Submitted:
06 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. CO2 Capture
2.1. CO2 capture technologies
2.2. Criteria for Selecting CO2 Sorbent Material
- Adsorption capacity for CO2:
- Selectivity for CO2:
- Adsorption and desorption kinetics:
- Mechanical strength of sorbent particles:
- Chemical stability/tolerance towards impurities:
- Regeneration of sorbents:
- Sorbent costs:
2.3. Liquid amine for CO2 capture
| Criteria | Alkanolamines | Sterically hindered Amines | ||
| Primary | Secondary | Tertiary | ||
| Examples | Monoethanolamine (MEA) | Diethanolamine (DEA) | N-methyldiethanolamine (MEDA) | 2-amino-2-methyl-1- propanol (AMP) |
| Structure | 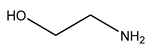 |
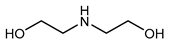 |
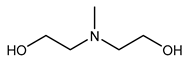 |
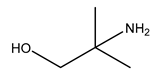 |
|
CO2 loading at 59.85 °C (mol CO2/mol amine) |
0.426 (MEA 30 wt %) [33] |
0.404 (DEA 30 wt %) [33] |
0.141 (TEA 30 wt %) [33] |
0.466 (AMP 30 wt %) [33] |
| Regeneration efficiency (%) at 90 °C | 75.5 [34] |
84.89 [34] |
95.09 [34] |
|
| Advantages | ● Inexpensive solvent ● Reversible absorption ● High selectively (between acid and other gases) ● Reacts with CO2 more rapidly [33] |
● Inexpensive solvent ● Reversible absorption ● High selectively (between acid and other gases) ● Reacts with CO2 more rapidly [33] |
