1. Introduction
Irreversible electroporation (IRE) is a non-thermal tumor ablation technique that induces nanoscale pores in the plasma membrane, disrupting cellular homeostasis and leading to cell death while preserving extracellular matrix and vascular structures [
1]. Conventional IRE systems, such as NanoKnife and Galvanize Knife, deliver high-voltage pulses via direct electrode insertion, which can produce thermal effects and carry procedural risks, including venous thrombosis, biliary complications, gastrointestinal injuries, and rectal fistula, depending on the tumor location [
2,
3,
4]. Moreover, histologic and imaging studies provide only indirect evidence that NanoKnife or similar platforms achieve true non-thermal IRE, highlighting the need for alternative modalities that combine precision, safety, and mechanistic clarity [
5]. Traditional thermal-based surgical and ablative modalities also pose risks of collateral tissue damage and delayed healing [
6], emphasizing the importance of developing approaches that selectively target malignant cells while sparing surrounding normal tissue.
Cold atmospheric plasma (CAP) has rapidly emerged as a transformative modality in cancer therapy, offering a non-thermal approach through the generation of reactive oxygen and nitrogen species (RONS) that selectively target malignant cells [
7]. Over the past decade, our group has pioneered both the preclinical and clinical translation of CAP technologies, culminating in the development of the Canady Helios Cold Plasma (CHCP) system, the first FDA-approved CAP device(K240297) in the United States for intraoperative use [
8,
9,
10,
11]. Preclinical studies have demonstrated CAP’s ability to induce apoptosis, disrupt cell cycles7, and inhibit proliferation in a variety of cancer cell types, including breast [
9], head and neck [
12], colon, glioblastoma [
13], and soft tissue sarcoma [
8], while sparing normal tissue. The first Phase I clinical trial of CHCP for advanced solid tumors (NCT04267575) established safety and feasibility, with evidence of effective margin sterilization, exceptional local regional recurrence control, and promising survival outcomes without adverse effects [
14]. Previous studies showed that applying gas plasma increased the membrane permeability, allowing macromolecules to enter cells and disrupt homeostasis [
15]. While conventional IRE relies solely on direct electrode-delivered pulses [
16], CHCP integrates a high frequency electric charge with an inert gas which creates a localized Plasma Treated Electromagnetic Field (PTEF
TM) that includes reactive oxygen and nitrogen species including Hydrogen peroxide (H
2O
2), Atomic oxygen (O
- ),Ozone (O
3),Singlet oxygen (
1O
2), Superoxide anion (O
⋅−2),Carbonate radicals (CO
3⋅−),Nitric oxide (NO
⋅),Nitrogen dioxide (NO
2⋅),Nitrite (NO
2−),Nitrate (NO
3−),Peroxynitrite (ONOO
−) and Peroxynitric acid (ONOOH) [
14,
17]. PTEF provides a quantitative, reproducible framework for assessing membrane permeabilization during plasma treatment, distinguishing CHCP from tumor treating fields (TTF) and electrode-based IRE platforms. Notably, CHCP offers a non-contact, non-thermal, and controllable method for inducing IRE, enabling selective targeting of microscopic cancer cells while preserving surrounding normal tissue.
In this study, we systematically investigate CHCP-induced PTEF-mediated IRE across four biologically distinct breast cancer cell lines: triple-negative (MDA-MB-231, Hs578T), ER⁺/PR⁺/HER2⁻ (MCF-7), and ER⁺/PR⁺/HER2⁺ (BT-474). Using propidium iodide (PI) uptake as a sensitive marker of membrane integrity, we quantify field-strength and time dependent responses to CHCP treatment. PI is a membrane impermeant dye that fluoresces upon binding nucleic acids, providing a direct readout of membrane permeabilization and IRE induced cell death. We further demonstrate functional effects of CHCP induced electroporation, that includes intracellular delivery of small interfering RNA (siRNA) targeting the anti-apoptotic gene BCL2A1. We also showed that CHCP induced IRE by suppression of clonogenic potential of breast cancer cell lines. Moreover, morphological analyses of breast cancer cells and pathological analysis of patient tumor samples demonstrated selective induction of tumor cell death after CHCP treatment. Together, these findings establish CHCP induced PTEF as a novel, non-thermal (240 C), and non-contact IRE (NTNC-IRETM)platform for selective IRE based cancer therapy, with significant translational effect for selective tumor cell death at surgical margin and recurrence prevention.
2. Materials and Methods
2.1. Cold Plasma Device
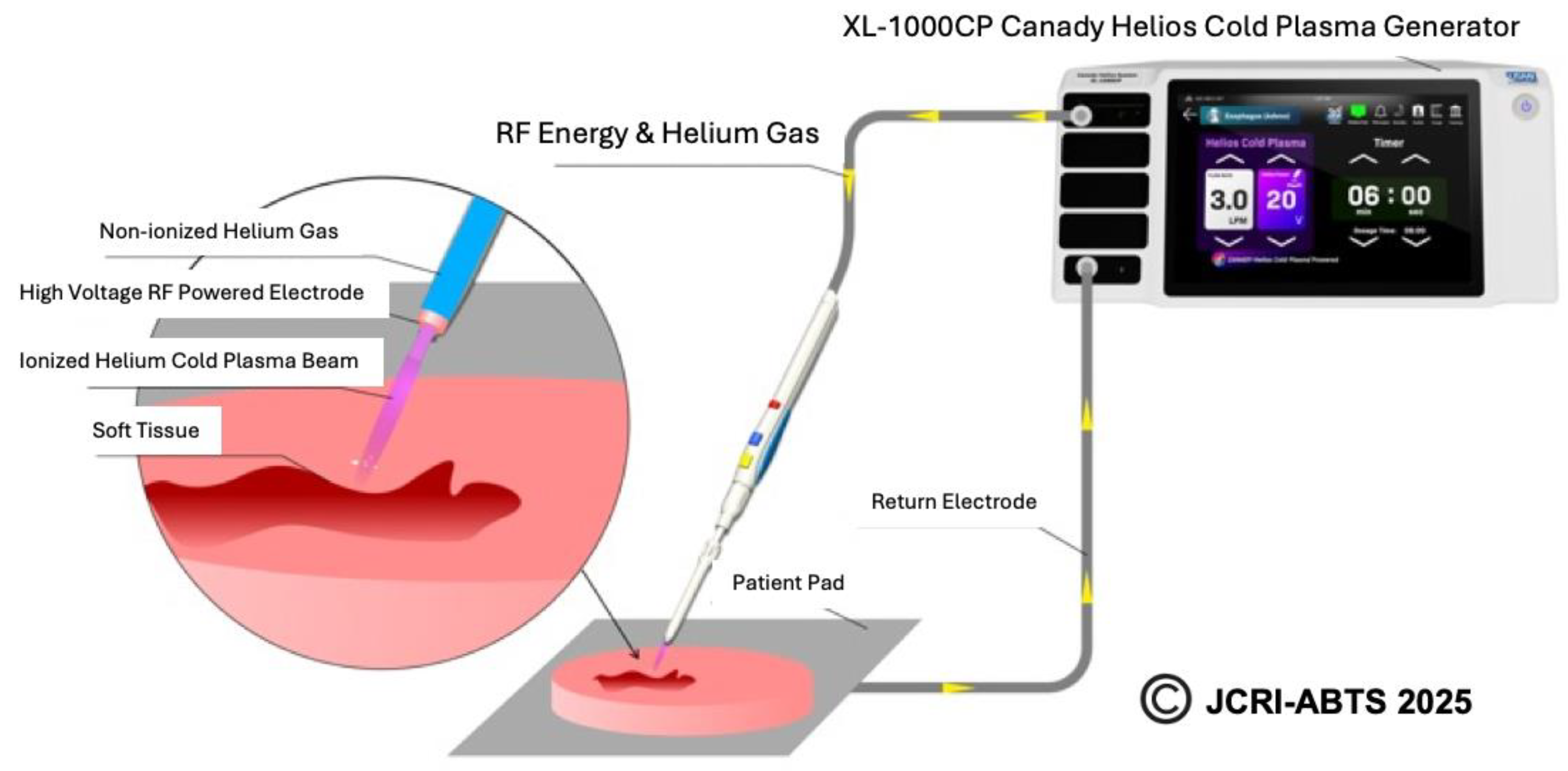
The Canady Helios Cold Plasma (CHCP) XL-1000 CP System was used for performing all experiments at Jerome Canady Research Institute for Advanced Biological and Technological Sciences (JCRI-ABTS), Takoma Park, MD, USA. Briefly, the CHCP XL-1000CP device (FDA cleared in 2024) integrates the high-frequency power generator, helium gas regulation system, and timer functions into a single unit. The system delivers an output voltage of up to 6 kV at a frequency of approximately 300 kHz with a power output of less than 40 W. The CHCP system is designed to operate with the FDA-cleared Canady Helios Cold Plasma Ablator, a disposable handpiece used for the ablation of tumor tissue. Details and schematics on plasma generation by CHCP used in this study is shown in
Figure 1. The helium flow rate was set to a constant 3 L/min and the power was set to 25V (~1675 V/cm PTEF) and 30V (~2010 V/cm PTEF). The plasma scalpel tip was placed 1.5 cm above the surface of the cell media and remained unmoved for the duration of the treatment. The CAP treatment was performed in a laminar airflow tissue culture hood, Purifier Logic + Class II, Type A2 Biosafety Cabinet (Labconco, Kansas City, MO, USA) at room temperature.
