Submitted:
17 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
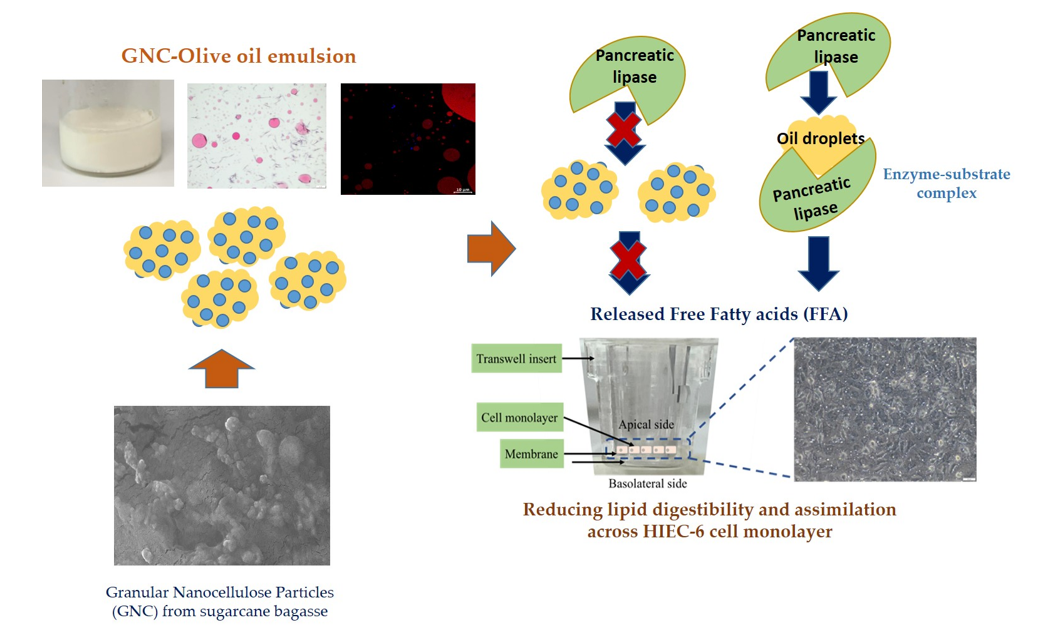
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Granular Nanocellulose Particle (GNC) Preparation
2.3. Simulated Gastrointestinal Tract (GIT) Fluids and Enzyme Solutions Preparation
2.4. In Vitro Simulated GIT Digestion System
2.5. The Role of GNC in Releasing FFA Content in the Simulated GIT System
2.5.1. GNC Concentrations
2.5.2. The Simulation Mixture of the GNC and Olive Oil
2.6. The Characteristics of GNC During In Vitro GIT Simulation
2.6.1. The Particle Size Distribution and Zeta Potential Determination
2.6.2. The GNC-Olive Oil Droplets Characterization
2.6.3. The Interfacial of GNC and Olive Oil Emulsion Observation
2.7. Cytotoxicity of GNC
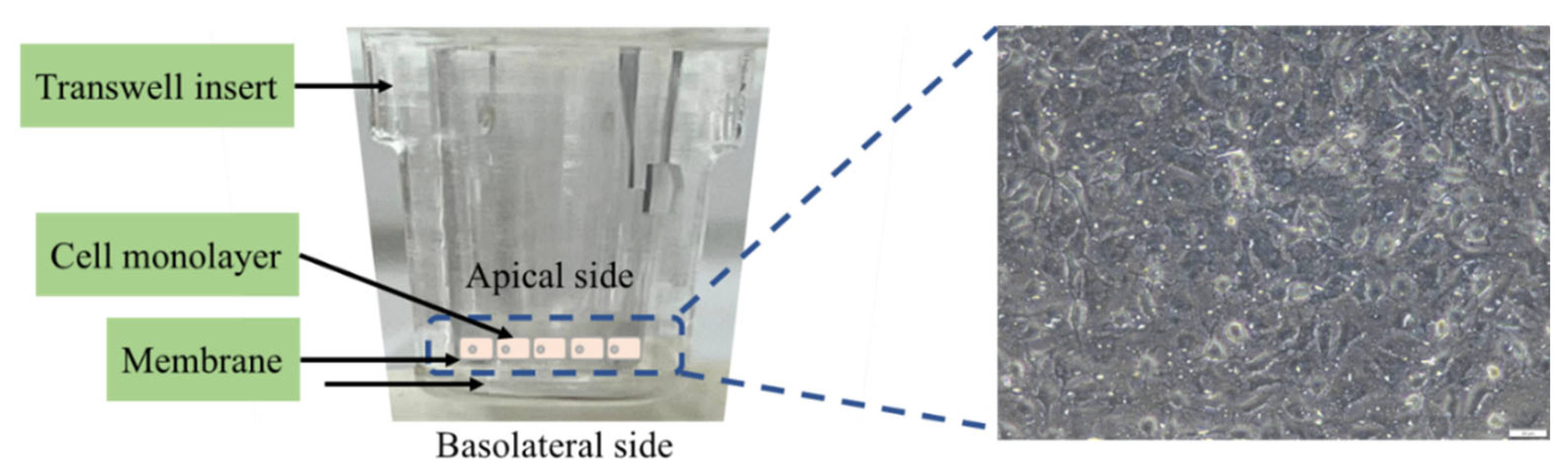
2.8. The Lipid Digestibility and Permeability of HIEC-6 Cell Monolayer
2.9. Statistic Analysis
3. Results and Discussion
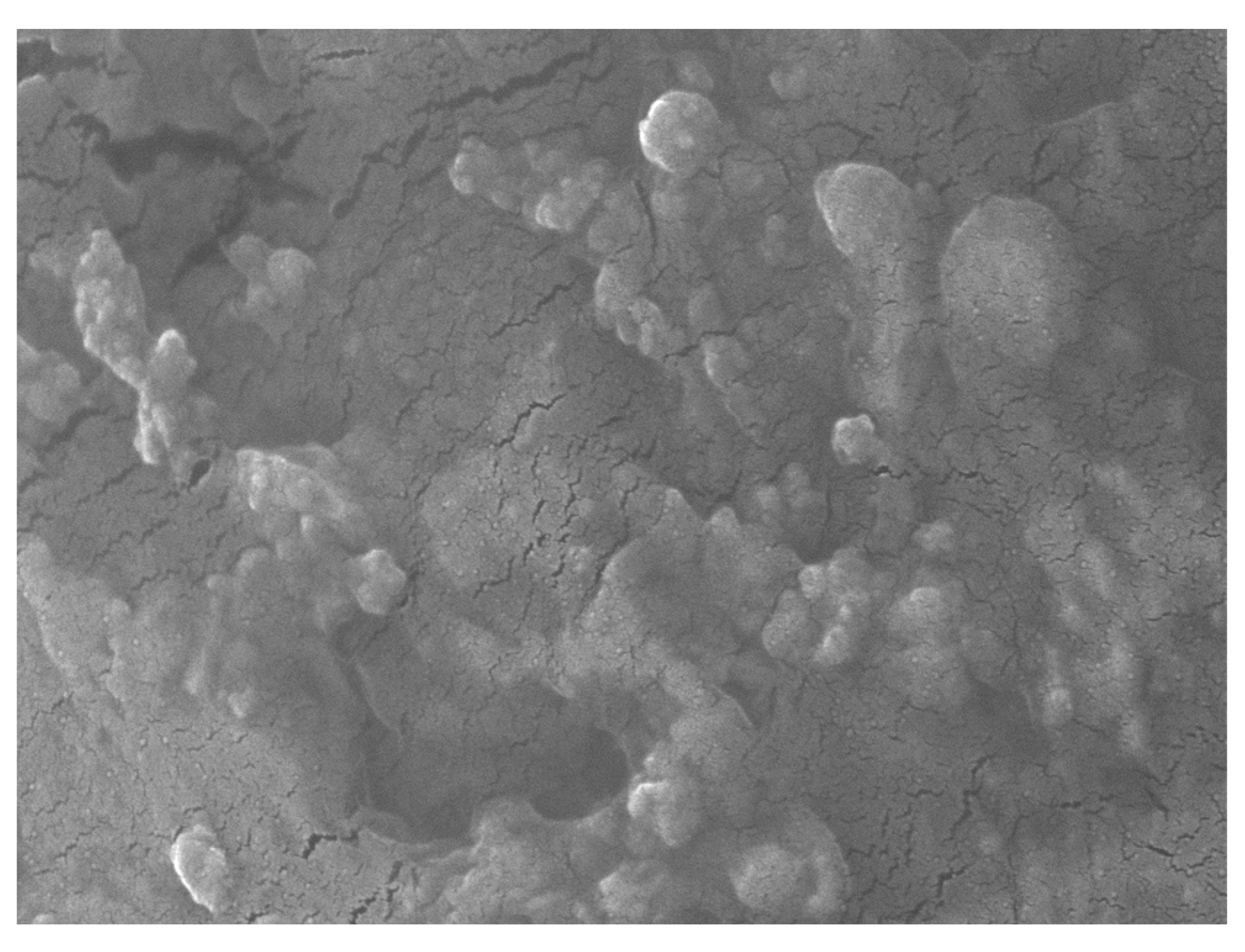
3.1. The Characteristics of Granular Nanocellulose Particles (GNC) from Sugarcane Bagasse
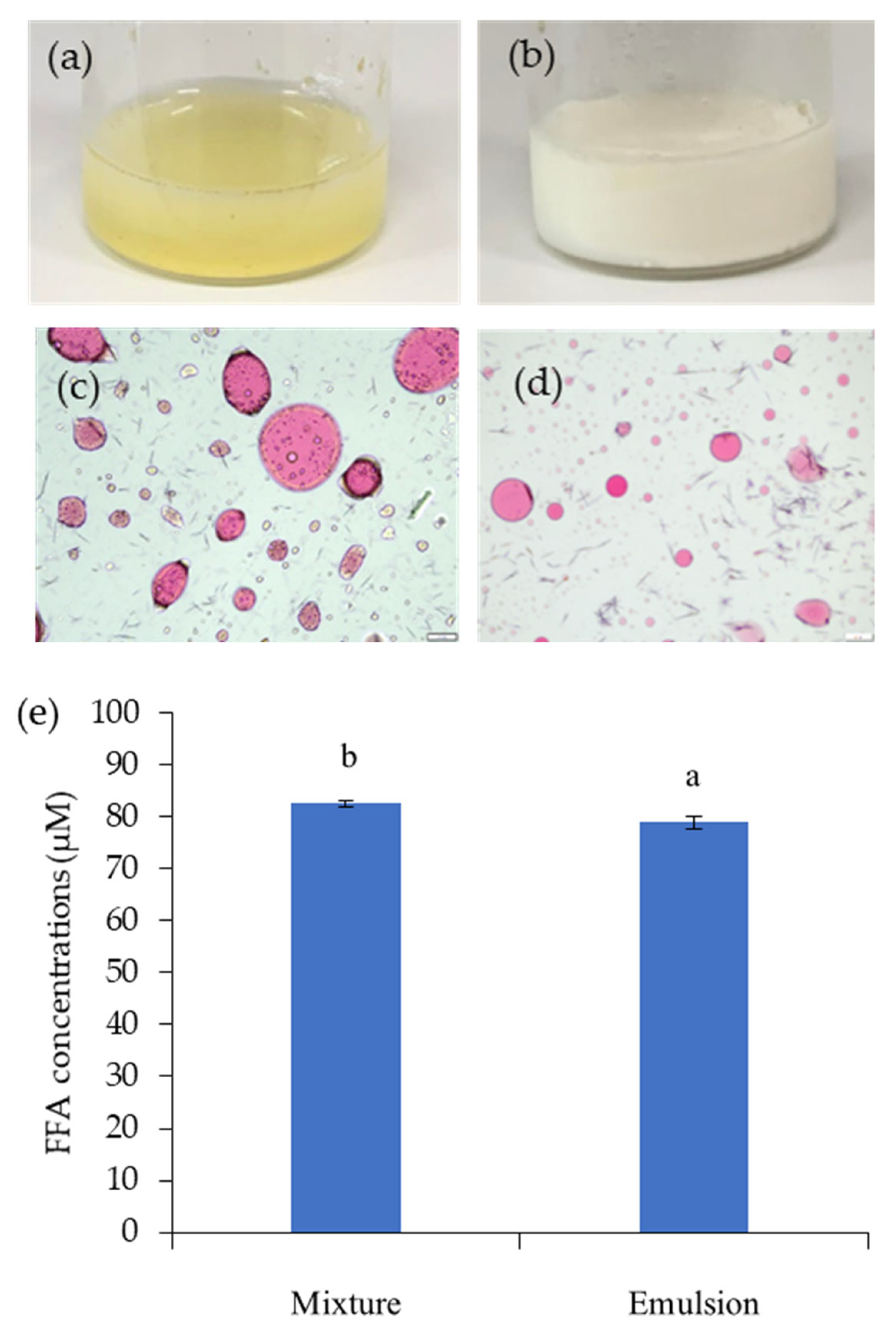
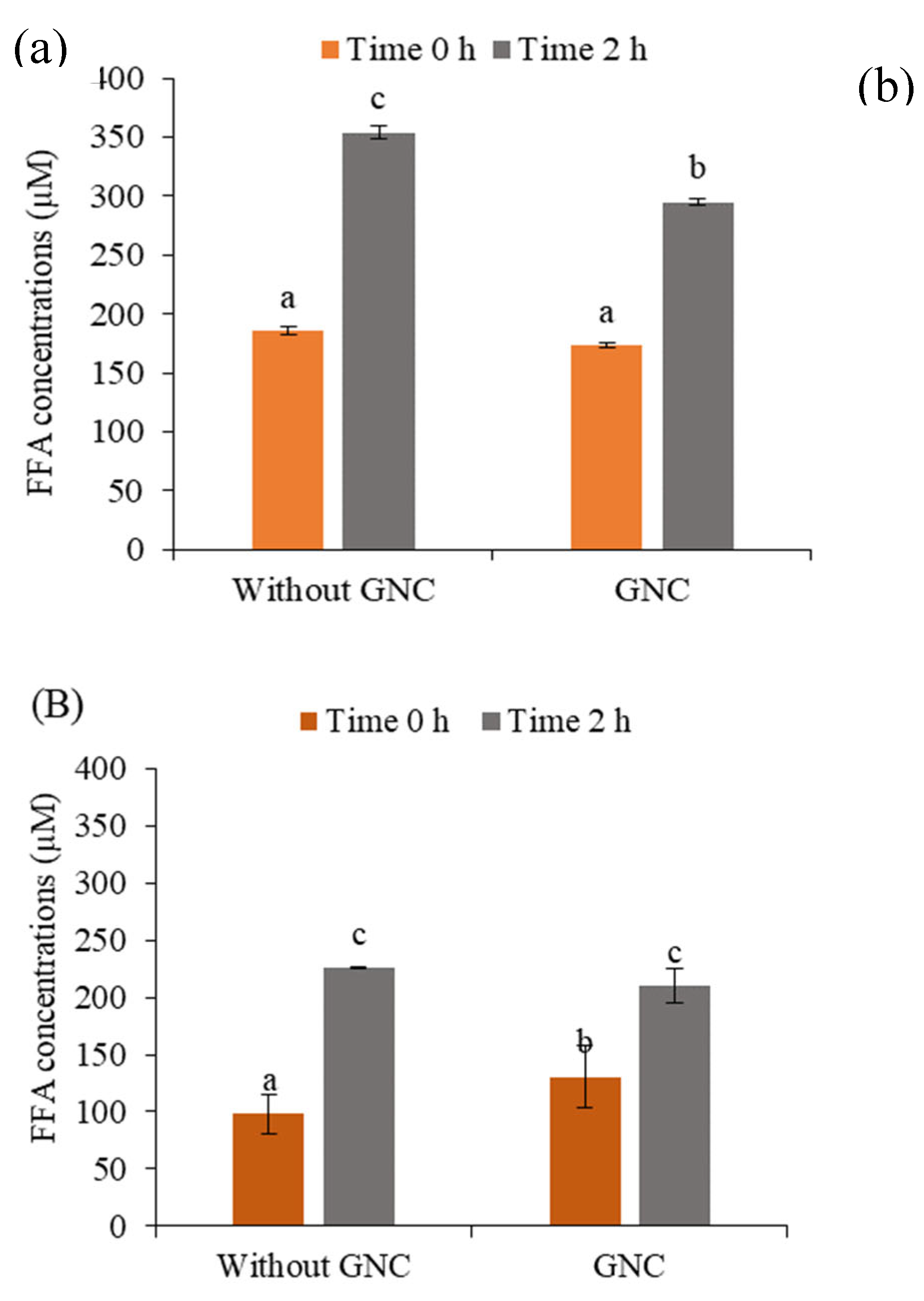
3.2. The Role of GNC in Releasing FFA Content after In Vitro Gastrointestinal Digestion
3.2.1. The Role of GNC Concentration
3.2.2. The Role of Simulation Mixture of the GNC and Olive Oil
3.3. Characteristics of GNC During In Vitro Simulated Gastrointestinal System
3.3.1. The Particle Size Distribution and Zeta Potential Value
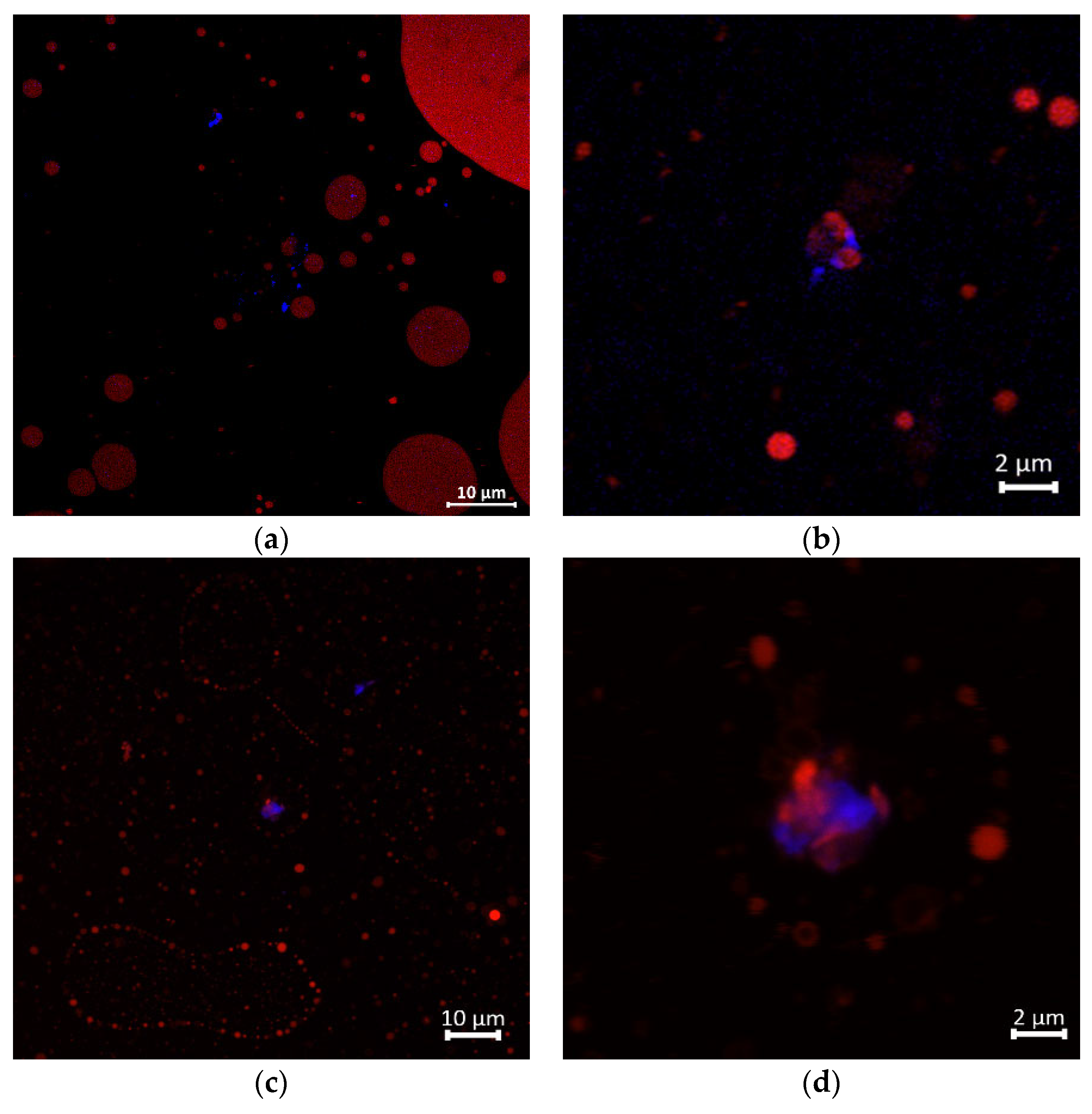
3.3.2. The Interfacial Properties of GNC-Olive Oil Emulsion During the Simulated GIT System
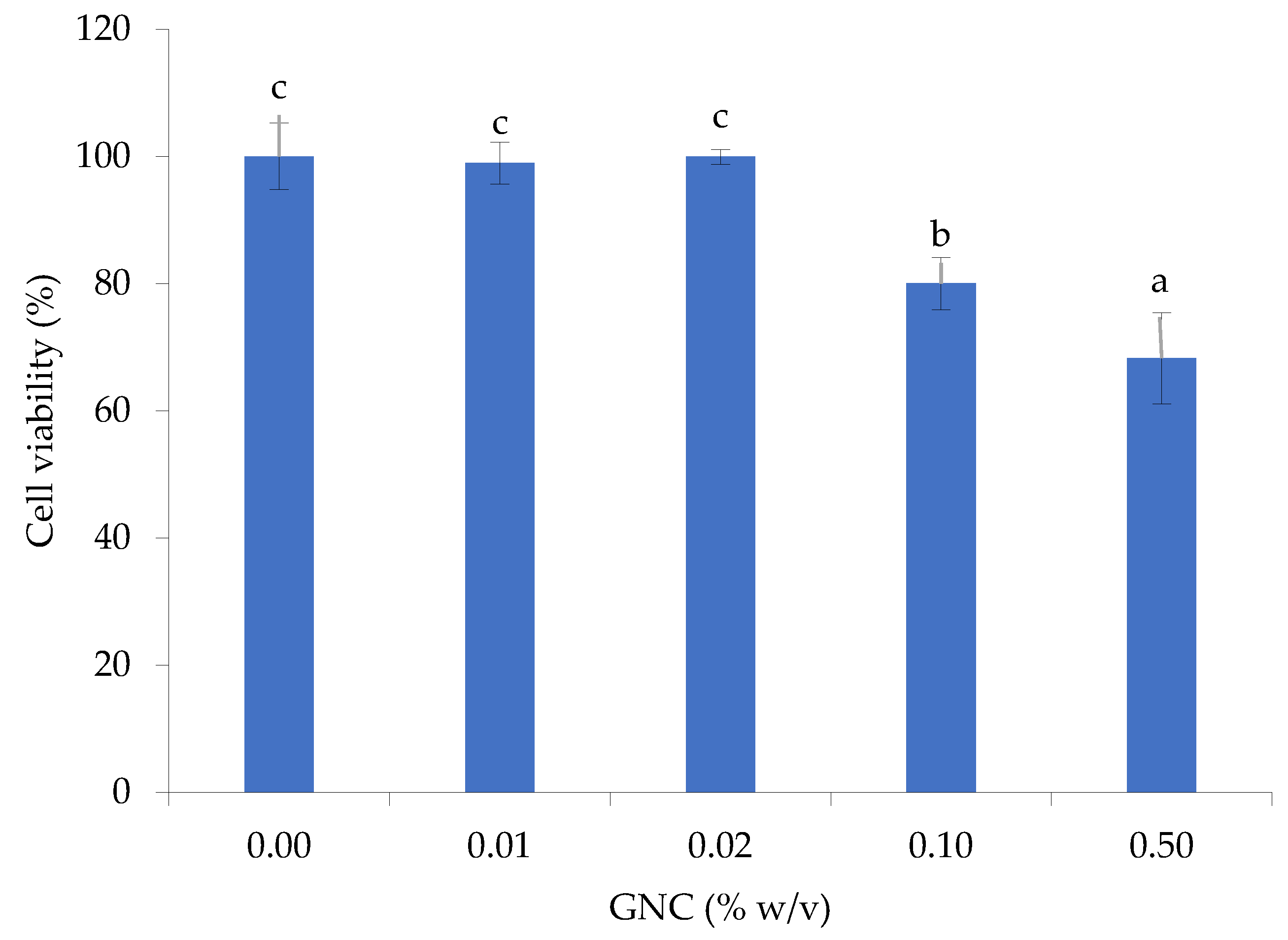
3.4. Cytotoxicity of GNC along the GIT Simulation
3.5. The Releasing FFA in Simulated GIT System
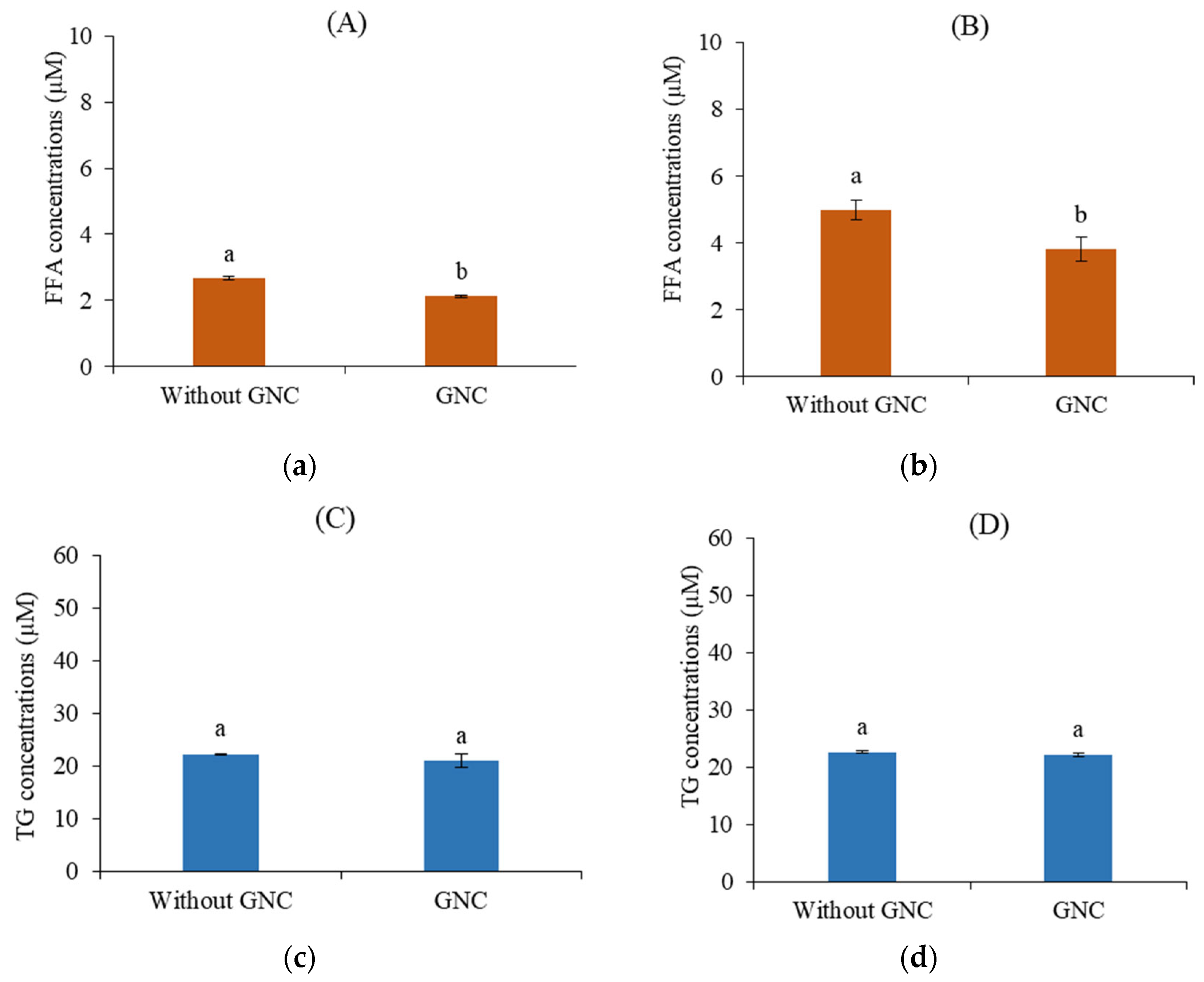
