1. Introduction
Metabolic diseases are increasing in relevance in health and the economic expenditure in most countries. Among them, diabetes has been identified as a prevalent and enduring chronic condition in humans that is also associated with a variety of health complications, including but not limited to heart disease, stroke, kidney disease, nerve damage, and vision impairment. These complications may stem from fluctuating blood glucose levels. Thus, it is imperative to engage people in regular monitoring of blood glucose levels to ensure they remain within the optimal range. This practice is essential for effectively managing diabetes and facilitating the progression toward optimal disease control. It is noteworthy that a correlation exists between diabetes and obesity. Also, early diagnosis is representing a key action requiring an observation period with regular monitoring before taking the most appropriate therapeutic decisions.
Current technologies used for the monitoring of glucose levels can be classified into two distinct categories: invasive (requiring the analysis of blood drops) and non-invasive (by analyzing other biological fluids).
Conventionally, blood analysis in medical facilities entails a multifaceted process involving the manual extraction and examination of blood samples. This method is both time-consuming and producing a certain discomfort for the patient, in addition to the need for the proper management of biological contaminated waste. [
1]
Recent technological advancements have led to the development of methods for the continuous and real-time monitoring of glucose levels in different fluids (sweat, tears, saliva and interstitial fluids) other than blood. This development addresses the requirement of mitigating the aforementioned drawbacks [
2]. Non-invasive technologies are showing to have considerable potential, in particular with sweat. Sweat represents the more interesting and suitable medium for the non-invasive sensing and monitoring of glucose than other fluids, such as saliva, tears, or urine, because it is easy to be collected and available in large body areas (also without relevant privacy issues that impact on compliance and adherence to the clinical procedure). However, the measurement of glucose levels requires the use of highly precise and sensitive sensors, given the low glucose concentration in sweat. Indeed, the normal range of glucose levels in blood is between 3.9 and 6.1 millimoles per liter (mM) [
3], while in sweat, the concentration is considerably lower, ranging from 0.02 to 0.6 mM [
4]. Therefore, it is imperative that sensors exhibit superior sensitivity.
Due to the social and economic relevance regarding the possible exploitation, a patent landscape of the field is important to be considered. A patent landscape, also known as patent mapping or state-of-the-art analysis, is an in-depth analysis of patents within a specific technology domain to systematically review and extract useful insights from patent search results. It assists researchers, investors, and policymakers in comprehending innovation trends and research and development (R&D) opportunities. This paper provides an overview of the patent landscape related to wearable biosensors for the monitoring of glucose levels in sweat.
2. Materials and Methods
Espacenet [
5] has been selected as the most appropriate database for the purposes of this research. The comprehensive coverage and free availability of the database are key factors in this decision. The bibliographic coverage is extensive, encompassing over 150 million documents, while the full text comprises more than 127 million documents.
In order to identify all relevant applications, we have conducted a search on the Espacenet database using the following parameters: the Title Abstract Claims (TAC) and the full-text fields, in addition to some specific Cooperative Patent Classification (CPC) codes (G01N27/327, referred to biochemical electrodes and A61B5/1486 referred to devices for measuring bodily fluids using enzyme electrodes).
The final query, reported in
Appendix A, may be copied and pasted into the Smart search field of Espacenet in order to retrieve the results obtained.
The data retrieved from Espacenet were imported into Orbit Patent Intelligence platform [
6] and subsequently analyzed. Orbit is equipped with a more extensive array of analytical tools for the examination of patent data in comparison to Espacenet.
The inclusion criteria set for patents selection were for time and typology of application:
1. patent applications or granted using sweat as biological fluid dedicate to measurement of glucose level.
2. patent applications or granted filed in a 20-year interval, starting from January 2005 and before December 16, 2024.
3. patents or patent applications pertaining to a wearable device designed for the continuous monitoring of glucose levels in sweat.
3. Results and Discussion
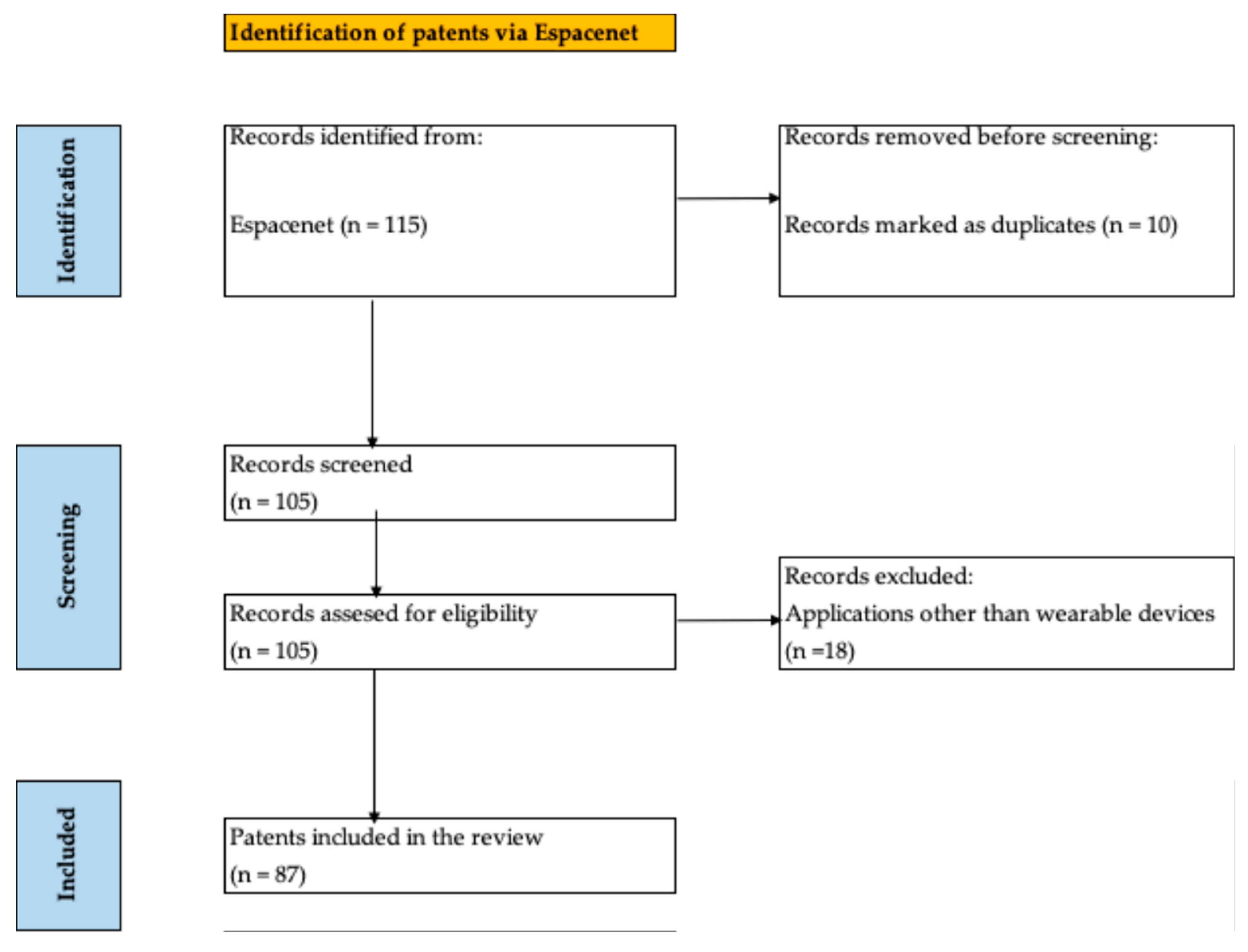
A total of 115 records were identified in Espacenet.
