Submitted:
21 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
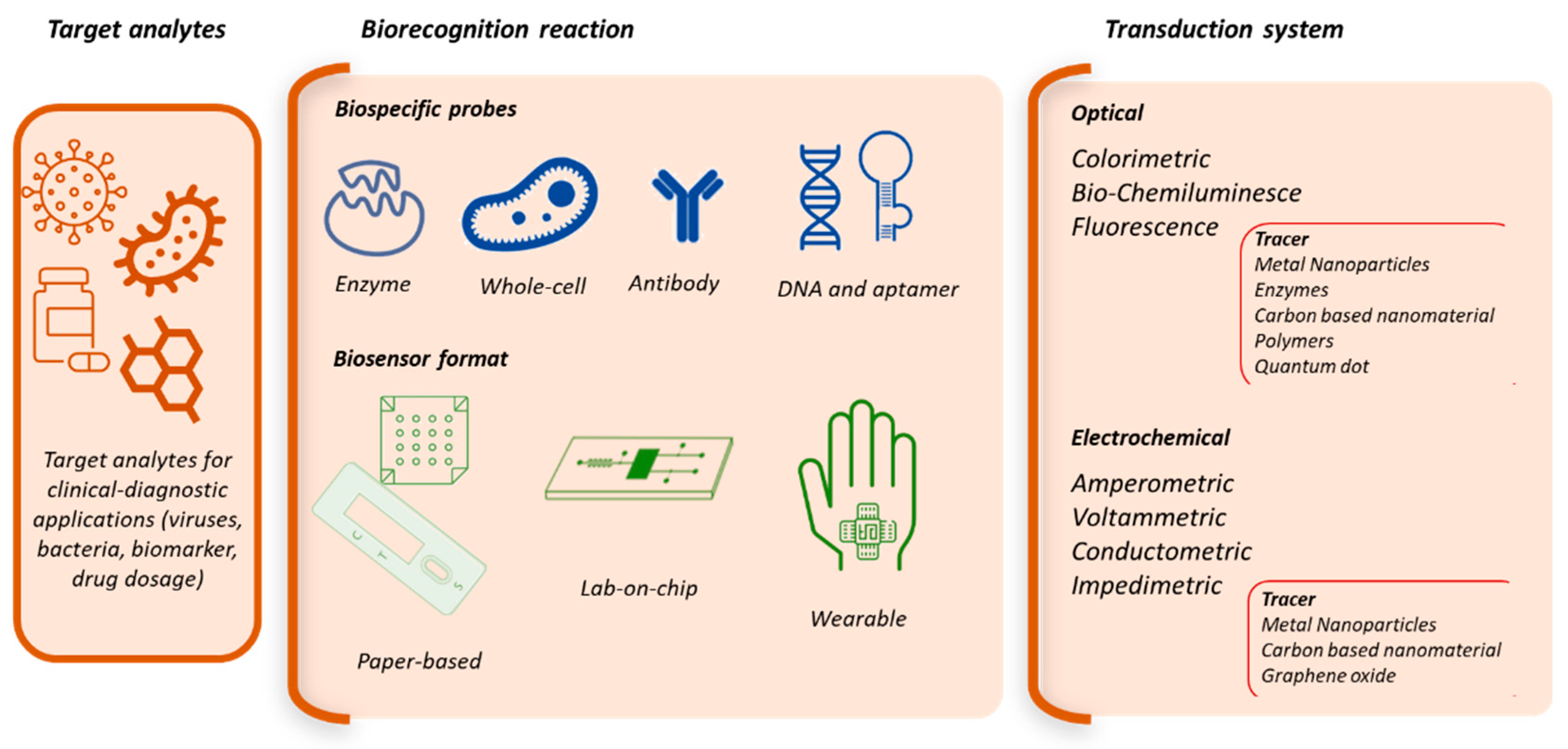
1. Introduction
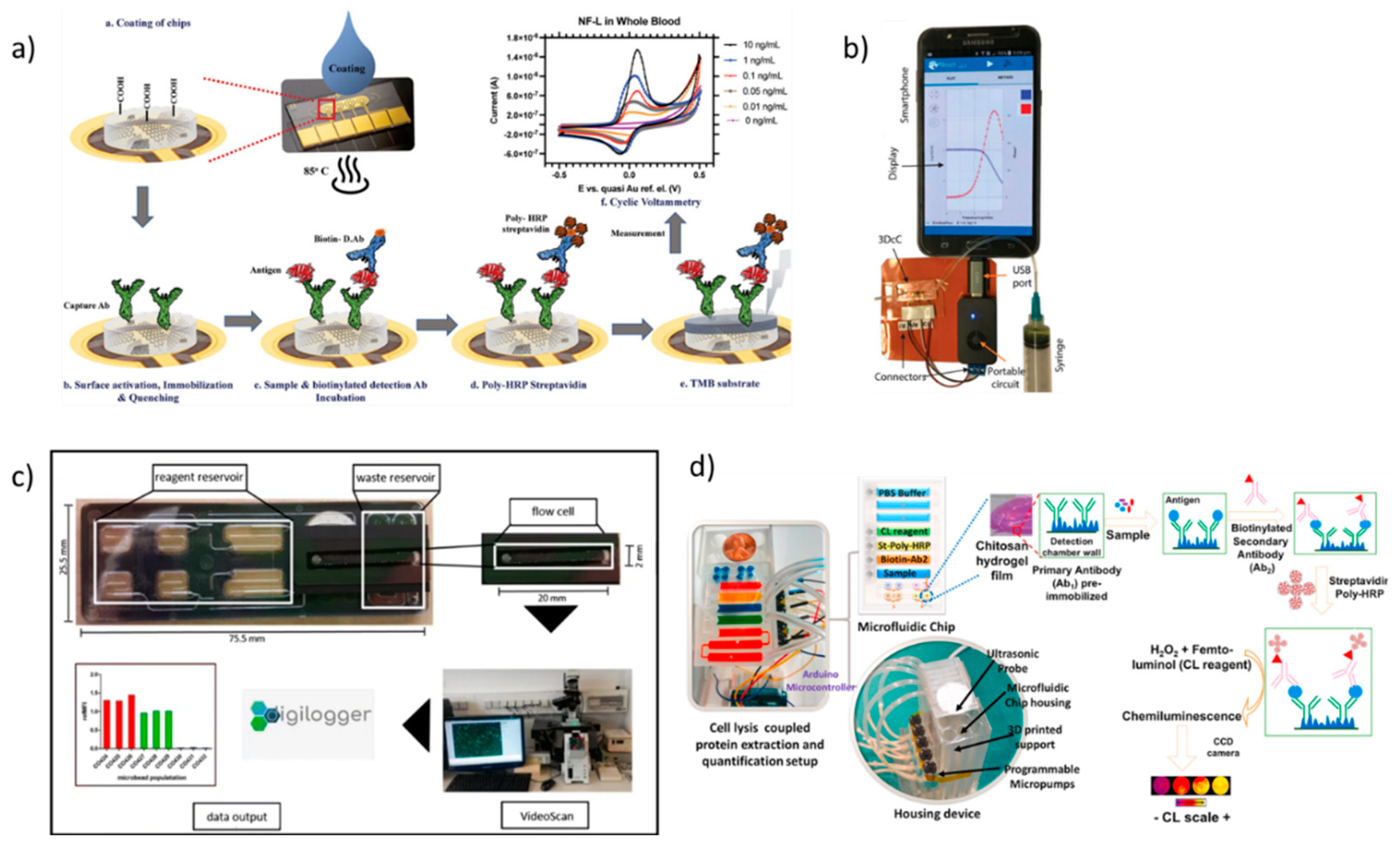
2. Lab-on-chip based biosensors
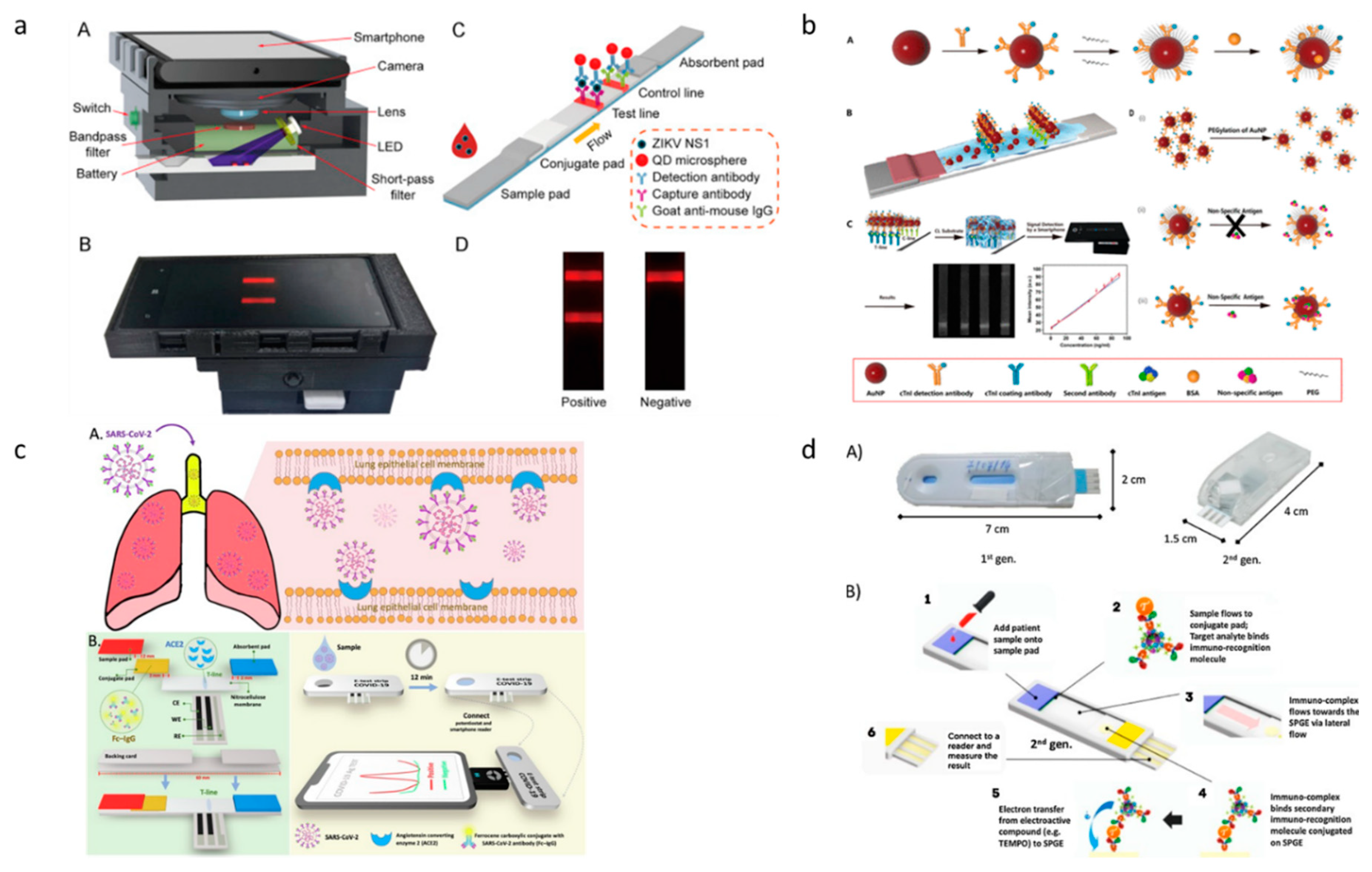