3.6. The FFA and TG Permeability in HIEC-6 Cell Monolayer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.-Y.; Wang, M.M.C.; Hsieh, S.-K. ; Hsieh, M-H.; Chen, W.-Y, Tzen J.T.C. Pancreatic lipase inhibition of strictinin isolated from Pu’er tea (Cammelia sinensis) and its anti-obesity effects in C57BL6 mice. J. Funct. Foods. [CrossRef]
- Calcaterra, V.; Rossi, V. ; Mari. A.; Casini, F. Bergamaschi F, Zuccotti G.V, Fabiano V. Medical treatment of weight loss in children and adolescents with obesity. Pharmacol. Res, 1064. [Google Scholar] [CrossRef]
- Iqbal, S.; Zhang, P.; Wu, P.; Yin, Q.; Hidayat, K.; Chen, X.D. Modulation of viscosity, microstructure and lipolysis of W/O emulsions by cellulose ethers during in vitro digestion in the dynamic and semi-dynamic gastrointestinal models. Food. Hydrocoll. 2022, 128, 107584. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, J.; Lyu, J.; Chen, J.; Wang, R.; Liu, X.; Richel, A. Structural conversion of pectin fractions during heat processing in relation to the ability of inhibiting lipid digestion: A case study of hawthorn pectin. Food. Hydrocoll. 2021, 117, 106721. [Google Scholar] [CrossRef]
- Peng, F.; Ren, X.; Du, B.; Niu, K.; Yu, Z.; Yang, Y. Insoluble dietary fiber of pear fruit pomace (Pyrus ussuriensis Maxim) consumption ameliorates alterations of the obesity-related features and gut microbiota caused by high-fat diet. J. Funct. Foods. 2022, 99, 105354. [Google Scholar] [CrossRef]
- Low, D.Y.; Pluschke, A.M.; Gerrits, W.J.J.; Zhang, D.; Shelat, K.J.; Gidley, M.J.; Williams, B.A. Cereal dietary fibres influence retention time of digesta solid and liquid phases along the gastrointestinal tract. Food. Hydrocoll. 2020, 104, 105739. [Google Scholar] [CrossRef]
