Submitted:
08 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
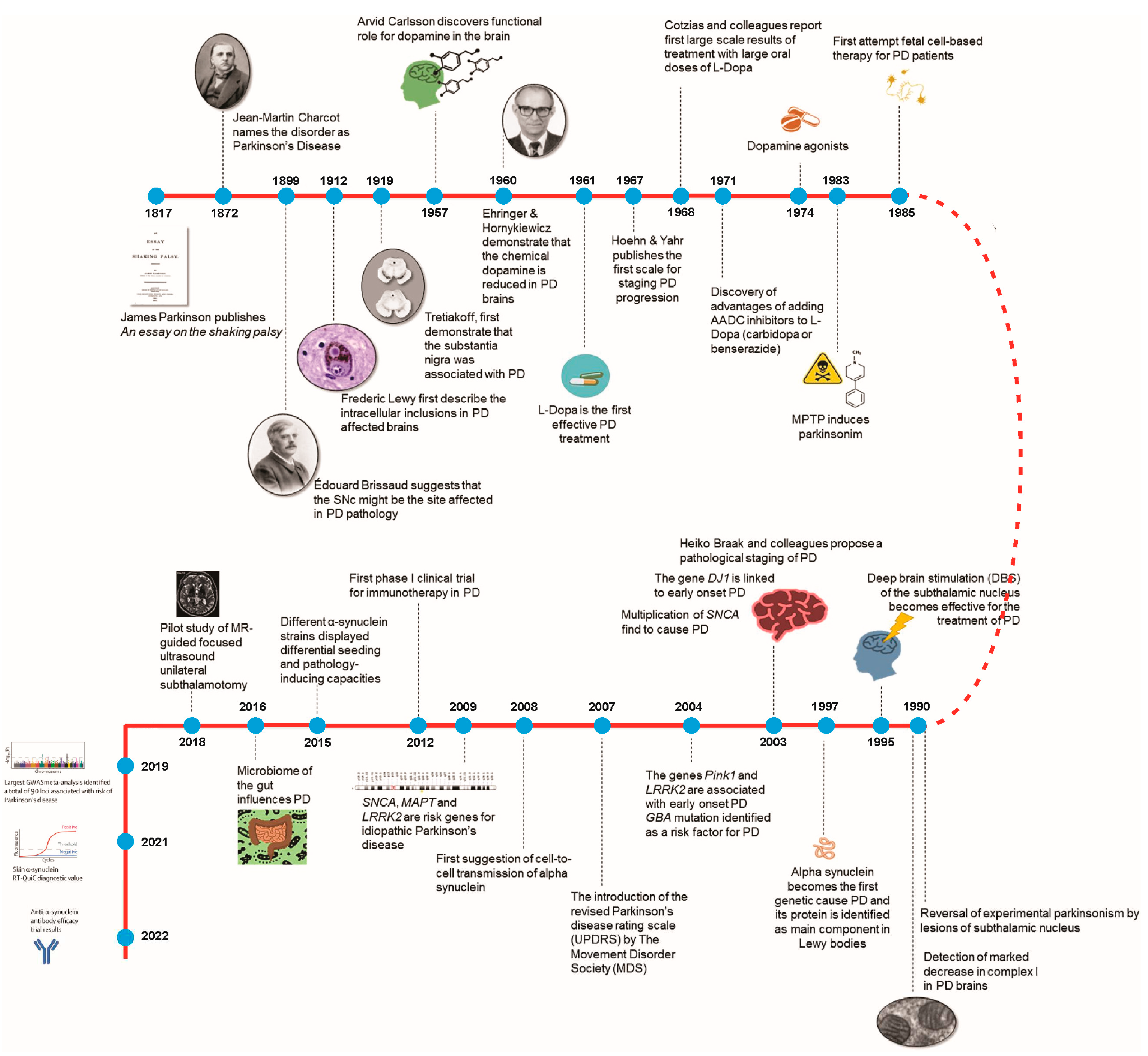
2. Two Centuries of Parkinson's Disease: Insights and Innovations
3. Genetic Mysteries of Parkinson's Disease
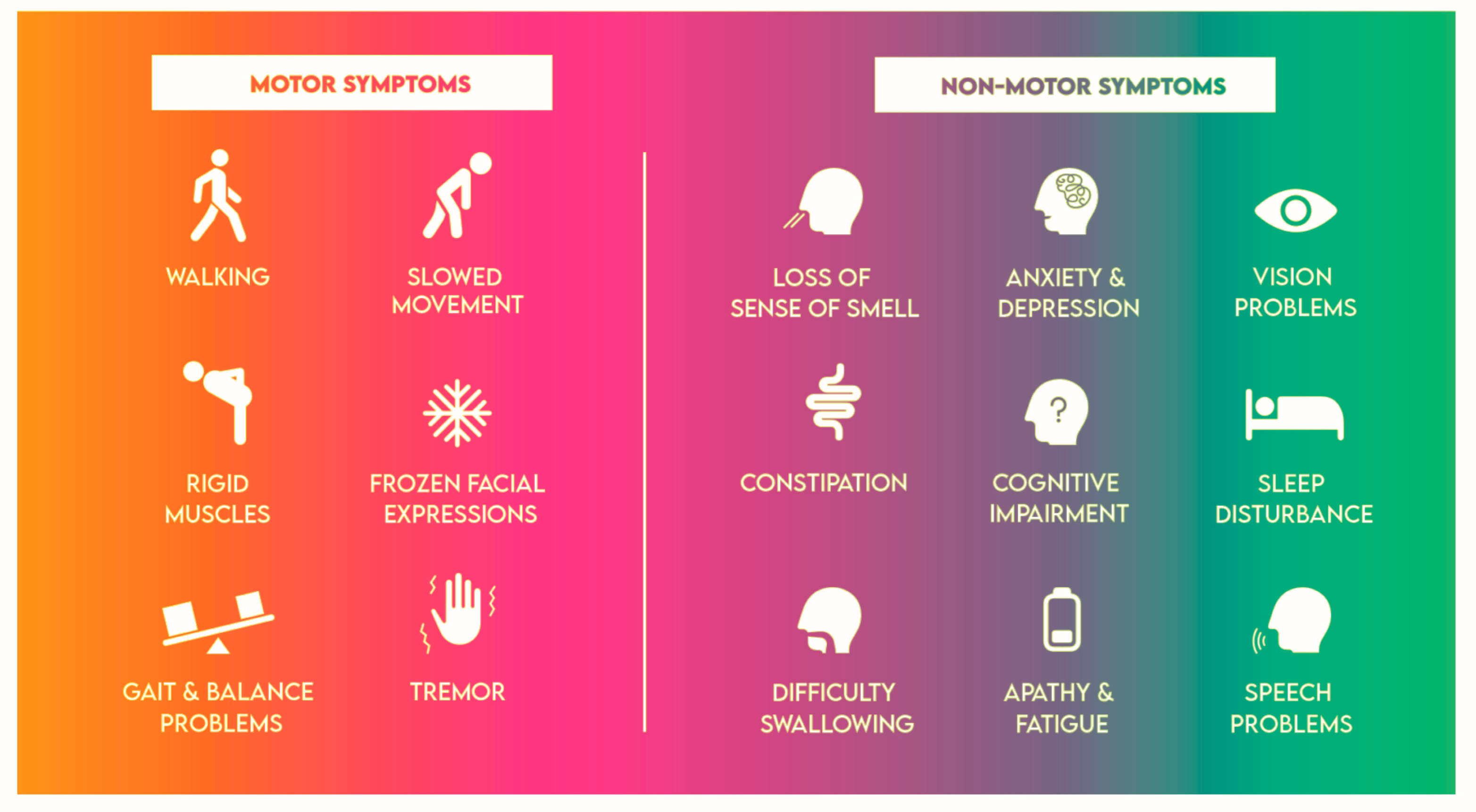
4. Multifaceted Challenges of Parkinson's Disease: Navigating Motor and Non-Motor Symptoms
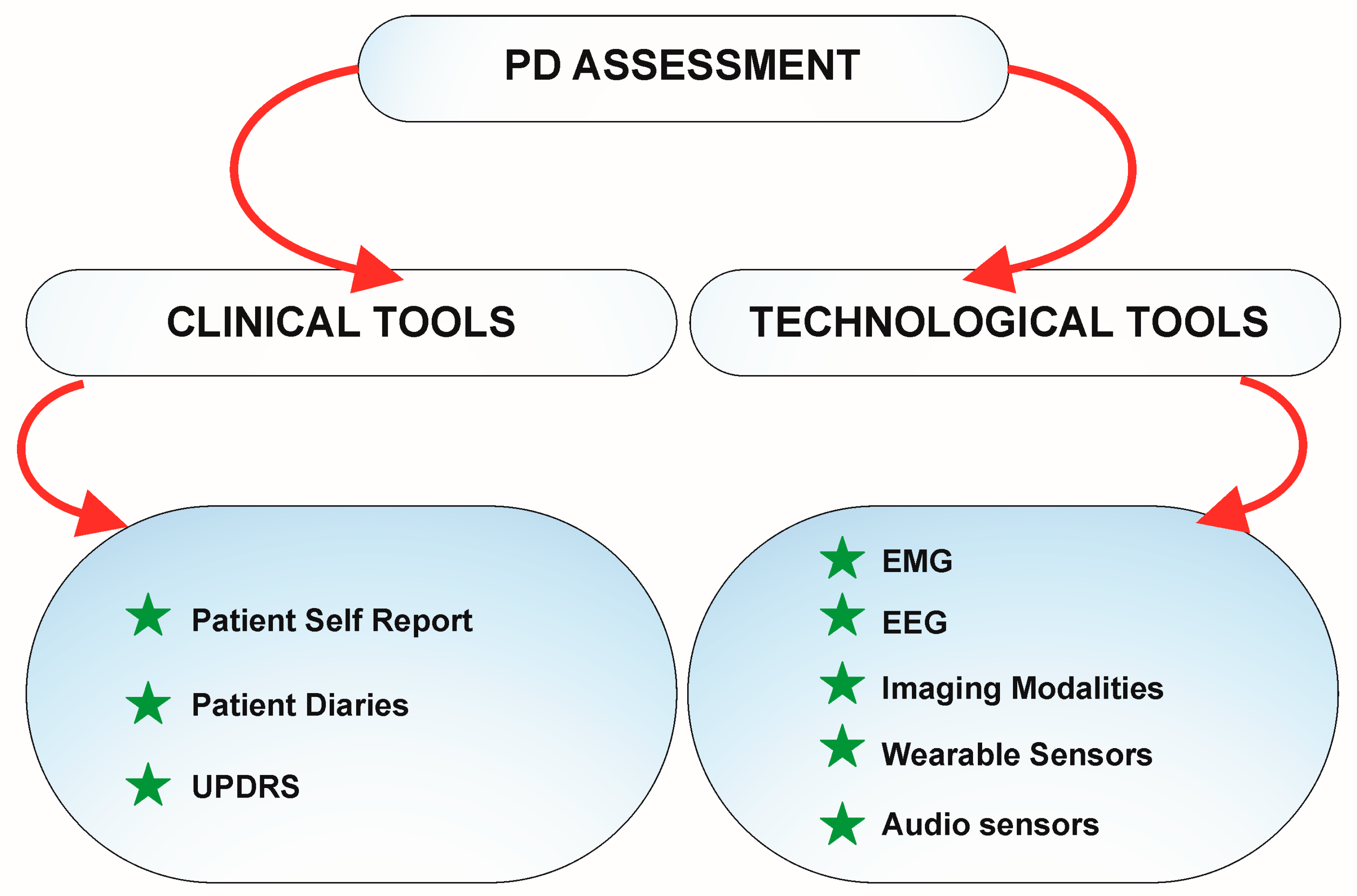
5. Advancing Parkinson's Care: Technology's Transformative Impact
6. Revolutionary Advancements: Cutting-Edge Technologies Transforming the Clinical Evaluation, and Therapies for Parkinson's Disease"
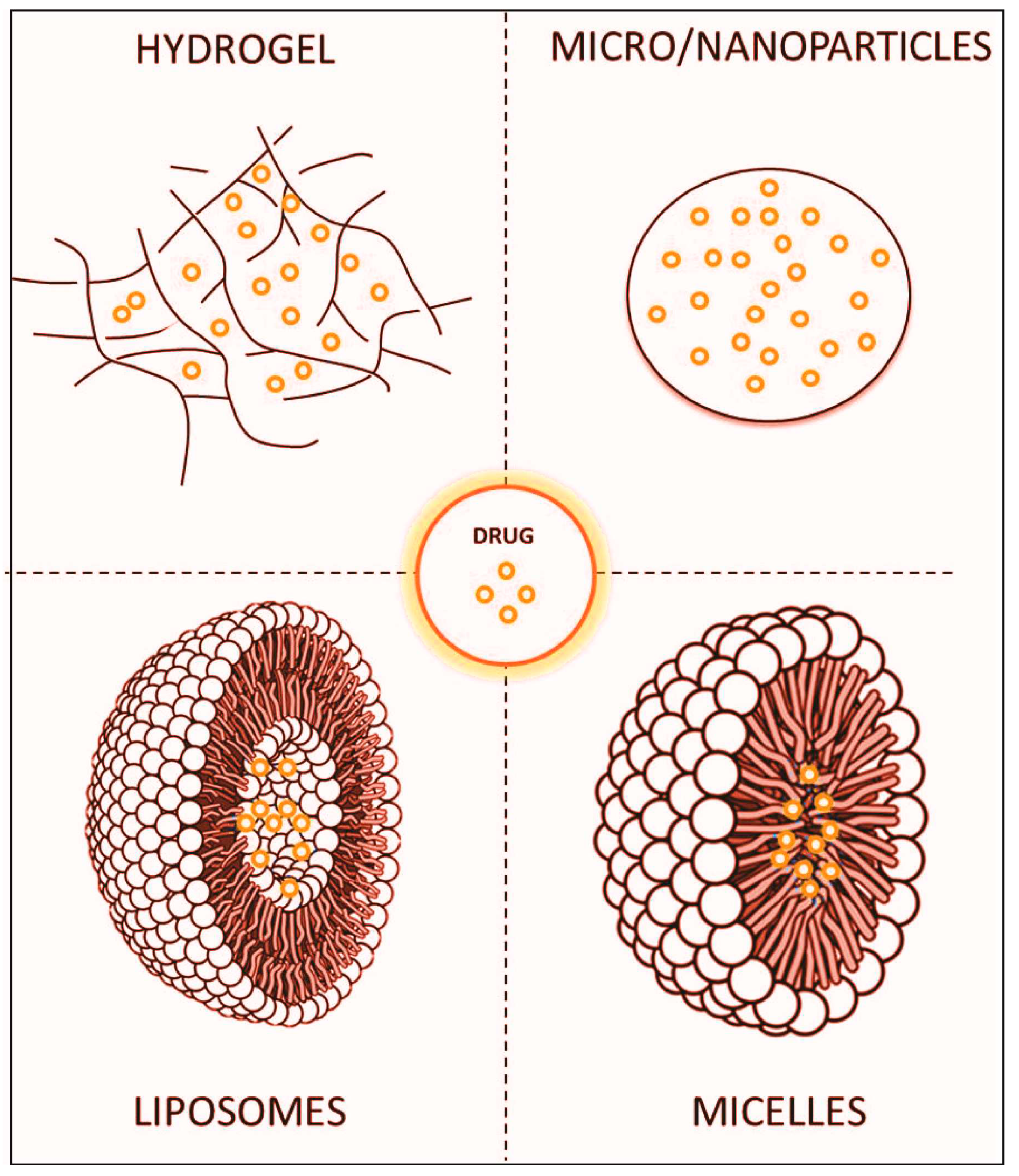
6.1. Innovative Approaches for Enhanced Parkinson's Disease Therapy: Targeted Drug Delivery Systems
6.2. Focused Ultrasound: Pioneering Non-Invasive Solutions for Neurological Disorders
6.3. Advancements in Deep Brain Stimulation (DBS) for Parkinson's Disease
7. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Del Rey, N.L.; Quiroga-Varela, A.; Garbayo, E.; Carballo-Carbajal, I.; Fernandez-Santiago, R.; Monje, M.H.G.; Trigo-Damas, I.; Blanco-Prieto, M.J.; Blesa, J. Advances in Parkinson's Disease: 200 Years Later. Front Neuroanat 2018, 12, 113. [Google Scholar] [CrossRef]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease - repurposed drugs and new approaches. Nat Rev Neurol 2019, 15, 204–223. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson's disease: risk factors and prevention. The Lancet Neurology 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Tangamornsuksan, W.; Lohitnavy, O.; Sruamsiri, R.; Chaiyakunapruk, N.; Norman Scholfield, C.; Reisfeld, B.; Lohitnavy, M. Paraquat exposure and Parkinson’s disease: A systematic review and meta-analysis. Archives of Environmental and Occupational Health 2019, 74, 225–238. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H.V. Non-motor symptoms of Parkinson's disease: Diagnosis and management. Lancet Neurology 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C. miR-124 and Parkinson's disease: A biomarker with therapeutic potential. Pharmacological Research 2019, 150. [Google Scholar] [CrossRef]
- Batistela, M.S.; Josviak, N.D.; Sulzbach, C.D.; de Souza, R.L.R. An overview of circulating cell-free microRNAs as putative biomarkers in Alzheimer's and Parkinson's Diseases. International Journal of Neuroscience 2017, 127, 547–558. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Berry, C.; Chang, E.T.; Sielken, R.L., Jr.; Mandel, J.S. Association between Parkinson's disease and cigarette smoking, rural living, well-water consumption, farming and pesticide use: Systematic review and meta-analysis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Paul, K.C.; Bronstein, J.M.; Bordelon, Y.; Horvath, S.; Ritz, B. Parkinson's disease is associated with DNA methylation levels in human blood and saliva. Genome Medicine 2017, 9. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson's disease. The Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Caggiu, E.; Paulus, K.; Mameli, G.; Arru, G.; Sechi, G.P.; Sechi, L.A. Differential expression of miRNA 155 and miRNA 146a in Parkinson's disease patients. eNeurologicalSci 2018, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Baghi, M.; Rostamian Delavar, M.; Yadegari, E.; Peymani, M.; Pozo, D.; Hossein Nasr-Esfahani, M.; Ghaedi, K. Modified level of miR-376a is associated with Parkinson's disease. Journal of Cellular and Molecular Medicine 2020, 24, 2622–2634. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nature Medicine 1998, 4, 1318–1320. [Google Scholar] [CrossRef]
- Borsche, M.; König, I.R.; Delcambre, S.; Petrucci, S.; Balck, A.; Bruggemann, N.; Zimprich, A.; Wasner, K.; Pereira, S.L.; Avenali, M.; et al. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 2020, 143, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.J.; Borges, A.F.; Souza, P.O.; De Souza, P.R.; De Barros Cardoso, C.R.; Dorta, M.L.; De Oliveira, M.A.P.; Teixeira, A.L.; Ribeiro-Dias, F. Decreased toll-like receptor 2 and toll-like receptor 7/8-induced cytokines in Parkinson's disease patients. NeuroImmunoModulation 2016, 23, 58–66. [Google Scholar] [CrossRef]