Following the elimination of 10 duplicate patents, a total of 105 patents were selected for title and abstract screening. Following the screening process, a total of 87 records were selected for inclusion in this study (see
Figure 1).
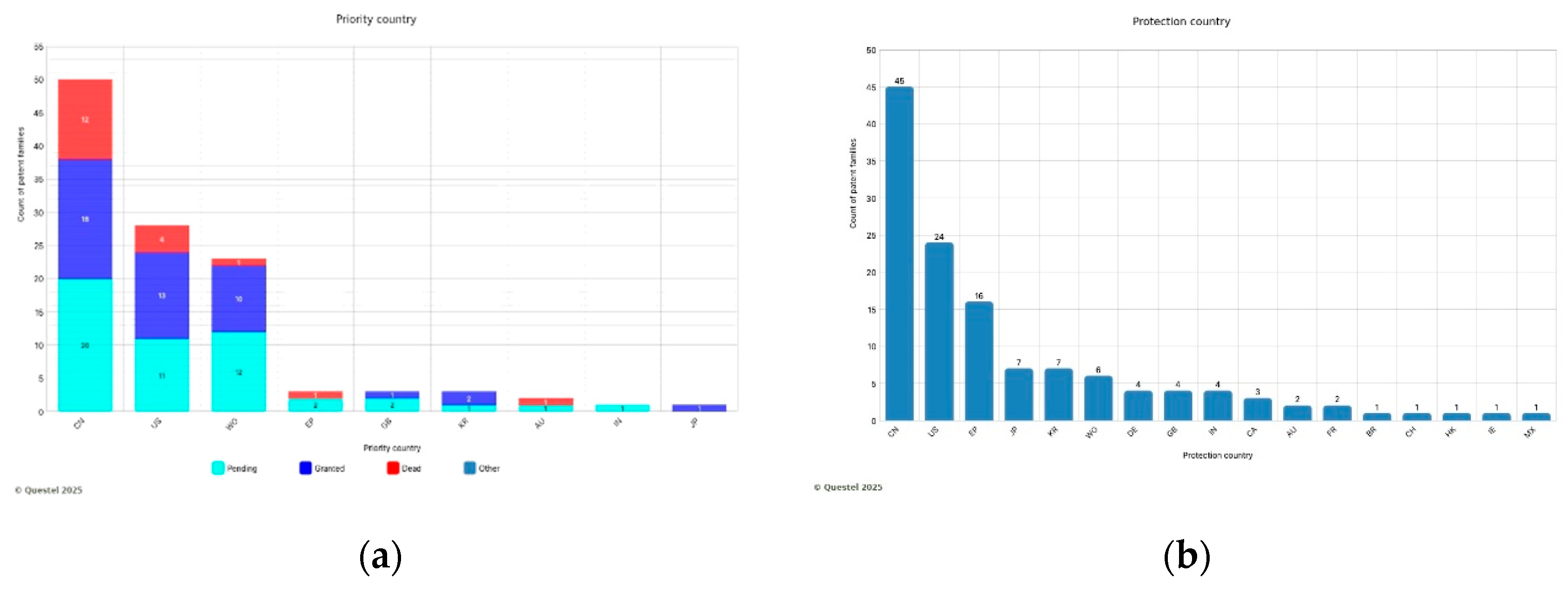
China has the highest number of priority patent applications, with 50, followed by the USA with 28.
In addition, the PCT is a frequently utilized system by assignees to extend priority filings. Indeed, it occupies third place with 26 entries (
Figure 2a).
The top markets are China, the USA, and Europe. In contrast, Japan and South Korea exhibit a lower level of patent protection (
Figure 2b).