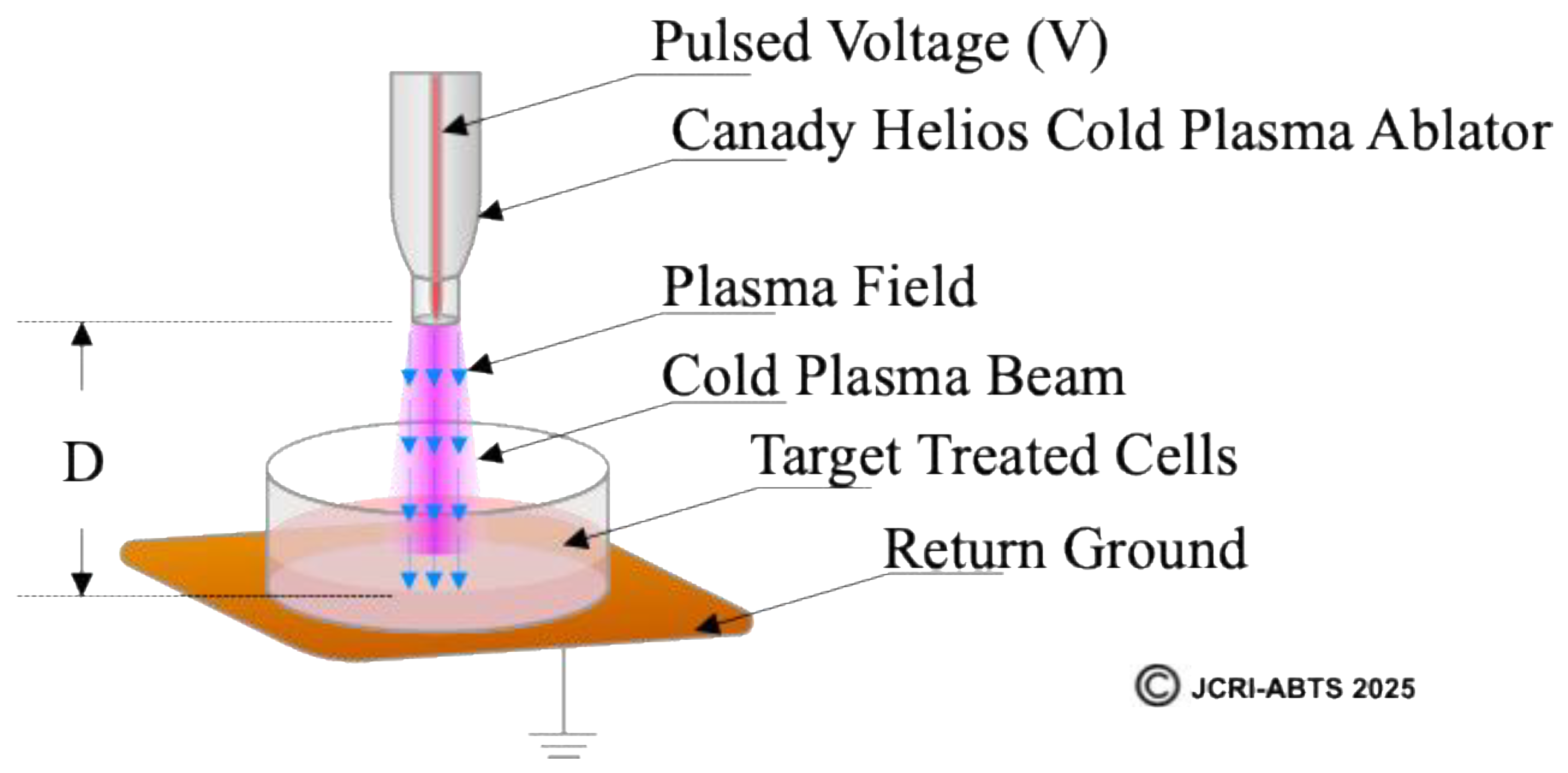
2.2. Definition and Calculation of Plasma Treated Electromagnetic Field (PTEFTM)
To quantitatively characterize the electrical component of CHCP treatment, we define the Plasma Treated Electromagnetic Field (PTEF
TM) as the effective electric field strength generated between the CHCP central electrode and the return ground pad beneath the cell culture. PTEF field strength was calculated using the following equation:
where
is the input voltage supplied by the CHCP XL-1000 CP generator,
is the voltage conversion coefficient (experimentally determined as 100), and D is the fixed distance between the plasma electrode tip and the cell culture surface (1.5 cm in this study). It should be noted that the reported values represent average electric field strengths; the peak electric field may be higher
18.
| Input Setting Voltage (V) |
PTEF Field Strength (V/cm) |
| 15 V |
~1005 V/cm |
| 20 V |
~1340 V/cm |
| 25 V |
~1675 V/cm |
| 30V |
~2010 V/cm |
A schematic illustration of PTEF (
Figure 2) shows the relationship between the plasma electrode, target cells, and ground pad, with the field vector indicated by a blue arrow. This figure provides a visual definition of PTEF and its experimental geometry. The top is highlighting the Canady Helios Cold Plasma Ablator
TM, the plasma beam directed toward the cell culture, the fixed distance (D), and the return ground. This diagram visually defines the PTEF as the spatially directed electric field vector that integrates both the localized electromagnetic field and the plasma-generated species to induce membrane permeabilization in target cells.
2.3. Cell Culture
Cell culture experiments were carried out as described previously. The human breast cancer cell line BT-474 was purchased from ATCC (Manassas, VA, USA). MCF-7, MDA-MB-231, and Hs578T were generously donated by Professor Kanaan’s laboratory at Howard University. All cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 Medium supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 1% Pen Strep (Thermo Fisher Scientific, Waltham, MA, USA) in a 37 °C and 5% CO2 humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA). When cells reached approximately 80% confluence, cells were seeded at a concentration of 105 cells/well into 12-well plates (USA Scientific, Ocala, FL, USA) with a 1 mL media volume per well for all experiments.
2.4. Treatment Protocol
Cells were directly treated with CHCP at 15V, 20V, 25V (~1675 V/cm PTEF) and 30V (1005 - 2010 V/cm) for 5 minutes using helium as the carrier gas or with plasma-activated media (PAM). Helium-treated cells served as negative controls.
2.5. Electroporation Assay and Propidium Iodide Staining
Human breast cancer cell lines (Hs578T, BT-474, MCF-7, and MDA-MB-231) were seeded at a density of 1 × 10⁵ cells per well in 12-well tissue culture plates and incubated overnight at 37 °C in a humidified atmosphere containing 5% CO₂. Cells were treated with CHCP using a standardized system as explained above. Untreated and helium-only exposed cells served as controls. Post-treatment, cells were incubated for 0, 30, 60 and 120 minutes, followed by staining with propidium iodide (PI). PI uptake intensity, indicating membrane permeability, was quantified via fluorescence imaging and analyzed statistically (n=3 per condition). Briefly, the culture medium was removed, and cells were incubated with propidium iodide (PI; 1 - 5 μg/mL in phenol red-free RPMI medium) for 30 minutes at 37 °C, protected from light. PI selectively stains cells with compromised membranes by intercalating into DNA, emitting fluorescence upon binding (excitation: 535 nm; emission: 617 nm). After incubation, cells were gently washed with phosphate-buffered saline (PBS) to remove unbound dye and subsequently fixed with 4% paraformaldehyde for 10 minutes or formalin for 5 minutes at room temperature. Cells were then washed again with PBS. Fluorescence intensity was quantified using a microplate reader equipped with appropriate filters (excitation at 535 nm and emission at 617 nm) (BioTek Synergy HTX (Winooski, VT, USA)). The fluorescence signal corresponds to the proportion of membrane-compromised cells. Fluorescence data from treated and control groups were analyzed to assess plasma-induced membrane permeability. Statistical significance was determined using appropriate tests (e.g., Student’s t-test or ANOVA), with p-values < 0.05 considered significant. Data was plotted by Microsoft Excel 2024 (Redmond, WA, USA) as mean ± standard error of the mean.
2.6. Transfection of siRNA
Human BCL2A1 targeting MISSION® esiRNA and matching scrambled control esiRNA were purchased from Sigma-Aldrich. Scrambled control esiRNA that does not target any gene was used as the negative control siRNA. MCF-7, MDA-MB-231 and Hs578T cell were transfected with siRNA after 30 mins following CHCP treatment. Transfection of BCL2A1 esiRNA or control esiRNA was done at 13 pmol. Cells were seeded in a 12-well-plate at a density of 1 × 105 cells/well overnight in (RPMI) 1640 Medium supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 1% Pen Strep (Thermo Fisher Scientific, Waltham, MA, USA) in a 37 °C and 5% CO2 humidified incubator. After 24 hours cells were treated with CHCP as explained above and after 30 mins of incubation the media was replaced with 1 mL of antibiotics-free medium RPMI mixed with esiRNA at the required concentration of 13 pmol/mL and were incubated at 6 hours and total RNA was isolated from the cells.