- Masliah, E.; Rockenstein, E.; Adame, A.; Alford, M.; Crews, L.; Hashimoto, M.; Seubert, P.; Lee, M.; Goldstein, J.; Chilcote, T.; et al. Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron 2005, 46, 857–868. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. New England Journal of Medicine 2009, 361, 1651–1661. [Google Scholar] [CrossRef]
- Abuelezz, N.Z.; Nasr, F.E.; Abdel Aal, W.M.; Molokhia, T.; Zaky, A. Sera miR-34a, miR-29b and miR-181c as potential novel diagnostic biomarker panel for Alzheimers in the Egyptian population. Experimental Gerontology 2022, 169. [Google Scholar] [CrossRef]
- Adamczak, S.; Dale, G.; De Rivero Vaccari, J.P.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: Clinical article. Journal of Neurosurgery 2012, 117, 1119–1125. [Google Scholar] [CrossRef]
- Henderson-Smith, A.; Fisch, K.M.; Hua, J.; Liu, G.; Ricciardelli, E.; Jepsen, K.; Huentelman, M.; Stalberg, G.; Edland, S.D.; Scherzer, C.R.; et al. DNA methylation changes associated with Parkinson’s disease progression: outcomes from the first longitudinal genome-wide methylation analysis in blood. Epigenetics 2019, 14, 365–382. [Google Scholar] [CrossRef]
- Fan, Y.; Howden, A.J.M.; Sarhan, A.R.; Lis, P.; Ito, G.; Martinez, T.N.; Brockmann, K.; Gasser, T.; Alessi, D.R.; Sammler, E.M. Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochemical Journal 2018, 475, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, R.; Elbaz, A.; Sanft, K.R.; Peterson, B.J.; Bower, J.H.; Ahlskog, J.E.; Grossardt, B.R.; De Andrade, M.; Maraganore, D.M.; Rocca, W.A. Education and occupations preceding Parkinson disease: A population-based case-control study. Neurology 2005, 65, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Racette, B.A.; Nielsen, S.S.; Criswell, S.R.; Sheppard, L.; Seixas, N.; Warden, M.N.; Checkoway, H. Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology 2017, 88, 344–351. [Google Scholar] [CrossRef]
- Goldman, J.E.; Yen, S.H.; Chiu, F.C.; Peress, N.S. Lewy bodies of Parkinson's disease contain neurofilament antigens. Science 1983, 221, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Doherty, K.M.; Silveira-Moriyama, L.; Parkkinen, L.; Healy, D.G.; Farrell, M.; Mencacci, N.E.; Ahmed, Z.; Brett, F.M.; Hardy, J.; Quinn, N.; et al. Parkin disease: A clinicopathologic entity? JAMA Neurology 2013, 70, 571–579. [Google Scholar] [CrossRef]
- Martin, I.; Kim, J.W.; Dawson, V.L.; Dawson, T.M. LRRK2 pathobiology in Parkinson's disease. Journal of Neurochemistry 2014, 131, 554–565. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. α-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nature Medicine 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Torra, A.; Parent, A.; Cuadros, T.; Rodríguez-Galván, B.; Ruiz-Bronchal, E.; Ballabio, A.; Bortolozzi, A.; Vila, M.; Bové, J. Overexpression of TFEB Drives a Pleiotropic Neurotrophic Effect and Prevents Parkinson's Disease-Related Neurodegeneration. Molecular Therapy 2018, 26, 1552–1567. [Google Scholar] [CrossRef]
- Kenborg, L.; Funch Lassen, C.; Hansen, J.; Olsen, J.H. Parkinson's disease and other neurodegenerative disorders among welders: A Danish cohort study. Movement Disorders 2012, 27, 1283–1289. [Google Scholar] [CrossRef]
- Cao, F.; Liu, Z.; Sun, G. Diagnostic value of miR-193a-3p in Alzheimer's disease and miR-193a-3p attenuates amyloid-β induced neurotoxicity by targeting PTEN. Experimental Gerontology 2020, 130. [Google Scholar] [CrossRef]
- Andersen, J.V.; Schousboe, A.; Verkhratsky, A. Astrocyte energy and neurotransmitter metabolism in Alzheimer's disease: Integration of the glutamate/GABA-glutamine cycle. Progress in Neurobiology 2022, 217. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tang, Y.; Yu, M.; Wu, L.; Liu, F.; Ni, J.; Wang, Z.; Wang, J.; Fei, J.; Wang, W.; et al. Downregulation of blood serum microRNA 29 family in patients with Parkinson's disease. Scientific Reports 2017, 7. [Google Scholar] [CrossRef]
- Chinta, S.J.; Mallajosyula, J.K.; Rane, A.; Andersen, J.K. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neuroscience Letters 2010, 486, 235–239. [Google Scholar] [CrossRef]
- Guzman, J.N.; Sanchez-Padilla, J.; Wokosin, D.; Kondapalli, J.; Ilijic, E.; Schumacker, P.T.; Surmeier, D.J. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 2010, 468, 696–700. [Google Scholar] [CrossRef]
