Submitted:
17 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Fluid Milk, and Cultured Dairy Products
Cultured Dairy Products
| Milk type | Mineral constituent | Health benefits |
| Cow milk | 3-4% fat, 3.5% protein, 5% lactose, a good source of calcium, proteins, and nutrients such as vitamin B12 | weight loss, building strong bones and teeth, boosting the immune system, reducing fat. |
| Buffalo milk | twice of cow milk, 2:1 fat to protein ratio in buffalo milk, high calcium content | supports bone health, and antioxidant activity, and improves heart health. |
| Camel milk | similar composition to cow milk, Rich in vitamin C, unsaturated fatty acids, and B vitamins | lower blood sugar and improve insulin sensitivity, immune boosting |
| Sheep milk | higher fat and protein contents than goat and cow milk, higher lactose content than milk from cows, buffaloes, and goats | boosts immunity, regulates blood pressure, and prevents birth defects, and bone mineral density. |
| Goat milk | similar composition to cow milk | built strong bones, anti-inflammatory properties, metabolism booster, and improve nutrient uptake efficiency. |
| Yak milk | 15 and 18 percent solid content, 5.5 to 9 percent fat, and 4 to 5.9 percent protein | The antioxidative effect, Favorable for infants, helps to gain weight, Absorbs Calcium & Vitamin B |
| Equine milk | low in proteins (particularly caseins) and ashes and rich in lactose, low levels of fat and protein | Smooth digestion, beauty treatments, overcoming eczema, good for bone health, maintaining blood pressure, and detoxifying the body. |
| Standardized milk | 4.5% fat | Nutritive value |
| Toned milk | 3.0 % fat | Reduces high blood pressure, and promotes easy digestion. |
| Double Toned milk | 1.5 % fat | Rich in vitamin D, helps in weight loss |
| Skimmed milk | Not more than.0.5 %, Potassium source, | helps in lowering blood pressure |
| Full Cream | 6.0 %, a Good source of calcium | maintain healthy teeth. |
Adulteration of Milk
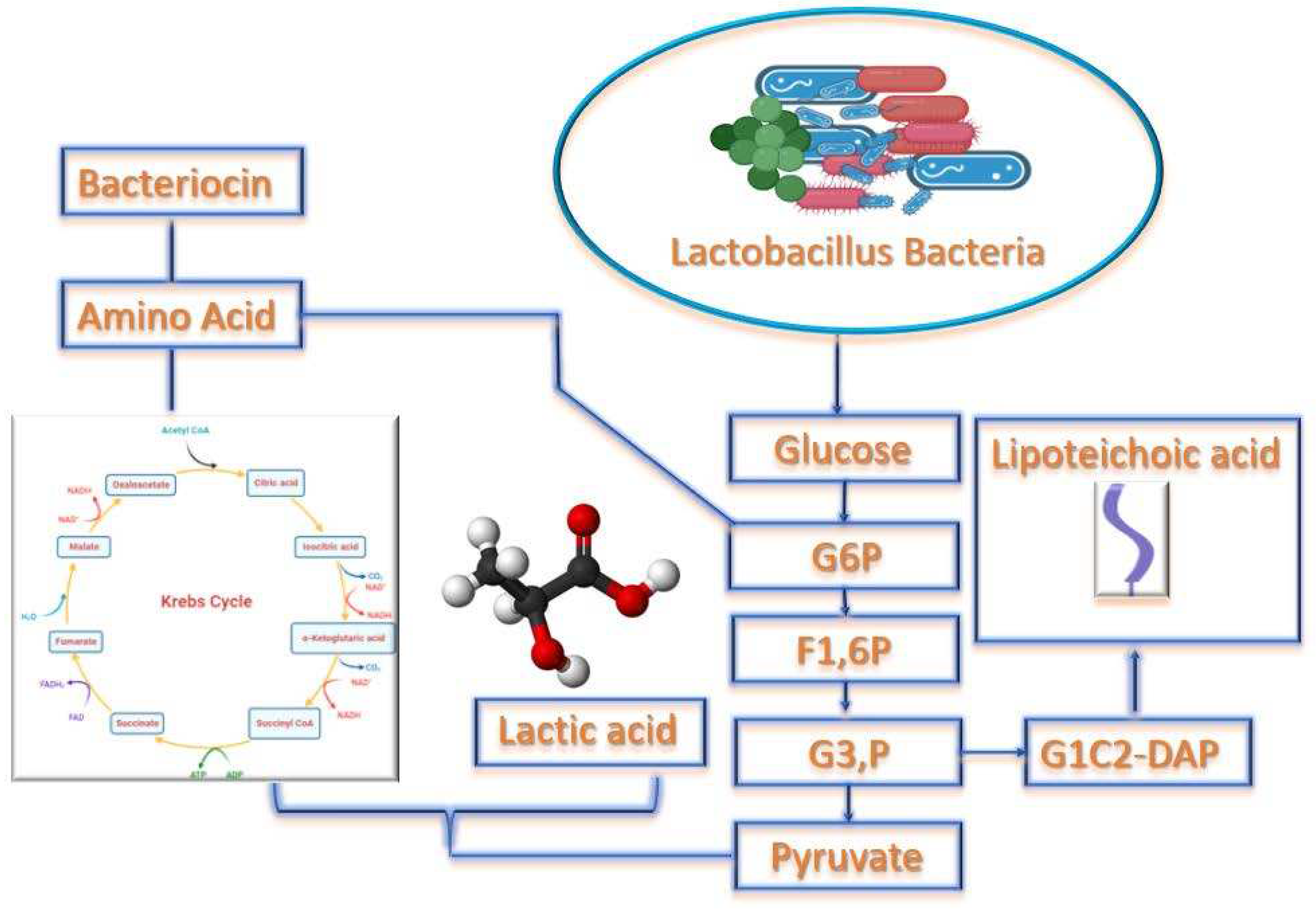
Activity of Lactic Acid Bacteria (LAB)
Proteolytic System and Properties of LAB for Milk Fermentation
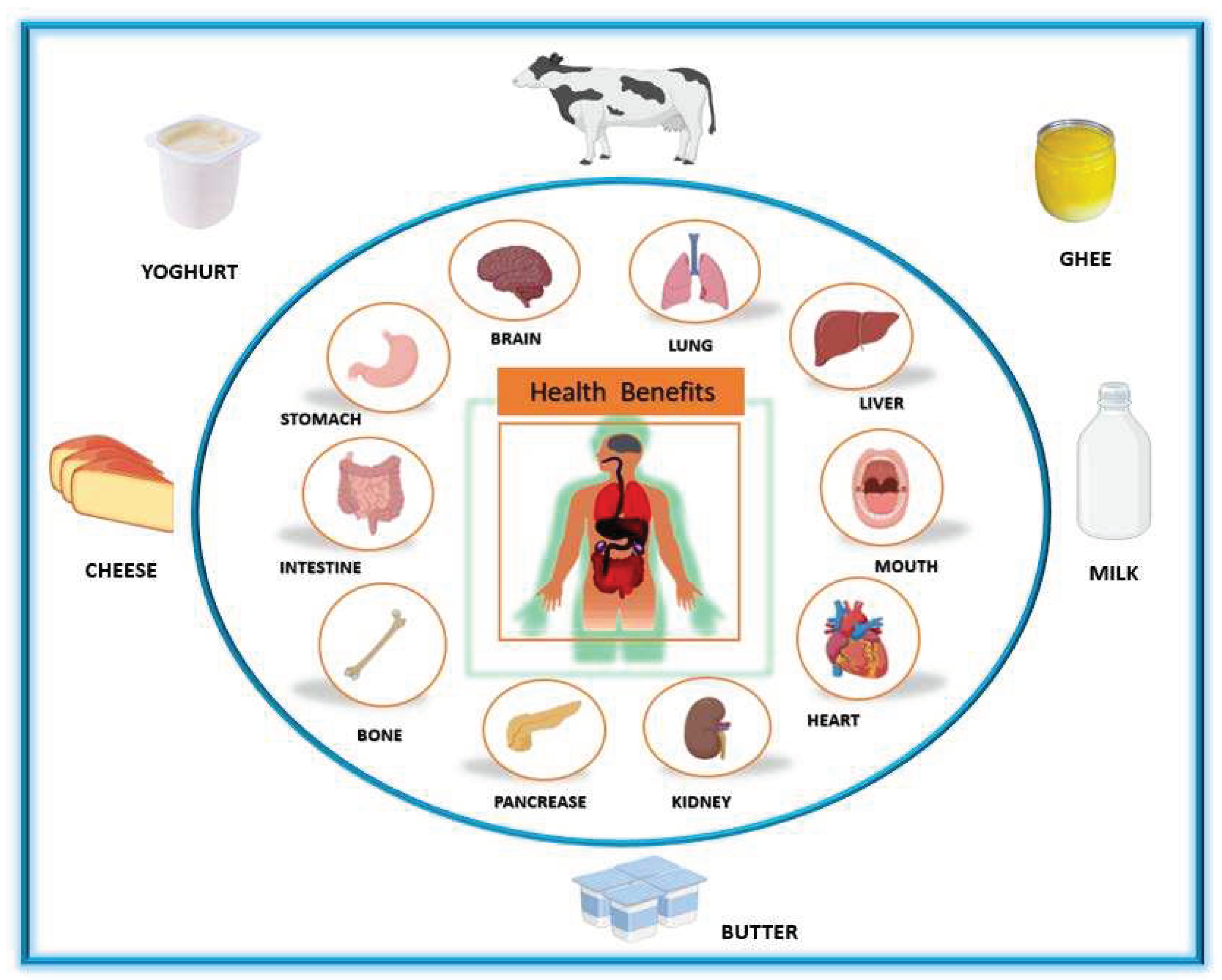
Health Benefits of Dairy Products
Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de los Reyes-Gavilán, C.G.; Fernández, M.; Hudson, J.A.; Korpela, R. Role of microorganisms present in dairy fermented products in health and disease. Biomed Res Int 2015, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wouters, J.T.; Ayad, E.H.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Intl Dairy J 2002, 12, 91–109. [Google Scholar]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial yogurt Manufacture: Monitoring of fermentation process and improvement of Final product quality. J Dairy Sci 2007, 90, 2641–2654. [Google Scholar] [PubMed]
- Button, J.E.; Dutton, R.J. Cheese microbes. Curr Biol 2012, 22, 587–589. [Google Scholar] [CrossRef]
- Visioli, F.; Strata, A. Milk, Dairy Products, and Their Functional Effects in Humans: A Narrative Review of Recent Evidence. Adv. Nutr. Int. Rev. J. 2014, 5, 131–143. [Google Scholar] [CrossRef]
- Kapaj, A.; Deci, E. World Milk Production and Socio-Economic Factors Effecting Its Consumption. In Dairy in human health and disease across the lifespan; Academic Press: Cambridge, MA, USA, 2017; pp. 107–115. [Google Scholar] [CrossRef]
- Wodajo, H.D.; Gemeda, B.A.; Kinati, W.; Mulem, A.A.; van Eerdewijk, A.; Wieland, B. Contribution of small ruminants to food security for Ethiopian smallholder farmers. Small Rumin. Res. 2020, 184, 106064. [Google Scholar] [CrossRef]
- Borawski, P.; Kalinowska, B.; Mickiewicz, B.; Parzonko, A.; Klepacki, B. ; James Changes in the Milk Market in the United States on the Background of the European Union and the World. Eur. Res. Stud. J. 2021, XXIV, 1010–1033. [Google Scholar] [CrossRef]
- Douphrate, David, I. ; Hagevoort, G.R.; Nonnenmann, M.W.; Kolstrup, C.L.; Reynolds, S.J.; Jakob, M.; Kinsel, M. The dairy industry: a brief description of production practices, trends, and farm characteristics around the world. Journal of agromedicine 2013, 18, 187–197. [Google Scholar] [CrossRef]
- Witthuhn, R.C.; Schoeman, T.; Britz, T.J. Characterisation of the microbial population at different stages of Kefir production and Kefir grain mass cultivation. Intl Dairy J 2005, 15, 383–389. [Google Scholar] [CrossRef]