2.7. Quantitative Real-Time RT-PCR
Total RNA was extracted from MCF-7, MDA-MB-231 and Hs578T cell pellets using the TRI reagent and Direct-sol MiniPrep kit (Zymo Research) with DNAse treatment according to the manufacturer's instructions. First-strand cDNA was synthesized with 1 μg of total RNA from these cells using Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science). Real-time RT-PCR reactions were performed according to the MIQE Guidelines. Quantitative PCR was performed using 1 µL (diluted 1:20 using PCR grade water) of first strand cDNA under the conditions of 95 °C for 15 s, annealing at 60 °C for 60 s, extension at 72 °C for 30 s for 40 cycles, and a final extension at 72 °C for 10 min using SYBR Green Master Mix (Applied Biosciences). Primer sequences for analyzing 18s RNA were used for normalization and relative mRNA expression was calculated with 2 − ΔΔCT method.
2.8. Plate Colony Formation Assay
MDA-MB-231, MCF-7, and Hs578T cells were seeded at a density of 1 × 10⁵ cells per well in 6-well culture plates and incubated for 24 hours under standard culture conditions (37 °C, 5% CO₂). Cells were subsequently treated with CHCP at voltages of 15 V, 20 V, 25 V, or 30 V for 5 minutes. Following treatment, cells were harvested at three different post-treatment incubation times (30, 60, and 120 minutes), diluted 1:1000 in fresh RPMI medium, and reseeded into 12-well plates to allow colony formation. After 7-10 days of incubation, colonies were fixed with 10% neutral-buffered formalin for 30 minutes and stained with 1% crystal violet for 15 minutes. Excess dye was removed by washing three times with phosphate buffered saline (PBS), and plates were air-dried at room temperature. Colony formation was quantified by counting visible colonies containing ≥50 cells under a light microscope. Colony numbers were recorded as mean ± SEM from three independent experiments.
2.9. Hematoxylin and Eosin (H&E) Staining of Tissue Specimens
Fresh tissue specimens, with or without ex vivo CHCP treatment, were fixed in 10% neutral-buffered formalin for 24-48 hours. Fixed tissues were dehydrated through a graded ethanol series, cleared in xylene, and embedded in paraffin wax. Serial sections of 6-7μm thickness were prepared using a microtome and mounted on glass slides. Sections were deparaffinized in xylene, rehydrated through descending ethanol concentrations, and subjected to standard H&E staining. Briefly, slides were stained with hematoxylin to visualize nuclei, differentiated in acid-alcohol, and counterstained with eosin to highlight cytoplasmic and extracellular components. Following staining, sections were dehydrated with ethanol, and cover slipped using a permanent mounting medium. Stained slides were examined under a light microscope (63× objective) to assess cellular morphology, tissue architecture, and pathological alterations, including plasma-induced cell death and membrane compromise.
2.10. Statistical Analysis
Data are presented as mean ± SEM. Comparisons between CHCP-treated and helium control groups were analyzed using Student’s t-test and one-way ANOVA followed by Post hoc Tukey’s tests. A p-value < 0.05 was considered statistically significant.
4. Discussion
Irreversible electroporation (IRE) has been investigated for over two decades as a non-thermal method of tumor ablation, with foundational studies by Robinsky [
20,
21] who demonstrated its potential to increase plasma membrane permeability through externally applied electric fields. Conventional clinical platforms, such as NanoKnife [
22] and Galvanize Knife [
23], rely on direct electrode insertion to deliver high-voltage pulses, creating transient nanopores that induce cell death. However, these systems are invasive, can produce unintended thermal effects, and often lack direct evidence of non-thermal electroporation activity [
2,
3,
4,
24].
In contrast, the CHCP system induces electroporation through a combination of localized electromagnetic fields and reactive plasma species, enabling NTNC-IRETM-mediated membrane disruption. Our in vitro studies demonstrate that CHCP produces voltage and time dependent electroporation across multiple breast cancer subtypes. At higher PTEF values (~2010 V/cm, corresponding to 30 V), robust and sustained PI uptake was observed in MDA-MB-231, MCF-7, Hs578T, and BT-474 cells, whereas intermediate PTEF (~1675 V/cm, 25 V) resulted in only transient membrane permeability that largely resolved within 30 to 60 minutes. Notably, triple-negative Hs578T and HER2-positive BT-474 cells exhibited prolonged PI uptake at 30 V, while hormone receptor-positive MCF-7 cells returned to baseline PI intensity. The reduction of cells in the CHCP treated samples is due to the stained severely membrane compromised cells that are removed during the washing process in the PI assay protocol. The difference in PI uptake in these cell lines, is due to the membrane composition, cytoskeletal organization, and intrinsic repair mechanisms which modulate electroporation dynamics.
These results are corroborated by morphological analyses, which revealed voltage dependent membrane blebbing, cell shrinkage, and detachment. Cells treated at 30 V detached more rapidly (4 to 6 hours for MDA-MB-231 and Hs578T) compared to those treated at 25 V (24 hours), indicating irreversible electroporation with increasing voltage. No morphological changes were observed in untreated or helium-only controls, confirming that CHCP-induced effects are specifically driven by the applied electric field in combination with plasma-mediated oxidative stress.
Functionally, CHCP-mediated electroporation permits intracellular delivery of macromolecules. In vitro and in vivo studies have demonstrated that cold atmospheric plasma can successfully deliver plasmids, linear DNA, siRNA, and miRNA into multiple established cell lines various cell lines as well as into live mouse models [
15]. Using BCL2A1 expression as a readout, we demonstrated that esiRNA delivery and subsequent gene silencing occurred only at higher PTEF levels (~1675-2010 V/cm), whereas lower voltages (~1005-1340 V/cm) were insufficient to induce RNA uptake. These findings establish a clear voltage threshold for stable pore formation and provide mechanistic evidence that CHCP can be used as a controlled biophysical tool for intracellular access. Our study corroborates with Experimental studies using dielectric barrier discharge (DBD) sources of CAP have shown that microsecond-pulsed plasma treatments induce robust and stable pore formation in biological membranes, similar to electroporation, significantly reducing transepithelial electrical resistance (TEER). This effect is power-density and voltage-dependent, with higher power microsecond DBDs producing more uniform and sustained permeabilization compared to nanosecond DBDs, which rely more on lipid oxidation mechanisms [
25].
CHCP induced tumor cell death arises from the synergistic interplay of IRE and plasma generated RONS. IRE exploits tumor cell membrane vulnerabilities such as altered lipid composition, heightened metabolic stress, and depolarized resting potentials to achieve enhanced selectivity and efficacy in tumor ablation [
26]. Tumor membranes, with disordered lipid organization and reduced stability, are more easily destabilized by electric fields, lowering the threshold for pore formation and promoting irreversible disruption. Depolarized potentials further reduce the critical field strength required for permeabilization, giving tumor cells greater susceptibility than normal cells [
16]. In addition, metabolic stress and diminished repair capacity limit the ability of tumor membranes to reseal after pore formation, while ATP depletion and disrupted ionic gradients rapidly push cells toward apoptosis. The acidic and hypoxic tumor microenvironment further sensitizes malignant cells, amplifying the cytotoxic impact of electroporation and RONS. Meanwhile, RONS generated by cold plasma overwhelm the already fragile redox balance of tumor cells, driving lipid peroxidation, protein oxidation, mitochondrial collapse, and DNA damage. Clinical and experimental evidence confirms that these physicochemical vulnerabilities make tumor cells predictably more sensitive to IRE than surrounding healthy tissues, an effect supported by mathematical modeling of electric field thresholds [
27].
In parallel, short-lived reactive species generated by plasma including hydroxyl radicals (∙OH), atomic oxygen (O∙), and hypochlorite (OCl⁻) synergize with the electric field to destabilize membranes [
17,
28]. These species trigger lipid peroxidation, altering fluidity and integrity of the bilayer, while also oxidizing membrane proteins and inactivating protective enzymes such as catalase [
28]. Such oxidative modifications further weaken the tumor membrane and promote sustained pore formation. Direct physical contributions of plasma (electric fields and photons) act alongside chemical oxidation, producing a dual mode of action. Furthermore, membrane damage induced by irreversible electroporation (IRE) promotes immunogenic cell death through the exposure and release of calreticulin, ATP, HMGB1, and other damage-associated molecular patterns (DAMPs), which recruit and activate antigen-presenting cells at the tumor site [
29]. These signals facilitate uptake of tumor debris, cross-priming of cytotoxic T cells, and amplification of innate and adaptive immune responses [
30], thereby linking CHCP-induced electroporation with antitumor immunity.
Importantly, the translational relevance of CHCP was confirmed in ex vivo histopathology analyses of resected human zone 0 specimens from our Phase I clinical CHCP trial [
14]. H&E-stained sections revealed selective tumor cell death at surgical margins (Zone 0), characterized by plasma membrane disruption, cytoplasmic leakage, and nuclear condensation, consistent with an IRE mechanism. Surrounding non-malignant and vascular structures were preserved, demonstrating the tumor-selective nature of CHCP treatment. Notably, CHCP treatment extended the survival of the R0 patients who had microscopic residual disease, highlighting the potential of CHCP for tumor cell death at the surgical margin and reduction of microscopic residual disease which is a critical factor in preventing local recurrence and improving long-term survival in advanced solid tumors.
CHCP offers several advantages including non-contact application, precise control over voltage and exposure duration, non-thermal (22-24
0 C) tumor cell death, and sustained membrane permeability compared to the classical IRE techniques, such as probe-based high-voltage pulses or nanosecond pulsed electric fields (i.e, NanoKnife [
22] and Galvanize Knife [
23]). The integration of electromagnetic field effects with reactive plasma species contributes to prolonged pore stability, amplify selective tumor cell death in a cell-type dependent manner. These unique properties make CHCP a versatile platform of NTNC-IRETM for selective tumor cell death.
