Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
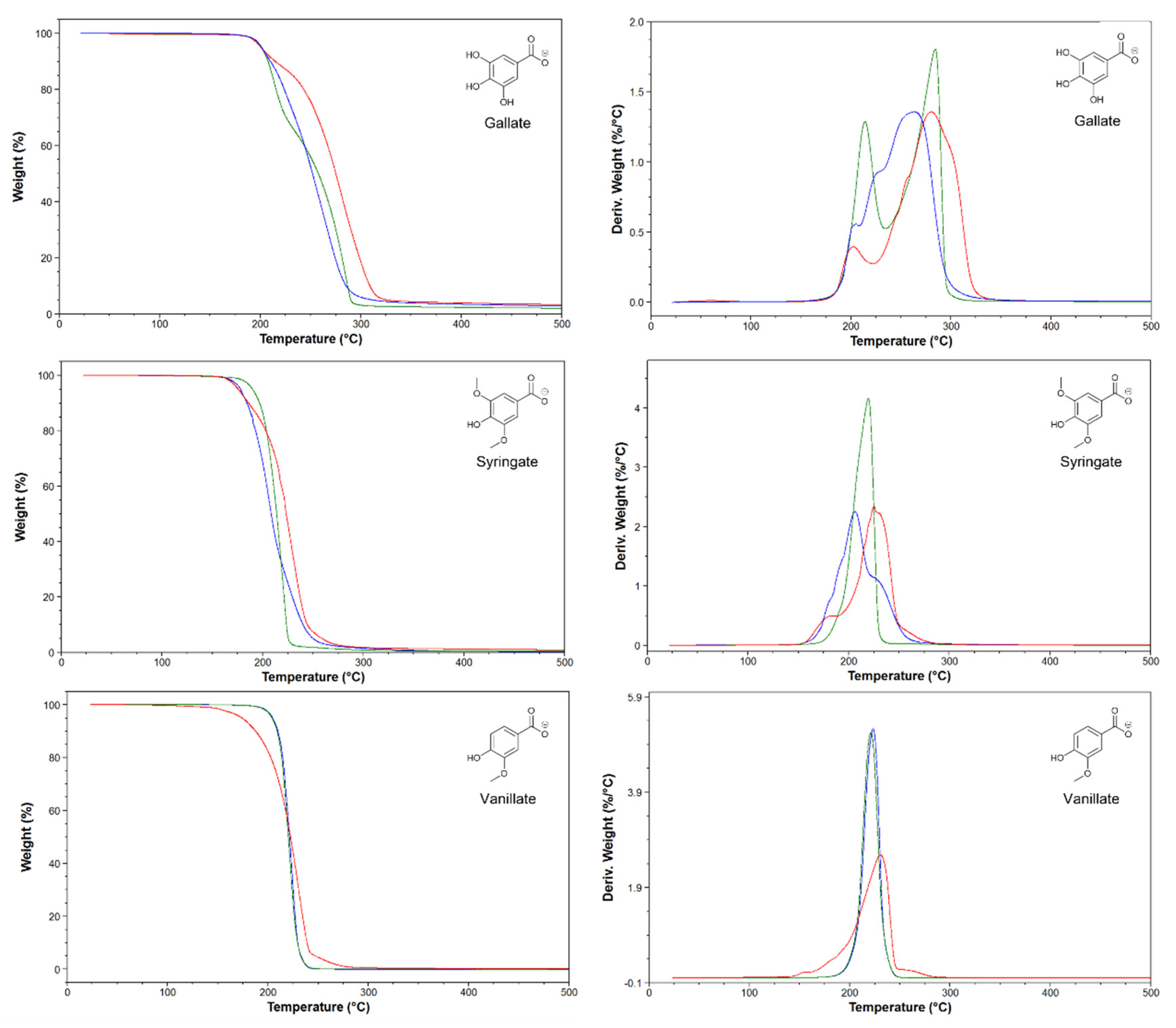
2.1. Thermal characterization
2.2. Electrochemical characterization
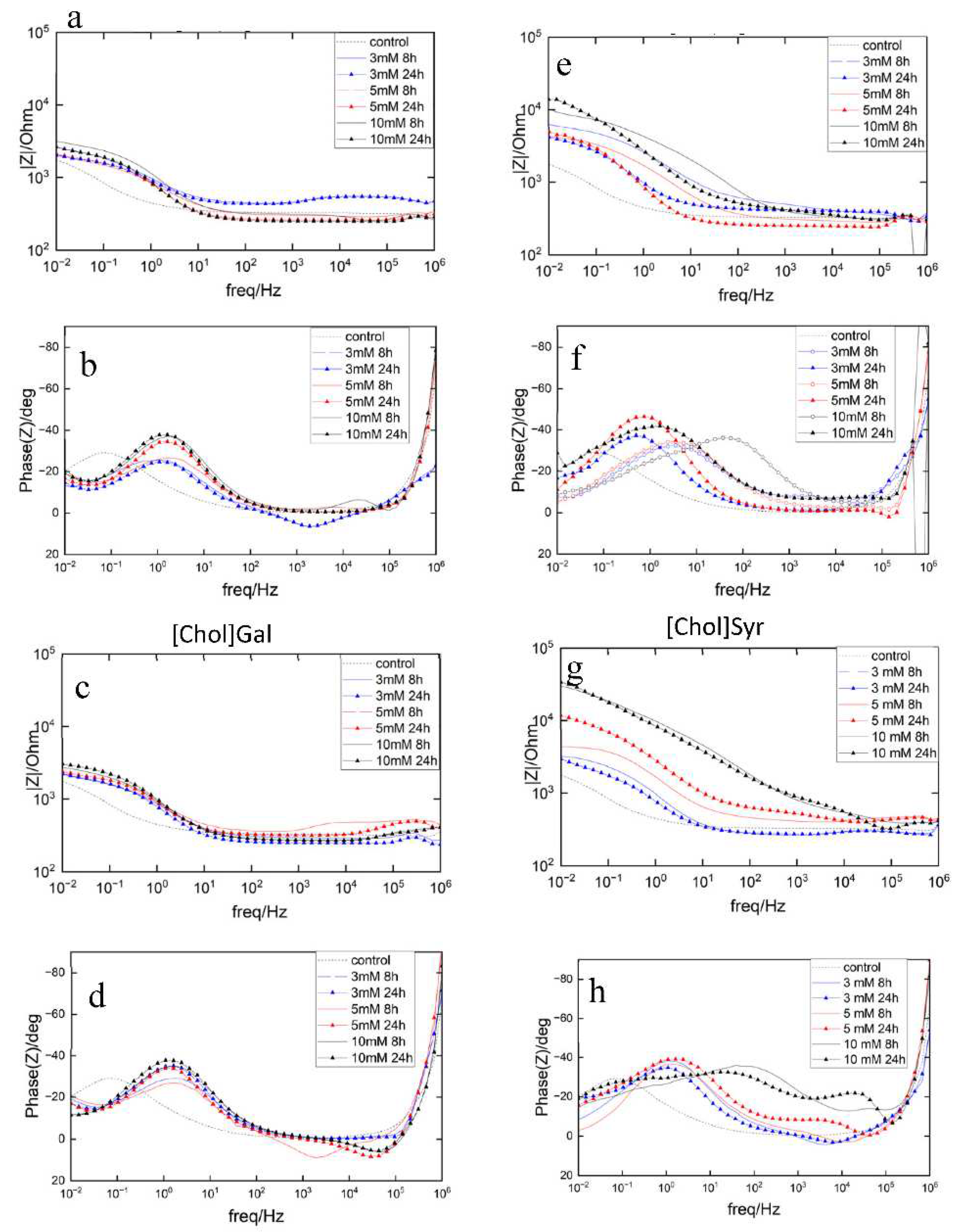
2.2.1. Potentiostatic Electrochemical Impedance Spectroscopy (PEIS)
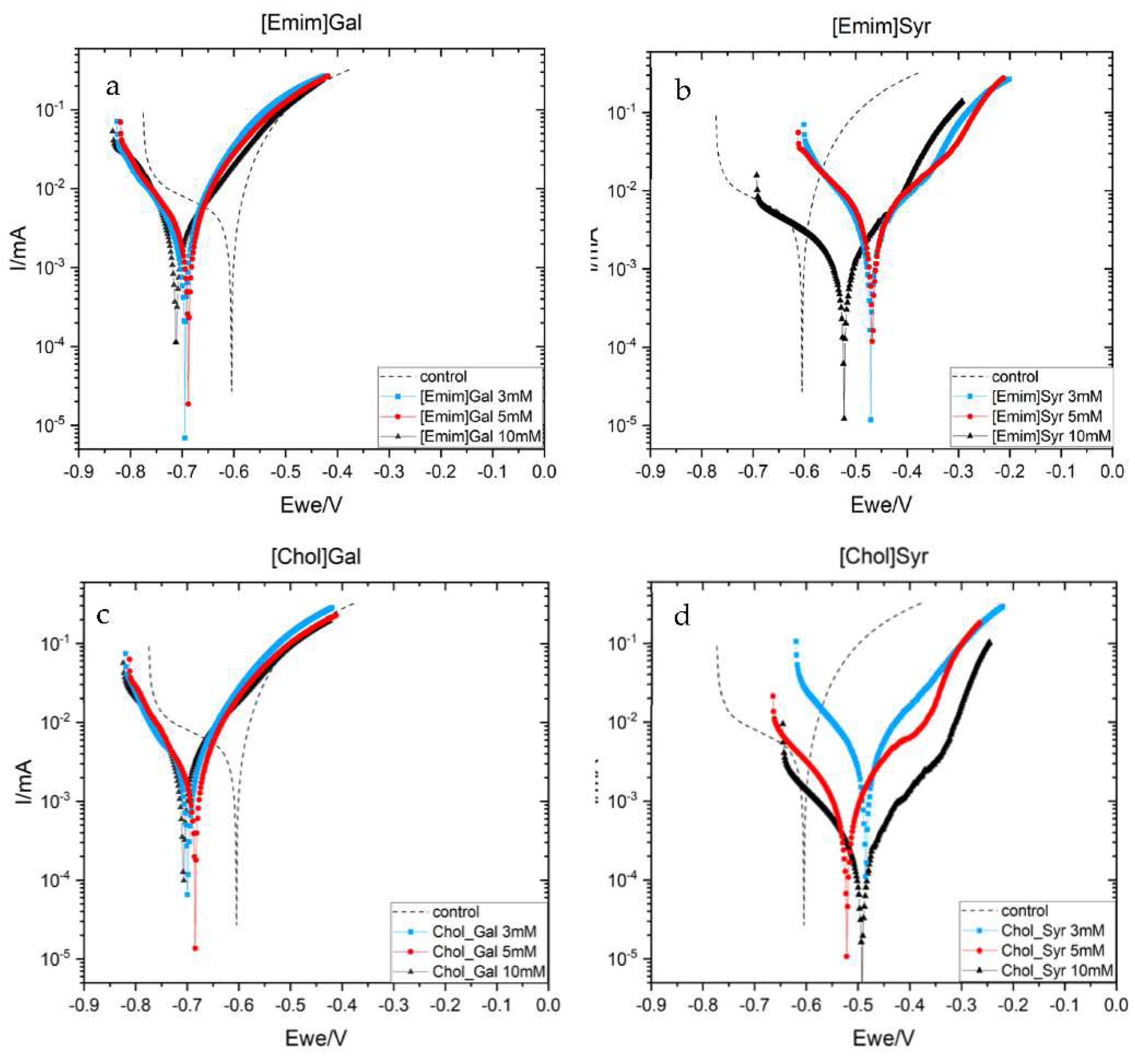
2.2.2. Cyclic Potentiodynamin Polarization (CPP)
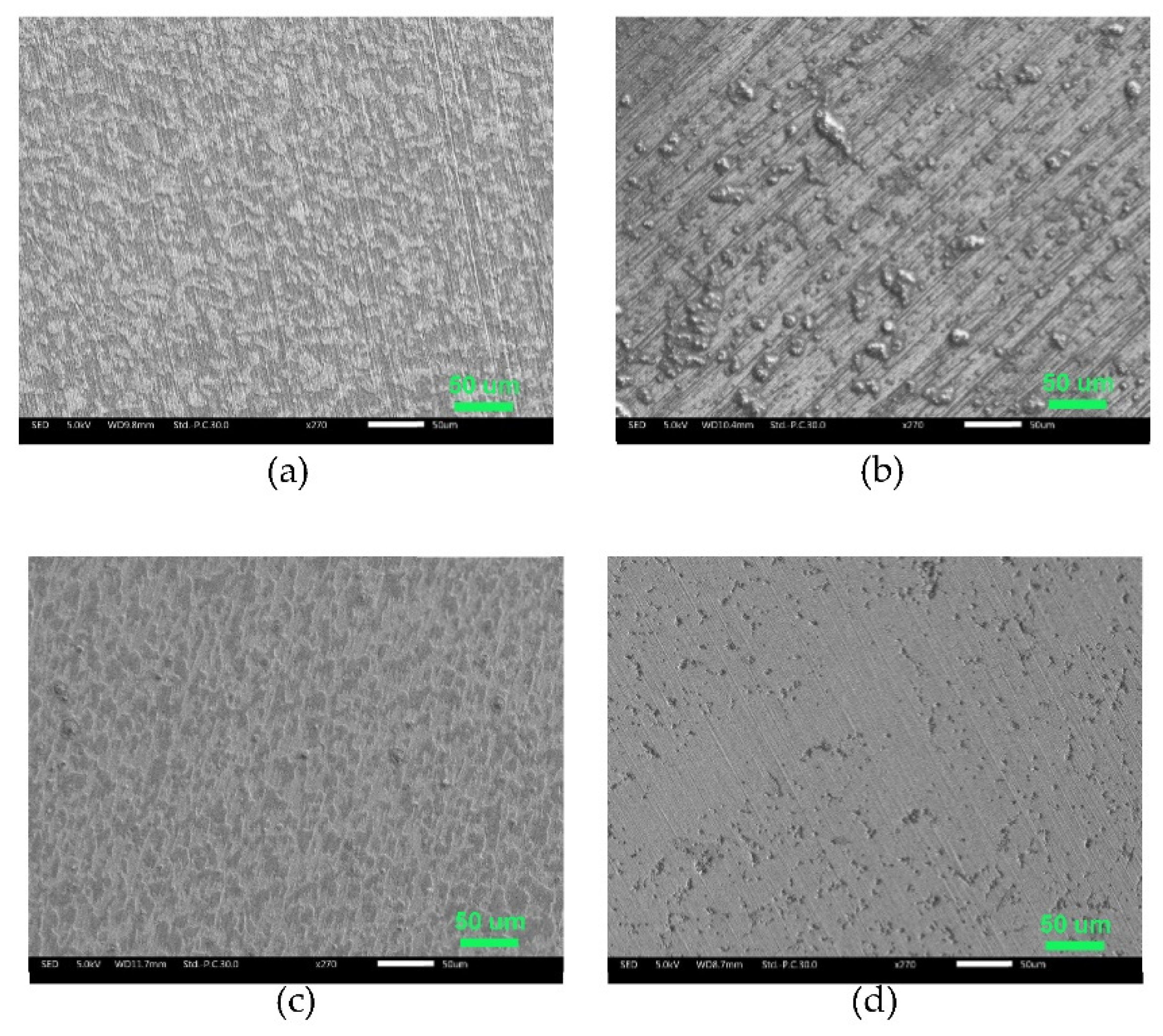
2.3. Surface characterization
3. Materials and Methods
3.1. Materials
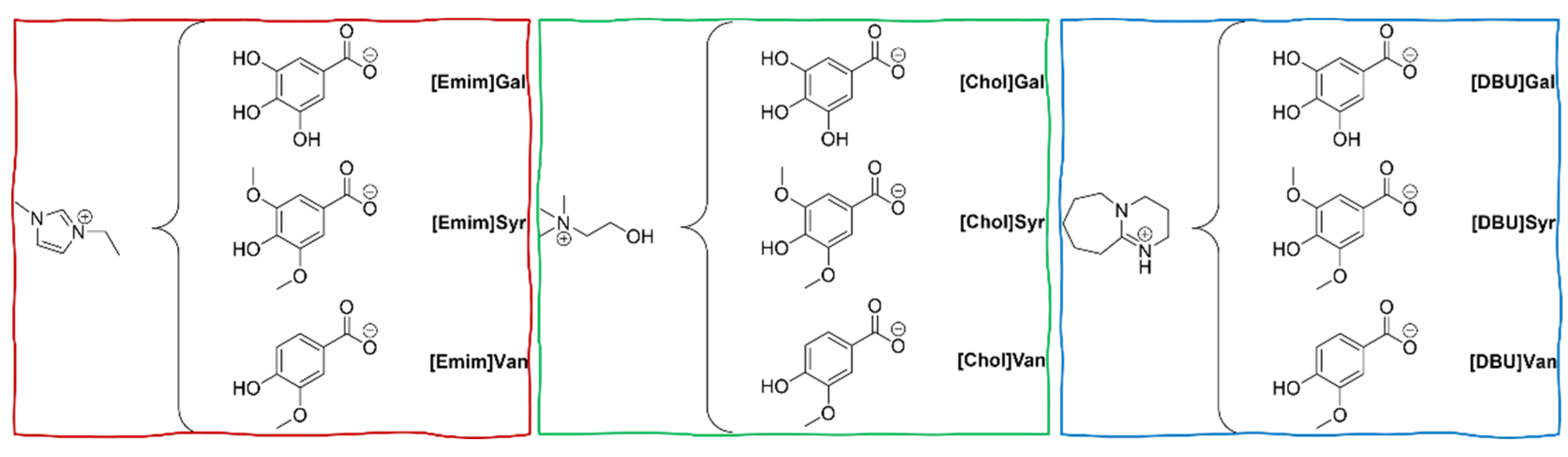
3.2. Synthetic pathways
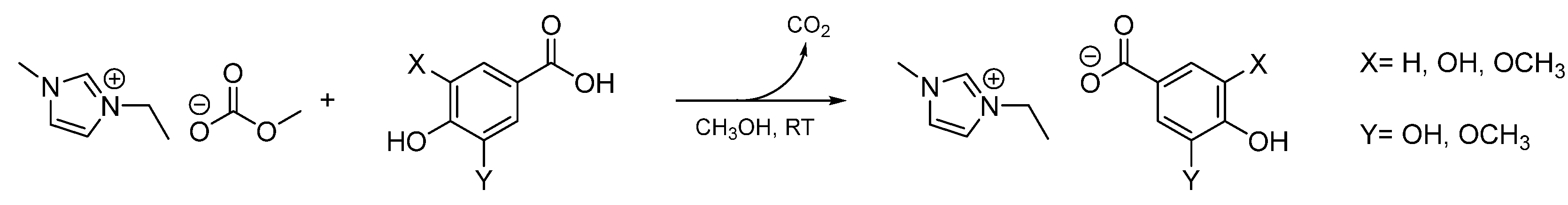
3.2.1. General procedure for the synthesis of imidazolium lignin-based ILs
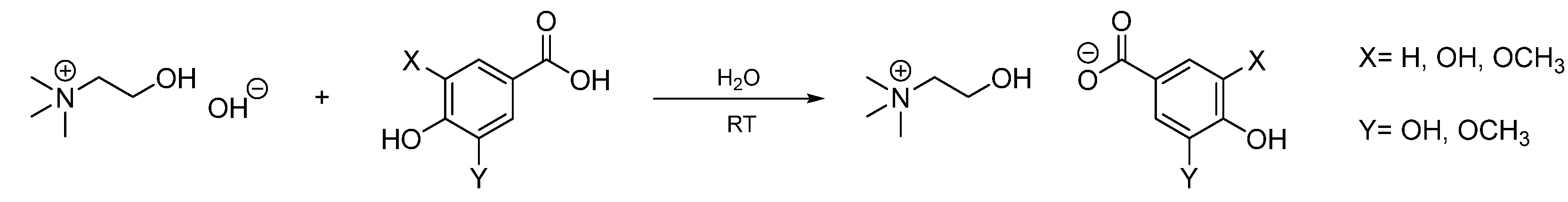
3.2.2. General procedure for the synthesis of cholinium lignin-based ILs
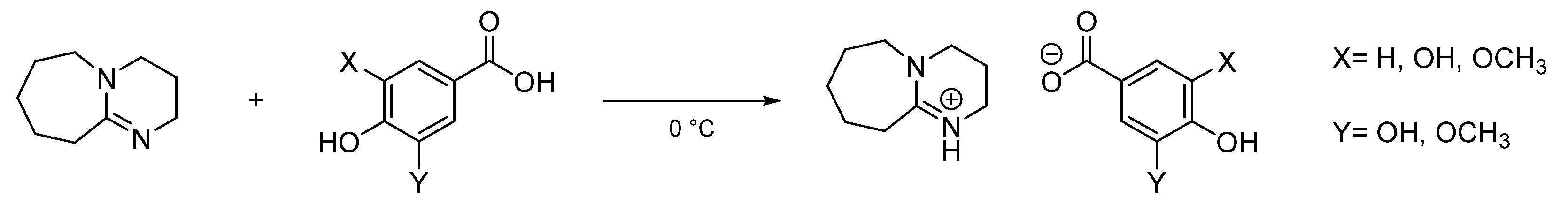
3.2.3. General procedure for the synthesis of protic lignin-based ILs
3.3. Characterization methods
