1. Introduction
Hund’s rule of maximum multiplicity is a powerful empirical tool for determining the electron population of electronic shells. It was recently reported [
1] that the real driving force behind Hund’s rule appears to be the second law of infodynamics [
2]
where
in this time derivative is the information entropy proportional to Shannon entropy
of a discrete random variable that attains maximum
if the events are equiprobable (i.e., if the probabilities
) and vanishes for certain and impossible events
1 In other words, electron populations within an orbital minimize Shannon entropy (
2).
It is now generally accepted [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13] that information in the universe evolves, decreasing the information entropy
. Assuming that the total entropy of the universe
S is constant and is the sum of the information entropy and the physical entropy
, we obtain [
1]
This study extends the findings of [
1] to the Aufbau rule and discusses them from the perspective of emergent dimensionality [
7,
8,
9,
10,
11,
12,
13,
14].
2. Hund’s rule and Shannon entropy
A simple but inventive procedure for calculating Shannon entropies of electron populations was disclosed in [
1]. Any set of
N electrons satisfies
, where
and
denote, respectively, the number of up- and down-spin electrons. Thus, the probabilities of finding ↑ and ↓ electrons within the set of
N electrons are
and
.
Definition 1 (Electron set entropy).
An electron set entropy is
This definition was disclosed in [
1] but is given here for clarity. Electrons are fermions, so electron sets populating chemical elements’ orbitals must satisfy the Pauli exclusion principle. For the s orbital, for example, which can accommodate a maximum of
