used to determine the nitrogen monolayer saturation adsorption capacity, so as to estimate the specific surface area of the experimental coal sample [
29]. The BJH method is used to analyze the pore size distribution [
30], and the capillary condensation theory and equivalent exchange principle are used to measure the volume of liquid nitrogen in the pore and then estimate the pore size, and then the total pore volume and average pore size of the coal sample can be calculated by formula.
3.1.1. Calculate the Specific Surface Area
By applying BET equation, the specific surface area of each layer on the isothermal adsorption line can be calculated, and the interaction between the single-layer adsorption amount Vm and the multi-layer adsorption amount V
m can be deduced [
27].
P: partial pressure of nitrogen, Pa;
P0: Saturated vapor pressure of nitrogen at adsorption temperature, Pa;
V: The actual adsorption amount of nitrogen on the sample surface, cm2/g;
Vm: nitrogen monolayer saturation adsorption capacity, cm
2/g;
D: constant related to the adsorption capacity of the sample;
:Specific surface area, m
2/g;
Using BET equation, P/P0 as the X-axis and (P/P0)/(V(1-P/P0)) as the Y-axis, the linear equation y=Ax+B can be obtained, and then Vm can be determined by combining the calculated results with slope A and intercept B.
3.1.2. Calculate Aperture Distribution
The coal pore size division method proposed by IUPAC has become the most commonly used method in the world today, which divides the pores of coal into three categories according to the changes in the pore size of coal and the influence of solid substances: micropores (<2nm), mesoporous (2~50nm) and macroporous (>50nm) to better meet the needs of different applications. At present, BJH has become the most widely used and mature calculation model of aperture distribution, which is based on the Kelvin capillary condensation theory and has been widely used in a variety of theories. In this paper, the BJH method is used to analyze the pore size distribution, where dv (r) represents the differential relationship between pore volume and pore size, reflecting the function of pore density distribution, that is, the proportion of pore volume. The cumulative pore volume refers to the cumulative volume of coal sample with the increase of pore volume.
Using capillary condensation theory and equivalent exchange principle, the volume of liquid nitrogen in the hole can be accurately measured by adsorption technology, and then the size of the hole can be accurately estimated. The following formula can be used to calculate the total pore volume and average pore diameter:
Where, 𝑟 is the pore range, nm; 𝛾 is liquid nitrogen surface tension, N/m; 𝑇 is the absolute temperature, K; R is the universal constant of gas,8.314 J∙mol-1∙K-1;𝑉 is the adsorption angle, liquid nitrogen adsorption r=0.
Aperture size calculation formula:
Where, 𝑉 is the total pore volume of the adsorbent, mL; 𝑑 is the average aperture, nm.
3.3. Fourier Infrared Spectroscopy Experiment
The content of C, H, N and S in lignite can be determined by industrial analysis and elemental analysis, while the content of O can be calculated by subtraction. Test results are shown in the following
Table 1:
Bruker VERTEX 80v infrared spectrometer is used in the experiment. According to the relevant regulations [
21], the experimental environment was clean and free of strong electromagnetic field interference sources, and the coal sample was preheated for 30min. The coal sample was tested according to the standard experimental measurement process. A sample of 1g solid coal powder with a particle size between 200 and 300 mesh was prepared. The test sample was made by mixing KBr with the coal sample and pressing. The scanning range was between 4000 and 400 cm
-1, and 64 sample scans were completed.
4. Results
4.1. Scanning Electron Microscope Analysis of Lignite Surface Structure
The SEM images of the four groups of samples are shown in
Figure 3.
From top to bottom, the images are (a) raw coal samples, (b) water-immersed coal samples, (c) chemically inhibited coal samples and (d) biologically inhibited coal samples respectively. By observation, it can be found that in the figure, there are a large number of pores formed by gas generation and gas accumulation due to low metamorphism in the process of coal production, exogenous pores formed by geological structure after consolidation of diagenesis, and some solution pores. These are secondary pores formed by the dissolution of soluble minerals in coal after long-term immersion in water. Under electron microscope, there is almost no difference in surface structure between lignite treated with water and lignite raw coal.
Under electron microscope, the surface structure of coal sample (c) and coal sample (d) treated with chemical and biological inhibitors is significantly different from that of raw coal in morphology. This is because the solid product calcium carbonate covered on the surface of coal sample produced by the two inhibition methods leads to a more dense and compact surface of coal sample after treatment than that of raw coal sample and water immersed coal sample. By enlarging the coal sample (c) and coal sample (d), it can be found that the calcium carbonate produced by chemical inhibition treatment and covered on the surface of the coal sample mainly presents a massive distribution, while the calcium carbonate produced by biological inhibition treatment presents a more dense spherical distribution. The main reason for this phenomenon is that the biological inhibition process uses microorganisms as the intermediate medium. The resulting calcium carbonate combined with the viscosity of the microorganisms themselves can more firmly seal the pores generated by various factors in the coal seam, significantly reducing the contact area between the coal surface and oxygen.
