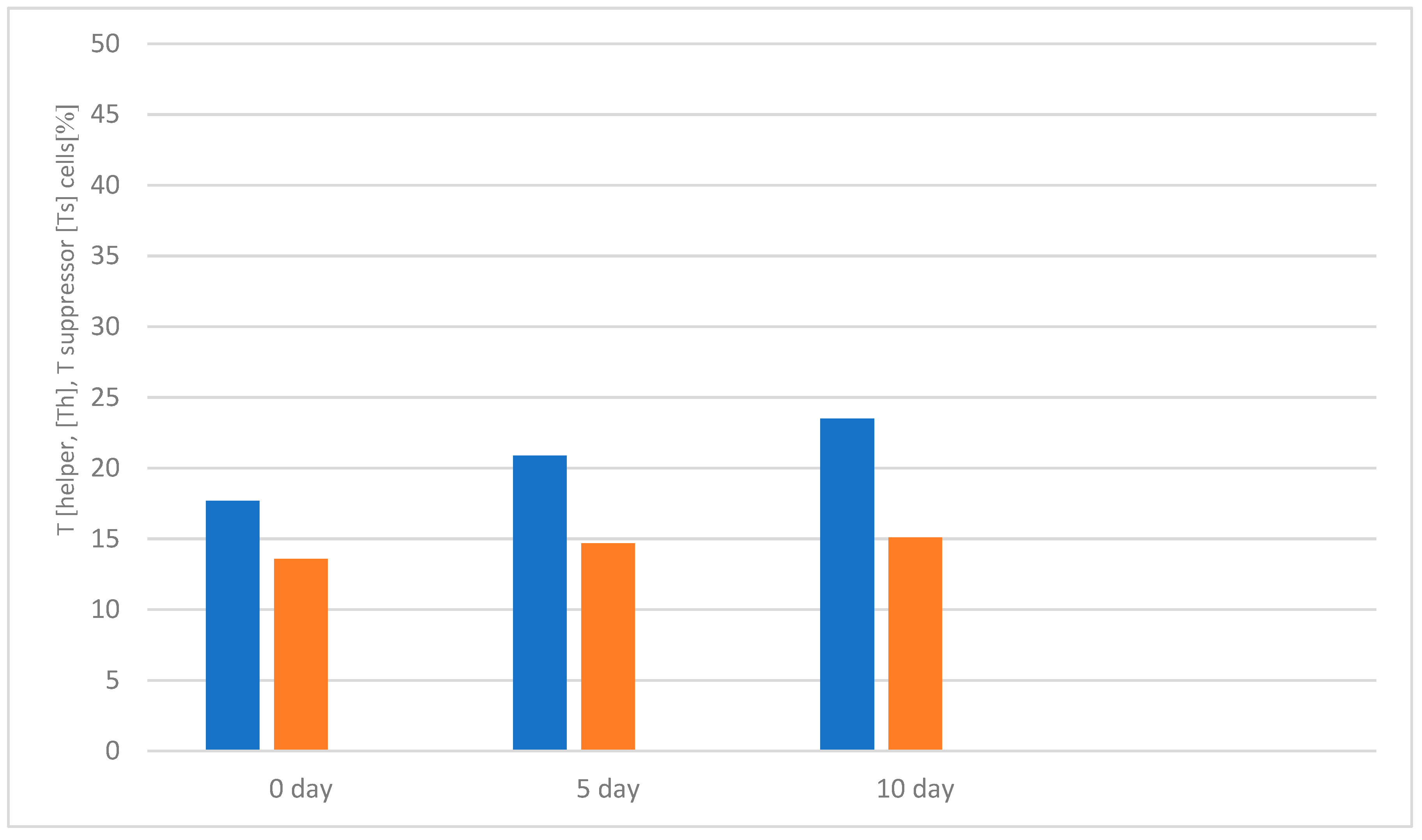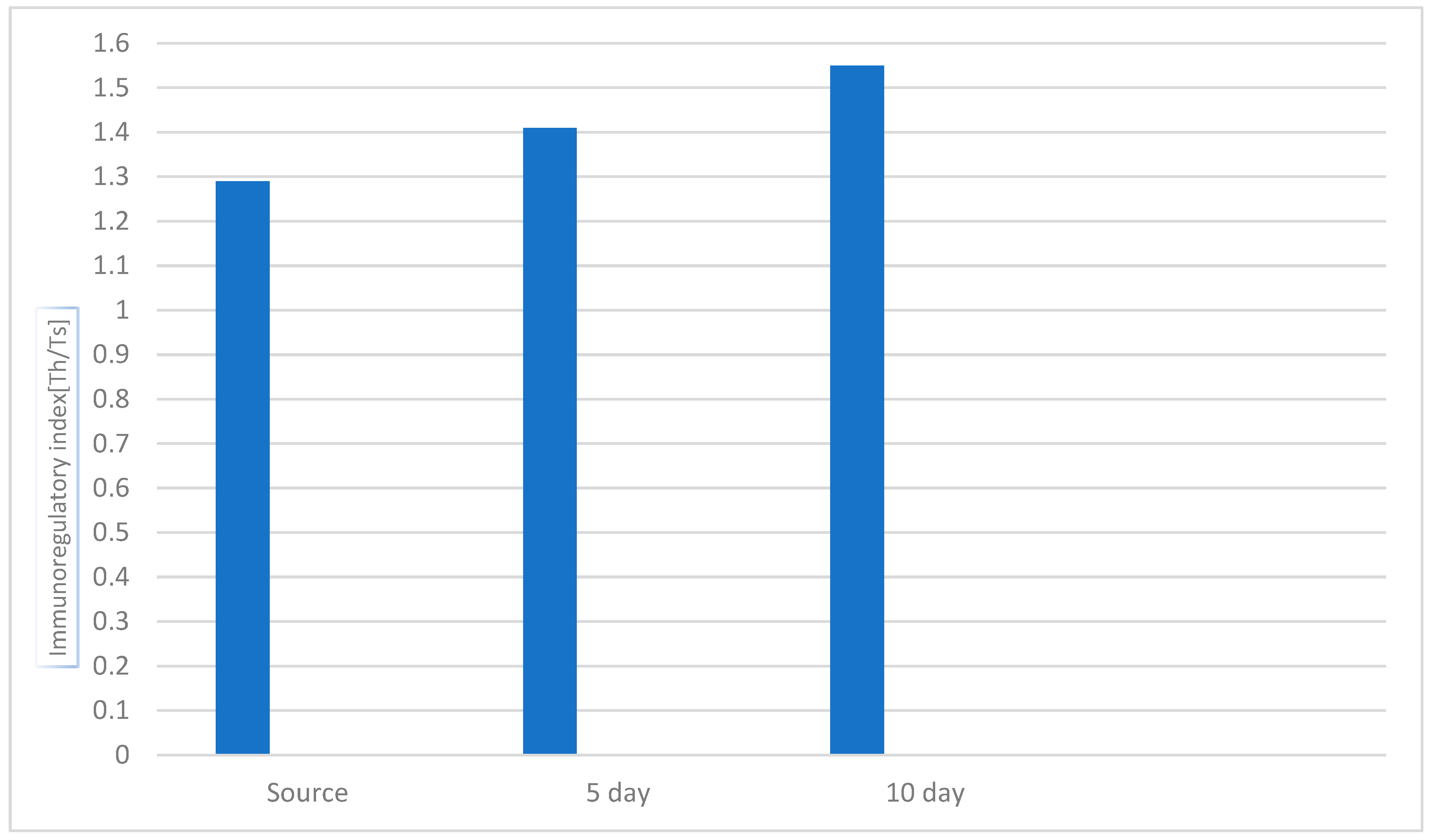1. Introduction
Deterioration of the ecological situation caused by the influence on animals of xenobiotics (heavy metals, dioxins, peritroids, etc.), radionuclides (α-, β-, γ-emitters) and biological agents (viruses, bacteria, parasites) causes the development of an immunodeficiency state and ecopathology (Miglani et al., 2022; Cabassi, 2007).
Despite the extreme diversity and difference of environmental agents of chemical, biological and physical nature, they are united by one fundamental property - immunotoxicity, implemented through the pituitary-adrenal gland system, which automatically includes a highly specific defense system of immunity from a specific pathogenic agent through the synthesis of anti-infectious, antitoxic and antiradiotoxic antibodies (Galbiati et al., 2021; Bou Zerdan et al., 2021).
Taking into account, the mechanisms of the immunotoxic action of polyfactorial pathological agents, methods and means of extraimmune and immunotherapy in conditions of ecological trouble have been proposed, which provide for a decrease in the antigenic load on the body, neutralization of toxins, antigens, allergens and their elimination, specific (desensitizing) immunotherapy, normalization of immune imbalance through the use of substances of phytogenic (herbal infusions, apiproducts), zoogenic (γ-globulins, histoglobulins, organ and tissue eluates) and microbial (toxoid, lacto-, bifidumbacterin, etc.) origin (Rasheed N., 2021). Continuing research in this direction, we have developed and tested patented preparations of zoogenic (anti-radiation therapeutic and prophylactic immunoglobulin, Patent RU No. 2169572, A61K 35/25, 2001) and phyto-apisogenic origin (preparation «Vita-Force», Patent RU 2324361, A23K 1/100, 2008), which have an immunotherapeutic effect in radiation and biological damage to the body, both in isolated and combined action of these factors.
However, the mechanism of the protective action of the aforementioned immunotropic drugs has not been finally clarified. In particular, the role of lymphocyte subpopulations taking an active part in radio-induced immunodeficiency and autoimmunity against the background of exposure to radiation and biological factors on the body has not been clarified, since autoimmune diseases, including radio-induced autoimmune reactions, are accompanied by a sharp change in the ratio of T-helpers (CD4) / T-suppressors (CD8), which is called the immunomodulatory index (II) or CD4 / CD8 ratio (Cox et al., 2013; Curtsinger, Mescher, 2010; O Shea, Paul, 2010).
From a practical and theoretical point of view, it is of interest to activate lymphocytes against the background of radio-induced depletion of immunity using potential activators based on substances of zoogenic and apisogenic origin. Although the exact role of lymphocyte subpopulations in normal tissue response may be unclear but their accumulation (especially T lymphocytes) after damage by ionizing radiation suggests that they may be critical targets for radiotherapic intervention (Hong et al., 2003; Chiang et al. 2005; 2008; Maravan et al., 2011; Tegmoortash et al., 2005; Toma et al., 2010).
Considering that a subpopulation of lymphocytes, especially T-helpers (CD4) and T-suppressors (CD8), play a key role in the adaptation of the body to the effects of pathological agents of biological (pathogenic agents), toxic (ecotoxicants) and physical (ionizing radiation) nature, which induce the development of autoimmune pathology in the body (Peterson, 2012) and the possibility of a therapeutic effect on the specified pathology with therapeutic and prophylactic agents. These studies were carried out the purpose of which is to activate the immune system against the background of its imbalance caused by agents of a radiation nature.
2. Material and Methods
2.1. Research Objects. Outbred White Mice Weighing 18-20 g Were Used as a Biological Model in the Experiments. The Formed Strain of Esherichia coli «PL-6» No. 1154115 was Used as Microorganisms
2.2. Obtaining an Immunizing Agent - a Protein-Quinoid Complex (conjugated) Antigen Used as an Inducer of Immune (Therapeutic) Antiradiation Serum
In order to obtain a complex antigen, at the 1st stage, the bacterial part of the complex antigen - Escherichiosis antitoxin (EAT) was prepared. To obtain it, we used the industrial strain Esherichia coli PL-6, which was grown on Hottinger's medium for 48 h at 37 ° C, followed by the addition of formalin (0.4-0.5%) and thermostating the mixture at 37 ° C for 10-12 days and precipitation of mixture of toxoids with sterile solution of aluminum hydroxide (GOA) at the rate of 1% for the entire volume.
After settling of the toxoid for 2-3 days, the supernatant was decanted, centrifuged (added mixture of toxoids in the form of a gel) using the haptenic part of the conjugated antigen as a carrier protein.
At the second stage of the technological cycle, a microbial radiotoxin (RT) was obtained from the produced Esherichia coli strain PL-6, which was grown on meat-peptone agar (MPA) for 48 h at 37 ° C, then the biomass was washed off with physiological solution of pH 7.2 which was washed three times by centrifugation, centrifugate was resuspended in physiological solution at a concentration of 1.2 × 1010 MC / cm3 and is used to isolate radiotoxin.
