1. Introduction
Morus, an essential ligneous organism, possesses multifaceted values encompassing its significance as a vital sustenance for silkworms, as well as its economic, medicinal, and ecological merits. Mulberry, an integral facet of Chinese agrarian culture, boasts an extensive history of cultivation, yielding profound cultural accretion since the early stages of civilization. Numerous studies have elucidated the formidable challenge of propagating the mulberry tree species, with the meager seedling rooting rate posing a significant predicament in mulberry cultivation1. While the optimization of cultivation methods has proven efficacious in augmenting the mulberry seedling rooting rate, there remains a dearth of mature agricultural technologies to enhance seedling survival rates. The growth of mulberry seedlings is predominantly sustained by the absorption and transportation of nutrients through the roots2, making them susceptible to adverse soil conditions that can impede growth and development, even jeopardizing the survival of mulberry seedlings3. It is evident that enhancing the physiological parameters governing mulberry seedling growth, as well as fortifying root capabilities in soil moisture and nutrient absorption, can instigate robust growth and development throughout the seedlings' lifespan, thereby bolstering mulberry yield4.
Drawing upon prior investigations, cutting techniques have shown promise in ameliorating mulberry survival rates. For tree species notorious for their resistance to rooting, the judicious application of plant growth regulators can expedite crucial processes such as cell division, rhizome elongation, and floral and fruit maturation[5-7]. This study ascertained that exogenous hormones promote the growth and differentiation of the basal root of mulberry twig cuttings, facilitating the emergence of adventitious roots8. The findings pertaining to mulberry cuttings revealed that the employment of ABT1 solution expedited wound healing at the lower incision, curtailed the rooting duration, and substantially augmented the post-transplantation rooting rate9. Notably, hormone concentration emerged as the primary determinant affecting twig cutting rooting, as verified by previous experimental results. Consequently, cutting propagation engenders the formation of intact new plants, while external plant growth regulators facilitate superior root development in seedlings, thereby ensuring robust fruit mulberry yield. However, investigations exploring the interplay between endogenous hormones10 and related enzyme activities11 during mulberry rooting in the context of cutting propagation remain scarce. Thus, this study delved into the impact of various ABT1 concentrations on alterations in endogenous hormone content and oxidase activity throughout the mulberry twig cutting rooting process, thereby elucidating the physiological and biochemical underpinnings of mulberry rooting. Such endeavors hold immense significance in providing technical support and theoretical groundwork for mulberry cutting propagation and elucidating its rooting mechanism.
2. Materials and methods
2.1. Test materials
The mulberry cultivars under examination encompassed the esteemed ‘Yueshenda 10’, meticulously chosen by the Sericulture and Agricultural Products Processing Institute of Guangdong Academy of Agricultural Sciences. These selections were introduced and meticulously cultivated within the mulberry orchard situated in the third residential zone of Henan Agricultural University, located in Henan Province. As the maternal source, vigorously thriving 3-year-old seedlings were employed, and semi-lignified branches from the current year were carefully obtained to prepare the cuttings. The cuttings were standardized in terms of diameter (0.6-0.8cm) and length (13-15cm), with a flat cut made at the upper end and a 45° oblique cut at the lower end. Bundled together, a total of 80 plants comprised a bundle. Each cutting retained two complete buds and leaves, and only 1/2 of each leaf is retained. The experimental plant growth regulator utilized was ABT rooting powder (ABT1), sourced from the Forestry Research Institute of Beijing Academy of Forestry.
2.2. Test method
2.2.1. Cutting method
The cutting facility is equipped with an automatic intermittent spray apparatus, complemented by a sunshade net structure top. The cutting pool spans a length of 20m and a width of 1.5m, with the upper section layered with a 30-centimeter-thick bed of pristine river sand serving as the cutting substrate. Preceding the cutting procedure, a carbendazim solution with a 50% mass fraction was evenly sprayed onto the substrate at a concentration of 800 times, ensuring thorough sand incorporation. Subsequently, the treated sand was left to dry for a period of 3 days. On the 4th day, the meticulously prepared cuttings underwent an initial rapid immersion in a 50% mass fraction carbendazim wettable powder solution, diluted at a concentration of 800 times, for a duration of 10 seconds. Thereafter, a completely randomized block design was adopted, wherein the cuttings were immersed in an ABT1 hormone solution for a period of 30 minutes. The hormone concentrations were set at 200,500,800 and 1000 mg/L, while the control group was subjected to treatment solely with clear water. Each treatment constituted a single block, with three replicate samples per treatment, amounting to a total of 80 cuttings per replicate sample. Overall, five block groups encompassed a grand total of 1200 cuttings. The spacing between rows measured 15 cm, while the plant spacing was maintained at approximately 12cm. The cutting depth ranged between 5 and 6 cm, with the morphological lower end of the cuttings oriented downward and the buds and leaves positioned upward. Following the cutting procedure, the seedlings were subjected to weekly disinfection utilizing carbendazim, with the spray interval and duration thoughtfully adjusted in accordance with prevailing weather conditions. The greenhouse temperature was regulated within a range of 20-29 °C, while the air's relative humidity was maintained at 80-90%.
2.2.2. Dynamic observation and index observation of rooting
Following the cutting procedure, at intervals of 4 days, 2-3 cuttings were carefully extracted to capture photographic documentation of their root morphological transformations, thereby documenting the progression of rooting. Concurrently, a sampling investigation was conducted, facilitating the statistical analysis of adventitious root emergence and development. After a 48-day period of cutting, the complete root system of each plant was extracted from the substrate and meticulously cleansed to eliminate any extraneous matter. Subsequently, the number of roots, the length of each root, and the rooting rate were meticulously tallied, with root length measurements precise to 0.01 cm.
2.2.3. Determination of physiological and biochemical indexes
At specific time points, namely 0,12,24,36 and 48 d after the initial cutting, a total of twelve cuttings, all at the same developmental stage, were selected. Subsequent to a thorough water wash, a 2-centimeter segment of the cutting's base cortex was carefully collected, followed by the swift removal of the phloem, which was then sectioned into pieces. The collected samples should be thoroughly amalgamated and expeditiously immersed in liquid nitrogen, followed by their preservation within an ultra-low temperature freezer at -80℃ within 2h, in order to facilitate subsequent utilization. The endogenous hormone content and oxidase activity of the cuttings treated with ABT1 at concentrations of 200,500,800 and 1000 mg/L, as well as the control group (CK), were determined. The determination of endogenous hormone levels and oxidase activity in the cuttings was performed utilizing an ELISA kit, whereby the mass fractions were precisely quantified. A panel of five endogenous hormones, namely indoleacetic acid (IAA), abscisic acid (ABA), jasmonic acid (JA), cytokinin (ZR) and gibberellin (GA3) and three oxidase activities such as peroxidase (POD), oxygen polyphenol oxidase (PPO) and indoleacetic acid oxidation (IAAO) were dynamically detected by double antibody one-step sandwich enzyme-linked immunosorbent assay (ELISA).