- Story, M.; French, S. Food Advertising and Marketing Directed at Children and Adolescents in the US. Int. J. Behav. Nutr. Phys. Act. 2004, 1, 3–3. [Google Scholar] [CrossRef]
- Dairy processing and quality assurance; Chandan, Ramesh, C., Kilara, A., Shah, N.P., Eds.; John Wiley & Sons, 2009. [Google Scholar]
- Teneva-Angelova, T.; Balabanova, T.; Boyanova, P.; Beshkova, D. Traditional Balkan fermented milk products. Eng. Life Sci. 2018, 18, 807–819. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Logrieco, A.F.; Cho, G.; Kabisch, J.; Böhnlein, C.; Franz, C.M.A.P. Microbial quality and safety of milk and milk products in the 21st century. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2013–2049. [Google Scholar] [CrossRef]
- Wouters, J.T.; Ayad, E.H.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar] [CrossRef]
- Mehta, B.M. Chemical Composition of Milk and Milk Products. In Handbook of food chemistry; Springer: Berlin, Heidelberg, 2015; pp. 511–553. [Google Scholar] [CrossRef]
- Lusk, J.L. External validity of the food values scale. Food Qual. Preference 2011, 22, 452–462. [Google Scholar] [CrossRef]
- Martin, N.H.; Ranieri, M.L.; Murphy, S.C.; Ralyea, R.D.; Wiedmann, M.; Boor, K.J. Results from raw milk microbiological tests do not predict the shelf-life performance of commercially pasteurized fluid milk. J. Dairy Sci. 2011, 94, 1211–1222. [Google Scholar] [CrossRef]
- Serafeimidou, A.; Zlatanos, S.; Laskaridis, K.; Sagredos, A. Chemical characteristics, fatty acid composition and conjugated linoleic acid (CLA) content of traditional Greek yogurts. Food Chem. 2012, 134, 1839–1846. [Google Scholar] [CrossRef]
- Chandan, R.C. Dairy ingredients for food processing: an overview. Dairy ingredients for food processing 2011, 3–33. [Google Scholar]
- Chandan, R.C. Dairy ingredients for food processing: an overview. Dairy ingredients for food processing 2011, 3–33. [Google Scholar]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 184–217. [Google Scholar]
- Wouters, J.T.; Ayad, E.H.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar] [CrossRef]
- Floros, J.D.; Newsome, R.; Fisher, W.; Barbosa-Cánovas, G.V.; Chen, H.; Dunne, C.P.; German, J.B. Feeding the world today and tomorrow: the importance of food science and technology: an IFT scientific review. Comprehensive Reviews in Food Science and Food Safety 2010, 9, 572–599. [Google Scholar] [PubMed]
- Tamime, A. Fermented milks: a historical food with modern applications–a review. Eur. J. Clin. Nutr. 2002, 56, S2–S15. [Google Scholar] [CrossRef]
- Fernández, L.; Escobedo, S.; Gutiérrez, D.; Portilla, S.; Martínez, B.; García, P.; Rodríguez, A. Bacteriophages in the Dairy Environment: From Enemies to Allies. Antibiotics 2017, 6, 27. [Google Scholar] [CrossRef]
- Abdelgadir, W.S.; Ahmed, T.K.; Dirar, H.A. The traditional fermented milk products of the Sudan. International Journal of Food Microbiology 1998, 44, 1–13. [Google Scholar] [CrossRef]
- Shiby, V.K.; Mishra, H.N. Fermented Milks and Milk Products as Functional Foods—A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Shiby, V.K.; Mishra, H.N. Fermented Milks and Milk Products as Functional Foods—A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef]
- Kalsoom, M.; Rehman, F.U.; Shafique, T.A.L.H.A.; Junaid, S.A.N.W.A.L.; Khalid, N.; Adnan, M.; Zafar, I.R.F.A.N.; et al. Biological importance of microbes in agriculture, food and pharmaceutical industry: A review. Innovare Journal Life Sciences 2020, 8, 1–4. [Google Scholar] [CrossRef]
- Robinson, R.K.; Lucey, J.A.; Tamime, A.Y. Manufacture of yoghurt. Fermented milks 2006, 53–75. [Google Scholar]
- Marshall, V.M.; Tamime, A.Y. Starter cultures employed in the manufacture of biofermented milks. Int. J. Dairy Technol. 1997, 50, 35–41. [Google Scholar] [CrossRef]
- Hickisch, A.; Beer, R.; Vogel, R.; Toelstede, S. Influence of lupin-based milk alternative heat treatment and exopolysaccharide-producing lactic acid bacteria on the physical characteristics of lupin-based yogurt alternatives. Food Res. Int. 2016, 84, 180–188. [Google Scholar] [CrossRef]
- Wouters, J.T.; Ayad, E.H.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef]
- Mc Mahon, D.J.; Oberg, C.J.; Mc Manus, W. Functionality of Mozzarella cheese. Aust. J. Dairy Technol. 1993, 48, 99–104. [Google Scholar]