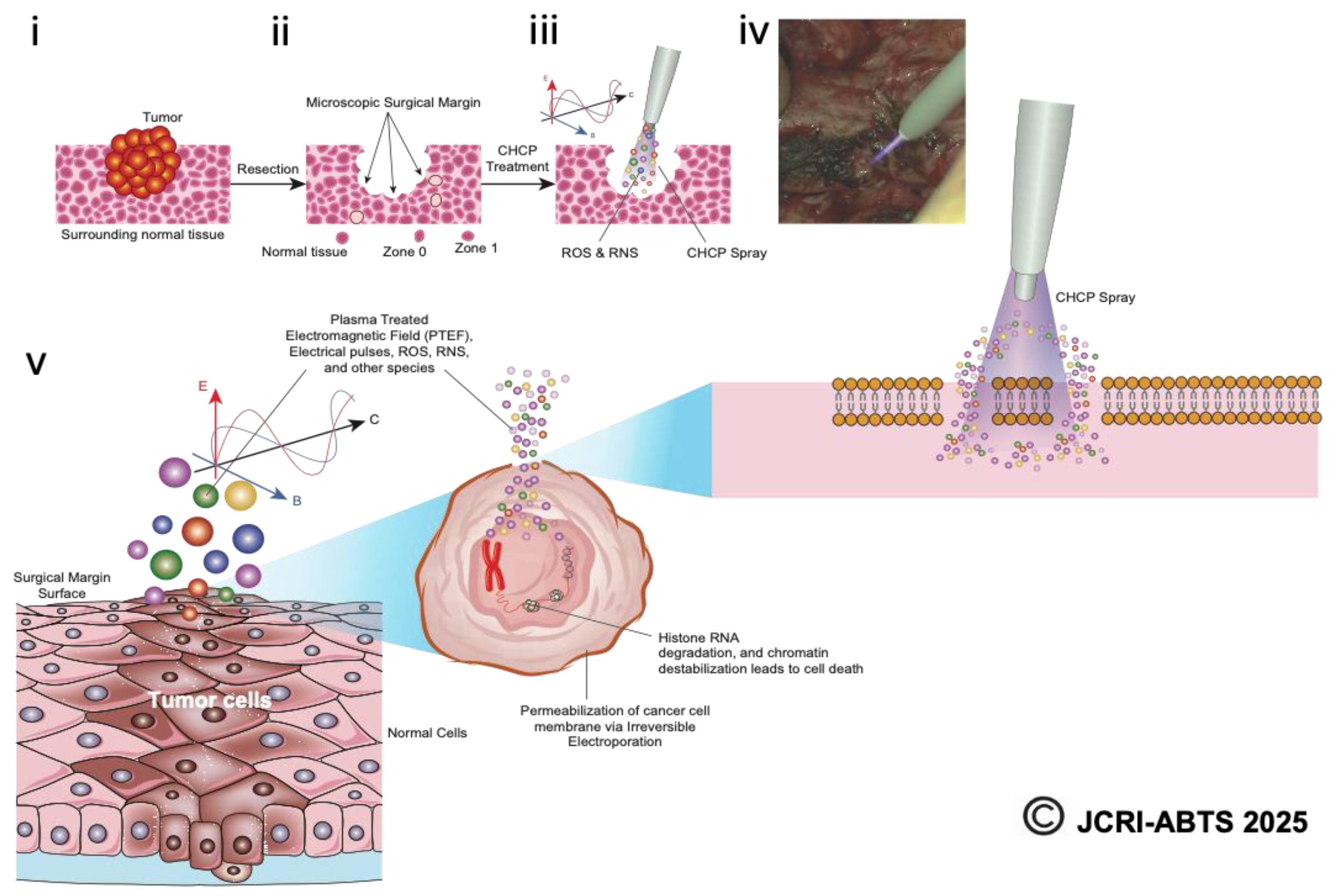
Figure 11.
Schematic representation of the Canady Helios Cold Plasma (CHCP) treatment workflow. The illustration depicts (i) surgical resection of the tumor using standard oncologic procedures, (ii) collection of specimens from the tumor core, surgical margin (Zone 0), peritumoral tissue (Zone 1), and adjacent normal tissue, and (iii) intraoperative application of CHCP spray directly to the surgical margin. (iv) A representative intraoperative image shows CHCP treatment of a pancreatic surgical margin following a Whipple procedure. (v) The schematic further highlights CHCP-generated PTEF™, where E denotes the electric field, B the magnetic field, and C the three-dimensional vector reference of the magnetic field. Reactive oxygen and nitrogen species (ROS/RNS), including hydroxyl radicals (OH*), are shown entering the cancer cell membrane through transient “pores” formed by CHCP-induced electroporation, ultimately disrupting cellular integrity and promoting selective tumor cell death.
Figure 11.
Schematic representation of the Canady Helios Cold Plasma (CHCP) treatment workflow. The illustration depicts (i) surgical resection of the tumor using standard oncologic procedures, (ii) collection of specimens from the tumor core, surgical margin (Zone 0), peritumoral tissue (Zone 1), and adjacent normal tissue, and (iii) intraoperative application of CHCP spray directly to the surgical margin. (iv) A representative intraoperative image shows CHCP treatment of a pancreatic surgical margin following a Whipple procedure. (v) The schematic further highlights CHCP-generated PTEF™, where E denotes the electric field, B the magnetic field, and C the three-dimensional vector reference of the magnetic field. Reactive oxygen and nitrogen species (ROS/RNS), including hydroxyl radicals (OH*), are shown entering the cancer cell membrane through transient “pores” formed by CHCP-induced electroporation, ultimately disrupting cellular integrity and promoting selective tumor cell death.
In summary, our findings establish CHCP as a novel, NTNC-IRETM modality with both mechanistic and clinical significance. Voltage and cell type dependent membrane permeabilization, supported by functional RNA delivery and histopathological evidence, underscores its potential for intraoperative margin selective tumor cell death. By selectively targeting residual malignant cells while preserving healthy tissue, CHCP reduce recurrence risk and improve surgical outcomes in patients with advanced or recurrent solid tumors.
Author Contributions
Conceptualization, J.C.; Methodology, J.C. and S.M.; Software, T.Z.; Validation, J.C. T.Z. S.M., A.N., O.J., and Y.D.; Formal Analysis, J.C., S.M., and T.Z.; Investigation, J.C. and S.M.,; Resources, J.C.; Data Curation, J.C. ,S.M., O.J., and Y.D.; Writing – Original Draft Preparation, J.C. ,S.M., and T.Z.; Writing – Review & Editing, J.C. ,S.M., O.J., M.K., Y.D., and A.N.; Visualization, J.C. T.Z. and S.M.; Supervision, J.C.; Project Administration, J.C. T.Z. and S.M.; Funding Acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Illustration showing the CHCP system.
Figure 1.
Illustration showing the CHCP system.
Figure 2.
Schematic representation of the Plasma Treated Electromagnetic Field (PTEFTM) generated by the Canady Helios Cold Plasma (CHCP) system.
Figure 2.
Schematic representation of the Plasma Treated Electromagnetic Field (PTEFTM) generated by the Canady Helios Cold Plasma (CHCP) system.
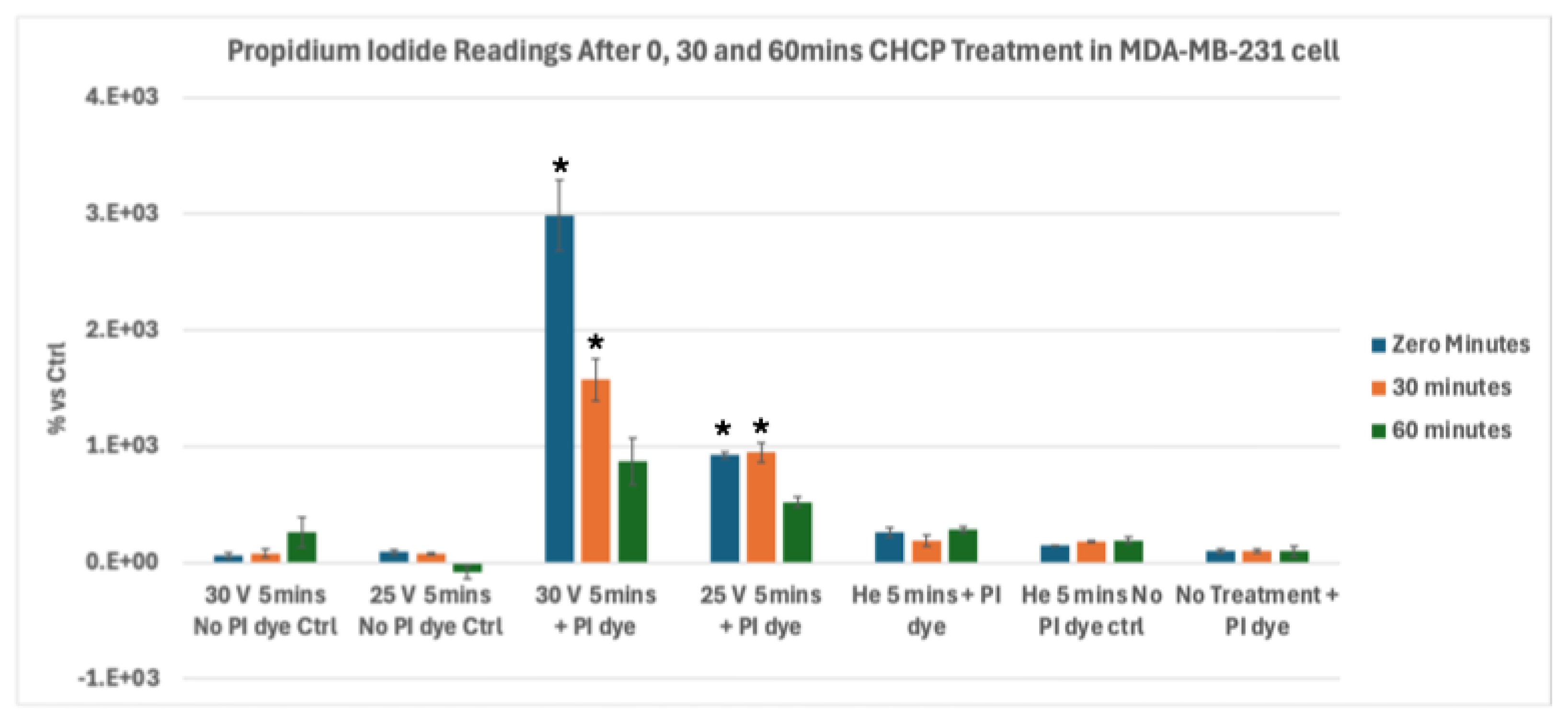
Figure 3.