- Ren, L.; Yi, J.; Yang, J.; Li, P.; Cheng, X.; Mao, P. Nonsteroidal anti-inflammatory drugs use and risk of Parkinson disease: A dose–response meta-analysis. Medicine (United States) 2018, 97. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Kang, X.; Lin, B.; Yu, Q.; Song, B.; Gao, G.; Chen, Y.; Sun, X.; Li, X.; et al. One-Step Biallelic and Scarless Correction of a β-Thalassemia Mutation in Patient-Specific iPSCs without Drug Selection. Molecular Therapy - Nucleic Acids 2017, 6, 57–67. [Google Scholar] [CrossRef]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin Jr, W.G. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef]
- Goldman, S.M.; Marek, K.; Ottman, R.; Meng, C.; Comyns, K.; Chan, P.; Ma, J.; Marras, C.; Langston, J.W.; Ross, G.W.; et al. Concordance for Parkinson's disease in twins: A 20-year update. Annals of Neurology 2019, 85, 600–605. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Chen, J.F.; Ascherio, A. Caffeinated clues and the promise of adenosine A2a antagonists in PD. Neurology 2002, 58, 1154–1160. [Google Scholar] [CrossRef]
- Nalls, M.A.; Pankratz, N.; Lill, C.M.; Do, C.B.; Hernandez, D.G.; Saad, M.; Destefano, A.L.; Kara, E.; Bras, J.; Sharma, M.; et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nature Genetics 2014, 46, 989–993. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Lu, A.T.; Paul, K.C.; Folle, A.D.; Bronstein, J.M.; Bordelon, Y.; Horvath, S.; Ritz, B. Longitudinal Epigenome-Wide Methylation Study of Cognitive Decline and Motor Progression in Parkinson's Disease. Journal of Parkinson's Disease 2019, 9, 389–400. [Google Scholar] [CrossRef]
- Pagan, F.; Hebron, M.; Valadez, E.H.; Torres-Yaghi, Y.; Huang, X.; Mills, R.R.; Wilmarth, B.M.; Howard, H.; Dunn, C.; Carlson, A.; et al. Nilotinib effects in Parkinson's disease and dementia with lewy bodies. Journal of Parkinson's Disease 2016, 6, 503–517. [Google Scholar] [CrossRef]
- Blanz, J.; Saftig, P. Parkinson's disease: acid-glucocerebrosidase activity and alpha-synuclein clearance. Journal of Neurochemistry 2016, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Guhathakurta, S.; Evangelista, B.A.; Ghosh, S.; Basu, S.; Kim, Y.S. Hypomethylation of intron1 of α-synuclein gene does not correlate with Parkinson’s disease. Molecular Brain 2017, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jacobs, E.; Schwarzschild, M.A.; McCullough, M.L.; Calle, E.E.; Thun, M.J.; Ascherio, A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Annals of Neurology 2005, 58, 963–967. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Brahmachari, S.; Lee, Y.; Dawson, V.L.; Dawson, T.M.; Ko, H.S. The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson's disease. Scientific Reports 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Stubblefield, B.; Cookson, M.R.; Goldin, E.; Velayati, A.; Tayebi, N.; Sidransky, E. Aggregation of α-synuclein in brain samples from subjects with glucocerebrosidase mutations. Molecular Genetics and Metabolism 2011, 104, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Lindersson, E.; Beedholm, R.; Højrup, P.; Moos, T.; Gai, W.; Hendil, K.B.; Jensen, P.H. Proteasomal Inhibition by α-Synuclein Filaments and Oligomers. Journal of Biological Chemistry 2004, 279, 12924–12934. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Stafanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 2004, 305, 1292–1295. [Google Scholar] [CrossRef]
- Bressan, E.; Reed, X.; Bansal, V.; Hutchins, E.; Cobb, M.M.; Webb, M.G.; Alsop, E.; Grenn, F.P.; Illarionova, A.; Savytska, N.; et al. The foundational data initiative for Parkinsons disease (FOUNDIN-PD): enabling efficient translation from genetic maps to mechanism. bioRxiv 2021. [Google Scholar]