For this purpose, the microbial suspension with the indicated concentration was irradiated on the Isledovatel gamma device at a dose of 150 Gy, followed by thermostating the culture for 4 h at 37 ° C, then the microbial biomass was precipitated by centrifugation at 3000 rpm for 30 min, the centrifugate was added to 96% ethanol in a ratio of 1: 5 and extracted at room temperature for 2 h.The resulting extract was evaporated on a vacuum rotary evaporator to the original volume and neutralized with 0.1 n KOH to pH 7.4, diluted with physiological solution to 2.7% concentration (27 mg / ml) and was used as one of the components of the conjugated antigen.
The resulting antigenic components (Escherichiosis toxoid - EAT) and radiotoxin (RT) were mixed in a ratio of 27.2: 72.8, and the mixture was conjugated overnight at room temperature.
The obtained stable complex antigen - protein-quinoid radiotoxin (BCRA), including haptenic (o-quinone) and protein (Esherichia coli endotoxin) parts, is a 14% solution containing 140 mg of antigenic substance in 1 ml of the preparation, of which the share of Esherichia coli toxoid is 38 mg, radioantigen - 108 mg, with a volume ratio of components 27.2: 72.8.
2.3. Obtaining hyperimmune anti-radiating polyclonal antibodies. As an inducer of the synthesis of polyclonal antibodies with bifunctional properties, the conjugated complex protein-quinoid radiotoxin (BCRA) obtained by the above technology was used.
In experiments, to determine the optimal scheme of hyperimmunization, white mice were used, which were immunized with BCRA according to various schemes. An indirect competitive ELISA method was used to test the antisera.
The results of ELISA testing of the obtained antisera showed that the optimal scheme of hyperimmunization is 4-fold subcutaneous, administered with an interval of 2 weeks (2nd, 3rd) and 4 weeks (4th injection) of conjugated antigen (BCRA) in doses of 1 mg of conjugate (CG) + 500 μl of Freund's complete adjuvant (NAF) (2nd injection), 1 mg CG + 500 μl of saline (3rd and 4th injections, respectively), provided antisera with a maximum antibody titer of 1: 500.
The optimal hyperimmunization scheme tested in white mice was used for the scaled production of sera from large donors (cattle).
2.4. Obtaining metabolic products of Bifidobacterium bifidum and Bacillus subtilis and a highly dispersed fraction of bentonite.
To obtain metabolic products (PM), the commercial strain Bifidobacterium bifidum1 and the museum strain Bacillus subtilis 3, which were grown on appropriate nutrient media, were used as PM donors. The grown cultures were centrifuged at 5000 rpm for 30 min, the supernatants were decanted, and the concentration of biologically active substances (BAS) was determined by measuring the optical density on an SF-4B spectrophotometer. The concentration of biologically active substances was calculated according to the formula K = OPs - OPc, where OPs is the optical density of the supernatant, OPc is the optical density of the culture medium (MPB) and Blourock's medium.
The obtained supernatants were poured into 10 ml ampoules, subjected to radiating sterilization on a Puma gamma unit at a dose of 5 × 103 Gy and lyophilized on a Lausanne unit (Switzerland) and used them as components of the Polyapisogen composite preparation.
To obtain a highly dispersed fraction of bentonite, the latter was subjected to a special treatment with hydrochloric acid (HCL) in order to decompose the carbonates. The treatment was carried out for 40 min with 1N HCL, then the supernatant was removed, poured over with 0.1 N HCL, periodically stirred for 35 min. The supernatant was decanted, the precipitate was washed with distilled water to remove neutralized quartz and soluble salts. Then the sediment was subjected to fractionation in labeled pots with appropriate divisions into fractions. When the suspension was stratified into 3 fractions: 1st at a height of 3 cm, 2nd - 7 cm higher than the first and 3rd (7 cm higher than the second). The upper (3rd) fraction was carefully (without dispersing) elutriated into clean glasses. The particle size of the fraction was determined by microscopy. It was found that the size of the bentonite particles in the investigated fraction was 60-90 μm, i.e. microparticles that easily pass through the channel of needles of syringes for parenteral administration of bentonite suspension into the body.
The highly dispersed fraction of bentonite (VDFB) obtained by the described technology was used as one of the components of the developed multifunctional therapeutic composition and antibody bentonite diagnosticum (ATBD) for staging the reaction of bentonite flocculation (RBF) when indicating radiotoxin in the body of irradiated animals.
2.4.1. Setting up a Bentonite Flow Reaction (RBF) for the Indication of Radiotoxins
To indicate radiotoxins, blood samples were taken from animals in dynamics, sera were obtained, which were kept in a refrigerator for 30 min, heated in a water bath at 56 ° C for 10-15 min and studied as an antigen-containing material in RBF.
The setting of RBP was carried out on immunological plates, in the wells of which serial two-fold dilutions of the tested sera were added in distilled water in a volume of 0.1 ml. Then, 0.1 ml of the antibody variant of the bentonite diagnosticum (ATBD) was added to each well. The mixture was shaken thoroughly and left at room temperature for 1.5–2 h. The reaction was followed by an appropriate control, using normal serum from healthy (non-irradiated) animals as negative antigen.
The reaction was considered positive when the bentonite microparticles sensitized with antiradiotoxic antibodies were located at the bottom of the wells in the form of an «umbrella» (as in the case of the usual reaction of indirect hemagglutination - RNGA), with pronounced fistulous edges and complete clearing of the supernatant fluid, the whole bottom of the hole is in the form of a button (point) The method is protected by Patent RU (No. 2324176, GO 1N 33/02, publ. 05/10/2008, bull. No. 13).
2.5. Construction of a Composite Medicinal Preparation Based on Polyclonal Antibodies (PCA) of the Apiphyto-Preparation «Vita-Forci» (ACE), Bifidobacterium Bifidummetabolic Products (PM B.b) and Bacillus Subtilis (PM Bs) and a Highly Dispersed Fraction of Bentonite (VDFB).
The hyperimmune antiradiation serum obtained at the previous stage of the work, the apifito extract of the biologically active feed additive «Vita-Forci», the metabolic products of Bifidobacterium bifidum, Bacillus subtilis as well as the highly dispersed fraction of bentonite, were used as the main components of the developed polyfunctional therapeutic composition (PLC).
For the preparation of the PLC, the basic components were hyperimmune anti-radiation (anti-BChA serum- component I), and 4% ethanol extract of the apiphitopreparation «Vita-Forci» [component II], taken in a 1: 1 ratio, in which 20 g of a highly dispersed fraction were dissolved bentonite (VDFB), 2 g of Bifidobacterium bifidummetabolism powder (PM Bb) and 4 g of Bacillus subtilis metabolic products (PMBs).
In the resulting preparation, the concentration of active substances (ADV) was determined by determining the dry matter content after drying the liquid phase to constant weight. It has been established that the dry matter content in the composition ranges from 0.9-1.2 ∙ 104 mg / ml. Therefore, the concentration of ADV in the composition was standardized on dry matter by adding dry matter from the composition, bringing the concentration of ADV to 10%, i.e. 1 ∙ 104 mg / ml.
The resulting product was poured into vials of 200.0 cm3 and subjected to radiation sterilization by irradiation on a gamma device «Tracer» at a dose of 2.5 ∙ 107 Gy, stored in a refrigerator at 4 ± 2 °C, subsequently used as a remedy named by us conditionally as «Polyapisogen» for radiation damage to the body.
2.6. Simulation of Radiation Damage
To assess the radioprotective effect of the developed polyfunctional drug «Polyapisogen», we carried out a simulation of experimental radiation sickness (ARS).
Modeling of acute radiation sickness of mild, moderate and severe degree was carried out by irradiation of laboratory and agricultural animals on the Puma gamma installation at an exposure dose rate of 3.13 · 10-5 C (kg s) at doses of 6.0-8.0 Gy (white mice), 7.0-9.0 Gy (white rats) and 4.2 Gy (pigs), respectively.
2.7 Evaluation of the Effectiveness of the Test Drug on the Course and Outcome of Experimental Acute Radiation Sickness (ARS).
The therapeutic efficacy of the developed drug was assessed by the degree of correction of clinical and immunohemopoietic (course and outcome of ARS, escherichiosis infection, radio-induced pancytopenia, bone marrow cellularity, immunoregulatory index - Th / Ts), biochemical (concentration of quinoid - HRT, lipid - MDA radiotoxins - anti superoxide dismutase - SOD, cytokines - IL-1, TNF-α, IFN-γ) and microbiological (state of intestinal metabolism) indicators.
The course and outcome of ARS in irradiated animals was determined by dynamic monitoring of animals, recording the nature of the manifestation of the disease and its modification, food and water intake, behavioral reactions, the condition of the skin (coat), the timing of mortality and 30-day survival of the affected and treated animals.
To study the effect of the test drug on the state of the blood system, samples were taken from animals in dynamics, which in a volume of 3 ml were introduced into the cells of the «Coster» hemoanalyzer (France). Analysis and evaluation of the data obtained were carried out in accordance with the manufacturer's instructions.
The myeloprotective effect of the drug was assessed by the degree of recovery (inhibition of emptying-depopulation of bone marrow stem cells) of myelocytes in the bone marrow (Lefkovits, Waldman, 1979). For this, a suspension of myelocytes was obtained in the amount of 50 μl, it was introduced into the Goryaev chamber and the number of myelocytes in the bone marrow was counted.
Correction of the immune system was assessed by the value of the immunoregulatory index (IRI), which is the ratio of T-helpers and T-suppressors (Th / Ts). To calculate IRI, the content of Th and Ts cells in the peripheral blood of irradiated, Escherichiosis and animals treated with the test drug was determined.
The level of T-helpers and T-suppressors in the peripheral blood was determined using DM diagnostic kits for the determination of T-lymphocytes, T-helpers, T-suppressors, and B-lymphocytes developed by Vitebsk Medical University (Belarus).
Evaluation of the results was expressed as a percentage: the percentage of T-lymphocytes is equal to the percentage of rosette-forming lymphocytes with T3DM diagnostics; the percentage of T-helpers (Th) is equal to the percentage of rosette-forming lymphocytes with CD4 diagnostics. Norm 23-46% (average 40 ± 3.0%). The percentage of T-suppressors (Ts) is equal to the percentage of rosette-forming lymphocytes with a CD8 diagnosticum. The norm is 17-30% (average = 22 ± 1%). The percentage of B-lymphocytes is equal to the percentage of rosette-forming lymphocytes with a CD22 diagnostics (norm 23 ± 3.6%, mean 17-31%). Immunoregulatory index (IRI): Th / Ts = 1.4-2.0 (Toka E.N. et al., 2004).
The effect of the test drug on post-radiation cytokinesis was studied by determining the concentration of interleukin (IL-1) and tumor necrotic factor (TNF-α) according to Shver K. et al (1991), interferon-γ (INF-γ) according to Tyrinova T.V. et al (2013) in blood serum by enzyme-linked immunosorbent assay (ELISA).
Taking into account that ionizing radiation induces excessive formation of reactive oxygen species (ROS), which contribute to the development of oxidative stress, leading to an imbalance of the prooxidant-antioxidant system (POAS), and dismutation of superoxide anion and hydrogen peroxide is implemented by the antioxidant enzyme, superoxide dismutase (SOD), we studied the influence of the developed composite preparation on superoxide dismutase activity in ARS. SOD activity was determined according to the method described by Higashi T. et al. (1983). The principle of the method is based on the ability to compete with nitro blue tetrazolium (NBT) for superoxide anions formed as a result of the interaction of the reduced form of nicotinamide nucleotide (NAD ∙ H) and phenazine methosulfate (PMS). The amount of SOD was determined on an SF-46 spectrophotometer (St. Petersburg, Russia) by measuring the optical density of the reaction mixture at a wavelength of 540 nm; the calculation was carried out using the formula: Eo-Epr / Eo ∙ 100%, where Eo is the extension of the reaction mixture in the absence of SOD (zero sample), Epr - investigated sample (experimental sample). The unit of activity was defined as the amount of the enzyme required to reduce the optical density in the course of NBT reduction by 50%. SOD activity was expressed in μmol / ml.
Statistical data processing was performed using the Statistic 6.0 software package for Windows. Data are presented as mean values (M) and standard error (SE). To identify significant differences between the compared indicators, the nonparametric Wilcoxon-Mann-Whitney U-test was used. Differences were considered significant at a significance level of p <0.05.
3. Results of Studies
3.1. Study of the Effect of the Drug «Polyapisogen» on the System of Immunohemopoiesis in Health and Disease
At the first stage of the work, studies were carried out to evaluate the tested drugs for the immunohemopoiesis system of intact animals, i.e. in natural (normal) conditions of ecological well-being.
