Submitted:
01 October 2025
Posted:
02 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
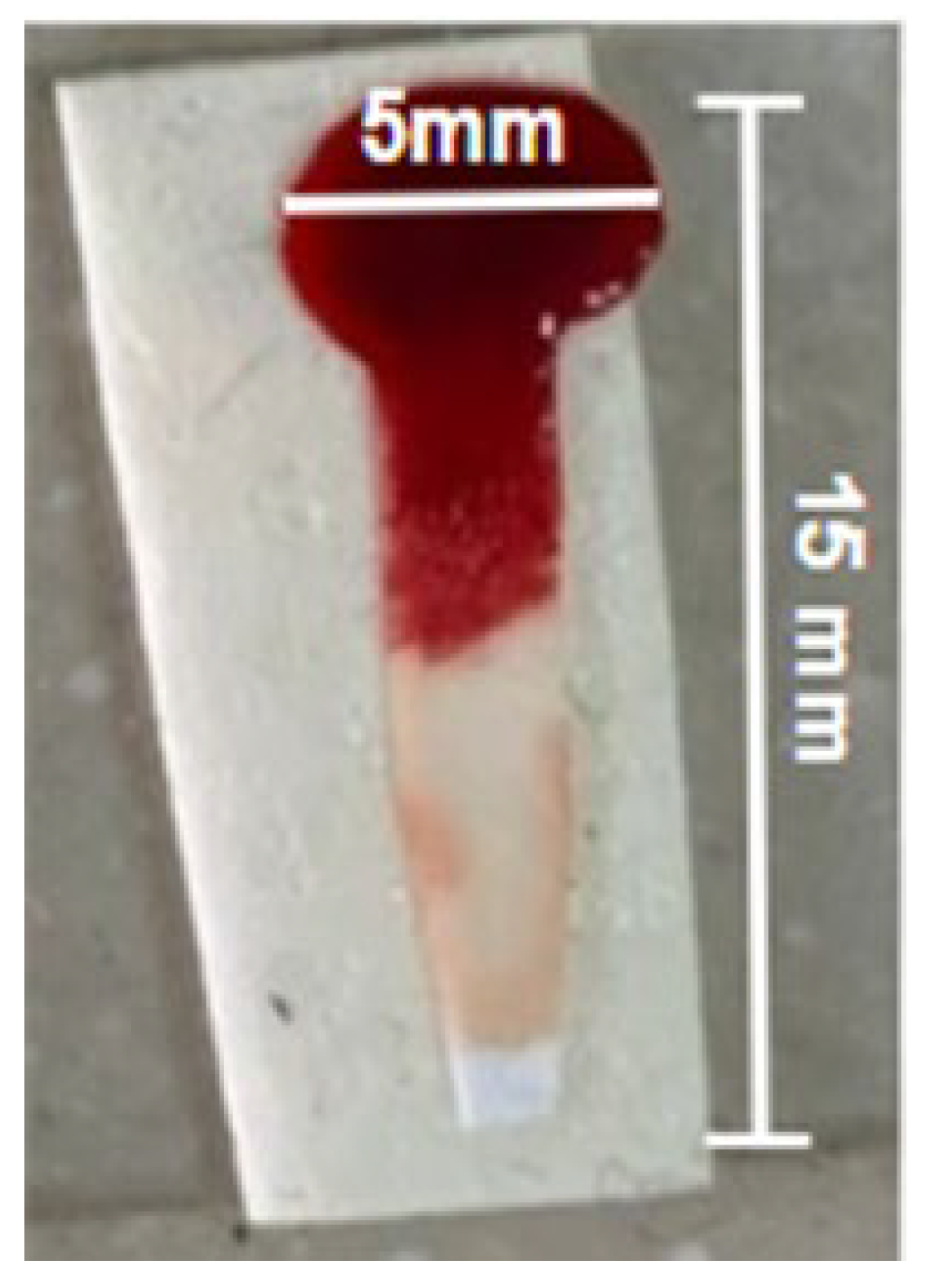
2.1. Substrate Selection and Characterization
- − Whatman Paper N°1: with a pore size of 11 µm and a thickness of 180 µm. Its intermediate porous structure allows partial retention of blood cells, especially leukocytes, and provides a good balance between flow and mechanical support.