- Santarelli, M.; Bottari, B.; Malacarne, M.; Lazzi, C.; Sforza, S.; Summer, A.; Neviani, E.; Gatti, M. Variability of lactic acid production, chemical and microbiological characteristics in 24-hour Parmigiano Reggiano cheese. Dairy Sci. Technol. 2013, 93, 605–621. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS microbiology reviews 2005, 29, 591–610. [Google Scholar] [PubMed]
- Liu, S. Milk Fermentation with Kefir Grains and Health Benefits. 2021, 152–170. [CrossRef]
- Kabak, B.; Dobson, A.D.W. An Introduction to the Traditional Fermented Foods and Beverages of Turkey. Crit. Rev. Food Sci. Nutr. 2011, 51, 248–260. [Google Scholar] [CrossRef]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Intl Dairy J 2001, 11, 185–201. [Google Scholar]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol Adv 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.-E.; Lyck, S.; Tamime, A.Y. Production of drinking products; 2006; pp. 95–127. [Google Scholar] [CrossRef]
- Köksoy, A.; Kılıç, M. Effects of water and salt level on rheological properties of ayran, a Turkish yoghurt drink. International Dairy Journal 2003, 13, 835–839. [Google Scholar] [CrossRef]
- Makwana, M.; Hati, S. Fermented Beverages and Their Health Benefits. In Fermented beverages; Woodhead Publishing, 2019; pp. 1–29. [Google Scholar] [CrossRef]
- Nilsson, L.-E.; Lyck, S.; Tamime, A.Y. Production of Drinking Products; 2006; pp. 95–127. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Tiwari, S.; Kumar, A.; Raman, R.K.; Kadyan, S. Review on factors affecting and control of post-acidification in yoghurt and related products. Trends Food Sci. Technol. 2021, 109, 499–512. [Google Scholar] [CrossRef]
- Pădureţ, S. The Effect of Fat Content and Fatty Acids Composition on Color and Textural Properties of Butter. Molecules 2021, 26, 4565. [Google Scholar] [CrossRef]
- Budhkar, Y.A.; Bankar, S.B.; Singhal, R.S. Microbiology of cream and butter. Encyclopedia of Food Microbiology 2014, 2, 728–737. [Google Scholar]
- Sharma, A.; Noda, M.; Sugiyama, M.; Ahmad, A.; Kaur, B. Production of functional buttermilk and soymilk using Pediococcus acidilactici BD16 (alaD+). Molecules 2021, 26, 4671. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Beede, D.K.; Thatcher, W.W.; Israel, L.A.; Wilcox, C.J. Influences of Environment and Its Modification on Dairy Animal Health and Production. J. Dairy Sci. 1982, 65, 2213–2227. [Google Scholar] [CrossRef]
- Sruamsiri, S. Agricultural wastes as dairy feed in Chiang Mai. Anim. Sci. J. 2007, 78, 335–341. [Google Scholar] [CrossRef]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. European journal of lipid science and technology 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Ozdemir, U.; Kilic, M. INFLUENCE OF FERMENTATION CONDITIONS ON RHEOLOGICAL PROPERTIES AND SERUM SEPARATION OF AYRAN. J. Texture Stud. 2004, 35, 415–428. [Google Scholar] [CrossRef]
- Sserunjogi, M.L.; Abrahamsen, R.K.; Narvhus, J. A Review Paper: Current Knowledge of Ghee and Related Products. Int. Dairy J. 1998, 8, 677–688. [Google Scholar] [CrossRef]
- Mehta, B.M. Butter, butter oil, and ghee. In Gourmet and health-promoting specialty oils; AOCS Press, 2009; pp. 527–559. [Google Scholar]
- Wani, A.D.; Prasad, W.; Khamrui, K.; Jamb, S. A review on quality attributes and utilization of ghee residue, an under-utilized dairy by-product. Future Foods 2022, 100131. [Google Scholar]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed. Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Kwak, H.S.; Ganesan, P.; Mijan, A.M. Butter, ghee, and cream products. Milk and Dairy Products in Human Nutrition: Production, Composition and Health 2013, 390–411. [Google Scholar]
- Sindhuja, S.; Prakruthi, M.; Manasa, R.; Shivananjappa, M. Health benefits of ghee (clarified butter) - A review from ayurvedic perspective. IP J. Nutr. Metab. Heal. Sci. 2020, 3, 64–72. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Bosarge, A. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am. J. Clin. Nutr. 2008, 87, 621–626. [Google Scholar] [CrossRef]