3. Paper-based diagnostic devices
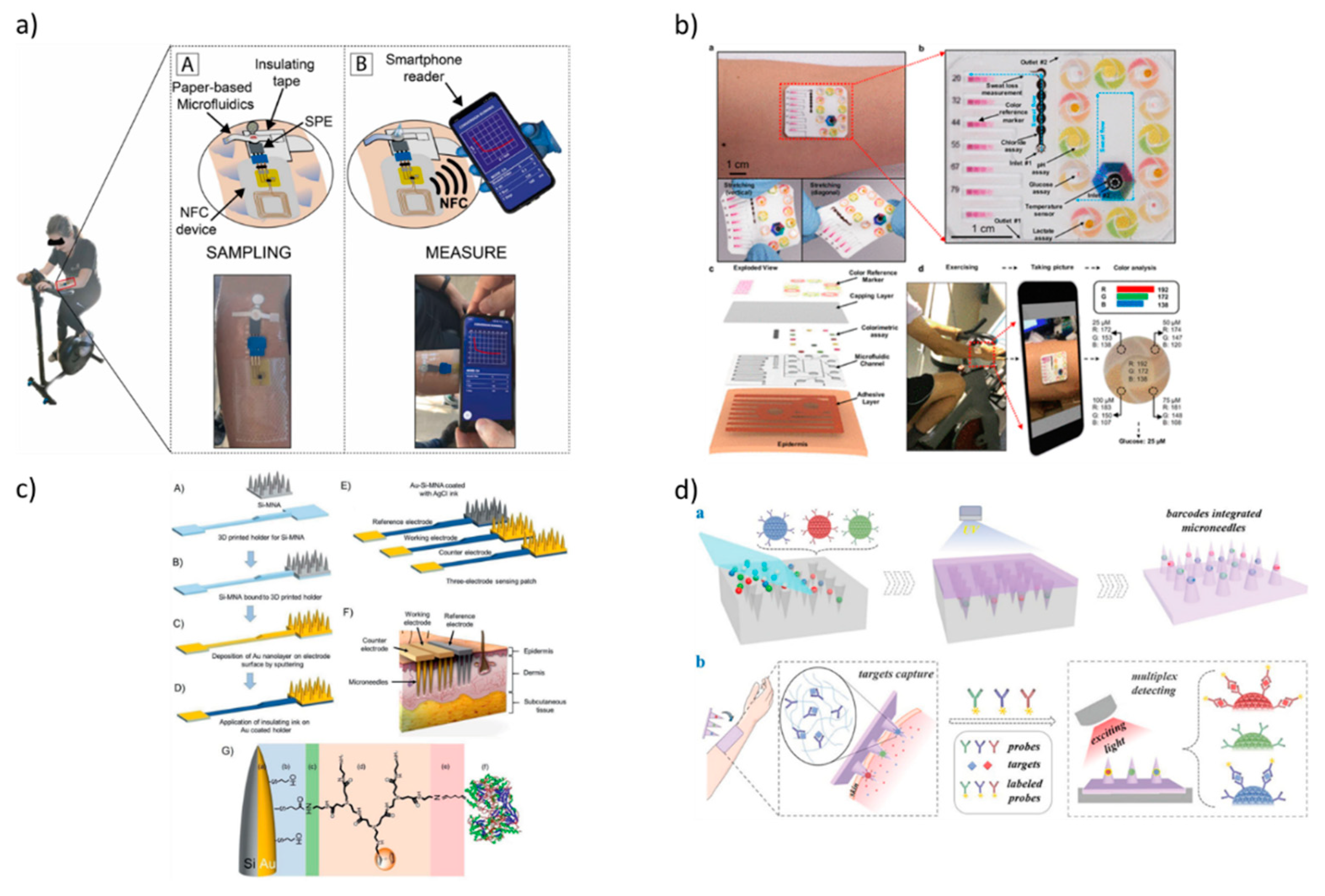
4. Wearable technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- ISO. ISO 22870:2016 Point-of-care testing (POCT) - Requirements for quality and competence. 2016.