electrons, the corresponding probabilities are
if the orbital accommodates one electron and
if the orbital accommodates two electrons. Thus, possible electron set entropies (
4) are
.
We do not assume any particular logarithm base
b, noting that nature uses natural logarithm in Landauer’s principle [
15] and Boltzmann/Gibbs physical entropy
. Furthermore, the author of [
1] assumes that the electron set entropies of states s
1 and s
2 are the same, but this assumption is unjustified, as we shall discuss later.
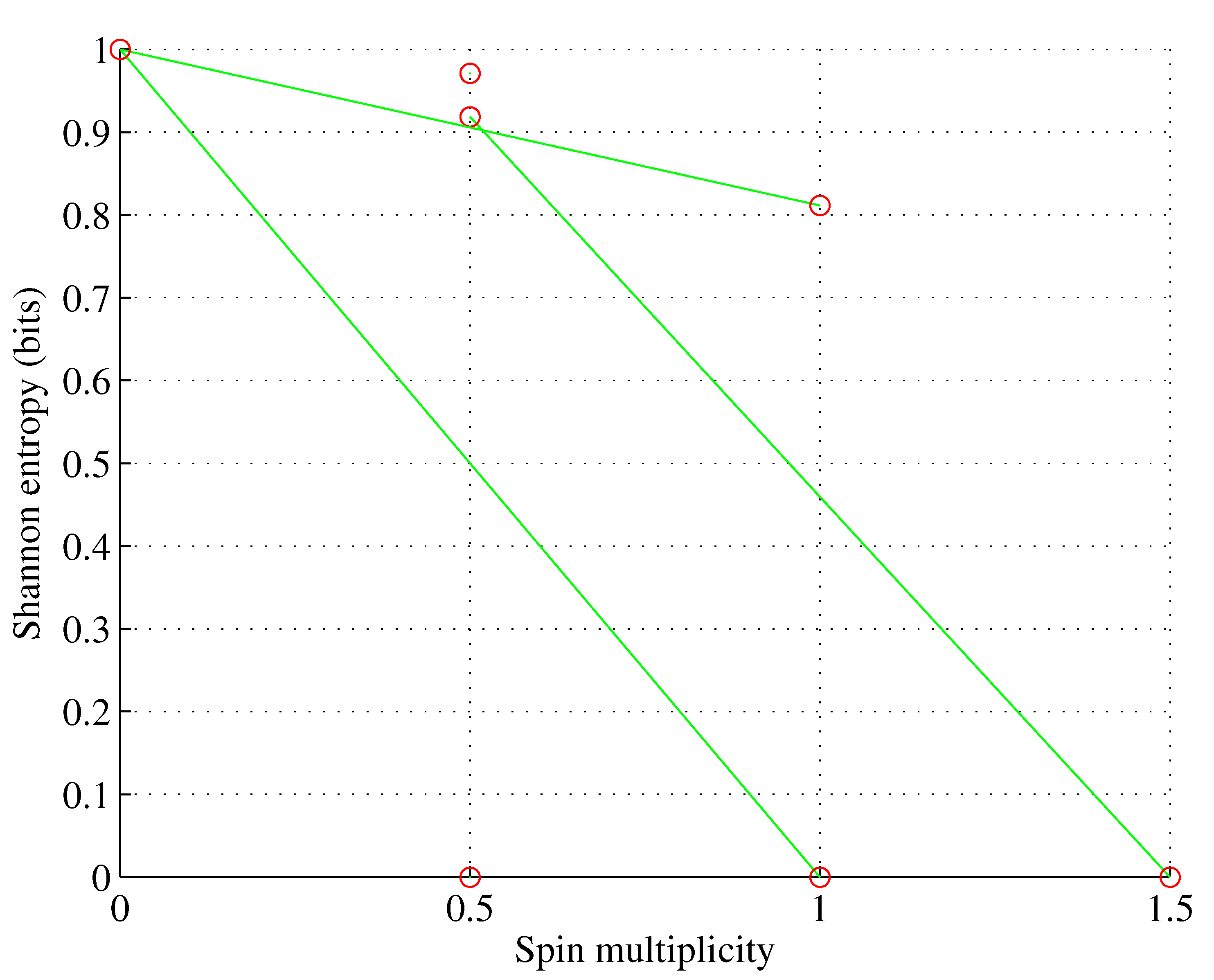
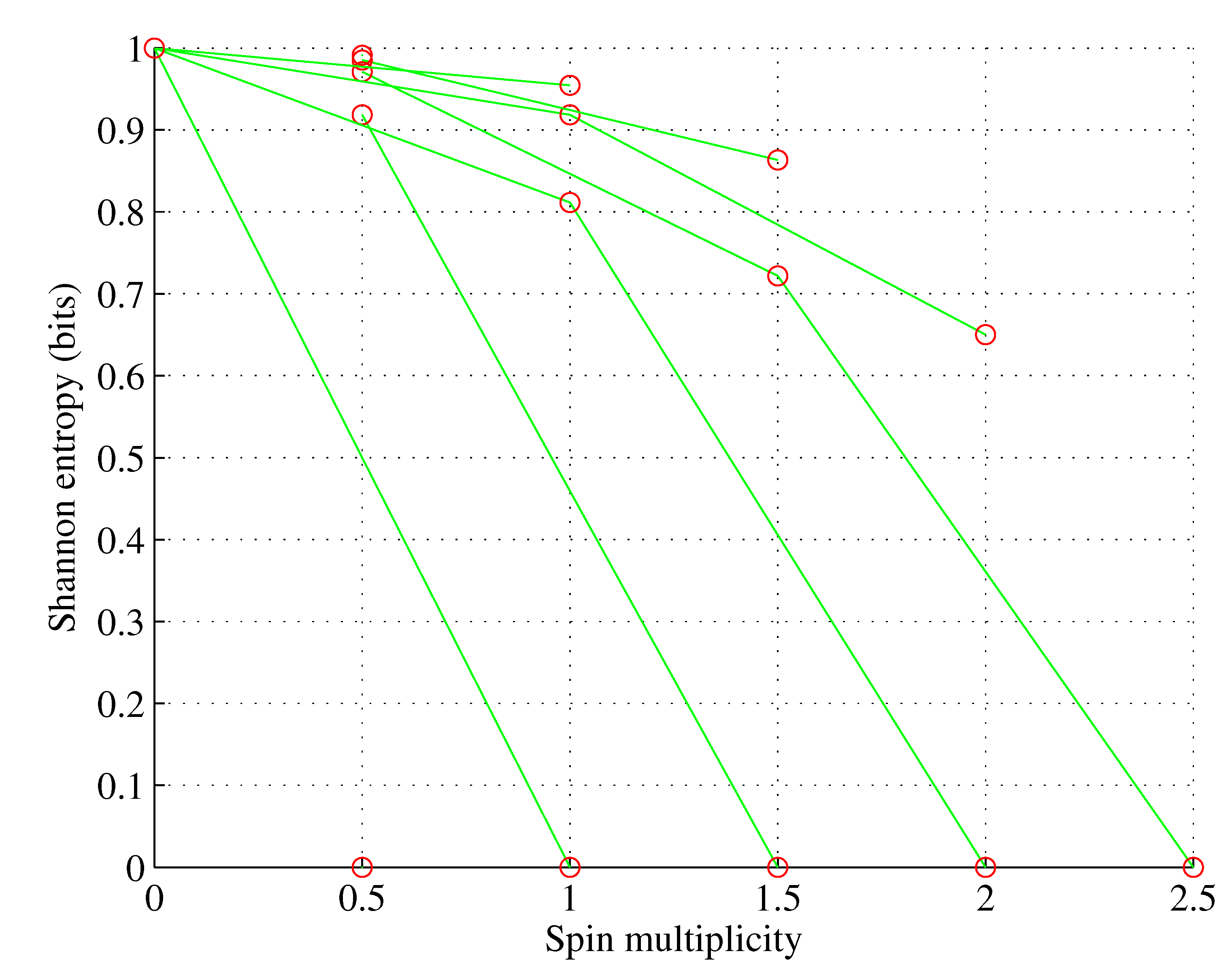
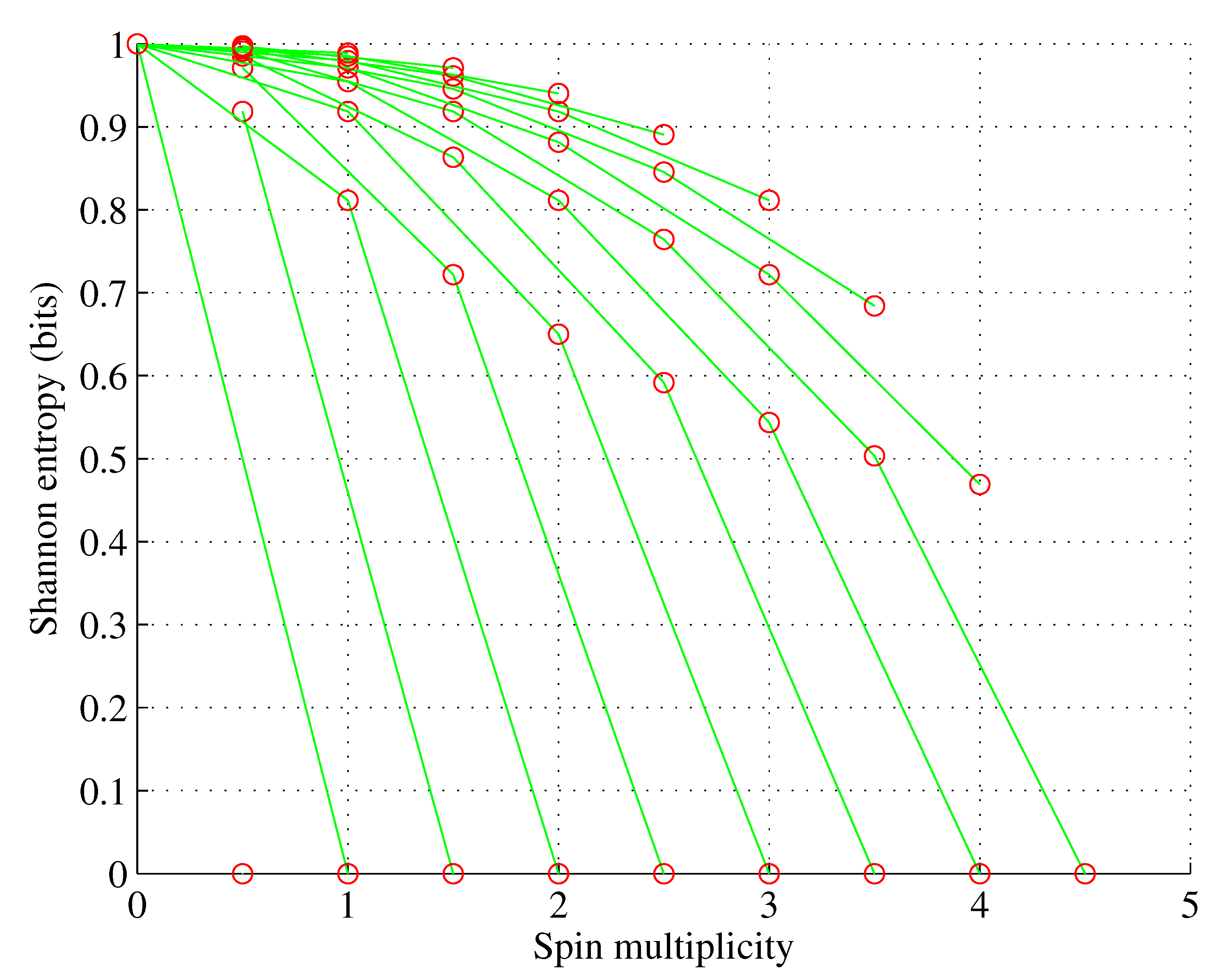
The situation becomes more diverse for orbitals with a larger angular momentum quantum number
ℓ as multiple sets with different electron set entropies and spin multiplicities are possible, as shown in
Figure 1,
Figure 2,
Figure 3 and
Figure 4 for orbitals p-g.