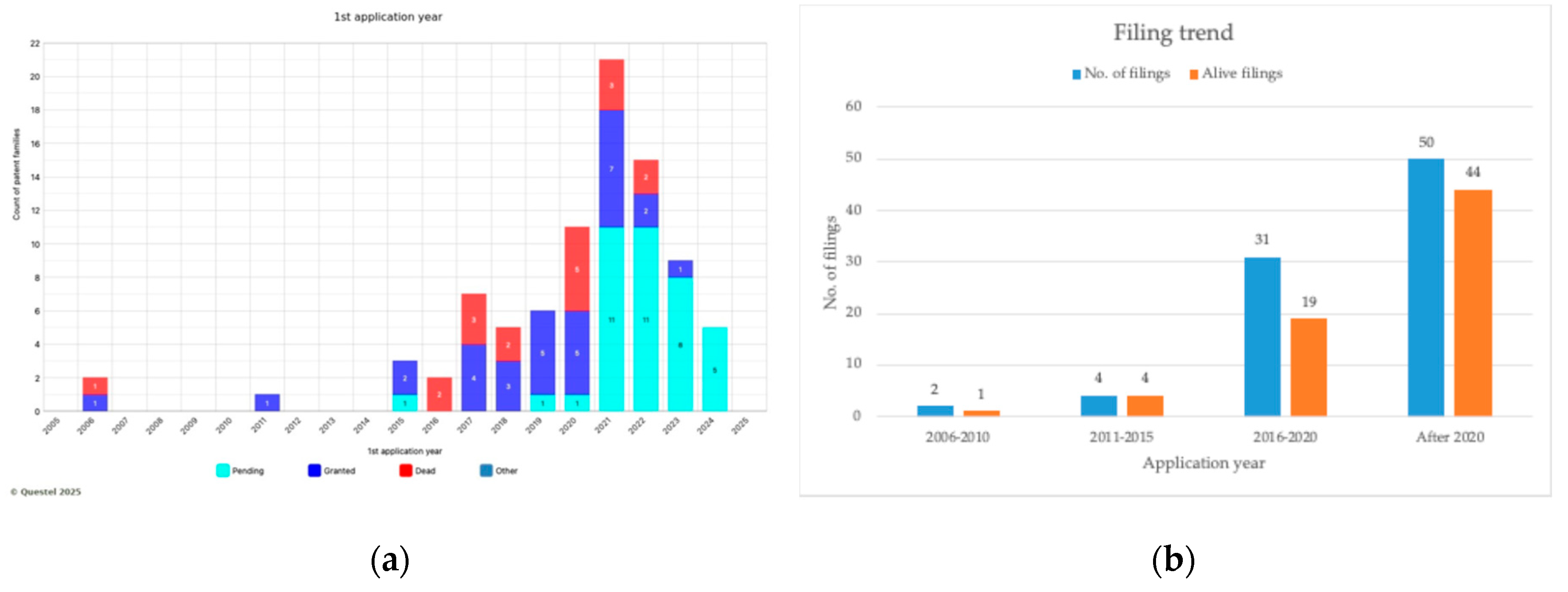
The graphs in
Figure 3a and 3b illustrate the filing trend over the past two decades.
The majority of applications were submitted subsequent to 2020, with a peak in filings observed in 2021. However, this trend underwent a decline in subsequent years.
The patents that were granted in 2021 and 2022 were predominantly Chinese, with two utility granted models among them.
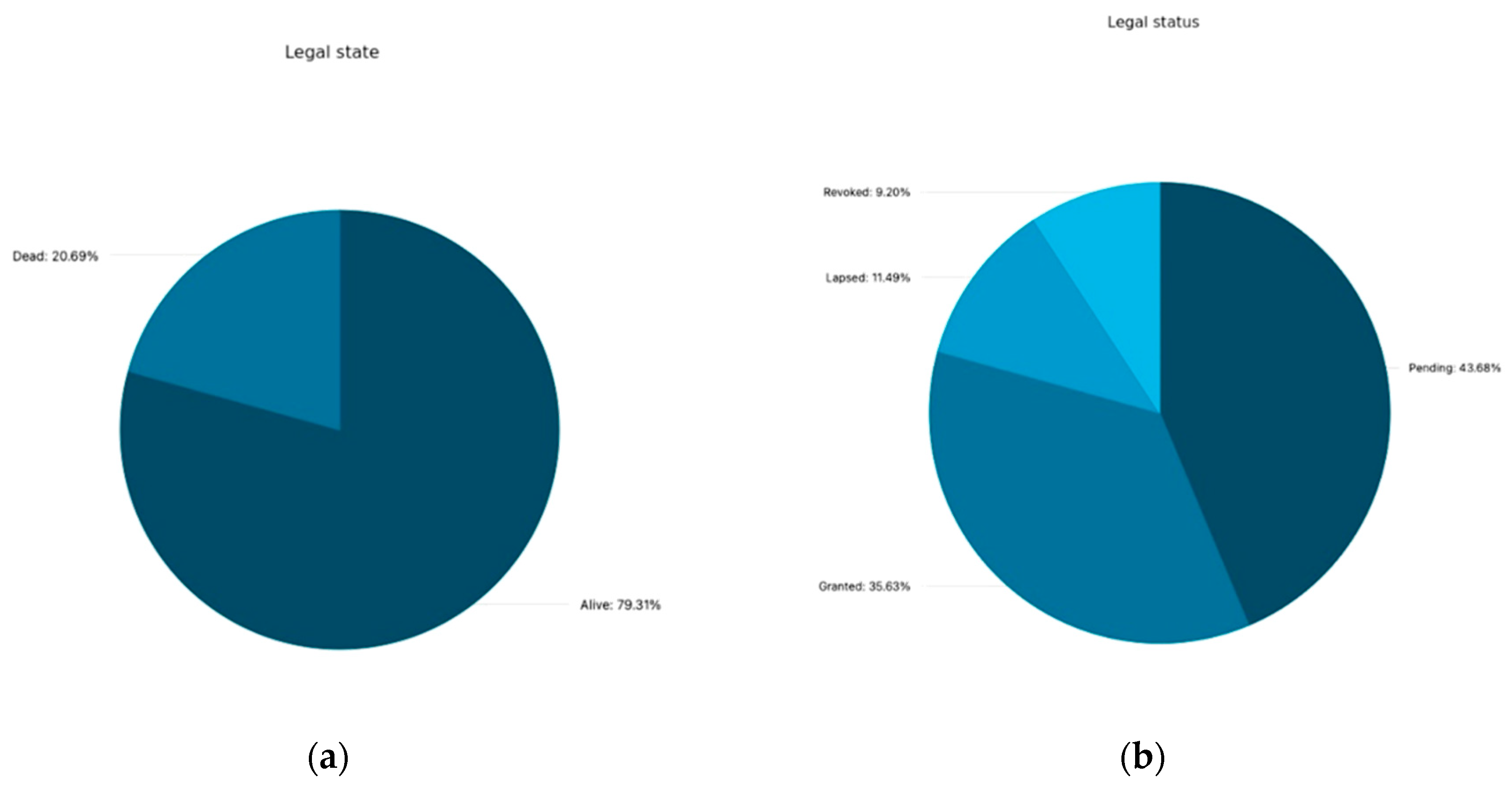
Figure 4a and 4b illustrate the legal status of filings. A substantial proportion of the applications that are retrieved are found to be active.
