1. Introduction
Zeolites, a category of microporous crystalline solid catalysts, played important roles in petroleum refining, fine chemicals and biomass conversion [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. Zeolites are usually synthesized in basic condition under hydrothermal treatment [
4,
12,
13,
14]. Inorganic alkali, e.g. NaOH or KOH, was widely used to provide basic condition during zeolite synthesis, because it was a type of available cheap basic source. Unfortunately, these cheap inorganic bases were strictly restricted during synthesizing Lewis acid zeolites [
15,
16,
17,
18,
19], especially for Ti-doped TS-1 zeolites[
20,
21,
22,
23,
24,
25,
26,
27,
28] that were widely used in numerous industrially significant green oxidation processes with hydrogen peroxide as an oxidant under mild conditions [
29,
30,
31,
32,
33,
34,
35]. The reason was that alkali metal cations, e.g. Na
+, from inorganic alkali had an adverse effect on the incorporation of active Ti sites. The presence of alkali-metal cations even a trace amount of ~0.1 wt.% in the synthetic system could lead to no catalytic activity of TS-1 catalyst [
36,
37]. Therefore, quaternary ammonium hydroxide of tetrapropylammonium hydroxide (TPAOH) was first chosen as both organic structure directing agent ((TPA
+) and basic source (OH
−) in traditional synthesis, leading to the high production cost of TS-1 zeolites. So as to reduce the cost, a various of strategies were designed by using tetrapropylammonium bromide (TPABr) that had the same cation TPA
+ with TPAOH as structure directing agent combining with ammonia or organic amines to provide basic synthetic condition [
38,
39,
40,
41,
42]. These methods significantly decreased the production cost due to the much cheaper of TPABr than TPAOH. However, the low alkalinity of ammonia and organic bases led to the increase usage amounts in the synthetic system. Moreover, the volatile properties of these bases made some environmental problems.
Considering the high alkalinity, low-cost, and non-volatile of NaOH, it is still greatly disable to synthesize TS-1 by using NaOH as base combined cheap TPABr as template. But, it is still very challenging due to crucial effects of Na+ during the synthesis as mentioned above. Therefore, if NaOH was chosen as base in the synthesis, the key point to make active TS-1 zeolite was to solve the adverse effect the Na+ on the active Ti sites.
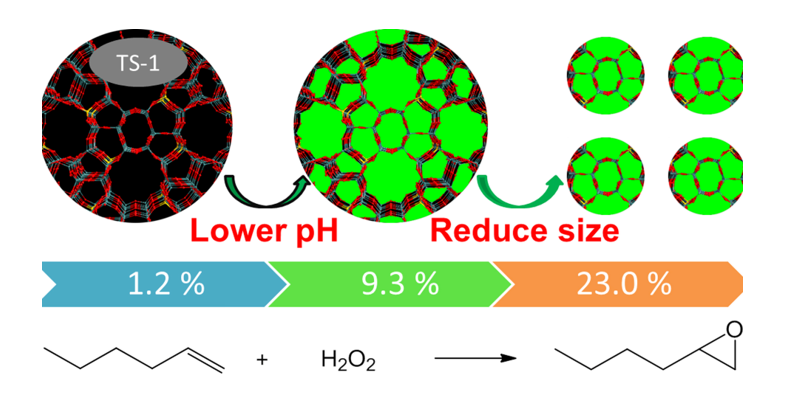
Here, a novel strategy was developed to solve the problem of the adverse effects of Na+ ions via precisely controlling pH value. Under the optimal recipe, the active TS-1 zeolites for 1-hexene epoxidation with H2O2 could be obtained by using NaOH as the base and TPABr as the template. A higher catalytic activity could be achieved by further reducing the size of the TS-1 crystal by seed-added strategy, which could reach a similar catalytic performance of current commercial TS-1 catalyst prepared by using TPAOH as templates.
2. Materials and Methods
2.1. Materials
Titanium tetra-n-butoxide, colloidal silica, NaOH, Tetrapropylammonium bromide, hydrogen peroxide, 1-hexene, cyclohexene, and Ce(SO4)2 were bought purchased from Macklin. All the chemical reagents were used without further purifications.
2.2. Syntheses of TS-1 catalysts
The colloidal silica and the titanium tetra-n-butoxide were employed as silicon and titanium sources, respectively. Tetrapropylammonium bromide (TPABr) was used as the template and NaOH as the base. The molar composition of the mixture was: SiO2 :TiO2 :TPABr :H2O :NaOH = 1 :0.035 :0.10 :30 :x. The x varied from 0.02 to 0.07. The raw materials were thoroughly mixed by stirring. Then the mixture was transferred into a tumbled autoclave and treated at 443 K for 2 days. After treatment, the solid obtained by filtration or centrifugation, dried, and calcined at 550 ℃ for 6 h. The small sized zeolite (denoted as TS-1-small) was synthesized by using the same procedure except adding 1.0 wt. % silicalite-1 zeolite as seeds in the starting mixture. The solid obtained was calcined at 823 K for 6 h, designated as TS-1-a, TS-1-b, TS-1-c and TS-1-d when x value was 0.02, 0.03, 0.05, and 0.07, respectively.