- Cai, M.; Liu, Z.; Li, W.; Wang, Y.; xie, A. Association between rs823128 polymorphism and the risk of Parkinson's disease: A meta-analysis. Neuroscience Letters 2018, 665, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Corces, M.R.; Shcherbina, A.; Kundu, S.; Gloudemans, M.J.; Frésard, L.; Granja, J.M.; Louie, B.H.; Eulalio, T.; Shams, S.; Bagdatli, S.T.; et al. Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer’s and Parkinson’s diseases. Nature Genetics 2020, 52, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.N.; Chew, E.G.Y.; Chung, S.J.; Peng, R.; Blauwendraat, C.; Nalls, M.A.; Mok, K.Y.; Satake, W.; Toda, T.; Chao, Y.; et al. Identification of Risk Loci for Parkinson Disease in Asians and Comparison of Risk between Asians and Europeans: A Genome-Wide Association Study. JAMA Neurology 2020, 77, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, D.; Cheng, Q.; Zhang, P.; Zhao, C.; Min, J.; Wang, F. Association of Levels of Physical Activity With Risk of Parkinson Disease: A Systematic Review and Meta-analysis. JAMA network open 2018, 1, e182421. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Di Fonzo, A.B. GBA, Gaucher disease, and parkinson’s disease: From genetic to clinic to new therapeutic approaches. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Bustos, B.I.; Billingsley, K.; Blauwendraat, C.; Raphael Gibbs, J.; Gan-Or, Z.; Krainc, D.; Singleton, A.B.; Lubbe, S.J. For the International Parkinson’s Disease Genomics Consortium (IPDGC). Genome-Wide Contribution of Common Short-Tandem Repeats to Parkinson’s Disease Genetic Risk. 0000.
- Desplats, P.; Spencer, B.; Coffee, E.; Patel, P.; Michael, S.; Patrick, C.; Adame, A.; Rockenstein, E.; Masliah, E. α-synuclein sequesters Dnmt1 from the nucleus: A novel mechanism for epigenetic alterations in Lewy body diseases. Journal of Biological Chemistry 2011, 286, 9031–9037. [Google Scholar] [CrossRef]
- Goldman, J.S. Predictive Genetic Counseling for Neurodegenerative Diseases: Past, Present, and Future. Cold Spring Harb. Perspect. Med 2019, 23, a036525. [Google Scholar] [CrossRef]
- Nativio, R.; Lan, Y.; Donahue, G.; Sidoli, S.; Berson, A.; Srinivasan, A.R.; Shcherbakova, O.; Amlie-Wolf, A.; Nie, J.; Cui, X.; et al. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nature Genetics 2020, 52, 1024–1035. [Google Scholar] [CrossRef]
- Tan, A.H.; Noyce, A.; Carrasco, A.M.; Brice, A.; Reimer, A.; Illarionova, A.; Singleton, A.; Schumacher-Schuh, A.; Stecher, B.; Siddiqi, B.; et al. GP2: The Global Parkinson's Genetics Program. Movement Disorders 2021, 36, 842–851. [Google Scholar] [CrossRef]
- Brockmann, K.; Srulijes, K.; Pflederer, S.; Hauser, A.K.; Schulte, C.; Maetzler, W.; Gasser, T.; Berg, D. GBA-associated Parkinson's disease: Reduced survival and more rapid progression in a prospective longitudinal study. Movement Disorders 2015, 30, 407–411. [Google Scholar] [CrossRef]
- Cilia, R.; Tunesi, S.; Marotta, G.; Cereda, E.; Siri, C.; Tesei, S.; Zecchinelli, A.L.; Canesi, M.; Mariani, C.B.; Meucci, N.; et al. Survival and dementia in GBA-associated Parkinson's disease: The mutation matters. Annals of Neurology 2016, 80, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.S.; LaMarca, M.E.; Scott, C.R.; Sidransky, E. Gaucher disease: Mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Human Mutation 2008, 29, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Lee, C.Y.; Menozzi, E.; Schapira, A.H.V. Genetic variations in GBA1 and LRRK2 genes: Biochemical and clinical consequences in Parkinson disease. Frontiers in Neurology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Samocha, K.E.; Robinson, E.B.; Sanders, S.J.; Stevens, C.; Sabo, A.; McGrath, L.M.; Kosmicki, J.A.; Rehnström, K.; Mallick, S.; Kirby, A.; et al. A framework for the interpretation of de novo mutation in human disease. Nature Genetics 2014, 46, 944–950. [Google Scholar] [CrossRef]
- Cornejo-Olivas, M.; Torres, L.; Velit-Salazar, M.R.; Inca-Martinez, M.; Mazzetti, P.; Cosentino, C.; Micheli, F.; Perandones, C.; Dieguez, E.; Raggio, V.; et al. Variable frequency of LRRK2 variants in the Latin American research consortium on the genetics of Parkinson's disease (LARGE-PD), a case of ancestry /692/617/375/1718 /631/208/1516 article. npj Parkinson's Disease 2017, 3. [Google Scholar] [CrossRef]
- Cresto, N.; Gardier, C.; Gubinelli, F.; Gaillard, M.C.; Liot, G.; West, A.B.; Brouillet, E. The unlikely partnership between LRRK2 and α-synuclein in Parkinson's disease. European Journal of Neuroscience 2019, 49, 339–363. [Google Scholar] [CrossRef]