35.6% of patent applications have been granted, while 43.7% are currently under examination.
11.5% have lapsed due to the failure to pay the annual fees, and 9.2 % have been revoked by the Patent Office for failing to meet the patentability requirements or have been withdrawn by the applicant. Therefore, currently in total only 69 patents are still alive (31 granted, and 38 pending).
As illustrated in
Table 1, the CPC codes for the 87-patent data are documented. The classification system is a tool that provides insight into the evolution of a technical field.
The most frequently utilized patent classification systems are the International Patent Classification (IPC) [
7] and the Cooperative Patent Classification (CPC) [
8].
The IPC is a hierarchical classification system. The highest level comprises eight sections (A–H), which are further divided into 80,000 subdivisions. The updated version is 2025.01.
The CPC represents an enhanced version of the IPC, featuring a greater number of subdivisions, a feature that is constantly augmented as novel technical domains emerge.
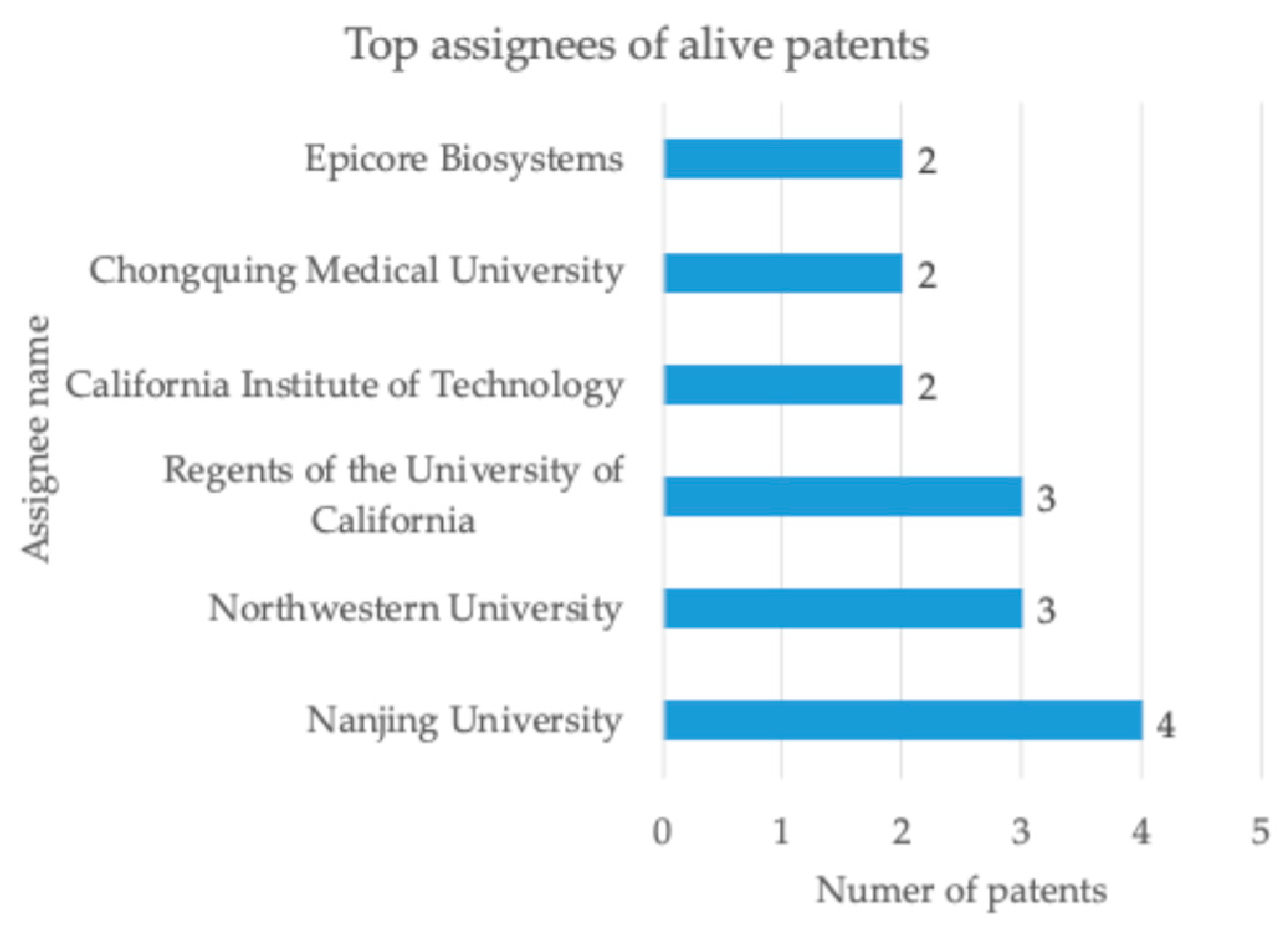
The majority of the leading patent assignees are Chinese or American universities and public research institutions, as illustrated in
Figure 5.