3. Results and analysis
3.1. Growth and development of adventitious roots
The development of adventitious roots in mulberry cuttings originates from the callus region, thereby classifying it as callus rooting in terms of morphology.
Figure 1 depicts the morphological transformations occurring at the base of the rooting cuttings in mulberry. Based on the observation of the base morphology, these transformations can be broadly categorized into five stages. The initial stage involves the preparation of the cuttings for rooting. The period from 1 to 12 days represents the germination stage, characterized by varying degrees of basal swelling and partial callus formation. From 12 to 24 days, the induction stage takes place, during which a substantial amount of callus tissue forms, with the majority of cutting sites exhibiting swelling accompanied by white protrusions at the callus tissue, ultimately giving rise to root primordia. The period from 24 to 36 days corresponds to the expression stage, wherein root primordia differentiate and visible adventitious roots emerge, reaching a length of approximately 2-3 centimeters. Lastly, the elongation stage occurs from 36 to 48 days, during which adventitious roots continue to elongate.
Table 1 illustrates the rooting outcomes of mulberry cuttings treated with different concentrations of ABT1 after 48 days of cutting. The results indicate that the treatment group subjected to 800mg/L ABT1 exhibited a significant impact on both the rooting rate and average number of roots, surpassing the other treatment groups. Additionally, notable variations in root length were observed among different hormone concentrations. In general, the rooting parameters of mulberry under varying ABT1 concentrations exhibited an initial increase followed by a subsequent decrease with increasing concentration, with the most favorable outcomes observed at a concentration of 800mg/L ABT1.
3.2. Endogenous hormone content during adventitious root formation
3.2.1. Dynamic changes of endogenous hormone IAA in the process of cuttings rooting
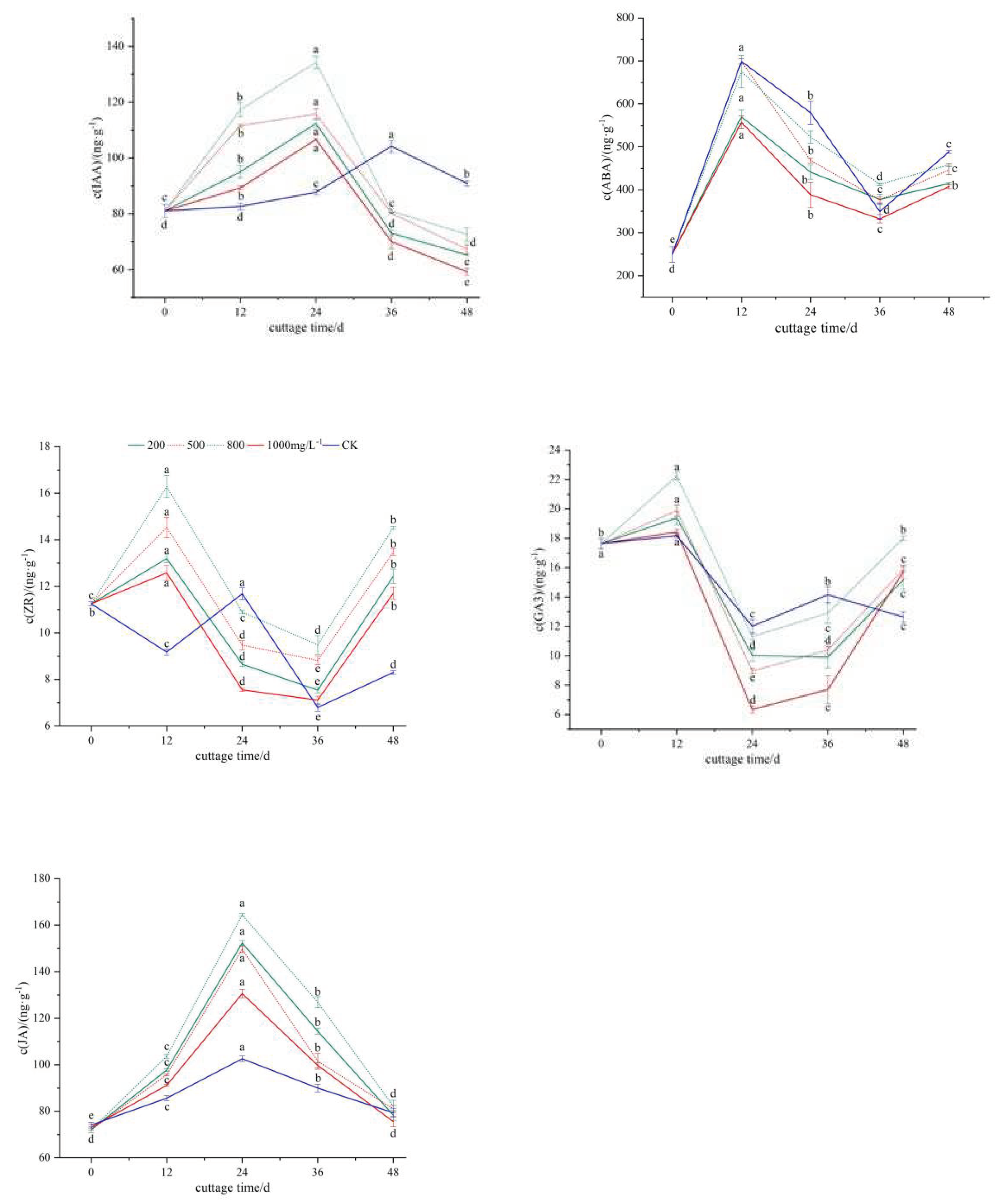
The IAA content of the cuttings subjected to ABT1 treatment and the control group exhibited a 'rise-fall' pattern(
Figure 2, A). In the treatment group, the IAA increased rapidly from the day of cutting and reached its peak on the 24th day, followed by a rapid decrease. Among the treatment groups, the cuttings treated with 800mg/L ABT1 displayed the highest IAA content (134.27 ng·g
-1). This indicates that the IAA content increased from the initial cutting stage until the formation of root primordia during callus differentiation, significantly decreased during the formation of adventitious roots, and decreased further during the formation and elongation of a substantial number of adventitious roots. This trend exhibited a stable pattern of change. In the control group, the IAA content increased gradually and reached its peak on the 36th day, which was 12 days later than that of the treatment group. These results suggest that a lower level of endogenous IAA is conducive to promoting the growth of adventitious roots. Previous studies have highlighted the requirement for higher levels of endogenous IAA to induce the formation of root primordia during adventitious root development. However, the IAA content decreases during the growth and differentiation of root primordia, indicating a correlation between increased IAA content and the formation and occurrence of adventitious roots
[12-14]. This experiment supports the same observation, affirming that IAA serves as a crucial endogenous hormone in the induction of adventitious root formation in mulberry cuttings.