3.3.1. Thermal Gravimetric Analysis (TGA)
3.3.2. Differential Scanning Calorimetry (DSC)
3.3.2. PEIS and CPP
3.3.3. Scanning electron microscopy (SEM) and Energy-dispersive X-ray spectroscopy (EDS)
3.3.4. Optical microscope
3.3.5. Optical Profilometer
4. Conclusions
Supplementary Materials
References
- C. Verma, E. E. Ebenso, M. A. Quraishi, and C. M. Hussain, ‘Recent developments in sustainable corrosion inhibitors: design, performance and industrial scale applications’, Mater Adv, vol. 2, no. 12, pp. 3806–3850, 2021. [CrossRef]
- A. W. Ma, Sh. Ammar, S. S. A. Kumar, K. Ramesh, and S. Ramesh, ‘A concise review on corrosion inhibitors: types, mechanisms and electrochemical evaluation studies’, J Coat Technol Res, vol. 19, no. 1, pp. 241–268, Jan. 2022. [CrossRef]
- S. Marzorati, L. Verotta, and S. P. Trasatti, ‘Green corrosion inhibitors from natural sources and biomass wastes’, Molecules, vol. 24, no. 1, 2019. [CrossRef]
- H. Wei, B. Heidarshenas, L. Zhou, G. Hussain, Q. Li, and K. (Ken) Ostrikov, ‘Green inhibitors for steel corrosion in acidic environment: state of art’, Materials Today Sustainability, vol. 10. Elsevier Ltd, Dec. 01, 2020. [CrossRef]
- P. A. Thomas and B. B. Marvey, ‘Room temperature ionic liquids as green solvent alternatives in the metathesis of oleochemical feedstocks’, Molecules, vol. 21, no. 2. MDPI AG, Feb. 01, 2016. [CrossRef]
- V. Zullo, A. Iuliano, and L. Guazzelli, ‘Sugar-based ionic liquids: Multifaceted challenges and intriguing potential’, Molecules, vol. 26, no. 7. MDPI AG, Apr. 01, 2021. [CrossRef]
- M. L. Picchio et al., ‘Natural Deep Eutectic Solvents Based on Choline Chloride and Phenolic Compounds as Efficient Bioadhesives and Corrosion Protectors’, ACS Sustainable Chemistry & Engineering, vol. 10, no. 25, pp. 8135–8142, Jun. 2022. [CrossRef]