Analysis of electroporation effects of CHCP treatment on triple-negative breast cancer MDA-MB-231 cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF), 25V (~1675 V/cm PTEF) and Helium for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.016, * p < 0.020 and p < 0.062 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.001, *p < 0.008 and p < 0.618 respectively.
Figure 3.
Analysis of electroporation effects of CHCP treatment on triple-negative breast cancer MDA-MB-231 cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF), 25V (~1675 V/cm PTEF) and Helium for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.016, * p < 0.020 and p < 0.062 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.001, *p < 0.008 and p < 0.618 respectively.
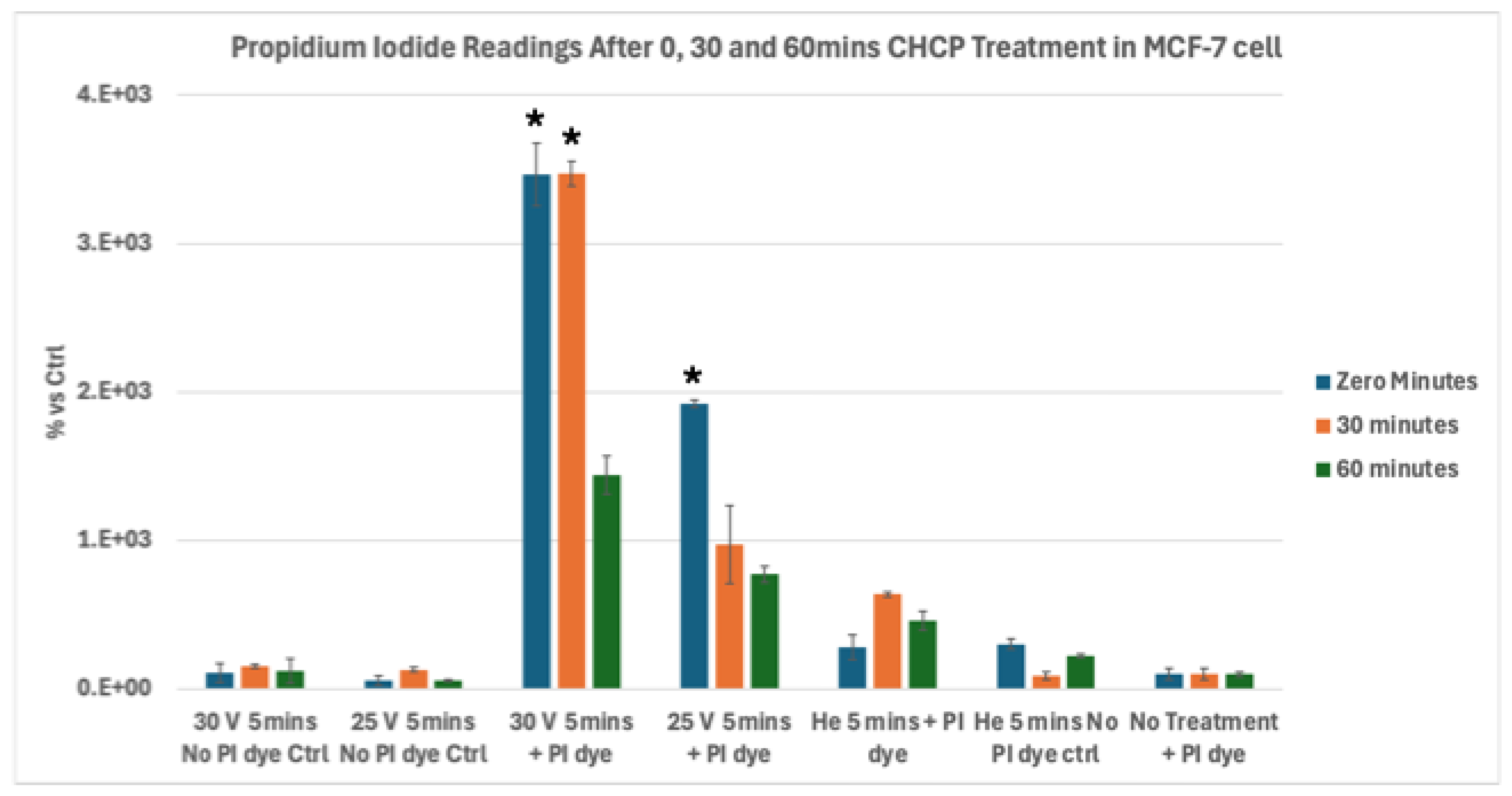
Figure 4.
Analysis of electroporation effects of CHCP treatment on ER+ PR+ HER2- breast cancer MCF-7 cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF) (A) and 25V (~1675 V/cm PTEF) (B) for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as a mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0015, * p < 0.0089 and *p < 0.013 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.014, p < 0.203 and p < 0.168 respectively.
Figure 4.
Analysis of electroporation effects of CHCP treatment on ER+ PR+ HER2- breast cancer MCF-7 cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF) (A) and 25V (~1675 V/cm PTEF) (B) for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as a mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0015, * p < 0.0089 and *p < 0.013 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.014, p < 0.203 and p < 0.168 respectively.
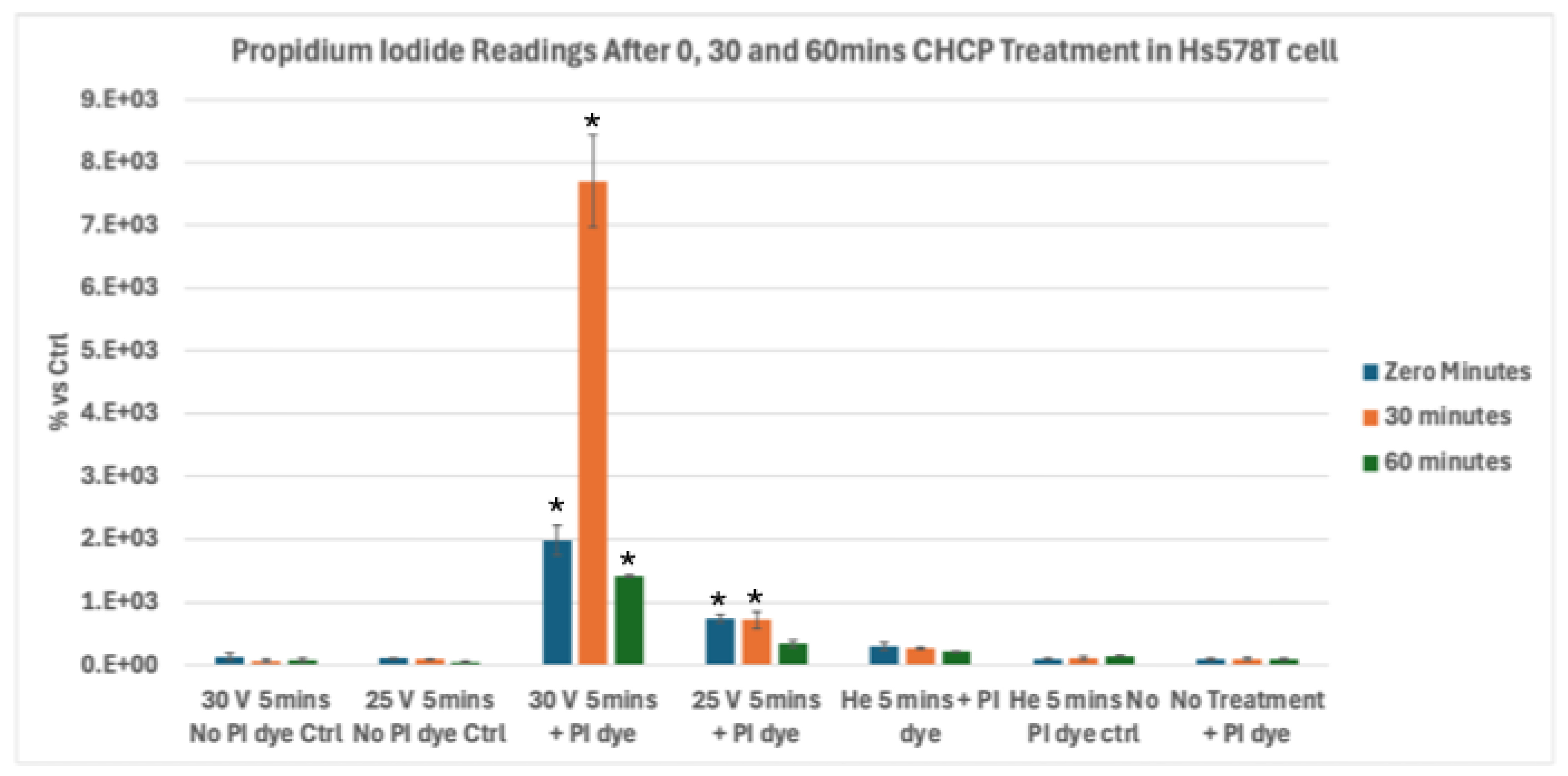
Figure 5.
