Submitted:
25 July 2023
Posted:
26 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Sericin Properties Relevant to Bone Grafting
3. Studies on Sericin in Bone Grafting
4. Comparative Analysis
5. Potential Applications and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.I.; Lee, J.H.; Lee, S.J. The comprehensive on-demand 3D bio-printing for composite reconstruction of mandibular defects. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G. Multiple ways for the same destination: Bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 9. [Google Scholar] [CrossRef] [PubMed]
- Nelke, K.; Łuczak, K.; Janeczek, M.; Pasicka, E.; Żak, K.; Łukaszewski, M.; Jadach, R.; Dobrzyński, M. Fresh–frozen allogenic bone graft usage in treatment of an odontogenic keratocyst in the mandible. Appl. Sci. 2023, 13, 1234. [Google Scholar] [CrossRef]
- Salah, M.; Naini, F.B. Exosomes in craniofacial tissue reconstruction. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, J.; Cho, B.H.; Kim, Y.; Lee, J.Y. Three-dimensional analysis of bone volume change at donor sites in mandibular body bone block grafts by a computer-assisted automatic registration method: A retrospective study. Appl. Sci. 2022, 12, 7261. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, J.H.; Kim, G.C.; Hwang, D.S.; Kim, C.H.; Kim, B.J.; Kim, J.H.; Kim, U.K. The effect of autogenous tooth bone graft material without organic matter and type I collagen treatment on bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 17. [Google Scholar] [CrossRef]
- Choi, W.H.; Kim, Y.D.; Song, J.M.; Shin, S.H. Comparative study of bone regeneration using fibrin sealant with xenograft in rabbit sinus: Pilot study. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 5. [Google Scholar] [CrossRef]
- Pfaffeneder-Mantai, F.; Meller, O.; Schneider, B.; Bloch, J.; Bytyqi, D.; Sutter, W.; Turhani, D. Specially designed and CAD/CAM manufactured allogeneic bone blocks using for augmentation of a highly atrophic maxilla show a stable base for an all-on-six treatment concept: A case report. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 21. [Google Scholar] [CrossRef]
- Vani, T.M.; Paramashivaiah, R.; Prabhuji, M.L.; Peeran, S.W.; Fageeh, H.; Tasleem, R.; Bahamdan, G.K.; Aldosari, L.I.; Bhavikatti, S.K.; Scardina, G.A. Regeneration of intrabony defects with nano hydroxyapatite graft, derived from eggshell along with periosteum as barrier membrane under magnification—An interventional study. Appl. Sci. 2023, 13, 1693. [Google Scholar] [CrossRef]
- Negut, I.; Ristoscu, C. Bioactive glasses for soft and hard tissue healing applications—A short review. Appl. Sci. 2023, 13, 6151. [Google Scholar] [CrossRef]