2.3. Characterization
The X-ray diffraction (XRD) measurements were performed on a Bruker Powder D8 Advance diffractometer at 40 kV and 40 mA using CuKa radiation (λ=1.5418 Angstrom). DRUV/Vis spectra were recorded on a Shimadzu UV-2450 spectrophotometer at 298 K using BaSO4 as a reference. FTIR spectra were recorded as KBr pellets on a Shimadzu IRPrestige-21 spectrometer. Nitrogen adsorption-desorption isotherms were measured on a TriStar II 3020 sorption analyzer at 77 K. Elemental analyses (Si, Ti and Na) were performed on an inductively coupled plasma optical emission spectrometer (Shimadzu ICPE-9000 spectrometer). The scanning electron microscopy (SEM) images were obtained on a JEOL JSM-7600F microscope operated at 20 kV. Particle sizes were measured on Malvern Zetasizer Nano ZS90 analyzer.
2.4. Catalytic Reaction
The oxidation reactions with catalyst (25 mg), 1-hexene (5 mmol) and H2O2 (5 mmol ) in methanol (5 mL) in a 20 mL glass reactor with 60℃ oil bath with stirring for 2 h. After reaction, the mixture was analyzed by gas chromatography. The H2O2 was determined with Ce(SO4)2 solution(0.1 M).
3. Results and discussion
The optimal synthetic system was composed of tetrapropylammonium bromide (TPABr) as structure directing agent, colloidal silica as silicon source, titanium tetra-n-butoxide as titanium source and NaOH as pH-adjusting agent.
The active TS-1 zeolites could be synthesized at pH value range from 8.9 to 10.2, as shown in
Table 1. With the increase of pH value, the sodium content in TS-1 products increased, meanwhile, the titanium content decreased. This implied the competitive relationship of Na
+ and Ti
+ cations to incorporate into the TS-1 product. More sodium in TS-1 would lead to the decrease of cooperated Ti content, which was consistent with previous reports [
43,
44]. The slight different of TS-1-a was due to some amorphous raw materials contained in TS-1-a sample as indicated from the lower BET surface area and smaller microporous volume.
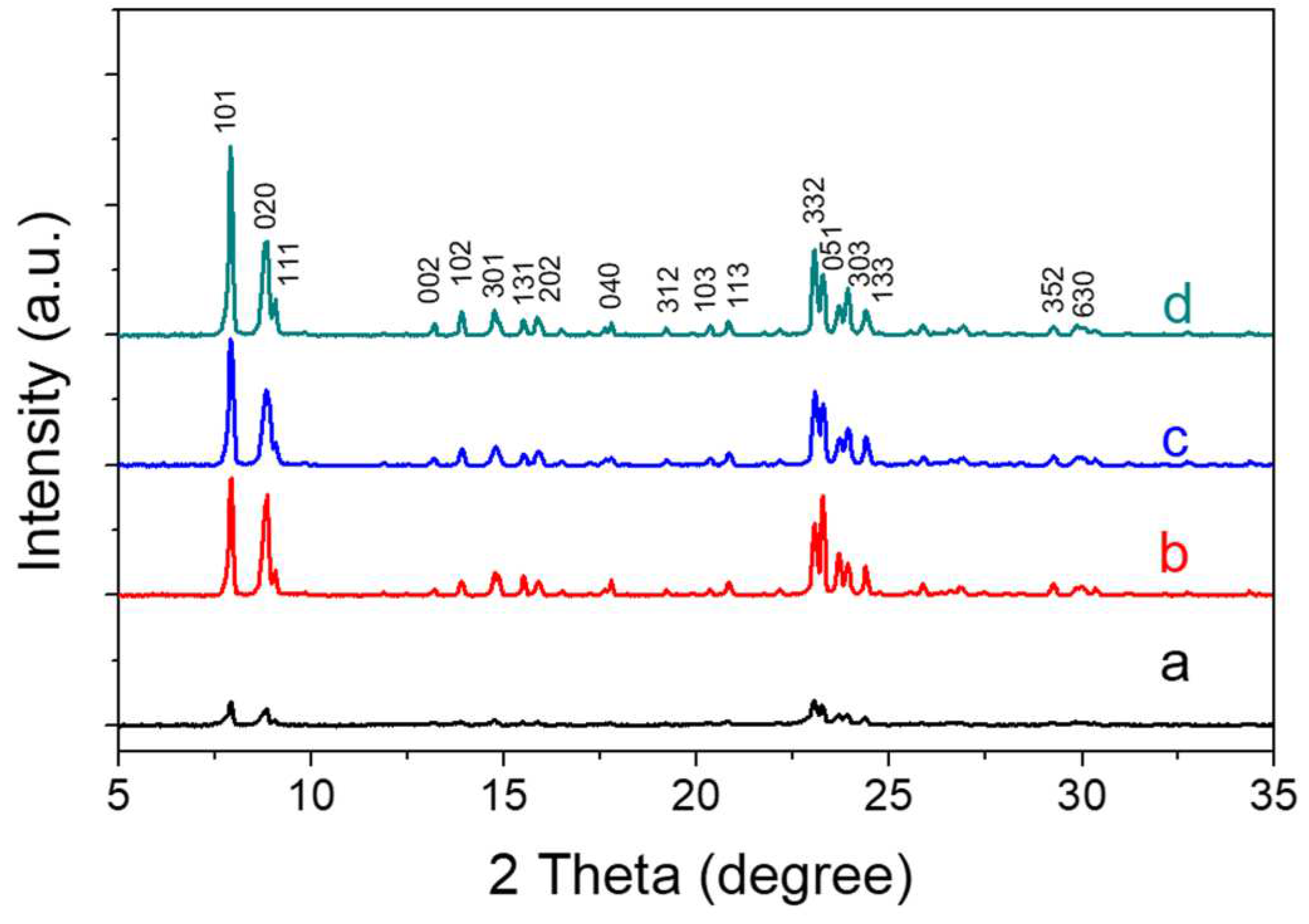
XRD patterns (
Figure 1) showed all the samples synthesized at different pH value had the pure MFI structure, but the TS-1-a sample showed weak peaks. This suggested that TS-1-a did not completely crystallize at such lower different pH value of 8.9, which was supported by the lower BET surface area and smaller microporous volume obtained by nitrogen adsorption characterization.