- Nosaka, N.; Suzuki, Y.; Nagatoishi, A.; Kasai, M.; Wu, J.; Taguchi, M. Effect of Ingestion of Medium-Chain Triacylglycerols on Moderate- and High-Intensity Exercise in Recreational Athletes. J. Nutr. Sci. Vitaminol. 2009, 55, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Upadhyay, N.; Padghan, P.V.; Gandhi, K.; Lal, D.; Sharma, V. Detection of vegetable oil and animal depot fat adulteration in anhydrous milk fat (Ghee) using fatty acid composition. MOJ Food Processing & Technology 2015, 1, 00013. [Google Scholar]
- Bugaut, M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1987, 86, 439–472. [Google Scholar] [CrossRef]
- Ongol, M.P.; Asano, K. Main microorganisms involved in the fermentation of Ugandan ghee. Int. J. Food Microbiol. 2009, 133, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ongol, M.P.; Asano, K. Main microorganisms involved in the fermentation of Ugandan ghee. Int. J. Food Microbiol. 2009, 133, 286–291. [Google Scholar] [CrossRef]
- Afzal, A.; Mahmood, M.; Hussain, I.; Akhtar, M. Adulteration and Microbiological Quality of Milk (A Review). Pak. J. Nutr. 2011, 10, 1195–1202. [Google Scholar] [CrossRef]
- Hattersley, J.G. The Negative Health Effects of Chlorine. The Journal of Orthomolecular Medicine 2000, 15, 2nd. [Google Scholar]
- Afzal, A.; Mahmood, M.; Hussain, I.; Akhtar, M. Adulteration and Microbiological Quality of Milk (A Review). Pak. J. Nutr. 2011, 10, 1195–1202. [Google Scholar] [CrossRef]
- E. Watt, B.; Proudfoot, A.T.; Vale, J.A. Hydrogen Peroxide Poisoning. Toxicol. Rev. 2004, 23, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, Y.; Wu, J.; Yang, X.; Bai, H.; Zheng, H.; Ren, D.; Zou, Y.; Li, M. Screening melamine adulterant in milk powder with laser Raman spectrometry. J. Food Compos. Anal. 2010, 23, 199–202. [Google Scholar] [CrossRef]
- Guan, R.-F.; Liu, D.-H.; Ye, X.-Q.; Yang, K. Use of fluorometry for determination of skim milk powder adulteration in fresh milk. J. Zhejiang Univ. B 2005, 6, 1101–1106. [Google Scholar] [CrossRef]
- Haasnoot, W.; Marchesini, G.R.; Koopal, K. Spreeta-Based Biosensor Immunoassays to Detect Fraudulent Adulteration in Milk and Milk Powder. J. AOAC Int. 2006, 89, 849–855. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Synthetic milk: a threat to Indian dairy industry. Carpathian J Food Sci Technol 2013, 5, 64–8. [Google Scholar]
- Jha, S.N.; Matsuoka, T. Detection of adulterants in milk using near infrared spectroscopy. Journal of Food Science and Technology 2004, 41, 313–316. [Google Scholar]
- Sukumaran, M.K.; Singuluri, H. Milk Adulteration in Hyderabad, India–A comparative study on the levels of different adulterants present in milk. Indian Journal of Dairy Science 2014, 68. [Google Scholar]
- Haasnoot, W.; Smits, N.G.; Kemmers-Voncken, A.E.; Bremer, M.G. Fast biosensor immunoassays for the detection of cows' milk in the milk of ewes and goats. J. Dairy Res. 2004, 71, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Pappas, C.; Tarantilis, P.; Moschopoulou, E.; Moatsou, G.; Kandarakis, I.; Polissiou, M. Identification and differentiation of goat and sheep milk based on diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) using cluster analysis. Food Chem. 2008, 106, 1271–1277. [Google Scholar] [CrossRef]
- Das, S.; Goswami, B.; Biswas, K. Milk Adulteration and Detection: A Review. Sens. Lett. 2016, 14, 4–18. [Google Scholar] [CrossRef]
- Sukumaran, M.K.; Singuluri, H. Milk Adulteration in Hyderabad, India–A comparative study on the levels of different adulterants present in milk. Indian Journal of Dairy Science 2014, 68. [Google Scholar]
- Qi, X.; Tester, R.F. Fructose, galactose and glucose–In health and disease. Clinical nutrition ESPEN 2019, 33, 18–28. [Google Scholar] [CrossRef]
- Stich, E. Food color and coloring food: quality, differentiation and regulatory requirements in the European Union and the United States. In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing, 2016; pp. 3–27. [Google Scholar]
- Nyakayiru, J.; van Lieshout, G.A.A.; Trommelen, J.; van Kranenburg, J.; Verdijk, L.B.; Bragt, M.C.E.; van Loon, L.J.C. The glycation level of milk protein strongly modulates post-prandial lysine availability in humans. Br. J. Nutr. 2019, 123, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gandhi, N. Milk preservatives and adulterants: processing, regulatory and safety issues. " Food Reviews International 2015, 31, 236–261. [Google Scholar] [CrossRef]