- Esteves, A.R.; Swerdlow, R.H.; Cardoso, S.M. LRRK2, a puzzling protein: Insights into Parkinson's disease pathogenesis. Experimental Neurology 2014, 261, 206–216. [Google Scholar] [CrossRef]
- Herzig, M.C.; Kolly, C.; Persohn, E.; Theil, D.; Schweizer, T.; Hafner, T.; Stemmelen, C.; Troxler, T.J.; Schmid, P.; Danner, S.; et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Human Molecular Genetics 2011, 20, 4209–4223. [Google Scholar] [CrossRef]
- West, A.B. Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Experimental Neurology 2017, 298, 236–245. [Google Scholar] [CrossRef]
- Ozelius, L.J.; Senthil, G.; Saunders-Pullman, R.; Ohmann, E.; Deligtisch, A.; Tagliati, M.; Hunt, A.L.; Klein, C.; Henick, B.; Hailpern, S.M.; et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews [14]. New England Journal of Medicine 2006, 354, 424–425. [Google Scholar] [CrossRef]
- Ness, D.; Ren, Z.; Gardai, S.; Sharpnack, D.; Johnson, V.J.; Brennan, R.J.; Brigham, E.F.; Olaharski, A.J. Leucine-Rich Repeat Kinase 2 (LRRK2)-Deficient Rats Exhibit Renal Tubule Injury and Perturbations in Metabolic and Immunological Homeostasis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Crook, A.; Jacobs, C.; Newton-John, T.; O’Shea, R.; McEwen, A. Genetic Counseling and Testing Practices for Late-Onset Neurodegenerative Disease: A Systematic Review. J. Neurol 2021. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Kehoe, C.; Shorr, E.; Battista, R.; Hall, A.; Simuni, T.; Marder, K.; Wills, A.M.; Naito, A.; Beck, J.C.; et al. Genetic testing for Parkinson disease: current practice, knowledge, and attitudes among US and Canadian movement disorders specialists. Genetics in Medicine 2020, 22, 574–580. [Google Scholar] [CrossRef]
- Payne, K.; Walls, B.; Wojcieszek, J. Approach to Assessment of Parkinson Disease with Emphasis on Genetic Testing. Medical Clinics of North America 2019, 103, 1055–1075. [Google Scholar] [CrossRef]
- Blauwendraat, C.; Heilbron, K.; Vallerga, C.L.; Bandres-Ciga, S.; von Coelln, R.; Pihlstrøm, L.; Simón-Sánchez, J.; Schulte, C.; Sharma, M.; Krohn, L.; et al. Parkinson's disease age at onset genome-wide association study: Defining heritability, genetic loci, and α-synuclein mechanisms. Movement Disorders 2019, 34, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Balint, E.; Ashkar, A.A. Remote hyperinflammation drives neurological disease via T-cell-mediated innate-like cytotoxicity. Cellular and Molecular Immunology 2021, 18, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, P.; Shabbott, B.; Cortés, J.C. Motor control abnormalities in Parkinson's disease. Cold Spring Harbor Perspectives in Medicine 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Subramaniam, S.R.; Magen, I.; Lee, P.; Hayes, J.; Attar, A.; Zhu, C.; Franich, N.R.; Bove, N.; De La Rosa, K.; et al. A Molecular Tweezer Ameliorates Motor Deficits in Mice Overexpressing α-Synuclein. Neurotherapeutics 2017, 14, 1107–1119. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nature Reviews Neuroscience 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson's disease. Parkinsonism and Related Disorders 2016, 22, S119–S122. [Google Scholar] [CrossRef]
- Cruz Hernández, J.C.; Bracko, O.; Kersbergen, C.J.; Muse, V.; Haft-Javaherian, M.; Berg, M.; Park, L.; Vinarcsik, L.K.; Ivasyk, I.; Rivera, D.A.; et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nature Neuroscience 2019, 22, 413–420. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer's Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Williams, U.; Bandmann, O.; Walker, R. Parkinson’s disease in sub-Saharan Africa: A review of epidemiology, genetics and access to care. J Mov Disord 2018, 11, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chiriches, C.; Guillen, N.; Rokicki, M.; Guy, C.; Mian, A.; Ottmann, O.G.; Ruthardt, M. Subtractive Interaction Proteomics Reveal a Network of Signaling Pathways Activated By an Oncogenic Transcription Factor in High Risk AML. Blood 2018, 132, 3917–3917. [Google Scholar] [CrossRef]
- Ghanouni, P.; Pauly, K.B.; Elias, W.J.; Henderson, J.; Sheehan, J.; Monteith, S.; Wintermark, M. Transcranial MRI-guided focused ultrasound: A review of the technologic and neurologic applications. American Journal of Roentgenology 2015, 205, 150–159. [Google Scholar] [CrossRef]