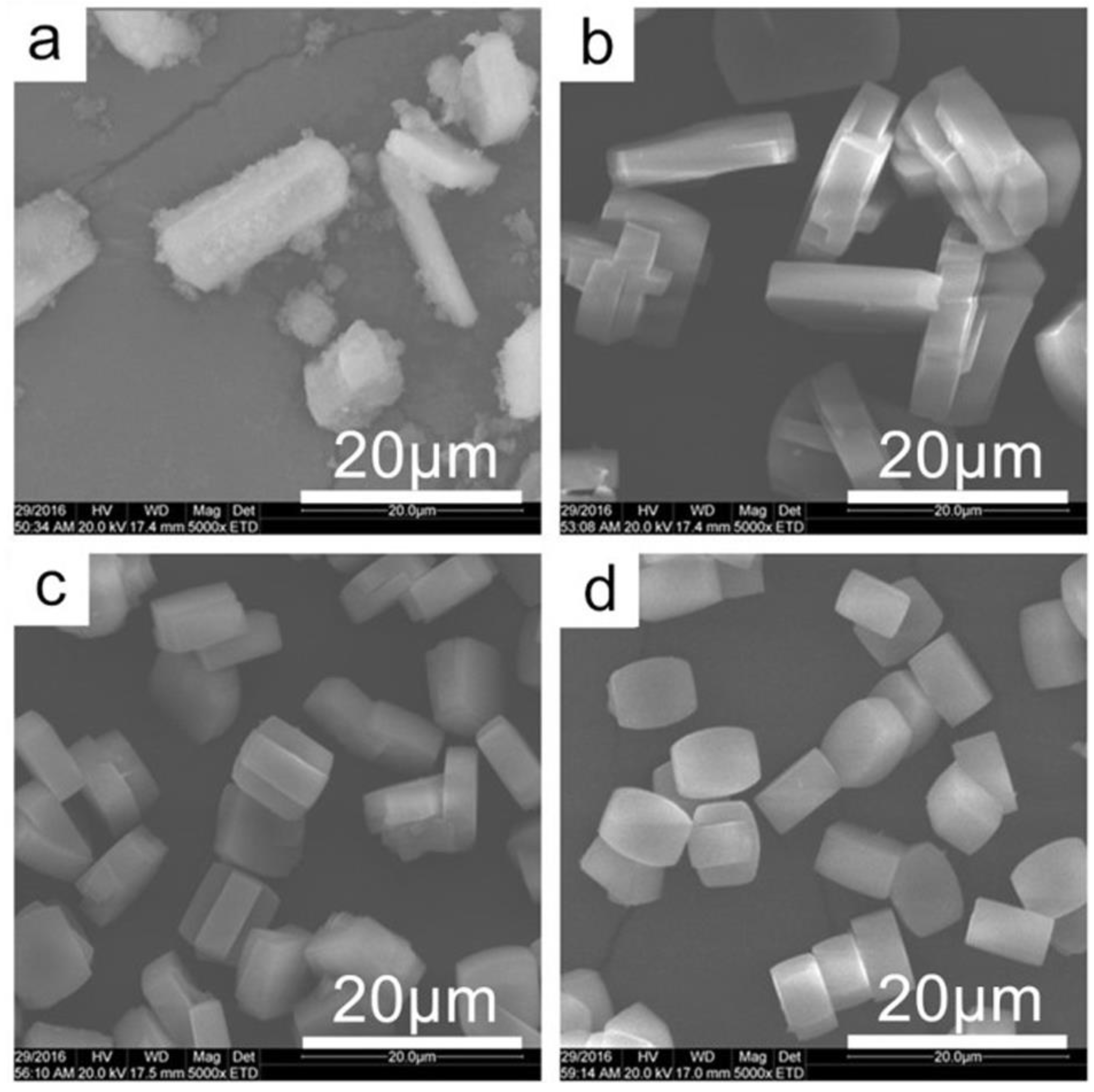
The SEM images of samples synthesized at different pH value are shown in
Figure 2. It revealed that sample of TS-1-a was composed of large crystals and amorphous small particles, confirming the incomplete crystallization at such lower pH value. This is also consistent with above-mentioned results of lower microporous volume in nitrogen adsorption characterization and lower intensity in XRD pattern. When the pH value was higher than 9.3, no amorphous particles were observed, indicating all the raw materials became crystalline TS-1 zeolites. And with the increase of pH value, it was found that the particle size of TS-1 zeolite decrease gradually.