- Khorasaniha, R.; Olof, H.; Voisin, A.; Armstrong, K.; Wine, E.; Vasanthan, T.; Armstrong, H. Diversity of fibers in common foods: Key to advancing dietary research. Food. Hydrocoll. 2023, 139, 108495. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food. Hydrocoll. 2010, 24(1), 42–48. [Google Scholar] [CrossRef]
- Fabek, H.; Goff, H.D. Simulated intestinal hydrolysis of native tapioca starch: Understanding the effect of soluble fibre. Bioact. Carbohydr. Diet. Fibre. 2015, 6(2), 83–98. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, X.; Yagoub, A.E.; Wahia, H.; Zhou, C. Application and challenge of nanocellulose in the food industry. Food. Biosci. 2021, 43, 101285. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; Fox, D.M.; Hamad, W.Y.; Heux, L.; Jean, B.; Korey, M.; Nieh, W.; Ong, K.J.; Reid, M.S.; Renneckar, S.; Roberts, R.; Shatkin, J.A.; Simonsen, J.; Stinson-Bagby, K.; Wanasekara, N.; Youngblood, J. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47(8), 2609–2679. [Google Scholar] [CrossRef]
- Fitri, I.; Mitbumrung, W.; Akanitkul, P.; Rungraung, N.; Kemsawasd, V.; Jain, S.; Winuprasith, T. Encapsulation of β-Carotene in oil-in-water emulsions containing nanocellulose: Impact on emulsion properties, In vitro digestion and bioaccessibility. Polym. 2022, 14(7), 1414. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of vitamin D3 in pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food. Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Tangsrianugul, N.; Winuprasith, T.; Suphantharika, M.; Wongkongkatep, J. Effect of hydrocolloids on physicochemical properties, stability, and digestibility of Pickering emulsions stabilized by nanofibrillated cellulose. Food. Funct. 2022, 13(2), 990–999. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological effects of ingested nanocellulose in in vitro intestinal epithelium and in vivo rat models. Environ. Sci. Nano. 2019, 6(7), 2105–2115. [Google Scholar] [CrossRef]