However:
for , ;
for , only one electron set is possible; and
for , ;
wherein nature selects this electron set among the ones allowed by the Pauli exclusion principle that maximizes spin multiplicity. However, as shown in [
1], for
, nature also selects this electron population among the allowed ones, which minimizes the orbital Shannon entropy. This rule of populating the sublevels can be stated in the simple theorem illustrated in
Figure 5.
Theorem 1 (Orbital entropy).
For any orbital capable of storing , electrons and storing N electrons and populated to maximize spin multiplicity (i.e. according to Hund’s rule), the orbital Shannon entropy vanishes iff and amounts
otherwise.
Proof. According to Hund’s rule, for the spin multiplicity is equal to , while for is equal to . For electrons can freely populate available sublevels to maximize the spin multiplicity and therefore (the same for ↓). For the electrons will begin to repopulate the available sublevels following the Pauli exclusion principle up to , where , which completes the proof. □
Some researchers postulate that certain elements are exceptions to Hund’s rule. Chromium, for example, having the atomic number is between vanadium (, electron configuration [Ar]3d34s2) and manganese (, [Ar]3d54s2) within the periodic table of elements. Thus, it should have electron configuration [Ar]3d44s2, following Hund’s rule, but instead, it has [Ar]3d54s1, as one electron from 4s moves to 3d to make it more stable. But it is not Hund’s rule that is violated. It is the Aufbau rule. Hund’s rule governs the electron population of a solitary orbital only.
3. Aufbau rule and Shannon entropy
The Aufbau or Madelung energy ordering rule is another powerful empirical tool for predicting the electron configurations of chemical elements corresponding to the ground state. It correctly predicts the electron configurations of most of the elements. However, about twenty chemical elements violate the Aufbau rule, leading to intriguing exceptions and anomalies. Chromium and copper violations are attributed to a delicate balance of electron-electron repulsion and the energy gap between the 3d and 4s orbitals [
16]. Palladium, often called a "double anomaly" [
17], exhibits a rare electron configuration, [Kr]4d
105, where all ten electrons fill the 4d orbital. This exceptional behavior is attributed to the compactness of 3d orbitals and complex electron interactions [
17]. In addition, there are no chemical elements that have orbital f
8 in their electron configurations, although the Aufbau rule predicts f8 for gadolinium (
) as [Xe]+6s
2+4f
8 and for curium (
) as [Rn]+7s
2+5f
8. Furthermore, there are only two nonsingleton sets of consecutive elements violating the Aufbau rule (Nickel has a disputed configuration). We note that for
, actual ground states are predicted. Thus, perhaps other elements, such as darmstadtium (
) and roentgenium (
) also violate the Aufbau rule.
The elements that violate the Aufbau rule are listed in
Table 1, showing their actual electron configurations, and the configurations predicted by the Aufbau rule. Furthermore,
Table 1 shows the spin multiplicities of the elements, i.e.,
.
if
Z is even and
otherwise. Furthermore, all but one of the violating elements satisfy a higher or equal multiplicity rule; i.e., actual spin multiplicity is mostly greater or equal to the spin multiplicity predicted using the Aufbau and Hund rules. The only exception is palladium (
).