- Recio, I.; Garcı́a-Risco, M.R.; López-Fandiño, R.; Olano, A.; Ramos, M. Detection of rennet whey solids in UHT milk by capillary electrophoresis. International Dairy Journal 2000, 10, 333–338. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Borriello, S.P.; Pokludová, L. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef]
- Das, S.; Goswami, B.; Biswas, K. Milk Adulteration and Detection: A Review. Sens. Lett. 2016, 14, 4–18. [Google Scholar] [CrossRef]
- Singh, P.; Gandhi, N. Milk preservatives and adulterants: processing, regulatory and safety issues. Food Reviews International 2015, 31, 236–261. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, Pesticide, and Microbial Contaminants of Honey: Human Health Hazards. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Sharma RO HI, T.; Sanodiya, B.S.; Bagrodia DE EP IK, A.; Pandey MU KE SH WA, R.; Sharma AN JA, N.A.; Bisen, P.S. Efficacy and potential of lactic acid bacteria modulating human health. International Journal of Pharma and Bio Sciences 2012, 3, 935–948. [Google Scholar]
- Steele, J.; Broadbent, J.; Kok, J. Perspectives on the contribution of lactic acid bacteria to cheese flavor development. Curr. Opin. Biotechnol. 2013, 24, 135–141. [Google Scholar] [CrossRef]
- Liu, S.-N.; Han, Y.; Zhou, Z.-J. Lactic acid bacteria in traditional fermented Chinese foods. Food Res. Int. 2011, 44, 643–651. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Febrisiantosa, A. The Role of Lactic Acid Bacteria in Milk Fermentation. Food Nutr. Sci. 2014, 05, 435–442. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: the proteolytic system. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Kongo, *!!! REPLACE !!!*; Marcelino, J. Kongo; Marcelino, J. Lactic acid bacteria as starter-cultures for cheese processing: past, present and future developments. Lactic acid bacteria-R & D for food, health and livestock purposes 2013, 1–22. [Google Scholar]
- Kok, D.J.; De Vos, W.M. The proteolytic system of lactic acid bacteria. Genetics and biotechnology of lactic acid bacteria 1994, 169–210. [Google Scholar]
- Sheeladevi, A.; Ramanathan, N. Lactic acid production using lactic acid bacteria under optimized conditions. Int. J. Pharm. Biol. Arch 2011, 2. [Google Scholar]
- Sandine, W.E.; Elliker, P.R. Microbially induced flavors and fermented foods. Flavor in fermented dairy products. Journal of Agricultural and Food Chemistry 1970, 18, 557–562. [Google Scholar] [CrossRef]
- Law, B.A.; Sezgin, E.; Sharpe, M.E. Amino acid nutrition of some commercial cheese starters in relation to their growth in peptone-supplemented whey media. J. Dairy Res. 1976, 43, 291–300. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Hugenholtz, J.; Kleerebezem, M. Metabolic engineering of lactic acid bacteria: overview of the approaches and results of pathway rerouting involved in food fermentations. Curr. Opin. Biotechnol. 1999, 10, 492–497. [Google Scholar] [CrossRef]
- Harutoshi, T. Exopolysaccharides of Lactic Acid Bacteria for Food and Colon Health Applications:. In Lactic acid bacteria-R & D for food, health and livestock purposes; IntechOpen, 2013. [Google Scholar] [CrossRef]
- Thomas, T.D.; Pritchard, G.G. (). Proteolytic enzymes of dairy starter cultures. FEMS Microbiol. Rev. 1987, 46, 245–268. [Google Scholar] [CrossRef]
- Kok, J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiology Reviews 1990, 7, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Law, B.A.; Kolstad, J. Proteolytic systems in lactic acid bacteria. Antonie van Leeuwenhoek 1983, 49, 225–245. [Google Scholar] [CrossRef]
- Crow, V.; Coolbear, T.; Gopal, P.; Martley, F.; McKay, L.; Riepe, H. The role of autolysis of lactic acid bacteria in the ripening of cheese. Int. Dairy J. 1995, 5, 855–875. [Google Scholar] [CrossRef]
- Law, B.A.; Kolstad, J. Proteolytic systems in lactic acid bacteria. Antonie van Leeuwenhoek 1983, 49, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.D.; Pritchard, G.G. Proteolytic enzymes of dairy starter cultures. " FEMS Microbiology Reviews 1987, 3, 245–268. [Google Scholar] [CrossRef]
- Dhasmana, S.; Das, S.; Shrivastava, S. Potential nutraceuticals from the casein fraction of goat’s milk. J. Food Biochem. 2021, 46, e13982. [Google Scholar] [CrossRef]
- Coolbear, T.; Reid, J.R.; Pritchard, G.G. Stability and specificity of the cell wall-associated proteinase from Lactococcus lactis subsp. cremoris H2 released by treatment with lysozyme in the presence of calcium ions. Applied and Environmental Microbiology 1992, 58, 3263–3270. [Google Scholar] [CrossRef]