- Li, X.; Kuang, Y.; Jiang, Y.; Dong, H.; Han, W.; Ding, Q.; Lou, J.; Wang, Y.; Cao, T.; Li, J.; Jiao, W. In vitro gastrointestinal digestibility of corn oil-in-water Pickering emulsions stabilized by three types of nanocellulose. Carbohydr. Polym. 2022, 277, 118835. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; Ng, K.W.; Loo, S.C.J.; Bell, D.C.; Brain, J.; Demokritou, P. Reducing intestinal digestion and absorption of fat using a nature-derived biopolymer: Interference of triglyceride hydrolysis by nanocellulose. ACS. Nano. 2018, 12(7), 6469–6479. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 1. Formation and stability. Food. Hydrocoll. 2019, 96, 699–708. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.; Murray, B.; Russell, J.A.; Boxal, S. Modulating in vitro gastric digestion of emulsions using composite whey protein-cellulose nanocrystal interfaces. Colloids. Surf. B. Biointerfaces. 2017, 158, 137–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, W.; Wu, M.; Rahmaninia, M.; Xu, C.; Li, B. Tailoring functionality of nanocellulose: Current status and critical challenges. Nanomater. 2023, 13(9), 1489. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Preparation and liquid crystalline properties of spherical cellulose nanocrystals. Langmuir. 2008, 24(1), 5–8. [Google Scholar] [CrossRef]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. J. Chem. Eng. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Jirathampinyo, S.; Chumchoochart, W.; Tinoi, J. Integrated biobased processes for nanocellulose preparation from rice straw cellulose. Processes. 2023, 11(4), 1006. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Matić, P.; Skendrović Babojelić, M. Interactions of polyphenols from traditional apple varieties ‘Bobovac’, ‘Ljepocvjetka’ and ‘Crvenka’ with β-Glucan during in vitro simulated digestion. Food. Chem. 2021, 363, 130283. [Google Scholar] [CrossRef] [PubMed]