For the catalytic applications of TS-1 zeolites, it has been proven that only tetrahedral Ti species, namely, framework Ti species, provide the catalytic activity [
20,
30,
44]. Other species including extraframework Ti species and anatase-like TiO
2 particles could not contribute to the catalytic performance.
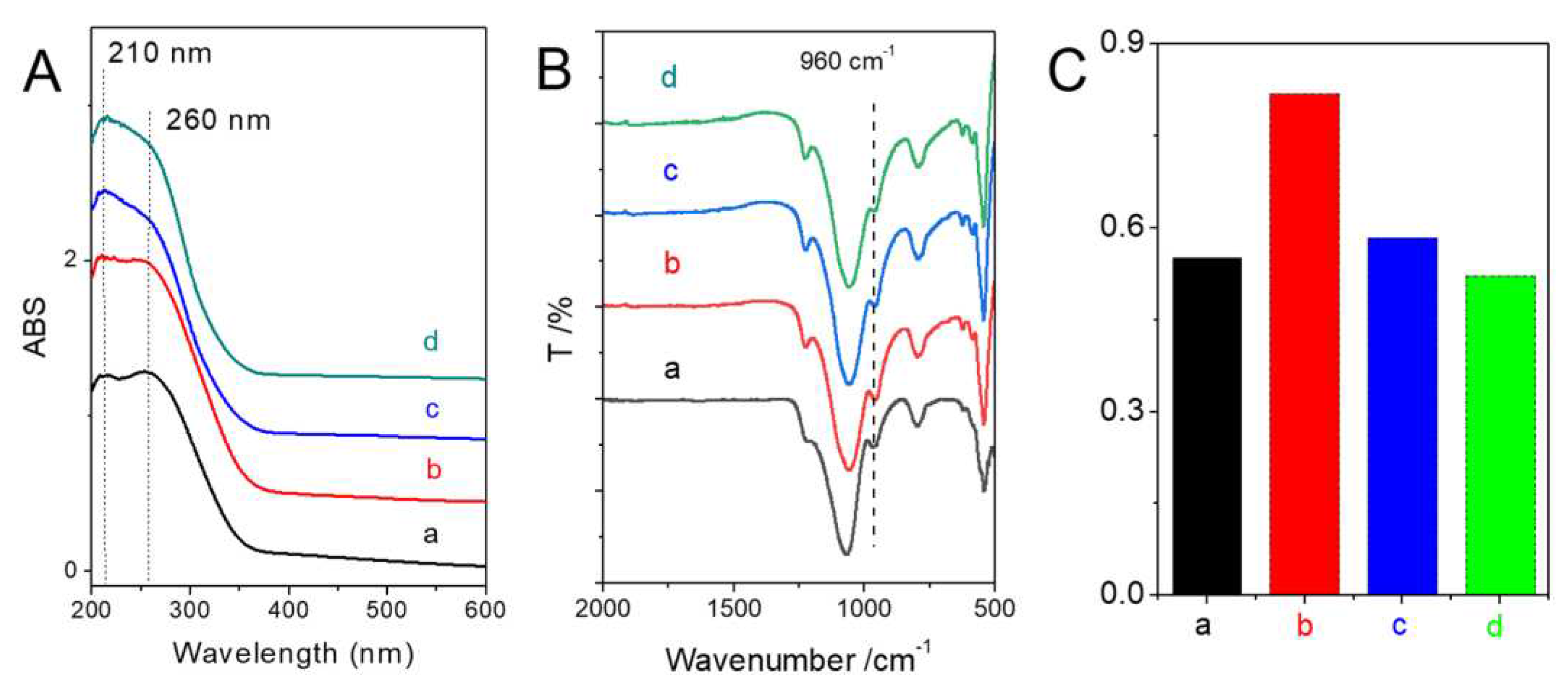
Diffuse reflectance UV-visible (DRUV-visible) and FTIR spectroscopy are powerful techniques to detect the coordination states of Ti species in TS-1. The absorbance peak at approximately 210 nm in the DRUV-visible spectrum and the band at 960 cm
−1 in FTIR have been widely accepted as a proof of the presence of framework Ti species [
20,
35,
45]. As shown in
Figure 3A, all the samples showed the band of 210 nm and 260 nm, indicating the presence of both framework Ti species and extra-framework Ti species. No obvious adsorption band at about 330 nm, implying no anatase-like TiO
2 particles present in these samples. FTIR spectra (
Figure 3B,C) showed that all the samples had the peak at 960 cm
−1, which was attributed to a stretching vibration mode of [SiO
4] perturbed by adjacent framework Ti species [
45,
46]. Importantly, the band intensity at 960 cm
−1 proportionally increases if the amount of the framework Ti in TS-1 increases. As shown in
Figure 3C, TS-1-b displayed the relatively high intensity of this characteristic band, suggesting the higher content of framework Ti species in this sample.