- Larsson, A.; Greig-Pylypczuk, R.; Huisman, A. ; The state of point-of-care testing: A European perspective. Ups J Med Sci 2015, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kozel, T.R.; Burnham-Marusich, A.R. Point-of-Care Testing for Infectious Diseases: Past, Present, and Future. J Clin Microbiol 2017, 55, 2313. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, K.; Li, Z.; Wang, P. Point of care testing for infectious diseases. Clin Chim Acta. 2019, 493, 138–147. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Public health guidance on screening and vaccination for infectious diseases in newly arrived migrants within the EU/EEA. 2018.
- Reali, S.; Najib, E.Y.; Balázs, K.E.T.; Tan, A.C.H.; Váradi, L.; Hibbs, D.E.; Groundwater, P.W. Novel diagnostics for point-of-care bacterial detection and identification. RSC advance 2019, 9, 21486–21497. [Google Scholar] [CrossRef]
- Gopal, A.; Yan, L.; Kashif, S.; Munshi, T.; Roy, V.A.; Voelcker, N.H.; Chen, X. Biosensors and Point-of-Care Devices for Bacterial Detection: Rapid Diagnostics Informing Antibiotic Therapy. Advanc Health Mat 2022, 11, 2101546. [Google Scholar] [CrossRef]
- Nath, P.; Kabir, A.; Khoubafarin Doust, S.; Kreais, Z.J.; Ray, A. Detection of bacterial and viral pathogens using photonic point-of-care devices. Diagnostics 2020, 10, 841. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, P.; Ahmed, R.; Wang, J.; Akin, D.; Soto, F.; Demirci, U. Advanced point-of-care testing technologies for human acute respiratory virus detection. Advanc Mat 2022, 34, 2103646. [Google Scholar] [CrossRef]
- Xiao, M.; Tian, F.; Liu, X.; Zhou, Q.; Pan, J.; Luo, Z.; Yi, C. Virus Detection: From State-of-the-Art Laboratories to Smartphone-Based Point-of-Care Testing. Advanc Sci 2022, 9, 2105904. [Google Scholar] [CrossRef]
- Zhao, V.X.T.; Wong, T.I.; Zheng, X.T.; Tan, Y.N.; Zhou, X. Colorimetric biosensors for point-of-care virus detections. Mat sci energy technol 2020, 3, 237–249. [Google Scholar] [CrossRef]
- Lu, S.; Lin, S.; Zhang, H.; Liang, L.; Shen, S. Methods of respiratory virus detection: Advances towards point-of-care for early intervention. Micromachines 2021, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Bolandi, N.; Hemmati, A.; Eyvazi, S.; Ghasemzadeh, S.; Baradaran, B.; Mokhtarzadeh, A. State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: A critical review. Microchem J 2022, 107248. [Google Scholar] [CrossRef]
- Suntornsuk, W.; Suntornsuk, L. Recent applications of paper-based point-of-care devices for biomarker detection. Electrophoresis 2020, 41, 287–305. [Google Scholar] [CrossRef]
- Mahmoudi, T.; de la Guardia, M.; Baradaran, B. Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends. TrAC Trends Anal Chem 2020, 125, 115842. [Google Scholar] [CrossRef]
- Ouyang, M.; Tu, D.; Tong, L.; Sarwar, M.; Bhimaraj, A.; Li, C.; Di Carlo, D. A review of biosensor technologies for blood biomarkers toward monitoring cardiovascular diseases at the point-of-care. Biosens Bioelectron 2021, 171, 112621. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic point-of-care testing: Commercial landscape and future directions. Frontiers Bioengineering Biotechnol 2021, 8, 602659. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the therapeutics and diagnostics pipelines. Theranostics. 2018, 8, 4016. [Google Scholar] [CrossRef]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The application of whole cell-based biosensors for use in environmental analysis and in medical diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef]
- Mitchell, K.R.; Esene, J.E.; Woolley, A.T. Advances in multiplex electrical and optical detection of biomarkers using microfluidic devices. Anal Bioanal Chem 2022, 414, 167–180. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Liu, P.Y.; Nguyen, B.T.T.; Wu, W.; Zhao, Q.; Liu, A.Q. On-Chip Optical Detection of Viruses: A Review. Adv Photon Res 2021, 2, 2000150. [Google Scholar] [CrossRef]
- Sezgintürk, M.K. Introduction to commercial biosensors. In Commercial Biosensors and Their Applications; Sezgintürk, M. K., Ed.; Elsevier: Netherlands, 2020; pp. 1–28. [Google Scholar]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sensors Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Russo, A.; Cavalera, S.; Giovannoli, C.; Spano, G.; Baggiani, C. Silver and gold nanoparticles as multi-chromatic lateral flow assay probes for the detection of food allergens. Anal bioanal chem 2019, 411, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Baggiani, C.; Giovannoli, C.; Spano, G.; Anfossi, L. Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchim Acta 2017, 184, 1295–1304. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens Bioelectron 2016, 76, 164–179. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Lopreside, A.; Montali, L.; Zangheri, M.; Evangelisti, L.; D’Elia, M.; Michelini, E. Portable light detectors for bioluminescence biosensing applications: A comprehensive review from the analytical chemist’s perspective. Anal Chim Acta 2022, 339583. [Google Scholar] [CrossRef]
- Calabria, D.; Trozzi, I.; Lazzarini, E.; Pace, A.; Zangheri, M.; Iannascoli, L.; Mirasoli, M. AstroBio-CubeSat: A lab-in-space for chemiluminescence-based astrobiology experiments. Biosens Bioelectron 2023, 226, 115110. [Google Scholar] [CrossRef] [PubMed]
- Zangheri, M.; Mirasoli, M.; Nascetti, A.; Caputo, D.; Bonvicini, F.; Gallinella, G.; Roda, A. Microfluidic cartridge with integrated array of amorphous silicon photosensors for chemiluminescence detection of viral DNA. Sens bio-sens res 2016, 7, 127–132. [Google Scholar] [CrossRef]
- Mirasoli, M.; Bonvicini, F.; Lovecchio, N.; Petrucci, G.; Zangheri, M.; Calabria, D.; Nascetti, A. On-chip LAMP-BART reaction for viral DNA real-time bioluminescence detection. Sens Actuat B Chem 2018, 262, 1024–1033. [Google Scholar] [CrossRef]
- Lazzarini, E.; Pace, A.; Trozzi, I.; Zangheri, M.; Guardigli, M.; Calabria, D.; Mirasoli, M. An Origami Paper-Based Biosensor for Allergen Detection by Chemiluminescence Immunoassay on Magnetic Microbeads. Biosensors 2022, 12, 825. [Google Scholar] [CrossRef]
- Sciutto, G.; Zangheri, M.; Anfossi, L.; Guardigli, M.; Prati, S.; Mirasoli, M.; Roda, A. Miniaturized biosensors to preserve and monitor cultural heritage: From medical to conservation diagnosis. Angew Chem 2018, 130, 7507–7511. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Calabria, D.; Marchegiani, E.; Anfossi, L.; Guardigli, M.; Roda, A. Smartphone biosensor for point-of-need chemiluminescence detection of ochratoxin A in wine and coffee. Anal Chim Acta 2021, 1163, 338515. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Calabretta, M.M.; Calabria, D.; Caliceti, C.; Cevenini, L.; Lopreside, A.; Zangheri, M. Smartphone-based biosensors. Past, Present and Future Challenges of Biosensors and Bioanalytical Tools in Analytical Chemistry: A Tribute to Professor Marco Mascini. Compr. Anal. Chem 2017, 77, 237–286. [Google Scholar]
- Calabria, D.; Zangheri, M.; Trozzi, I.; Lazzarini, E.; Pace, A.; Mirasoli, M.; Guardigli, M. Smartphone-based chemiluminescent origami µPAD for the rapid assessment of glucose blood levels. Biosensors 2021, 11, 381. [Google Scholar] [CrossRef]
- Di Fusco, M.; Quintavalla, A.; Lombardo, M.; Guardigli, M.; Mirasoli, M.; Trombini, C.; Roda, A. Organically modified silica nanoparticles doped with new acridine-1, 2-dioxetane analogues as thermochemiluminescence reagentless labels for ultrasensitive immunoassays. Anal. Bioanal. Chem 2015, 407, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAC Trends Anal Chem 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Electrochemical biosensors for hormone analyses. Biosens Bioelectron 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Chakraborty, A.; Tibarewala, D.N.; Barui, A. Impedance-based biosensors, in: Bioelectronics and Medical Devices, 2019, pp. 97e122.