Analysis of Electroporation effects of CHCP treatment on triple-negative breast cancer Hs578T cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF) and 25V (~1675 V/cm PTEF) for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0030, * p < 0.0105 and *p < 0.00014 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0087, *p < 0.03 and p < 0.783 respectively.
Figure 5.
Analysis of Electroporation effects of CHCP treatment on triple-negative breast cancer Hs578T cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF) and 25V (~1675 V/cm PTEF) for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0030, * p < 0.0105 and *p < 0.00014 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0087, *p < 0.03 and p < 0.783 respectively.
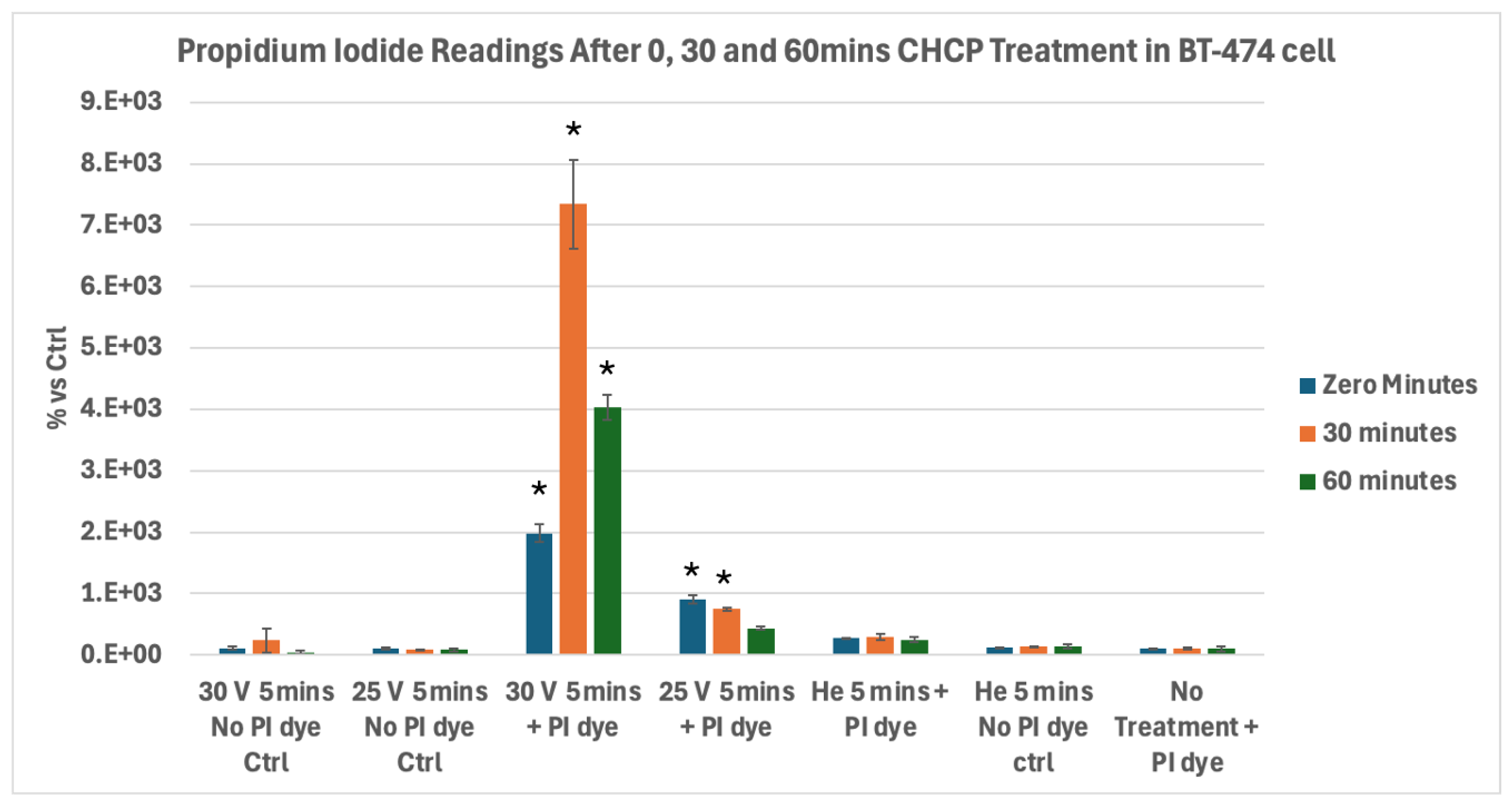
Figure 6.
Analysis of Electroporation effects of CHCP treatment on ER+ PR+ HER2+ breast cancer BT-474 cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF) and 25V (~1675 V/cm PTEF) for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0005, * p < 0.0010 and *p < 0.0001 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0008, *p < 0.0135 and p < 0.273 respectively.
Figure 6.
Analysis of Electroporation effects of CHCP treatment on ER+ PR+ HER2+ breast cancer BT-474 cells using PI intake intensity measurements. Cells were treated with CHCP at 30V (~2010 V/cm PTEF) and 25V (~1675 V/cm PTEF) for 5 minutes, followed by analysis at multiple incubation time points (0, 30 and 60 minutes) to assess plasma-induced membrane permeability. Percentage of PI intensity indicates the degree of cell membrane electroporation, with data represented as mean ± SEM (n = 3). Statistical significance was analyzed between CHCP-treated and helium-treated samples using Student’s t-test, where 30V (~2010 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0005, * p < 0.0010 and *p < 0.0001 respectively. where 25V (~1675 V/cm PTEF), at Zero, 0.5 and 1 hour were *p < 0.0008, *p < 0.0135 and p < 0.273 respectively.
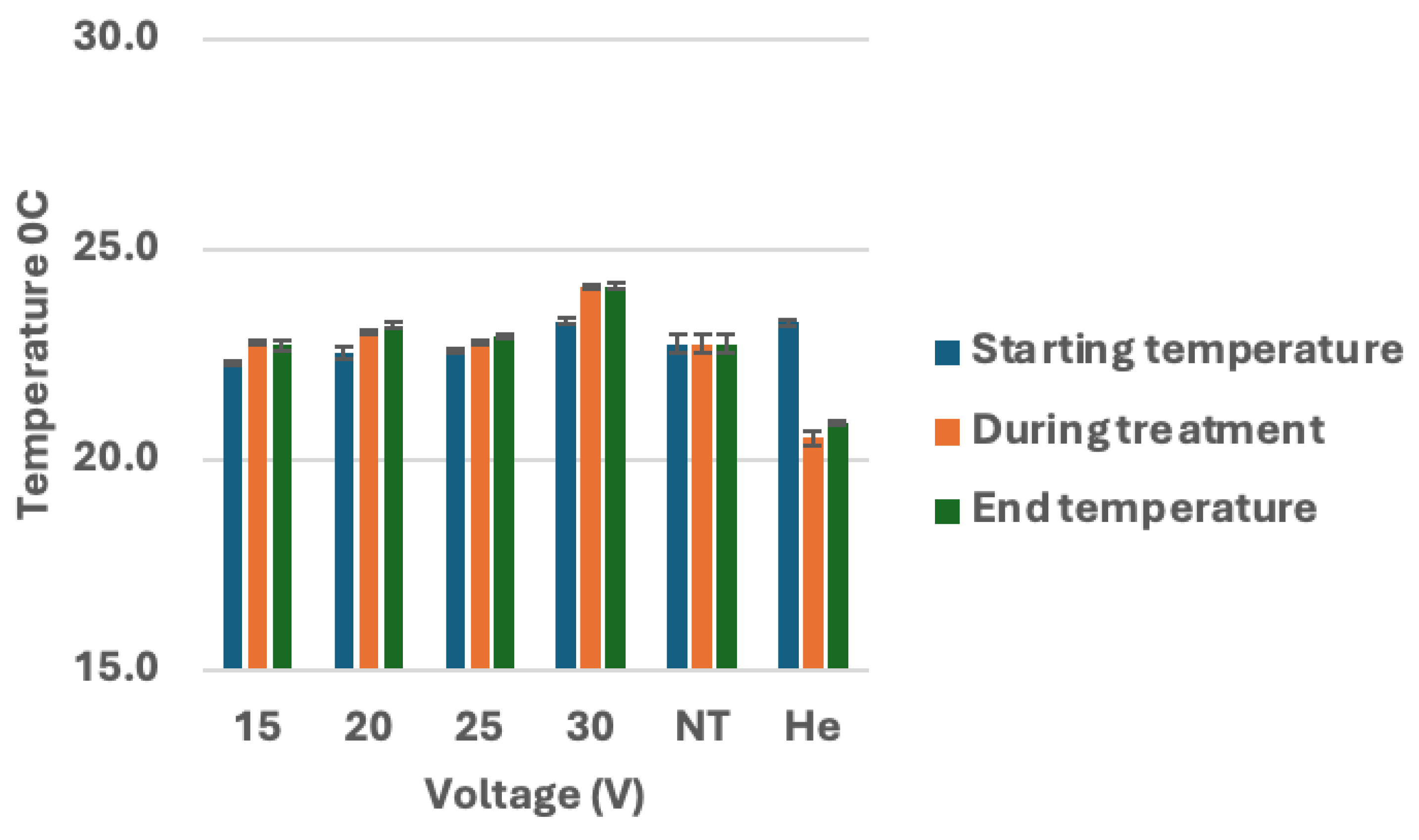
Figure 7.
