Submitted:
18 August 2023
Posted:
22 August 2023
Read the latest preprint version here
Abstract
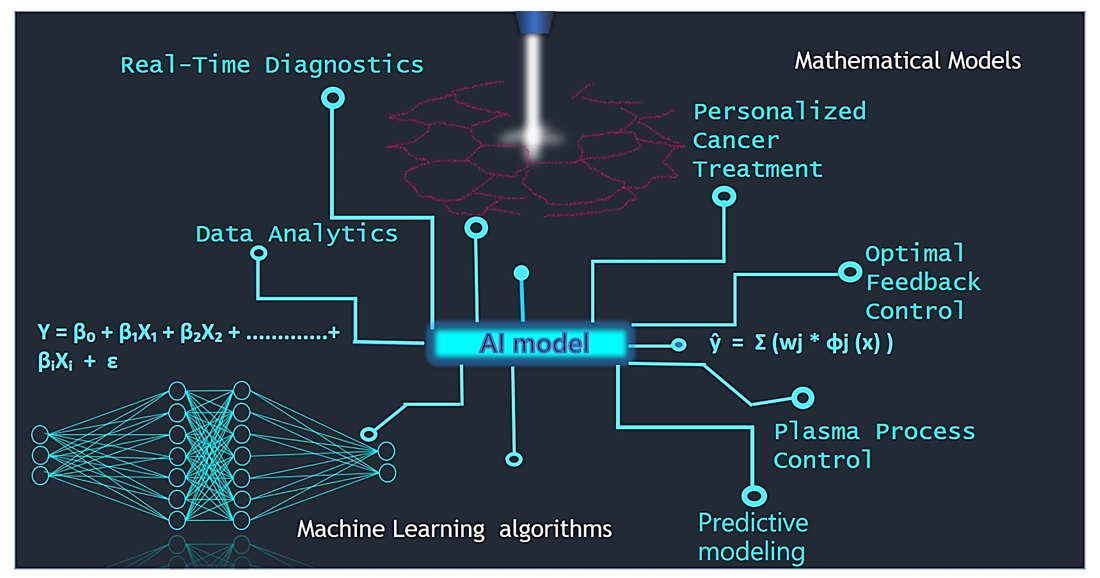
Keywords:
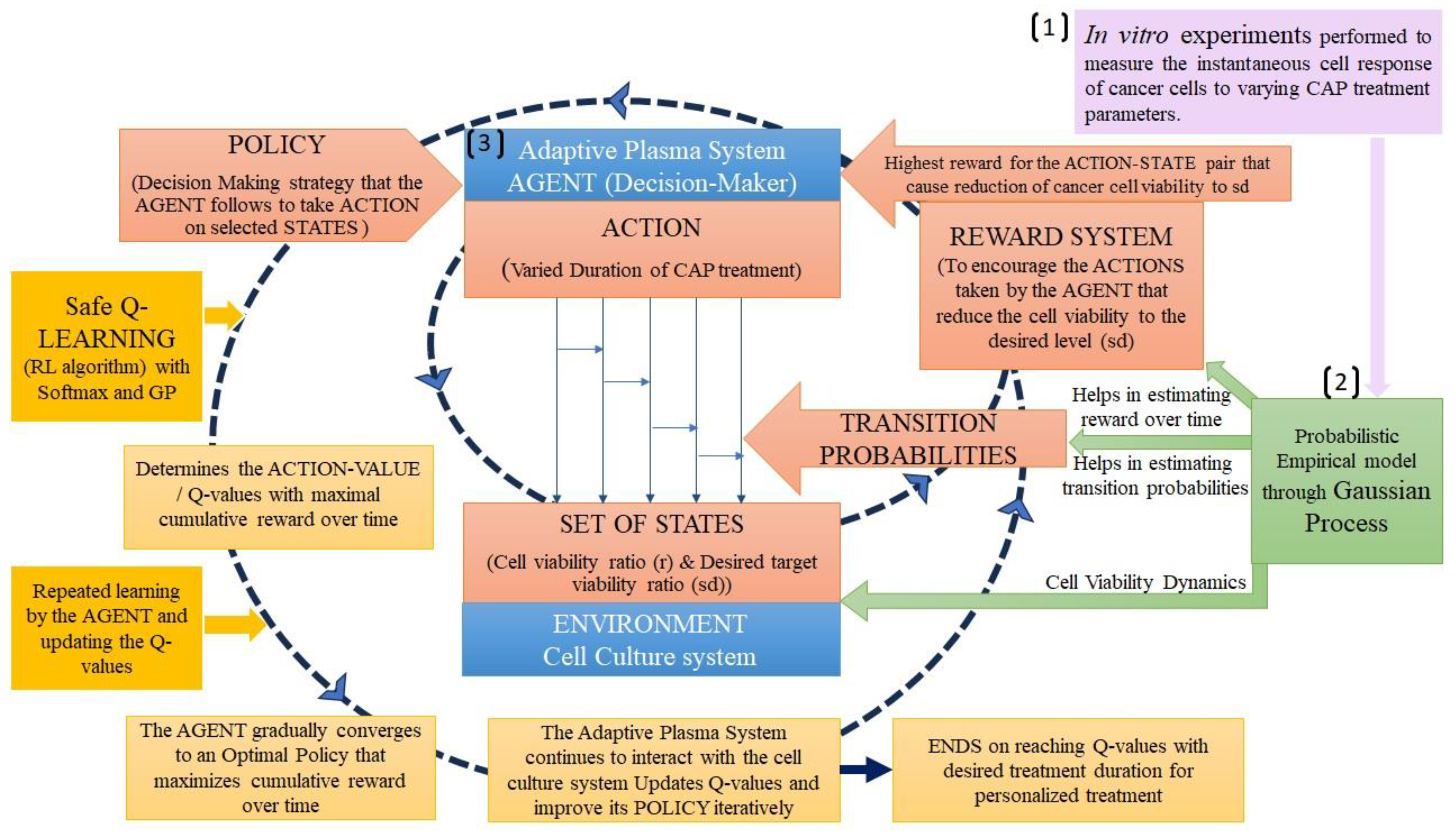
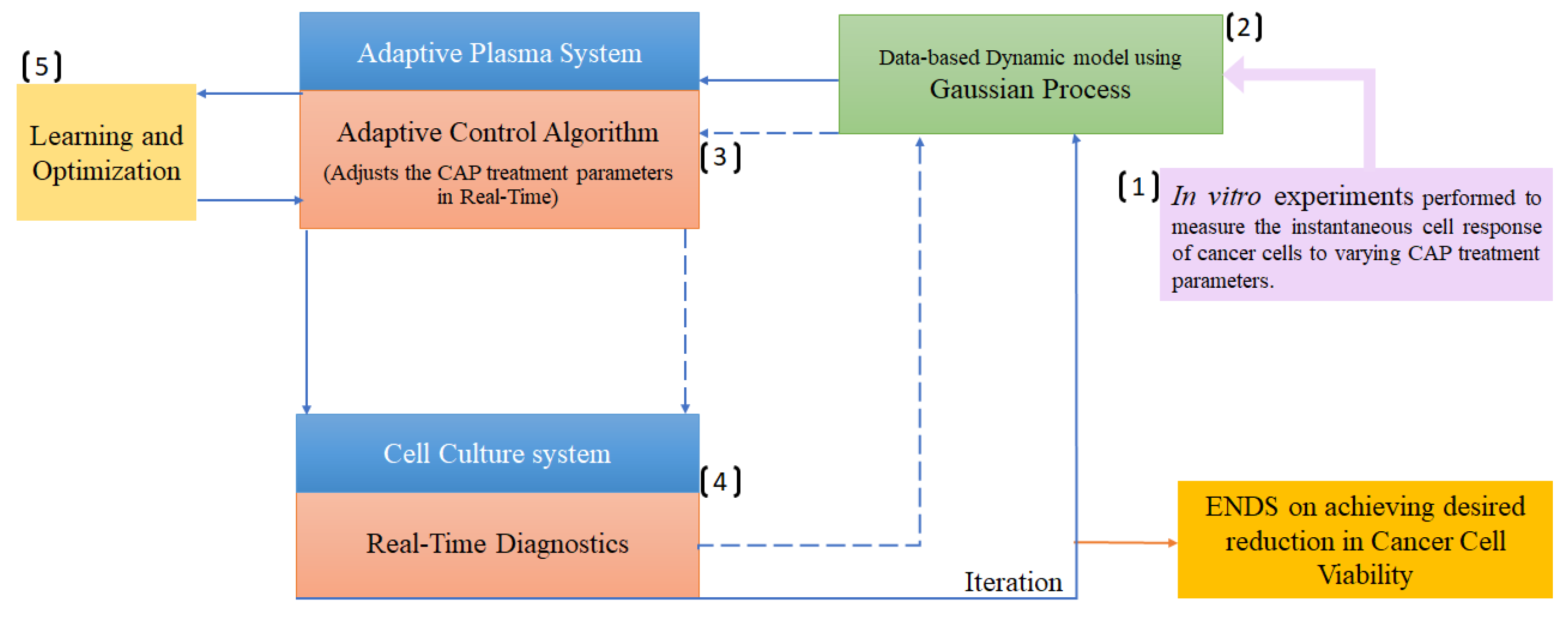
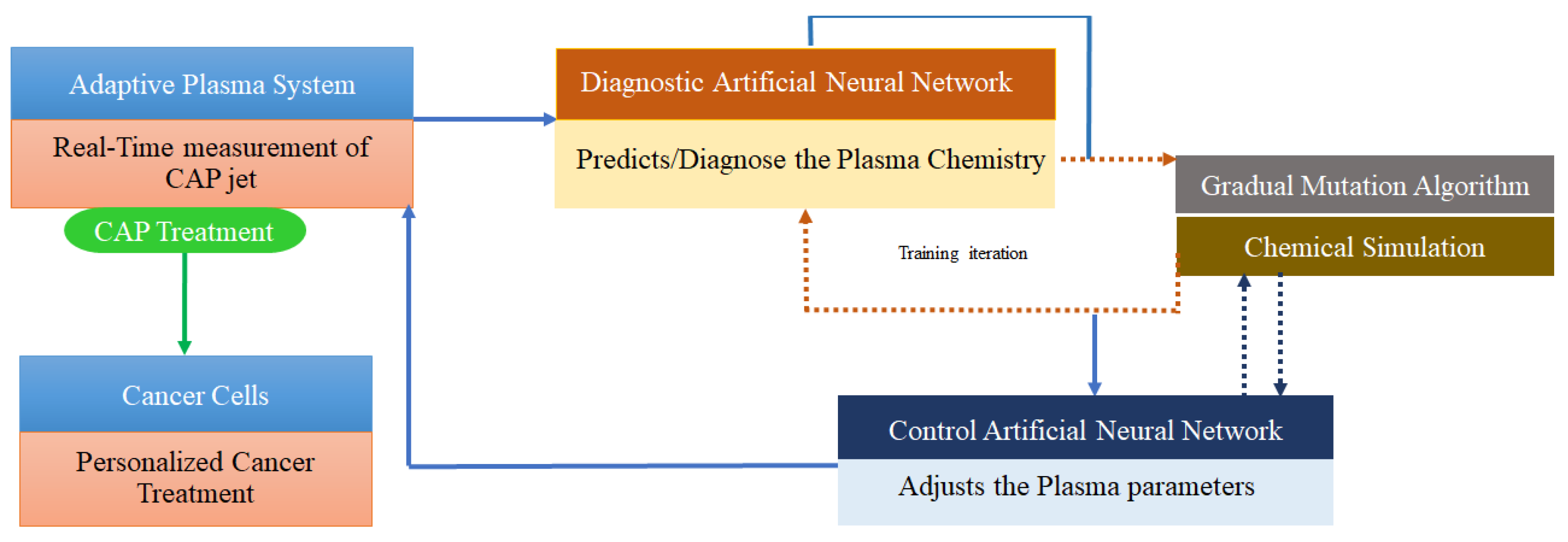
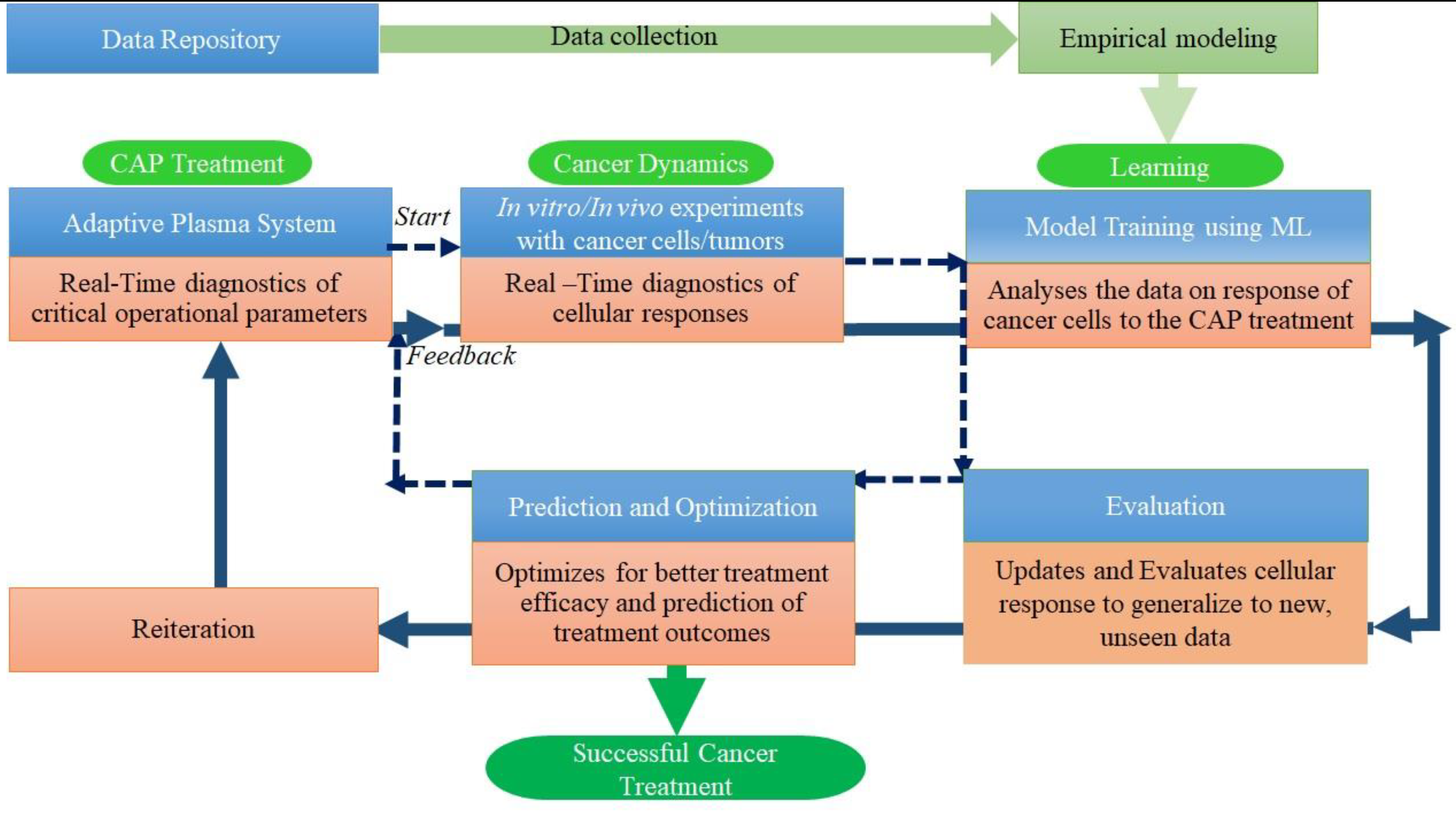
Graphical Abstract
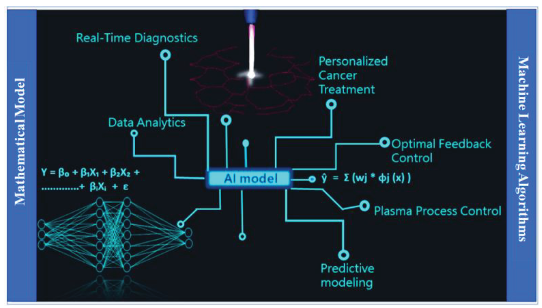
1. Introduction
2. Mathematical models for CAP treatment response in cancer
3. AI for CAP treatment response in cancer
3.1. Reinforcement learning
3.2. Gaussian process
3.3. Deep learning
4. AI in Real-Time diagnostics
4.1. Real-time diagnosis of operational parameters of CAP sources
4.2. Real-time diagnosis of the cell responses to CAP treatment
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; Reuter, S.; Laux, C.; Mesbah, A.; Walsh, J.; Jiang, C.; Thagard, S. M.; Tanaka, H.; Liu, D.; Yan, D.; Yusupov, M. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6(2), 127–157. [Google Scholar] [CrossRef]
- Keidar, M. Plasma Cancer Therapy, 1st ed.; Keidar, M., Ed.; Springer: Cham, 2020. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Q.; Adhikari, M.; Malyavko, A.; Lin, L.; Zolotukhin, D. B.; Yao, X.; Kirschner, M.; Sherman, J. H.; Keidar, M. A Physically Triggered Cell Death via Transbarrier Cold Atmospheric Plasma Cancer Treatment. ACS Appl. Mater. Interfaces 2020, 12(31), 34548–34563. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for Cancer Treatment. Plasma Sources Sci. Technol. 2015, 24(3), 033001. [Google Scholar] [CrossRef]
- Suenaga, Y.; Takamatsu, T.; Aizawa, T.; Moriya, S.; Matsumura, Y.; Iwasawa, A.; Okino, A. Influence of Controlling Plasma Gas Species and Temperature on Reactive Species and Bactericidal Effect of the Plasma. Appl. Sci. 2021, 11(24), 11674. [Google Scholar] [CrossRef]
- Feibel, D.; Golda, J.; Held, J.; Awakowicz, P.; Schulz-von der Gathen, V.; Suschek, C. V.; Opländer, C.; Jansen, F. Gas Flow-Dependent Modification of Plasma Chemistry in ΜAPP Jet-Generated Cold Atmospheric Plasma and Its Impact on Human Skin Fibroblasts. Biomed. 2023, 11(5), 1242. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Keidar, M. A Map of Control for Cold Atmospheric Plasma Jets: From Physical Mechanisms to Optimizations. Appl. Phys. Rev. [CrossRef]
- Yan, D.; Malyavko, A.; Wang, Q.; Lin, L.; Sherman, J. H.; Keidar, M. Cold Atmospheric Plasma Cancer Treatment, a Critical Review. Appl. Sci. 2021, 11(16), 7757. [Google Scholar] [CrossRef]
- Jung, J. M.; Yoon, H. K.; Jung, C. J.; Jo, S. Y.; Hwang, S. G.; Lee, H. J.; Lee, W. J.; Chang, S. E.; Won, C. H. Cold Plasma Treatment Promotes Full-Thickness Healing of Skin Wounds in Murine Models. Int. J. Low. Extrem. Wounds 2023, 22(1), 77–84. [Google Scholar] [CrossRef]
- Rached, N. A.; Kley, S.; Storck, M.; Meyer, T.; Stücker, M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J. Clin. Med. 2023, 12(15), 5121. [Google Scholar] [CrossRef]
- Muto, R.; Hayashi, N. Sterilization Characteristics of Narrow Tubing by Nitrogen Oxides Generated in Atmospheric Pressure Air Plasma. Sci. Rep. [CrossRef]