- van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M.A.C. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods. 1994, 174(1), 311–320. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-fibrillated cellulose on lipid digestion and absorption. Int. J. Biol. Macromol. 2019, 139, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kerr, W.L.; Kong, F. Characterization of lipid emulsions during in vitro digestion in the presence of three types of nanocellulose. J. Colloid. Interface. Sci. 2019, 545, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, R.; Yokoyama, W.; Zhong, F. Investigation of the effect of nanocellulose on delaying the in vitro digestion of protein, lipid, and starch. Food. Hydrocoll. For. Health. 2022, 2, 100098. [Google Scholar] [CrossRef]
- Zhai, H.; Gunness, P.; Gidley, M.J. Effects of cereal soluble dietary fibres on hydrolysis of p -nitrophenyl laurate by pancreatin. Food. Funct. 2016, 7(8), 3382–3389. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Yao, R.; Yan, S.; Wang, Q. Mechanism of lipid metabolism regulation by soluble dietary fibre from micronized and non-micronized powders of lotus root nodes as revealed by their adsorption and activity inhibition of pancreatic lipase. Food. Chem. 2020, 305, 125435. [Google Scholar] [CrossRef]
- Skjold-Jørgensen, J.; Bhatia, V.K.; Vind, J.; Svendsen, A.; Bjerrum, M.J.; Farrens, D. The enzymatic activity of lipases correlates with polarity-induced conformational changes: A Trp-induced quenching fluorescence study. Biochem. 2015, 54(27), 4186–4196. [Google Scholar] [CrossRef]