3.2.2. Dynamic changes of endogenous hormone ABA in the rooting process of cuttings
During the rooting process of mulberry twig cuttings, both the control group and the treatment group exhibited a similar change trend in ABA content, following a pattern of 'first increase, then decrease, and then increase'(
Figure 2, B). In the early stage of cutting (0-12 days), the endogenous ABA content showed a significant increase, indicating that hormone treatment did not have a notable impact on the initial ABA content. From 12 to 24 days, there was a significant decrease in endogenous ABA content, with the control group displaying higher levels compared to the treatment group. Furthermore, the endogenous ABA content continued to decrease from 36 to 48 days. This observation suggests that the decrease in ABA content during the transition from the initial callus stage to the large callus stage facilitates the transport of IAA or other rooting substances from leaves to the base of the plant
15. Subsequently, the ABA content increased from the formation to the elongation of adventitious roots, potentially due to accelerated cell division resulting in reduced ABA production. Once callus formation occurred, entering the rooting stage was accompanied by reduced cell division, leading to increased ABA production. Additionally, the endogenous ABA content exhibited an inverse relationship with the rooting rate, indicating that high levels of ABA content can inhibit the formation of adventitious roots.
3.2.3. Dynamic changes of endogenous hormone ZR in the rooting process of cuttings
The change trend of ZR content in the cuttings of the treatment group and the control group exhibited inconsistencies(
Figure 2, C). In the ABT1-treated cuttings, the ZR content showed an initial increase, followed by a decrease and then another increase. Conversely, the control group displayed a decrease, followed by an increase, and then a subsequent decrease. The peak ZR content in the ABT1-treated cuttings was observed at 12 days after cutting, reaching a maximum of 16.28ng/g
-1. During the early stage of cutting, the formation of callus benefited from the accumulation of ZR, promoting cytoplasmic differentiation. The process of cell division and differentiation requires a significant amount of ZR, hence the decreasing trend in ZR content during the induction and formation stages of adventitious roots in the treated cuttings. On the other hand, tissues such as root tips stimulate the synthesis of ZR during the formation and elongation of adventitious roots, leading to an increase in ZR content. In the control group, the peak ZR content appeared 24 days after cutting, which was 12 days later than the treated cuttings, aligning with the delayed formation of callus. Furthermore, the ZR content in the control group was lower than that in the treatment group, indicating weaker cell division ability, which is not conducive to the induction of rooting.
3.2.4. Dynamic changes of endogenous hormone GA3 in the rooting process of cuttings
During the rooting process of mulberry cuttings, the change in GA3 content in the control group exhibited a double-peak curve of 'increase-decrease-increase-decrease', while the change of GA3 content in the treatment group showed a single-peak curve of 'decrease-increase-decrease'(
Figure 2, D). The first peak in GA3 content appeared at 12d, and the peak level was higher in the hormone-treated group compared to the control group. This indicates that a high level of GA3 content is favorable for callus induction, and ABT1 treatment can expedite the formation of callus. During the critical rooting period of 12~24d, the GA3 content of cuttings in all treatments exhibited a significant decrease followed by an increase. In the control group, the second peak occurred on the 36th day. However, the GA3 content of cuttings in the treatment group was lower than that in the control group between 24d and 36d.This suggests that exogenous hormone treatment effectively reduced the endogenous GA3 content of cuttings during the adventitious root formation stage in the twig cutting process, and a lower concentration of GA3 content was conducive to root formation.
3.2.5. Dynamic changes of endogenous hormone JA in the rooting process of cuttings
Jasmonate (JA) has the ability to induce the rapid accumulation of auxin at the injured site, thereby altering the function of plant stem cells and initiating lateral primordium formation
16. Throughout the entire process of adventitious root formation, the JA content exhibited an 'up-down' trend(
Figure 2, E). The change in JA content was consistent between the cuttings of the treatment group and the control group, with the treatment group showing higher levels of JA compared to the control group. The JA content accumulated rapidly during callus formation. Similar to IAA, the JA content reached its peak during the differentiation of callus into root primordia, which provided a basis for the initiation and elongation of adventitious roots. After the formation of a substantial amount of callus, the JA content decreased sharply as root primordia developed and adventitious roots formed. The analysis indicates that the rapid accumulation of JA in the early stages promotes adventitious root formation, while the low level of JA content in the later stages facilitates the formation of secondary lateral roots.