Analysis of temperature during CHCP treatment on breast cancer cells. Breast cancer cell lines MDA-MB-231, MCF-7, and Hs578T were treated with CHCP at 15 V, 20 V, 25 V, and 30 V for 5 minutes and temperature measurements of media were recorded before, during and after the treatment. Figure showing bar graphs showing the temperatures. The error bars represent means ± SEM. (n = 3). No Treatment (NT) and Helium control (He) at zero volts.
Figure 7.
Analysis of temperature during CHCP treatment on breast cancer cells. Breast cancer cell lines MDA-MB-231, MCF-7, and Hs578T were treated with CHCP at 15 V, 20 V, 25 V, and 30 V for 5 minutes and temperature measurements of media were recorded before, during and after the treatment. Figure showing bar graphs showing the temperatures. The error bars represent means ± SEM. (n = 3). No Treatment (NT) and Helium control (He) at zero volts.
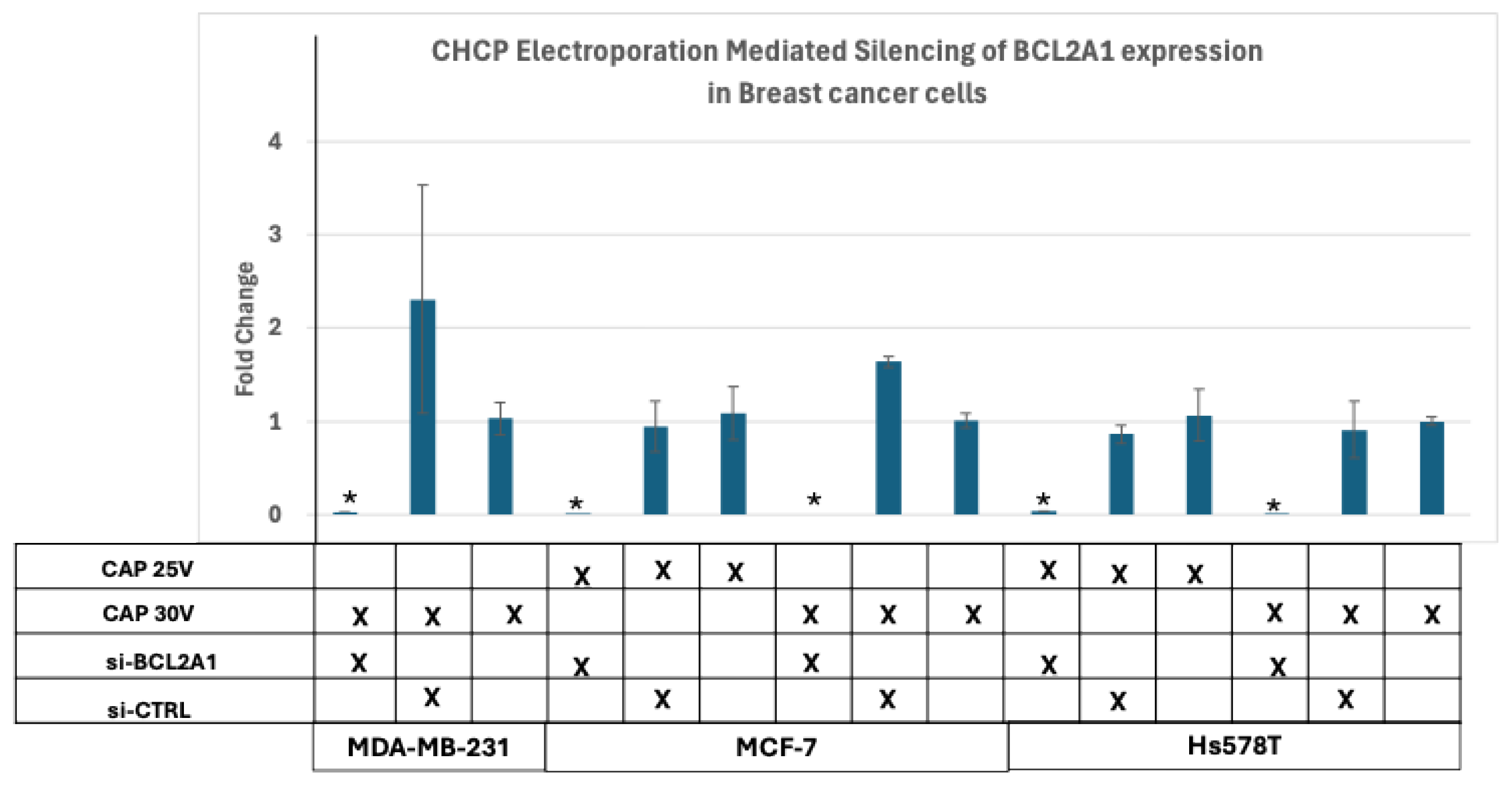
Figure 8.
Analysis of Electroporation effects of CHCP treatment on breast cancer cells by silencing of BCL2A1 mRNA. Transfection of siBCL2A1 by CHCP treatment. Breast cancer cell lines MDA-MB-231, MCF-7, and Hs578T were treated with CHCP at 15 V, 20 V, 25 V, and 30V (~2010 V/cm PTEF) for 5 minutes. Bar graphs showing the expression of BCL2A1 mRNA with esiRNA for BCL2A1 silencing with CAP treatment. The error bars represent means ± SEM. (n = 3). (MDA-MD-231 at 30V (~2010 V/cm PTEF) *p < 0.0055, MCF-7 at 25V (~1675 V/cm PTEF) *p < 0.012 and 30V *p < 5.76253E-06; Hs578T at 25V (~1675 V/cm PTEF) *p < 0.0005, and 30V (~2010 V/cm PTEF) *p < 0.02, Student’s t-test for siBCL2A1 versus control siRNA.
Figure 8.
Analysis of Electroporation effects of CHCP treatment on breast cancer cells by silencing of BCL2A1 mRNA. Transfection of siBCL2A1 by CHCP treatment. Breast cancer cell lines MDA-MB-231, MCF-7, and Hs578T were treated with CHCP at 15 V, 20 V, 25 V, and 30V (~2010 V/cm PTEF) for 5 minutes. Bar graphs showing the expression of BCL2A1 mRNA with esiRNA for BCL2A1 silencing with CAP treatment. The error bars represent means ± SEM. (n = 3). (MDA-MD-231 at 30V (~2010 V/cm PTEF) *p < 0.0055, MCF-7 at 25V (~1675 V/cm PTEF) *p < 0.012 and 30V *p < 5.76253E-06; Hs578T at 25V (~1675 V/cm PTEF) *p < 0.0005, and 30V (~2010 V/cm PTEF) *p < 0.02, Student’s t-test for siBCL2A1 versus control siRNA.