- Masood, A.; Ahmed, N.; Razip Wee, M. F. M.; Patra, A.; Mahmoudi, E.; Siow, K. S. Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity. Polymers, 2023. [Google Scholar] [CrossRef]
- Matthes, R.; Jablonowski, L.; Miebach, L.; Pitchika, V.; Holtfreter, B.; Eberhard, C.; Seifert, L.; Gerling, T.; Schlüter, R.; Kocher, T.; Bekeschus, S. In-Vitro Biofilm Removal Efficacy Using Water Jet in Combination with Cold Plasma Technology on Dental Titanium Implants. Int. J. Mol. Sci. 2023, 24(2), 1606. [Google Scholar] [CrossRef]
- Liu, Z.; Du, X.; Xu, L.; Shi, Q.; Tang, X.; Cao, Y.; Song, K. The Therapeutic Perspective of Cold Atmospheric Plasma in Periodontal Disease. Oral Dis. 2023. [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P. K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P. K.; Verma, S. K.; Suar, M. Aurora Borealis in Dentistry: The Applications of Cold Plasma in Biomedicine. Mater. Today Bio. 2022, 13, 100200. [Google Scholar] [CrossRef]
- Nonnenmacher, L.; Fischer, M.; Haralambiev, L.; Bekeschus, S.; Schulze, F.; Wassilew, G. I.; Schoon, J.; Reichert, J. C. Orthopaedic Applications of Cold Physical Plasma. EFORT Open Rev. 2023, 8(6), 409–423. [Google Scholar] [CrossRef]
- Živanić, M.; Espona-Noguera, A.; Lin, A.; Canal, C. Current State of Cold Atmospheric Plasma and Cancer-Immunity Cycle: Therapeutic Relevance and Overcoming Clinical Limitations Using Hydrogels. Adv. Sci. 2023, 10(8), 2205803. [Google Scholar] [CrossRef]
- Förster, S.; Niu, Y.; Eggers, B.; Nokhbehsaim, M.; Kramer, F. J.; Bekeschus, S.; Mustea, A.; Stope, M. B. Modulation of the Tumor-Associated Immuno-Environment by Non-Invasive Physical Plasma. Cancers, 2023. [Google Scholar] [CrossRef]
- Chupradit, S.; Widjaja, G.; Radhi Majeed, B.; Kuznetsova, M.; Ansari, M. J.; Suksatan, W.; Turki Jalil, A.; Ghazi Esfahani, B. Recent Advances in Cold Atmospheric Plasma (CAP) for Breast Cancer Therapy. Cell Biol. Int. 2023, 47(2), 327–340. [Google Scholar] [CrossRef] [PubMed]
- Limanowski, R.; Yan, D.; Li, L.; Keidar, M. Preclinical Cold Atmospheric Plasma Cancer Treatment. Cancers 2022, 14(14), 3461. [Google Scholar] [CrossRef] [PubMed]
- Trelles, J. P. Pattern Formation and Self-Organization in Plasmas Interacting with Surfaces. J. Phys. D. Appl. Phys. 2016, 49(39), 393002. [Google Scholar] [CrossRef]
- Keidar, M. A Prospectus on Innovations in the Plasma Treatment of Cancer. Phys. Plasmas 2018, 25(8), 83504. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Tang, A.; Kirschner, M.; Tadvalkar, G.; Canady, J.; Stepp, M. A.; Keidar, M. Adaptation of Operational Parameters of Cold Atmospheric Plasma for in vitro Treatment of Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10(11), 9269–9279. [Google Scholar] [CrossRef]
- Martinez, L.; Dhruv, A.; Balaras, E.; Keidar, M.; Martinez, L.; Dhruv, A.; Balaras, E.; Keidar, M. On Self Organization: Model for Ionization Wave Propagation with Targets of Varying Electrical Properties. PSST 2022, 31(3), 035004. [Google Scholar] [CrossRef]
- Keidar,M. Adaptive and Self-Adaptive Plasma Cancer Therapeutic Platform. US patent, 11517366, 2022.
- Brady, R.; Enderling, H. Mathematical Models of Cancer: When to Predict Novel Therapies, and When Not To. Bull. Math. Biol. 2019, 81(10), 3722–3731. [Google Scholar] [CrossRef]