- − Polycarbonate membrane: with 3 µm pores and 22 µm thickness. Its small pore size makes it suitable for the retention of erythrocytes and platelets, allowing cleaner separation of plasma.
- − Glass fiber membrane: with a pore size of 3 µm and a thickness of 785 µm. Despite sharing the same pore size as the previous one, its greater thickness provides higher absorption and retention capacity, making it suitable for complementing the separation process.
2.2. Paper Functionalization and Plasma Separation
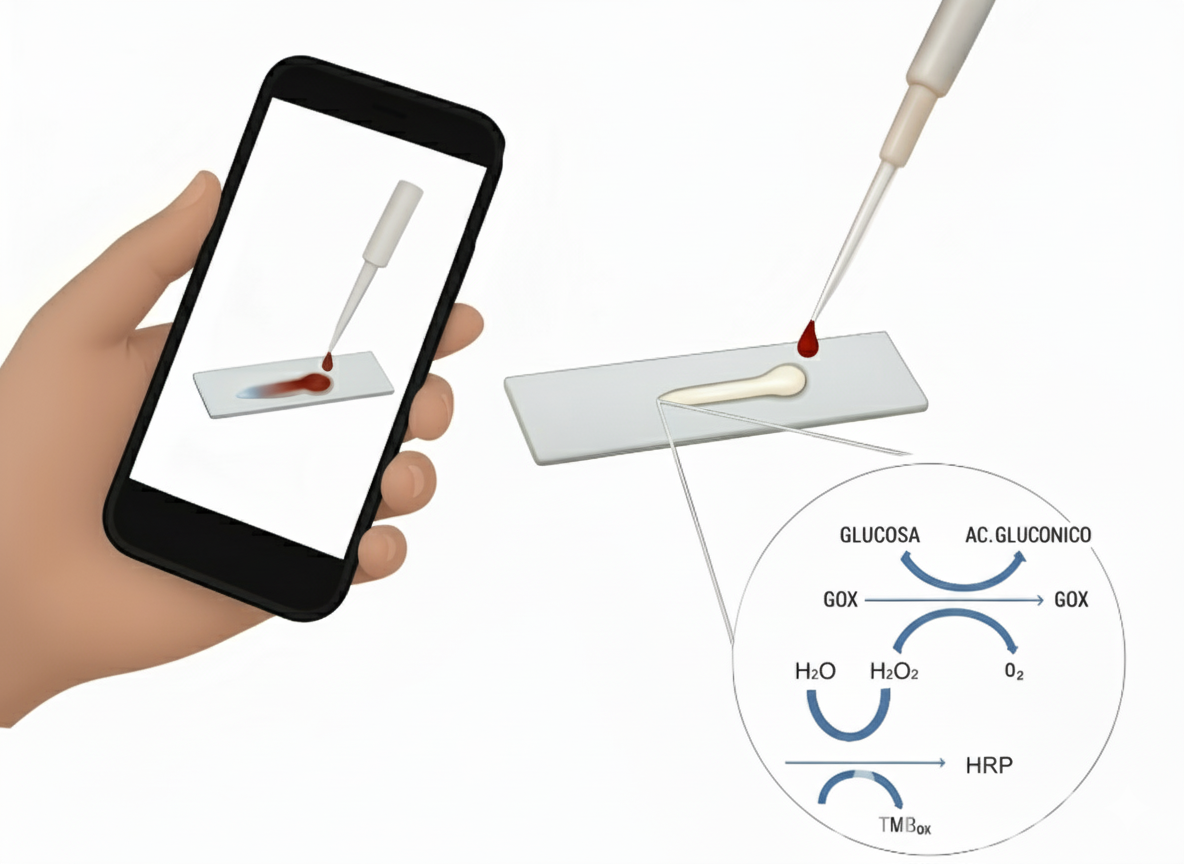
2.3. Colorimetric Enzymatic Assay for Glucose Detection.
- − A 1.2 μL layer of TMB solution (pre-prepared).