The experiments were carried out on 20 weaned rats, with an initial live weight of 41 ± 3 g, divided into 2 groups of 10 rat pups each. The rats of the 1st group received the usual diet for 30 days, the 2nd - the drug «Polyapisogen» at the rate of 100 mg / kg of feed. To study the immunomorphological composition of blood in animals in dynamics (outcome, after 5 and 10 days), blood samples were taken from the tail vein and the content of blood corpuscles was determined in a UniCel® DxH 600 hematological analyzer (Beckman Coulter, USA).
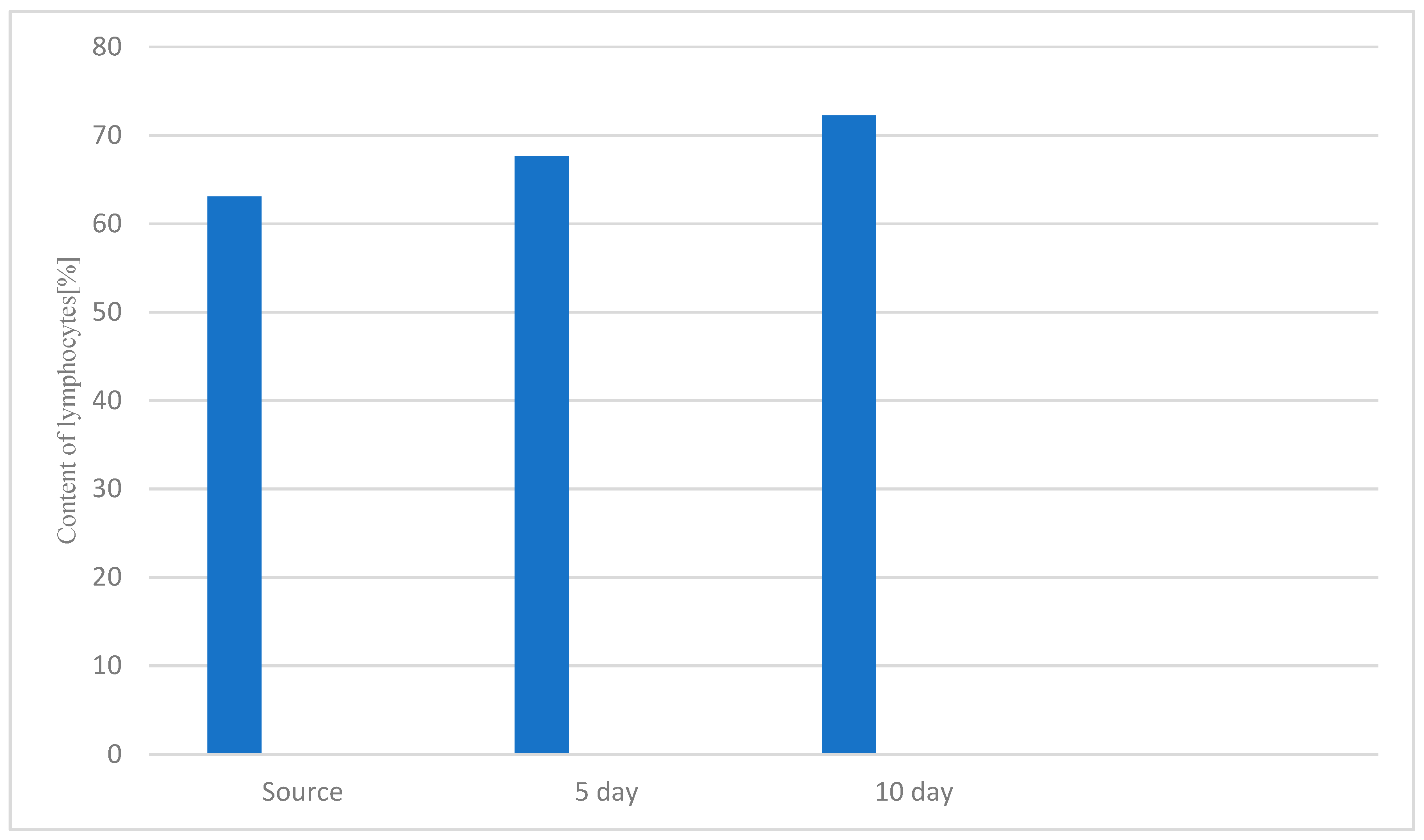
The results of hematological studies showed that the intake of the drug «Polyapisogen» into the body had a positive effect on the hematopoietic system. At the same time, there is a tendency to an increase in the content of erythrocytes, hemoglobin, leukocytes, lymphocytes and a decrease in the young forms of neutrophils, eosinophils and basophils, which approached that in healthy adult animals. The data obtained indicate a positive effect of the drug used on the hematopoietic system in growing animals.
Figure 1 shows the results of determining the content of lymphocytes in the peripheral blood of rat pups in dynamics after feeding them with the drug «Polyapisogen»
The data obtained indicate a positive effect of the drug used on the hematopoietic system in growing animals. In parallel, studies were carried out to study the effect of the test drug on the state of the body's immune system. Changes in the subpopulation were used as an indicator of the state of the immune system.
(T-helpers and T-suppressors) and their ratios (Tx / Tc), which determine the degree of activation or suppression of the immune system against the background of exposure to biologically active substances.
The results of determining the content of immunocytes (T-, B-lymphocytes, T-helpers and T-suppressors in growing rat pups against the background of the intake of the biologically active drug «Polyapisogen» in their body showed that the composition of lymphocytes circulating in the blood of growing rat pups was 11.9 ± 1.1 % B-lymphocytes and 57.5 ± 2.1% T-lymphocytes, which, in turn, contained 17.7 ± 2.8% of T-helpers (Th) and 13.9 ± 1.7% of T-suppressors (Ts).
The nature of changes in the content of immunocytes (Th and Ts) in dynamics (within 10 days) against the background of the intake of a composite immunotropic drug («Polyapisogen») is shown in
Figure 2.
The data in the figure show that the intake of the test drug in the body of growing rat pups causes not only quantitative, but also qualitative changes in immunocytes, which is accompanied by activation of the immune system (
Figure 3), expressed by an increase in the most important indicator of the state of the immune system, the immunoregulatory index (Th / Ts).
The data presented in
Figure 2 show that before the rat pups entered the body of the test drug, the average value of the immunoregulatory index (Th / Ts) was 1.29, and as the composite drug entered the body, this ratio (immunoregulatory index) constantly increased and by 10 days it was 1.55, which indicates a high level of activation of the immune system of a growing organism under the influence of the test drug and that the latter has an immunostimulating effect.
3.2. Study of the Stress-Protective Effect of the Drug «Polyapisogen»
It is known that long-term stay of animals in extreme conditions is accompanied by a violation of the adaptive mechanisms of the organism and the development of the so-called «Selye's Adaptation Syndrome» substances (BAS).
Taking the above into account, we carried out studies to assess the stress-protective (adaptogenic) activity of a multicomponent agent based on substances of zoogenic, phytogenic and apisogenic origin.
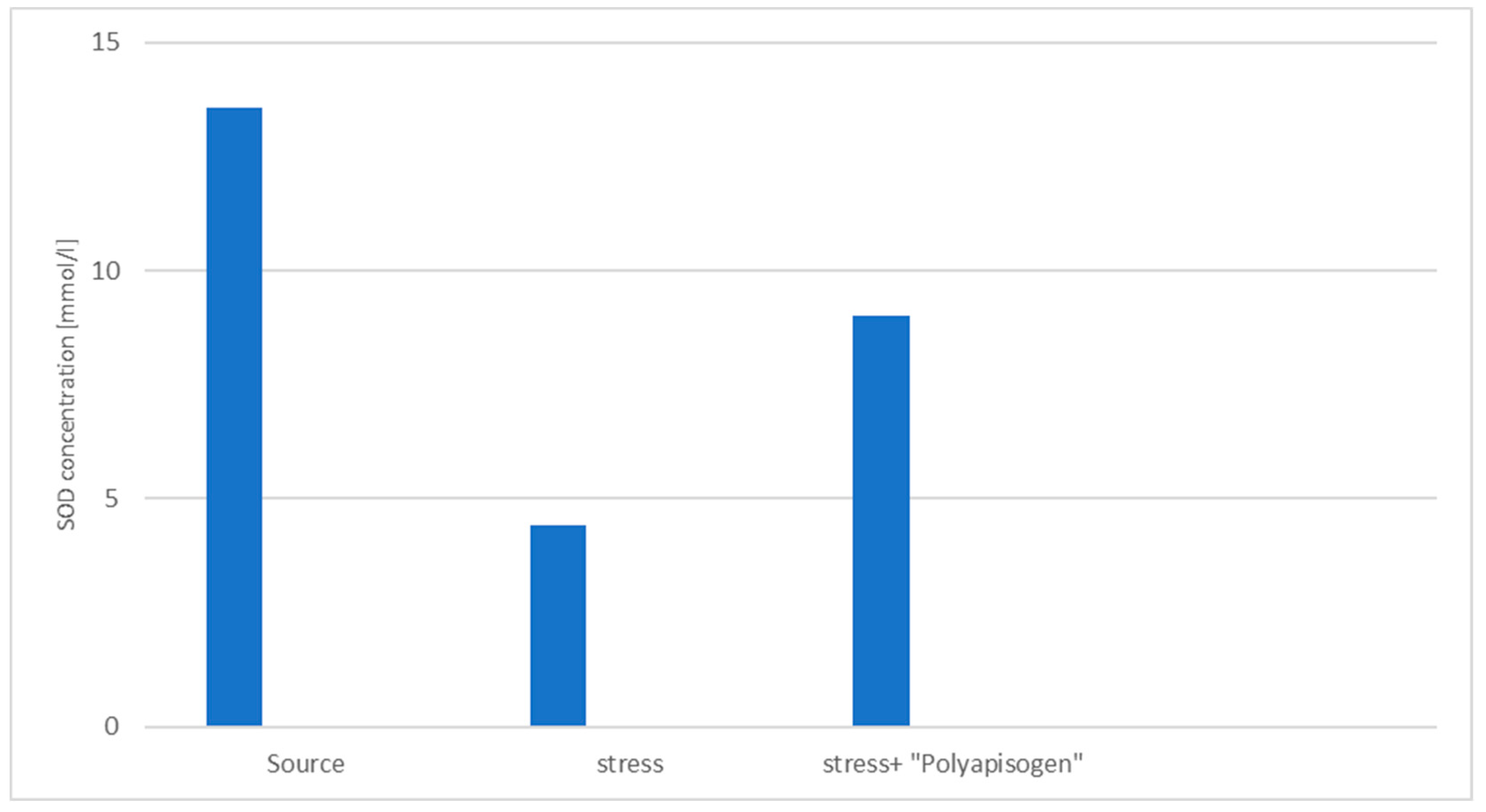
To assess the stress-protective effect of the studied drug, experiments were carried out on 32 white rats of both sexes and an initial live weight of 180-200 g. The stress-protective activity of the drug was assessed on the model of immobilization stress, carried out by fixing the animals in the supine position for 12 hours. Animals of the experimental group were intragastric, a 5% dealcoholized solution of an experimental sample of the studied drug was injected in a volume of 3 ml / kg (25 mg / kg) for 7 days before immobilization. Animals of the control group received an equal volume (0.6 ml) amount of distilled water according to a similar scheme. On the 8th day of the experiment, animals of the control and experimental groups were subjected to immobilized stress, after which they were decapitated under light ether anesthesia and the Selye triad was determined: the severity of adrenal hypertrophy and thymic involution, as well as destructive lesions in the gastric mucosa. For this, the stomach was cut along the greater curvature and the number of destructions was counted, which were subdivided into punctate hemorrhages, erosion and striped ulcers. The intensity of lipid peroxidation (LPO) processes was assessed by the accumulation of malondialdehyde (MDA), the state of the antioxidant system was judged by the activity of superoxide dismutase (SOD).
The results of the studies showed that immobilization stress in animals (control - stress + H2O) causes the development of a complex of dystrophic changes in internal organs (characteristic of a stress reaction): involution of lymphoid organs, adrenal hypertrophy, the appearance of destructive changes in the gastric mucosa. Prophylactic administration of the drug «Polyapisogen» at a dose of 25 mg/kg caused a pronounced antistress effect, as evidenced by the inhibition (by 21%) of hypercortisolism, inhibition of thymic involution and a decrease in the spleen mass caused by immobilization stress in animals.
Advanced oral administration of the test drug to animals prior to immobilization stress exerted a pronounced gastroprotective effect, preventing the development of deep destruction of the gastric mucosa in white rats (
Table 1). Thus, the number of erosions in prevented animals was 2 times less than in non-prevented (control) animals. At the same time, in the animals prophylactic with the test drug, in contrast to the non-prophylactic ones, pinpoint damage to the walls of the stomach (striped ulcers) were not found.