● Inexpensive solvent ● Reversible absorption ● High selectively (between acid and other gases) ● High CO2 absorption capacity ● Requires low regeneration energy [33] |
● High CO2 absorption capacity ● Requires low regeneration energy [34] |
| Disadvantages | ● Lower CO2 absorption capacity ● Requires high regeneration energy ● Oxidative degradation occurs in the presence of other gas components ● Corrosive ● High capital costs [33] |
● Lower CO2 absorption capacity ● Requires high regeneration energy ● Oxidative degradation occurs in the presence of other gas components ● Corrosive ● High capital costs [33] |
● Reaction rate with CO2 is low compared to MEA and DEA ● Corrosive ● High capital costs [33] |
● Low reaction rate [34] |
2.4. Comparison between major non-carbonaceous solid sorbents for CO2 capture and importance of silica materials
3. CO2 separation methods
4. CO2 adsorption using mesoporous silica materials (Physisorbents)
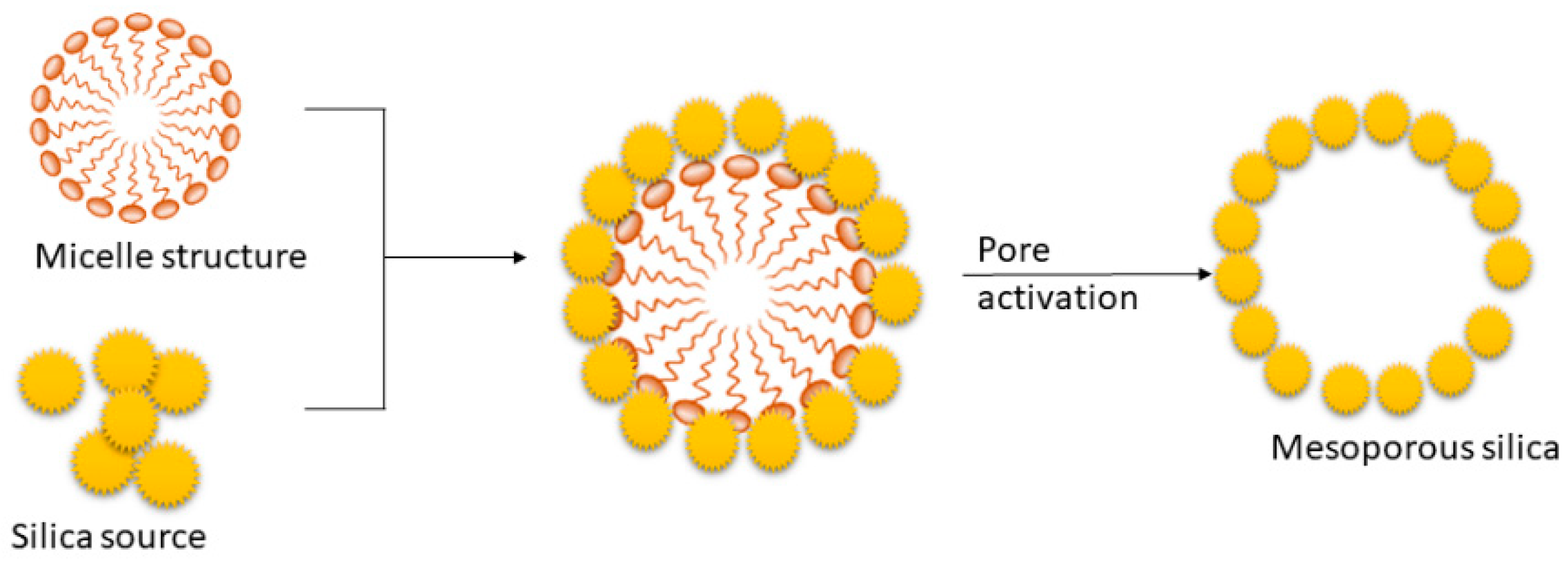
4.1. Description about mesoporous silica materials
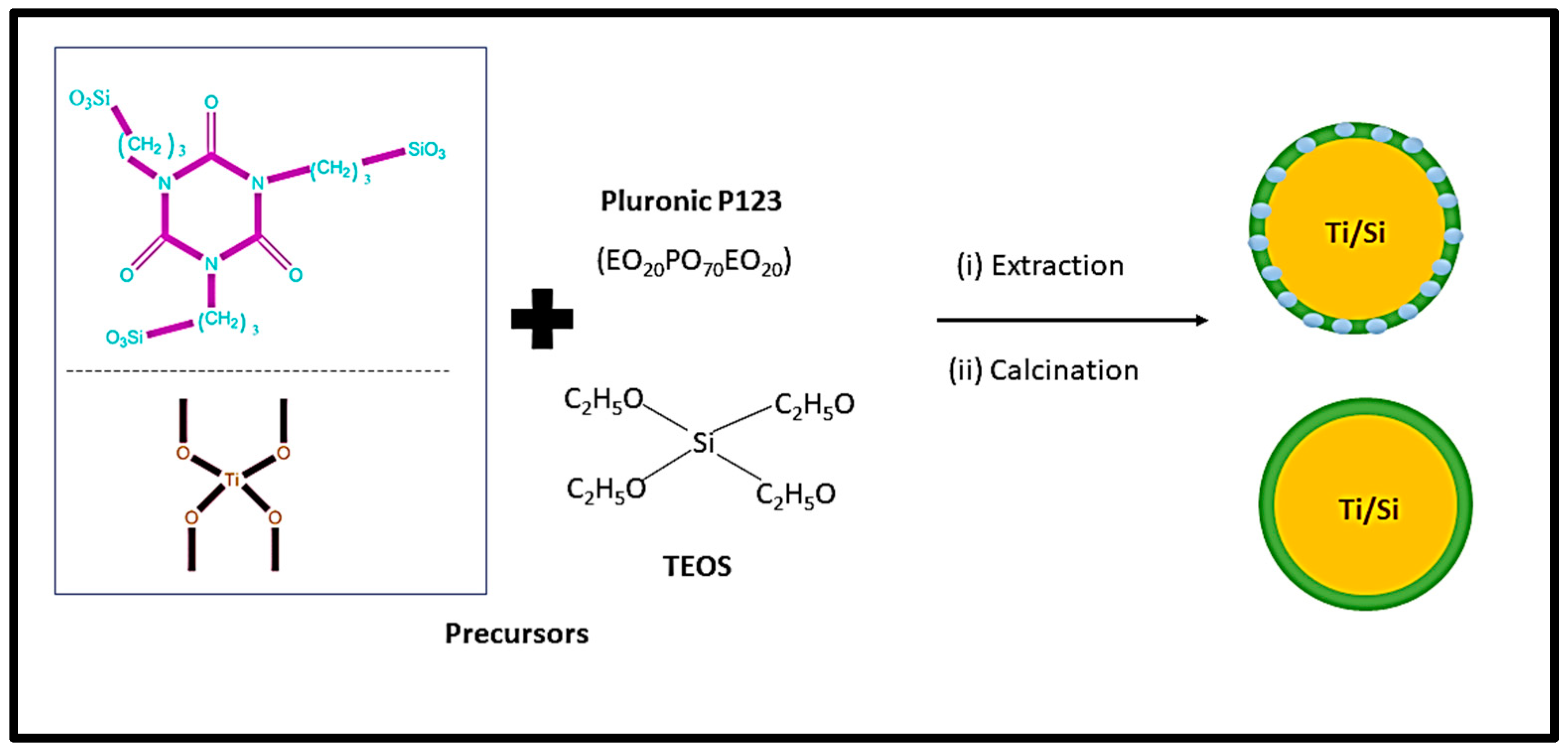
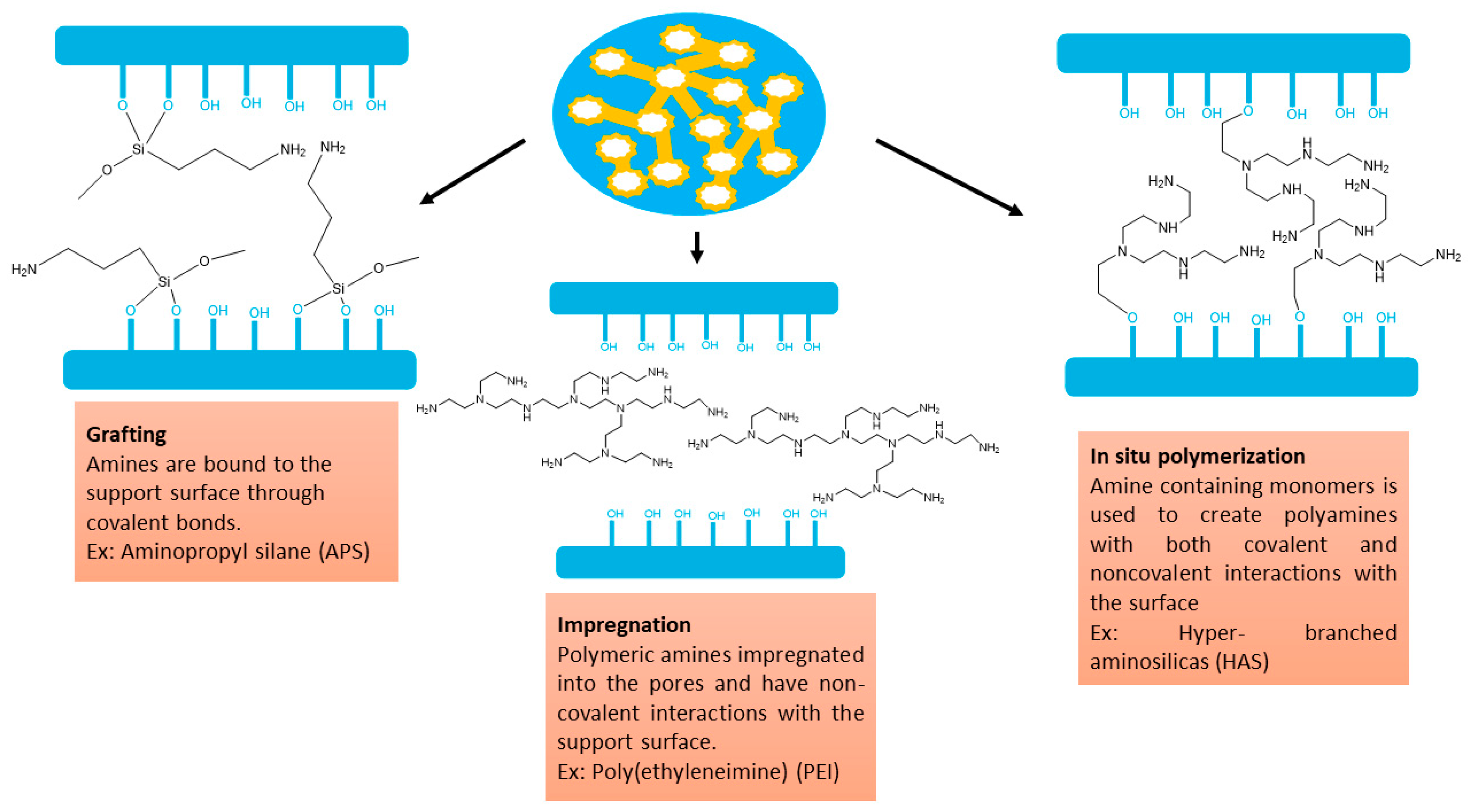
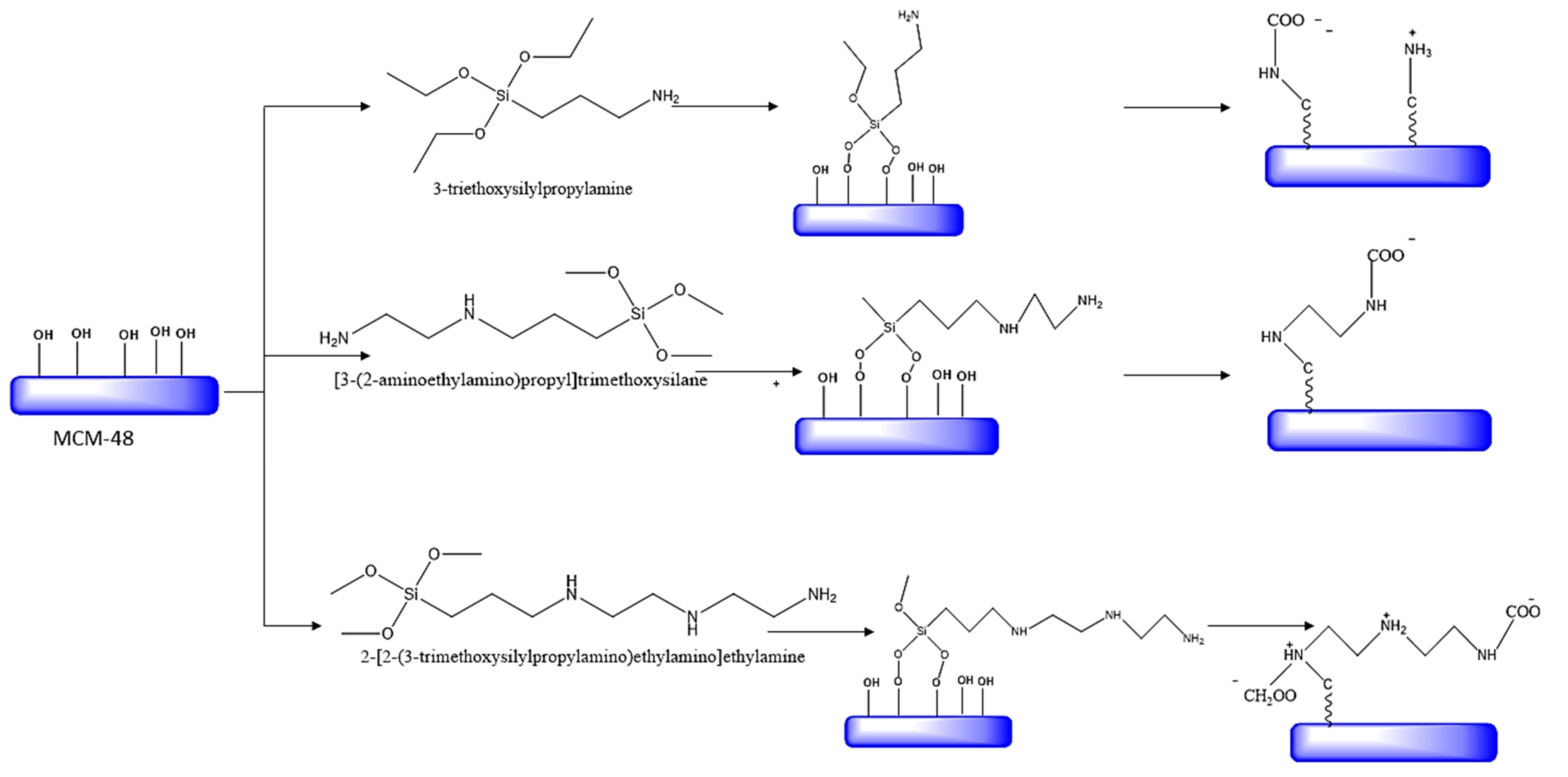
4.2. Synthesis procedures
4.3. Importance of micro-porosity and CO2 adsorption capacity of the different mesoporous silica materials
| Types of mesoporous silica | Structure |