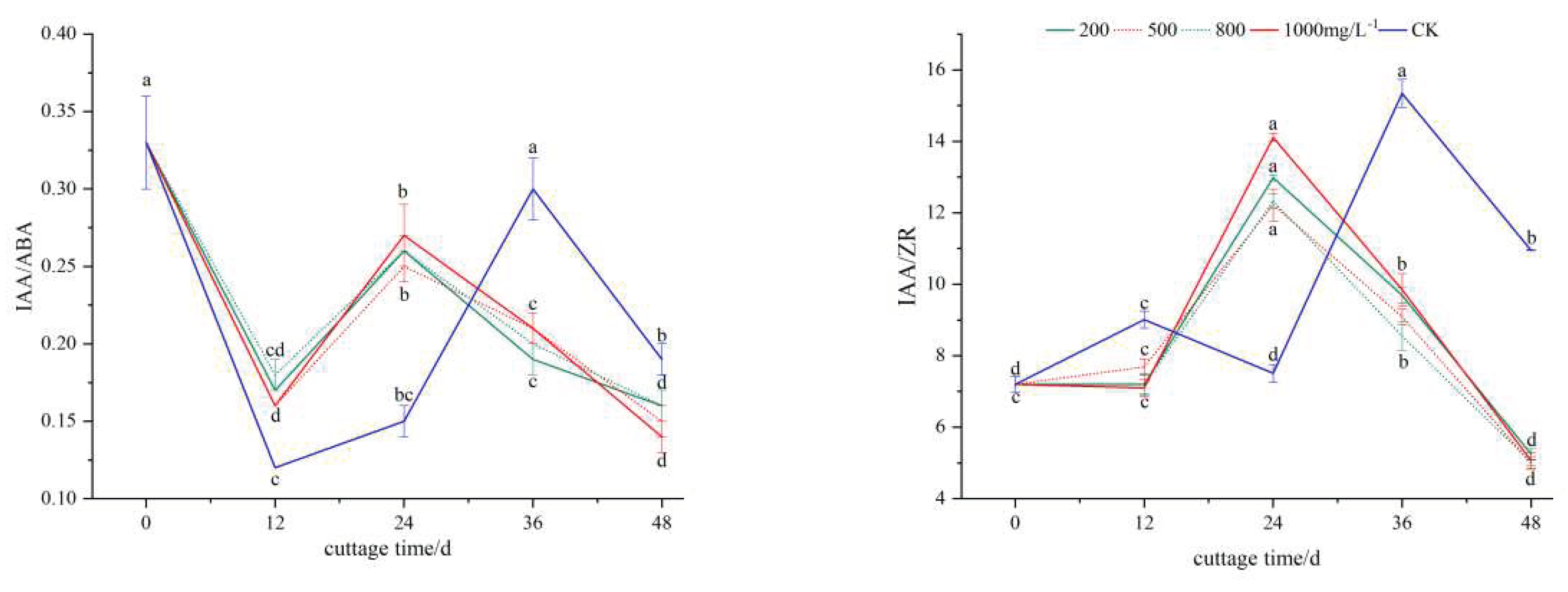
3.2.6. Changes of endogenous hormone ratio in cuttings treated with different concentrations of ABT1
The IAA/ABA values exhibited a 'decrease-increase-decrease' pattern within each group(
Figure 3, A). From 0 to 12 days, there was a decline, with the control group displaying a lower IAA/ABA value compared to the treatment group. This suggests that a lower initial IAA/ABA value was advantageous for callus formation. Between 12 and 36 days, the ratio increased, peaking at 24 days in the treatment group, 12 days earlier than in the control group. This indicates that during the formation phase of adventitious root primordia in the cuttings, the ABA content decreased while the IAA content increased, thereby promoting the differentiation of root primordia into adventitious roots. Subsequently, after 24 days, the IAA/ABA ratio experienced a rapid decline, favoring root development. From 0 to 24 days post-cutting, the IAA/ZR value in the ABT1 treatment group progressively rose, reaching a peak significantly higher than that of the control group(
Figure 3, B). This suggests a greater need for IAA/ZR during the early stages of callus formation. Based on the analysis, it can be concluded that the formation of callus, the generation of adventitious roots, and the elongation growth were primarily facilitated by the synergistic influence of IAA and ZR during the rooting process of mulberry cuttings.
3.3. Changes of oxidase activity during rooting of cuttings
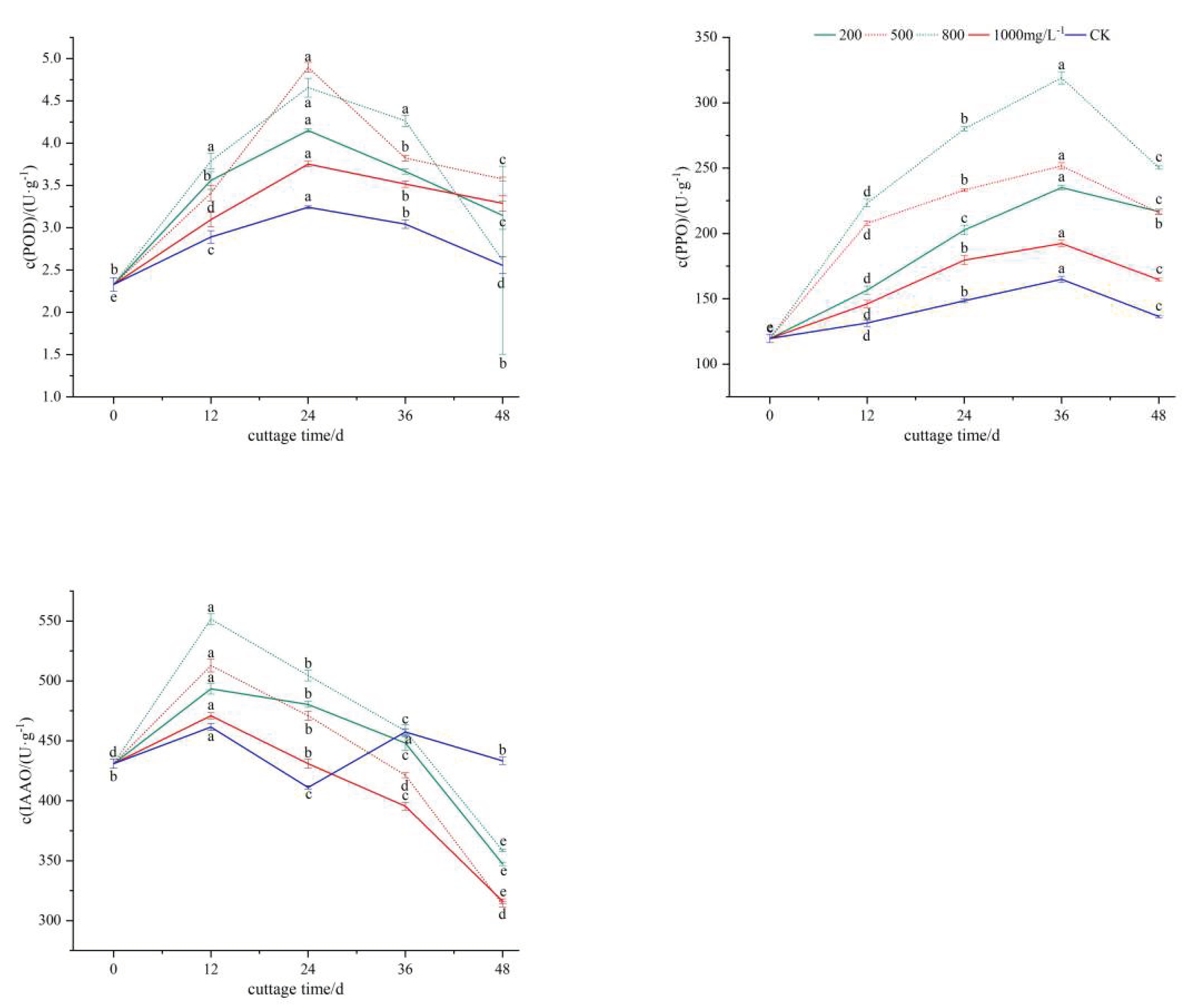
The POD activity of the cuttings displayed a unimodal trend characterized by an initial increase followed by a decrease(
Figure 4, A). Prior to the emergence of adventitious roots, it exhibited continuous growth, reaching its peak at 24 days and subsequently declining during the elongation phase of adventitious roots. From 0 to 12 days, the ABT1 treatment group demonstrated a rapid increase in POD activity, while the control group exhibited a slower increase. This stage corresponded to the separation of the cuttings from the parent plant, and POD played a role in enhancing the stress resistance of plant cells. Between 12 and 24 days, the POD activity of both the ABT1 treatment group and the control group reached its zenith, with the highest and lowest values recorded as 4.90U·g-1and 3.24U·g-1, respectively. From 24 to 48 days, the IAA treatment group and the control group displayed a downward trend in POD activity. This can be attributed to the POD enzyme's ability to oxidize and decompose indoleacetic acid, thereby regulating plant growth and development
[17-18] and promoting callus formation. Throughout the entire rooting process, the POD activity of the ABT1 treatment group consistently surpassed that of the control group, indicating that ABT1 enhanced POD activity in the cuttings, and a heightened level of POD activity was beneficial for mulberry twig cutting rooting.