- − A 1.5 μL layer of HRP (7.11 U/mL) was prepared in phosphate-buffered saline (PBS, pH 7.4).
- − A 1.5 μL layer of GOx (2 mg/mL) prepared in PBS (pH 7.4).
- − A final 1.5 μL layer of TMB to complete the multilayer detection platform.
2.4. Glucose Calibration
2.5. Measurements Validation
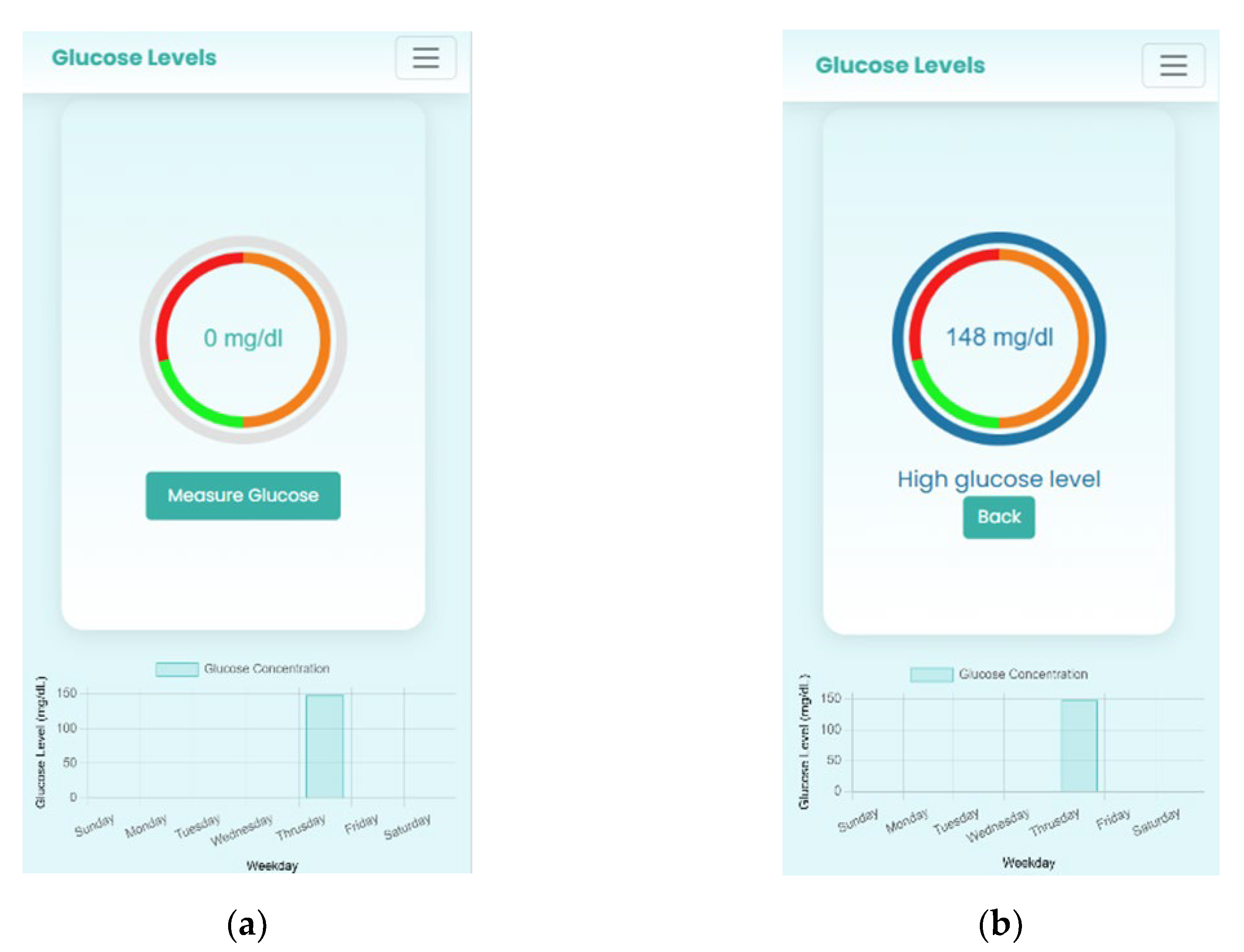
2.6. Software Design for Glucose Detection
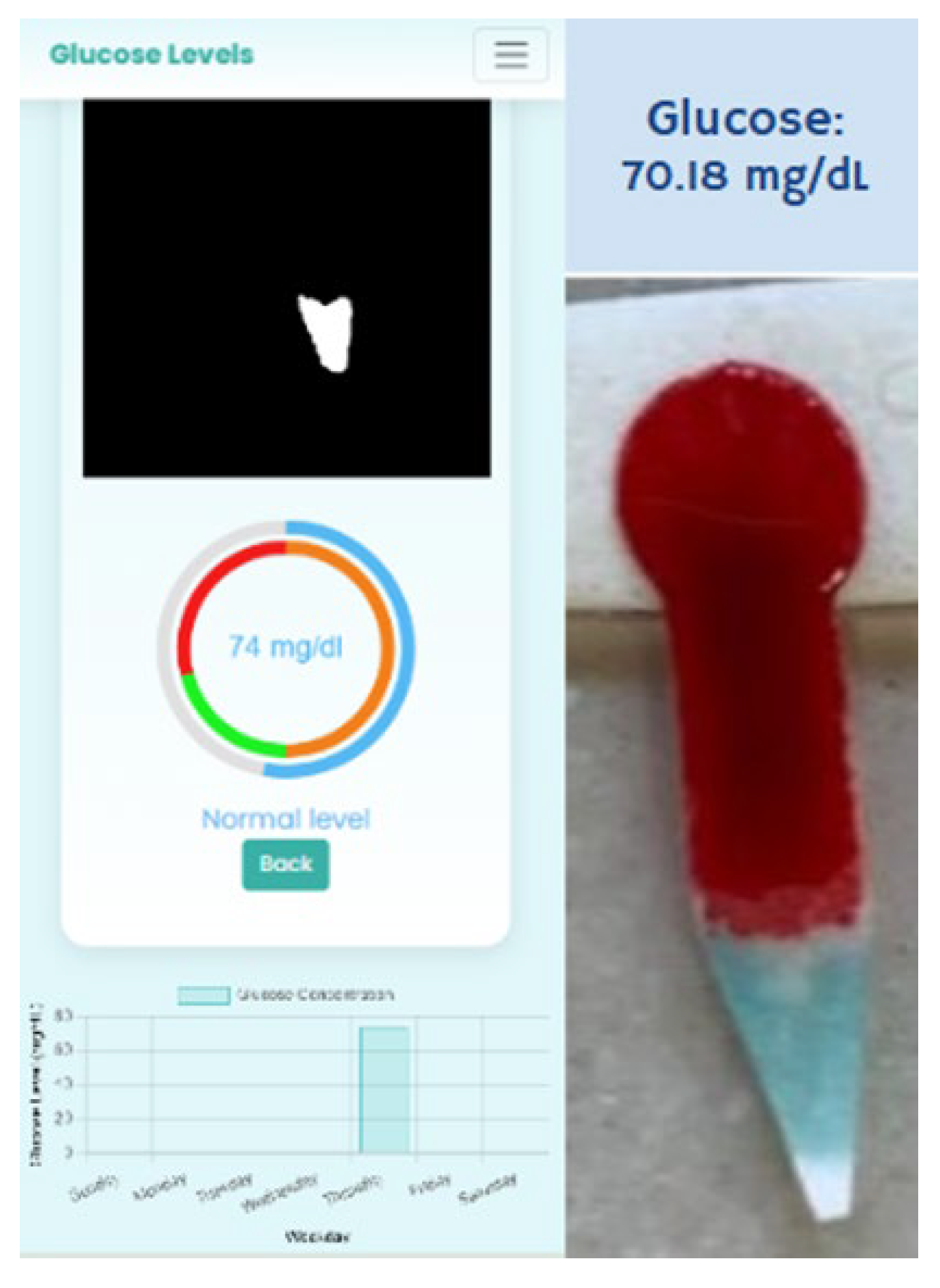
2.7. Image Processing and Colorimetric Analysis
2.7.1. Step 1: Definition of HSV Ranges
2.7.2. Step 2: Object Segmentation and Mask Generation
2.7.3. Step 3: Color Intensity Calculation
2.7.4. Step 4: Quantitative Analysis
3. Results
4. Conclusions
References
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y. Review of Noninvasive Continuous Glucose Monitoring in Diabetics. ACS Sens. 2023, 8, 3659–3679. [Google Scholar] [CrossRef] [PubMed]
- Hajimiri, H.; Safiabadi Tali, S.H.; Al-Kassawneh, M.; Sadiq, Z.; Jahanshahi-Anbuhi, S. Tablet-Based Sensor: A Stable and User-Friendly Tool for Point-of-Care Detection of Glucose in Urine. Biosensors 2023, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, Flow Control, and Applications of Microfluidic Paper-Based Analytical Devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.I.; Sindi, H.; Whitesides, G.M. Simple Telemedicine for Developing Regions: Camera Phones and Paper-Based Microfluidic Devices for Real-Time, Off-Site Diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef]