- M. Goyal, S. Kumar, I. Bahadur, C. Verma, and E. E. Ebenso, ‘Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review’, J Mol Liq, vol. 256, no. March 2021, pp. 565–573, Apr. 2018. [CrossRef]
- B. E. Brycki, I. H. Kowalczyk, A. Szulc, O. Kaczerewska, and M. Pakiet, ‘Organic Corrosion Inhibitors’, in Corrosion Inhibitors, Principles and Recent Applications, InTech, 2018, p. 13. [CrossRef]
- S. Malinowski, M. Wróbel, and A. Woszuk, ‘Quantum chemical analysis of the corrosion inhibition potential by aliphatic amines’, Materials, vol. 14, no. 20, Oct. 2021. [CrossRef]
- T. E. Sintra, D. O. Abranches, J. Benfica, B. P. Soares, S. P. M. Ventura, and J. A. P. Coutinho, ‘Cholinium-based ionic liquids as bioinspired hydrotropes to tackle solubility challenges in drug formulation’, European Journal of Pharmaceutics and Biopharmaceutics, vol. 164, pp. 86–92, Jul. 2021. [CrossRef]
- Sales et al., ‘Selection of hydrotropes for enhancing the solubility of artemisinin in aqueous solutions’, Fluid Phase Equilib, vol. 562, p. 113556, Nov. 2022. [CrossRef]
