1. Introduction
Estrogen is important for the protection of women's health, since women are prone to various problems after menopause, such as osteoporosis, abnormal lipid metabolism, hypertension, and glucose intolerance [
1,
2,
3]. It is also well known that postmenopausal women with decreased estrogen tend to gain body weight and fat mass [
4,
5]. Both male and female estrogen receptor α (ERα) and ERβ knockout mice showed significant levels of obesity and glucose intolerance [
2,
6] even though their energy intake had not changed, suggesting that estrogen is important for regulating fat accumulation and energy balance in the whole body. One proposed explanation for the anti-obesity effect is that energy expenditure is reduced during estrogen deficiency [
7,
8]. ERα is the predominant modulator of estrogenic effects regulating the size and number of adipocytes in adipose tissue [
6]. Although estrogen replacement therapy is beneficial, care should be taken regarding the risk of side effects, such as breast cancer and thrombosis [
9,
10,
11].
Piceatannol (PIC, 3',4',3,5-tetrahydroxy stilbene), a structurally related analog of resveratrol (RES, 3,4,5’-trihydroxy stilbene), is a natural compound found in high concentrations in passion fruit (Passiflora edulis) seeds [
12]. As with RES, PIC is known to have beneficial effects, including estrogenic, anti-inflammatory, antioxidant, and anticancer actions [
13,
14,
15]. PIC is also reported to activate sirtuin1 (SIRT1) [
16]. One study, using a rat model, demonstrated that the oral absorption of PIC was superior to that of RES and that its metabolites were more stable in plasma than those of RES [
17]. Although there are fewer studies on PIC than on RES, especially in vivo, the research thus far suggests PIC may be more effective than RES.
We previously reported that the addition of PIC to a high-fat diet inhibited body weight gain and visceral fat accumulation in ovariectomized (OVX) female mice but not in sham-operated mice [
18]. PIC activated uncoupling protein 1 (UCP1) in brown adipose tissue in both OVX and sham mice, possibly through the mediation of SIRT1 activation by PIC. We also found that the phosphorylation of hormone-sensitive lipase (HSL) in white adipose tissue was extremely low in OVX mice, and that PIC restored HSL activation. This is a specific effect of PIC that is observed only during estrogen deficiency.
HSL is, like adipose triglyceride lipase (ATGL), an enzyme that breaks down triglyceride (TG) stored in adipose tissue into fatty acids to provide an energy source in the blood in response to the body's energy needs. HSL is activated through the β-adrenergic receptor (β-AR) and protein kinase A (PKA) pathway [
19]. The phosphorylation of HSL by PKA facilitates its translocation from the cytosol to the surface of lipid droplets (LDs), where it cooperates with ATGL and other lipid-droplet associated proteins [
20,
21]. HSL mainly catalyzes diacylglycerol (DG), and the degraded free fatty acids (FFA) are released into the bloodstream.
Estrogen is recognized for its ability to downregulate lipogenic genes in adipocytes, the liver, and skeletal muscle through classical ERα genomic action as a nuclear receptor. However, there is a growing interest in its non-genomic actions [
22]. The G protein-coupled estrogen receptor (GPER) plays a role in mediating non-genomic, rapid-signaling responses to estrogen [
23]. GPER is involved in several intracellular signaling pathways in addition to adenylyl cyclase and the PKA pathway, including the phosphatidylinositol 3-kinase (PI3K)-Akt and mitogen-activated protein kinase (MAPK) pathways, which exert cell growth, NOS production, and anti-inflammatory effects [
24,
25,
26]. Therefore, GPER is recognized as a new target for estrogen-related diseases such as breast cancer, cardiovascular disease, and diabetes [
27,
28]. GPER knockout mice showed the development of pathological conditions such as obesity, dyslipidemia, and insulin resistance in males as well as females [
29]. Studies with 3T3-L1 adipocytes suggest that GPER activation inhibits adipogenesis via the disrupting mitotic clonal expansion [
30].
RES and phytoestrogens, such as genistein and daidzein, are suggested to act as agonists of GPER [
31]. However, there are no reports that estrogen promotes HSL phosphorylation by PKA downstream of GPER signaling.
Therefore, to explore the mechanism by which PIC activates HSL during estrogen deprivation in mature adipocytes, we examined the effects of PIC using 3T3-L1 adipocytes.
2. Materials and Methods
2.1. Materials
Piceatannol was obtained from Tokyo Chemical Industry Co, Ltd. (TCI, Tokyo, Japan). Propranolol (Sigma-Aldrich, St. Louis, MO, USA) was used for β-AR signaling experiments. H89, a PKA inhibitor, was obtained from Sigma-Aldrich, and G15 (GRB-G15), a selective GPER antagonist, was purchased from Selleck Biotech (Tokyo, Japan). These reagents were dissolved in dimethyl sulfoxide (DMSO) and added to the culture medium for each experiment. All other reagents were of the highest commercial analytical grade.
2.2. Cell cultures
3T3-L1 preadipocytes were obtained from the American Type Culture Collection (ATCC), seeded at 1×10⁵ cells/well into 6-well plates, and cultured in Dulbecco’s modified Eagle medium high glucose (DMEM, Sigma-Aldrich, D6429) containing 10% fetal bovine serum (FBS, BioWest, Rennes, France) and 1% penicillin streptomycin (P/S, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). After 2 days of confluency, 3T3-L1 preadipocytes were induced by adipogenic agents (0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone, and 10 μg/mL insulin) in DMEM containing 10% FBS (day 0). After 48 hours of induction, the medium was changed to differentiation medium, which consisted of DMEM containing 10% FBS, 1% P/S, and 10 μg/ml insulin. This change was made again every other day. On day 6 from the start of induction, mature adipocytes were used for the experiments. Conditions for PIC treatment of the cells are described in the figure legends. For inhibitor experiments, adipocytes were preincubated with each inhibitor for 60 minutes and then incubated with 25 µM PIC for another 60 minutes, after which cells were harvested.