- Di-Stefano, D.A.; Orlando, F.; Ottobelli, M.; Fiori, D.; Garagiola, U. A comparison between anorganic bone and collagen-preserving bone xenografts for alveolar ridge preservation: Systematic review and future perspectives. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 24. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, X.; Chen, Z.; Xiao, Y.; Li, S.; Gu, J.; Hu, H.; Cheng, G. Silk sericin enrichment through electrodeposition and carbonous materials for the removal of methylene blue from aqueous solution. Int. J. Mol. Sci. 2022, 23, 1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Lin, W.S.; Shih, C.H.; Chen, C.Y.; Kuo, S.H.; Li, W.L.; Lin, Y.S. Functionality of silk cocoon (Bombyx mori L.) sericin extracts obtained through high-temperature hydrothermal method. Materials (Basel) 2021, 14, 5314. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Das, G.; Shin, H.S.; Patra, J.K. Silk sericin protein materials: Characteristics and applications in food-sector industries. Int. J. Mol. Sci. 2023, 24, 4951. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.I.; Brancalhão, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, 19. [Google Scholar] [CrossRef]
- Jo, Y.N.; Um, I.C. Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int. J. Biol. Macromol. 2015, 78, 287–295. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, X.; Zhao, D.; Qin, L.; Jiang, W.; Hu, W.; Liu, X.; Xia, Q.; Dong, Z.; Zhao, P. Identification and characterization of sericin5 reveals non-cocoon silk sericin components with high β-sheet content and adhesive strength. Acta Biomater. 2022, 150, 96–110. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk sericin: A promising sustainable biomaterial for biomedical and pharmaceutical applications. Polymers (Basel) 2022, 14, 4931. [Google Scholar] [CrossRef]
- Nayak, S.; Talukdar, S.; Kundu, S.C. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012, 347, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, M.; Qiu, H.; Dai, B.; Qureshi, R.F.; Hussain, S.; Yousif, M.; Gao, P.; Khatri, Z. Green synthesis and incorporation of sericin silver nanoclusters into electrospun ultrafine cellulose acetate fibers for anti-bacterial applications. Polymers (Basel) 2021, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.H.; Chang, S.J.; Chiu, Y.K.; Chiu, Y.H.; Fang, T.J.; Chen, H.C. Low molecular weight sericin enhances the in vitro of immunological modulation and cell migration. Front. Bioeng. Biotechnol. 2022, 10, 925197. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Kweon, H.; Oh, J.H. Sericin for tissue engineering. Appl. Sci. 2020, 10, 8457. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, Y.S.; Kim, S.J.; Song, D.W.; Park, Y.H.; Bae, D.G.; Choi, J.H.; Um, I.C. Preparation of new natural silk non-woven fabrics by using adhesion characteristics of sericin and their characterization. Int. J. Biol. Macromol. 2018, 106, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Recent advances in silk sericin/calcium phosphate biomaterials. Front. Mater. 2020, 7, 24. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Zhang, C.; Chen, Y.; Zhu, L.; Mao, C.; OuYand, H. Biomimetic nucleation of hydroxyapatite crystals mediated by Antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biomacromolecules 2014, 15, 1185–1193. [Google Scholar] [CrossRef]
- Aramwit, P. “Bio-response to silk sericin. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; England, Woodhead Publishing; Elsevier, 2014; pp. 299–329. [Google Scholar] [CrossRef]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface. 2016, 13, 20160462. [Google Scholar] [CrossRef]
- Nayak, S.; Dey, T.; Naskar, D.; Kundu, S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials 2013, 34, 2855–2864. [Google Scholar] [CrossRef]
- Noosak, C.; Jantorn, P.; Meesane, J.; Voravuthikunchai, S.; Saeloh, D. Dual-functional bioactive silk sericin for osteoblast responses and osteomyelitis treatment. PLoS ONE. 2022, 17, e0264795. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.Y.; Kweon, H.; Kim, D.W.; Baek, K.; Kim, M.K.; Kim, S.G.; Chae, W.S.; Choi, J.Y.; Rotaru, H. Bone regeneration is associated with the concentration of tumour necrosis factor-α induced by sericin released from a silk mat. Sci. Rep. 2017, 7, 15589. [Google Scholar] [CrossRef]
- Ha, Y.Y.; Park, Y.W.; Kweon, H.; Jo, Y.Y.; Kim, S.G. Comparison of the physical properties and in vivo bioactivities of silkworm-cocoon-derived silk membrane, collagen membrane, and polytetrafluoroethylene membrane for guided bone regeneration. Macromol. Res. 2014, 22, 1018–1023. [Google Scholar] [CrossRef]
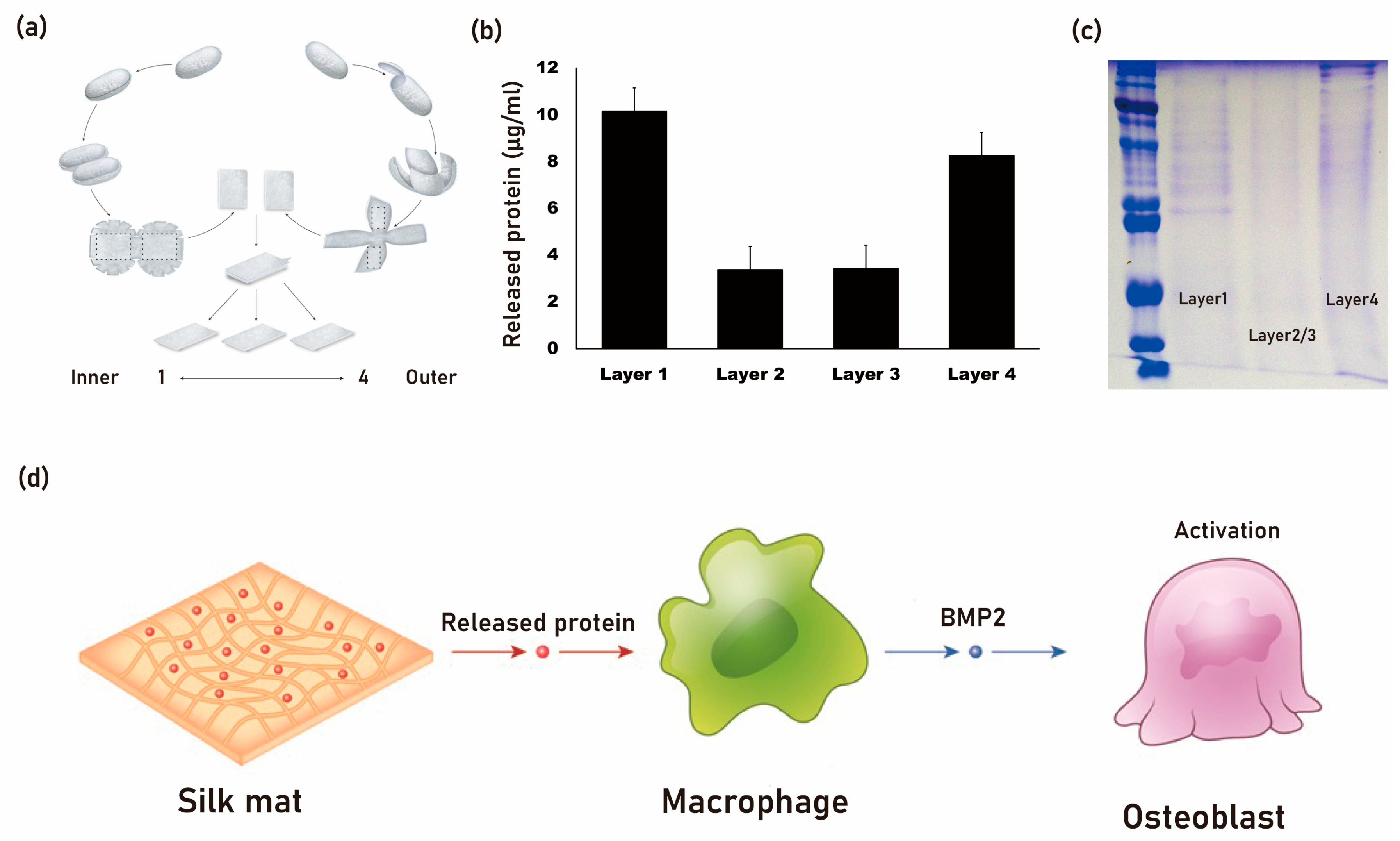
- Kim, J.W.; Jo, Y.Y.; Kweon, H.Y.; Kim, D.W.; Kim, S.G. The effects of proteins released from silk mat layers on macrophages. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 10. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Chang, A.R.; Ahn, J.; Jung, S.; Hong, J.; Oh, H.K.; Hwang, S.J. Efficacy and safety of rhBMP/β-TCP in alveolar ridge preservation: A multicenter, randomized, open-label, comparative, investigator-blinded clinical trial. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 42. [Google Scholar] [CrossRef]