- Richter, M.M. Electrochemiluminescence (ecl). Chem Rev 2004, 104, 3003–3036. [Google Scholar] [CrossRef]
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem rev 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Du, F.; Chen, Y.; Meng, C.; Lou, B.; Zhang, W.; Xu, G. Recent advances in electrochemiluminescence immunoassay based on multiple-signal strategy. Current Opin Electrochem 2021, 28, 100725. [Google Scholar] [CrossRef]
- Nasrollahpour, H.; Khalilzadeh, B.; Naseri, A.; Sillanpaa, M.; Chia, C.H. Homogeneous electrochemiluminescence in the sensors game: What have we learned from past experiments? Anal Chem 2021, 94, 349–365. [Google Scholar] [CrossRef]
- Ma, X., Gao; Xu, G. Rational design of electrochemiluminescent devices. Accounts of Chemical Research 2021, 54, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, P.; Sciuto, E.L.; Zanut, A.; Petralia, S.; Valenti, G.; Paolucci, F.; Conoci, S. Ultrasensitive PCR-Free detection of whole virus genome by electrochemiluminescence. Biosens Bioelectron 2022, 209, 114165. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Jeon, Y.M.; Heo, S.Y. Electrochemiluminescence Systems for the Detection of Biomarkers: Strategical and Technological Advances. Biosensors 2022, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Nascetti, A.; Mirasoli, M.; Marchegiani, E.; Zangheri, M.; Costantini, F.; Porchetta, A.; Roda, A. Integrated chemiluminescence-based lab-on-chip for detection of life markers in extraterrestrial environments. Biosens Bioelectron 2019, 123, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T., Chakrabarty. Microelectrofluidic systems: Modeling and simulation; CRC Press: Florida, US, 2018. [Google Scholar]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.A.; Manz, P.A. Micro total analysis systems: 1. Introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef]
- Auroux, A.; Reyes, D.R.; Iossifidis, D.; Manz, P.A. Micro total analysis systems: 2. Analytical standard operations and applications. Anal. Chem. 2002, 74, 2637–2652. [Google Scholar] [CrossRef]
- West, J.; Becker, M.; Tombrink, S.; Manz, A. Micro total analysis systems: Latest achievements. Anal. Chem. 2008, 80, 4403–4419. [Google Scholar] [CrossRef]
- Giannitsis, A.T. Microfabrication of biomedical lab-on-chip devices. A review. Est J Eng 2011, 17, 109. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chinese Chem Let 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Ainla, A.; Mousavi, M.P.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal chem 2018, 90, 6240–6246. [Google Scholar] [CrossRef]
- Dryden, M.D.; Wheeler, A.R. DStat: A versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 2015, 10, e0140349. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.A.; Bonham, A.J.; White, R.J.; Zimmer, M.P.; Yadgar, R.J.; Hobza, T.M.; Plaxco, K.W. CheapStat: An open-source,“Do-It-Yourself” potentiostat for analytical and educational applications. PLoS ONE 2011, 6, e23783. [Google Scholar] [CrossRef] [PubMed]
- Dobbelaere, T.; Vereecken, P.M.; Detavernier, C. A USB-controlled potentiostat/galvanostat for thin-film battery characterization. HardwareX 2017, 2, 34–49. [Google Scholar] [CrossRef]
- Lopin, P.; Lopin, K.V. PSoC-Stat: A single chip open source potentiostat based on a Programmable System on a Chip. PLoS ONE 2018, 13, e0201353. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.D.; Conlan, R.W.; Godwin, M.C.; Moussy, F. Towards a miniature implantable in vivo telemetry monitoring system dynamically configurable as a potentiostat or galvanostat for two-and three-electrode biosensors. IEEE T INSTRUM MEAS 2005, 54, 61–72. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Amor-Gutiérrez, O.; Costa-Rama, E.; Fernández-Abedul, M.T. Batch injection electroanalysis with stainless-steel pins as electrodes in single and multiplexed configurations. Sens Actuat B Chem 2017, 253, 1207–1213. [Google Scholar] [CrossRef]
- Foster, C.W.; Kadara, R.O.; Banks, C.E. Screen-printing electrochemical architectures; Springer: Berlin, Germany, 2016. [Google Scholar]
- Giacomelli, C.E.; Vermeer, A.W.P.; Norde, W. Adsorption of immunoglobulin G on core-shell latex particles precoated with chaps. J. Colloid Interf. Sci. 2000, 231, 283–288. [Google Scholar] [CrossRef]
- Charelier, R.C.; Gengenbach, T.R.; Griesser, H.J.; Brigham-Burke, M.; O’Shannessy, D.J. A general method to recondition and reuse BIAcore sensor chips fouled with covalently immobilized protein/peptide. Anal. Biochem. 1995, 229, 112–118. [Google Scholar] [CrossRef]
- Charles, P.T.; Goldman, E.R.; Rangasammy, J.G.; Schauer, C.L.; Chen, M.S.; Taitt, C.R. Fabrication and characterization of 3D hydrogel microarrays to measure antigenicity and antibody functionality for biosensor application. Biosens. Bioelectron. 2004, 20, 753–764. [Google Scholar] [CrossRef]
- Palma, R.; Borghs, G.; Declerck, P.; Goddeeris, B. Comparison of random and oriented immobilization of antibody fragments on mixed self-assembled monolayers. J. Immunol. Methods 2006, 312, 167–181. [Google Scholar]
- Hu, T.; Zhang, M.; Wang, Z.; Chen, K.; Li, X.; Ni, Z. Layer-by-layer self-assembly of MoS2/PDDA hybrid film in microfluidic chips for ultrasensitive electrochemical immunosensing of alpha-fetoprotein. Microchem J. 2020, 158, 105209. [Google Scholar] [CrossRef]
- Timilsina, S.S.; Ramasamy, M.; Durr, N.; Ahmad, R.; Jolly, P.; Ingber, D.E. Biofabrication of Multiplexed Electrochemical Immunosensors for Simultaneous Detection of Clinical Biomarkers in Complex Fluids. Advanc Health Mat 2022, 11, 2200589. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Gómez, V.; Hernández-Santos, D.; González-García, M.B.; Pingarrón-Carrazón, J.M.; Costa-García, A. Simultaneous detection of free and total prostate specific antigen on a screen-printed electrochemical dual sensor. Biosens Bioelectron. 2009, 24, 2678–2683. [Google Scholar] [CrossRef]
- Singh, R.; Hong, S.; Jang, J. Label-free detection of influenza viruses using a reduced Graphene oxide-based electrochemical immunosensor integrated with a microfluidic platform, Sci. Rep. 2017, 7, 4277. [Google Scholar]
- Roberts, A.; Chauhan, N.; Islam, S.; Mahari, S.; Ghawri, B.; Gandham, R.K.; Gandhi, S. Graphene functionalized field-effect transistors for ultrasensitive detection of Japanese encephalitis and Avian influenza virus. Sci Rep. 2020, 10, 14546. [Google Scholar] [CrossRef]
- Ono, T., Oe. Glycan-functionalized graphene-FETs toward selective detection of human-infectious avian influenza virus. Japanese Journal of Applied Physics 2017, 56, 030302. [Google Scholar] [CrossRef]