4.2. Experimental Analysis of Inhibited Lignite Pore Size
4.2.1. Aperture Distribution Characteristics
The pore size distribution of lignite samples after different treatments used in the experiment is shown in
Figure 4, where the horizontal coordinate represents the pore size and the vertical coordinate represents the number of holes. It can be seen from the figure that most of the internal pores of lignite raw coal are medium and large pores, and a few are micropores. Compared with raw coal, the pore size of lignite treated with water immersion increased slightly, and the change trend was consistent with that of raw coal. However, the number of macropores and mesoporous pores in the lignite samples after bioinhibition treatment has great changes. Compared with the raw coal, the number of macropores above 50nm has a significant decrease, while the number of micropores has little change. This phenomenon verifies that Bacillus pasteurelli lives well in macropores and produces high urease activity. It is found that the total number of pores of each pore in the bioinhibited coal sample is significantly less than that in the chemically inhibited coal sample by comparing the number of pores of each pore in the bioinhibited coal sample. The obvious change in the number of holes indicates that the effect of biological inhibition is better than that of chemical inhibition on the sealing of internal holes in coal samples.
4.2.2. Pore Structure Characteristic Parameter Analysis
The multilayer physical adsorption model of BET theory can effectively capture the adsorption characteristics in the range of 0.05~0.35, but when the range exceeds 0.05, the formation of multilayer physical adsorption will be affected by capillary condensation, thus making the surface non-uniformity become more obvious [
25]. Therefore, in this range, BET theory can capture the adsorption characteristics more accurately. In this paper, the BET equation is used to calculate the specific surface area and the relative pressure (P/P0) is set between 0.05 and 0.35.
According to IUPAC, coal pore size is divided into three types: micropores, mesoporous pores and macropores to better meet different application requirements. The BJH pore size distribution calculation model is developed from the Kelvin capillary condensation theory [
26].The adsorption properties of nitrogen in samples were studied by BJH method, and the pore size distribution was analyzed in detail. In
Table 2, the total pore volume P
V and pore specific surface area P
S of the four experimental coal samples are calculated using the above method. Two parameters, D
pv and D
ps, are introduced to represent the difference percentage of the PV and PS of the experimental coal samples compared with the raw lignite coal respectively. Positive values of the two parameters indicate that the value of the treated coal samples has increased compared with the raw coal. A negative value indicates that the value of the corresponding coal sample is lower than that of the original coal. Formulas (7) and (8) are the calculation formulas of D
pv and D
ps.
From the analysis of
Table 2, it can be seen that the total pore volume P
V and specific surface area P
S of the coal sample treated with the two inhibitors are significantly reduced compared with the raw coal sample, and the D
pv and D
ps of the bio-inhibited lignite sample are both smaller than those of the chemically inhibited lignite sample. This is because the adhesion of calcium carbonate induced by Bacillus pasteurelli is higher than that of ordinary calcium carbonate, which can better glue coal particles and seal coal seams. Therefore, both of the two inhibitors can effectively plug the pores in coal, and it is found that the effect of biological inhibition using Bacillus pasteuris is more obvious than that of chemical inhibition.