To evaluate the catalytic performance of the prepared TS- 1 zeolitic catalysts, epoxidation reactions of 1-hexene with hydrogen peroxide as oxidant were performed. As shown in
Table 2, as control sample, TS-1-Na0.02 synthesized with 0.02 of Na/Si molar ratio in the traditional synthetic system with pH about 12 showed almost no catalytic activity for 1-hexene epoxidation, indicating the great influence of Na
+ ions in the synthetic system. And it let we know why alkali metal cations should be strictly controlled in the traditional synthetic processes. After decreasing pH value, sample of TS-1-a without total crystallization gave a 7.8 % conversion of 1-hexene. TS-1-b showed the best conversion of 9.3 % among four synthesized samples. Although the crystal size samples of TS-1-c and TS-1-d were smaller than TS-1-b (
Figure 2), they showed the lower catalytic activities, mainly because of the slightly higher content of Na
+ in these two samples (
Table 1). Therefore, the catalytic activity was very susceptive to the present Na
+ cations in TS-1 zeolite. That is, a small amount of Na
+ cations present in the TS-1 zeolite would greatly decrease its activity, which was consistent with previous discoveries [
20,
26]. The crucial factor to synthesize active TS-1 catalyst was to control the content of Na cations in the final samples [
44]. As mentioned above in
Table 1, the sodium content in TS-1 products would increase with the increase of pH value. So, the key point to solve the problem of the adverse effects of Na
+ ions during synthesis processes was precisely to adjust the pH value as low as possible in the premise that the raw materials could crystallize to form crystals.
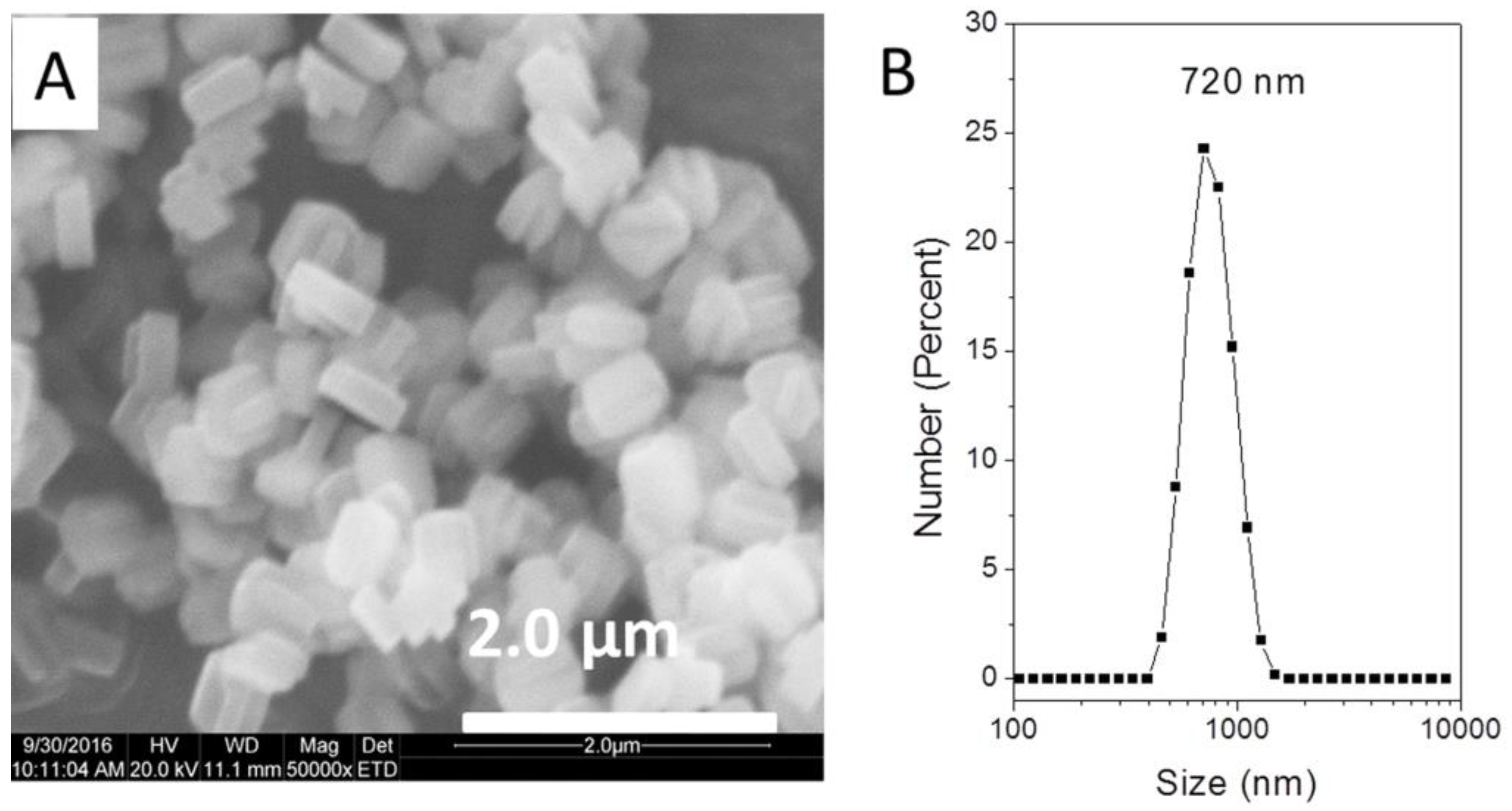
It was excited that active TS-1 zeolites were firstly synthesized by using common and cheap NaOH as base. This was a significant step to approach extremely low-cost synthesis of this important commercial catalyst. But the catalytic activity was still lower due to the large crystal size. Sample of TS-1-b with 9.3 % 1-hexene conversion had a crystal size of 15-18 μm in length and 4-5 μm in thickness, which was much larger than the sub-micrometer-sized TS-1 crystal size (100-500 nm) synthesized by traditional synthesis systems. Although increasing alkalinity could synthesize TS-1 zeolite with smaller particle sizes, the TS-1 zeolites prepared at high alkalinity have low catalytic activity using NaOH as the base to adjust alkalinity as above discussed. In order to reduce crystal size, a method of adding nano-sized silicalite-1 zeolites as seeds was employed based on the previous reported paper (detailed synthesis in the Materials and Methods section) [
30]. The catalytic performance could be improved to the level of current commercial TS-1 via decreasing the crystal sizes to less than 1 µm based on the above optimal synthetic condition of pH=9.3, as shown in
Table 2 and
Figure 4. Sample of TS-1-small with 720 nm sized crystal achieved similar conversion and higher epoxide selectivity to commercial TS-1 catalyst during catalytic oxidation of 1-hexene in the presence of H
2O
2, indicating the great potential for commercialization in consideration of its competence price and environmentally-benign issues.