- Lehman, V.T.; Lee, K.H.; Klassen, B.T.; Blezek, D.J.; Goyal, A.; Shah, B.R.; Gorny, K.R.; Huston, J., III; Kaufmann, T.J. MRI and tractography techniques to localize the ventral intermediate nucleus and dentatorubrothalamic tract for deep brain stimulation and MR-guided focused ultrasound: A narrative review and update. Neurosurgical Focus 2020, 49. [Google Scholar] [CrossRef]
- Xu, J.P. Progress in the stereotaxic technics. Chinese Journal of Neurology and Psychiatry 1983, 16, 60–62. [Google Scholar]
- Guilhon, E.; Voisin, P.; de Zwart, J.A.; Quesson, B.; Salomir, R.; Maurange, C.; Bouchaud, V.; Smirnov, P.; de Verneuil, H.; Vekris, A.; et al. Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound. Journal of Gene Medicine 2003, 5, 333–342. [Google Scholar] [CrossRef]
- Long, L.; Cai, X.; Guo, R.; Wang, P.; Wu, L.; Yin, T.; Liao, S.; Lu, Z. Treatment of Parkinson's disease in rats by Nrf2 transfection using MRI-guided focused ultrasound delivery of nanomicrobubbles. Biochemical and Biophysical Research Communications 2017, 482, 75–80. [Google Scholar] [CrossRef]
- Grüll, H.; Langereis, S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. Journal of Controlled Release 2012, 161, 317–327. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, Y.; Lin, J.; Lou, X. Target Selection for Magnetic Resonance-Guided Focused Ultrasound in the Treatment of Parkinson's Disease. Journal of Magnetic Resonance Imaging 2022, 56, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.P.; Horimoto, A.R.V.R.; Heilbron, K.; Sarihan, E.I.; Inca-Martinez, M.; Mason, E.; Cornejo-Olivas, M.; Torres, L.; Mazzetti, P.; Cosentino, C.; et al. Characterizing the Genetic Architecture of Parkinson's Disease in Latinos. Annals of Neurology 2021, 90, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Sesso, H.D.; Paffenbarger Jr, R.S.; Lee, I.M. Physical activity and risk of Parkinson's disease: A prospective cohort study. Journal of Neurology, Neurosurgery and Psychiatry 2006, 77, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vicente, M.; Talloczy, Z.; Kaushik, S.; Massey, A.C.; Mazzulli, J.; Mosharov, E.V.; Hodara, R.; Fredenburg, R.; Wu, D.C.; Follenzi, A.; et al. Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. Journal of Clinical Investigation 2008, 118, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Gallay, M.N.; Moser, D.; Rossi, F.; Magara, A.E.; Strasser, M.; Bühler, R.; Kowalski, M.; Pourtehrani, P.; Dragalina, C.; Federau, C.; et al. MRgFUS Pallidothalamic Tractotomy for Chronic Therapy-Resistant Parkinson's Disease in 51 Consecutive Patients: Single Center Experience. Frontiers in Surgery 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.S.; Woodworth, G.F.; Vujaskovic, Z.; Mishra, M.V. Radiosensitization of high-grade gliomas through induced hyperthermia: Review of clinical experience and the potential role of MR-guided focused ultrasound. Radiotherapy and Oncology 2020, 142, 43–51. [Google Scholar] [CrossRef]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nature Neuroscience 2014, 17, 322–329. [Google Scholar] [CrossRef]
- Schneeberger, A.; Tierney, L.; Mandler, M. Active immunization therapies for Parkinson's disease and multiple system atrophy. Movement Disorders 2016, 31, 214–224. [Google Scholar] [CrossRef]
- Iwaki, H.; Blauwendraat, C.; Leonard, H.L.; Kim, J.J.; Liu, G.; Maple-Grødem, J.; Corvol, J.C.; Pihlstrøm, L.; van Nimwegen, M.; Hutten, S.J.; et al. Genomewide association study of Parkinson's disease clinical biomarkers in 12 longitudinal patients' cohorts. Movement Disorders 2019, 34, 1839–1850. [Google Scholar] [CrossRef]
- Bracko, O.; Njiru, B.N.; Swallow, M.; Ali, M.; Haft-Javaherian, M.; Schaffer, C.B. Increasing cerebral blood flow improves cognition into late stages in Alzheimer’s disease mice. Journal of Cerebral Blood Flow and Metabolism 2020, 40, 1441–1452. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Research Letters 2013, 8. [Google Scholar] [CrossRef]