Silica Source |
Surfactant/ Block co-polymer |
BET Specific surface area (m2/g) | Pore volume (cm3/g) | Pore size (nm) |
Adsorption capacity (mmol/g) |
Adsorption Conditions | Ref. | |
| Temp. (°C) | Pressure (bar) | |||||||||
| KIT-5 | 3D-cubic | TEOS | Pluronic P123 | 711 | 1.05 | 8.04 | 0.48 | 30 | 1 | [99] |
| KIT-6 | 3D-cubic | TEOS | Pluronic P123 | 895 | 1.22 | 6.0 | - | - | - | [96] |
| MCM – 41 | Hexagonal | Na2SiO3 | CTAB | 994 | 1.00 | 3.03 | 0.63 | 25 | 1 | [95] |
| Na2SiO3 | CTAB | 993 | 1.00 | 3.1 | 0.63 | 25 | 1 | [100] | ||
| Na2SiO3 | CTAB | 980 | 0.92 | 4.08 | [92] | |||||
| MCM 48 | Cubic | SiO2 | CTAB | 1287 | 1.1 | 3.5 | 25 | 1 | [101] | |
| SBA-15 | 2D hexagonal | TEOS | P123 | 1254 | 2.44 | 11.4 | - | - | - | [102] |
| SBA-16 | Cubic cage | TEOS | Pluronic F127 | 736 | 0.75 | 4.1 | - | - | - | [96] |
| SNS | TEOS | Pluronic F127 | 394 | 0.10 | 21.1 | 2.06 | 25 | 1 | [103] | |
| SNT | TEOS | Pluronic F127 | 319 | 0.07 | 26.0 | 2.46 | 25 | 1 | [103] | |
| Silica-based sorbent | Amine types |
CO2 adsorption performance capacity (mmol/g) |
Conditions | BET Specific surface area (m2/g) | Pore volume (cm3/g) | Pore size (nm) | Preparation methods | Ref | |
| Temperature (°C) | Pressure (bar) | ||||||||
| DWSNT | - | 0.1 | 25 | 83 | 0.58 | Immobilization | [126] | ||
| DWSNT | APTMS | 1.0 | 25 | 112 | 0.72 | Immobilization | [126] | ||