Only 13% of alive patents and patent applications has valuable geographic protection, defined as a patent family comprising a minimum of three members. The preferred validation countries are China, the USA, Europe, and Japan.
Considering the most frequently cited applications combined with those that have a minimum geographic scope of protection, only 19% of active patents seem to have a potential economic value.
3.1. Academic and Industrial Research
From the perspective of academic research or industrial R&D, the main trend in the field is the development of glucose sensors that are enzyme-based and functionalized with carbon or metal nanoparticles.
Non-invasive glucose monitoring techniques can be classified into four categories: optical (photoplethysmography and surface-enhanced Raman scattering), biochemical, biomechanical (triboelectric and piezoelectric sensors), and thermal (thermosensitive sensors and wearable thermoelectric generators). [
9]
Biochemical sensing can be achieved through the implementation of two distinct approaches: the enzymatic approach, exemplified by first-to third generation glucose sensors, and the non-enzymatic approach, typified by fourth-generation glucose sensors.
In the case of an enzyme-based sweat sensor, the glucose oxidase enzyme catalyzes the oxidation of glucose, resulting in the formation of gluconic acid. Glucose can be oxidized by oxygen (1
st type), resulting in the production of hydrogen peroxide, by a redox mediator, such as Prussian blue or ferrocene (2
nd type), or by engineered enzymes (3
rd type). For instance, electrodes treated with carbon nanotubes (CNTs) can be coupled to glucose oxidase (GOx), enabling direct electron transfer from flavin adenine dinucleotide (FAD) to the electrodes. [
10,
11,
12,
13]
In a non-enzymatic sweat sensor (4
th type), glucose reacts directly with nanomaterials, including metals, alloys, and metal oxides. These nanocatalysts function as a replacement for GOx, facilitating the direct oxidation of glucose on the electrode. [
14,
15]
An analysis of patent data reveals a concerted effort in research and development aimed at the development of glucose sensors of the third and fourth types.
Graphene represents the most prevalent carbon material utilized in the electrode, followed by rGO and carbon nanotubes. The employment of MXenes and MOF is comparatively limited.
For example, in CN118830835A [
16] the glucose sensor is made by depositing gold nanoparticles and titanium carbide multilayer nano-sheets on a screen-printed working electrode using cyclic voltammetry. Prussian blue is then deposited using cyclic voltammetry, and glucose oxidase (GOx) is dripped on to form a glucose sensor.
In CN106923842B [
17] the glucose sensor is a graphene flexible patch sensor.
In CN118191059A [
18] a polydimethylsiloxane/carbon nanotube/glucose oxidase sensor is claimed.
A patent of particular interest (No. CN115950942B) [
19] pertains to the methodology of fabricating an organic electrochemical transistor, with the innovative technique being based on laser-induced graphene.
The technical scheme involves the utilization of dodecylbenzene sulfonic acid (DBSA) as a surfactant, which facilitates the effective combination of poly (3, 4-ethylenedioxythiophene) (PEDOT: PSS) with a porous laser induced graphene (LIG) electrode. The sensing capability is achieved through the modification of platinum nano particles (PtNPs) and glucose oxidase (GOx) on the surface of a porous LIG gate.
The flexible, wearable, self-powered sensor system claimed in CN117269261A [
20] comprises an anode and a cathode and is characterized by the following features: At the anode, the LIG nano-enzyme Au NPs with simulated glucose oxidase activity catalyze glucose oxidation in sweat, thereby generating electrons that reach the cathode through an external circuit. At the cathode, the LIG Pt NPs nano-enzyme with laccase-simulating activity obtain electrons and catalyze oxygen reduction, thus forming a loop.
The glucose electrode described in CN112697857A [
21] is constituted by a matrix electrode, and a polyaniline film and a glucose oxidase film which are sequentially coated on the surface of the aforementioned matrix electrode.
In US10722160B2 [
22] the redox mediator of the non- invasive epidermal electrochemical sensor device includes glucose oxidase (GOx) or glucose dehydrogenase (GDH).
The smart wristband described in US20180263539A1 [
23] comprises one or more sensors comprising glucose oxidase (GOx) and/or lactate oxidase (LOx) immobilized within a chitosan film.
In the year 2024, Chongqing Medical University patented a flexible non-enzymatic electrochemical sensor. [
24,
25] The sensor was synthesized using hydrothermal and one-pot preparation methods, incorporating gold nanoparticles (AuNPs) functionalized onto aminated multi-walled carbon nanotubes (AMWCNTs) as an efficient catalyst, and crosslinked with carboxylated styrene butadiene rubber (XSBR) and PEDOT:PSS. Subsequently, the sensors were integrated onto screen-printed electrodes (SPEs) to create flexible glucose sensors (XSBR-PEDOT:PSS-AMWCNTs/AuNPs/SPE).