- Awasthi, R.; Roseblade, A.; Hansbro, P.M.; Rathbone, M.J.; Dua, K.; Bebawy, M. Nanoparticles in cancer treatment: Opportunities and obstacles. Current Drug Targets 2018, 19, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. International Journal of Nanomedicine 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Chandra Bhatt, P.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Advances 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Singh, A.; Mewar, S.; Sharma, U.; Jagannathan, N.R.; Sahoo, S.K. The transport of non-surfactant based paclitaxel loaded magnetic nanoparticles across the blood brain barrier in a rat model. Biomaterials 2012, 33, 2936–2951. [Google Scholar] [CrossRef] [PubMed]
- Dudhipala, N.; Gorre, T. Neuroprotective effect of ropinirole lipid nanoparticles enriched hydrogel for parkinson’s disease: In vitro, ex vivo, pharmacokinetic and pharmacodynamic evaluation. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Gelperina, S.; Maksimenko, O.; Khalansky, A.; Vanchugova, L.; Shipulo, E.; Abbasova, K.; Berdiev, R.; Wohlfart, S.; Chepurnova, N.; Kreuter, J. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: Influence of the formulation parameters. European Journal of Pharmaceutics and Biopharmaceutics 2010, 74, 157–163. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, H.; Zhao, H.; Yang, W.; Song, Y.; Li, X.; Wang, Y.; Du, D.; Liao, H.; Pan, W.; et al. New insight into brain disease therapy: nanomedicines-crossing blood–brain barrier and extracellular space for drug delivery. Expert Opinion on Drug Delivery 2022, 19, 1618–1635. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Advances 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of Dual Drug Loaded Lipid Based Nanoparticles across the Blood-Brain Barrier Impart Enhanced Neuroprotection in a Rotenone Induced Mouse Model of Parkinson's Disease. ACS Chemical Neuroscience 2016, 7, 1658–1670. [Google Scholar] [CrossRef]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering–ordered nanostructured small molecule amphiphile self-assembly materials by design. Chemical Society Reviews 2012, 41, 1297–1322. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine: Nanotechnology, Biology, and Medicine 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nature Communications 2019, 10. [Google Scholar] [CrossRef]
- Bond, A.E.; Shah, B.B.; Huss, D.S.; Dallapiazza, R.F.; Warren, A.; Harrison, M.B.; Sperling, S.A.; Wang, X.Q.; Gwinn, R.; Witt, J.; et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease a randomized Clinical trial. JAMA Neurology 2017, 74, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Dallapiazza, R.F.; Timbie, K.F.; Holmberg, S.; Gatesman, J.; Lopes, M.B.; Price, R.J.; Miller, G.W.; Elias, W.J. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. Journal of Neurosurgery 2018, 128, 875–884. [Google Scholar] [CrossRef]
- Jung, N.Y.; Park, C.K.; Kim, M.; Lee, P.H.; Sohn, Y.H.; Chang, J.W. The efficacy and limits of magnetic resonance–guided focused ultrasound pallidotomy for Parkinson’s disease: A Phase I clinical trial. Journal of Neurosurgery 2019, 1306, 1853–1861. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. New England Journal of Medicine 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; Fernández-Rodríguez, B.; Pineda-Pardo, J.A.; Rodríguez-Rojas, R.; Obeso, I.; Hernández-Fernández, F.; del Álamo, M.; Mata, D.; Guida, P.; Ordás-Bandera, C.; et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nature Communications 2021, 12. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, J.; Pan, L.; Zong, R.; Bian, X.; Duan, C.; Zhang, D.; Lou, X. Pretherapeutic functional connectivity of tractography-based targeting of the ventral intermediate nucleus for predicting tremor response in patients with Parkinson's disease after thalamotomy with MRI-guided focused ultrasound. Journal of Neurosurgery 2022, 137, 1135–1144. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer disease in a mouse model: Mr imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 2014, 273, 736–745. [Google Scholar] [CrossRef]