- Belkhir, S.; Thomas, F.; Roche, B. Darwinian Approaches for Cancer Treatment: Benefits of Mathematical Modeling. Cancers, 2021. [Google Scholar] [CrossRef]
- Altrock, P. M.; Liu, L. L.; Michor, F. The Mathematics of Cancer: Integrating Quantitative Models. Nat. Rev. Cancer 2015, 15(12), 730–745. [Google Scholar] [CrossRef]
- Bull, J. A.; Byrne, H. M. The Hallmarks of Mathematical Oncology. Proc. IEEE. 2022, 110(5), 523–540. [Google Scholar] [CrossRef]
- Farayola,F. M.; Shafie, S.; Siam, F. M.; Mahmud, R.; Ajadi, S. O. Mathematical Modeling of Cancer Treatments with Fractional Derivatives: An Overview. Mal J Fund Appl Sci, 2021, 17, 389–401. [Google Scholar] [CrossRef]
- Murphy, W.; Carroll, C.; Keidar, M. Simulation of the Effect of Plasma Species on Tumor Growth and Apoptosis. J. Phys. D. Appl. Phys. 2014, 47(47), 472001. [Google Scholar] [CrossRef]
- Ryser, M. D.; Komarova, S. V. Mathematical Modeling of Cancer Metastases. Comp. Bioeng. [CrossRef]
- Chamani, F.; Pyle, M. M.; Shrestha, T. B.; Sebek, J.; Bossmann, S. H.; Basel, M. T.; Sheth, R. A.; Prakash, P. In Vitro Measurement and Mathematical Modeling of Thermally-Induced Injury in Pancreatic Cancer Cells. Cancers (Basel). 2023, 15(3), 655. [Google Scholar] [CrossRef]
- Engeland, C. E.; Heidbuechel, J. P. W.; Araujo, R. P.; Jenner, A. L. Improving Immunovirotherapies: The Intersection of Mathematical Modelling and Experiments. ImmunoInformatics 2022, 6, 100011. [Google Scholar] [CrossRef]
- Hormuth, D. A.; Farhat, M.; Christenson, C.; Curl, B.; Chad Quarles, C.; Chung, C.; Yankeelov, T. E. Opportunities for Improving Brain Cancer Treatment Outcomes through Imaging-Based Mathematical Modeling of the Delivery of Radiotherapy and Immunotherapy. Adv. Drug Deliv. Rev. 2022. [CrossRef]
- Wei, H. C. Mathematical Modeling of Tumor Growth and Treatment: Triple Negative Breast Cancer. Math. Comput. Simul. 2023, 204, 645–659. [Google Scholar] [CrossRef]
- Gatenby, R. A.; Silva, A. S.; Gillies, R. J.; Frieden, B. R. Adaptive Therapy. Cancer Res. 2009, 69(11), 4894–4903. [Google Scholar] [CrossRef]
- Elharrar, X.; Barbolosi, D.; Ciccolini, J.; Meille, C.; Faivre, C.; Lacarelle, B.; André, N.; Barlesi, F. A Phase Ia/Ib Clinical Trial of Metronomic Chemotherapy Based on a Mathematical Model of Oral Vinorelbine in Metastatic Non-Small Cell Lung Cancer and Malignant Pleural Mesothelioma: Rationale and Study Protocol. BMC Cancer. [CrossRef]
- Smalley, I.; Kim, E.; Li, J.; Spence, P.; Wyatt, C. J.; Eroglu, Z.; Sondak, V. K.; Messina, J. L.; Babacan, N. A.; Maria-Engler, S. S.; De Armas, L.; Williams, S. L.; Gatenby, R. A.; Chen, Y. A.; Anderson, A. R. A.; Smalley, K. S. M. Leveraging Transcriptional Dynamics to Improve BRAF Inhibitor Responses in Melanoma. EBioMedicine 2019, 48, 178–190. [Google Scholar] [CrossRef]
- Guerreiro, N.; Jullion, A.; Ferretti, S.; Fabre, C.; Meille, C. Translational Modeling of Anticancer Efficacy to Predict Clinical Outcomes in a First-in-Human Phase 1 Study of MDM2 Inhibitor HDM201. AAPS J. 2021, 23(2), 1–17. [Google Scholar] [CrossRef] [PubMed]