4.3. Experimental Analysis of Infrared Spectrum
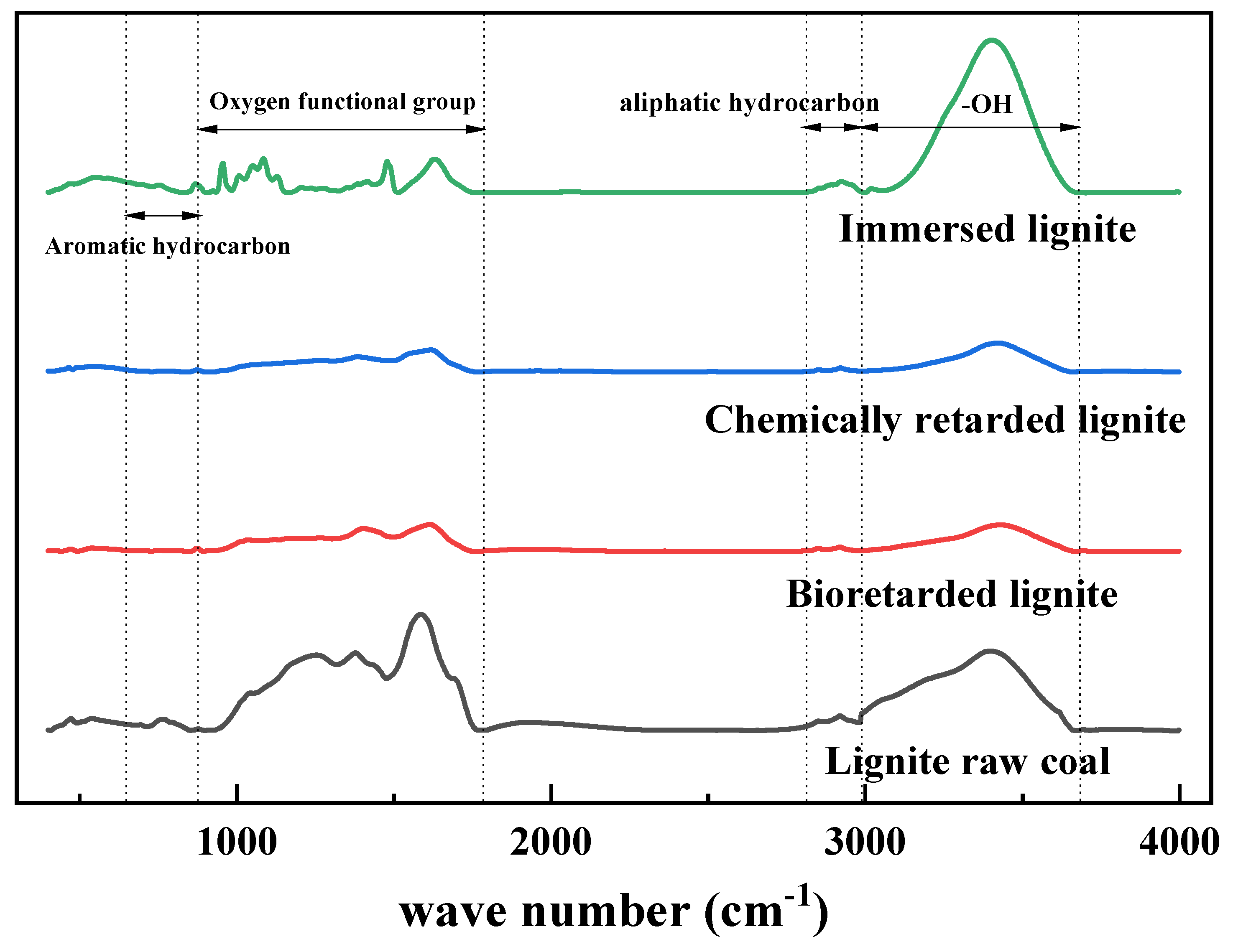
The classification of functional groups in coal molecules is shown in
Table 3. The spontaneous combustion of coal is due to the heat accumulation generated by the reaction between the active groups and oxygen in the coal molecules, which makes the temperature of the coal seam rise and reach the combustion point. The addition of biological inhibitors prevents the natural microstructure of coal by reducing the number of active groups to reduce the reaction rate. Combined with the infrared spectrum of coal samples, the main functional groups in coal can be divided into four categories: hydroxyl, oxygen-containing functional groups, aliphatic hydrocarbons, and aromatic hydrocarbons [
33]. In this paper, the content variation is analyzed by comparing the size of the peak area of the functional group.
1. Hydroxyl group
Hydroxyl groups in coal are the main components of hydrogen bonds, and their type and strength affect the risk of spontaneous combustion of coal. The characteristic peaks of hydroxyl group in coal are mainly distributed in the range of 3800~3000 cm
-1, and the wave numbers are as follows: free hydroxyl group, intermolecular hydrogen bond, phenol hydroxyl group, alcohol hydroxyl group and amino group intermolecular hydrogen bond. According to
Figure 6, the distribution and intensity of characteristic peaks are quite different in this band, and the strength of hydroxyl characteristic peaks of coal samples treated with water immersion is higher than that of raw coal, and the hydrogen bonds concluded between molecules are mainly used, indicating that the number of hydrogen bonds binding coal and water molecules increases after water immersion treatment. The characteristic peak strength of hydroxyl group of coal samples after inhibited treatment is lower than that of raw coal, and the type of hydroxyl group is also different. After inhibited treatment, lignite has no characteristic peak of free hydroxyl group, the number of intermolecular hydrogen bonds is increased, and the number of intermolecular hydrogen bonds between amino groups is reduced, and the effect of biological inhibited treatment is more obvious than that of chemical inhibited treatment.