- Maity, A.; Sui, X.; Jin, B.; Pu, H.; Bottum, K.J.; Huang, X.; Chen, J. Resonance-frequency modulation for rapid, point-of-care Ebola-Glycoprotein diagnosis with a graphene-based field-effect biotransistor. Anal chem. 2018, 90, 14230–14238. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, H.; Li, Y.T.; Xiao, M.M.; Zhang, Z.L.; Pang, D.W.; Zhang, G.J. A field effect transistor modified with reduced graphene oxide for immunodetection of Ebola virus. Microchim Acta. 2019, 186, 1–9. [Google Scholar] [CrossRef]
- Sengupta, J.; Adhikari, A.; Hussain, C.M. Graphene-based analytical lab-on-chip devices for detection of viruses: A review. Carbon Trends 2021, 4, 100072. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Kim, S.I. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.C.; OuYang, J.; Jin, L.; Li, C.P. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens Actuat B Chem 2021, 327, 128899. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Panat, R. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Adv Mat. 2021, 33, 2006647. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, M., Kadoya; Kuroda, A. Photon counting system for high-sensitivity detection of bioluminescence at optical fiber end. Bioluminescence: Methods and Protocols 2016, 299–310. [Google Scholar]
- Calabria, D.; Trozzi, I.; Lazzarini, E.; Pace, A.; Zangheri, M.; Iannascoli, L.; Mirasoli, M. AstroBio-CubeSat: A lab-in-space for chemiluminescence-based astrobiology experiments. Biosens Bioelectron 2023, 226, 115110. [Google Scholar] [CrossRef]
- Dinter, F.; Burdukiewicz, M.; Schierack, P.; Lehmann, W.; Nestler, J.; Dame, G.; Rödiger, S. Simultaneous detection and quantification of DNA and protein biomarkers in spectrum of cardiovascular diseases in a microfluidic microbead chip. Anal bioanal chem 2019, 411, 7725–7735. [Google Scholar] [CrossRef]
- Chang, N.; Zhai, J.; Liu, B.; Zhou, J.; Zeng, Z.; Zhao, X. Low cost 3D microfluidic chips for multiplex protein detection based on photonic crystal beads. Lab Chip 2018, 18, 3638–3644. [Google Scholar] [CrossRef]
- Yuan, X.; Garg, S.; De Haan, K.; Fellouse, F.A.; Gopalsamy, A.; Tykvart, J.; Aitchison, J.S. Bead-based multiplex detection of dengue biomarkers in a portable imaging device. Biomed Opt Express 2020, 11, 6154–6167. [Google Scholar] [CrossRef]
- Dai, B.; Yin, C.; Wu, J.; Li, W.; Zheng, L.; Lin, F.; Zhuang, S. A flux-adaptable pump-free microfluidics-based self-contained platform for multiplex cancer biomarker detection. Lab Chip 2021, 21, 143–153. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Chen, T.; Ozkaya, G.U.; Choudhary, D.; Molinolo, A.A.; Gutkind, J.S.; Rusling, J.F. Detecting cancer metastasis and accompanying protein biomarkers at single cell levels using a 3D-printed microfluidic immunoarray. Biosens Bioelectron. 2021, 171, 112681. [Google Scholar] [CrossRef] [PubMed]
- Mirasoli, M.; Bonvicini, F.; Lovecchio, N.; Petrucci, G.; Zangheri, M.; Calabria, D.; Nascetti, A. On-chip LAMP-BART reaction for viral DNA real-time bioluminescence detection. Sens Actuat B Chem 2018, 262, 1024–1033. [Google Scholar] [CrossRef]
- Calabria, D.; Lazzarini, E.; Pace, A.; Trozzi, I.; Zangheri, M.; Cinti, S.; Mirasoli, M. Smartphone-based 3D-printed electrochemiluminescence enzyme biosensor for reagentless glucose quantification in real matrices. Biosens Bioelectron 2023, 227, 115146. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2020, 20, 9–34. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Fu, J.Z.; Wu, W.B. Fabrication of paper-based microfluidic analysis devices: A review. Rsc Advan 2015, 5, 78109–78127. [Google Scholar] [CrossRef]
- Lee, V.B.C.; Mohd-Naim, N.F.; Tamiya, E.; Ahmed, M.U. Trends in paper-based electrochemical biosensors: From design to application. Anal Sci 2018, 34, 7–18. [Google Scholar] [CrossRef]
- Tran, V.K.; Ko, E.; Geng, Y.; Kim, M.K.; Jin, G.H.; Son, S.E.; Seong, G.H. Micro-patterning of single-walled carbon nanotubes and its surface modification with gold nanoparticles for electrochemical paper-based non-enzymatic glucose sensor. J Electroanal Chem. 2018, 826, 29–37. [Google Scholar] [CrossRef]
- Punjiya, M.; Moon, C.H.; Matharu, Z.; Nejad, H.R.; Sonkusale, S. A three-dimensional electrochemical paper-based analytical device for low-cost diagnostics. Analyst 2018, 143, 1059–1064. [Google Scholar] [CrossRef]
- Amor-Gutiérrez, O.; Costa-Rama, E.; Fernández-Abedul, M.T. Sampling and multiplexing in lab-on-paper bioelectroanalytical devices for glucose determination. Biosens Bioelectron 2019, 135, 64–70. [Google Scholar] [CrossRef]
- He, X.; Chang, S.J.; Settu, K.; Chen, C.J.; Liu, J.T. An anti-HCT-interference glucose sensor based on a fiber paper-based screen-printed carbon electrode. Sens Actua B Chem. 2019, 297, 126763. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Tahernia, M.; Choi, S. An equipment-free, paper-based electrochemical sensor for visual monitoring of glucose levels in urine. SLAS TECHNOL Transl Life Sci Inn 2019, 24, 499–505. [Google Scholar] [CrossRef]
- Calabria, D.; Pace, A.; Lazzarini, E.; Trozzi, I.; Zangheri, M.; Guardigli, M.; Mirasoli, M. Smartphone-Based Chemiluminescence Glucose Biosensor Employing a Peroxidase-Mimicking, Guanosine-Based Self-Assembled Hydrogel. Biosensors 2023, 13, 650. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, B.; Yu, C.; Fang, L.; Tu, T.; Wei, J.; Ye, X. High accuracy determination of multi metabolite by an origami-based coulometric electrochemical biosensor. J Electroanal Chem 2020, 873, 114358. [Google Scholar] [CrossRef]
- Rafatmah, E.; Hemmateenejad, B. Dendrite gold nanostructures electrodeposited on paper fibers: Application to electrochemical non-enzymatic determination of glucose. Sens Actuat B: Chem 2020, 304, 127335. [Google Scholar] [CrossRef]
- Pesaran, S.; Rafatmah, E.; Hemmateenejad, B. An all-in-one solid state thin-layer potentiometric sensor and biosensor based on three-dimensional origami paper microfluidics. Biosensors 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Towards lateral flow quantitative assays: Detection approaches. Biosensors 2019, 9, 89. [Google Scholar] [CrossRef]
- Urusov, A.E.; Jerdev, A.V.; Starovoitova, T.A.; Vengerov, Y.Y.; Dzantiev, B.B. The device registration of immune chromatographic express-tests. Klinicheskaia Laboratornaia Diagnostika 2016, 61, 173–179. [Google Scholar]
- Zangheri, M.; Mirasoli, M.; Guardigli, M.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; Roda, A. Chemiluminescence-based biosensor for monitoring astronauts’ health status during space missions: Results from the International Space Station. Biosens Bioelectron 2019, 129, 260–268. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Xu, H.; Yan, W.; Jin, Q.; Cui, D. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: A review. Talanta 2019, 202, 96–110. [Google Scholar] [CrossRef]