2.3. Western blotting
The Western blot procedure was conducted using methods previously described [
18]. Briefly, proteins were extracted from cells using a buffer containing a protease inhibitor cocktail (P8340; Sigma-Aldrich) and phosphatase inhibitor cocktails (P5726 and P0044; Sigma-Aldrich). After measurement of protein concentrations using the BCA assay (FUJIFILM Wako Pure Chemical Corporation), equivalent amounts of proteins were separated by 8% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). After blocking treatment (5% w/v nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20, for 1 h at room temperature), membranes were incubated overnight at 4 ° C with the following primary antibodies (all from Cell Signaling Technology, Danvers, MA, USA): rabbit anti-phospho-HSL (Ser563) (CST#4139), rabbit anti-phospho-HSL (Ser565) (CST#4137), rabbit anti-HSL (CST#18381), rabbit anti-Akt (CST #9272), rabbit anti-phospho-Akt (Ser473) (CST #9272), and rabbit anti-β-actin (CST#8457). The membranes were then incubated with peroxidase-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA; 111-035-144). Immunoreactivity was detected using ECL Prime Western blot detection reagents (Cytiva, Tokyo, Japan), with β-actin serving as the loading control. The molecular weight marker used was the 3-Color prestained XL-ladder (APRO Science, Tokushima, Japan).
2.4. Oil-red O staining
Cells in a 6-well plate were washed twice with phosphate-buffered saline (PBS (-)) and fixed with 10% formalin. Intracellular LDs were stained by 0.2% Oil-red O solution (Sigma-Aldrich) and examined microscopically.
2.5. Measurement of intracellular triglyceride concentration
After washing with PBS (-), the adipocytes were scraped with 2 ml of methanol, and the total lipid was extracted using the Bligh and Dyer method [
32]. The extracted and dried lipid was dissolved in 50 µL isopropanol, and the concentration of triglyceride was measured by an enzymatic method (Labo AssayTM Triglyceride, FUJIFILM Wako Pure Chemical Corporation).
2.6. Statistics
All data are presented as mean ± standard deviation (M ± SD). Statistical analysis was performed using Microsoft Excel for Mac version 16.70 and one-way ANOVA was performed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA), followed by Student’s t-test. A p-value below 0.05 was considered statistically significant.
3. Results
3.1. Inhibition effects of PIC on fat accumulation in 3T3-L1 adipocytes
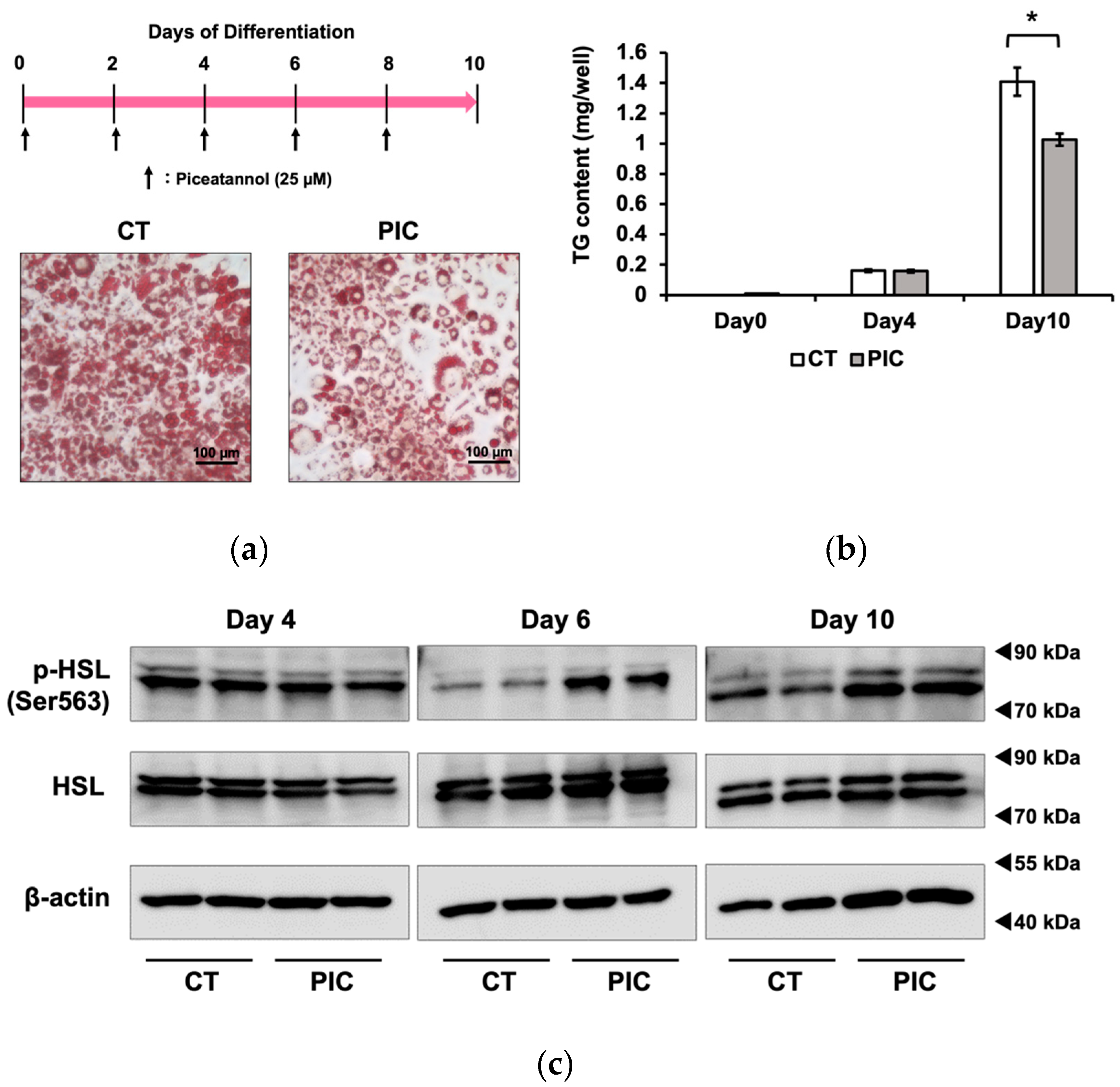
We investigated the inhibitory effects of PIC on fat accumulation using 3T3-L1 adipocytes. The differentiation of 3T3-L1 cells into adipocytes was induced with IBMX, dexamethasone, and insulin 2 days after confluence was reached. Beginning on day 2, the medium with insulin was replaced every other day. PIC was added during the differentiation induction and freshly added during each medium change. After 10 days of differentiation induction, intracellular LDs in 3T3-L1 cells were stained with Oil Red O (
Figure 1a). The results showed a suppression of fat accumulation in the PIC-treated group, particularly in the reduction of small to medium-sized LDs. Subsequently, the intracellular triacylglycerol content was measured. There was no significant difference between the two groups on day 4, but on day 10, the PIC-treated group exhibited inhibition of fat accumulation (
Figure 1b).