| DWSNT | MAPTMS | 1.5 | 25 | 114 | 0.79 | Immobilization | [126] | ||
| DWSNT | DEAPTMS | 1.8 | 25 | 68.9 | 0.49 | Immobilization | [126] | ||
| DWSNT | AEAPTMS | 2.25 | 25 | 60.9 | 0.45 | Immobilization | [126] | ||
| HAS | Aziridines | 3.25 | 25 | 71 | 5 | 0.15 | [127] | ||
| HPS | PEI | 2.44 | 75 | 1 | 0.5 | 0.009 | Impregnation | [128] | |
| HVMCM-41 | PEHA | 4.07 | 105 | 1 | Impregnation | [125] | |||
| KIT-6 | PEHA | 4.48 | 105 | 1 | Impregnation | [125] | |||
| MCM-41 | EDA | 1.19 | 35 | Impregnation | [129] | ||||
| MCM-41 | DETA | 1.43 | 35 | Impregnation | [129] | ||||
| MCM-41 | TEPA | 1.96 | 35 | Impregnation | [129] | ||||
| MCM-41 | PEHA | 2.34 | 35 | Impregnation | [129] | ||||
| MCM-41 | MEA (3%) | 11.39 | 25 | 426 | 0.42 | 3.12 | Impregnation | [130] | |
| MCM-41 | PEI | 0.39 | 40 | 0.15 | 443 | 0.340 | 2.95 | Impregnation | [49] |
| MCM-41 | PEI | 0.22 | 75 | 1 | 590 | 1.4 | 13.6 | Impregnation | [122] |
| MCM-41 | PEIAziridine | 0.98 | 75 | 1 | In-situ grafted polymerization | [131] | |||
| MCM-41 | APTS | 94 | 25 | 1 | 10 | 0.01 | Grafting | [116] | |
| MCM-41 | APTS | 2.48 | 20 | 1 | 17 | 0.04 | 20.1 | Grafting | [133] |
| MCM-41 | PEHA | 4.5 | 105 | 1 | Impregnation | [122] | |||
| MCM-41 | MEA | 0.89 | 25 | 1 | 19 | 0.82 | Impregnation | [100] | |
| MCM-41 | DEA | 0.80 | 25 | 1 | 13 | 0.07 | Impregnation | [100] | |