4. Conclusions
In summary, for synthesizing Lewis acid zeolites, especially Ti-containing zeolites, the amounts of Na+ in the raw materials were strictly restricted in the traditional process because trace amounts would greatly decrease the catalytic activity of final zeolites. In this paper, we reported a method for synthesizing low-cost and high-performance TS-1 zeolites by directly using sodium hydroxide (NaOH) as the base. The key point was to solve the problem of the adverse effects of Na+ ions during synthesis processes via precisely adjusting the pH value as low as possible in the premise that the raw materials could crystallize to form crystals. TS-1 zeolites with 9.3 % 1-hexene conversion could be directly synthesized under pH value of about 9.3. Furthermore, assisted with nano-seeds, sub-micrometer-sized TS-1 zeolites with 23.0 % 1-hexene conversion and 95% epoxide selectivity could be obtained, which approached the similar catalytic performance to current commercial TS-1 zeolites, indicating great commercial potential. Moreover, the present report opens the possibility of preparing other high-performance Lewis acid zeolites (e.g. Sn, Zr, etc.) by using cheap, stable and non-volatility inorganic alkali.
Author Contributions
Conceptualization, J.W. and Y.W.; methodology, G.L., K.F., J.W. and T.L.; formal analysis, G.L., K.F., F.X.; investigation, G.L., K.F., F.X. Y.W. and T.L.; data curation, K.F., G.L.; writing—original draft preparation, G.L., K.F., F.X., J.W.; writing—review and editing, J.W. Y.W. and T.L.; supervision, J.W., Y.W. and T.L.; project administration, J.W. and Y.W.; funding acquisition, J.W. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52072190), and the Science, Education and Industry Integration Innovation Pilot Project from Qilu University of Technology (Shandong Academy of Sciences) (No. 2022JBZ02-04), and the Hundred Talents Programs in Chinese Academy of Science, and the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province.
Data Availability Statement
We would like to share our data upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz, K.A.; Agrawal, K.V.; Al Wahedi, Y.; Bhan, A.; Al Hashimi, S.; Terasaki, O.; Thommes, M.; Tsapatsis, M. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 2012, 336, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, P.; Yan, W.; Boronat, M.; Li, X.; Su, J.H.; Wang, J.; Li, Y.; Corma, A.; Xu, R.; Yu, J. Accelerated crystallization of zeolites via hydroxyl free radicals. Science 2016, 351, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; Basahel, S.N.; Al-Thabaiti, S.; Xu, W.Q.; Cho, H.J.; Fetisov, E.O.; Thyagarajan, R.; DeJaco, R.F.; Fan, W.; Mkhoyan, K.A.; Siepmann, J.I.; Tsapatsis, M. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef]
- Snyder, B.E.R.; Vanelderen, P.; Bols, M.L.; Hallaert, S.D.; Bottger, L.H.; Ungur, L.; Pierloot, K.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. The active site of low-temperature methane hydroxylation in iron-containing zeolites. Nature 2016, 536, 317–321. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; van Bokhoven, J.A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E. Clicking zeolites together A new mechanism to synthesize catalytic zeolites gives a previously unknown topology. Science 2023, 379, 236–237. [Google Scholar] [CrossRef]
- Li, J.; Gao, Z.R.; Lin, Q.F.; Liu, C.X.; Gao, F.X.; Lin, C.; Zhang, S.Y.; Deng, H.; Mayoral, A.; Fan, W.; Luo, S.; Chen, X.B.; He, H.; Camblor, M.A.; Chen, F.J.; Yu, J.H. A 3D extra-large-pore zeolite enabled by 1D-to-3D topotactic condensation of a chain silicate. Science 2023, 379, 283–287. [Google Scholar] [CrossRef]
- Tan, X.Y.; Robijns, S.; Thuer, R.; Ke, Q.L.; De Witte, N.; Lamaire, A.; Li, Y.; Aslam, I.; Van Havere, D.; Donckels, T.; Van Assche, T.; Van Speybroeck, V.; Dusselier, M.; Vankelecom, I. Truly combining the advantages of polymeric and zeolite membranes for gas separations. Science 2022, 378, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.G.; Dong, T.; Yang, S.Y.; Chen, H.; Yang, Z.Z.; Liu, W.M.; He, C.; Wu, P.; Tian, J.S.; Peng, Y.; Chu, X.F.; Wu, D.S.; An, T.C.; Wang, Y.; Dai, S. Intra-crystalline mesoporous zeolite encapsulation-derived thermally robust metal nanocatalyst in deep oxidation of light alkanes. Nature Commun. 2022, 13, 295. [Google Scholar] [CrossRef]