The PPO activity of the cuttings demonstrated a unimodal pattern characterized by an initial increase followed by a decrease, peaking at 36 days after cutting(
Figure 4, B). The PPO content varied across different treatments in each period, with the order being CK<1000mg/L<200mg/L<500mg/L<800mg/L. However, the PPO activity exhibited differences among the various treatments during each period. This indicates that elevated levels of PPO activity contribute to the formation of the 'IAA-phenolic acid complex,' which facilitates callus and root formation and development. Cutting for 36 days, the PPO activity of the cuttings demonstrated varying degrees of decline. In the control group, this decrease may be attributed to a reduction in its activity and metabolic levels, while in the treatment group, it may be due to the elongation growth of the roots, no longer necessitating higher PPO activity. Studies have shown that PPO activity increases during the expansion of callus and the staggered rooting period, promoting the development of root primordia and the induction of adventitious roots. Subsequently, PPO activity decreases, thereby facilitating the elongation of adventitious roots
19. The findings of this study are consistent with these observations.
IAAO modulates the formation and development of adventitious roots by regulating the level of IAA content in cuttings. The IAAO activity of the cuttings in the treatment group exhibited a unimodal pattern characterized by an initial increase followed by a subsequent decrease. Conversely, the overall dynamics of IAAO activity in the cuttings from the control group demonstrated a bimodal trend of 'rising-falling-rising-falling'(
Figure 4, C). A peak was observed at 12 days after cutting. In the treatment group, cuttings treated with 800mg/L ABT1 was 504.51U·g
-1, surpassing the control group by a factor of 1.2. This observation suggests that hormone treatment can induce heightened IAAO activity, thereby fostering callus formation. However, IAAO activity declines during the process of root formation and elongation. This decrease may be attributed to the fact that reduced IAAO activity results in elevated IAA levels, which in turn facilitate root development. The control group exhibited a second peak at 36 days, with the IAAO content in the cuttings surpassing that of the treatment group. This occurrence can be ascribed to the decay of certain roots from the mulberry twigs during the cutting process, leading to a continuous increase in IAAO activity. Consequently, the majority of cuttings perished between 36 and 48 days, resulting in a decline in IAAO activity.
4. Discussion
The application of exogenous plant growth regulators can influence the stress tolerance of plants by exerting the activity of endogenous plant hormones. The responsiveness of root-related oxidase activity to growth regulators varies depending on the concentration of the hormones. In this study, we aimed to investigate the impact of ABT1 on root growth parameters, endogenous hormones related to root development, and oxidase activity in mulberry cuttings. Additionally, we examined the response of seedling roots to varying concentrations of root regulatory factors.
4.1. The relationship between exogenous hormones Treatment and rooting of cuttings
The selection of growth regulators constitutes the primary determinant influencing root growth parameters. In certain investigations, ABT1 has been acknowledged as a potent growth regulator, capable of enhancing both the rooting rate and rooting effect of cuttage[20-22], aligning with prior experimental findings23. Thus, ABT1 was employed as an exogenous hormone in this study to investigate the physiological and chemical responses of cuttings under hormonal influence. Within our study, the impact of root growth parameters varied according to the concentration of ABT1. The optimal concentration of ABT1(800mg/L) demonstrated a significant enhancement across all root growth parameters, surpassing the other treatments. Conversely, an excessive concentration of ABT1 impeded root growth. Similar outcomes have been observed in horticultural studies focusing on adventitious root development experiments24.
4.2. The relationship between adventitious root formation and endogenous hormones in twig cuttings of mulberry
It was observed that the morphological transformations associated with adventitious root development were concomitant with the modulation of endogenous hormones. The content of IAA exhibited a positively correlation with the rooting rate, whereas the level of ABA displayed a negative correlation. These findings align with the research outcomes of Buxus microphylla, which indicate that disparities in post-cutting rooting ability correspond with alterations in endogenous hormone levels such as auxin, abscisic acid, and gibberellin25. According to reports, IAA serves as the principal endogenous hormone responsible for promoting the rooting of plant cuttings26. High concentration of IAA facilitate increased cell division within the root meristem, while lower concentrations expedite cell differentiation in the root elongation zone27. The results obtained in this study revealed that the endogenous hormone IAA reached its highest level when treated with 800mg/L, followed by 500mg/L and 200mg/L, with 1000mg/L yielding intermediate levels, and the control group (CK) displaying the lowest concentration. The IAA content in the mulberry cuttings exhibited a significant increase during the callus production and root primordium differentiation phases, peaking at 24 days, which closely corresponded with the observed timing of root primordium formation. Comparatively, the cuttings in the treatment group demonstrated a rapid accumulation of IAA, enhancing the induction and differentiation of root primordia in contrast to the control. However, during the period of adventitious root expression, a declining trend was observed, possibly due to the gradual consumption of IAA as the adventitious roots penetrated the epidermis, resulting in decreased IAA content at the base of the new shoot. Notably, the peak time of IAA in the treatment group's cuttings occurred 12 days earlier than in the control group, suggesting that the swift surge in IAA content within the cuttings may primarily contribute to their early rooting. Consequently, IAA is deemed the primary endogenous hormone promoting the formation of adventitious roots in mulberry cuttings, corroborating the outcomes of previous investigations[28-30]. However, further verification is required to determine whether low concentrations of IAA exert a positive influence on adventitious root elongation.