- Law, B.A. Peptide Utilization by Group N Streptococci. J. Gen. Microbiol. 1978, 105, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, A.; Wincker, P.; Mauger, S.; Jaillon, O.; Malarme, K.; Weissenbach, J.; Ehrlich, S.D.; Sorokin, A. The Complete Genome Sequence of the Lactic Acid Bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001, 11, 731–753. [Google Scholar] [CrossRef]
- Rice, G.H.; Stewart, F.H.C.; Hillier, A.J.; Jago, G.R. The uptake of amino acids and peptides byStreptococcus lactis. J. Dairy Res. 1978, 45, 93–107. [Google Scholar] [CrossRef]
- Smith; Selby, J. ; Hillier, A.J.; Lees, G.J.; Jago, G.R.. The nature of the stimulation of the growth of Streptococcus lactis by yeast extract. Journal of Dairy Research 1975, 42, 123–138. [Google Scholar] [CrossRef]
- Monnet, V.; Bockelmann, W.; Gripon, J.C.; Teuber, M. Comparison of cell wall proteinases from Lactococcus lactis subsp. cremoris AC1 and Lactococcus lactis subsp. lactis NCDO 763." Applied microbiology and biotechnology 1989, 31, 112–118. [Google Scholar] [CrossRef]
- Monnet, V.; Le Bars, D.; Gripon, J.-C. Specificity of a cell wall proteinase from Streptococcus lactis NCDO763 towards bovine β-casein. FEMS microbiology letters 1986, 36, 127–131. [Google Scholar] [CrossRef]
- Monnet, V.; Bockelmann, W.; Gripon, J.C.; Teuber, M. Comparison of cell wall proteinases from Lactococcus lactis subsp. cremoris AC1 and Lactococcus lactis subsp. lactis NCDO 763." Applied microbiology and biotechnology 1989, 31, 112–118. [Google Scholar] [CrossRef]
- Reid, J.R.; Ng, K.H.; Moore, C.H.; Coolbear, T.; Pritchard, G.G. Comparison of bovine β-casein hydrolysis by PI and PIII-type proteinases from Lactobacillus lactis subsp. cremoris. Applied Microbiology and Biotechnology 1991, 36, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.; Exterkate, F.A.; Slangen, C.J.; de Veer, G.J.C. Comparative study of action of cell wall proteinases from various strains of Streptococcus cremoris on bovine αs1-, β-, and κ-casein. Applied and Environmental Microbiology 1986, 52, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.; Robben, A.J.P.; Slangen, C.J. Specificity of a cell-envelope-located proteinase (PIII-type) from Lactococcus lactis subsp. cremoris AM1 in its action on bovine β-casein. Applied microbiology and biotechnology 1991, 35, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Neviani, E.C.Y.B.; Boquien, C.-Y.; Monnet, V.; Thanh, L.P.; Gripon, J.-C. Purification and characterization of an aminopeptidase from Lactococcus lactis subsp. cremoris AM2. Applied and Environmental Microbiology 1989, 55, 2308–2314. [Google Scholar] [CrossRef]
- Wohlrab, Y.; Bockelmann, W. Purification and characterization of a second aminopeptidase (PepC-like) from Lactobacillus delbrueckii subsp. bulgaricus B14. International Dairy Journal 1993, 3, 685–701. [Google Scholar] [CrossRef]
- Exterkate, F.A.; de Veer, G.J.C.M. Purification and Some Properties of a Membrane-Bound Aminopeptidase A from Streptococcus cremoris. Appl. Environ. Microbiol. 1987, 53, 577–583. [Google Scholar] [CrossRef]
- Hwang, I.K. .; Kaminogawa, S.; Yamauchi, K. Purification and properties of a dipeptidase from Streptococcus cremoris. Agricultural and Biological Chemistry 1981, 45, 159–165. [Google Scholar]
- van Boven, A.; Tan, P.S.T.; Konings, W.N. Purification and Characterization of a Dipeptidase from Streptococcus cremoris Wg2. Appl. Environ. Microbiol. 1988, 54, 43–49. [Google Scholar] [CrossRef]
- Wohlrab, Y.; Bockelmann, W. Purification and characterization of a dipeptidase from Lactobacillus delbrueckii subsp. bulgaricus. Int. Dairy J. 1992, 2, 345–361. [Google Scholar] [CrossRef]
- Bacon, C.L.; Wilkinson, M.; Jennings, P.V.; Ni Fhaolain, I.; O'Cuinn, G. Purification and characterisation of an aminotripeptidase from cytoplasm of Lactococcus lactis subsp. cremoris AM2. Int. Dairy J. 1993, 3, 163–177. [Google Scholar] [CrossRef]
- Bosman, B.W.; Tan, P.S.T.; Konings, W.N. Purification and characterization of a tripeptidase from Lactococcus lactis subsp. cremoris Wg2. Applied and environmental microbiology 1990, 56, 1839–1843. [Google Scholar] [CrossRef]