2. Aliphatic hydrocarbon
The characteristic peaks of aliphatic hydrocarbons in coal macromolecular structure range from 3000 to 2800 cm
-1, in which methyl and methylene are the most common types. The antisymmetric stretching band of methyl group can be observed at 2950 cm
-1, the antisymmetric stretching band of methylene group can be observed at 2915 cm
-1, the stretching vibration band of -CH can be observed at 2890 cm
-1, and the symmetric stretching band of methylene group can be observed at 2850 cm
-1. As can be seen from
Figure 6, the distribution trend of aliphatic hydrocarbon characteristic peaks in coal samples is roughly the same. The methylene content is higher than the methyl content at 2950 cm
-1, indicating that the macromolecular structure of coal is mainly in the form of long chain alkyl. Methyl and methylene are one of the main reactive groups in the macromolecular structure of coal. The peak area of methyl and methylene in the coal sample after bioretarding treatment is significantly lower than that of raw coal, indicating that the methyl and methylene in the molecular structure of coal can be consumed after bioretarding treatment, the number of active groups involved in the coal-oxygen reaction can be reduced, the reaction rate can be reduced, and the effect of coal spontaneous combustion can be prevented.
3. Oxygen-containing functional groups
The oxygen-containing functional groups in coal are mainly composed of carbonyl group, carboxyl group and ether bond, and their spectral peak band ranges from 1800 to 900 cm
-1. The carboxyl group and carbonyl group in the oxygen-containing functional group can combine with oxygen to produce more active small molecules to participate in the coal-oxygen reaction. Therefore, the higher the content of carboxyl group and carbonyl group in the oxygen-containing functional group, the easier the coal-oxygen reaction is to occur. By observing the comparison of activity spectrum peaks in
Figure 6, it is found that the area and quantity of carboxyl group spectrum peaks of the coal samples after bioinhibited treatment are reduced, and the reduction is greater than that of the coal samples after chemically inhibited treatment. The results show that bioinhibition can reduce the active sites of oxygen-containing functional groups in coal structure and delay the reaction rate.
4. Aromatic hydrocarbon