- Wei, Y.; Parmentier, T.E.; de Jong, K.P.; Zecevic, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Korde, A.; Min, B.; Kapaca, E.; Knio, O.; Nezam, I.; Wang, Z.Y.; Leisen, J.; Yin, X.Y.; Zhang, X.Y.; Sholl, D.S.; Zou, X.D.; Willhammar, T.; Jones, C.W.; Nair, S. Single-walled zeolitic nanotubes. Science 2022, 375, 62–66. [Google Scholar] [CrossRef]
- van der Graaff, W.N.P.; Li, G.; Mezari, B.; Pidko, E.A.; Hensen, E.J.M. Synthesis of Sn-Beta with Exclusive and High Framework Sn Content. ChemCatChem 2015, 7, 1152–1160. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Odermatt, C.; Romero, K.; Prasomsri, T.; Román-Leshkov, Y. Solid Lewis Acids Catalyze the Carbon–Carbon Coupling between Carbohydrates and Formaldehyde. ACS Catal. 2015, 5, 972–977. [Google Scholar] [CrossRef]
- Guo, Q.; Fan, F.; Pidko, E.A.; van der Graaff, W.N.; Feng, Z.; Li, C.; Hensen, E.J. Highly active and recyclable Sn-MWW zeolite catalyst for sugar conversion to methyl lactate and lactic acid. ChemSusChem 2013, 6, 1352–1356. [Google Scholar] [CrossRef]
- Hammond, C.; Conrad, S.; Hermans, I. Simple and scalable preparation of highly active Lewis acidic Sn-beta. Angew. Chem. Int. Ed. 2012, 51, 11736–11739. [Google Scholar] [CrossRef]
- Gunther, W.R.; Wang, Y.; Ji, Y.; Michaelis, V.K.; Hunt, S.T.; Griffin, R.G.; Roman-Leshkov, Y. Sn-Beta zeolites with borate salts catalyse the epimerization of carbohydrates via an intramolecular carbon shift. Nat. Commun. 2012, 3, 1109. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Duan, R.G.; Yokoi, T.; Wu, P.; Kubota, Y.; Tatsumi, T. Synthesis, Crystallization Mechanism, and Catalytic Properties of Titanium-Rich TS-1 Free of Extraframework Titanium Species. J. Am. Chem. Soc. 2008, 9, 10150–10164. [Google Scholar] [CrossRef] [PubMed]
- Reichinger, M.; Schmidt, W.; Berg, M.W.E.V.D.; Aerts, A.; Martens, J.A.; Kirschhock, C.E.A.; Gies, H.; Grünert, W. Alkene epoxidation with mesoporous materials assembled from TS-1 seeds – Is there a hierarchical pore system? J. Catal. 2010, 269, 367–375. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, X.Y.; Tian, G.; Li, Y.; Rooke, J.C.; Zhu, G.-S.; Qiu, S.L.; Yang, X.Y.; Su, B.L. Highly Stable and Reusable Multimodal Zeolite TS-1 Based Catalysts with Hierarchically Interconnected Three-Level Micro-Meso-Macroporous Structure. Angew. Chem. Int. Ed. 2011, 50, 11156–11161. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Ahn, W.-S.; Ryoo, R. MFI Titanosilicate Nanosheets with Single-Unit-Cell Thickness as an Oxidation Catalyst Using Peroxides. ACS Catal. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Huang, D.-G.; Zhang, X.; Liu, T.-W.; Huang, C.; Chen, B.-H.; Luo, C.-W.; Ruckenstein, E.; Chao, Z.-S. Synthesis of High-Performanced Titanium Silicalite-1 Zeolite at Very Low Usage of Tetrapropyl Ammonium Hydroxide. Ind. Eng. Chem. Res. 2013, 52, 3762–3772. [Google Scholar] [CrossRef]
- Liu, M.; Wei, H.J.; Li, B.J.; Song, L.Y.; Zhao, S.Z.; Niu, C.C.; Jia, C.F.; Wang, X.Y.; Wen, Y.Q. Green and efficient preparation of hollow titanium silicalite-1 by using recycled mother liquid. Chem. Eng. J. 2018, 331, 194–202. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P. Recent Progresses in Titanosilicates. Chinese J. Chem. 2017, 35, 836–844. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhai, Y.; Zhang, X.B.; Wang, F.M.; Lv, G.J.; Rosine, A.; Li, M.Y.; Zhang, Q.; Liu, Y.K. (NH4)(2)SO4-assisted synthesis of thin-walled Ti-rich hollow titanium silicalite-1 zeolite for 1-hexene epoxidation. Microporous Mesoporous Mater. 2022, 331, 111655. [Google Scholar] [CrossRef]
- Pan, D.; Kong, L.T.; Zhang, H.B.; Zhang, Y.H.; Tang, Y. TS-1 Synthesis via Subcrystal Aggregation: Construction of Highly Active Hydrogen-Bonded Titanium Species for Alkene Epoxidation. ACS Appl. Mater. Interfaces 2023, 15, 28125–28134. [Google Scholar] [CrossRef]