- P. Kwolek, K. Dychtoń, B. Kościelniak, A. Obłój, A. Podborska, and M. Wojnicki, ‘Gallic Acid as a Potential Green Corrosion Inhibitor for Aluminum in Acidic Solution’, Metals (Basel), vol. 12, no. 2, Feb. 2022. [CrossRef]
- B. Obot and A. Madhankumar, ‘Enhanced corrosion inhibition effect of tannic acid in the presence of gallic acid at mild steel/HCl acid solution interface’, Journal of Industrial and Engineering Chemistry, vol. 25, pp. 105–111, May 2015. [CrossRef]
- Y. Cao and T. Mu, ‘Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis’, Industrial & Engineering Chemistry Research, vol. 53, no. 20, pp. 8651–8664, May 2014. [CrossRef]
- Y. J. Tan, S. Bailey, and B. Kinsella, ‘An investigation of the formation and destruction of corrosion inhibitor films using electrochemical impedance spectroscopy (EIS)’, Corros Sci, vol. 38, no. 9, pp. 1545–1561, Sep. 1996. [CrossRef]
- E. Fazary, M. Taha, and Y. H. Ju, ‘Iron complexation studies of gallic acid’, J Chem Eng Data, vol. 54, no. 1, pp. 35–42, Jan. 2009. [CrossRef]
- Mero et al., ‘Influence of the cation partner on levulinate ionic liquids properties’, J Mol Liq, vol. 354, p. 118850, May 2022. [CrossRef]
- T. E. Sintra et al., ‘Enhancing the Antioxidant Characteristics of Phenolic Acids by Their Conversion into Cholinium Salts’, ACS Sustainable Chemistry & Engineering, vol. 3, no. 10, pp. 2558–2565, Sep. 2015. [CrossRef]
- Sales et al., ‘Selection of hydrotropes for enhancing the solubility of artemisinin in aqueous solutions’, Fluid Phase Equilib, vol. 562, p. 113556, Nov. 2022. [CrossRef]

| TGA | DSC | ||||
|---|---|---|---|---|---|
| ILs | Tstart (°C) | Tonset (°C) | Tpeak (°C) | Tm (°C) | Tg (°C) |
| [Emim]Gal | 201.0 | 189.8 253.5 |
202.4 280.33 |
- | - |
| [Emim]Syr | 175.8 | 204.2 | 225.21 | 148.0 | 5.99 |