The out-of-plane bending vibration of various substituted aromatic hydrocarbons is mainly distributed in the band 900~650 cm-1. The aromatic structure is the core structure of coal molecules, and the molecular skeleton structure will fracture only at high temperature. It was found that there was no significant change in the aromatic substitution structure spectrum peak between the treated coal sample and the raw coal sample, indicating that the molecular skeleton structure of the coal was not changed after the biological inhibition treatment.
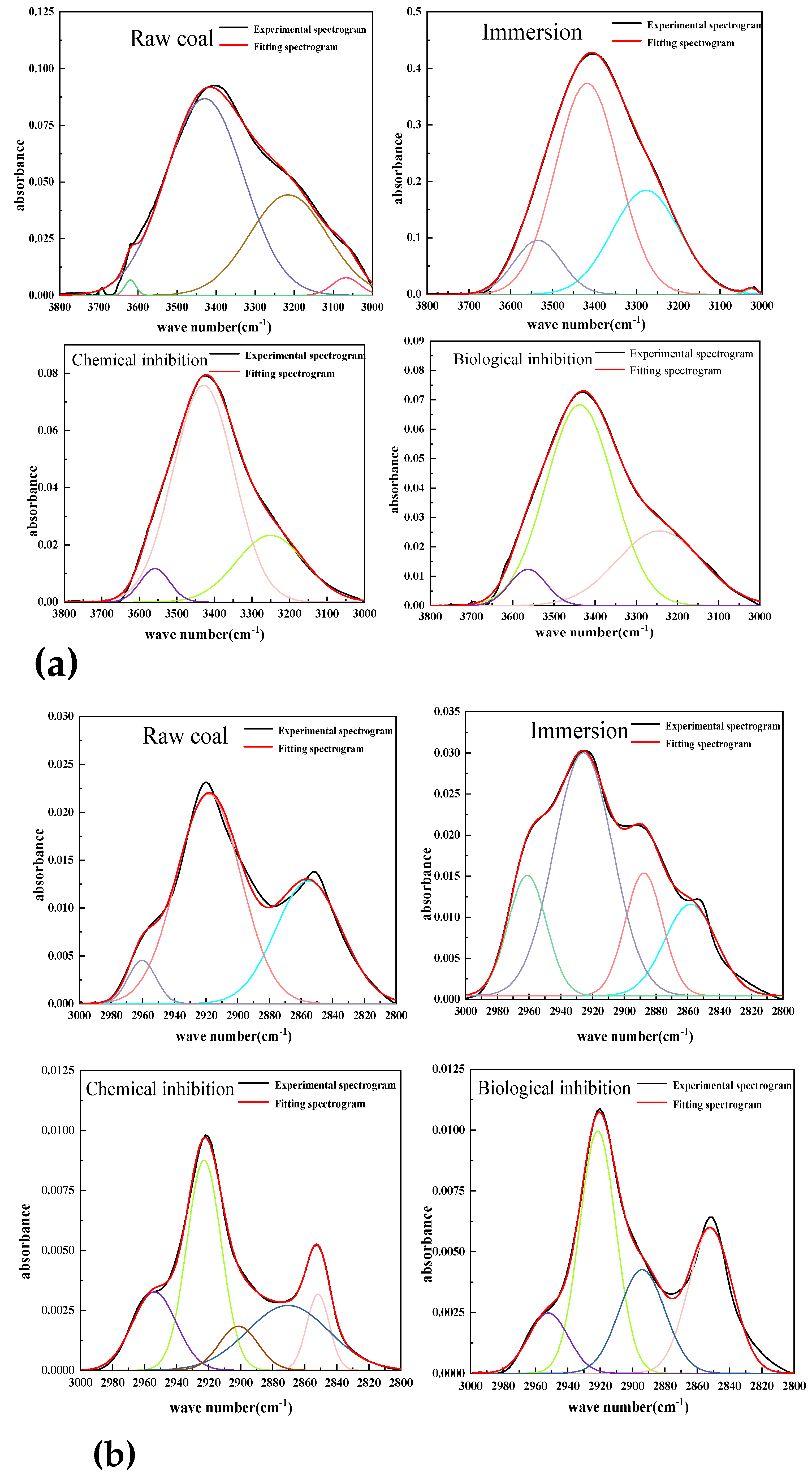
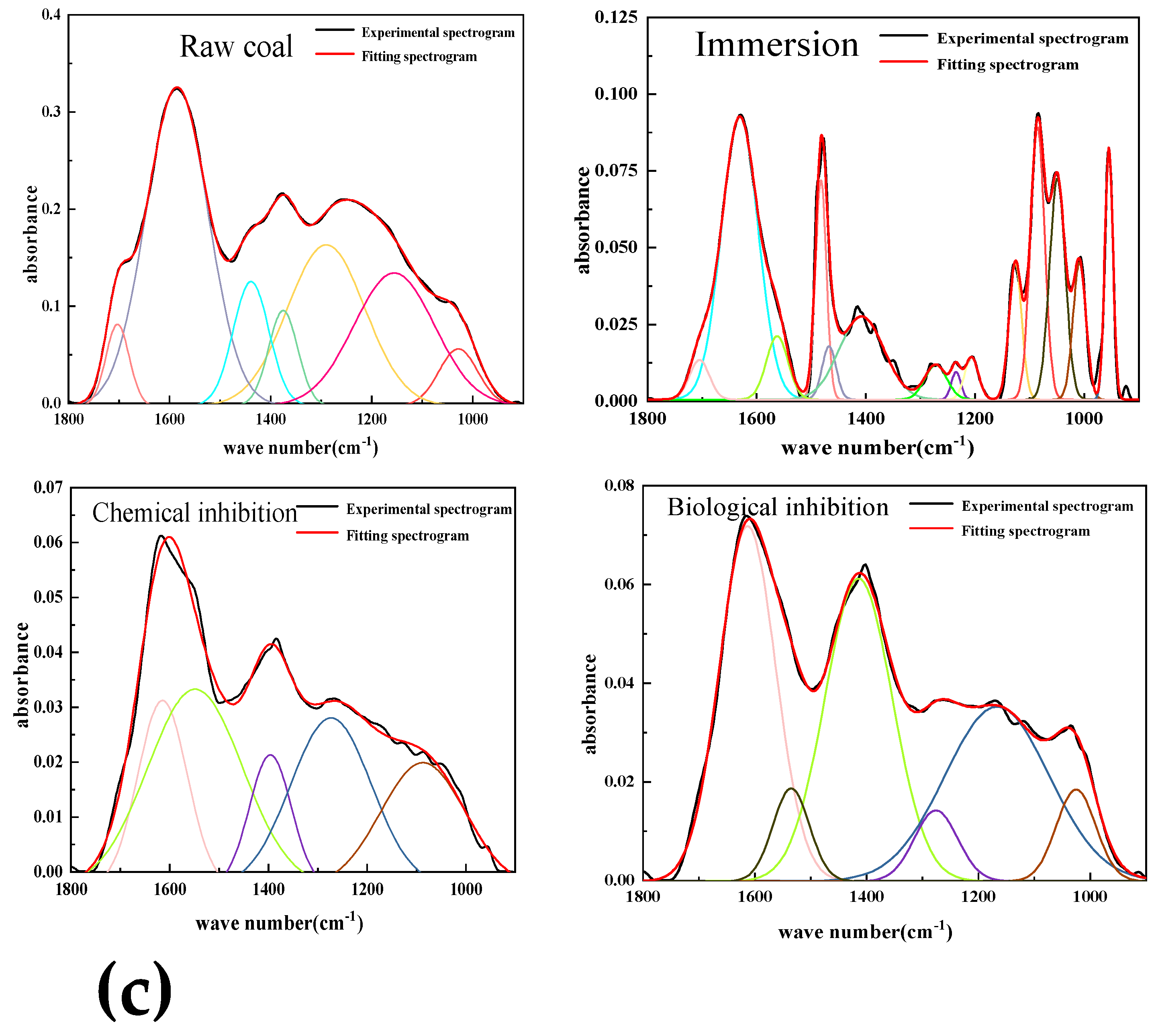
4.4. Analysis of Spectral Peak Characteristics of Functional Groups
The main causes of coal spontaneous combustion are aliphatic hydrocarbons, hydroxyl groups and oxide functional groups. By using Origin software, the infrared spectral data is fitted peak-to-peak to determine the number and proportion of different types of functional groups. The functional group changes of lignite after different experimental treatments were accurately detected by sectional fitting. The absorption bands of hydroxyl, aliphatic, oxygen-containing functional groups and aromatic hydrocarbons were 3600~3000 cm
-1, 3000~2800 cm
-1, 1780~900 cm
-1 and 900~650 cm
-1, respectively. Understanding these common functional groups is helpful to better understand the changes of functional groups after lignite treatment. The following parameters are introduced to characterize the change of the content of different active groups in lignite after resistance treatment.
In the formula, EC represents the content of corresponding active groups in coal samples treated in different ways, RC represents the initial content of corresponding functional groups in raw lignite coal, and D
-CH3/-CH2、D
-OH and D
-COOH respectively represent the changes in the content of methyl/methylene, hydroxyl and oxygen-containing functional groups (carboxyl groups) in coal samples treated compared with raw coal. The detection shows that the hydroxyl content of the coal sample after the resistance treatment is reduced, indicating that the two treatment methods will combine with the hydroxyl group in the coal structure, reducing the chance of hydroxyl group and oxygen contact. Methyl and methylene groups in aliphatic hydrocarbons are important groups involved in heat accumulation during oxidation.
Table 4 lists the calculation results.