- Ellerbee, A.K.; Phillips, S.T.; Siegel, A.C.; Mirica, K.A.; Martinez, A.W.; Striehl, P.; Jain, N.; Prentiss, M.; Whitesides, G.M. Quantifying Colorimetric Assays in Paper-Based Microfluidic Devices by Measuring the Transmission of Light through Paper. Anal. Chem. 2009, 81, 8447–8452. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, C. A Novel Screen-Printed Microfluidic Paper-Based Electrochemical Device for Detection of Glucose and Uric Acid in Urine. Biomed. Microdevices 2016, 18, 92. [Google Scholar] [CrossRef]
- Madrid, R.E.; Ashur Ramallo, F.; Barraza, D.E.; Chaile, R.E. Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering 2022, 9, 101. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, X.; Li, N. Smartphone-Based Mobile Biosensors for the Point-of-Care Testing of Human Metabolites. Mater. Today Bio 2022, 14, 100254. [Google Scholar] [CrossRef]
- Horta-Velázquez, A.; Ramos-Ortiz, G.; Morales-Narváez, E. The Optimal Color Space Enables Advantageous Smartphone-Based Colorimetric Sensing. Biosens. Bioelectron. 2025, 273, 117089. [Google Scholar] [CrossRef] [PubMed]
- AuYoung, B.; Gutha Ravichandran, A.; Patel, D.; Dave, N.; Shah, A.; Wronko-Stevens, B.; Bettencourt, F.; Rajan, R.; Menon, N. A Low-Cost Paper-Based Device for the Colorimetric Quantification of Bilirubin in Serum Using Smartphone Technology. Front. Chem. 2022, 10. [Google Scholar] [CrossRef]
- Gautam, N.; Verma, R.; Ram, R.; Singh, J.; Sarkar, A. Development of a Biodegradable Microfluidic Paper-Based Device for Blood-Plasma Separation Integrated with Non-Enzymatic Electrochemical Detection of Ascorbic Acid. Talanta 2024, 266, 125019. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, F.; Hemmateenejad, B. Pronounced Effect of Lamination on Plasma Separation from Whole Blood by Microfluidic Paper-Based Analytical Devices. Anal. Chim. Acta 2023, 1279, 341767. [Google Scholar] [CrossRef]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Henry, C.S.; Laiwattanapaisal, W. Blood Separation on Microfluidic Paper-Based Analytical Devices. Lab. Chip 2012, 12, 3392–3398. [Google Scholar] [CrossRef]
- Yang, X.; Forouzan, O.; Brown, T.P.; Shevkoplyas, S.S. Integrated Separation of Blood Plasma from Whole Blood for Microfluidic Paper-Based Analytical Devices. Lab. Chip 2011, 12, 274–280. [Google Scholar] [CrossRef]
- Al-Tamimi, M.; Altarawneh, S.; Alsallaq, M.; Ayoub, M. Efficient and Simple Paper-Based Assay for Plasma Separation Using Universal Anti-H Agglutinating Antibody. ACS Omega 2022, 7, 40109–40115. [Google Scholar] [CrossRef]
- Rodríguez, A.; Burgos-Flórez, F.; Posada, J.D.; Cervera, E.; Zucolotto, V.; Sanjuán, H.; Sanjuán, M.; Villalba, P.J. Electrochemical Immunosensor for the Quantification of S100B at Clinically Relevant Levels Using a Cysteamine Modified Surface. Sensors 2021, 21, 1929. [Google Scholar] [CrossRef]
- Burgos-Flórez, F.; Rodríguez, A.; Cervera, E.; De Ávila, M.; Sanjuán, M.; Villalba, P.J. Microfluidic Paper-Based Blood Plasma Separation Device as a Potential Tool for Timely Detection of Protein Biomarkers. Micromachines 2022, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Nilghaz, A.; Shen, W. Low-Cost Blood Plasma Separation Method Using Salt Functionalized Paper. 2015. [CrossRef]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral Anticoagulants. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Cai, Z.; Liu, K.; Li, J.; Zhang, B.; He, J. Sensitive Colorimetric Assay for Uric Acid and Glucose Detection Based on Multilayer-Modified Paper with Smartphone as Signal Readout. Anal. Bioanal. Chem. 2018, 410, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
- Skonta, A.; Bellou, M.G.; Matikas, T.E.; Stamatis, H. Colorimetric Glucose Biosensor Based on Chitosan Films and Its Application for Glucose Detection in Beverages Using a Smartphone Application. Biosensors 2024, 14, 299. [Google Scholar] [CrossRef]
- Hema, D.; Kannan, Dr.S. ; Department of Computer Applications, School of Information Technology, Madurai Kamaraj University, Madurai Interactive Color Image Segmentation Using HSV Color Space. Sci. Technol. J. 2019, 7, 37–41. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, H.S.; Chung, E.; Lee, D.Y. Nanozyme-Based Colorimetric Biosensor with a Systemic Quantification Algorithm for Noninvasive Glucose Monitoring. Theranostics 2022, 12, 6308–6338. [Google Scholar] [CrossRef]
- Holbrook, A.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Fish, J.; Kovacs, M.J.; Svensson, P.J.; Veenstra, D.L.; Crowther, M.; Guyatt, G.H. Evidence-Based Management of Anticoagulant Therapy. Chest 2012, 141, e152S–e184S. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-Based Microfluidic Point-of-Care Diagnostic Devices. Lab. Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Boga, L.B.A.; Madrid, R.E. Optimización de Estrategias de Separación de Suero Sanguíneo Para Aplicaciones Con Dispositivos Point Of Care (POC) de Papel. 32° Jorn. Jóvenes Investig. AUGM 2025.