- Yu, B.; Tang, Q.; Fu, C.; Regenstein, J.; Huang, J.; Wang, L. Effects of different particle-sized insoluble dietary fibre from citrus peel on adsorption and activity inhibition of pancreatic lipase. Food. Chem. 2023, 398, 133834. [Google Scholar] [CrossRef]
- Souza, A.G.d.; Ferreira, R.R.; Aguilar, E.S.F.; Zanata, L.; Rosa, D.d.S. Cinnamon essential oil nanocellulose-based pickering emulsions: Processing parameters effect on their formation, stabilization, and antimicrobial activity. Polysaccharides. 2021, 2(3), 608–625. [Google Scholar] [CrossRef]
- Wen, C.; Yuan, Q.; Liang, H.; Vriesekoop, F. Preparation and stabilization of d-limonene Pickering emulsions by cellulose nanocrystals. Carbohydr. Polym. 2014, 112, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci. Technol. Adv. Mate. 2017, 18(1), 959–971. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules. 2012, 13(1), 267–275. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, J.; Bondancia, T.J.; Claro, P.I.C.; Mattoso, L.H.C.; Farinas, C.S.; Marconcini, J.M. Enzymatic deconstruction of sugarcane bagasse and straw to obtain cellulose nanomaterials. ACS. Sustain. Chem. Eng. 2020, 8(5), 2287–2299. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid. Interface. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Ni, Y.; Gu, Q.; Li, J.; Fan, L. Modulating in vitro gastrointestinal digestion of nanocellulose-stabilized pickering emulsions by altering cellulose lengths. Food. Hydrocoll. 2021, 118, 106738. [Google Scholar] [CrossRef]
- Rungraung, N.; Jain, S.; Mitbumrung, W.; Khomein, P.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Controlling the in vitro gastrointestinal digestion of emulsified lipids by encapsulation within nanocellulose-fortified alginate beads. Food. Struct. 2022, 32, 100266. [Google Scholar] [CrossRef]