Table 4 shows the content changes of hydroxyl, carboxyl and aliphatic groups (methyl and methylene) in the three treated experimental coal samples compared with the raw coal. It can be seen from the table that the content of three active groups in the coal samples added with biological inhibitors is reduced compared with the raw coal, among which the content of methyl and methylene is reduced the most.
Through the above comparative analysis, it can be found that whether chemical or biological inhibitors are added, the content of three main active groups in the coal sample will decrease, among which the methyl and methylene content in aliphatic hydrocarbons is the most obvious, and these two active groups are also the key groups for heat accumulation in the coal oxidation reaction. The decrease in the content of active functional groups indicates that the number of active groups involved in the reaction is correspondingly reduced, and thus the reaction rate is reduced. The D-CH3/-CH2 of chemically retarded coal samples and biologically retarded coal samples are -94.4% and -96.6%, respectively, indicating that the effect of biological retarders on the consumption of these two active groups is better than that of chemical retarders.
5. Conclusion
In this paper, the modification of lignite before and after the addition of retardants was analyzed from the perspective of microscopic structure, and the change of flame retardant performance of lignite treated with two retardants compared with that of raw coal was explored. It was concluded by comparison that the oxidation and self-heating capacity of lignite after microbial retardation was effectively curbed in the initial stage of spontaneous combustion, and the retardation performance was better than that of chemical retardants.
(1) Based on SEM images, the change characteristics of the surface micro-morphology of the treated coal samples were analyzed. The surface of lignite is mainly composed of dissolution caves and pores. Compared with raw coal, a large number of deposited calcium carbonate particles are obviously attached to the surface of lignite after resistance treatment, which plays a role of physical oxygen insulation.
(2) The pore size distribution, total pore volume and specific surface area of lignite after different treatments were studied. The holes of lignite mainly exist in the form of parallel slate-like slit holes and open wedge holes on all sides. Based on the pore distribution map, it is found that the primary pores of lignite can be effectively blocked by microbial inhibitor compared with raw coal.