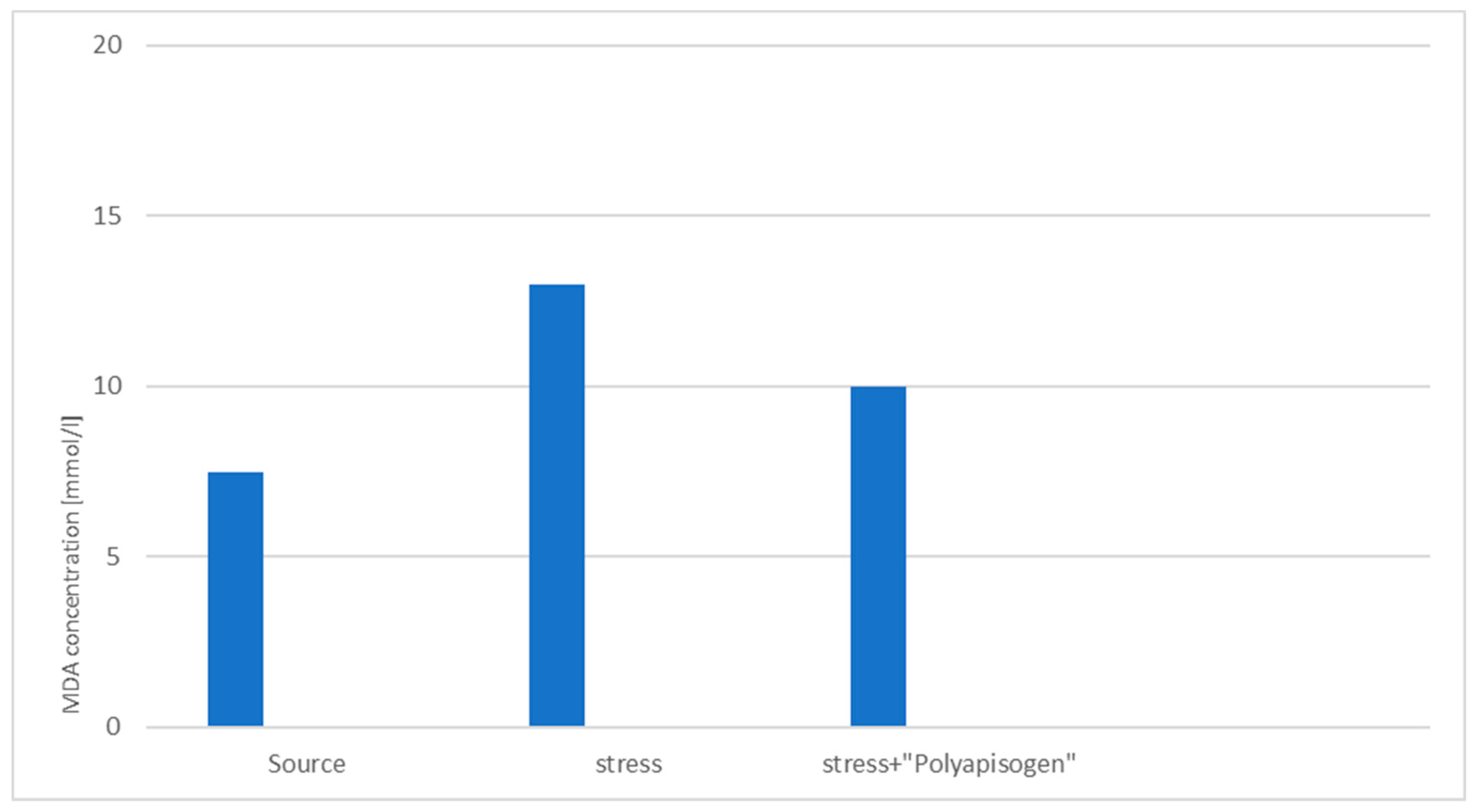
Considering that post-stress hypercortisolism is accompanied by excessive activation of free radical oxidation with hyperproduction of toxic lipid peroxidation products (MDA), parallel biochemical studies of blood sera of stressed and prophylactic animals with a potential antistressor drug “Polyapisogen” were conducted to determine the concentration of TBA-active compounds and the concentration of antioxidant enzyme - (SOD).
The results of studying the state of the prooxidant-antioxidant system (POAS) in stressed and prophylactic animals with the study drug are presented in
Figure 4 and
Figure 5.
The figures show that immobilization stress is accompanied by an increase in the process of lipid peroxidation in the body, which is expressed by a sharp increase in the concentration of toxic products of lipid oxidation - malondialdehyde (MDA): in rats of the control group, the concentration of these products by the end of stress exceeded the initial level by 2.01 times. Post-stress enhancement of the function of the prooxidant system led to reciprocal inhibition of the function of the antioxidant defense system, which was accompanied by a simultaneous decrease in the activity of the antiradical enzyme SOD by a factor of 3.91.
Preliminary administration of the drug «Polyapisogen» had a corrective effect on the prooxidant-antioxidant system (POAS) of stressed animals, which was accompanied by a decrease in the concentration of MDA by 1.61 times while maintaining the activity of the antioxidant enzyme (SOD), the content of which in prevented animals slightly differed from that in intact animals, yielding the level of the initial indicators 1.13 times.
The results of studying the effect of the experimental sample of the multicomponent composite preparation «Polyapisogen» on the organism of intact (healthy) animals showed that it is low-toxic (IV hazard class), does not possess cumulative, allergenic, teratogenic, embryotoxic properties and irritating effect on the skin and mucous membrane of the stomach of animals. It has a beneficial effect on the offspring, increases the physiological and immunohemopoietic status, has a stress-protective (adaptogenic) and antioxidant effect, which together can provide effective protection of the body under conditions of exposure to environmental agents of physical, chemical and biological nature. The results obtained were the basis for testing the developed composite preparation as an immunotherapeutic agent on animals affected by ionizing radiation.
3.3. Study of the Immunotherapeutic Activity of the Drug in Radiation Pathology
As a biological model, 180 adult white mice were used in the experiments with a live weight of 18-20 g (white rats 180-200 g) and rabbits of the Chinshila breed with a live weight of 3-4 kg of both sexes.
In experiments on white mice, 108 animals were used in 3 replicates. The first series of experiments was carried out on 36 white mice, divided into 3 groups of 12 animals each. The animals of the 1st and 2nd groups were irradiated on a gamma device «Puma» at a dose of 7.7 Gy (LD100 / 30), and 24 h after irradiation, the animals of the 2nd group were injected subcutaneously with the test drug at a dose of 0.1 ml (25 mg / kg live weight). The animals of the 1st and 3rd groups were not injected with the drug, and they served as a radiation and biological control, respectively. The animals were monitored for 30 days after irradiation, recording the form of the course of acute radiation sickness (ARS), the time of death and the number of dead and surviving animals. As a criterion for the effectiveness of the test drug, 30-day survival rate and average life expectancy (ALE) of animals was used.
The results of dynamic observations of the animals showed that in the white mice of the 1st group irradiated at a dose of 7.7 Gy, radiation sickness proceeded in a severe form and on days 9th, 11th, and 14th after irradiation, 3, 5 and 4 animals died, respectively, the lifespan was 3 days (AOI = 3 days).