- Brüningk, S. C.; Peacock, J.; Whelan, C. J.; Brady-Nicholls, R.; Yu, H. H. M.; Sahebjam, S.; Enderling, H. Intermittent Radiotherapy as Alternative Treatment for Recurrent High Grade Glioma: A Modeling Study Based on Longitudinal Tumor Measurements. Sci. Rep. [CrossRef]
- Mathur, D.; Barnett, E.; Scher, H. I.; Xavier, J. B. Optimizing the Future: How Mathematical Models Inform Treatment Schedules for Cancer. Trends Cancer 2022, 8(6), 506. [Google Scholar] [CrossRef] [PubMed]
- Dean, J. A.; Tanguturi, S. K.; Cagney, D.; Shin, K. Y.; Youssef, G.; Aizer, A.; Rahman, R.; Hammoudeh, L.; Reardon, D.; Lee, E.; Dietrich, J.; Tamura, K.; Aoyagi, M.; Wickersham, L.; Wen, P. Y.; Catalano, P.; Haas-Kogan, D.; Alexander, B. M.; Michor, F. Phase I Study of a Novel Glioblastoma Radiation Therapy Schedule Exploiting Cell-State Plasticity. Neuro. Oncol. 2023, 25(6), 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Leder, K.; Pitter, K.; Laplant, Q.; Hambardzumyan, D.; Ross, B. D.; Chan, T. A.; Holland, E. C.; Michor, F. Mathematical Modeling of Pdgf-Driven Glioblastoma Reveals Optimized Radiation Dosing Schedules. Cell. [CrossRef]
- Keidar, M. Therapeutic Approaches Based on Plasmas and Nanoparticles. J. Nanomedicine Res. 2016, 3 (2). [CrossRef]
- Keidar, M.; Yan, D.; Beilis, I. I.; Trink, B.; Sherman, J. H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36(6), 586–593. [Google Scholar] [CrossRef]
- Lyu, Y.; Lin, L.; Gjika, E.; Lee, T.; Keidar, M. Mathematical Modeling and Control for Cancer Treatment with Cold Atmospheric Plasma Jet. J. Phys. D. J. Phys. D. Appl. Phys. 2019, 51(18), 185202. [Google Scholar] [CrossRef]
- Tanaka, H.; Bekeschus, S.; Yan, D.; Hori, M. ; Keidar,M; Laroussi,M. Plasma-Treated Solutions (PTS) in Cancer Therapy. Cancers, 1737. [Google Scholar] [CrossRef]
- Tampieri, F.; Gorbanev, Y.; Sardella, E. Plasma-Treated Liquids in Medicine: Let’s Get Chemical. Plasma Process. Plasma Process. Polym. 2023, e2300077. [Google Scholar] [CrossRef]
- Solé-Martí, X.; Vilella, T.; Labay, C.; Tampieri, F.; Ginebra, M. P.; Canal, C. Thermosensitive Hydrogels to Deliver Reactive Species Generated by Cold Atmospheric Plasma: A Case Study with Methylcellulose. Biomater. Sci. 2022, 10(14), 3845–3855. [Google Scholar] [CrossRef]
- Malyavko, A.; Yan, D.; Wang, Q.; Klein, A.L.; Patel, K.C.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma Cancer Treatment, Direct versus Indirect Approaches. Mater. Adv. 2020, 1, 1494–1505. [Google Scholar] [CrossRef]
- Poramapijitwat, P.; Thana, P.; Sukum, P.; Liangdeng, Y.; Kuensaen, C.; Boonyawan, D. Selective Cytotoxicity of Lung Cancer Cells—A549 and H1299—Induced by Ringer’s Lactate Solution Activated by a Non-Thermal Air Plasma Jet Device, Nightingale®. Plasma Chem. Plasma Process. 2023, 43(4), 805–830. [Google Scholar] [CrossRef]
- Miebach, L.; Mohamed, H.; Wende, K.; Miller, V.; Bekeschus, S. Pancreatic Cancer Cells Undergo Immunogenic Cell Death upon Exposure to Gas Plasma-Oxidized Ringers Lactate. Cancers. [CrossRef]