- Lee, K.S.; Clennell, B.; Steward, T.G.J.; Gialeli, A.; Cordero-Llana, O.; Whitcomb, D.J. Focused Ultrasound Stimulation as a Neuromodulatory Tool for Parkinson’s Disease: A Scoping Review. Brain Sciences 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Fry, F.J.; Ades, H.W.; Fry, W.J. Production of reversible changes in the central nervous system by ultrasound. Science 1958, 127, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proceedings of the National Academy of Sciences of the United States of America 2011, 108, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, R.; Rodríguez-Rojas, R.; del Álamo, M.; Hernández-Fernández, F.; Pineda-Pardo, J.A.; Dileone, M.; Alonso-Frech, F.; Foffani, G.; Obeso, I.; Gasca-Salas, C.; et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson's disease: a pilot study. The Lancet Neurology 2018, 17, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Moosa, S.; Martínez-Fernández, R.; Elias, W.J.; del Alamo, M.; Eisenberg, H.M.; Fishman, P.S. The role of high-intensity focused ultrasound as a symptomatic treatment for Parkinson's disease. Movement Disorders 2019, 34, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Tufail, Y.; Finsterwald, M.; Tauchmann, M.L.; Olson, E.J.; Majestic, C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Au, K.L.K.; Wong, J.K.; Tsuboi, T.; Eisinger, R.S.; Moore, K.; Lemos Melo Lobo Jofili Lopes, J. Globus Pallidus Internus (GPi) deep brain stimulation for Parkinson's disease: expert review and commentary. Neurol Ther 2021, 10. [Google Scholar] [CrossRef]
- Ligaard, J.; Sannæs, J.; Pihlstrøm, L. Deep brain stimulation and genetic variability in Parkinson’s disease: a review of the literature. npj Parkinson's Disease 2019, 5. [Google Scholar] [CrossRef]
- Odekerken, V.J.J.; van Laar, T.; Staal, M.J.; Mosch, A.; Hoffmann, C.F.E.; Nijssen, P.C.G.; Beute, G.N.; van Vugt, J.P.P.; Lenders, M.W.P.M.; Contarino, M.F.; et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): A randomised controlled trial. The Lancet Neurology 2013, 12, 37–44. [Google Scholar] [CrossRef]
- Tisch, S.; Zrinzo, L.; Limousin, P.; Bhatia, K.P.; Quinn, N.; Ashkan, K.; Hariz, M. Effect of electrode contact location on clinical efficacy of pallidal deep brain stimulation in primary generalised dystonia. Journal of Neurology, Neurosurgery and Psychiatry 2007, 78, 1314–1319. [Google Scholar] [CrossRef]
- You, Z.; Wu, Y.Y.; Wu, R.; Xu, Z.X.; Wu, X.; Wang, X.P. Efforts of subthalamic nucleus deep brain stimulation on cognitive spectrum: From explicit to implicit changes in the patients with Parkinson's disease for 1 year. CNS Neuroscience and Therapeutics 2020, 26, 972–980. [Google Scholar] [CrossRef] [PubMed]

| GENE | Year of Discovery | Reported Variants | Frequency | Inheritance | Confidence as a PD Gene |
|---|---|---|---|---|---|
| SNCA * | 1997, 2003 | Missense or multiplication | Very rare | Dominant | Very high |
| PRKN * | 1998 | Missense or loss of function | Rare | Recessive | Very high |
| UCHL1 | 1998 | Missense | Unclear | Dominant | Low |
| PARK7 * | 2003 | Missense | Very rare | Recessive | Very high |
| LRRK2 * | 2004 | Missense | Common | Dominant | Very high |
| PINK1 * | 2004 | Missense or loss of function | Rare | Recessive | Very high |
| POLG | 2004 | Missense or loss of function | Rare | Dominant | High |
| HTRA2 | 2005 | Missense | Unclear | Dominant | Low |
| ATP13A2 * | 2006 | Missense or loss of function | Very rare | Recessive | Very high |
| FBXO7 * | 2008 | Missense | Very rare | Recessive | Very high |
| GIGYF2 | 2008 | Missense | Unclear | Dominant | Low |
| GBA * | 2009 | Missense or loss of function | Common | Dominant (incomplete penetrance) | Very high |
| PLA2G6 * | 2009 | Missense or loss of function | Rare | Recessive | Very high |
| EIF4G1 | 2011 | Missense | Unclear | Dominant | Low |
| VPS35 * | 2011 | Missense | Very rare | Dominant | Very high |
| DNAJC6 | 2012 | Missense or loss of function | Very rare | Recessive | High |
| SYNJ1 | 2013 | Missense or loss of function | Very rare | Recessive | High |
| DNAJC13 | 2014 | Missense | Unclear | Dominant | Low |
| TMEM230 | 2016 | Missense | Unclear | Dominant | Low |
| VPS13C | 2016 | Missense or loss of function | Rare | Recessive | High |
| LRP10 | 2018 | Missense or loss of function | Unclear | Dominant | Low |
| NUS1 | 2018 | Missense | Unclear | Recessive | Low |
| COL6A3 | 2022 | Missense | Rare | Dominant | High |
| TH | 2022 | Missense | Unclear | Dominant | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