Previously, we reported that PIC reduced visceral fat accumulation in estrogen-deficient postmenopausal obese mice; we attributed this effect to the increased activation of hormone-sensitive lipase (HSL) by HSL phosphorylation (pHSL) at Ser563 in white adipose tissue [
18]. Therefore, we investigated whether PIC could enhance pHSL (Ser563) in 3T3-L1 cells. As a result, we observed an increase in pHSL (Ser563) after PIC treatment on day 6 and day 10 of differentiation induction in 3T3-L1 cells (
Figure 1c).
3.2. Phosphorylation of HSL by PIC is regulated independently of β-adrenergic receptors
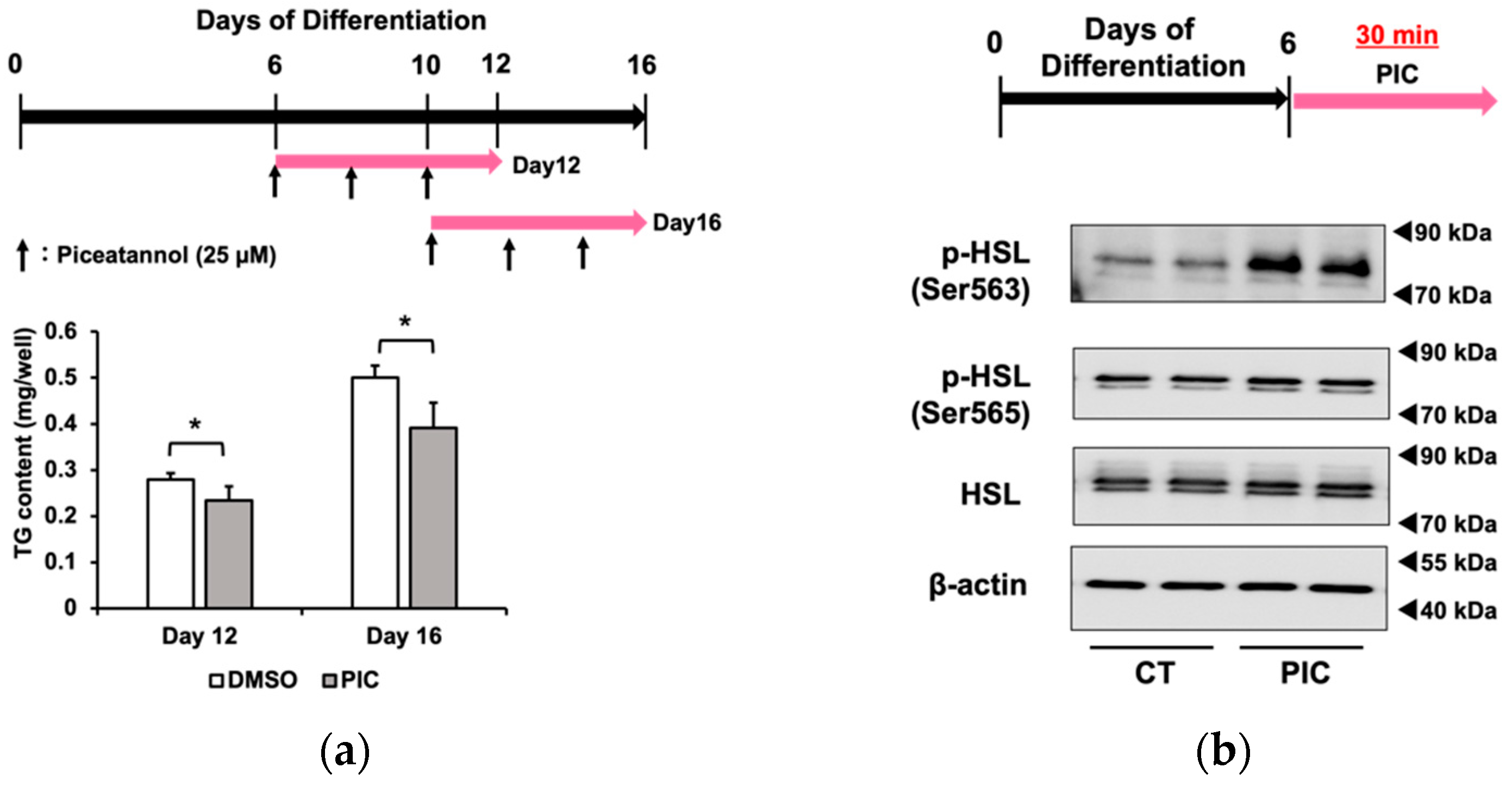
Previous studies have found that PIC influences mitotic clonal expansion (MCE) in 3T3-L1 cells and reduces adipogenesis by inhibiting differentiation into adipocytes. However, our results in OVX mice suggest that the promotion of fat decomposition also plays a role in suppressing fat accumulation. Therefore, we conducted further investigations into the effects of PIC on fat accumulation in 3T3-L1 adipocytes that had accumulated fat post-differentiation.
In
Figure 2a, we added PIC for 6 days beginning on day 6 or day 10 after the initiation of differentiation and measured the accumulation of triacylglycerol. The results demonstrated that PIC had an inhibitory effect on fat accumulation in mid- and late-stage differentiated 3T3-L1 adipocytes. Although it was indicated that PIC inhibits adipocyte differentiation factors that are upregulated in the early stages of differentiation, such as PPARγ, C/EBPα, β, and δ, our results suggested that the inhibition of fat accumulation by PIC after adipocyte differentiation might occur through pathways independent of these factors.