- Atlan, D.; Laloi, P.; Portalier, R. X-prolyl-dipeptidyl aminopeptidase of Lactobacillus delbrueckii subsp. bulgaricus: characterization of the enzyme and isolation of deficient mutants. Applied and Environmental Microbiology 1990, 56, 2174–2179. [Google Scholar]
- Bockelmann, W.; Fobker, M.; Teuber, M. Purification and characterization of the X-prolyl-dipeptidyl-aminopeptidase from Lactobacillus delbrueckii subsp. bulgaricus and Lactobacillus acidophilus. International Dairy Journal 1991, 1, 51–66. [Google Scholar]
- Booth, M.; Ni Fhaoláin, I.; Jennings, P.V.; O'Cuinn, G. Purification and characterization of a post-proline dipeptidyl aminopeptidase fromStreptococcus cremorisAM2. J. Dairy Res. 1990, 57, 89–99. [Google Scholar] [CrossRef]
- El Abboudi, M.; El Soda, M.; Pandian, S.; Simard, R.; Olson, N. Purification of X-prolyl dipeptidyl aminopeptidase from Lactobacillus casei subspecies. Int. J. Food Microbiol. 1992, 15, 87–98. [Google Scholar] [CrossRef]
- Khalid, N.M.; Marth, E.H. Purification and Partial Characterization of a Prolyl-Dipeptidyl Aminopeptidase from Lactobacillus helveticus CNRZ 32. Appl. Environ. Microbiol. 1990, 56, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kiefer-Partsch, B.; Bockelmann, W.; Geis, A.; Teuber, M. Purification of an X-prolyl-dipeptidyl aminopeptidase from the cell wall proteolytic system of Lactococcus lactis subsp. cremoris. Appl. Microbiol. Biotechnol. 1989, 31. [Google Scholar] [CrossRef]
- Lloyd, R.J.; Pritchard, G.G. Characterization of X-prolyl dipeptidyl aminopeptidase from Lactococcus lactis subsp. lactis. Microbiology 1991, 137, 49–55. [Google Scholar] [CrossRef]
- Meyer, J.; Jordi, R. Purification and Characterization of X-Prolyl-Dipeptidyl-Aminopeptidase from Lactobacillus lactis and from Streptococcus thermophilus. J. Dairy Sci. 1987, 70, 738–745. [Google Scholar] [CrossRef]
- Miyakawa, H.; Kobayashi, S.; Shimamura, S.; Tomita, M. Purification and Characterization of an X-Prolyl Dipeptidyl Aminopeptidase from Lactobacillus delbrueckii ssp. bulgaricus LBU-147. J. Dairy Sci. 1991, 74, 2375–2381. [Google Scholar] [CrossRef]
- Zevaco, C.; Monnet, V.; Gripon, J.-C. Intracellular X-prolyl dipeptidyl peptidase from Lactococcus lactis spp. lactis: purification and properties. Journal of Applied Bacteriology 1990, 68, 357–366. [Google Scholar] [CrossRef]
- Kaminogawa, S.; Azuma, N.; Hwang, I.-K.; Suzuki, Y.; Yamauchi, K. Isolation and characterization of a prolidase from Streptococcus cremoris H61. " Agricultural and biological chemistry 1984, 48, 3035–3040. [Google Scholar] [CrossRef]
- Baankreis, R.; Exterkate, F.A. Characterisation of a Peptidase from Lactococcus lactis ssp* cremoris HP that Hydrolyses Di- and Tripeptides Containing Proline or Hydrophobic Residues as the Aminoterminal Amino Acid. Syst. Appl. Microbiol. 1991, 14, 317–323. [Google Scholar] [CrossRef]
- Driessen, A.J.; Poolman, B.; Kiewiet, R.; Konings, W. Arginine transport in Streptococcus lactis is catalyzed by a cationic exchanger. . Proc. Natl. Acad. Sci. 1987, 84, 6093–6097. [Google Scholar] [CrossRef] [PubMed]
- Poolman, B.; Driessen, A.J.; Konings, W.N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J. Bacteriol. 1987, 169, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J. Ornithine transport and exchange in Streptococcus lactis. J. Bacteriol. 1987, 169, 4147–4153. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.; Lovitt, R. Bacteriocins Produced by Lactic Acid Bacteria a Review Article. APCBEE Procedia 2012, 2, 50–56. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Moon, S.K.; Wee, Y.-J.; Choi, G.-W. A novel lactic acid bacterium for the production of high purity L-lactic acid, Lactobacillus paracasei subsp. paracasei CHB2121. Journal of bioscience and bioengineering 2012, 114, 155–159. [Google Scholar] [CrossRef]
- Zalán, Z.; Hudáček, J.; Štětina, J.; Chumchalová, J.; Halász, A. Production of organic acids by Lactobacillus strains in three different media. European Food Research and Technology 2010, 230, 395–404. [Google Scholar] [CrossRef]
- Mufandaedza, J.; Viljoen, B.; Feresu, S.; Gadaga, T. Antimicrobial properties of lactic acid bacteria and yeast-LAB cultures isolated from traditional fermented milk against pathogenic Escherichia coli and Salmonella enteritidis strains. Int. J. Food Microbiol. 2006, 108, 147–152. [Google Scholar] [CrossRef]