| MCM-41 | TEA | 0.63 | 25 | 1 | 213 | 0.17 | Impregnation | [100] | |
| MCM-41 | Branched PEI | 1.08 | 100 | 1 | 6 | 0 | - | Impregnation | [95] |
| MCM-41 | Branched PEI | 0.79 | 100 | 1 | 12 | 0.04 | - | Impregnation | [95] |
| MCM-41 | Branched PEI – (30 wt%) | 0.70 | 100 | 1 | 80 | 0.14 | - | Impregnation | [95] |
| MCM-41 | Branched PEI | 28 | 100 | 1 | 104 | 0.12 | 2.05 | Impregnation | [95] |
| MCM-41 | Branched PEI | 17.5 | 100 | 1 | 291 | 0.17 | 2.05 | Impregnation | [95] |
| MCM-41 | TEPA | 1.24 | 25 | 1 | 11 | 0.05 | 1.8 | Impregnation | [134] |
| MCM-48 | APTES | 0.62 | 25 | 1.01 | 1072 | 0.52 | 2.9 | Grafting | [101] |
| MCM-48 | TRI | 0.46 | 25 | 1.01 | 698 | 0.39 | 2.6 | Grafting | [101] |
| MCM-48 | TRI | 0.44 | 25 | 1.01 | 463 | 0.23 | 2.5 | Grafting | [101] |
| MsiNTs | PEI | 2.75 | 92 | 52.4 | 0.17 | 12.4 | Impregnation | [135] | |
| OMS | PEI | 1.4 | 25 | 352 | 0.79 | Grafting | [122] | ||
| SAB-15 | PEHA | 4.0 | 105 | 1 | Impregnation | [125] | |||
| SBA-15 | PEI | 0.65 | 25 | 683 | 1.19 | 8.5 | Impregnation | [124] | |
| SBA-15 | PEI/Zr4 | 1.34 | 25 | 642 | 1.08 | 8.6 | Impregnation | [124] | |
| SBA-15 | PEI/Zr7 | 1.56 | 25 | 674 | 1.23 | 9.5 | Impregnation | [124] | |
| SBA-15 | PEI/Zr14 | 1.41 | 25 | 601 | 0.69 | 7.0 | Impregnation | [124] | |
| SBA-15 | PEI/Ti1.4 | 0.24 | 25 | 510 | 0.39 | 4.4 | Impregnation | [124] | |