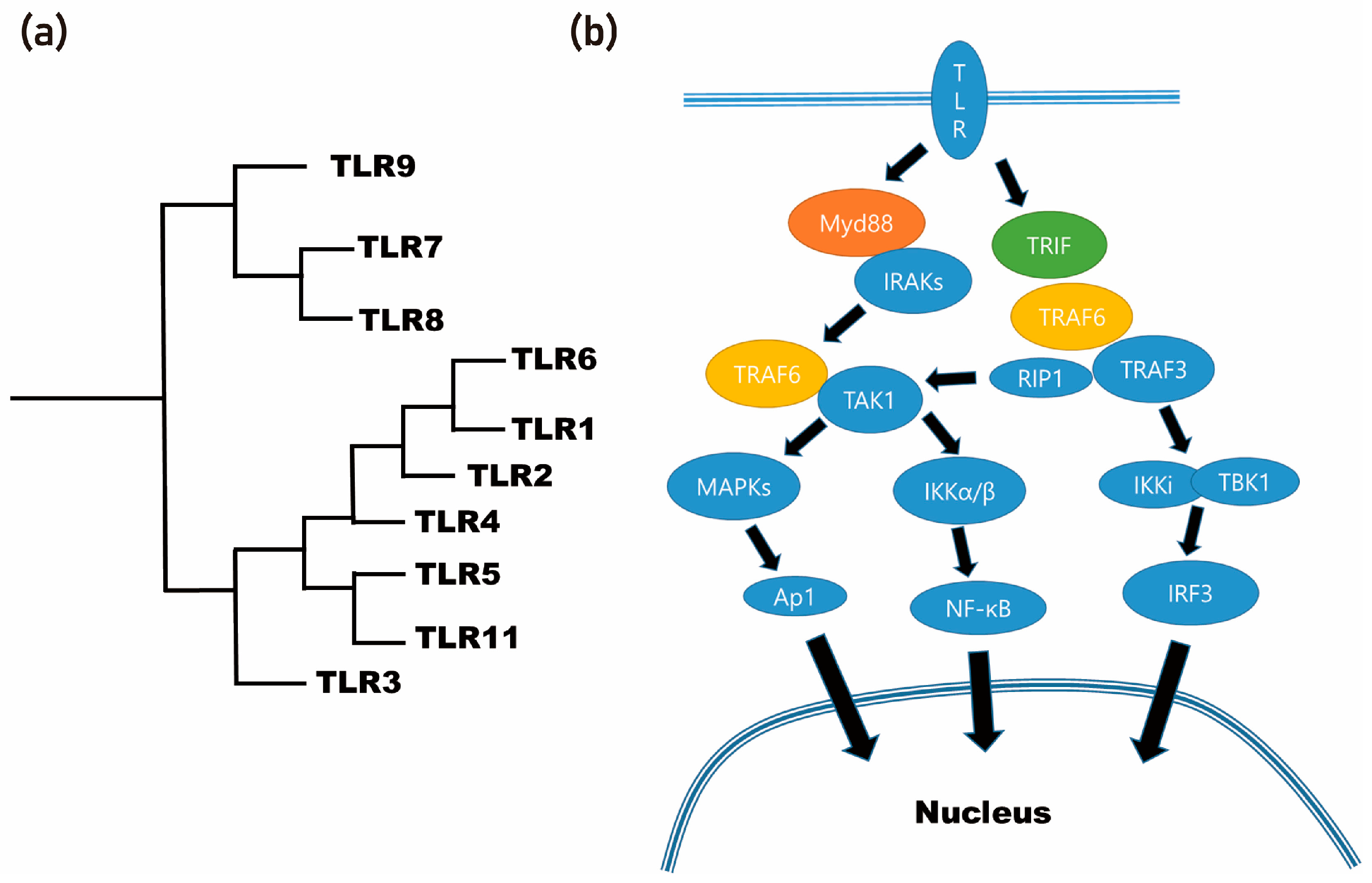
- Jo, Y.Y.; Kweon, H.; Kim, D.W.; Baek, K.; Chae, W.S.; Kang, Y.J.; Oh, J.H.; Kim, S.G.; Garagiola, U. Silk sericin application increases bone morphogenic protein-2/4 expression via a toll-like receptor-mediated pathway. Int. J. Biol. Macromol. 2021, 190, 607–617. [Google Scholar] [CrossRef]
- Kim, S.G.; Kweon, H.; Jo, Y.Y. Toll-like receptor and silk sericin for tissue engineering. Int. J. Indust. Entomol. 2021, 42, 1–6. [Google Scholar]
- Lee, J.H.; Kweon, H.; Oh, J.H.; Kang, Y.J.; Kim, D.W.; Yang, W.G.; Chae, W.S.; Kim, S.G. 4-Hexylresorcinol treatment before degumming increases the β-sheet structure of silk sericin and BMP-2 expression in RAW264.7 cells. Int. J. Mol. Sci. 2022, 24, 150. [Google Scholar] [CrossRef]
- Jo, Y.N.; Park, B.D.; Um, I.C. Effect of storage and drying temperature on the gelation behavior and structural characteristics of sericin. Int. J. Biol. Macromol. 2015, 81, 936–941. [Google Scholar] [CrossRef]
- Jo, Y.N.; Um, I.C. Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int. J. Biol. Macromol. 2015, 78, 287–295. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, W.G.; Chae, W.S.; Kim, D.W.; Kim, S.G.; Rotaru, H. Administration of 4-hexylresorcinol increases p53-mediated transcriptional activity in oral cancer cells with the p53 mutation. Oncol. Rep. 2022, 48, 160. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G. Immunomodulation for maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 5. [Google Scholar] [CrossRef] [PubMed]
- Weivoda, M.M.; Bradley, E.W. Macrophages and bone remodeling. J. Bone Miner. Res. 2023, 38, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, J.H.; Kim, D.W.; Kim, S.G.; Kweon, H. The difference in bone morphogenic protein-2 expression level among Bombyx mori subspecies. Int. J. Indust. Entomol. 2022, 45, 78–83. [Google Scholar]
- Kweon, H.; Yeo, J.H.; Kim, K.Y.; Kim, Y.S.; Song, H.S.; Kim, S.J.; Woo, S.; Han, S.; Lee, K.G. Characteristics of silk sericin extracted from Sericinjam. Int. J. Indust. Entomol. 2009, 18, 121–124. [Google Scholar]
- Choi, Y.Y.; Kim, S.W.; Kim, K.Y.; Um, I.C. Dissolution, crystallilnity, and mechanical properties of silk sericin from Sericinjam silkworm cocoons. Int. J. Indust. Entomol. 2023, 46, 9–15. [Google Scholar]
- Yu, W.; Zheng, Y.; Li, H.; Lin, H.; Chen, Z.; Tian, Y.; Chen, H.; Zhang, P.; Xu, X.; Shen, Y. The Toll-like receptor ligand, CpG oligodeoxynucleotides, regulate proliferation and osteogenic differentiation of osteoblast. J. Orthop. Surg. Res. 2020, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gilbert, J.R.; Shaw, M.A.; Shakir, S.; Losee, J.E.; Billiar, T.R.; Cooper, G.M. Toll-like receptor 4 mediates the regenerative effects of bone grafts for calvarial bone repair. Tissue Eng. Part A. 2015, 21, 1299–1308. [Google Scholar] [CrossRef]