Most studies indicate a close association between GA3 and ABA with the inhibition of root formation31. There are multiple potential mechanisms through which gibberellin may impede adventitious root formation. Firstly, gibberellin restrains the division of root primordium cells32. Secondly, gibberellin impedes the further growth and development of auxin-induced root primordia33. In the present study, the GA3 content within the cuttings exhibited a significant increase during the 0-12 day period, thereby inhibiting the formation of adventitious root callus. During the induction and initiation of adventitious roots, the control treatment (CK) displayed the highest level of endogenous GA3, followed by the 800mg/L, 200mg/L or 500 mg/L, with the 1000mg/L treatment yielding the lowest concentration. This finding aligns with a previous investigation, which demonstrated that high concentrations of GA3 inhibited the formation of adventitious roots in cuttings34. The study also revealed that ABA content was higher in the early stage of cutting and subsequently decreased during the formation of adventitious roots. This pattern facilitated the hydrolysis of starch into sugars within the cuttings, promoting the development of root primordia and rooting of the cuttings35. The experimental results corroborated this, indicating an initial increase in ABA content following cutting, likely attributed to the stress response triggered by the incision injury, leading to the production of substantial amounts of ABA to enhance the resilience of the cuttings. Within the 30-day period following cutting, the highest ABA levels were observed in the control group, followed by the 800mg/L or 500mg/L treatments, while the 200mg/L and 1000mg/L treatments exhibited the lowest concentrations. Furthermore, during the phase spanning from callus formation to adventitious root initiation, the ABA content progressively decreased, underscoring the beneficial impact of lower ABA levels on rooting, which aligns with the conclusion that higher concentrations of ABA inhibit rooting.
According to reports, CTKs, including ZR and CTK, are a class of cytokinins that primarily promote cell division and root elongation36. Significant variations in ZR concentration were observed across different treatment concentrations and stages. Through research, it was found that the treatment group exhibited much higher levels of ZR than the control group during callus formation and the formation and elongation of adventitious roots. The appropriate concentration of ABT1 was able to induce ZR production. Specifically, the highest endogenous ZR level was observed when treated with 800mg/L, followed by 500mg/L, 200mg/L or 1000mg/L, with the control group exhibiting the lowest concentration. Similar to ABA, JA (jasmonic acid) also exerts an effect on rooting. Studies have demonstrated that the stimulation of wounds in cuttings leads to an increase in JA levels at the base of the cuttings, promoting cell division and differentiation37. In this experiment, the JA content increased during the initial stage of cutting and callus formation, followed by a varying degree of decrease, consistent with the findings that high JA levels facilitate callus formation during the callus formation phase38. In addition to the influence of individual endogenous hormones, multiple endogenous hormones work in conjunction to regulate rooting39. Studies have highlighted that the endogenous IAA/ABA ratio serves as a reliable indicator for the successful development of adventitious roots in vitro after regeneration treatment40. In this experiment, the IAA/ABA ratio significantly increased during callus formation and root primordium induction in the treatment group, surpassing the levels observed in the control group. A higher IAA/ABA ratio emerged as a key factor for the initiation and growth of adventitious roots. The IAA/ZR ratio initially increased and then decreased in the treatment group, exhibiting a significant difference between the two groups. The control group displayed a fluctuating pattern of "up-down-up-down." According to the rooting process, the IAA/ZR value increased during callus formation and adventitious root induction, followed by a decrease during adventitious root expression and elongation. These findings demonstrate that a higher IAA/ZR value is conducive to the formation of adventitious roots.
4.3. Relationship between adventitious root formation and related enzyme activity in twig cutting
The response of root-related oxidase activity to growth regulators exhibited variability depending on the concentration of hormones. Different concentrations of ABT1 demonstrated varying degrees of improvement in the antioxidant enzyme activity of mulberry roots, effectively enhancing their overall activity. According to reports, PPO and POD are involved in numerous physiological and biochemical processes in plants, playing a crucial role in cell division, differentiation, and the formation and growth of root primordia41. One significant function of PPO is its ability to catalyze phenolic substances, which can synthesize a complex with IAA: IAA-phenolic acid complex. This complex promotes callus differentiation and stimulates the formation of adventitious roots42. Research has confirmed that high PPO activity during the rooting process is an indicator of better rooting ability of cuttings43. POD, on the other hand, acts to neutralize inhibitory substances that impede the occurrence of adventitious roots. An increase in POD activity during the induction and initiation stages of adventitious roots signifies an enhanced rooting capacity in cuttings[44-45]. The study revealed a consistent pattern of "up-down" changes in the activities of POD, PPO, and IAAO in cuttings treated with ABT1. Specifically, the enzyme activities significantly increased during the callus formation stage, effectively promoting the rooting of mulberry twig cuttings, albeit with slight variations in the later stages. The PPO activity in the ABT1 treatment group displayed a similar trend to that of the control group, continuously increasing during the adventitious root induction and occurrence stages, and decreasing during the root elongation stage. ABT1 treatment resulted in a significant increase in PPO activity at the base of the cuttings compared to the control group, with significant differences observed at different stages. This was conducive to the induction and occurrence of roots, aligning with research findings on Lycium barbarum twig cuttings46. Previous studies have demonstrated that lower IAAO activity during the induction and development stages of adventitious roots facilitates the accumulation of IAA in cuttings, promoting the occurrence and development of adventitious roots47, consistent with the results of this experiment. Furthermore, compared to the other two root-related enzymes, POD activity displayed less sensitivity to plant growth regulators. Within a concentration range of 800mg/L-1, POD activity in the roots remained highly stable.