- Le, H.D.; Loveday, S.M.; Singh, H.; Sarkar, A. Gastrointestinal digestion of pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Release of short-chain fatty acids. Food. Chem. 2020, 320, 126650. [Google Scholar] [CrossRef]
- Li, K.; Xie, L.; Wang, B.; Yan, J.; Tang, H.; Zhou, D. Mechanistic investigation of surfactant-free emulsion polymerization using magnetite nanoparticles modified by citric acid as stabilizers. Langmuir. 2020, 36(28), 8290–8300. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Minařík, A.; Kašpárková, V. Pickering oil-in-water emulsions stabilized by carboxylated cellulose nanocrystals – Effect of the pH. Food. Hydrocoll. 2018, 80, 60–67. [Google Scholar] [CrossRef]
- Du Le, H.; Loveday, S.M.; Singh, H.; Sarkar, A. Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Responsiveness to pH and ionic strength. Food. Hydrocoll. 2020, 99, 105344. [Google Scholar] [CrossRef]
- Scheuble, N.; Schaffner, J.; Schumacher, M.; Windhab, E.J.; Liu, D.; Parker, H.; Steingoetter, A.; Fischer, P. Tailoring emulsions for controlled lipid release: establishing in vitro–in vivo correlation for digestion of lipids. ACS. Appl. Mater. Interfaces. 2018, 10(21), 17571–17581. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Encapsulation of vitamin D(3) in pickering emulsion stabilized by nanofibrillated mangosteen cellulose: Effect of environmental stresses. J. Food. Sci. 2019, 84(11), 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H.; Demokritou, P. Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Adv. Colloid. Interface. Sci. 2017, 246, 165–180. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-fibrillated cellulose on lipid digestion and absorption. Int. J. Biol. Macromol. 2019, 139, 361–366. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid. Interface. Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Mun, S.; Choi, Y.; Kim, Y.