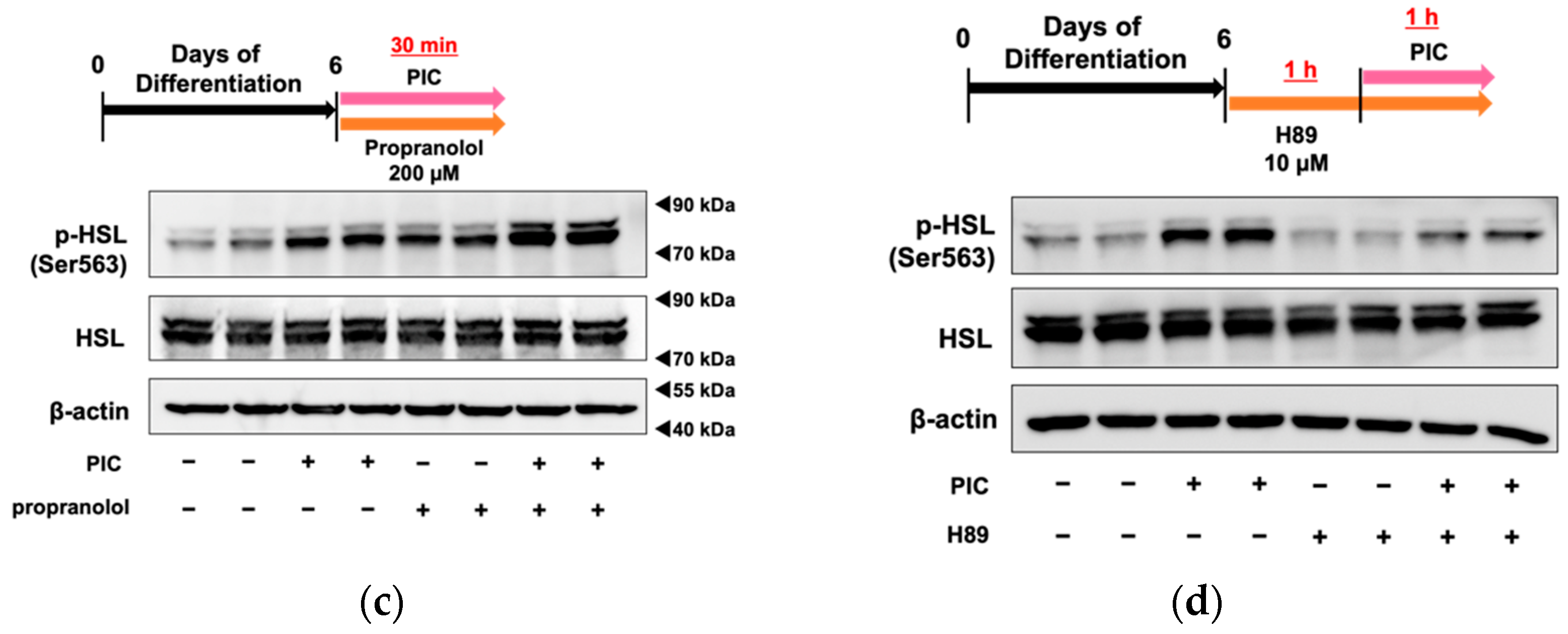
When PIC was administered for a short duration (30 minutes) to 3T3-L1 cells on day 6 of differentiation, HSL phosphorylation was enhanced at Ser563, similar to the results obtained with continuous treatment from differentiation initiation (
Figure 2c). On the other hand, PIC did not change phosphorylation at the Ser565 site. HSL is a lipase that provides energy by lipolysis during times of energy deficiency. In fasting conditions, catecholamines like adrenaline bind to β3-adrenergic receptors on the cell membrane in response to declining blood glucose levels. This binding activates downstream adenylate cyclase, leading to the production of cAMP from ATP. As intracellular cAMP levels rise, protein kinase A (PKA) is activated and phosphorylates HSL at Ser563. Phosphorylated HSL (Ser563) then accesses the LDs in white adipocytes, where it cleaves ester bonds in triacylglycerol molecules, releasing fatty acids into the bloodstream for use as an energy source. The phosphorylation site Ser565 of HSL is phosphorylated by another pathway, and access to LDs is conversely inhibited [
33]. Therefore, we investigated whether PIC's phosphorylation of HSL depended on β-AR by employing the β-AR inhibitor propranolol (
Figure 2c). The increase in pHSL (Ser563) due to PIC was not inhibited when propranolol was used to block β-AR, suggesting that PIC induces HSL phosphorylation independently of β-AR. We verified the efficacy of positive control experiments involving the addition of the β-AR agonist isoproterenol, which led to an increase in pHSL (Ser563). Additionally, we found that 200 µM propranolol demonstrated sufficient inhibitory effects.
Subsequently, we used the PKA inhibitor H89 to determine whether PIC-induced HSL phosphorylation involved PKA (
Figure 2d). In cells pretreated with H89 followed by PIC addition, we observed a reduction in HSL phosphorylation, indicating that PKA mediated the induction of pHSL (Ser563) by PIC.
These results suggested that PIC activates PKA without affecting β-AR and increases HSL phosphorylation.
3.3. Piceatannol phosphorylates HSL via the GPER pathway
We next focused on the G protein-coupled estrogen receptor (GPER) as an alternative factor involved in PKA activation, apart from β-AR. GPER differs in signaling mechanisms from classical estrogen receptors (ERs) such as ERα and ERβ. While ERα and ERβ are nuclear receptors, GPER is known to transmit signals through pathways involving second messengers within seconds to minutes of stimulation. Prior studies have primarily associated GPER with stimulating PKA activation through Gα subunit protein-mediated adenylate cyclase activation, leading to the proliferation of estrogen-sensitive cancer cells [
34]. However, no previous reports have linked GPER to HSL phosphorylation in adipocytes.