- Pavlik, T.; Gudkova, V.; Razvolyaeva, D.; Pavlova, M.; Kostukova, N.; Miloykovich, L.; Kolik, L.; Konchekov, E.; Shimanovskii, N. The Role of Autophagy and Apoptosis in the Combined Action of Plasma-Treated Saline, Doxorubicin, and Medroxyprogesterone Acetate on K562 Myeloid Leukaemia Cells. Int. J. Mol. Sci. 2023, 24(6), 5100. [Google Scholar] [CrossRef]
- Wang, X.; Liu, N.; Liu, J.; Cui, Y.; Wang, X.; Lu, J.; Kang, C.; Gao, L.; Shi, X.; Zhang, G. Comparison of Direct and Indirect Low-Temperature Plasma Triggering Immunogenic Cell Death in B16F10 Melanoma. Plasma Process. Polym. 2023, 20(8), e2200206. [Google Scholar] [CrossRef]
- Patrakova, E. A.; Biryukov, M. M.; Troitskaya, O. S.; Novak, D. D.; Milakhina, E. V.; Gugin, P. P.; Zakrevsky, D. E.; Schweigert, I. V.; Koval, O. A. Cytotoxic Activity of a Cold Atmospheric Plasma Jet in Relation to a 3D Cell Model of Human Breast Cancer. Cell tissue biol. [CrossRef]
- Bengtson, C.; Bogaerts, A. On the Anti-Cancer Effect of Cold Atmospheric Plasma and the Possible Role of Catalase-Dependent Apoptotic Pathways. Cells. [CrossRef]
- Bengtson, C.; Bogaerts, A. The Quest to Quantify Selective and Synergistic Effects of Plasma for Cancer Treatment: Insights from Mathematical Modeling. Int. J. Mol. Sci. 2021, 22(9), 5033. [Google Scholar] [CrossRef] [PubMed]
- Xu, M. Modeling and Simulation of Cancer Treatment Using Cold Atmospheric Plasma. 2021. [CrossRef]
- Lin, L.; Yan, D.; Gjika, E.; Sherman, J. H.; Keidar, M. Atmospheric Plasma Meets Cell: Plasma Tailoring by Living Cells. Appl. Mater. Interfaces, 3062. [Google Scholar] [CrossRef]
- Lin, L.; Hou, Z.; Yao, X.; Liu, Y.; Sirigiri, J. R.; Lee, T.; Keidar, M. Introducing Adaptive Cold Atmospheric Plasma: The Perspective of Adaptive Cold Plasma Cancer Treatments Based on Real-Time Electrochemical Impedance Spectroscopy. Phys. Plasmas 2020, 27(6), 063501. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Scally, L.; Manaloto, E.; Gunes, S.; Ng, S. W.; Maher, M.; Tiwari, B.; Byrne, H. J.; Bourke, P.; Tian, F.; Cullen, P. J.; Curtin, J. F. Cold Atmospheric Plasma Stimulates Clathrin-Dependent Endocytosis to Repair oxidized Membrane and Enhance Uptake of Nanomaterial in Glioblastoma Multiforme. Cells. Sci. Rep. [CrossRef]
- Michor, F.; Beal, K. Improving Cancer Treatment via Mathematical Modeling: Surmounting the Challenges Is Worth the Effort. Cell, 1059. [Google Scholar] [CrossRef]
- Bekisz, S.; Geris, L. Cancer Modeling: From Mechanistic to Data-Driven Approaches, and from Fundamental Insights to Clinical Applications. J. Comput. Sci. 2020, 46, 101198. [Google Scholar] [CrossRef]
- Sun, X.; Hu, B. Mathematical Modeling and Computational Prediction of Cancer Drug Resistance. Brief. Bioinform. 2017, 19(6), 1382–1399. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Wang, H. Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach. J. Multidiscip. Healthc. 2023, 16, 1779. [Google Scholar] [CrossRef]
- Bonzanini, A. D.; Shao, K.; Graves, D. B.; Hamaguchi, S.; Mesbah, A. Foundations of Machine Learning for Low-Temperature Plasmas: Methods and Case Studies. Plasma Sources Sci. Technol. 2023, 32(2), 024003. [Google Scholar] [CrossRef]