The use of the test drug against the background of irradiation had a modifying effect on the course and outcome of acute radiation sickness (ARS). Irradiation at a lethal dose and receiving a single subcutaneous dose of Polyapisogen at a dose of 25 mg / kg, white mice tolerated ARS in a milder form, which was reflected in the survival rate and average life span (ALE). At the same time, out of 12 irradiated and treated animals, 3 animals died on the 19th, 23rd, and 29th days, which is 75% survival at ALE = 24.0.
To check the reliability of the data obtained, the 2nd and 3rd series of experiments were carried out using 36 white mice and divided into 3 groups of 12 white mice, respectively. Irradiation, treatment of animals of the corresponding groups and evaluation of the drug's effectiveness were carried out according to the above scheme.
The results of the 2nd and 3rd replication of identical experiments showed that the survival rates of lethally irradiated animals and those treated with the test drug were 66.7 and 83.3%.
Thus, the results of studying the radioprotective activity of the test drug, based on the results of 3 replicates, averaged 74.96%.
Considering that ionizing radiation is a strong stressing agent leading to inhibition of adaptive reactions, and the use of phytoadaptogens promotes the development of adaptive reactions by correcting the antioxidant defense system (AOD), a second series of experiments was carried out on white mice.
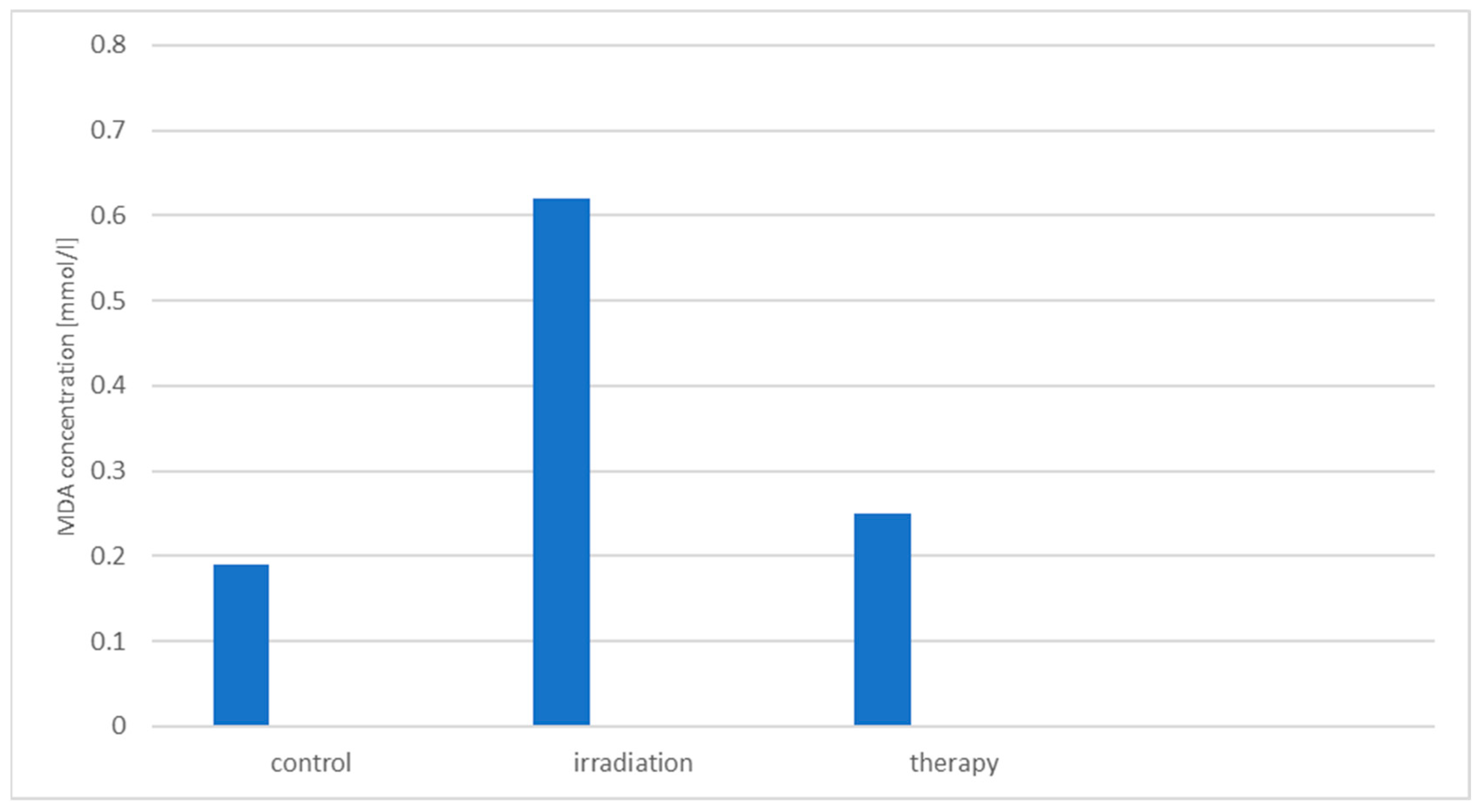
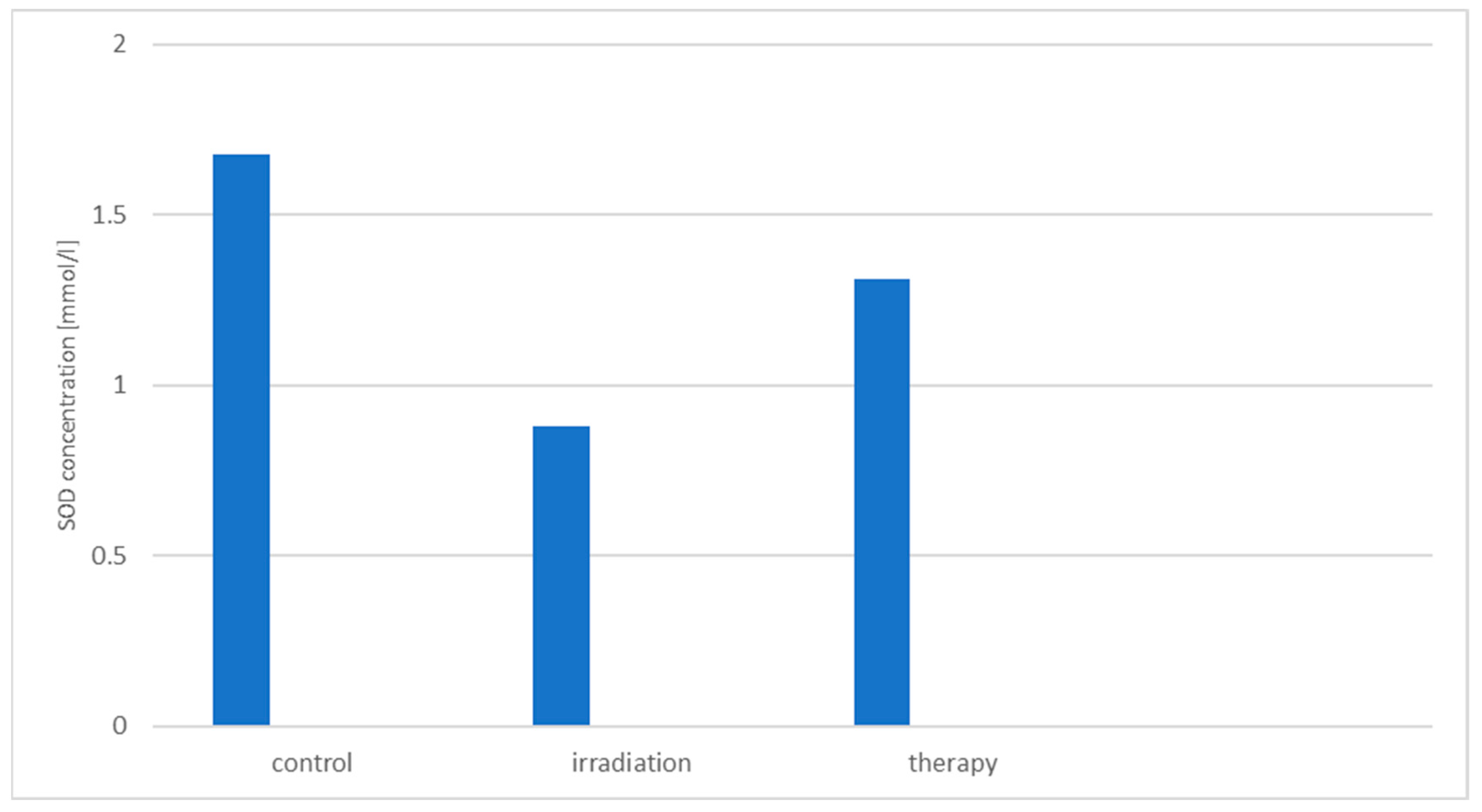
The experiments used 18 white mice weighing 17-20 g, divided into 3 groups of 6 animals each. Animals of the 1st and 2nd groups were subjected to radiation stress by irradiation with gamma rays at a dose of 7.7 Gy (LD100 / 30). 24 hours after irradiation, the irradiated animals of the 2nd group were injected subcutaneously once at a therapeutic dose of 25 mg / kg (in a volume of 0.1 ml) of the test drug. The irradiated animals of the 1st group and non-irradiated animals of the 3rd group were not injected with the drug, and they served as a control of irradiation (1st group) and biological control (3rd group). After 8 days - during the height of ARS and the development of an adaptive syndrome, animals of the intact and experimental groups are sacrificed under ether anesthesia, blood was taken in total, the content of lipid peroxidation products - MDA was determined in the blood plasma and erythrocyte hemolysate, and in the blood serum - the content of quinoid radiotoxins ( HRT) using the antibody variant of the bentonite diagnosticum (ATBP), while the activity of the antioxidant enzyme superoxide dismutase (SOD) was determined in the blood serum.
From the data in
Figure 7, it can be seen that radiogenic stress is accompanied by enhanced synthesis of toxic products of oxidative modification of macromolecules (OMM), the concentration of which exceeded the control level by 3.47 times. Enhanced synthesis of prooxidants led to a sharp inhibition of the activity of the antiradical enzyme SOD (by 1.92 times).
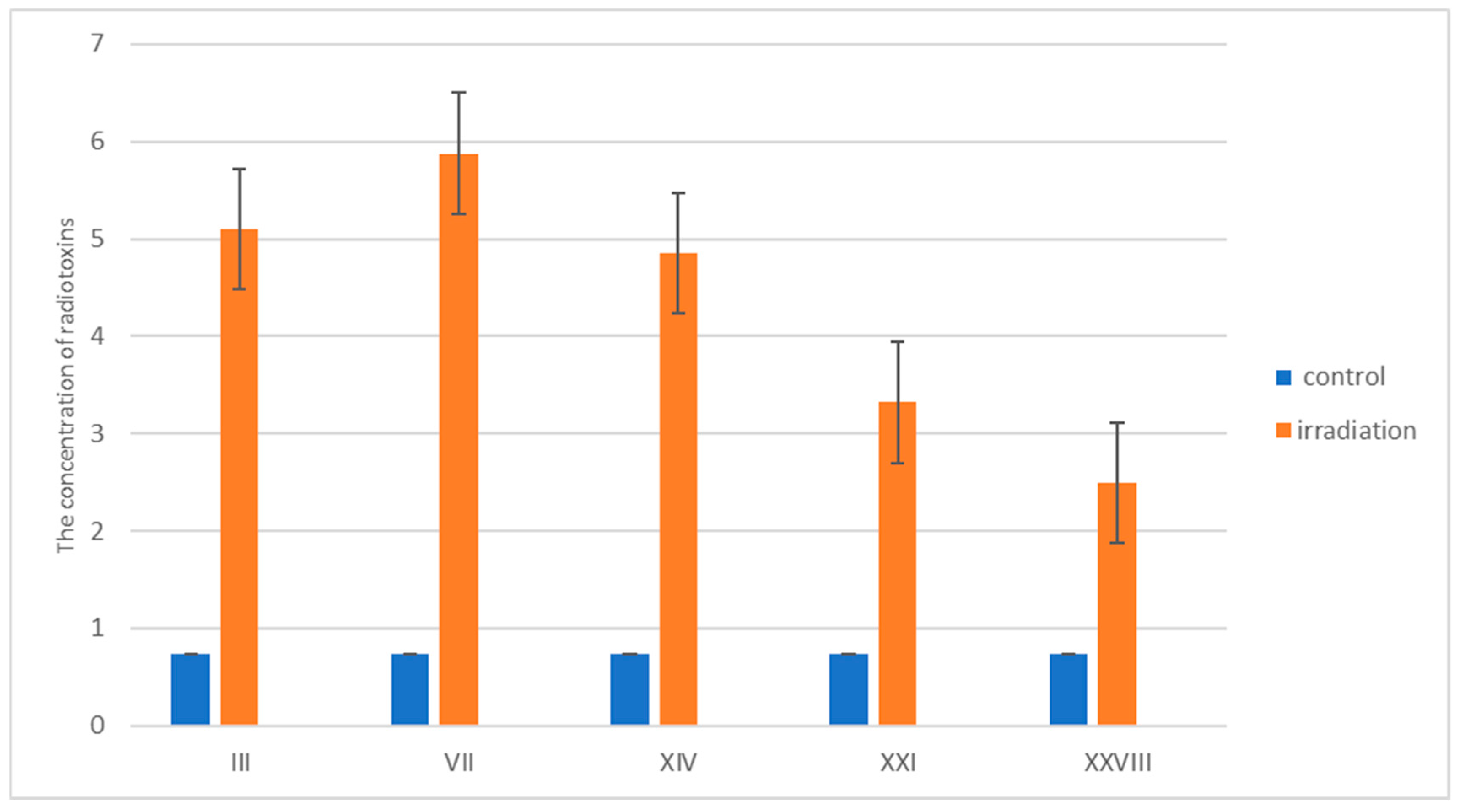
The results of the indication of radiotoxins in irradiated animals in dynamics (after 3, 7, 14, 21 and 28 days) showed (
Figure 8) that already 3 days after irradiation, quinoid radiotoxins (HRT) appear in the blood of animals in the RBP titer 4.7 log2 that reach a maximum (6.1 log2) are kept at this level (5.7 log2) and gradually decrease by day 21 (3.9 log2) and by day 30 their titers are 2.5 log2 (
Figure 8).
A single subcutaneous injection of the test drug to lethally irradiated animals had a detoxifying effect, inhibiting the formation of HRT and antibodies containing it almost to control values.
A single subcutaneous injection of the drug «Polyapisogen» led to the correction of the antioxidant defense system, inhibiting the synthesis of TBA-active products of oxidative modification of macromolecules (MDA), while maintaining the activity of the key antiradical enzyme SOD (Fig. 7).