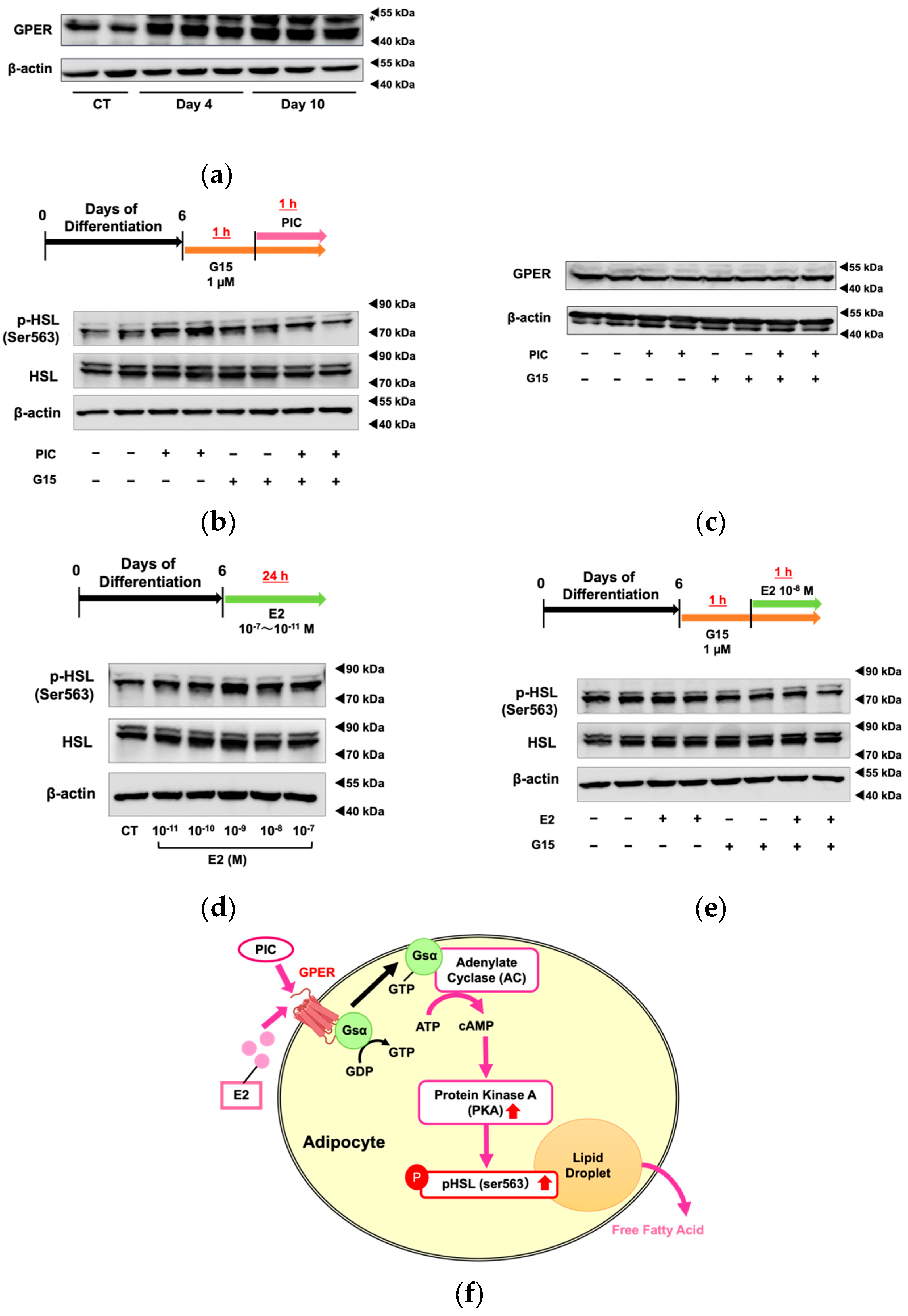
The GPER expression level significantly increased during differentiation of 3T3-L1 cells (
Figure 3a). Using G15, a specific GPER antagonist, we investigated whether PIC-induced HSL phosphorylation was mediated through GPER (
Figure 3b). G15 exhibits high affinity for GPER and binds only minimally to ERα and ERβ (Ki>10 µM) [
35]. Increased HSL phosphorylation in PIC-treated cells was suppressed when the cells were treated simultaneously with G15, the GPER inhibitor. These results suggest that PIC promotes HSL phosphorylation via GPER. The PIC and G15 treatments did not alter the expression level of GPER protein (
Figure 3c). Thus, PIC was shown to increase pHSL (Ser563) through the GPER signaling pathway, not by increasing GPER expression.
Next, we examined the effect of estradiol (E2) on HSL phosphorylation (
Figure 3d). Compared to the cells without treatment (control), pHSL (Ser563) increased in those with E2 at physiological concentrations of 10⁻⁸ M and adjacent concentrations. Since ERα and ERβ function as nuclear receptors, and GPER mediates rapid signal transduction as a membrane receptor, the increase in pHSL (Ser563) after 1 hour of short-term treatment with E2 suggests that HSL phosphorylation may be regulated through GPER rather than ERα or ERβ. The increase in HSL phosphorylation was shown to decrease when co-treated with the GPER-specific inhibitor G15 (
Figure 3e).
These findings suggest that PIC acts as an agonist of GPER and activates HSL downstream of the GPER signal transduction pathway in adipocytes.
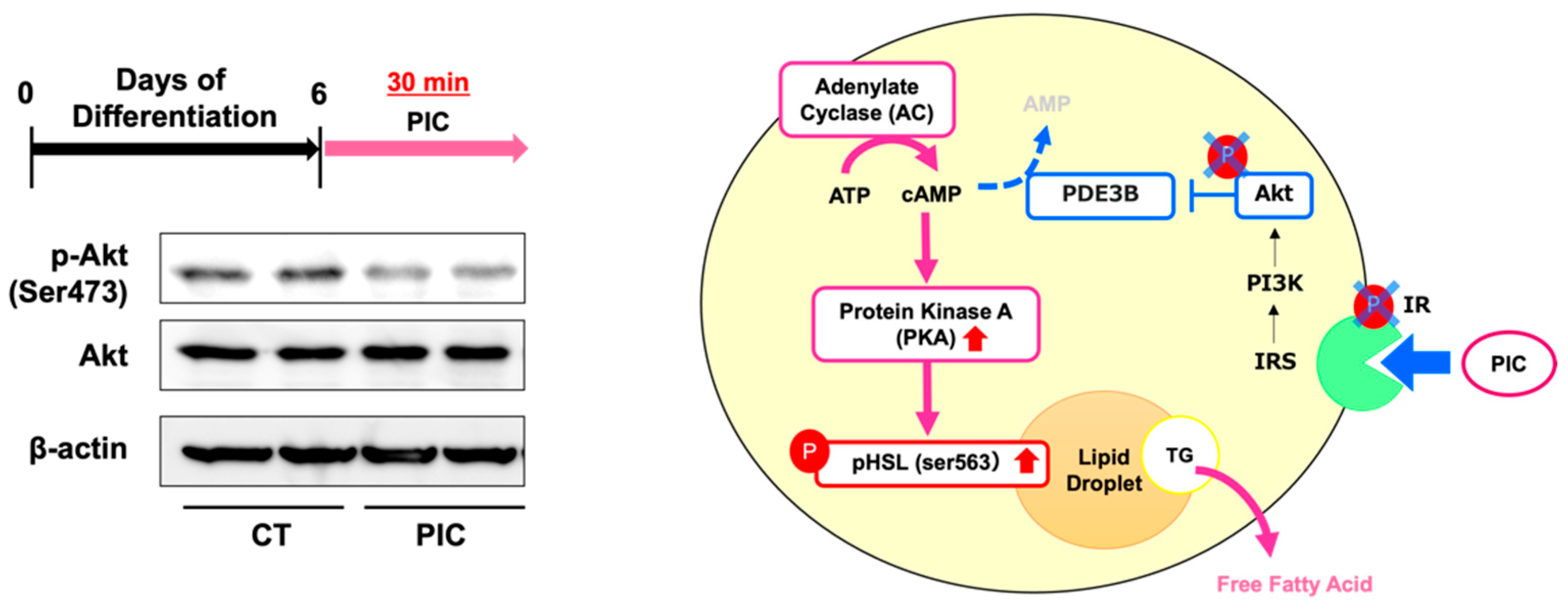
3.4. PIC inhibits Akt phosphorylation
Kwon et al. showed that PIC inhibited the phosphorylation of the insulin receptor (IR)/insulin receptor substrate-1 (IRS-1)/Akt pathway in the early phase of adipogenesis by direct binding to IR [
30]. We investigated whether PIC inhibits pAkt in “post-differentiated” 3T3-L1 adipocytes. Treatment with PIC in 3T3-L1 adipocytes differentiated up to day 6 resulted in a decrease in Akt phosphorylation (
Figure 4). PIC might increase cAMP levels by inhibiting the breakdown of cAMP to AMP via PDE-3B through the reduction in Akt phosphorylation. This could be considered a second pathway through which PIC increases pHSL (Ser563). It aligns well with the rapid signal transduction of the IR/Akt pathway, exhibiting a degree of consistency. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
4. Discussion
The purpose of this study was to determine how PIC activates hormone-sensitive lipase (HSL) phosphorylation as observed in white adipose tissue of OVX mice.