- Delavenne, E.; Mounier, J.; Déniel, F.; Barbier, G.; Le Blay, G. Biodiversity of antifungal lactic acid bacteria isolated from raw milk samples from cow, ewe and goat over one-year period. International Journal of Food Microbiology 2012, 155, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Mishra, H.N. Scientific and Technical Aspects of Yogurt Aroma and Taste: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 208–220. [Google Scholar] [CrossRef]
- Callanan, M.; Kaleta, P.; O'Callaghan, J.; O'Sullivan, O.; Jordan, K.; McAuliffe, O.; Sangrador-Vegas, A.; Slattery, L.; Fitzgerald, G.F.; Beresford, T.; et al. Genome Sequence of Lactobacillus helveticus, an Organism Distinguished by Selective Gene Loss and Insertion Sequence Element Expansion. J. Bacteriol. 2008, 190, 727–735. [Google Scholar] [CrossRef]
- Slattery, L.; O’callaghan, J.; Fitzgerald, G.; Beresford, T.; Ross, R. Invited review: Lactobacillus helveticus—A thermophilic dairy starter related to gut bacteria. J. Dairy Sci. 2010, 93, 4435–4454. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Routray, W.; Mishra, H.N. Scientific and Technical Aspects of Yogurt Aroma and Taste: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 208–220. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Dairy products and health : recent insights. ”Journal of agricultural and food chemistry 2015, 63, 9381–9388. [Google Scholar] [CrossRef]
- Sánchez, B.; Reyes-Gavilán, C.G.d.L.; Margolles, A.; Gueimonde, M. Probiotic fermented milks: Present and future. Int. J. Dairy Technol. 2009, 62, 472–483. [Google Scholar] [CrossRef]
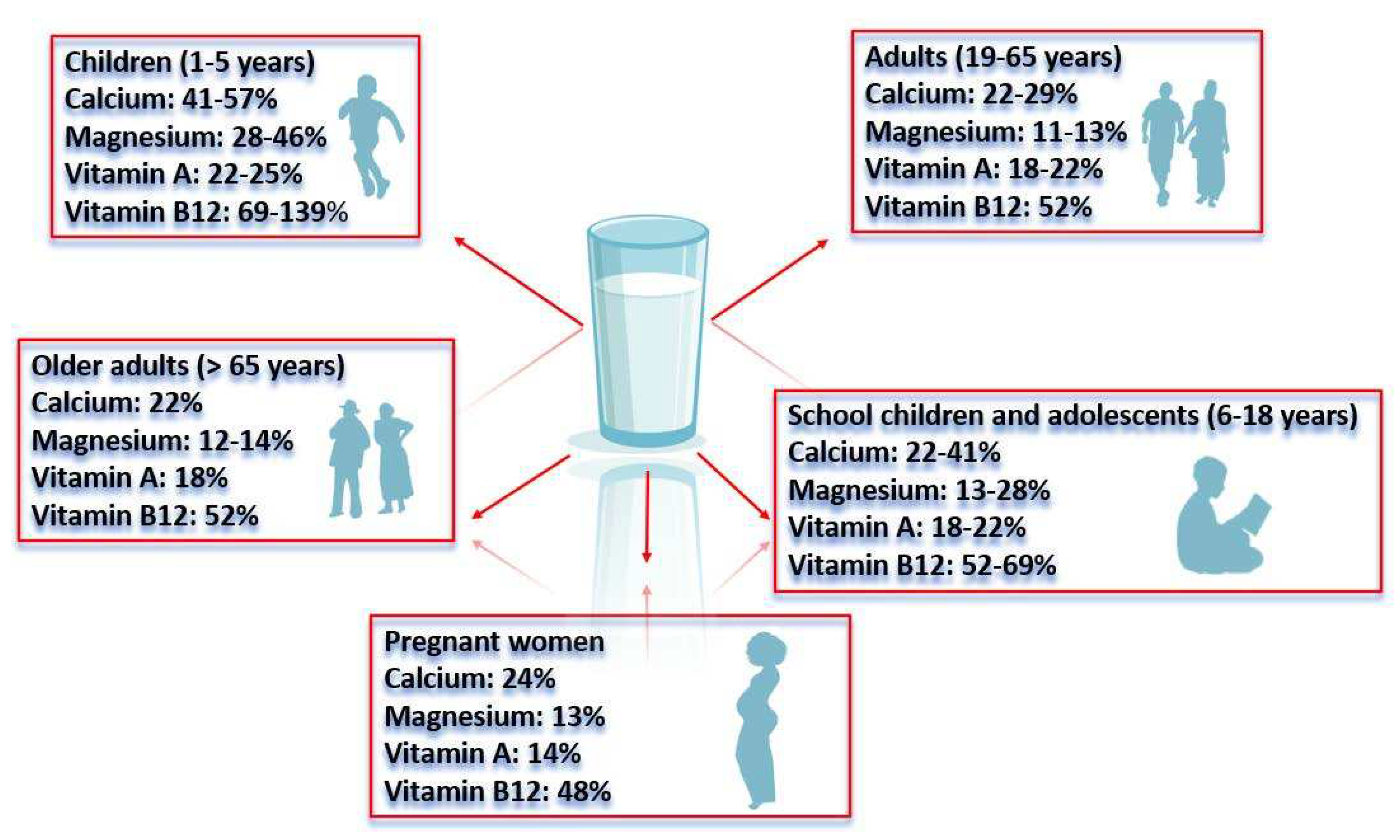
- Zamberlin, Š.; Antunac, N.; Havranek, J.; Samaržija, D. Mineral elements in milk and dairy products. Mljekarstvo: časopis za unaprjeđenje proizvodnje i prerade mlijeka 2012, 62, 111–125. [Google Scholar]
- Massey, L.K.; Whiting, S.J. Dietary salt, urinary calcium and bone loss. Journal of Bone and Mineral Research 1996, 11, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K. Magnesium Deficiency: A Cause of Heterogenous Disease in Humans. J. Bone Miner. Res. 1998, 13, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Paulina, G.; Bencini, R. Dairy Sheep Nutrition Wallington; CABI Publications: United Kingdom, 2004; p. 222. [Google Scholar]
- Ganpule, A.; Yajnik, C.S.; Fall, C.H.; Rao, S.; Fisher, D.J.; Kanade, A.; Cooper, C.; Naik, S.; Joshi, N.; Lubree, H.; Deshpande, V.; Joglekar, C. Bone mass in Indian children–relationships to maternal nutritional status and diet during pregnancy: the Pune Maternal Nutrition Study. J Clin Endocrinol Metab 2006, 91, 2994–3001. [Google Scholar] [CrossRef]
- Dekker, P.J.; Koenders, D.; Bruins, M.J. Lactose-free dairy products: market developments, production, nutrition and health benefits. " Nutrients 2019, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Cole, Z.A.; Gale, C.R.; Javaid, M.K.; Robinson, S.M.; Law, C.; Boucher, B.J.; Crozier, S.R.; Godfrey, K.M.; Dennison, E.M.; Cooper, C. Maternal dietary patterns during pregnancy and childhood bone mass: a longitudinal study. J Bone Miner Res 2009, 24, 663–668. [Google Scholar] [CrossRef]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2014, 39, 721–726. [Google Scholar] [CrossRef]
- Abargouei, A.S.; Janghorbani, M.; Salehi-Marzijarani, M.; Esmaillzadeh, A. Effect of dairy consumption on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Int. J. Obes. 2012, 36, 1485–1493. [Google Scholar] [CrossRef]
- Lampe, J.W. Dairy products and cancer. J Am Coll Nutr. 2011, 30 (5 Suppl 1), 464S–70S. [Google Scholar] [CrossRef]
- Xiong, L.; Ren, F.; Lv, J.; Zhang, H.; Guo, H. Lactoferrin attenuates high-fat diet-induced hepatic steatosis and lipid metabolic dysfunctions by suppressing hepatic lipogenesis and down-regulating inflammation in C57BL/6J mice. Food Funct. 2018, 9, 4328–4339. [Google Scholar] [CrossRef]
- Merritt, J.; Qi, F.; Shi, W. Milk Helps Build Strong Teeth and Promotes Oral Health. J. Calif. Dent. Assoc. 2006, 34, 361–366. [Google Scholar] [CrossRef]
- McKeever, T.M.; A Lewis, S.; A Cassano, P.; Ocké, M.; Burney, P.; Britton, J.; A Smit, H. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax 2007, 63, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; et al. Carbohydrate nutrition is associated with the 5-year incidence of chronic kidney disease. J Nutr 2011, 141, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