-R. Lipase digestibility of the oil phase in a water-in-oil-in-water emulsion. Food. Sci. Biotechnol. 2015, 24(2), 513–520. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food. Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- Vital, N.; Ventura, C.; Kranendonk, M.; Silva, M.J.; Louro, H. Toxicological assessment of cellulose nanomaterials: Oral exposure. Nanomater. 2022, 12(19), 3375. [Google Scholar] [CrossRef]
- Silbir, S.; Yekta Göksungur, M. Nanocellulose production and its food applications. 2nd Congress on Food Structure. Antalya, Turkey, 2016.
- Takenaka, T.; Harada, N.; Kuze, J.; Chiba, M.; Iwao, T.; Matsunaga, T. Human small intestinal epithelial cells differentiated from adult intestinal stem cells as a novel system for predicting oral drug absorption in humans. Drug. Metab. Dispos. 2014, 42(11), 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, Å.; Lutz, M.; Tannergren, C.; Wingolf, C.; Borde, A.; Ungell, A.L. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. Eur. J. Pharm. Sci. [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 2. In vitro lipid digestion. Food. Hydrocoll. 2019, 96, 709–716. [Google Scholar] [CrossRef]
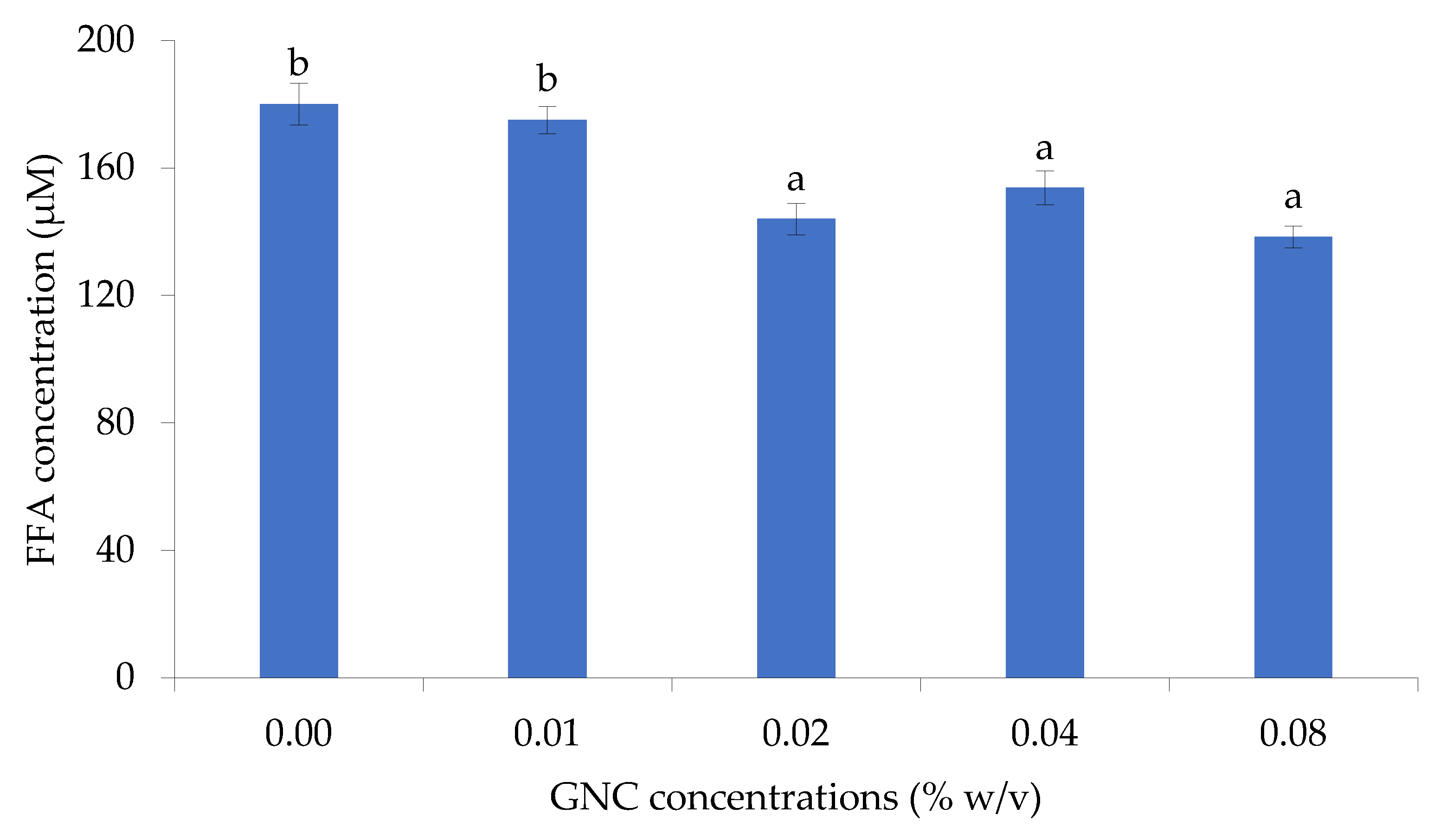
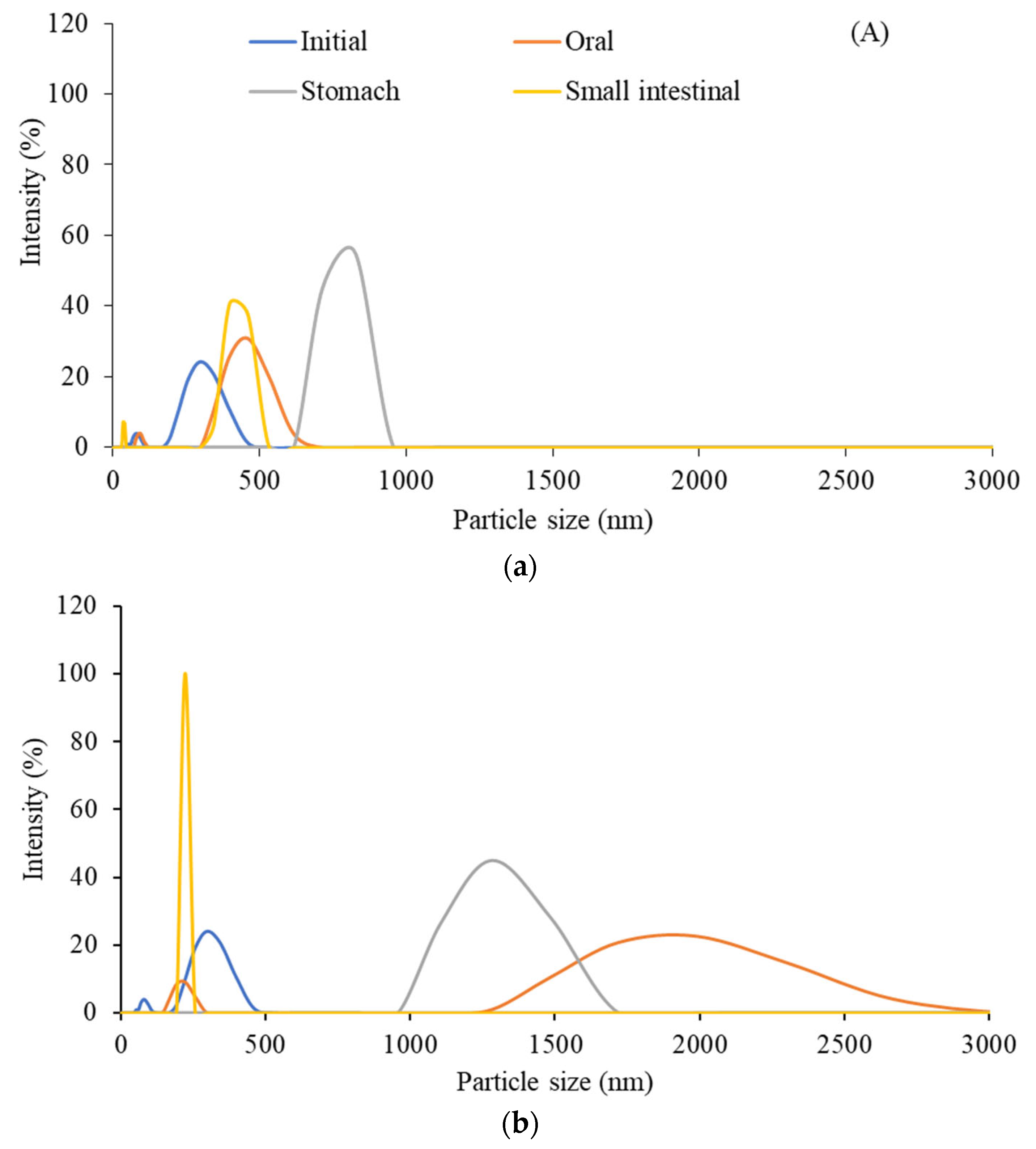
| Sample | Characteristics | Initial phase | Oral phase | Stomach phase | Small intestine phase |
|---|---|---|---|---|---|
|
Ingested GNC suspension |
Particle size (nm) | 295.3 | 458.7 | 825.0 | 458.7 |
| PDI | 0.551 | 0.835 | 0.211 | 0.826 | |
| Zeta potential (mV) | -38.2 | -33.8 | -10.5 | -31.4 | |
| GNC-olive oil emulsion | Particle size (nm) | 295.3 | 1990.0 | 1281.0 | 220 |
| PDI | 0.551 | 0.597 | 0.452 | 0.111 | |
| Zeta potential (mV) | -38.2 | -7.56 | -0.5 | -33.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