- Mesbah, A.; Graves, D. B. Machine Learning for Modeling, Diagnostics, and Control of Non-Equilibrium Plasmas. J. Phys. D. Appl. Phys. 2019, 52(30), 30LT02. [Google Scholar] [CrossRef]
- Goldenberg, S. L.; Nir, G.; Salcudean, S. E. A New Era: Artificial Intelligence and Machine Learning in Prostate Cancer. Nat. Rev. Urol. 2019, 16(7), 391–403. [Google Scholar] [CrossRef]
- Koh, D.-M.; Papanikolaou, N.; Bick, U.; Illing, R.; Charles, E. Kahn, J.; Kalpathi-Cramer, J.; Matos, C.; Martí-Bonmatí, L.; Miles, A.; Mun, S. K.; Napel, S.; Rockall, A.; Sala, E.; Strickland, N.; Prior, F. Artificial Intelligence and Machine Learning in Cancer Imaging. Commun. Med. [CrossRef]
- Savić, M.; Kurbalija, V.; Ilić, M.; Ivanović, M.; Jakovetić, D.; Valachis, A.; Autexier, S.; Rust, J.; Kosmidis, T. The Application of Machine Learning Techniques in Prediction of Quality of Life Features for Cancer Patients. Comput. Sci. Inf. Syst. 2023, 20(1), 381–404. [Google Scholar] [CrossRef]
- Singh, A. K.; Ling, J.; Malviya, R. Prediction of Cancer Treatment Using Advancements in Machine Learning. Recent Pat. Anticancer. Recent Pat. Anticancer. Drug Discov. 2023, 18(3), 364–378. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial Intelligence Assists Precision Medicine in Cancer Treatment. Front. Oncol. 2023, 12, 998222. [Google Scholar] [CrossRef]
- Tan, P.; Chen, X.; Zhang, H.; Wei, Q.; Luo, K. Artificial Intelligence Aids in Development of Nanomedicines for Cancer Management. Semin. Cancer Biol. 2023, 89, 61–75. [Google Scholar] [CrossRef]
- Charalambous, A.; Dodlek, N. Big Data, Machine Learning, and Artificial Intelligence to Advance Cancer Care: Opportunities and Challenges. Semin. Oncol. Nurs. [CrossRef]
- Wang, Z.; Liu, Y.; Niu, X. Application of Artificial Intelligence for Improving Early Detection and Prediction of Therapeutic Outcomes for Gastric Cancer in the Era of Precision Oncology. Semin. Cancer Biol. 2023, 93, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, L.; Lu, M.; Jin, R.; Ye, H.; Ma, T. The Artificial Intelligence and Machine Learning in Lung Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16(1), 55. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Keidar, M. Adaptive Plasma and Machine Learning. In: Keidar, M. (eds) Plasma Cancer Therapy. Springer Series on Atomic, Optical, and Plasma Physics, Springer, Cham.2020,223-250. [CrossRef]
- Hou, Z.; Lee, T.; Keidar, M. Reinforcement Learning with Safe Exploration for Adaptive Plasma Cancer Treatment. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6(4), 482–492. [Google Scholar] [CrossRef]
- Bonzanini, A. D.; Shao, K.; Stancampiano, A.; Graves, D. B.; Mesbah, A. Perspectives on Machine Learning-Assisted Plasma Medicine: Toward Automated Plasma Treatment. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6(1), 16–32. [Google Scholar] [CrossRef]
- Chan, K. J.; Makrygiorgos, G.; Mesbah, A. Towards Personalized Plasma Medicine via Data-Efficient Adaptation of Fast Deep Learning-Based MPC Policies. Am. Control Conf. 2023, 2769–2775. [Google Scholar] [CrossRef]
- Littman, M. L.; Szepesvári, C. A Generalized Reinforcement-Learning Model: Convergence and Applications. In ICML, 1996, 310–318.