| [Emim]Van | 174.0 | 204.1 | 230.69 | -1.17 | |
| [Chol]Gal | 201.5 | 215.4 | 263.0 | 135.8 | 22.29 |
| [Chol]Syr | 178.2 | 186.5 | 206.4 | 157.4 | 18.63 |
| [Chol]Van | 204.7 | 212.14 | 223.4 | 181.6 | - |
| [DBU]Gal | 201.0 | 201.03 264.91 |
214.2 284.3 |
76.37a | - |
| [DBU]Syr | 190.0 | 202.3 | 219.7 | - | - |
| [DBU]Van | 204.1 | 210.9 | 221.4 | - | - |
| Solution | Concentration (mM) |
Ecorr (mV) |
icorr (µA/cm2) |
IE (%) |
|---|---|---|---|---|
| control | 100 | -604 | 1.457 | - |
| [Emim]Gal | 3 | -695 | 1.032 | 29 |
| 5 | -689 | 0.923 | 37 | |
| 10 | -712 | 0.830 | 43 | |
| [Emim]Syr | 3 | -471 | 1.170 | 20 |
| 5 | -468 | 0.974 | 33 | |
| 10 | -522 | 0.312 | 79 | |
| [Chol]Gal | 3 | -696 | 1.051 | 28 |
| 5 | -684 | 0.733 | 49 | |
| 10 | -706 | 0.902 | 38 | |
| [Chol]Syr | 3 | -486 | 1.151 | 21 |
| 5 | -578 | 0.227 | 85 | |
| 10 | -491 | 0.066 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