5. Conclusion
The utilization of ABT1 at a concentration of 800mg/L significantly enhanced the rooting rate, average number of roots, average root length, and longest root length of mulberry twigs. ABT1 treatment resulted in increased levels of IAA and JA, while decreasing the levels of ABA, ZR and GA3 in mulberry cuttings. The insufficient endogenous IAA content during the critical rooting period is one of the primary factors contributing to the challenging rooting process in mulberry, as it hampers the induction of root primordium initiation cells. Although ABA inhibits rooting, it actively participates in signal transduction during adventitious root induction and enhances the resistance of cuttings. The content of ZR exhibits significantly increments during the elongation phase of adventitious roots. GA3 predominantly functions during the early stages of cutting and the formation and elongation of adventitious roots. Additionally, the interplay of endogenous hormones collectively influences the rooting of cuttings. A lower IAA/ABA ratio facilitates the formation and elongation of adventitious roots, while a higher IAA/ZR value fosters the rooting of cuttings. The activities of POD, PPO, and IAAO demonstrate an overall trend of 'increase-decrease', with ABT1 treatment generally augmenting the activities of POD and PPO, while inhibiting the activity of IAAO.
Author Contributions
Conceptualization, J.Q. and H.B.; methodology, J.Q. and J.S.; software, J.S. and H.D.; validation, Y.W., T.S. and H.D.; Data Curation, J.S. and H.D.; writing—original draft preparation, J.S. and Y.W.; writing—review and editing, J.Q. and H.B.; funding acquisition, J.Q. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received funding from the National Natural Science Foundation of China, grant number 31700549 and the Forestry Bureau of Henan Province, China, grant number 30802649.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qiu, X.Y.; Chen, L.; Hu, W.J.; Yu, C.L.; Shi, Y.B.; Chen, X.L.; Wang, J.S.; Hu, H.Q.; Shen, G.X. The expression changes of ABI4 and PIN1 genes and their correlation with rooting performance in mulberry green branch cuttings. Sericulture Sci. 2015, 41(02), 204–210. [Google Scholar]
- Sourati, R.; Sharifi, P.; Poorghasemi, M.; Alves Vieira, E.; Seidavi, A.; Anjum, N.A.; Sehar, Z.; Sofo, A. Effects of Naphthaleneacetic Acid, Indole-3-Butyric Acid and Zinc Sulfate on the Rooting and Growth of Mulberry Cuttings. Int. J. Plant Biol. 2022, 13, 245–256. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Luo, X. Research Progress of fruit tree Cutting Propagation (Literature Review). J. Hubei Agric Col. 1992(02), 57–62. [Google Scholar]
- Fang, C.G. Exploration and application of mulberry cuttage raising technology. China Agric. Inform. 2014, No.162(05), 221–222. [Google Scholar]
- Ahmad, I.; Kamran, M.; Ali, S.; Cai, T.; Bilegjargal, B.; Liu, T.L.; Han, Q.F. Seed filling in maize and hormones crosstalk regulated by exogenous application of uniconazole in semiarid regions. Environ. Sci. Pollut. Res. 2018, 25, 33225–33239. [Google Scholar]
- Hedden, P. Gibberellin metabolism and its regulation. Plant Growth Regul. 2001, 20, 317–318. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. The regulatory signaling of gibberellin metabolism and its crosstalk with phytohormones in response to plant abiotic stresses. Plant Signal. Mole. 2019, 333–339. [Google Scholar]
- Zhang, B.L.; Wang, W.; Wu, X.L. Application of ABT root powder on mulberry. Anhui Agric. Sci. 1993(04), 367–370. [Google Scholar]
- Reed, R.C.; Brady, S.R.; Muday, G.K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998, 118(4), 1369–1378. [Google Scholar] [CrossRef]
- Shang, C.Q. Comparative study on physiological and transcriptional regulation mechanism of different rooting types in mulberry hard branch cuttings. Jiangsu University of Science and Technology: Jiangsu, China, 2020.
- Du, W. Study on rooting mechanism of mulberry green branch cuttings by artificial induction. Jiangsu University of Science and Technology: Jiangsu, China, 2010.
- Wen, S.; Miao, D.; Cui, H.; Li, S.; Gu, Y.; Jia, R.; Leng, Y. Physiology and transcriptomic analysis of endogenous hormones regulating in vitro adventitious root formation in tree peony. Sci. Hortic. 2023. [Google Scholar]
- Quan, J.E; Ni, R.Y.; Wang, Y.G.; Sun, J.J.; Ma, M.Y.; Bi, H.T. Effects of Different Growth Regulators on the Rooting of Catalpa bignonioides Softwood Cuttings. Life. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Hu, D.; He, X.; Ma, Y.; Fei, Y. Effects of ABT on the morphogenesis and inclusions of Taxus chinensis (Pilger) Rehd f. baokangsis cutting rooting. NOT. BOT. HORTI AGROBO. 2021. [CrossRef]
- Pan, J. Research on rooting mechanism of cuttings of three species of Suzuki. Nanjing Forestry University: Jiangsu, China, 2007.
- Zhuo, M.; Sakuraba, Y.; Yanagisawa, S. A jasmonate-activated myc2-dof2.1-myc2 transcriptional loop promotes leaf senescence in Arabidopsis. The Plant Cell, 2019, 32(1), tpc.00297.2019.
- Intapruk, C.; Yamamoto, K.; Sekine, M.; Takano, M.; Shinmyo, A. Regulatory sequences involved in the peroxidase gene expression in Arabidopsis thaliana. Plant cell rep. 1994, 13(3-4), 123-129. [CrossRef]
- Graham, M.Y.; Graham, T.L. Rapid Accumulation of Anionic Peroxidases and Phenolic Polymers in Soybean Cotyledon Tissues following Treatment with Phytophthora megasperma f. sp. Glycinea Wall Glucan. Plant physiol. 1991, 97(4), 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Song, L.H.; Cao, B.H. Study on the changes of indole-acetic acid oxidase, polyphenol oxidase and peroxidase activities of cutting roots of Guangyoussonetia Broussonetia. J. Plant Sci. 2005, 23(4), 347–350. [Google Scholar]
- Ma, F.Q.; Yang, Q.; Guo, Q.S.; Zhang, G.J.; Qin, A.L.; Wu, H.; Cai. S.Y.; Wang, L. Effects of picking position, substrate and growth regulator on cuttings of Cypress cypress. J. For. Eng. 2003, 164, 901-909.