- Park, J. Lateral Flow Immunoassay Reader Technologies for Quantitative Point-of-Care Testing. Sensors 2022, 22, 7398. [Google Scholar] [CrossRef]
- Lee, A.S.; Kim, S.M.; Kim, K.R.; Park, C.; Lee, D.G.; Heo, H.R.; Kim, C.S. A colorimetric lateral flow immunoassay based on oriented antibody immobilization for sensitive detection of SARS-CoV-2. Sens Actuat B: Chem 2023, 379, 133245. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wang, C.; Yang, X.; Zheng, S.; Cheng, X.; Liu, Z.; Xiao, R. Rapid field determination of SARS-CoV-2 by a colorimetric and fluorescent dual-functional lateral flow immunoassay biosensor. Sens Actuat B: Chem 2022, 351, 130897. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Cavalera, S.; Di Nardo, F.; Calabria, D.; Rosati, S.; Simoni, P.; Anfossi, L. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens Bioelectron 2021, 172, 112765. [Google Scholar] [CrossRef]
- Li, H.; Ying, Y.; Cao, Z.; Liu, G.; Wang, J. Research progress on rapid detection technology based on smartphone and lateral flow immunoassay. Chinese J Anal Chem 2022, 50, 1–11. [Google Scholar]
- Pohanka, M. Point-of-care diagnoses and assays based on lateral flow test. Int J Anal Chem 2021, 1–9. [Google Scholar] [CrossRef]
- Calabria, D.; Calabretta, M.M.; Zangheri, M.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M. Recent advancements in enzyme-based lateral flow immunoassays. Sensors 2021, 21, 3358. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Tang, X.; Wu, Z.; Lu, J.; Liang, C.; Li, C. Development of a smartphone-based quantum dot lateral flow immunoassay strip for ultrasensitive detection of anti-SARS-CoV-2 IgG and neutralizing antibodies. Int J Infect Dis 2022, 121, 58–65. [Google Scholar] [CrossRef]
- Mahmoud, M.; Ruppert, C.; Rentschler, S.; Laufer, S.; Deigner, H.P. Combining aptamers and antibodies: Lateral flow quantification for thrombin and interleukin-6 with smartphone readout. Sens Actuat B Chem. 2021, 333, 129246. [Google Scholar] [CrossRef]
- Rong, Z.; Wang, Q.; Sun, N.; Jia, X.; Wang, K.; Xiao, R.; Wang, S. Smartphone-based fluorescent lateral flow immunoassay platform for highly sensitive point-of-care detection of Zika virus nonstructural protein 1. Anal chim acta 2019, 1055, 140–147. [Google Scholar] [CrossRef]
- Chabi, M.; Vu, B.; Brosamer, K.; Smith, M.; Chavan, D.; Conrad, J.C.; Kourentzi, K. Smartphone-read phage lateral flow assay for point-of-care detection of infection. Analyst 2023, 148, 839–848. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, L.; Yang, L.; Cui, Y. Minimizing Cross-Reactivity for the Chemiluminescent Lateral Flow Immunoassay of Cardiac Troponin I Based on PEGylation of Gold Nanoparticles. Anal Chem 2023, 95, 6646–6654. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Zangheri, M.; Calabria, D.; Mirasoli, M.; Caliceti, C.; Quintavalla, A.; Simoni, P. A simple smartphone-based thermochemiluminescent immunosensor for valproic acid detection using 1, 2-dioxetane analogue-doped nanoparticles as a label. Sens Actuat B Chem 2019, 279, 327–333. [Google Scholar] [CrossRef]
- Mazurkiewicz, W.; Podrażka, M.; Jarosińska, E.; Kappalakandy Valapil, K.; Wiloch, M.; Jönsson-Niedziółka, M.; Witkowska Nery, E. Paper-Based Electrochemical Sensors and How to Make Them (Work). ChemElectroChem 2020, 7, 2939–2956. [Google Scholar] [CrossRef]
- Arduini, F. Electrochemical paper-based devices: When the simple replacement of the support to print ecodesigned electrodes radically improves the features of the electrochemical devices. Curr Opin Electrochem 2022, 101090. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal. Chem 2009, 81, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab on a Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, G.; Guo, J.; Liu, S.; Guo, J. Integrated electrochemical lateral flow immunoassays (eLFIAs): Recent advances. Analyst 2022, 147, 554–570. [Google Scholar] [CrossRef]
- Deenin, W.; Yakoh, A.; Pimpitak, U.; Pasomsub, E.; Rengpipat, S.; Crespo, G.A.; Chaiyo, S. Electrochemical lateral-flow device for rapid COVID-19 antigen-diagnostic testing. Bioelectrochem 2023, 152, 108438. [Google Scholar] [CrossRef]
- Sinawang, P.D.; Fajs, L.; Elouarzaki, K.; Nugraha, J.; Marks, R.S. TEMPO-based immuno-lateral flow quantitative detection of dengue NS1 protein. Sens Actuat B Chem 2018, 259, 354–363. [Google Scholar] [CrossRef]
- Srisomwat, C.; Yakoh, A.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Amplification-free DNA sensor for the one-step detection of the hepatitis B virus using an automated paper-based lateral flow electrochemical device. Anal chem 2020, 93, 2879–2887. [Google Scholar] [CrossRef]
- Hong, D.; Jo, E.J.; Kim, K.; Song, M. B.; Kim, M.G. Ru (bpy)32+-loaded mesoporous silica nanoparticles as electrochemiluminescent probes of a lateral flow immunosensor for highly sensitive and quantitative detection of troponin I. Small 2020, 16, 2004535. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable chemical sensors: Present challenges and future prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab on a Chip. 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Guardigli, M.; Zangheri, M.; Caliceti, C.; Calabria, D.; Simoni, P. Advanced biosensors for monitoring astronauts’ health during long-duration space missions. Biosens Bioelectron 2018, 111, 18–26. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Zangheri, M.; Lopreside, A.; Marchegiani, E.; Montali, L.; Simoni, P.; Roda, A. Precision medicine, bioanalytics and nanomaterials: Toward a new generation of personalized portable diagnostics. Analyst 2020, 145, 2841–2853. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat biotechnol 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Sayson, D.; Nguyen, B.H.; Tran, S.D. Advances in medical wearable biosensors: Design, fabrication and materials strategies in healthcare monitoring. Molecules 2021, 27, 165. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.H.; Baeumner, A.J.; Alshareef, H.N. A MXene-based wearable biosensor system for high-performance in vitro perspiration analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, Y.; Wu, J.; Nyein, H.Y.Y.; Bariya, M.; Tai, L.C.; Javey, A. A fully integrated and self-powered smartwatch for continuous sweat glucose monitoring. ACS sensors 2019, 4, 1925–1933. [Google Scholar] [CrossRef]
- Lin, K.C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens Actuat B Chem 2020, 320, 128325. [Google Scholar] [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Arduini, F. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens Actuat B: Chemical 2023, 379, 133258. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Fiore, L.; Nappi, S.; Marrocco, G.; Arduini, F. Medium-distance affordable, flexible and wireless epidermal sensor for pH monitoring in sweat. Talanta 2021, 222, 121502. [Google Scholar] [CrossRef]