HSL was initially characterized as the hormonally regulated neutral lipase activity responsible for catalyzing the breakdown of triacylglycerols (TG) into fatty acids in adipose tissue. During lipolysis, HSL translocates to the surface of the lipid droplets (LDs) and participates, along with ATGL and monoacylglycerol lipase, in TG hydrolysis. In comparison with other neutral lipases, HSL has an important feature: its regulation by hormones through reversible phosphorylation of sites located within the regulatory domain [
36]. Therefore, we determined whether PIC activates HSL through the β-AR and PKA signaling pathways, which are well-known pathways for hormonal HSL activation. The results as shown in
Figure 2 indicate that activation by PIC was dependent on PKA but not via β-AR.
In our previous study using OVX mice, the increase in pHSL (ser563) by PIC was a specific effect seen only in OVX mice, suggesting that PIC’s effect might be related to estrogen function because of its structural similarity to estradiol.
The loss of ovarian hormones leads to an increase in adiposity and insulin resistance, thereby increasing the risk for cardiovascular and metabolic diseases [
4,
5]. Based on the findings obtained from many animal models, such as OVX, Erα, and ERβ knockout mice, estrogen deficiency-induced obesity is thought to be due to reduced energy expenditure resulting from the loss of central nervous system signaling, a reduced resting metabolic rate, and decreased spontaneous activity [
6,
37,
38]. 17β-Estradiol inhibits the formation of visceral fat and induces the expression of genes specific to brown adipose tissue [
39]. It is also well known that estrogen replacement therapy suppresses obesity [
9,
10].
Estrogen is involved in the expression of immune and female reproductive functions through estrogen receptors, ERα and ERβ, which regulate classical nuclear gene expression. Estrogen also regulates the expression of various genes related to lipid metabolism in adipocytes [
22]. It was reported that the addition of estradiol to adipocytes collected from abdominal fat of human women decreased the protein expression of LPL and increased HSL [
40]. However, the activation of HSL GPER-mediated by phosphorylation has not been previously reported.
Unlike classical estrogen receptors, GPER is a G protein-coupled receptor that orchestrates rapid responses through non-genomic actions. GPER reportedly can mediate estrogenic activation via various signaling pathways, such as MAPKs [
41,
42], cAMP [
24,
42], and intracellular calcium [
43]. GPER KO mice show obesity and insulin resistance [
44]. In the induction of 3T3-L1 adipocyte differentiation, E2 has been reported to inhibit adipogenesis by perturbating mitotic clonal expansion via GPER signaling [
45]. E2 and G1, an agonist of GPER, were shown to enhance mitochondrial functions in inflamed adipocytes through a PKA-dependent mechanism [
46]. However, there no reports have indicated that GPER could be associated with the activation of lipolytic enzymes in adipocytes via GPER’s involvement in the PKA signaling pathway.
The present study revealed that PIC also phosphorylated HSL in 3T3-L1‒differentiated adipocytes as shown in vivo, and that HSL phosphorylation was activated by the PKA pathway via GPER, but not β-AR. E2 also activated HSL phosphorylation. This is the first report to show that GPER, the non-genomic action of estrogen, may be implicated in lipolysis in adipose tissue through the phosphorylation of HSL by its downstream PKA pathway. On the other hand, GPER stimulation is known to activate EGFR, leading to downstream activation of signaling molecules, such as ERK1 and ERK2 [
41], and it is reported that ERK phosphorylates HSL (ser600) [
47]. Although we did not examine the MAPK pathway in this study, HSL phosphorylation by the ERK pathway may also need to be further studied.
HSL activity is also known to be inhibited by insulin signaling. When insulin signaling induces phosphorylation of Akt, phosphodiesterase (PDE)-3B, an enzyme that catalyzes the breakdown of cAMP to AMP, becomes activated. This leads to a decrease in intracellular cAMP levels and subsequently reduces PKA activity, resulting in the inhibition of HSL phosphorylation. In the early stages of differentiation induction in 3T3-L1 adipocytes, PIC has been reported to dose-dependently inhibit the phosphorylation of the insulin receptor (IR)/insulin receptor substrate-1 (IRS-1)/Akt pathway. The same study demonstrated that PIC directly binds to IR and non-competitively inhibits IR kinase activity [
30]. Our results shown in
Figure 4 suggested that PIC also inhibits insulin signaling in the post-differentiation phase. In addition to these mechanisms described previously, the present results suggest that PIC reduces the size of adipocytes by facilitating the breakdown of TG accumulated in differentiated adipocytes.
Several reports on the inhibition of adipogenesis by PIC have been reported, including those using 3T3-L1 and human visceral adipose-derived stem cells [
47], and some reports have shown that PIC is more effective than Res [
14]. RES, which is structurally similar to PIC, has been reported to be an agonist of GPER [
31]. However, whether PIC acts as a regulatory agonist needs further investigation.