- Chen, C. L.; Dong, D. Y.; Li, H. X.; Tarn, T. J. Hybrid MDP Based Integrated Hierarchical Q-Learning. Sci. China Inf. Sci. 2011, 54(11), 2279–2294. [Google Scholar] [CrossRef]
- Lin, L.; Yan, D.; Lee, T.; Keidar, M. Self-Adaptive Plasma Chemistry and Intelligent Plasma Medicine. Adv. Intell. Syst. 2022, 4(3), 2100112. [Google Scholar] [CrossRef]
- Lin, L.; Keidar, M. Artificial Intelligence without Digital Computers: Programming Matter at a Molecular Scale. Adv. Intell. Syst. 2022, 4(11), 2200157. [Google Scholar] [CrossRef]
- Gidon, D.; Pei, X.; Bonzanini, A. D.; Graves, D. B.; Mesbah, A. Machine Learning for Real-Time Diagnostics of Cold Atmospheric Plasma Sources. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3(5), 597–605. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Primc, G.; Vesel, A. Optical Emission Spectroscopy as a Diagnostic Tool for Characterization of Atmospheric Plasma Jets. Appl. Sci. 2021, 11(5), 2275. [Google Scholar] [CrossRef]
- Witman, M.; Gidon, D.; Graves, D. B.; Smit, B.; Mesbah, A. Sim-to-Real Transfer Reinforcement Learning for Control of Thermal Effects of an Atmospheric Pressure Plasma Jet. Plasma Sources Sci. Technol. [CrossRef]
- Zhang, Y. T.; Gao, S. H.; Ai, F. Efficient Numerical Simulation of Atmospheric Pulsed Discharges by Introducing Deep Learning. Front. Phys. 2023, 11. [Google Scholar] [CrossRef]
- van Der Gaag, T.; Onishi, H.; Akatsuka, H. Arbitrary EEDF Determination of Atmospheric-Pressure Plasma by Applying Machine Learning to OES Measurement. Phys. Plasmas. [CrossRef]
- van Der Gaag, T.; Nezu, A.; Akatsuka, H. Practical Considerations of the Visible Bremsstrahlung Inversion (VBI) Method for Arbitrary EEDF Determination in Cold Atmospheric-Pressure Plasma. Jpn. J. Appl. Phys. [CrossRef]
- van der Gaag, T.; Nezu, A.; Akatsuka, H. Partial EEDF Analysis and Electron Diagnostics of Atmospheric-Pressure Argon and Argon-Helium DBD Plasma. J. Phys. D. Appl. Phys. [CrossRef]
- Chang, J.; Niu, P. H.; Chen, C. W.; Cheng, Y. C. Using Deep Convolutional Neural Networks to Classify the Discharge Current of a Cold Atmospheric-Pressure Plasma Jet. IEEE Trans. Plasma Sci. 2023, 51(2), 311–319. [Google Scholar] [CrossRef]
- Starikovskiy, A.; Lazarus, M.; Yan, D.; Limanowski, R.; Lin, L.; Keidar, M. Recognizing Cold Atmospheric Plasma Plume Using Computer Vision. Plasma. [CrossRef]
- Lin, L.; Gershman, S.; Raitses, Y.; Keidar, M. Multi-Scale Plasma Chemistry Using Physics-Informed Neural Network. J. Phys D Appl. Phys.