- Promphet, N.; Hinestroza, J.P.; Rattanawaleedirojn, P.; Soatthiyanon, N.; Siralertmukul, K.; Potiyaraj, P.; Rodthongkum, N. Cotton thread-based wearable sensor for non-invasive simultaneous diagnosis of diabetes and kidney failure. Sens Actuat B: Chem. 2020, 321, 128549. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Rogers, J.A. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci advan. 2019, 5, eaav3294. [Google Scholar] [CrossRef]
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Rogers, J.A. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab on Chip 2018, 18, 2178–2186. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yan, B. A fluorescent wearable platform for sweat Cl− analysis and logic smart-device fabrication based on color adjustable lanthanide MOFs. J Mat Chem C 2018, 6, 1863–1869. [Google Scholar] [CrossRef]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Rogers, J.A. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS sensors 2019, 4, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Chen, Z.H.; Zeng, J.Z.; Fan, Q.X.; Fang, Z.Q.; Shi, G.; Zhang, M. Inorganic–organic hybrid tongue-mimic for time-resolved luminescent noninvasive pattern and chiral recognition of thiols in biofluids toward healthcare monitoring. ACS applied mat inter 2018, 10, 31725–31734. [Google Scholar] [CrossRef]
- Promphet, N.; Ummartyotin, S.; Ngeontae, W.; Puthongkham, P.; Rodthongkum, N. Non-invasive wearable chemical sensors in real-life applications. Anal Chim Acta 2021, 1179, 338643. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Jha, S.K. Smartphone based non-invasive salivary glucose biosensor. Anal chim acta 2017, 996, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Surana, R.K.; Jha, S.K. Smartphone based optical biosensor for the detection of urea in saliva. Sens Actuat B: Chem 2018, 269, 346–353. [Google Scholar] [CrossRef]
- Promphet, N.; Hinestroza, J.P.; Rattanawaleedirojn, P.; Soatthiyanon, N.; Siralertmukul, K.; Potiyaraj, P.; Rodthongkum, N. Cotton thread-based wearable sensor for non-invasive simultaneous diagnosis of diabetes and kidney failure. Sens Actuat B Chem 2020, 321, 128549. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Rodthongkum, N. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Siralertmukul, K.; Rodthongkum, N. Colorimetric sensor and LDI-MS detection of biogenic amines in food spoilage based on porous PLA and graphene oxide. Food chem. 2020, 329, 127165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, H.; Kim, S.B.; Wu, Y.; Ostojich, D.; Park, S.H.; Rogers, J.A. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab on Chip 2019, 19, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Yan, B. A fluorescent wearable platform for sweat Cl− analysis and logic smart-device fabrication based on color adjustable lanthanide MOFs. J Mat Chem C 2018, 6, 1863–1869. [Google Scholar] [CrossRef]
- Miller, P.R.; Taylor, R.M.; Tran, B.Q.; Boyd, G.; Glaros, T.; Chavez, V.H.; Polsky, R. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Comm biol. 2018, 1, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Lei, Y. Microneedle-based glucose monitoring: A review from sampling methods to wearable biosensors. Biomat Sci 2023. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Namjoshi, S.; Benson, H.A.E.; Mohammed, Y.; Kumeria, T. Dissolvable polymer microneedles for drug delivery and diagnostics. J. Control. Release 2022, 347, 561–589. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Cai, R.; Zheng, H.; Yu, J.; Zhang, Y.; Gu, Z. Microneedle-based transdermal detection and sensing devices. Lab Chip 2023, 23, 869–887. [Google Scholar] [CrossRef]
- Blicharz, T.M.; Gong, P.; Bunner, B.M.; Chu, L.L.; Leonard, K.M.; Wakefield, J.A.; Williams, R.E.; Dadgar, M.; Tagliabue, C.A.; El Khaja, R.; Marlin, S.L.; Haghgooie, R.; Davis, S.P.; Chickering, D. E.; Bernstein, H. Microneedle-based device for the one-step painless collection of capillary blood samples, Nat. Biomed. Eng. 2018, 2, 151–157. [Google Scholar]
- Kim, D.; Cao, Y.; Mariappan, D.; Bono Jr, M.S.; Hart, A.J.; Marelli, B. A microneedle technology for sampling and sensing bacteria in the food supply chain. Adv Funct Mat 2021, 31, 2005370. [Google Scholar] [CrossRef]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Wang, J. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat Biomed Engineer 2022, 6, 1214–1224. [Google Scholar] [CrossRef]
- Paul, R.; Saville, A.C.; Hansel, J.C.; Ye, Y.; Ball, C.; Williams, A.; Wei, Q. Extraction of plant DNA by microneedle patch for rapid detection of plant diseases. ACS nano 2019, 13, 6540–6549. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Kim, D.H. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat nanotechnol 2016, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, J.; Zhang, Y.; Chen, G.; Mao, W.; Ye, Y.; Gu, Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat biomed engineer 2020, 4, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem Soc Rev 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Peng, H. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat Biomed Engineer. 2020, 4, 159–171. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv health mat 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mat Sci Engineer C 2014, 41, 100–118. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens Bioelectron 2020, 165, 112331. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Shetti, N.P.; Malode, S.J.; Mishra, A.; Basu, S.; Aminabhavi, T.M. Skin patchable sensor surveillance for continuous glucose monitoring. ACS Applied Bio Mat 2022, 5, 945–970. [Google Scholar] [CrossRef]
- Sharma, S.; Huang, Z.; Rogers, M.; Boutelle, M.; Cass, A.E. Evaluation of a minimally invasive glucose biosensor for continuous tissue monitoring. Anal bioanal chem 2016, 408, 8427–8435. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Valdés-Ramírez, G.; Zhou, N.; Zhou, M.; Miller, P.R.; Jin, C.; Wang, J. Bicomponent microneedle array biosensor for minimally-invasive glutamate monitoring. Electroanal 2011, 23, 2302–2309. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Chen, W.; Chen, Y.; Zhang, J.X. PVDF-Nafion nanomembranes coated microneedles for in vivo transcutaneous implantable glucose sensing. Biosens Bioelectron. 2015, 74, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Yang, Y.; Zhao, Z.Q.; Guo, X.D. A gold nanoparticles deposited polymer microneedle enzymatic biosensor for glucose sensing. Electrochim Acta 2020, 358, 136917. [Google Scholar] [CrossRef]