| SBA-15 | NH2OH | 1.65 | 25 | 1 | 435.6 | 0.54 | 6.85 | Grafting | [136] |
| SBA-15 | APTMS | 1.46 | 25 | 0.15 | 82 | 0.16 | 5 | Grafting | [137] |
| SBA-15 | TEPA | 2.45 | 70 | 5 | 0.03 | Grafting | [102] | ||
| SBA-15 | AMP | 1.79 | 70 | 372 | 0.21 | Grafting | [122] | ||
| SBA-15(0.2µm) | PEI | 5.84 | 100 | 1 | 590 | 1.44 | 13.6 | Impregnation | [122] |
| SBA-15 (1.5µm) | PEI | - | 100 | 1 | 746 | 0.80 | 7.2 | Impregnation | [122] |
| SBA-15 (25µm) | PEI | 5.81 | 100 | 1 | 580 | 0.95 | 10.5 | Impregnation | [122] |
| SiO2 | APTES | 4.3 | 30 | 67 | 0.51 | In-situ polymerization | [29] | ||
| SiO2 | AEAPTMS | 5.7 | 30 | 45 | 0.37 | In-situ polymerization | [29] | ||
| SiO2 | TRI | 5.6 | 30 | 25 | 0.22 | In-situ polymerization | [29] | ||
| SiO2 | APTES | 0.5 | 30 | 216 | 1.11 | Grafting | [29] | ||
| SiO2 | AEAPTMS | 0.3 | 30 | 206 | 1.10 | Grafting | [29] | ||
| SiO2 | TRI | 0.8 | 30 | 172 | 0.99 | Grafting | [29] | ||
| SMCM-41 | MEA | 10.40 | 25 | 405 | 0.39 | 3.01 | Impregnation | [130] | |
| SBA-15 | TEPA | 4.5 | 75 | 1 | 121.1 | 0.327 | Impregnation | [138] | |
| MPSM | TEA | 4.27 | 75 | 1 | 34 | 0.08 | 9.5 | Impregnation | [50] |
| MCM-41 | TRI | 1.74 | 25 | 0.05 | 678.3 | 1.47 | Grafting | [139] | |
| MCM-41 | APTES | 1.20 | 30 | 1 | 1045.21 | 2.59 | 30 | Grafting | [140] |