3.4. Study of the Hemoprotective Effect of the Drug «Polyapisogen»
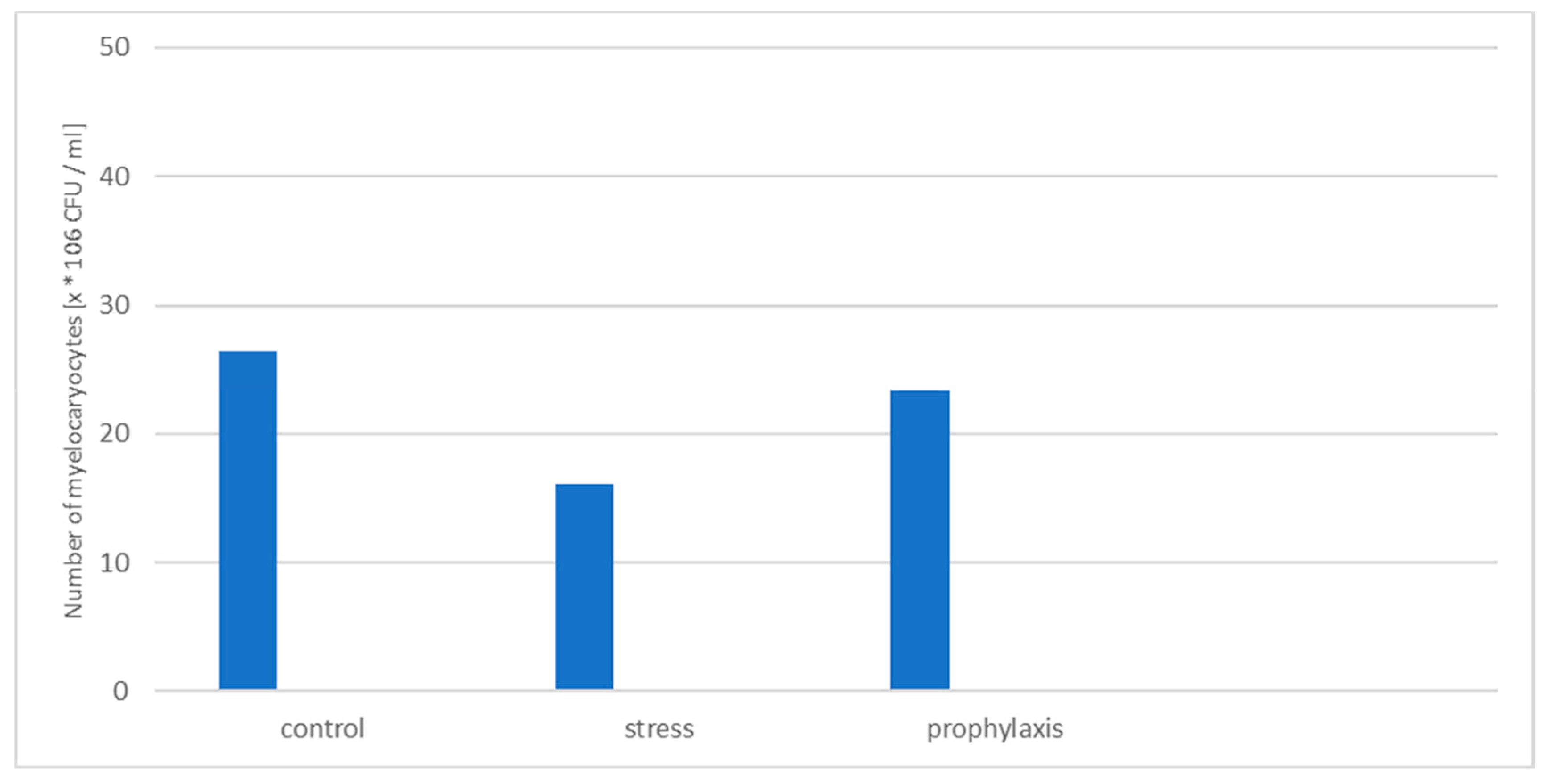
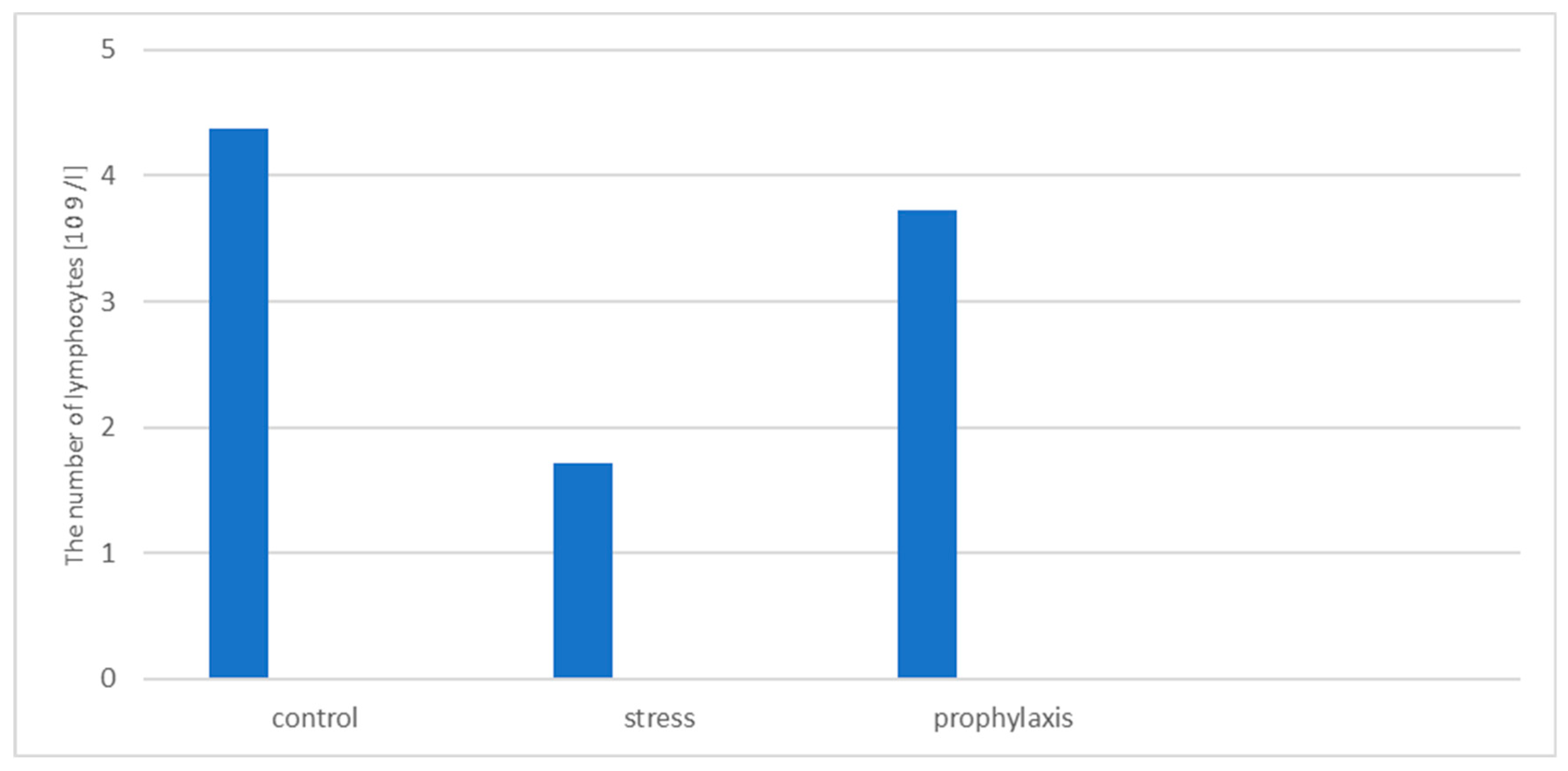
Considering that the main target of the attack of peroxyradicals in radiogenic stress is the hematopoietic system, in parallel, the state of the hematopoietic system was studied in stressed white mice against the background of the use of the drug «Polyapisogen». Blood for hematological studies was taken in total, and puncture for bone marrow examination was taken from the femur. On bone marrow smears, the percentage of individual populations of myelokaryocytes was calculated and then their absolute number in the femur was calculated. The mass of the thymus was also studied. In peripheral blood, the content of lymphocytes, the main target of free radical attacks, was determined.
The results of studies on the effect of the test drug based on bone marrow cells and peripheral blood of irradiated animals are presented in
Figure 9 and
Figure 10.
From the data in
Figure 8 and
Figure 9 it can be seen that radiogenic stress has a hemotoxic effect on the body, accompanied by inhibition of hematopoiesis with the suppression of the main sprouts of bone marrow hematopoiesis: erythroid, neutrophilic, lymphoid.
A single subcutaneous injection of Polyapisogen before irradiation had a hemo- and myeloprotective effect, preventing a decrease in the number of cells of lymphoid sprouts of bone marrow cells, as well as inhibiting the pancytopenic process in the peripheral blood, keeping the quantitative content of lymphocytes at the level of control, non-irradiated animals.
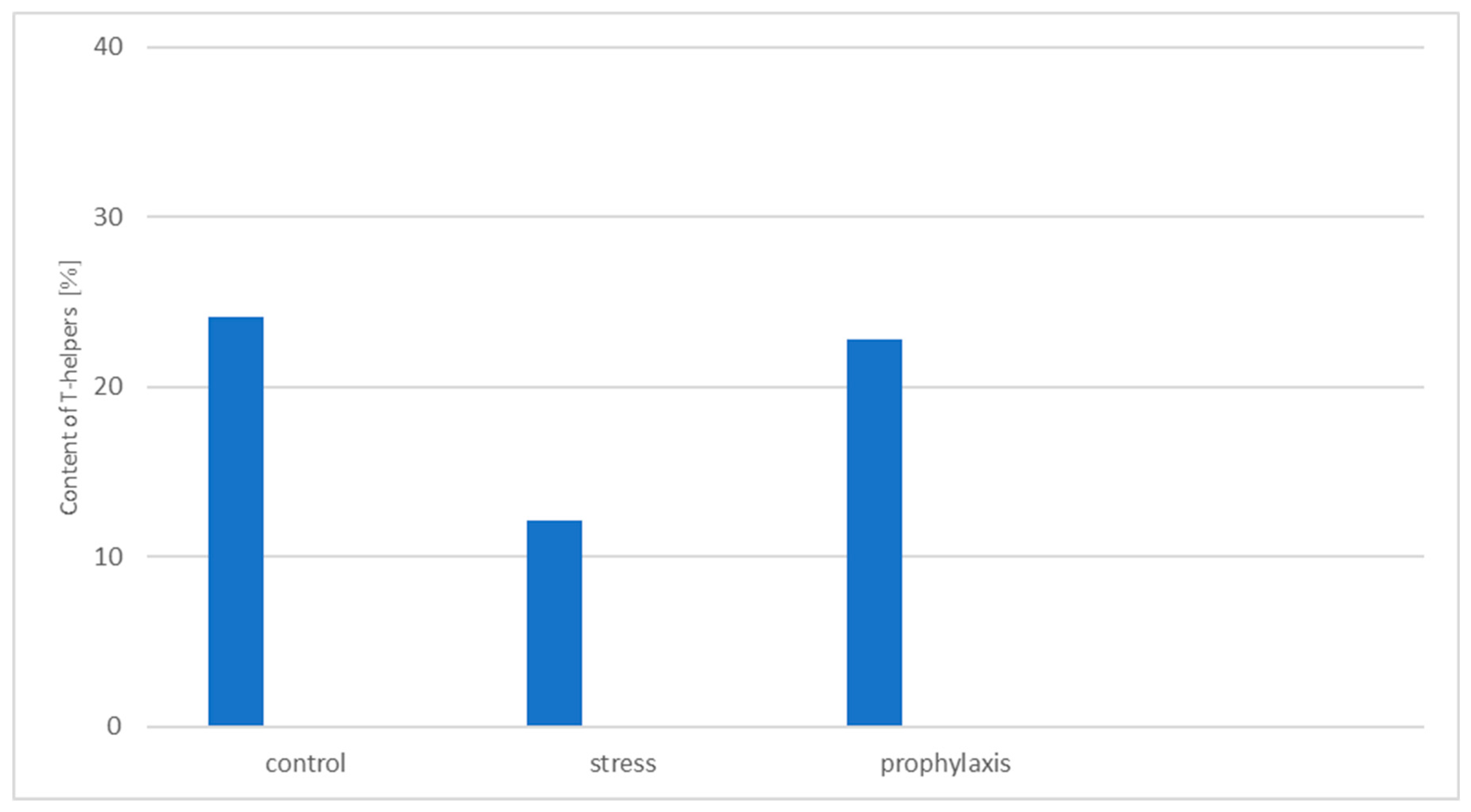
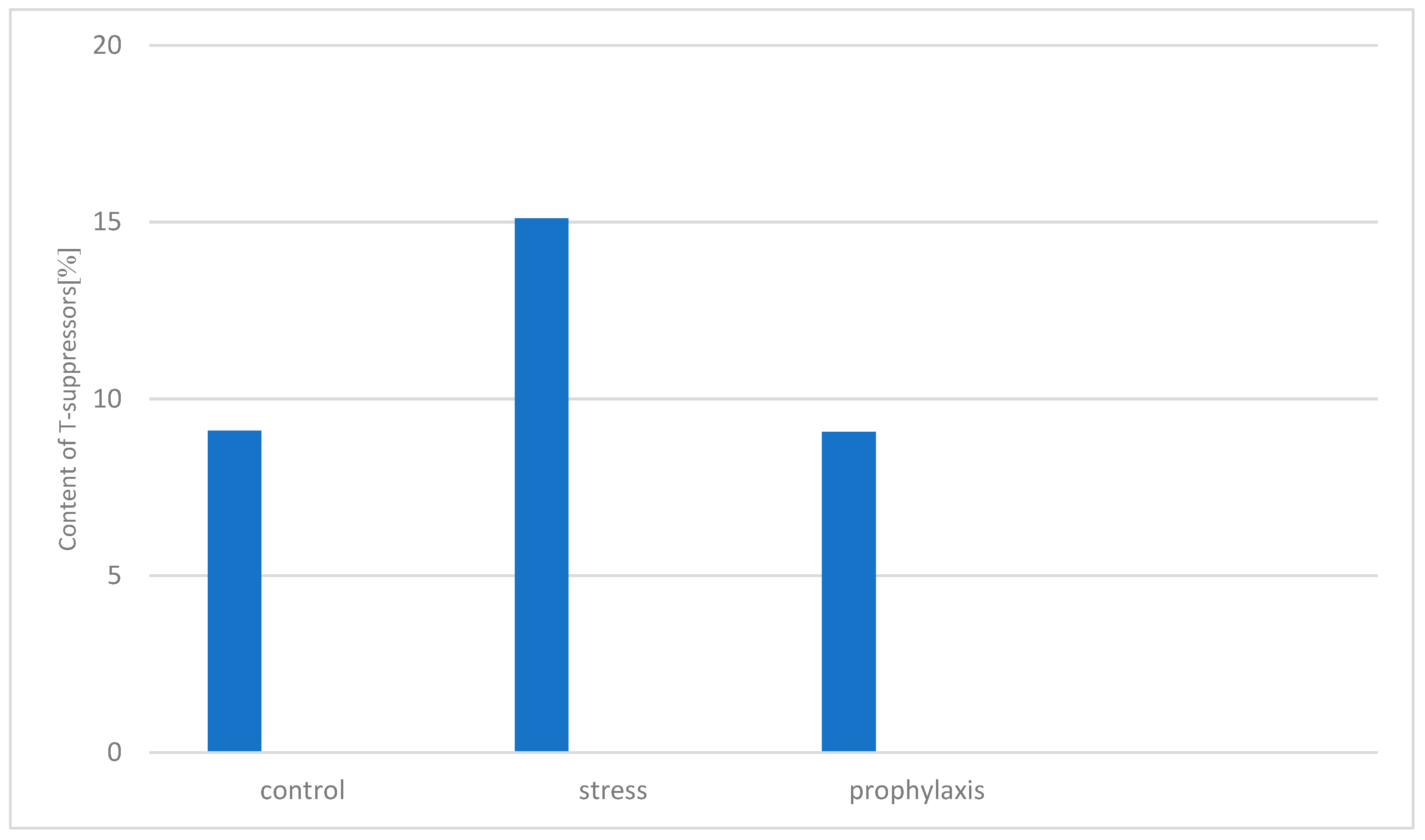
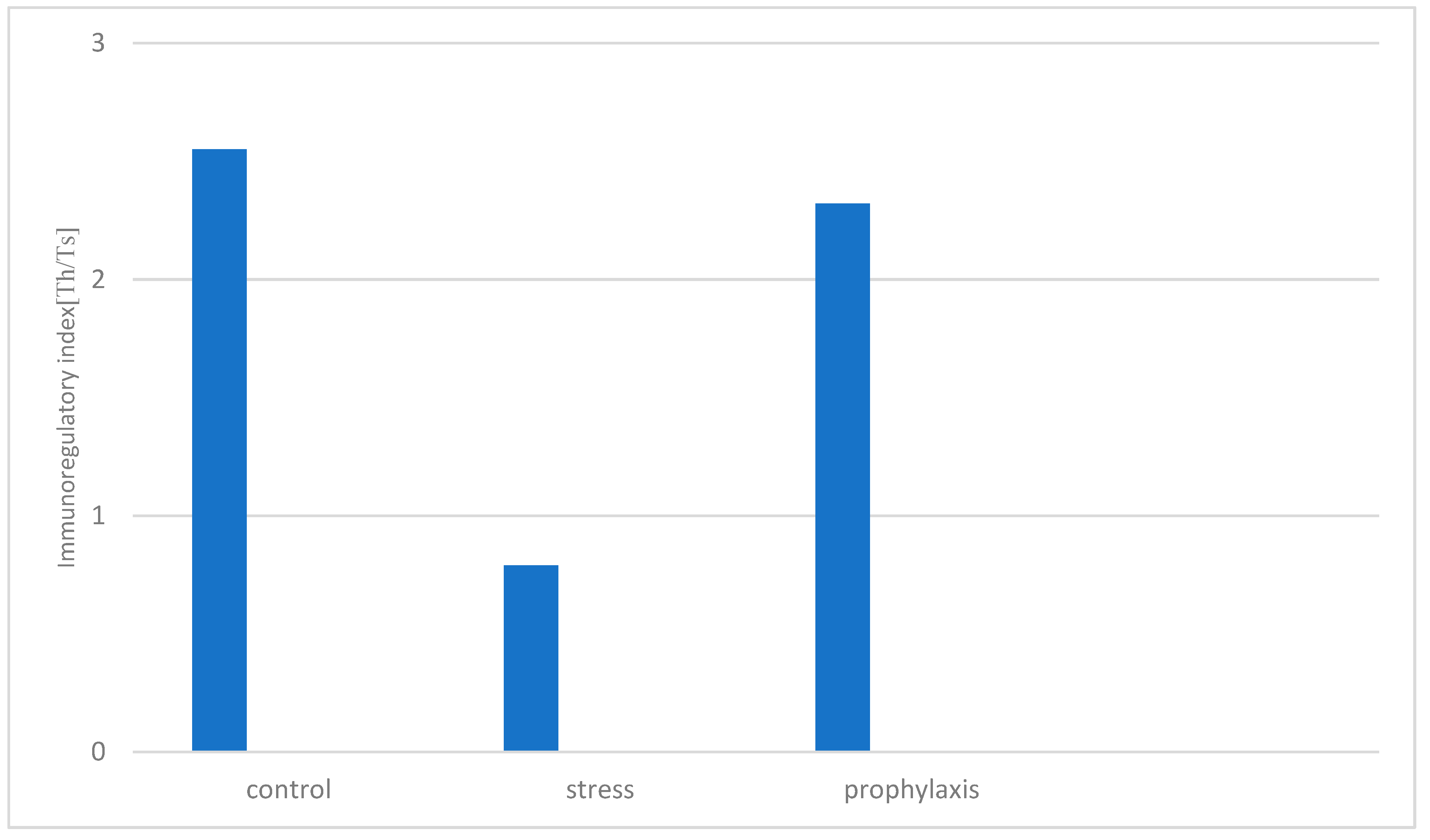
Considering that the hemoprotective effect of immunotherapeutic drugs is realized through the immunity system, namely, through the correction of the immunoregulatory index (Th / Ts), we conducted parallel studies to study the factors of cellular immunity in irradiated and treated (prophylactic) animals with the study drug.
The results of studying the state of immunity in animals irradiated and treated with the «Polyapisogen» preparation are presented in
Figure 11,
Figure 12 and
Figure 13.
From the data presented in
Figure 11,
Figure 12 and
Figure 13, it can be seen that the radiogenic stress of animals was accompanied by significant violations of the cellular immunity system. As early as 3 days after irradiation, there was a decrease in T-helpers with a simultaneous increase in the number of T-suppressors. The maximum decrease in the number of T-helpers (1.8 times in relation to the norm) and an increase in the number of T-suppressors (1.64 times in relation to the norm) occurred on days 7-21 (the peak period of ARS) after radiation stress.
The use of the composite drug «Polyapisogen» had a modifying effect on the course and outcome of ARS - it was characterized by a more favorable course of the disease against the background of inhibition of the immunohemotoxic effect, preventing the violation of the immunoregulatory (subpopulation) index (Th / Ts) (
Figure 10), thereby contributing to the maintenance of the functioning of T and B-cell immune system.
3.5. The Influence of the Drug «Polyapisogen» on Post-Radiation Cytokinesis