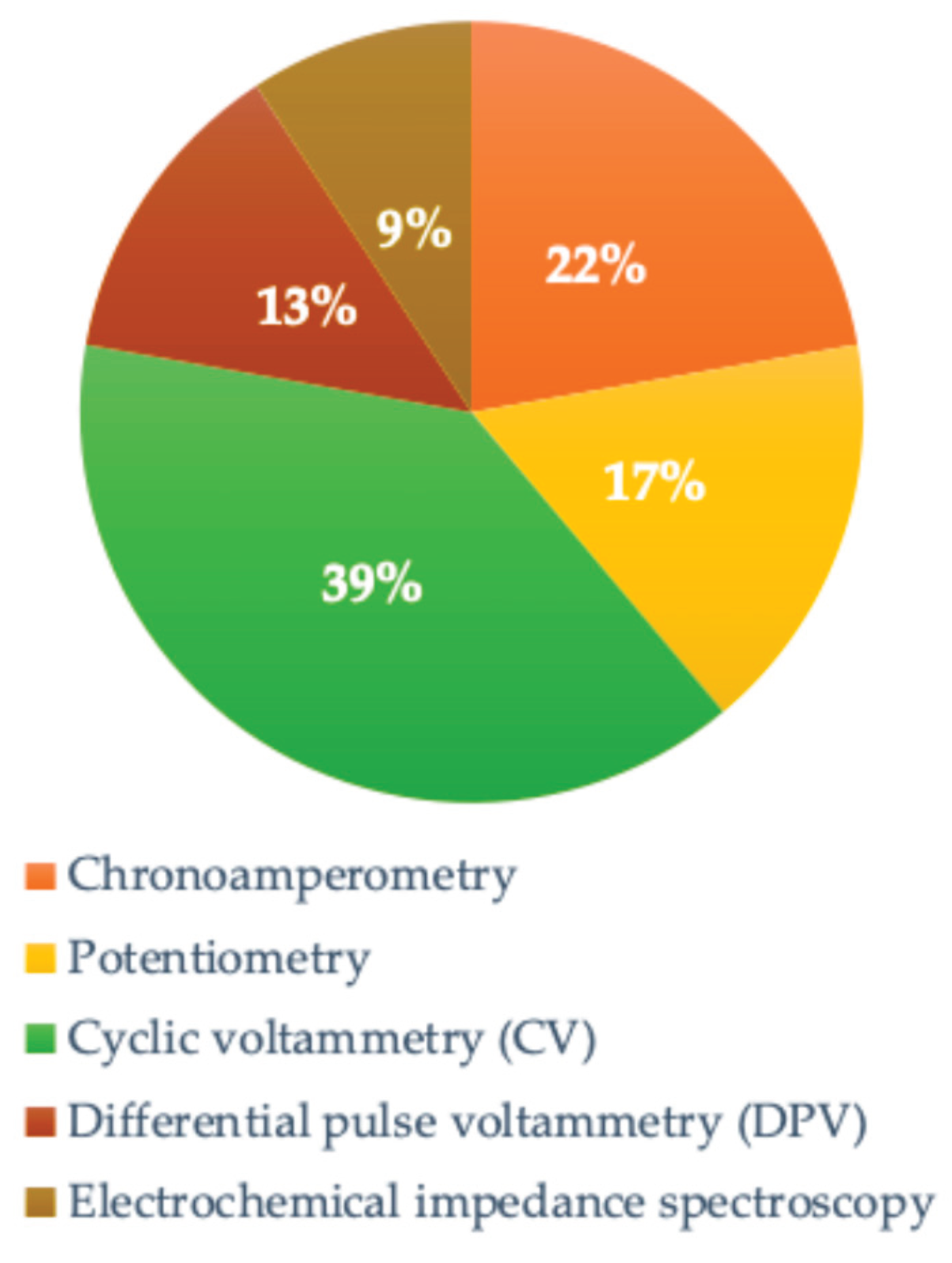
Cyclic voltammetry is the most frequently adopted electrochemical sensing technique, followed by chronoamperometry and potentiometry, as depicted in
Figure 6.
In contrast to biochemical techniques, a variety of other sensing methodologies are employed, including piezoelectric, optical, thermosensitive, and acoustic techniques. However, these alternative methods are not as frequently utilized as the aforementioned biochemical approach.
From the viewpoint of the preferred typology of device, the development of sensors is predominantly focused on their integration within patches.
3.2. Patented Products on the Market
From an investor's perspective, investing in wearable devices for glucose monitoring through sweat is a risky investment. This is due to the fact that the filing trend is in decline and because the products on the market do not measure glucose, but rather other parameters, such as lactate, sodium, and potassium.
The Smart Patch [
26], distributed by Epicore, is a microfluidic wearable device that captures sweat and analyzes fluid and electrolyte losses during exercise.
The OnaVital wearable device [
27], manufactured by Onalabs, is a certified medical equipment that facilitates the non-invasive, continuous and real time monitoring of vital signs, including blood pressure, oxygen saturation, heart rate, and skin temperature.
The technology developed by Limina
TM [
28] enables the measurement of hypoxanthine metabolite levels in an athlete's sweat.
The NIX hydration biosensor [
29], when used in conjunction with the NIX Solo app, has been demonstrated to quantitatively measure fluid and electrolyte losses in real time. The app provides precise information regarding the timing, quantity, and type of fluid intake necessary to maintain optimal hydration levels.
The HDROP wearable sensor [
30] has been developed to track more than just sweat rate and sweat loss. It has also been designed to monitor the loss of potassium and sodium, as well as the body's temperature.
3.3. Patent Strategies
From the perspective of technology transfer, the patent strategy is of paramount importance for the exploitation of the Intellectual Property.
The Technion R&D Foundation has implemented an effective patent strategy, having initiated two US provisional applications in 2020 pertaining to an amperometric enzyme-based biosensor. These provisional applications were subsequently consolidated into a unified PCT extension in 2021 [
31], followed by validation in both the US and EP applications.
The invention pertains to a carbon-based electrode for utilization in a biosensor, wherein the matrix material is polydopamine or agarose. The carbon allotrope is selected from a range of structures, including CNTs, fullerenes, and carbon nanobuds. The redox charge mediator is selected from substituted naphthoquinones.
Glucose dehydrogenase (GDH) is the enzyme used.
An illustration of a suboptimal patent strategy is provided by a patent application filed by Yangzhou University on a flexible glucose electrochemical sensor. [
32] A scholarly article was published on November 23, 2021. [
33]
The earliest priority date of the application in question is March 16, 2022. Consequently, the patent examiner determined that the application lacked novelty, as evidenced by the disclosure. The invention is currently in the public domain, and as such, it is available for use by anyone.
An illustration of inadequate geographic protection scope is provided by the patent issued for an organic electrochemist transistor based on laser-induced graphene, which was filed by Nanjing University of Technology.
The patent was filed in 2022 and granted in China [
34] but not extended abroad. A paper was published on 16 March 2023 [
35]. This is resulting in the fact that the implementation of this technology in a wearable device is a viable commercialization strategy, with the exception of the Chinese market.