| MCM-41 | PEI | 0.98 | 30 | 1 | 6.6 | 0.01 | 0.8 | Grafting | [141] |
| MCM-41 | PEI | 4.68 | 45 | 1 | 894 | 1.28 | 5.1 | Grafting | [118] |
| MCM-41 | PEI | 2.92 | 50 | 0.1 | 508 | 0.98 | 2.54 | Impregnation | [142] |
| MCM-41 | TEPA | 2.25 | 50 | 0.1 | 431 | 0.83 | 2.21 | Impregnation | [142] |
| MCM-41- KOH | PEI- | 3.38 | 50 | 0.1 | 391 | 1.08 | 2.33 | Impregnation | [142] |
| MCM-41- Ca(OH)2 | PEI- | 3.81 | 50 | 0.1 | 411 | 1.12 | 2.50 | Impregnation | [142] |
| MCM-41- CsOH | PEI- | 5.02 | 50 | 0.1 | 306 | 0.91 | 2.14 | Impregnation | [142] |
| MCM-41- KOH | TEPA- | 3.93 | 50 | 0.1 | 322 | 0.97 | 2.15 | Impregnation | [142] |
| MCM-41- Ca(OH)2 | TEPA- | 3.76 | 50 | 0.1 | 405 | 0.94 | 2.31 | Impregnation | [142] |
| PET- CsOH | TEPA- | 5.42 | 50 | 0.1 | 293 | 0.97 | 2.61 | Impregnation | [142] |
| MCM 48 | PEI | 1.09 | 80 | 0.24 | 79.3 | 0.02 | 1.68 | Impregnation | [143] |
| MCM-41 | PEI | 1.23 | 80 | 0.24 | 59.1 | 0.02 | 1.80 | Impregnation | [143] |
| SBA-15 | PEI | 1.07 | 80 | 0.24 | 62.1 | 0.01 | 5.2 | Impregnation | [143] |
| SBA-15 | PEI | 1.77 | 0 | 1 | 783 | 0.03 | 7.0 | Impregnation | [144] |
| SBA-15 | PEI | 1.26 | 45 | 0.15 | 399 | 0.79 | 8.2 | Impregnation | [145] |
| MCM 41 | PEI | 3.53 | 25 | 1 | 24 | 0.012 | Impregnation | [146] | |
| MCM 41 | APTS | 2.41 | 25 | 1 | 736 | 0.37 | Grafting | [146] | |