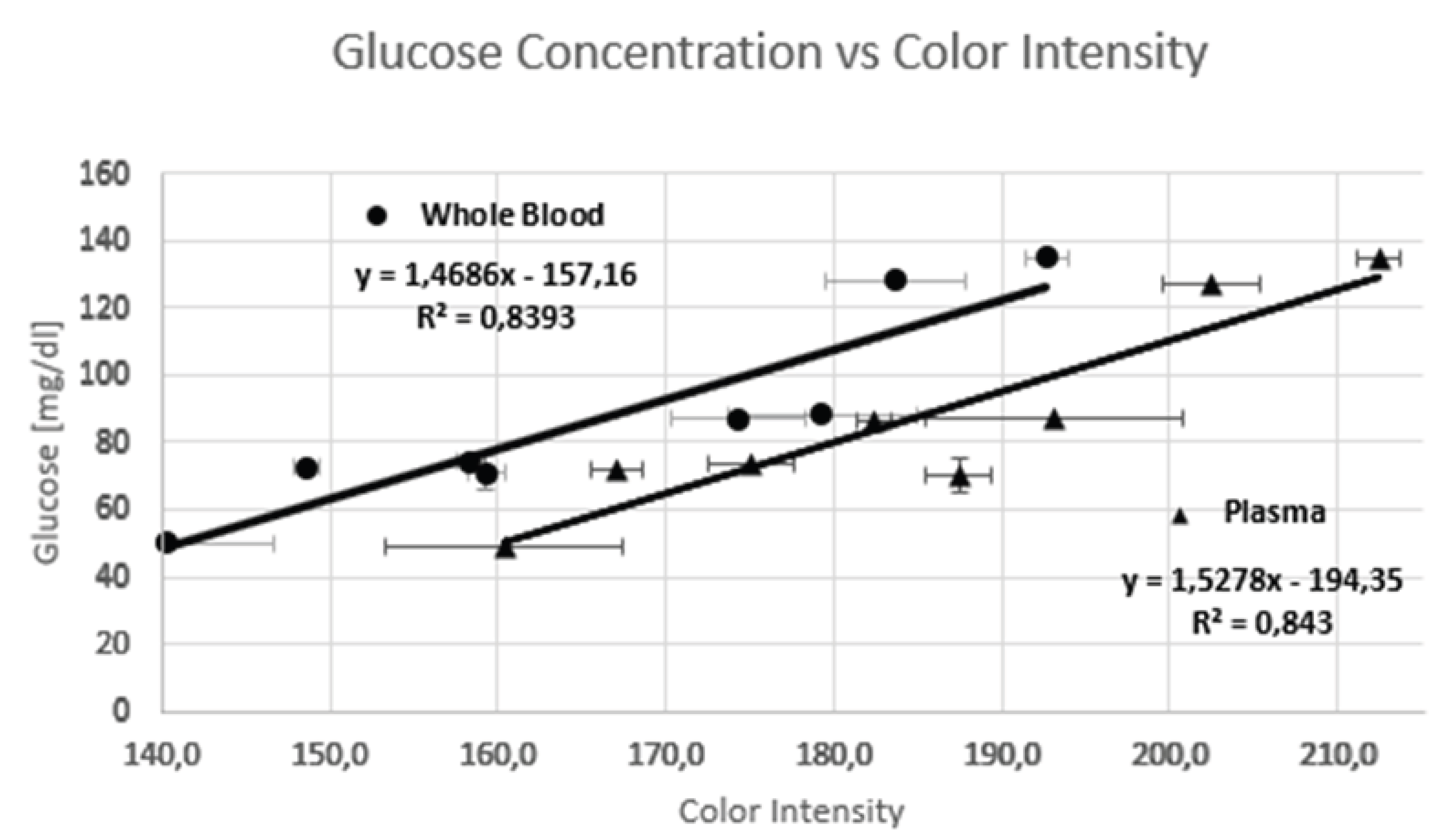
| N° | Glucose [mg/dl] (Commercial kit) | Average intensity of Whole Blood | Standard Dev. Whole Blood | Measured glucose [mg/dl] (Paper Chip) | Average of color intensity of Plasma | Standard Dev. Plasma | Measured glucose [mg/dl] (Paper Chip) |
| 1 | 50 | 140,3 | 12,7 | 48,9 | 160,3 | 14,0 | 50,6 |
| 2 | 71 | 159,3 | 2,1 | 76,8 | 158,0 | 3,8 | 47,0 |
| 3 | 87 | 148,7 | 1,5 | 61,2 | 165,0 | 3,0 | 57,7 |
| 4 | 88 | 158,3 | 1,5 | 75,4 | 187,3 | 5,0 | 91,9 |
| 5 | 128 | 174,3 | 8,1 | 98,9 | 167,0 | 2,1 | 60,8 |
| 6 | 72,48 | 179,3 | 11,2 | 106,2 | 175,0 | 15,4 | 73,0 |
| 7 | 74,31 | 183,7 | 8,4 | 112,6 | 182,3 | 5,9 | 84,2 |
| 8 | 135 | 192,7 | 2,5 | 125,8 | 193,0 | 2,5 | 100,5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