(3) Based on the fitting results of infrared spectra of coal samples, three main active groups, hydroxyl group, carboxyl group and methyl/methylene group, were selected for analysis. The results show that the content of active groups including hydroxyl group, carboxyl group and methyl/methylene group of lignite molecules after microbial inhibition is lower than that of raw coal. In particular, the content of methyl/methylene involved in the initial oxidation reaction is reduced by 96.5% compared with the baseline content of raw coal, indicating that the biological inhibitor can simultaneously block the oxidation of the three active groups.
(4) The performance difference between biological and chemical inhibition on coal spontaneous combustion was further compared, and it was found that calcium carbonate produced by biological inhibition showed a more dense spherical distribution than that produced by chemical inhibition, indicating that the adhesive of calcium carbonate produced by biological inhibition was better. Based on the pore size distribution map, it is found that the total pore volume and specific surface area of the coal sample after biological resistance treatment are reduced more than that after chemical resistance treatment, and the pore number is significantly reduced. The results show that biological inhibition is more effective than chemical inhibition in preventing spontaneous combustion of lignite in physical level. Combined with FTIR infrared spectrum analysis results, it can be seen that the consumption effect of biological inhibitors on methyl/methylene is better than that of chemical inhibitors, and the inhibition effect of spontaneous combustion is better.
Author Contributions
Conceptualization,Y.W.,X.C.and S.W.;Formal Analyses, Y.W.,and R.L.;Writing—original draft,R.L. and S.W.;Writing—review and editing, Y.W.,X.Z. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China, grant No. 2022YFC2904100.
References
- Bosikov, I.I.; Martyushev, N.V.; Klyuev, R.V.; Savchenko, I.A.; Kukartsev, V.V.; Kukartsev, V.A.; Tynchenko, Y.A. Modeling and Complex Analysis of the Topology Parameters of Ventilation Networks When Ensuring Fire Safety While Developing Coal and Gas Deposits. Fire 2023, 6, 95. [Google Scholar] [CrossRef]
- Cheng W, Hu X, Xie J, et al. An intelligent gel designed to control the spontaneous combustion of coal: fire prevention and extinguishing properties[J]. Fuel, 2017, 210: 826-835. [CrossRef]
- Xue D, Hu X, Cheng W, et al. Fire prevention and control using gel-stabilization foam to inhibit spontaneous combustion of coal: Characteristics and engineering applications[J]. Fuel, 2020, 264: 116903. [CrossRef]
- Zhong Y, Yang S, Hu X, et al. Whole process inhibition of a composite superabsorbent polymer-based antioxidant on coal spontaneous combustion[J]. Arabian journal for science and engineering, 2018, 43: 5999-6009. [CrossRef]
- Xue D, Hu X, Cheng W, et al. Development of a novel composite inhibitor modified with proanthocyanidins and mixed with ammonium polyphosphate[J]. Energy, 2020, 213: 118901. [CrossRef]
- Yang, Yi. Study on mechanism and properties of coal spontaneous combustion retarder based on oxidation characteristics [D]. Xi 'an University of Science and Technology, 2015.
- Ma Li, REN Lifeng, AI Shaowu, et al. Experimental study on the inhibition of primary and secondary oxidation of coal by chlorine salt inhibitors [J]. Mining Safety and Environmental Protection, 2015, 42(1): 1-4.Keyong C,Jie W,Lingyun M, et al. Synergistic Inhibitory Effect of Free Radical Scavenger/Inorganic Salt Compound Inhibitor on Spontaneous Combustion of Coal[J]. Combustion Science and Technology,2022,194(10). [CrossRef]
- Shuijun Y,Ruyi Z,Yunliang Y, et al. EXPERIMENTAL STUDY OF RUBBER ANTI-AGING AGENTS IN PREVENTING COAL SPONTANEOUS COMBUSTION[C]//Journal of Safety and Environment. Proceedings of the Second International Symposium on Safety Science and Technology (2000 ISSST) Part B. Chemical Industry Press,2000:212-217.
- Zheng Lanfang. Performance analysis of salt retarders for inhibiting spontaneous combustion of coal oxidation [J]. Coal Science and Technology, 2010 (5): 70-72.