This recommendation is directed towards researchers and entrepreneurs, emphasizing the crucial nature of conducting a freedom-to-operate (FTO) search prior to the commercialization of novel technologies.
4. Conclusions
China has the highest number of patent filings, characterized by a narrow scope of geographic protection.
A decline in filings was observed in 2023, following a period of significant growth that peaked in 2021.
A significant proportion of the retrieved patents and patent applications, constituting 80% of the total, are still active and remain subject to consideration for an FTO search.
A review of the most frequently cited applications, in conjunction with those that have a minimum geographic scope of protection, reveals that only approximately 19% of active patents appear to possess a potential economic value.
A review of the current market reveals that only five products have been granted a patent (though none of these products are designed for glucose measurement).
Glucose sensors that are enzyme-based represent the most prevalent type of glucose sensor in current patent filings.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
“Conceptualization, M.B. and G.A.; methodology, M.B.; data curation, M.B.; writing—original draft preparation, M.B.; writing—review and editing, G.A.; supervision, G.A. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FTO |
Freedom To Operate search |
| TAC |
Title Abstract Claims |
| CPC |
Cooperative Patent Classification |
| IPC |
International Patent Classification |
| PCT |
Patent Cooperation Treaty |
| CNTs |
Carbon nanotubes |
| GOx |
Glucose Oxidase |
| FAD |
flavin adenine dinucleotide |
| rGO |
Reduced Graphene Oxide |
| MOF |
Metal Organic Framework |
| GHD |
Glucose dehydrogenase |
| LIG |
Laser induced graphene |
| LOx |
Lactate oxidase |
Appendix A
This appendix provides the final query used for retrieving patent data in Espacenet.
(ctxt all "wearable" AND ctxt all "biosens*" AND ctxt all "glucose" AND ctxt all "sweat") OR (ctxt=("wearable" prox/distance<5 "biosens*") AND ctxt all "glucose" AND ctxt all "sweat") OR (ctxt all "wearable" AND ctxt all "glucose" AND ctxt all "sweat" AND (cl all "G01N27/327" OR cl all "A61B5/1486")) OR (ctxt all "wearable" AND ctxt=("glucose" prox/distance<3 "oxidase") AND ctxt all "sweat") OR (ctxt all "wearable" AND ctxt=("glucose" prox/distance<3 "dehydrogenase") AND ctxt all "sweat") OR ((ctxt all "wearable" AND ctxt all "sweat" AND ctxt all "nanozyme") OR (ctxt all "wearable" AND ctxt all "sweat" AND ctxt all "graphene" AND ctxt all "glucose") OR (ctxt all "wearable" AND ctxt all "sweat" AND (ctxt all "rGO" OR ctxt all "reduced graphene oxide") AND ctxt all "glucose") OR (ctxt all "wearable" AND ctxt all "sweat" AND ctxt all "MXene" AND ctxt all "glucose") OR (ctxt all "wearable" AND ctxt all "sweat" AND ctxt all "glucose" AND cl all "B82Y") OR (ctxt all "wearable" AND ctxt all "sweat" AND ctxt all "glucose" AND ctxt all "nanotube?") OR (ctxt all "wearable" AND ctxt all "sweat" AND ftxt=("carbon" prox/distance<3 "black") AND ctxt all "glucose")) OR (ctxt all "wearable" AND ctxt all "sweat" AND ftxt=("solar" prox/distance<3 "cell") AND ctxt all "glucose") OR (ctxt all "wearable" AND ctxt all "sweat" AND ftxt=("biofuel" prox/distance<3 "cell") AND ctxt all "glucose") OR (ctxt all "wearable" AND ctxt all "sweat" AND ftxt=("non" prox/distance<1 "-enzym*") AND ctxt all "glucose") OR (ctxt all "wearable" AND ctxt all "sweat" AND ftxt all "enzym*" AND ctxt all "glucose") OR ((ctxt all "wearable" AND ctxt all "sweat" AND ftxt all "enzym*" AND ctxt all "glucose" AND (ftxt all "optic*" OR ftxt all "colorim*")) NOT ftxt all "electrochem*")
References
- Ameen, S. Sh. M.; Omer, K.M.; Mansour, F. R.; Bedair, A.; Hamed, M. Non-invasive wearable electrochemical sensors for continuous glucose monitoring. Electrochemistry Comm. 2025, 173, 107894. [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanovic, G.M. Comprehensicve Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [CrossRef]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [CrossRef]
- Espacenet patent search. Available online: https://worldwide.espacenet.com (accessed on 16 December 2024).
- Orbit Patent Intelligence. Available online: https://www.orbit.com (accessed on 16 December 2024).
- WIPO IPC publication. Available online: https://ipcpub.wipo.int (accessed on 01 June 2025).
- CPC Scheme and Definitions. Available online: https://www.cooperativepatentclassification.org/cpcSchemeAndDefinitions/table (accessed 01 June 2025).
- Sunstrum, F.N.; Khan, J.U.; Li, N-W., Welch, A. W. Wearable textile sensors for continuous glucose monitoring. Biosensors and Bioelectronics 2025, 273, 117133. [CrossRef]
- Zhu, B.; Li, X.; Zhou, L.; Su, B. An Overview of Wearable and Implantable Electrochemical Glucose Sensors. Electroanalysis 2022, 34, 237 – 245. [CrossRef]
- Huang, Q; Chen, J. Zhao, Y.; Huang, J.; Liu, H. Advancements in electrochemical glucose sensors. Talanta 2025, 281, 126897. [CrossRef]
- Huang, X.; Yao, C.; Huang, S.; Zheng S.; Liu Z.; Liu, J.; Wang, J.; Chen, H-j; Xie, X. Technological Advances of Wearable Device for Continuous Monitoring of In Vivo Glucose. ACS Sens, 2024, 9, 1065 – 1088. [CrossRef]
- Saha, T.; Del Caño, R.; Mahato, K.; Dela Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854 – 7889. [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv Healthcare Mater. 2018, 7, 1701150. [CrossRef]
- Wang, K.; Liu, W.; Wu, J.; Li, H.; Peng, H.; Zhang, J.; Ding, K.; Wang, X.; Hou, C.; Zhang, H.; Luo, Y. Smart Wearable Sensor Fuels Noninvasive Body Fluid Analysis. ACS Appl. Mater. Interfaces 2025, 17, 13279 – 13301. [CrossRef]
- Zhang, Y; Lin, X; Li, Z.; Zhang, X.; Chen, Y.; Zheng, Z.; Liu, Y.; Xia, Y.; Mou, L. Wearable sweat detection device. Chine Patent Application No. CN118830835A. Available online: https://worldwide.espacenet.com/patent/search/family/093142176/publication/CN118830835A?q=CN118830835A (accessed 01 June 2025).