As stated above, a single subcutaneous application of the drug «Polyapisogen» had a hemo and myeloprotective effect against the background of exposure to gamma radiation by restoring the helper-suppressor ratio of T-lymphocytes (Th / Ts), as well as the number of T and B-lymphocytes . In this regard, it became necessary to clarify the possible relationship between the reaction of the cytokine system and the severity of hematological manifestations of the bone marrow syndrome of survival of animals affected by ionizing radiation and treated with a composite preparation.
The effect of the test agent on hematopoietic cytokinesis (HPC) on irradiated and treated animals with the composite preparation «Polyapisogen» was assessed by the level of synthesis of interleukin-1 (IL-1), tumor necrotic factor (TNF) and interferon (IFN). The concentration of cytokines in blood serum (diluted 10 times) and bone marrow supernatants was determined by the enzyme immunoassay using the Mouse TNF-α, Mouse IL-I kits (Endogen, USA).
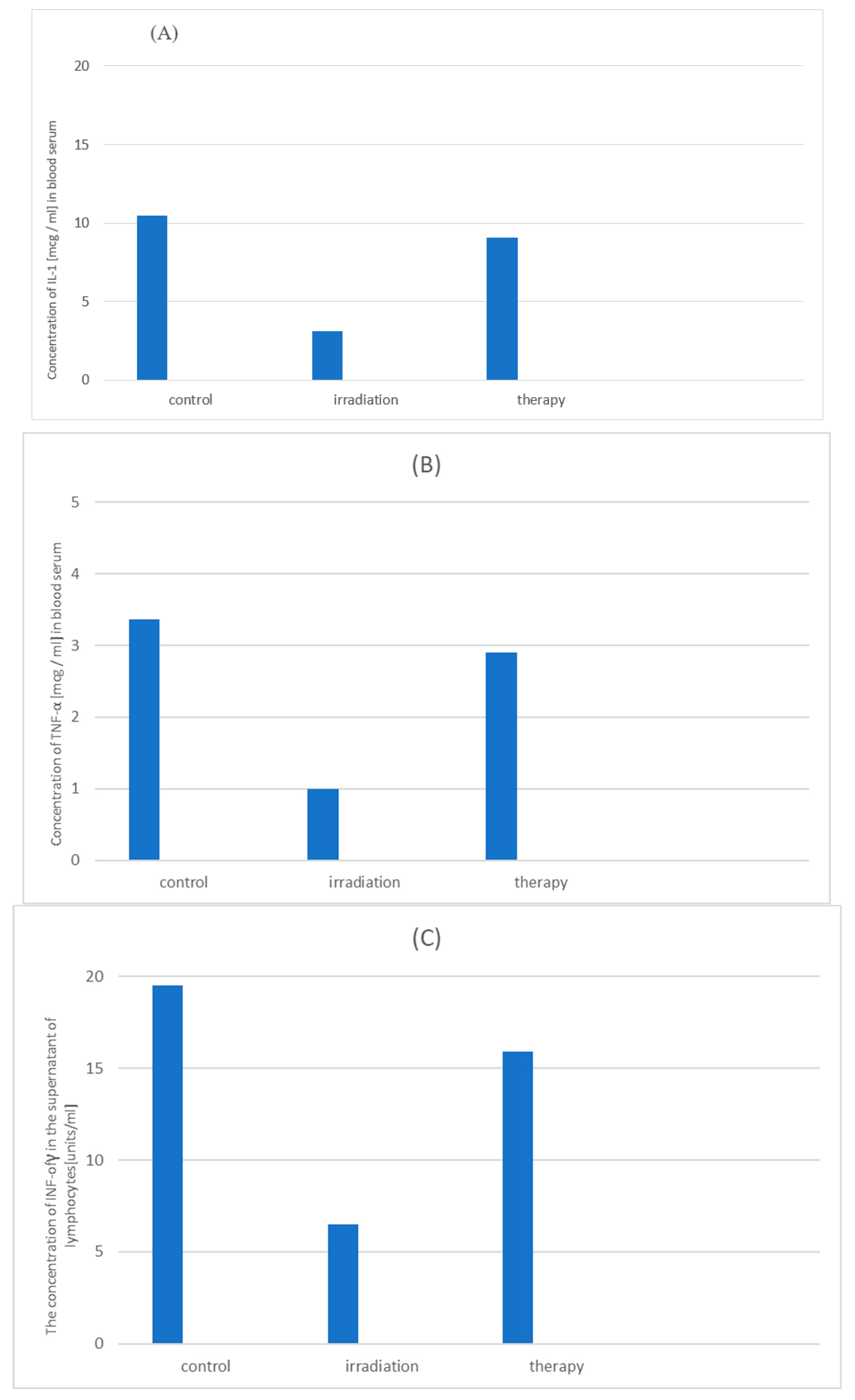
The results of determination of hemoregulatory cytokines in blood serum (IL-1, TNF) and lymphocyte supernatants (IFN) are shown in
Figure 14.
From the data in
Figure 14, it can be seen that under the influence of ionizing radiation, significant changes followed in the cytokine system of the body, which were characterized by inhibition of synergistic mediators of immunohemopoiesis, such as TNF, interleukin-1 (IL-1) and γ-interferon (INF-γ).
The inhibition of the content of these cytokines followed one day after the radiation exposure and reached maximum by the peak of ARS (by 8 days), when the concentration of IL-1 in the blood decreased by 3.10 times, TNF-α in blood serum decreased by 3.46 times, INF-γ in lymphocyte supernatants - 2.91 times.
A single subcutaneous injection of the test drug at a dose of 0.1 ml (25 mg / kg on dry matter) had a protective effect on the cytokine system of the irradiated organism, regulating the activity of T-helpers and macrophages, which leads to an increase in gene expression and biosynthesis of immunoregulatory cytokines. As can be seen from
Figure 12, during the period of maximum inhibition of the synthesis of immuno- and hemoregulatory cytokines (8 days after irradiation) in only irradiated animals, the use of the tested bioradioprotector had an immunomodulating effect on the T-cell and macrophage systems of immunity, providing the synthesis of key cytokines of immunohemopoiesis: IL -1, TNF-α and INF-γ, the concentration of which during the height of ARS (maximum inhibition of cytokine synthesis) did not differ significantly from that in control (only irradiated) animals.
Thus, the test agent for emergency (preventive) immunotherapy of ARS has the ability to regulate the immunomodulatory function of the T-cell and macrophage systems of immunohemopoiesis through the cytokine system.
Discussion
In conditions of a sharp deterioration of the ecological situation in a number of countries due to the constant influence on animals of chemical (xenobiotics), physical (ionizing radiation) and biological (viruses, microbes, parasites) factors, both in isolated and combined versions, the body is impaired immunoregulatory processes leading to the growth of infectious, allergic, autoimmune diseases, thus causing the development of an immunotoxic effect (Veraldi et al, 2008; Yadav et al., 2022; Segner et al., 2021).