- Li, C.G.; Lu, Y.; Wu, H.; Wu, P.; He, M. A hierarchically core/shell-structured titanosilicate with multiple mesopore systems for highly efficient epoxidation of alkenes. Chem. Commun. 2015, 51, 14905–14908. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, T.; Meng, C.; Guo, X.; Song, C. Enhanced Catalytic Performance of Titanium Silicalite-1 in Tuning the Crystal Size in the Range 1200–200 nm in a Tetrapropylammonium Bromide System. ChemCatChem 2015, 7, 2660–2668. [Google Scholar] [CrossRef]
- Zhou, J.; Hua, Z.; Cui, X.; Ye, Z.; Cui, F.; Shi, J. Hierarchical mesoporous TS-1 zeolite: A highly active and extraordinarily stable catalyst for the selective oxidation of 2,3,6-trimethylphenol. Chem. Commun. 2010, 46, 4994–4996. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.G.; Bellussi, G.; Romano, U. Synthesis of Propylene Oxide from Propylene and Hydrogen Peroxide Catalyzed by Titanium Silicalite. J. Catal. 1991, 129, 159–167. [Google Scholar] [CrossRef]
- Thangaraj, A.; Ratnasamy, P. Catalytic properties of crystalline titanium silicalites III Ammoximation of cyclohexanone. J. Catal. 1991, 131, 394–400. [Google Scholar] [CrossRef]
- Lin, M.; Xia, C.; Zhu, B.; Li, H.; Shu, X. Green and efficient epoxidation of propylene with hydrogen peroxide (HPPO process) catalyzed by hollow TS-1 zeolite: A 1. 0 kt/a pilot-scale study. Chem. Eng. J. 2016, 295, 370–375. [Google Scholar]
- Du, S.; Chen, X.; Sun, Q.; Wang, N.; Jia, M.; Valtchev, V.; Yu, J. A non-chemically selective top-down approach towards the preparation of hierarchical TS-1 zeolites with improved oxidative desulfurization catalytic performance. Chem. Commun. 2016, 52, 3580–3583. [Google Scholar] [CrossRef]
- Khouw, C.B.; Davis, M.E. Catalytic Activity of Titanium Silicates Synthesized in the Presence of Alkali-Metal and Alkaline-Earth Ions. J. Catal. 1995, 151, 77–86. [Google Scholar] [CrossRef]
- Fu, K.; Li, G.; Xu, F.; Dai, T.; Su, W.; Wang, H.; Li, T.; Wang, Y.; Wang, J. Nano-Cavities within Nano-Zeolites: The Influencing Factors of the Fabricating Process on Their Catalytic Activities. Nanomaterials 2023, 13, 1923. [Google Scholar] [CrossRef]
- Zuo, Y.; Song, W.C.; Dai, C.Y.; He, Y.P.; Wang, M.L.; Wang, X.S.; Guo, X.W. Modification of small-crystal titanium silicalite-1 with organic bases: Recrystallization and catalytic properties in the hydroxylation of phenol. Appl. Catal. A-Gen. 2013, 453, 272–279. [Google Scholar] [CrossRef]
- Wang, X.S.; Guo, X.W.; Li, G. Synthesis of titanium silicalite (TS-1) from the TPABr system and its catalytic properties for epoxidation of propylene. Catal. Today 2002, 74, 65–75. [Google Scholar] [CrossRef]
- Zhao, Q.; Bao, X.H.; Han, X.W.; Liu, X.M.; Tan, D.L.; Lin, L.W.; Guo, X.W.; Li, G.; Wang, X.S. Studies on the crystallization process of titanium silicalite-1 (TS-1) synthesized using tetrapropylammonium bromide as a template. Mater. Chem. Phys. 2000, 66, 41–50. [Google Scholar] [CrossRef]
- Wang, X.S.; Guo, X.W. Synthesis, characterization and catalytic properties of low cost titanium silicalite. Catal. Today 1999, 51, 177–186. [Google Scholar] [CrossRef]
- Li, G.; Guo, X.W.; Wang, X.S.; Zhao, Q.; Bao, X.H.; Han, X.W.; Lin, L.W. Synthesis of titanium silicalites in different template systems and their catalytic performance. Appl. Catal. A-Gen. 1999, 185, 11–18. [Google Scholar]
- Millini, R.; Massara, E.P.; Perego, G.; Bellussi, G. Framework composition of titanium silicalite. J. Catal. 1992, 137, 497–503. [Google Scholar] [CrossRef]
- Wang, J.G.; Wang, Y.B.; Tatsumi, T.; Zhao, Y.L. Anionic polymer as a quasi-neutral medium for low-cost synthesis of titanosilicate molecular sieves in the presence of high-concentration alkali metal ions. J. Catal. 2016, 338, 321–328. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhao, Y.L.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. High-Performance Titanosilicate Catalyst Obtained through Combination of Liquid-Phase and Solid-Phase Transformation Mechanisms. ChemCatchem 2014, 6, 2719–2726. [Google Scholar] [CrossRef]
- Ricchiardi, G.; Damin, A.; Bordiga, S.; Lamberti, C.; Spano, G.; Rivetti, F.; Zecchina, A. Vibrational Structure of Titanium Silicate Catalysts. A Spectroscopic and Theoretical Study. J. Am. Chem. Soc. 2001, 123, 11409–11419. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).