- Liu, H.; Liu, H. Blood glucose monitoring system. Chinese Patent No. CN106923842B. Available online: https://worldwide.espacenet.com/patent/search/family/059432939/publication/CN106923842B?q=CN106923842B (accessed 2 June 2025).
- Cai, K.; Wang, L. Preparation method of flexible wearable sensor for real-time monitoring of glucose in sweat. Chinese Patent Application No. CN118191059A. Available online: https://worldwide.espacenet.com/patent/search/family/091407985/publication/CN118191059A?q=CN118191059A (accessed 02 June 2025).
- Lyu. G.; Ren, G. Preparation method and application of all-carbon organic electrochemical transistor based on laser-induced graphene. Chine Patent No. CN115950942B. Available online: https://worldwide.espacenet.com/patent/search/family/087285522/publication/CN115950942B?q=CN115950942 (accessed 02 June 2025).
- Gu, C.; Liang, W.; Zhang, L.; Ge, P.; Li, F. Flexible wearable self-powered sensor system for in-situ detection of glucose in sweat and application of flexible wearable self-powered sensor system. Chinese Patent Application No. CN117269261A. Available online: https://worldwide.espacenet.com/patent/search/family/089220640/publication/CN117269261A?q=CN117269261A (accessed 02 June 2025).
- Jiang, X.; Mou, L. Glucose electrode, micro-fluidic chip, micro-fluidic passive sweat patch, and preparation method and application of micro-fluidic passive sweat patch. Chinese Patent Application No. CN112697857A. Available online: https://worldwide.espacenet.com/patent/search/family/075506318/publication/CN112697857A?q=CN112697857A (accessed 02 June 2025).
- Wang, J.; Bandodkar, A.J.; Mercier, P. Non-invasive and wearable chemical sensors and biosensors. US Patent No. US10722160B2. Available online: https://worldwide.espacenet.com/patent/search/family/056092499/publication/US10722160B2?q=US10722160B2 (accessed 02 June 2025).
- Javey, A.; Gao, W.; Davis, R.W.; Emaminejad, S.; Wearable sensor array for in-situ body fluid analysis. US patent Application No. US20180263539A1 (rejected in 2020). Available online: https://worldwide.espacenet.com/patent/search/family/058427319/publication/US2018263539A1?q=US20180263539A1 (accessed 02 June 2025).
- Yi, Y.; Chen, Y.; Zang, G.; Sun, Y; Wen, Z.; Zhang, Y. Compound for continuously detecting glucose content in sweat, sensor and application. Chinese patent Application No. CN118237080A. Available online: https://worldwide.espacenet.com/patent/search/family/091555891/publication/CN118237080A?q=pn%3DCN118237080A (accessed 02 June 2025).
- Chen, Y.; Sun, Y.; Li, Y.; Peng, X.; He, Y.; Hou, Y.; Fan, J; Zang, G., Zhang, Y. A wearable non-enzymatic sensor for continuous monitoring of glucose in human sweat. Talanta 2024, 278, 126499. [CrossRef]
- GX Sweat patch. Available online: https://www.epicorebiosystems.com/our-solutions/gx-sweat-patch (accessed 02 June 2025).
- OnaVital. Available online: https://onalabs.com/en/onavital/ (accessed 02 June 2025).
- Limina Muscular Biosensor. Available online: https://www.liminafitness.com/the-science (accessed 02 June 2025).
- NIX Hydration Biosensor. Available online: https://nixbiosensors.com (accessed 02 June 2025).
- HDROP Hydration Wearable Monitor Sensor. Available online: https://nixbiosensors.com (accessed 02 June 2025).
- Yehezkeli, O.; Cohen, R.; Cohen, Y.; Mukah, D. Oxygen insentive amperometric biosensors. PCT application No. WO2022054044A1. Available online: https://worldwide.espacenet.com/patent/search/family/077774957/publication/WO2022054044A1?q=pn%3DWO2022054044A1 (accessed on 02 June 2025).
- Su, T.; Xia, Y.; Zhang, S., Shu, Y. Preparation method of flexible wearable sweat glucose electrochemical sensor, Chinese Patent Application No. CN114674885A (now rejected). Available online: https://worldwide.espacenet.com/patent/search/family/082074779/publication/CN114674885A?q=pn%3DCN114674885A (accessed 02 June 2025).
- Shu, Y.; Su, T.; Shang, Z; Xu, Q.; Hu, X. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 2021, 93, 48 16222 – 16230. [CrossRef]
- Lyu, G.; Ren, G. Preparation method and application of all-carbon organic electrochemical transistor based on laser-induced graphene. Chinese Patent No. CN115950942B. Available online: https://worldwide.espacenet.com/patent/search/family/087285522/publication/CN115950942B?q=pn%3DCN115950942B (accessed 02 June 2025).
- Ren, G.; fan, H.; Zhang, L.; he, S.; Zhu, C.; Gao, K.; Zhang, Y.; Wang, J.; Kang, X.; Gong, Z.; Li, G.; Lu, G.; Yu, H-D. A laser-induced graphene-based flexible and all-carbon organic electrochemical transistor. J. Mater. Chem. C, 2023,11, 4916-4928. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).