At the same time, of the resolving factors for the development of these pathologies, the most important are various microorganisms (bacteria, viruses, mycoplasmas, fungi, etc.), which, along with classical ones, induce the appearance of little-studied diseases, as well as the development of gastrointestinal and respiratory diseases of young animals. , in which the body is dominated by E. coli, hemolytic streptococci, salmonella, pseudomonas (Carpena et al., 2021).
Of particular concern is the ecological disadvantage of the habitat of a number of regions of the country, in which feed, water, soil, air are polluted with heavy metals, pesticides and toxic chemicals, and household waste that are hazardous to human and animal health (Alengebawy 2021), as well as dioxins and mycotoxins (Wang et al., 2020; van den Brand 2022), which are immunosuppressants, allergens, contributing to the development of immune deficiency states.
Despite the extreme diversity and differences of agents of chemical, physical and biological nature, they are united by one fundamental property - immunotoxicity, which is realized through the pituitary-adrenal system, which automatically includes a highly specific defense system of immunity from specific pathogenic agents by synthesizing anti-infectious, antitoxic and antiradiotoxic antibodies (Silverman et al., 2005; Galbiati et al., 2021).
The above data allow us to state unequivocally that when the body is exposed to factors of biological (pathogenic agents), physical (ionizing radiation) and chemical (xenobiotics) nature, significant changes occur in the immune system in the form of an immunotoxic effect, which is the basis for the development of immunotherapy in isolated and the combined action of these factors on the body (Blake et al., 2021; Fessas et al., 2020).
Taking into account the mechanisms of immunotoxic action, polyfactorial pathological agents, extraimmune and immunotherapy in conditions of environmental disadvantage should be aimed at reducing the antigenic load on the body, neutralizing toxins, antigens, allergens and their elimination, carrying out specific (desensitizing) immunotherapy, normalizing the vitamin balance by using phytogenic substances. (herbal infusions, apiproducts), zoogenic (γ-globulins, histoglobulins, organ and tissue eluates) and microbial (toxoid, lacto-, bifidumbacterin, etc.) origin (Singh et al., 2015; Delia et al., 2007; Münstedt, Männle 2019; Zhang et al., 2021).
However, the use of a wide range of the above funds for the purpose of extraimmune therapy is not systematized, the existing recommendations for their use suggest long-term, course use of components of different composition without a specific dosage and sequence of their use, which creates certain difficulties and problems in assessing their effectiveness.
Therefore, in our opinion, it is promising and rational to create a composite monopreparation that combines the proposed components, thereby helping to unify both the composition of the drug and its therapeutic properties.
Taking into account the above, we have designed a polyfunctional preparation based on substances of zoogenic (polyglobulins), apisogenic («Vita-Force»), microbial (products of metabolism of Bifidobacterium bifidumand B. subtilts) and mineral (bentonite) origin (Patents RU No. 2169572, 2001 and No. 2324361, 2008).
When designing a unified drug with anti-radiation activity, we were guided, first of all, by the conceptual position of traditional medicine and radiation pharmacology that multicomponent mixtures of rough extracts, phytozoan extracts have universal properties, while simultaneously exerting a beneficial effect on the immune, hematopoietic, endocrine systems, removing the stress response to pathological agents and increasing the overall resistance of the body in various pathologies caused by agents of physical, chemical and biological nature (Khalil MJ, 2006; Miguel JA, et al., 2010; Olaitan PB et al., 2007; Paulino N. et al., 2008). The main condition for the design of multicomponent multifunctional drugs is, first of all, the correct selection of their compositional ratios and the exclusion of their antigonistic relationships with each other. At the same time, a wide spectrum of the bioprotective effect of multicomponent mixtures of substances of animal, plant and microbial origin is associated with the influence of not only one, but also many components of biologically active substances, which are often in complementary or reinforcing interactions, balanced by the nature of biogenic compounds.
The strategy of developing means of extraimmune and immune therapy in conditions of exposure to the body of environmental factors of anthropogenic nature (xenobiotics, ionizing radiation, pathogenic bioagents: viruses, bacteria, parasites) involves the use of immunotropic drugs (serum, globulins, tissue preparations - histoglobulins, vitamins and microelements), adaptogens (products of animal, plant and microbial origin, bee products), adjuvants and minerals (Khalid et al., 2022; Basak, Gokhale, 2022; Mazziotta et al., 2023, Nizamov R.N., et al., 2021.).
The results of preliminary experiments to study the effect of the experimental sample of the multicomponent composite preparation «Polyapisogen» on the organism of intact (healthy) animals showed that it is low-toxic (IV hazard class), does not possess cumulative, allergenic, teratogenic, embryotoxic properties and irritating effect on the skin and mucous membranes the lining of the stomach. It has a beneficial effect on the offspring, increases the physiological and immunohemopoietic status, has a stress-protective (adaptogenic) and antioxidant effect, which together can provide effective protection of the body under conditions of exposure to environmental agents of physical, chemical and biological nature.
The results obtained served as the basis for testing the developed composite preparation as an extra immunotherapeutic agent on animals affected by ionizing radiation.
In the first series of experiments on the study of the immunotherapeutic activity of the test agent under radiogenic stress, we used 108 white mice subjected to lethal γ-ray irradiation followed by the introduction of a potential radioprotective drug. The results of the studies showed that the use of the composite preparation «Polyapisogen» in 6 variants of the experiment had a pronounced radioprotective effect, ensuring the survival of lethally irradiated animals in 40 (variant VI), 55 (variant V), 65 (variant IV), 60 (variant III), 75 (option I) and 80% (option II) of cases with radiation death of all irradiated animals in the control (option VIII) group. At the same time, both oral and parenteral (subcutaneous) application of powder and liquid (oral and injection) forms of an experimental sample of the developed multicomponent composite preparation were tested.
The increase in the survival rate of lethally irradiated animals against the background of the use of the drug «Polyapisogen» was due to inhibition of the formation in the irradiated organism of radio-induced products of oxidative modification of macromolecules (OMM) with simultaneous stimulation (or preservation of the activity of the antiradical enzyme, superoxide dismutase (SOD), which is confirmed by the data of other researchers (Martinello M., Mutinelli F., 2021; Fischer N., Seo E.J., 2018). Antiradical activity of the drug «Polyapisogen» is due to the presence of preparation of natural bioantioxidants: phenols, tocopherols, carotenoid, vitamins C and E, which, acting synergistically, inhibit the formation of free radicals, inhibit them, destroy peroxides (dismutation), bind catalysts - metal ions of variable valence, by quenching (quenching) inactivate singlet oxygen (Fischer, Seo, 2018; Adnan, Rasul et al., 2022; Zhang et al., 2023).
Considering that the main target of peroxyradical attack in radiogenic stress is the hematopoietic system, in parallel, the state of the hematopoiesis system was studied in stressed white mice against the background of the use of the drug «Polyapisogen».
The results of studying the state of the hematopoiesis system in the irradiated and those who received the composite preparation «Polyapisogen» showed that radiogenic stress has a hemotoxic effect on the body, accompanied by inhibition of hematopoiesis with the suppression of all the main sprouts of bone marrow hematopoiesis: erythroid, neutrophilic, lymphoid sprouts. These changes in the bone marrow were accompanied by a reciprocal decrease in the content of hemoglobin (by 1.05 times), erythrocytes (by 1.01 times), and neutrophils (by 1.89 times, P <0.05) in the peripheral blood.
A single subcutaneous injection of the drug «Polyapisogen» both before and after irradiation had a hemo- and myeloprotective effect, preventing a decrease in the number of cells of both erythroid and lymphoid and neutrophilic sprouts of bone marrow cells, as well as inhibiting the pancytopenic process in the peripheral blood, maintaining the quantitative content of erythrocytes, lymphocytes and neutrophils at the level of control, non-irradiated animals. Hemo- and myeloprotective action of the drug «Polyapisogen» against the background of radiation damage is due to the content of substances of microbial origin (B. bifidum, B. subtilis) in the drug, which, being immunotropic agents, cause the activation of macrophages. Activated macrophages synthesize a complex of key humoral factors that have a regulatory effect on hematopoiesis: interleukin-1, tumor necrotic factor - TNF-α, granulation macrophage colony-stimulating factors, prostaglandin E, α-, β-, γ-interferons (Mazziotta et al., 2023; Azad et al., 2018).
Considering that the hemoprotective effect of immunotherapeutic drugs is realized through the immunity system, namely, through humoral (immunoglobulin) and cellular components of immunity (T-, B-lymphocytes, macrophages), we carried out parallel studies to study the factors of cellular and humoral immunity in exposed and animals treated (prophylactic) with the study drug.
The results of the studies showed that already from the 3rd day after irradiation, a significant decrease in the number of B-lymphocytes and T-helpers followed, while the number of T-suppressors increased. The maximum decrease in the number of B-lymphocytes and T-helpers by 1.8 times and 1.71 times and the increase in T-suppressors by 1.64 times occurred on days 7-21 after irradiation.
The use of the composite drug «Polyapisogen» had a modifying effect on the course and outcome of ARS - it was characterized by a more favorable course of the disease against the background of inhibition of the immunohemotoxic effect, preventing the inhibition of hematopoiesis, the immune system and disorders of the immunoregulatory (subpopulation) index (Th / Ts), thereby contributing to the preservation of the functioning of the T- and B-cell immune system (Dainiak, 2018; López, Martín, 2011).
Radiogenic stress caused a sharp suppression of the blast-transforming activity of lymphocytes - as early as 3 days after irradiation, RBTL-activity of lymphocytes decreased by more than 2 times. The indicated tendency persisted throughout the entire experiment, and by the time of death of the animals, this indicator was 4.41 times lower than the initial level.
A single subcutaneous injection of the tested composite preparation had a significant effect on the functional activity of immunocytes - the number of pyroninophilic cells in animals of the treated group did not have significant differences with control (non-irradiated) animals. Activation of immunity in animals affected by ionizing radiation against the background of the use of an immunotropic drug - «Polyapisogen», is due to the presence of immunoglobulins in the drug, which take an active part in stimulating the processes of restoring hematopoiesis and immunogenesis, stimulating nonspecific anti-infectious resistance, binding tissue decay products (detoxification and complement desensitizing ability and stimulate the proliferation of B-lymphocytes, thereby exerting a homeostatic effect on the irradiated organism (Akita, 2014; Reeves, 2014; C Jagetia, 2007).
According to the data in Fig. 14, it follows that under the influence of ionizing radiation, significant changes followed in the cytokine system of the body, accompanied by inhibition of the synthesis of synergistically acting mediators of immunohemopoiesis, such as TNF, interleukin-1 (IL-1) and γ-interferon (INF-γ).
The inhibition of the content of these cytokines followed one day after the radiation exposure and reached a maximum by the peak of ARS (by 8 days), when the concentration of IL-1 in the blood decreased by 3.10 times, in the bone marrow - by 8.94 times, TNF-α in the blood serum decreased 3.46 times, in the bone marrow - 6.11 times, INF-γ in the supernatants of lymphocytes - 2.91 times. A single subcutaneous injection of the test drug had a protective effect on the cytokine system of the irradiated organism, regulating the activity of T-helpers and macrophages, which leads to an increase in gene expression and biosynthesis of immunoregulatory cytokines (Schaue et al., 2012; Lierova et al. 2018).
Thus, the test agent for emergency (preventive) immunotherapy, ARS, has the ability to correct the immunomodulatory function of the T-cell and macrophage systems of immunohemopoiesis, which is carried out through the cytokine system.
Thus, the results of the studies showed that the composite preparation «Polyapisogen» obtained according to the technology developed by us, based on polyglobulins, beekeeping products, metabolic products of Bifidobacterium bifidum and Bacillus subtilis and natural minerals, has polyfunctional properties, providing protection of the body in case of radiation damage. A single subcutaneous injection of the drug at a dose of 25 mg / kg had a radioprotective effect, providing 70-75% protection of lethally irradiated animals. An increase in the survival rate of animals affected by γ-rays against the background of the use of the developed drug was accompanied by a modification of the course and outcome of acute radiation sickness (ARS) by neutralizing and eliminating radio-induced toxic products, autoantigens, radioantigens, microbial agents, allergens and toxins, radiosensitizers, and myeloprotective, immunoprotective and immunoregulatory (correction of the immunoregulatory index - the ratio of T-helpers and T-suppressors of CD4 / CD8) action.
The conducted research can serve as a basis for the creation of multifunctional (radioprotective, chemoprotective and bioprotective) agents with their isolated and combined effects on the body.