In conclusion, the findings presented here suggested that GPER and HSL activation were involved in fat accumulation in adipose tissue under estrogen deficiency. The results also showed that PIC acts as a phytoestrogen and promotes lipolysis by activating HSL. The GPER-mediated activation of HSL may play an important role in estrogen-induced adipose tissue lipolysis and the inhibition of obesity.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
In this study, we investigated the mechanisms by which PIC influences fat metabolism in 3T3-L1 adipocytes. Our findings revealed that PIC inhibits fat accumulation by enhancing HSL phosphorylation, independently of β-adrenergic receptors. Additionally, PIC's effect through GPER emerged as a novel pathway for HSL activation. This newfound insight into GPER's role in adipocytes may have profound implications for understanding obesity-related conditions. Furthermore, we discovered that PIC suppresses Akt phosphorylation in post-differentiated adipocytes. These findings demonstrate the multifaceted nature of PIC's impact on adipocyte metabolism, unveiling its potential as a therapeutic target for metabolic disorders.
Author Contributions
Author Contributions: Conceptualization, K.A. and Y.F.; investigation, K.A., A.M., and N.O.; writing—original draft preparation, K.A. and Y.F.; writing—review and editing, K.A., Y.F., T.I., and I.I.; visualization, K.A.; supervision, Y.F.; funding acquisition, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by Morinaga&Co., Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available upon request to the corresponding author.
Acknowledgments
The authors would like to thank Sadao Mori, Takayuki Yamamoto and Toshihiro Kawama, Research Institute, Morinaga and Company Ltd., for providing piceatannol and useful discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tella, S.H.; Gallagher, J.C. Prevention and Treatment of Postmenopausal Osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Maric-Bilkan, C.; Gilbert, E.L.; Ryan, M.J. Impact of Ovarian Function on Cardiovascular Health in Women: Focus on Hypertension. Int. J. Womens Health 2014, 6, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J. Minireview: The Year in Review of Estrogen Regulation of Metabolism. Mol. Endocrinol. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian Hormones and Obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased Adipose Tissue in Male and Female Estrogen Receptor-α Knockout Mice. Proceedings of the National Academy of Sciences 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Heine, P.A.; Taylor, J.A.; Lubahn, D.B. The Role of Estrogen and Estrogen Receptor-α in Male Adipose Tissue. Mol. Cell. Endocrinol. 2001, 178, 147–154. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Brown, T.R.; Russo, J. Regulation of Energy Metabolism Pathways by Estrogens and Estrogenic Chemicals and Potential Implications in Obesity Associated with Increased Exposure to Endocrine Disruptors. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2009, 1793, 1128–1143. [Google Scholar] [CrossRef]
- Babaei, P.; Mehdizadeh, R.; Ansar, M.M.; Damirchi, A. Effects of Ovariectomy and Estrogen Replacement Therapy on Visceral Adipose Tissue and Serum Adiponectin Levels in Rats. Menopause Int. 2010, 16, 100–104. [Google Scholar] [CrossRef]
- Papadakis, G.E.; Hans, D.; Gonzalez Rodriguez, E.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P.; Lamy, O. Menopausal Hormone Therapy Is Associated With Reduced Total and Visceral Adiposity: The OsteoLaus Cohort. J. Clin. Endocrinol. Metab. 2018, 103, 1948–1957. [Google Scholar] [CrossRef]
- Wunderle, M.; Pretscher, J.; Brucker, S.Y.; Volz, B.; Hartmann, A.; Fiessler, C.; Hein, A.; Häberle, L.; Jud, S.M.; Lux, M.P.; et al. Association between Breast Cancer Risk Factors and Molecular Type in Postmenopausal Patients with Hormone Receptor-Positive Early Breast Cancer. Breast Cancer Res. Treat. 2019, 174, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of Passion Fruit (Passiflora Edulis) Seed Containing High Amounts of Piceatannol Inhibits Melanogenesis and Promotes Collagen Synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological Activity of Piceatannol: Leaving the Shadow of Resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Han, Y.; Jo, H.; Lee, K.W.; Song, Y.S. Piceatannol Is Superior to Resveratrol at Suppressing Adipogenesis in Human Visceral Adipose-Derived Stem Cells. Plants 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Gomez, M.; Galano, A.; Raúl Alvarez-Idaboy, J. Piceatannol, a Better Peroxyl Radical Scavenger than Resveratrol. RSC Adv. 2013, 3, 20209–20218. [Google Scholar] [CrossRef]
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and Its Metabolite, Isorhapontigenin, Induce SIRT1 Expression in THP-1 Human Monocytic Cell Line. Nutrients 2014, 6, 4794–4804. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and Metabolism of Piceatannol in Rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef]
- Arisawa, K.; Kaneko, M.; Matsuoka, A.; Ozawa, N.; Kawawa, R.; Ishikawa, T.; Ichi, I.; Fujiwara, Y. Piceatannol Prevents Obesity and Fat Accumulation Caused by Estrogen Deficiency in Female Mice by Promoting Lipolysis. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Mottillo, E.P.; Granneman, J.G. Intracellular Fatty Acids Suppress β-Adrenergic Induction of PKA-Targeted Gene Expression in White Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E122–E131. [Google Scholar] [CrossRef]
- Anthonsen, M.W.; Rönnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of Novel Phosphorylation Sites in Hormone-Sensitive Lipase That Are Phosphorylated in Response to Isoproterenol and Govern Activation Properties in Vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [CrossRef]