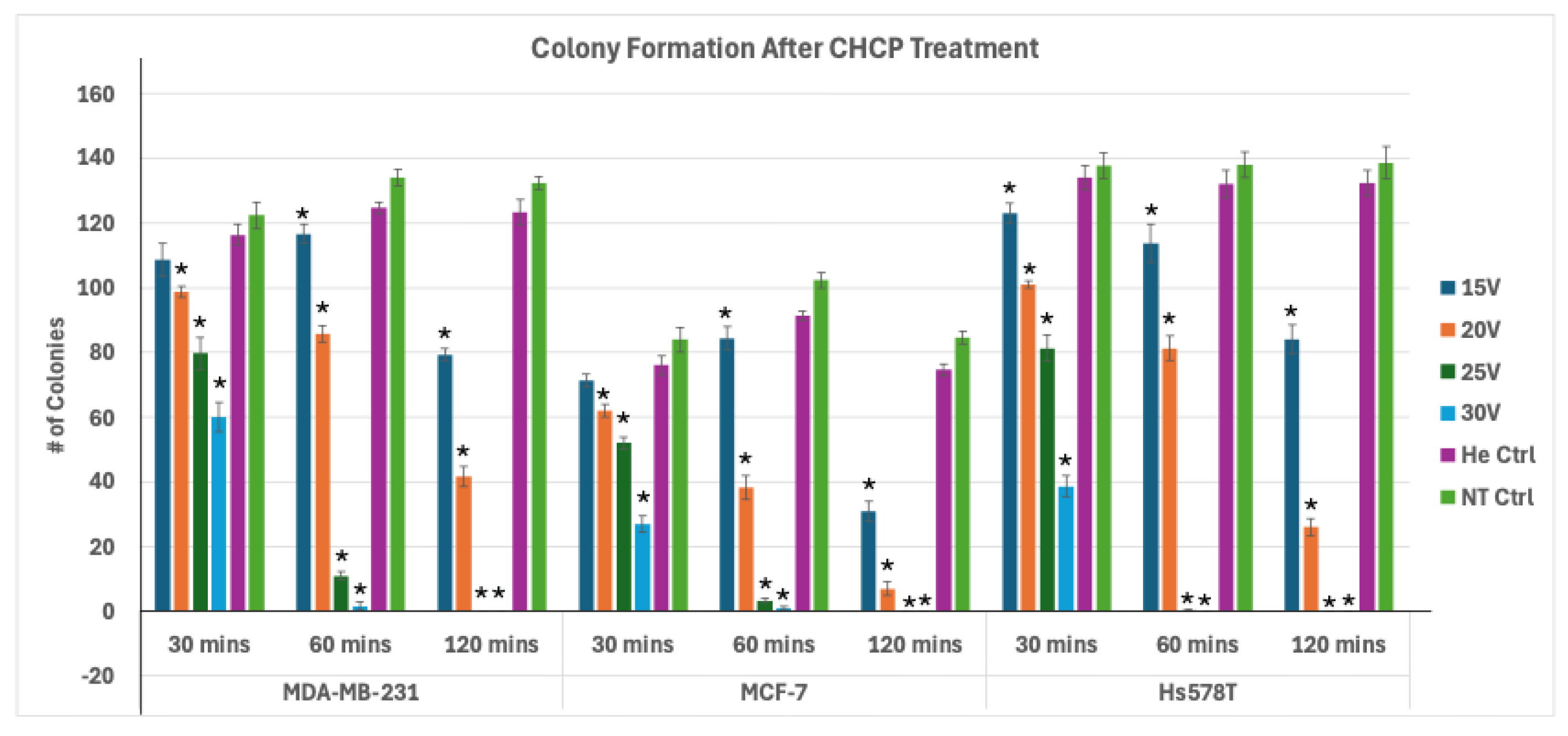
Figure 9.
Analysis of Electroporation effects of CHCP treatment on breast cancer cells on colony formation. Breast cancer cell lines MDA-MB-231, MCF-7, and Hs578T were treated with CHCP at 15 V, 20 V, 25 V, and 30 V for 5 minutes and incubated for 30, 60 and 120 mins followed by colony formation assay. Figure showing bar graphs showing the number of colonies with CAP treatment. The error bars represent means ± SEM. (n = 3). Significance was set at p < 0.05, One-way ANOVA followed by Post hoc Tukey’s tests for treated samples versus Helium control. All p-values < 0 (*), indicating highly significant.
Figure 9.
Analysis of Electroporation effects of CHCP treatment on breast cancer cells on colony formation. Breast cancer cell lines MDA-MB-231, MCF-7, and Hs578T were treated with CHCP at 15 V, 20 V, 25 V, and 30 V for 5 minutes and incubated for 30, 60 and 120 mins followed by colony formation assay. Figure showing bar graphs showing the number of colonies with CAP treatment. The error bars represent means ± SEM. (n = 3). Significance was set at p < 0.05, One-way ANOVA followed by Post hoc Tukey’s tests for treated samples versus Helium control. All p-values < 0 (*), indicating highly significant.
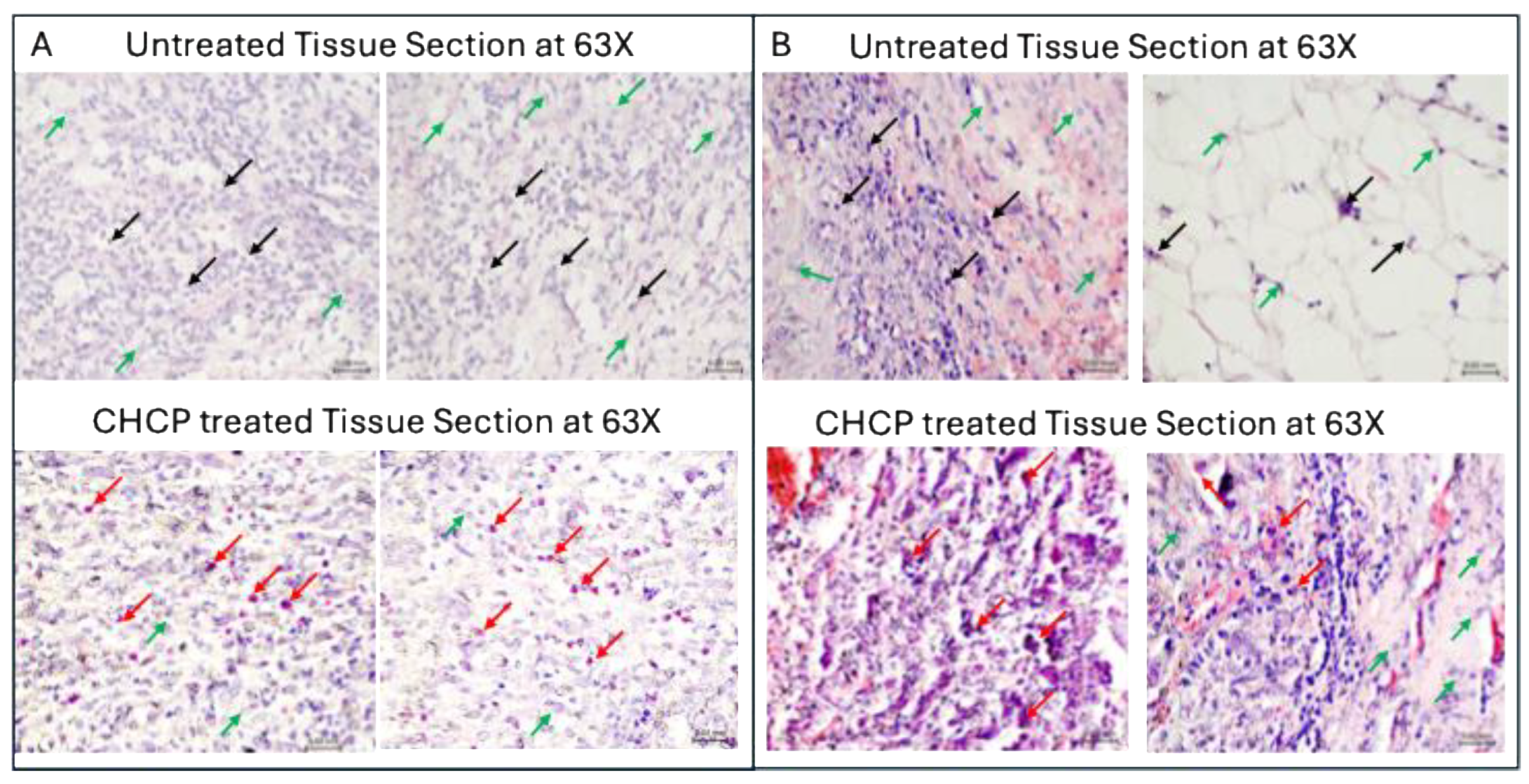
Figure 10.
Light micrographs of H&E-stained Zone 0 tissue samples from patient (A) R0009 (metastatic pleomorphic sarcoma of the left distal femur) and (B) R0004 (metastatic recurrent non-small cell lung adenocarcinoma of the left hip/proximal femur) with or without Canady Helios Cold Plasma (CHCP) treatment, imaged at 63× objective magnification. Black arrows indicate viable untreated tumor cells, red arrows denote CHCP-treated tumor cells exhibiting apoptosis with cellular cytosolic content leakage and while green arrows represent normal cells. Scale bars = 0.2 mm.
Figure 10.
Light micrographs of H&E-stained Zone 0 tissue samples from patient (A) R0009 (metastatic pleomorphic sarcoma of the left distal femur) and (B) R0004 (metastatic recurrent non-small cell lung adenocarcinoma of the left hip/proximal femur) with or without Canady Helios Cold Plasma (CHCP) treatment, imaged at 63× objective magnification. Black arrows indicate viable untreated tumor cells, red arrows denote CHCP-treated tumor cells exhibiting apoptosis with cellular cytosolic content leakage and while green arrows represent normal cells. Scale bars = 0.2 mm.
Table 1.
ANOVA Results for Colony Formation Across Cell Lines and Time Points.
Table 1.
ANOVA Results for Colony Formation Across Cell Lines and Time Points.
| Cell Line |
Time Point |
F-statistic |
p-value |
| MDA-MB-231 |
30 min |
33.3 |
1.23 × 10⁻⁶ |
| MDA-MB-231 |
60 min |
742.1 |
1.64 × 10⁻¹⁴ |
| MDA-MB-231 |
120 min |
626.3 |
4.52 × 10⁻¹⁴ |
| MCF-7 |
30 min |
63.1 |
3.38 × 10⁻⁸ |
| MCF-7 |
60 min |
353.2 |
1.37 × 10⁻¹² |
| MCF-7 |
120 min |
410.7 |
5.6 × 10⁻¹³ |
| Hs578T |
30 min |
131.1 |
4.84 × 10⁻¹⁰ |
| Hs578T |
60 min |
289.1 |
4.52 × 10⁻¹² |
| Hs578T |
120 min |
358.4 |
1.26 × 10⁻¹² |