As the entropies of independent systems are additive quantities, we can calculate the Shannon entropies of chemical elements by summing the orbital entropies of electron configurations using the relation (
5).
Definition 2. An element’s ground-state Shannon entropy is the sum of the orbital entropies in the electron configuration of this element, neglecting the principal quantum number.
For example, the electron configuration of oxygen is [He]2s
22p
4→ s
2s
2p
4, where
and
is given by the relation (
5). Thus, the Shannon entropy for oxygen is equal to
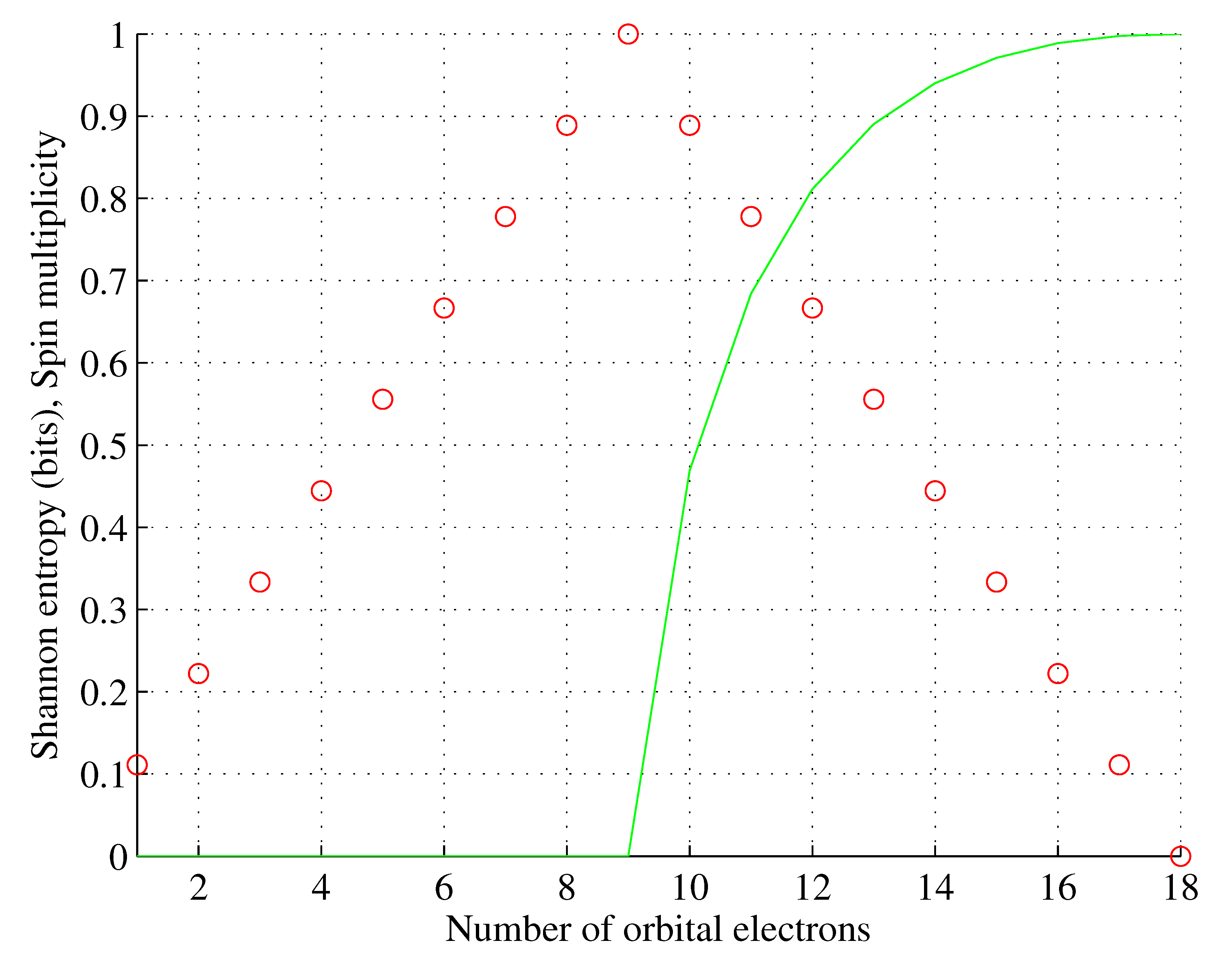
Similarly, for chromium , and so on.