- Su, C.-L.; Sztalryd, C.; Contreras, J.A.; Holm, C.; Kimmel, A.R.; Londos, C. Mutational Analysis of the Hormone-Sensitive Lipase Translocation Reaction in Adipocytes*. J. Biol. Chem. 2003, 278, 43615–43619. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen Regulation of Adiposity and Fuel Partitioning: EVIDENCE OF GENOMIC AND NON-GENOMIC REGULATION OF LIPOGENIC AND OXIDATIVE PATHWAYS*. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Maggiolini, M. Mechanisms of Estrogen Signaling and Gene Expression via GPR30. Mol. Cell. Endocrinol. 2009, 308, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R.; Bland, K.I. Estrogen Action Via the G Protein-Coupled Receptor, GPR30: Stimulation of Adenylyl Cyclase and cAMP-Mediated Attenuation of the Epidermal Growth Factor Receptor-to-MAPK Signaling Axis. Mol. Endocrinol. 2002, 16, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.S.; Ma, S.; Miao, L.; Li, R.; Yin, Y.; Raj, G.V. Androgen Receptor-Mediated Non-Genomic Regulation of Prostate Cancer Cell Proliferation. Transl. Androl. Urol. 2013, 2, 187–196. [Google Scholar]
- Lin, B.C.; Suzawa, M.; Blind, R.D.; Tobias, S.C.; Bulun, S.E.; Scanlan, T.S.; Ingraham, H.A. Stimulating the GPR30 Estrogen Receptor with a Novel Tamoxifen Analogue Activates SF-1 and Promotes Endometrial Cell Proliferation. Cancer Res. 2009, 69, 5415–5423. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G-Protein-Coupled Estrogen Receptor GPER in Health and Disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G Protein-Coupled Oestrogen Receptor GPER in Health and Disease: An Update. Nat. Rev. Endocrinol. 2023, 19, 407–424. [Google Scholar] [CrossRef]
- Sharma, G.; Hu, C.; Brigman, J.L.; Zhu, G.; Hathaway, H.J.; Prossnitz, E.R. GPER Deficiency in Male Mice Results in Insulin Resistance, Dyslipidemia, and a Proinflammatory State. Endocrinology 2013, 154, 4136–4145. [Google Scholar] [CrossRef]
- Kwon, J.; Seo, S.; Heo, Y.-S.; Yue, S.; Cheng, J.-X.; Lee, K.; Kim, K.-H. Piceatannol, Natural Polyphenolic Stilbene, Inhibits Adipogenesis via Modulation of Mitotic Clonal Expansion and Insulin Receptor-dependent Insulin Signaling in Early Phase of Differentiation. J. Biol. Chem. 2012, 287, 11566–11578. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Arterburn, J.B. International Union of Basic and Clinical Pharmacology. XCVII. G Protein–Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol. Rev. 2015, 67, 505–540. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- McDonough, P.M.; Maciejewski-Lenoir, D.; Hartig, S.M.; Hanna, R.A.; Whittaker, R.; Heisel, A.; Nicoll, J.B.; Buehrer, B.M.; Christensen, K.; Mancini, M.G.; et al. Differential Phosphorylation of Perilipin 1A at the Initiation of Lipolysis Revealed by Novel Monoclonal Antibodies and High Content Analysis. PLoS One 2013, 8, e55511. [Google Scholar] [CrossRef] [PubMed]
- Bauzá-Thorbrügge, M.; Rodríguez-Cuenca, S.; Vidal-Puig, A.; Galmés-Pascual, B.M.; Sbert-Roig, M.; Gianotti, M.; Lladó, I.; Proenza, A.M. GPER and ERα Mediate Estradiol Enhancement of Mitochondrial Function in Inflamed Adipocytes through a PKA Dependent Mechanism. J. Steroid Biochem. Mol. Biol. 2019, 185, 256–267. [Google Scholar] [CrossRef]
- Dennis, M.K.; Burai, R.; Ramesh, C.; Petrie, W.K.; Alcon, S.N.; Nayak, T.K.; Bologa, C.G.; Leitao, A.; Brailoiu, E.; Deliu, E.; et al. In Vivo Effects of a GPR30 Antagonist. Nat. Chem. Biol. 2009, 5, 421–427. [Google Scholar] [CrossRef]
- Recazens, E.; Mouisel, E.; Langin, D. Hormone-Sensitive Lipase: Sixty Years Later. Prog. Lipid Res. 2021, 82, 101084. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Shimomura, Y.; Nakanishi, Y.; Futawatari, T.; Ohtani, K.; Sato, N.; Mori, M. Estrogen Increases in Vivo Leptin Production in Rats and Human Subjects. J. Endocrinol. 1997, 154, 285–292. [Google Scholar] [CrossRef]
- Zidon, T.M.; Padilla, J.; Fritsche, K.L.; Welly, R.J.; McCabe, L.T.; Stricklin, O.E.; Frank, A.; Park, Y.; Clegg, D.J.; Lubahn, D.B.; et al. Effects of ERβ and ERα on OVX-Induced Changes in Adiposity and Insulin Resistance. J. Endocrinol. 2020, 245, 165–178. [Google Scholar] [CrossRef]
- Al-Qahtani, S.M.; Bryzgalova, G.; Valladolid-Acebes, I.; Korach-André, M.; Dahlman-Wright, K.; Efendić, S.; Berggren, P.-O.; Portwood, N. 17β-Estradiol Suppresses Visceral Adipogenesis and Activates Brown Adipose Tissue-Specific Gene Expression. Horm. Mol. Biol. Clin. Investig. 2016, 29, 13–26. [Google Scholar] [CrossRef]
- Palin, S.L.; McTernan, P.G.; Anderson, L.A.; Sturdee, D.W.; Barnett, A.H.; Kumar, S. 17β-Estradiol and Anti-Estrogen ICI:Compound 182,780 Regulate Expression of Lipoprotein Lipase and Hormone-Sensitive Lipase in Isolated Subcutaneous Abdominal Adipocytes. Metabolism 2003, 52, 383–388. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, P.; Sham, K.W.Y.; Yuen, J.M.L.; Xie, C.; Zhang, Y.; Liu, Y.; Li, S.; Huang, X.; Cheng, C.H.K.; et al. Identification of a Membrane Estrogen Receptor in Zebrafish with Homology to Mammalian GPER and Its High Expression in Early Germ Cells of the Testis. Biol. Reprod. 2009, 80, 1253–1261. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Hathaway, H.J. What Have We Learned about GPER Function in Physiology and Disease from Knockout Mice? J. Steroid Biochem. Mol. Biol. 2015, 153, 114–126. [Google Scholar] [CrossRef]
- Zhu, P.; Yuen, J.M.L.; Sham, K.W.Y.; Cheng, C.H.K. GPER Mediates the Inhibitory Actions of Estrogen on Adipogenesis in 3T3-L1 Cells through Perturbation of Mitotic Clonal Expansion. Gen. Comp. Endocrinol. 2013, 193, 19–26. [Google Scholar] [CrossRef]
- Sharma, G.; Mauvais-Jarvis, F.; Prossnitz, E.R. Roles of G Protein-Coupled Estrogen Receptor GPER in Metabolic Regulation. J. Steroid Biochem. Mol. Biol. 2018, 176, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Shen, W.-J.; Muliro, K.; Patel, S.; Souza, S.C.; Roth, R.A.; Kraemer, F.B. Stimulation of Lipolysis and Hormone-Sensitive Lipase via the Extracellular Signal-Regulated Kinase Pathway*. J. Biol. Chem. 2001, 276, 45456–45461. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Pejenaute, H.; del Moral, R.; Boulet, N.; Hijona, E.; Andrade, F.; Villanueva-Millán, M.; Aguirre, L.; Arbones-Mainar, J.M. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int. J. Mol. Sci. 2018, 19, 2081. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).