- Xiaowei Z,Yujie Z,Bobo S, et al. Comparative study on the inhibiting effect of dissolvable tiny-foam extinguishing agent and chlorine salts on coal spontaneous combustion.[J]. Environmental science and pollution research international,2023,30(33). [CrossRef]
- Bilin D,Ling Q,Yansheng W, et al. Study on the effect of inorganic and organic sodium on coal spontaneous combustion[J]. Fuel,2023,353. [CrossRef]
- Kuźnia, M.; Zygmunt-Kowalska, B.; Szajding, A.; Magiera, A.; Stanik, R.; Gude, M. Comparative Study on Selected Properties of Modified Polyurethane Foam with Fly Ash. Int. J. Mol. Sci. 2022, 23, 9725. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Seo, D.-J.; Kim, Y.-J.; Cho, S.-S.; Han, Y.-J.; Yang, I.; Kim, C.-W.; Oh, K.; An, J.-C.; Park, J.-I. Molecular Characteristics of Catalytic Nitrogen Removal from Coal Tar Pitch over γ-Alumina-Supported NiMo and CoMo Catalysts. Int. J. Mol. Sci. 2023, 24, 11793. [Google Scholar] [CrossRef] [PubMed]
- Xu Hongying, Miao Mengmeng, Chen Peng. Experimental study on inhibition of spontaneous combustion of coal by composite inhibitor by infrared spectrum [J]. Safety and Environmental Protection of Mining Industry,2019,46(04):40-44. (in Chinese). [CrossRef]
- Pandey J, Mohalik N K, Mishra R K, et al. Investigation of the role of fire retardants in preventing spontaneous heating of coal and controlling coal mine fires[J]. Fire Technology, 2015, 51: 227-245. [CrossRef]
- Kewei G,Pierre B,T. M S, et al. Wind Erosion Mitigation Using Microbial-Induced Carbonate Precipitation[J]. Journal of Geotechnical and Geoenvironmental Engineering,2023,149(8). [CrossRef]
- Lu J,Hua X,Wenjing W, et al. Applications of microbially induced calcium carbonate precipitation in civil engineering practice: A state-of-the-art review[J]. Construction and Building Materials,2023,404. [CrossRef]
- GB474-2008, Coal sample preparation method [s].
- Remonatto, D.; Lerin, L.A. Biocatalysis and Bioactive Molecules: Future and Development. Int. J. Mol. Sci. 2023, 24, 5571. [Google Scholar] [CrossRef] [PubMed]
- GBT21186-2007, Fourier Transform infrared spectrometer [s].
- Wang, H.; Wen, H.; Li, Z.; Mi, W. Influence of Liquid CO2 Extraction and Dissolution on Coal Adsorption Characteristics. Minerals 2023, 13, 650. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Cielecka-Piontek, J.; Nosal-Wiercińska, A.; Pietrzak, R. Microporous Biocarbons Derived from Inonotus obliquus Mushroom and Their Application in the Removal of Liquid and Gaseous Impurities. Int. J. Mol. Sci. 2022, 23, 15788. [Google Scholar] [CrossRef] [PubMed]
- et al. Effect of solvent swelling with different enhancement methods on the microstructure and pyrolysis performance of Hefeng subbituminous coal[J]. Fuel, 2023, 332(P1). [CrossRef]
- et al. Energy Calculation and Simulation of Methane Adsorbed by Coal with Different Metamorphic Grades.[J]. ACS omega, 2020, 5(25) : 14976-14989. [CrossRef]
- Meng W,Zhuo L,Zhikai L, et al. Method Selection for Analyzing the Mesopore Structure of Shale—Using a Combination of Multifractal Theory and Low-Pressure Gas Adsorption[J]. Energies,2023,16(5). [CrossRef]
- Wu, G.; Ye, Z.; Zhang, L.; Tang, J. Bituminous Coal Sorption Characteristics and Its Modeling of the Main Coal Seam Gas Component in the Huaibei Coalfield, China. Sustainability 2023, 15, 9822. [Google Scholar] [CrossRef]
- et al. Experimental Study on Coastal Sediment Reinforcement by Induced Carbonate Precipitation by Different Enzyme Sources[J]. 2023. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E. P. ; Joyner, G.; Halenda, P.P. The determination of pore volume and area distribution in porous substances. I. Computa-tions from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Zhou Y,Li S,Bai Y, et al. Joint Characterization and Fractal Laws of Pore Structure in Low-Rank Coal[J]. Sustainability,2023,15(12). [CrossRef]
- Qu L,Wang Z,Liu L. Molecular Simulation Study Based on Adsorption of Gas (CO 2,O 2,CH 4 ) on Coal[J]. Fire,2023,6(9). [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Yanni Z,Pan S,Shaokang C, et al. Preparation and properties of hydrotalcite microcapsules for coal spontaneous combustion prevention[J]. Process Safety and Environmental Protection,2021(prepublish). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).