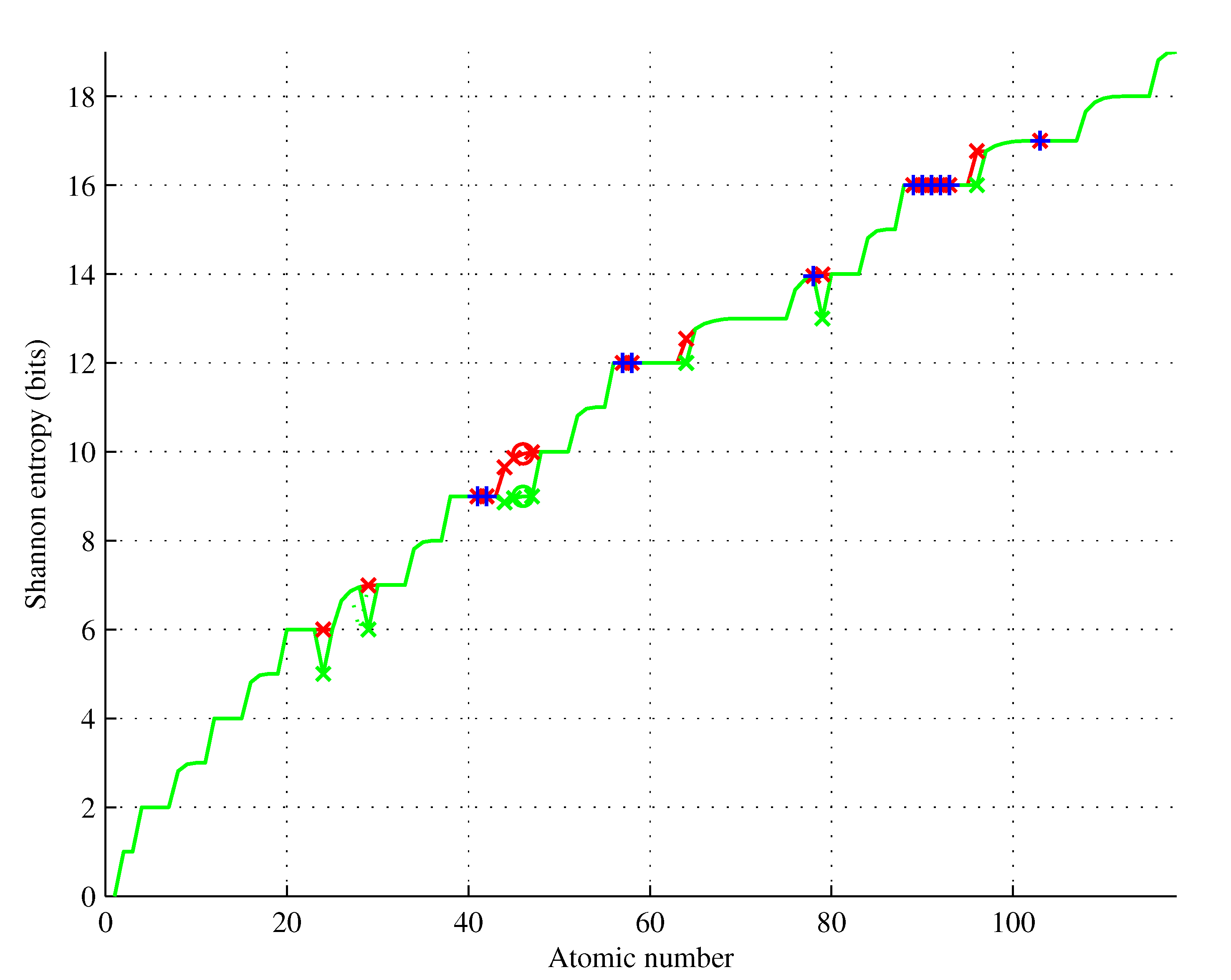
Shannon entropies
and
for
are shown in
Figure 6 based on actual ground state configurations and configurations obtained using the Aufbau rule.