- Rodrigues, D.; Chan, K. J.; Mesbah, A. Data-Driven Adaptive Optimal Control Under Model Uncertainty: An Application to Cold Atmospheric Plasmas. IEEE Trans. Control Syst. Technol. 2023, 31(1), 55–69. [Google Scholar] [CrossRef]
- Kim, D. H.; Hong, S. J. Use of Plasma Information in Machine-Learning-Based Fault Detection and Classification for Advanced Equipment Control. IEEE Trans. Semicond. Manuf. 2021, 34(3), 408–419. [Google Scholar] [CrossRef]
- Sebastian, A.; Lipa, D.; Ptasinska, S. DNA Strand Breaks and Denaturation as Probes of Chemical Reactivity versus Thermal Effects of Atmospheric Pressure Plasma Jets. ACS Omega 2022. [CrossRef]
- Sabrin, S.; Karmokar, D. K.; Karmakar, N. C.; Hong, S. H.; Habibullah, H.; Szili, E. J. Opportunities of Electronic and Optical Sensors in Autonomous Medical Plasma Technologies. ACS Sensors 2023, 8(3), 974–993. [Google Scholar] [CrossRef]
- Trieschmann, J.; Vialetto, L.; Gergs, T. Machine Learning for Advancing Low-Temperature Plasma Modeling and Simulation. 2023. [CrossRef]
- Hoeben, A.; Joosten, E. A. J.; van den Beuken-Van Everdingen, M. H. J. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers (Basel). 2021, 13(2), 1–3. [Google Scholar] [CrossRef]
- Dey, A.; Mitra, A.; Pathak, S.; Prasad, S.; Zhang, A. S.; Zhang, H.; Sun, X. F.; Banerjee, A. Recent Advancements, Limitations, and Future Perspectives of the Use of Personalized Medicine in Treatment of Colon Cancer. Technol. Cancer Res. Treat. 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhu, K. Cold Atmospheric Plasma: Novel Opportunities for Tumor Microenvironment Targeting. Cancer Med. 2023, 12(6), 7189–7206. [Google Scholar] [CrossRef] [PubMed]
- Canady, J.; Murthy, S. R. K.; Zhuang, T.; Gitelis, S.; Nissan, A.; Ly, L.; Jones, O. Z.; Cheng, X.; Adileh, M.; Blank, A. T.; Colman, M. W.; Millikan, K.; O’Donoghue, C.; Stenson, K. M.; Ohara, K.; Schtrechman, G.; Keidar, M.; Basadonna, G. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer. Cancers, 3688. [Google Scholar] [CrossRef]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomed. 2023, 11(1), 208. [Google Scholar] [CrossRef]
- Boeckmann, L.; Berner, J.; Kordt, M.; Lenz, E.; Schäfer, M.; Semmler, M.; Frey, A.; Sagwal, S.; Rebl, H.; Miebach, L.; Niessner, F.; Sawade, M.; Hein, M.; Ramer, R.; Grambow, E.; Seebauer, C.; von Woedtke, T.; Nebe, B.; Metelmann, H.-R.; Langer, P.; Hinz, B.; Vollmar, B.; Emmert, S.; Bekeschus, S. Synergistic Effect of Cold Gas Plasma and Experimental Drug Exposure Exhibits Skin Cancer Toxicity in Vitro and in Vivo. J. Adv. Res. 2023. [CrossRef] [PubMed]
- Bekeschus, S. Medical Gas Plasma Technology: Roadmap on Cancer Treatment and Immunotherapy. Redox Biol. 2023, 65, 102798. [Google Scholar] [CrossRef]
- Ercan, U. K.; Özdemir, G. D.; Özdemir, M. A.; Güren, O. Plasma Medicine: The Era of Artificial Intelligence. Plasma Process. Plasma Process. Polym. 2023, e2300066. [Google Scholar] [CrossRef]
| Mathematical models employed | Response parameter measured/assumed | Parameters influencing cancer treatment | Significance of the model | Reference |
|---|---|---|---|---|
| Linear model | Cell viability using cell counting kit-8 | Treatment duration, liquid surface, thickness of the medium, number of cells | Provides an insight into the relationship between various parameters and the efficiency of PAM in treating cancer cells. | [60] |
| General mathematical model | Permittivity of the cells by capacitance imaging. | Electron density of CAP bullet with cell lines as targets | Permittivity of cancer cells can influence the behavior (eg. electron density, electric field) of CAPJ. Implies the significance in selective cancer treatment and adaptive plasma control. | [61] |
| Exponential growth model, Net proliferative rate function together with MPC | Cell viability using Real Time-Glo assay providing real-time feedback | Plasma discharge voltage, treatment duration | Improves the effectiveness and selectivity of CAP treatment on cancer cells by optimizing the treatment parameters. | [48] |
| Net proliferativerate function,Gaussian process regression model together with MPLC | Electrical properties of cells using EIS/FPR providing real-time feedback | Plasma discharge voltage, treatment duration, flow rate, cell viability | Improves the effectiveness and selectivity by adapting and optimizing the treatment parameters of SACAPJ | [62] |
| Phenomenological rate equation model | Lipid peroxidation by measuring MDA, RONS using OES. | Gold nanoparticles, RONS in CAP | CAP treatment enhanced the uptake of gold nanoparticles by the brain cancer cells through clathrin-mediated endocytosis | [63] |
| A custom mathematical model to analyze the catalase-dependent kinetics | Concentration of hydroxyl radicals | Singlet oxygen exposure, catalase concentration | Demonstrates that catalase inactivation by singlet oxygen would not be sufficient to reactivate the apoptotic pathway. Hence, catalase-dependent apoptotic pathways are unlikely to be the primary cause of the anti-cancer effect of CAP. Needs experimental validations. | [58] |
| Predictive model | Concentration of H2O2 and NO2- | Membrane diffusion rate of H2O2, intracellular catalase concentration | Predicts how varying properties of PTLs, such as the concentration of H2O2 and NO2-, influence their anti-cancer efficacy and selectivity. | [59] |
| Selected Parameters of the CAP sources for Real-Time diagnostics | Input Data obtained from | AI algorithms employed | Reference |
|---|---|---|---|
| Rotational and Vibrational temperatures | OES | Linear regression (Supervised ML) | [87] |
| Substrate characteristics | OES | k-Means Clustering (Unsupervised ML) | [87] |
| Separation distance between the electrodes | Electro-Acoustic Emission | Gaussian Process Regression (Supervised probabilistic ML) | [87] |
| Electron energy distribution function (EEDF) | OES | Genetic Algorithm (metaheuristic algorithm) | [91] |
| EEDF | OES, Momentum-transfer cross section | Visible Bremmsstrahlung Inversion (Supervised ML) | [92,93] |
| Time-series current signals from APPJ (discharge type and working gas) | Sensors/Probes | Convolutional neural networks (DL) | [94] |
| Plasma Plume length | Video frames of the plasma plume captured using a camera (iPhone 11) | Computer Vision algorithms | [95] |
| Temperature setpoint | Simulated data from thermal dynamics model of plasma-substrate interactions | Reinforcement learning | [89] |
| Self-Adaptive Plasma ChemistryGas input densities and Energy levels | OES | Artificial Neural Networks (DL), Gradual Mutation Algorithm | [85] |
| Pulse Discharge characteristics (current density and gap voltage) | Simulated fluid model data of time and pulse rise rate | Deep neural networks (DL) | [90] |
| Plasma chemistry (tokamak) | FTIR | Physics Informed Neural Networks | [96] |
| Input data | Real-time diagnostics | Algorithms used | Reference |
|---|---|---|---|
| CAP treatment duration and Discharge voltage applied | Cell viability Luminescence Assay | Model Predictive Control (MPC) | [48] |
| Cancer Cell viability ratio | Electrochemical Impedance Spectroscopy (EIS), operational parameters | Gaussian Process (GP), MPLC | [62] |
| Cancer Cell viability ratio | EIS, Cell viability assays, operational parameters | GP Regression (Supervised probabilistic ML), Safety Q – Reinforcement learning | [80] |
| Voltage applied, irradiation time, frequency of the plasma and flow rate of the feed gas on the extent of DNA damage | Agarose gel electrophoresis, UV fluorescence Imaging | Artificial Neural Networks (supervised DL)Physics Guided Neural Network (supervised DL | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