- Gao, J.; Huang, W.; Chen, Z.; Yi, C.; Jiang, L. Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sens Actuat B: Chem. 2019, 287, 102–110. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Luo, X.; Yang, L.; Cui, Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst nanoeng 2021, 7, 75. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Wang, J. Microneedle-based detection of ketone bodies along with glucose and lactate: Toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal chem 2019, 92, 2291–2300. [Google Scholar] [CrossRef]
- Trzebinski, J.; Sharma, S.; Moniz, A.R.B.; Michelakis, K.; Zhang, Y.; Cass, A.E. Microfluidic device to investigate factors affecting performance in biosensors designed for transdermal applications. Lab on Chip 2012, 12, 348–352. [Google Scholar] [CrossRef]
- Valdés-Ramírez, G.; Li, Y.C.; Kim, J.; Jia, W.; Bandodkar, A.J.; Nuñez-Flores, R.; Wang, J. Microneedle-based self-powered glucose sensor. Electrochem Comm 2014, 47, 58–62. [Google Scholar] [CrossRef]
- Caliò, A.; Dardano, P.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.; Iuele, H.; De Stefano, L. Polymeric microneedles based enzymatic electrodes for electrochemical biosensing of glucose and lactic acid. Sens Actuat B Chem 2016, 236, 343–349. [Google Scholar] [CrossRef]
- Kim, K.B.; Choi, H.; Jung, H.J.; Oh, Y.J.; Cho, C.H.; Min, J.H.; Cha, H.J. Mussel-inspired enzyme immobilization and dual real-time compensation algorithms for durable and accurate continuous glucose monitoring. Biosens Bioelectron 2019, 143, 111622. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Yan, L.; Senel, M.; Gengenbach, T.R.; Prieto-Simon, B.; Voelcker, N.H. Transdermal electrochemical monitoring of glucose via high-density silicon microneedle array patch. Adv Func Mat 2022, 32, 2009850. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.; Tasca, F.; Antiochia, R. Minimally invasive glucose monitoring using a highly porous gold microneedles-based biosensor: Characterization and application in artificial interstitial fluid. Catalysts 2019, 9, 580. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive microneedle-based biosensor array for simultaneous lactate and glucose monitoring in artificial interstitial fluid. Electroanal 2019, 31, 374–382. [Google Scholar] [CrossRef]
- Kim, K.B.; Lee, W.C.; Cho, C.H.; Park, D.S.; Cho, S.J.; Shim, Y.B. Continuous glucose monitoring using a microneedle array sensor coupled with a wireless signal transmitter. Sens Actuat B Chem 2019, 281, 14–21. [Google Scholar] [CrossRef]
- Invernale, M.A.; Tang, B.C.; York, R.L.; Le, L.; Hou, D.Y.; Anderson, D.G. Microneedle electrodes toward an amperometric glucose-sensing smart patch. Adv health mat 2014, 3, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Rasheed, T.; Rizwan, K. Metal-organic frameworks based hybrid nanocomposites as state-of–the-art analytical tools for electrochemical sensing applications. Biosens Bioelectron 2022, 199, 113867. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, G.S.; Yoo, K.; Lee, J.B. Fabrication of a microneedle/CNT hierarchical micro/nano surface electrochemical sensor and its in-vitro glucose sensing characterization. Sensors 2013, 13, 16672–16681. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, H.S.; Xuan, X.; Park, J.Y.; Paik, S.J.; Allen, M.G. A patch type non-enzymatic biosensor based on 3D SUS micro-needle electrode array for minimally invasive continuous glucose monitoring. Sens Actuat B: Chem 2016, 222, 1144–1151. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, I.; Cho, S. Nonenzymatic determination of glucose at near neutral pH values based on the use of nafion and platinum black coated microneedle electrode array. Microchim Acta 2018, 185, 1–8. [Google Scholar] [CrossRef]
- He, R.; Niu, Y.; Li, Z.; Li, A.; Yang, H.; Xu, F.; Li, F. A hydrogel microneedle patch for point-of-care testing based on skin interstitial fluid. Adv Health Mat 2020, 9, 1901201. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Joung, H.A.; Noh, H.; Song, M.B.; Kim, M.G.; Jung, H. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab on Chip 2015, 15, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, D.; Logan, K.A.; Sheng, Y.; Gao, J.; Farrell, S.; Dixon, D.; Callan, J.F. Rapid paper based colorimetric detection of glucose using a hollow microneedle device. Int j pharma 2018, 47, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bonfante, G.; Sasaki, Y.; Takama, N.; Minami, T.; Kim, B. Porous microneedles on a paper for screening test of prediabetes. Med Devices Sensors 2020, 3, e10109. [Google Scholar] [CrossRef]
- Zhu, D.D.; Duong, P.K.; Cheah, R.H.; Liu, X.Y.; Wong, J.R.; Wang, W.J.; Chen, P. Colorimetric microneedle patches for multiplexed transdermal detection of metabolites. Biosens Bioelectron 2022, 212, 114412. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Wang, J.; Chen, Z.; Chen, G.; Wen, D.; Chan, A.; Gu, Z. Transdermal colorimetric patch for hyperglycemia sensing in diabetic mice. Biomat 2020, 237, 119782. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Huang, C.-Y.; Hsu, Y.-P.; Hwang, T.-L.; Chang, S.-H.; Wang, H.-Y. J.; Feng, L.-Y.; Tzou, S.-J.; Wei, K.-C.; Yang, H.-W. On-skin glucose-biosensing and on-demand insulin-zinc hexamers delivery using microneedles for syringe-free diabetes management, Chem. Eng. J. 2020, 398, 125536. [Google Scholar]
- Zhang, X.X.; Chen, G.P.; Bian, F.K.; Cai, L. J.; Zhao, Y.J. Encoded microneedle arrays for detection of skin interstitial fluid biomarkers, Adv. Mater. 2019, 31, 1902825. [Google Scholar]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; Zhang, X.; Gupta, R.; Zhou, Q.; Morrissey, J. J; . Scheller, E.L.; Rudra, J. S.; Singamaneni, S. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid, Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar]
- Zheng, H.; GhavamiNejad, A.; GhavamiNejad, P.; Samarikhalaj, M.; Giacca, A.; Poudineh, M. Hydrogel microneedle-assisted assay integrating aptamer probes and fluorescence detection for reagentless biomarker quantification. ACS Sens 2022, 7, 2387–2399. [Google Scholar] [CrossRef]
- Sang, M.; Cho, M.; Lim, S.; Min, I.S.; Han, Y.; Lee, C.; Shin, J.; Yoon, K.; Yeo, W.-H.; Lee, T.; Won, S.M.; Jung, Y.; Heo, Y.J.; Yu, K.J. Fluorescent-based biodegradable microneedle sensor array for tether-free continuous glucose monitoring with smartphone application, Sci. Adv. 2023, 9, 1765. [Google Scholar]
- Zou, Y., Chu; Guo, J., Liu. Minimally invasive electrochemical continuous glucose monitoring sensors: Recent progress and perspective. Biosens Bioelectron 2023, 115103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