- Hou, L.; Sasaki, H.; Stashenko, P. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infect. Immun. 2000, 68, 4681–4687. [Google Scholar] [CrossRef]
- Matsumoto, C.; Oda, T.; Yokoyama, S.; Tominari, T.; Hirata, M.; Miyaura, C.; Inada, M. Toll-like receptor 2 heterodimers, TLR2/6 and TLR2/1 induce prostaglandin E production by osteoblasts, osteoclast formation and inflammatory periodontitis. Biochem. Biophys. Res. Commun. 2012, 428, 110–115. [Google Scholar] [CrossRef]
- Kim, D.W.; Jo, Y.Y.; Garagiola, U.; Choi, J.Y.; Kang, Y.J.; Oh, J.H.; Kim, S.G. Increased level of vascular endothelial growth factors by 4-hexylresorcinol is mediated by transforming growth factor-β1 and accelerates capillary regeneration in the burns in diabetic animals. Int. J. Mol. Sci. 2020, 21, 3473. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Kim, D.W.; Lee, S.K.; Kim, S.G.; Kim, T.W. The administration of 4-hexylresorcinol accelerates orthodontic tooth movement and increases the expression level of bone turnover markers in ovariectomized rats. Int. J. Mol. Sci. 2020, 21, 1526. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jo, Y.Y.; Kim, J.Y.; Oh, J.H.; Yang, B.E.; Kim, S.G. Clinical study for silk mat application into extraction socket: A split-mouth, randomized clinical trial. Appl. Sci. 2019, 9, 1208. [Google Scholar] [CrossRef]
- Kim, J.W.; Jo, Y.Y.; Kim, J.Y.; Oh, J.H.; Yang, B.E.; Kim, S.G. Retrospective comparative clinical study for silk mat application into extraction socket. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kweon, H.; Oh, J.H.; Kim, S.G. The optimal scaffold for silk sericin-based bone graft: Collagen versus gelatin. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 2. [Google Scholar] [CrossRef] [PubMed]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Abdul-Aziz, K.K.; Tamer, T.M.; Augustyniak, M.; El-Wakil, A. Carboxymethyl cellulose/sericin-based hydrogels with intrinsic antibacterial, antioxidant, and anti-inflammatory properties promote re-epithelization of diabetic wounds in rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef]
- Nayak, S.; Talukdar, S.; Kundu, S.C. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012, 347, 783–794. [Google Scholar] [CrossRef]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B Biointerfaces. 2019, 181, 994–1003. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