- Zhang, Z.W.; Wang, Z.H. Effects of different concentrations of ABT root powder on cuttage rooting of 3 tea cultivars. Molecular plant breeding 2021, 19(19), 6574–6580. [Google Scholar]
- Yan, T.W.; Chen, G.H.; Li, G. Effects of ABT1 on rooting, nutrient content and enzyme activity in cuttings of Quercus mongolicus. J. Inner Mongolia Agric. Univ. (Social Science Edition), 2022, 43(06), 34-39.
- Sun, J.J.; Li, H.Y.; Chen, H.L.; Wang, T.T.; Quan, J.E; Bi, H.T. The Effect of Hormone Types, Concentrations, and Treatment Times on the Rooting Traits of Morus ‘Yueshenda 10’ Softwood Cuttings. Life 2023, 13, 1032. [Google Scholar] [CrossRef]
- Owusu Adjei, M.; Xiang, Y.; He, Y.; Zhou, X.; Mao, M.; Liu, J.; Hu, H.; Luo, J.; Zhang, H.; Feng, L.; Yang, W.; Li, X.; Ma, J. Adventitious root primordia formation and development in the stem of Ananas comosus var. bracteatus slip. Plant Signal Behav. 2021, 16(11), 1949147. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, Ks; Zhai, Jr. Relationship between rooting ability and endogenous phytohormone changes in successive continuous generation cuttings of Buxus sinica var. parvifolia, an endangered woody species in China. For. Stud. China 9, 2007, 189-197. [CrossRef]
- Kumar, P.; Patel, P.K.; Sonkar, M.K. Propagation through juvenile shoot cuttings in difficult-to-root Dalbergia latifolia – examining role of endogenous IAA in adventitious rooting. Plant Physiol. Rep. 2022, 27, 242–249. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of arabidopsis. Ann. Bot, 2013, 112, 1395-1407.
- Tahir, M.M.; Chen, S.; Ma, X.; Li, S.; Zhang, X.; Shao, Y.; Shalmani, A.; Zhao, C.; Bao, L.; Zhang, D. Transcriptome analysis reveals the promotive effect of potassium by hormones and sugar signaling pathways during adventitious roots formation in the apple rootstock. Plant Physiol Biochem. 2021, 165, 123–136. [Google Scholar] [CrossRef]
- Wang, Q.M.; Peng, W.X.; Zhang, J.P.; Pei, D. Study on the morphological structure and hormone regulation of root of young stem of walnut in vitro. J. of hortic. 2006, 33(2), 5. [Google Scholar]
- Gao, J.; Zeng, X.F.; Liu, X.H.; Yang, S.X. Cutting propagation of Periploca forrestii and dynamic analyses of physiological and biochemical characteristitics related to adventitious roots formation. J. Chinese medicinal materials, 2011, 34(6), 841-845.
- Pfaff, W.; Schopfer, P. Hormones are no causal links in phytochrome-mediated adventitious root formation in mustard seedlings (Sinapis alba L.). Planta, 1980, 150(4), 321-329. [CrossRef]
- Brian, P.W.; Hemming, H.G.; Lowe, D. Inhibition of rooting of cuttings by gibberellic acid. Ann. Bot. 1960, 24. [Google Scholar]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78(3), 372–384. [Google Scholar] [CrossRef]
- Chen, L.Y.; Zheng. Y.; Chen, L.G. Study on root culture and changes of endogenous hormone content of
tissue culture seedlings of Cuckoo. J. Fujian For. Col. 2021, 31(2), 5.
- Zhang, F.; Wang, H. Research progress on rooting mechanism of peach hardwood cuttings. J. Plant Physiol. 2019, 55(11), 1595–1606. [Google Scholar]
- Sipes, D.L; Einset, J.W. Cytokinin stimulation of abscission in lemon pistil explants. J. Plant Growth Regul 1983, 2,(1) 73–80. [CrossRef]
- Lup, S. D.; Tian, X.; Xu, J.; Pérez-Pérez; J. M. Wound signaling of regenerative cell reprogramming. Plant
Sci. : an international J. experimental plant biology, 2016, 250, 178–187. [CrossRef]
- Song, P.F.; Chen, H.J.; Jiang, Y.Q.; Wei, J.G.; Li, J.H. Effect of IBA on Rooting and Endogenous Hormone Changes of Rabbit Eyed Blue Berry Twig Cutting. Chinese Agri. Sci. Bulletin, 2014, 30(16), 117-122.
- Guo, S.J.; Ling, H.Q.; Li, F.L. A Study on the Physiological and Biochemical Basis of Rooting of Pinus bungeana cuttings. J. Beijing Fore. Univ. 2004, 26(2), 43–47. [Google Scholar]
- Wang, Y.; Yao, R.L. Increased endogenous indole-3-acetic acid:abscisic acid ratio is a reliable marker of Pinus massoniana rejuvenation. Biotech Histochem. 2019, 94(7), 546–553. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; Taşkin, T.; Otludil, B. Polyphenol Oxidase Activity during Rooting in Cuttings of Grape (Vitis vinifera L.) Varieties. Turk J. Bot, 2003, 27, 495-498.
- Bassuk, N.L.; Hunter, L.D.; Howard, B.H. The apparent involvement of polyphenol oxidase and phloridzin in the production of apple rooting cofactors. J. Hortic. Sci. 1981, 56(4), 313–322. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, K.; Zhang, A.; Zhu, W.; Zhang, H.; Tan, F.; Huang, Q.; Wu, X.; Zha, D. Metabolomic Analysis, Combined with Enzymatic and Transcriptome Assays, to Reveal the Browning Resistance Mechanism of Fresh-Cut Eggplant. Foods (Basel, Switzerland), 2022, 11(8), 1174. [CrossRef]
- Rout, G.R. Effect of Auxins on Adventitious Root Development from Single Node Cuttings of Camellia sinensis (L.) Kuntze and Associated Biochemical Changes. Plant Growth Regul. 2006, 48, 111-117. [CrossRef]
- Gaspar, T.; Kevers, C.; Hausman, J.; Berthon, J.; Ripetti, V. Practical uses of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomie, 1992, 12, 757-765.
- Su, C.F.; Duan, G.Z.; Fan, G.H. Physiological and biochemical analysis of Lycium barbarum cutting rooting. Northern Hortic. 2023(03), 520(01), 90–97. [Google Scholar]
- Nag, S.; Saha, K.; Choudhuri, M.A. Role of Auxin and Polyamines in Adventitious Root Formation in Relation to Changes in Compounds Involved in Rooting. J. Plant Growth Regul. 2001, 20, 182–194. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).