4. Conclusions
We observed that the following holds for the elements violating the Aufbau rule:
for less than half () of them, the element’s entropy is lower for the actual and Aufbau configurations, the remaining ones have the same entropies in actual and Aufbau configurations;
the first nonsingleton set contains four elements having lower entropies and the same spin multiplicities, with the exception of , the only element that violates the higher or equal multiplicity rule;
the second nonsingleton set contains five elements having the same entropies and spin multiplicities.
Overall, these results significantly strengthen the meaning of the Aufbau rule.
We finally note that the Shannon entropy
of hydrogen is zero, not
. In emergent dimensionality [
7,
8,
9,
10,
11,
12,
13,
14], neither one nor two electrons
exist. Therefore, s
1 is populated before s
2 not because one electron is
less than two electrons, but because the Shannon entropy for one electron
is lower than the Shannon entropy
for two electrons.
Acknowledgments
I truly thank my wife Magdalena Bartocha for her unwavering support and motivation. I thank my partner and friend, Renata Sobajda, for her prayers. I truly thank my godson, Wawrzyniec Bieniawski, for the state-of-the-art input.
References
- Vopson, M.M. The second law of infodynamics and its implications for the simulated universe hypothesis. AIP Advances 2023, 13, 105308. [Google Scholar] [CrossRef]
- Vopson, M.M.; Lepadatu, S. Second law of information dynamics. AIP Advances 2022, 12, 075310. [Google Scholar] [CrossRef]
- de Chardin, P.T. The Phenomenon of Man; Harper: New York, 1959. [Google Scholar]
- I. Prigogine and I. Stengers, Order out of Chaos: Man’s New Dialogue with Nature. Bantam Books, 1984.
- R. Melamede, “Dissipative structures and the origins of life,” in Unifying Themes in Complex Systems IV (A. A. Minai and Y. Bar-Yam, eds.), (Berlin, Heidelberg), pp. 80–87, Springer Berlin Heidelberg, 2008.
- V. Vedral, Decoding Reality: The Universe as Quantum Information. Oxford University Press, 2010.
- S. Łukaszyk, “Four Cubes,” 2020.
- S. Łukaszyk, Black Hole Horizons as Patternless Binary Messages and Markers of Dimensionality, ch. 15, pp. 317–374. Nova Science Publishers, 2023.
- S. Łukaszyk, “Life as the Explanation of the Measurement Problem,” tech. rep., Cornell University, 2018.
- Łukaszyk, S. A new concept of probability metric and its applications in approximation of scattered data sets. Computational Mechanics 2004, 33, 299–304. [Google Scholar]
- S. Łukaszyk, “Novel Recurrence Relations for Volumes and Surfaces of n-Balls, Regular n-Simplices, and n-Orthoplices in Real Dimensions,” Mathematics, vol. 10, no. 13, 2022.
- Łukaszyk, S.; Tomski, A. Omnidimensional Convex Polytopes. Symmetry 2023, 15. [Google Scholar]
- S. Łukaszyk, “The Imaginary Universe,” preprint, PHYSICAL SCIENCES, Mar. 2023.
- S. Lukaszyk, “A No-go Theorem for Superposed Actions (Making Schrödinger’s Cat Quantum Nonlocal),” in New Frontiers in Physical Science Research Vol. 3 (D. J. Purenovic, ed.), pp. 137–151, Book Publisher International (a part of SCIENCEDOMAIN International), Nov. 2022. arXiv:1801.08537 [quant-ph].
- Landauer, R. Irreversibility and Heat Generation in the Computing Process. IBM Journal of Research and Development 1961, 5, 183–191. [Google Scholar] [CrossRef]
- Eric Scerri, “The anomalous configuration of the chromium atom and related issues.”. 2012. Available online: http://ericscerri.blogspot.com/2012/07/anomalous-configuration-of-chromium.html (accessed on 16 October 2023).
- Eric Scerri, “The trouble with the aufbau principle.”. 2013. Available online: https://edu.rsc.org/feature/the-trouble-with-the-aufbau-principle/2000133.article (accessed on 16 October 2023).
| 1 |
In the latter case is not defined. It is, by convention, taken as 0. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).