| SBA-15 | PEI | 1.84 | 25 | 1.2 | 195 | 0.39 | 7.0 | Grafting | [147] |
| SBA-15- APES | 1.78 | 25 | 1.2 | 190 | 0.37 | 7.2 | Grafting | [147] | |
| SBA-15- APES | PEI | 1.54 | 25 | 1.2 | 24 | 0.21 | 2.7 | Grafting | [147] |
| OMS | PEI | 2.43 | 25 | 1.2 | 167 | 0.33 | 7.6 | Grafting | [147] |
| OMS- APES | 3.03 | 25 | 1.2 | 180 | 0.37 | 7.2 | Grafting | [147] | |
| OMS- APES | PEI | 1.18 | 25 | 1.2 | 39 | 0.18 | 2.3 | Grafting | [147] |
| OMS- NCC | Amidoxime | 5.54 | 120 | 1 | 315 | 0.69 | 9.3 | [148] | |
| MPS-MCC* | 2.41 | 120 | 302 | 0.44 | 7.0 | [149] | |||
| MPS-MCC** | 3.85 | 120 | 285 | 0.40 | 6.7 | [149] | |||
| OMS- MgO | 4.71 | 120 | 1 | 261 | 0.48 | 7.25 | [150] | ||
| OMS-CaO | 3.85 | 120 | 1 | 163 | 0.25 | 6.76 | [150] | ||
| SiO2- Al2O3 | APTS | 2.64 | 25 | 1 | 740 | 1.24 | 5.1 | Grafting | [151] |
| SiO2- Al(NO3)3 | APTS | 0.78 | 25 | 1 | 319 | 0.63 | 2.9 | Grafting | [151] |
| OMS-Ti | 0.81 | 25 | 1 | 487 | [90] | ||||
| MsiNTs | APTES | 2.87 | 25 | 1.2 | 293 | 0.79 | 22 | Grafting | [103] |
| SNS | APTES | 2.13 | 25 | 1.2 | 210 | 0.31 | 19.6 | Grafting | [103] |
| Al(NO3)3 | AP | 0.98 | 25 | 1 | 359 | 0.62 | 10.0 | [152] | |
| OMS-Al-Zr | 2.60 | 60 | 1 | 441 | 0.61 | 6.9 | [153] | ||
5. Chemisorbents (amine functionalized Si-based materials) – application at low and high temperature CO2 sorption
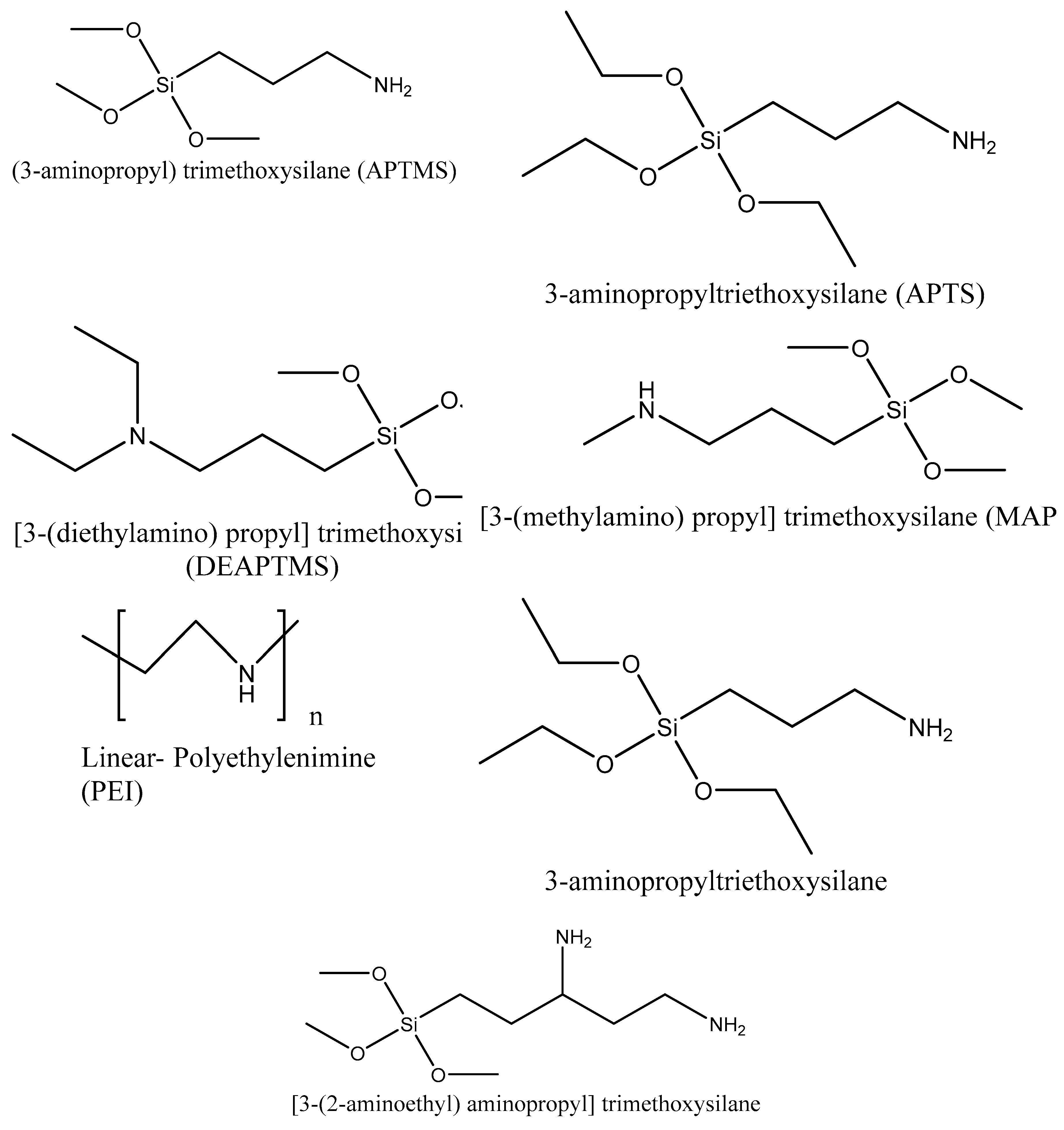
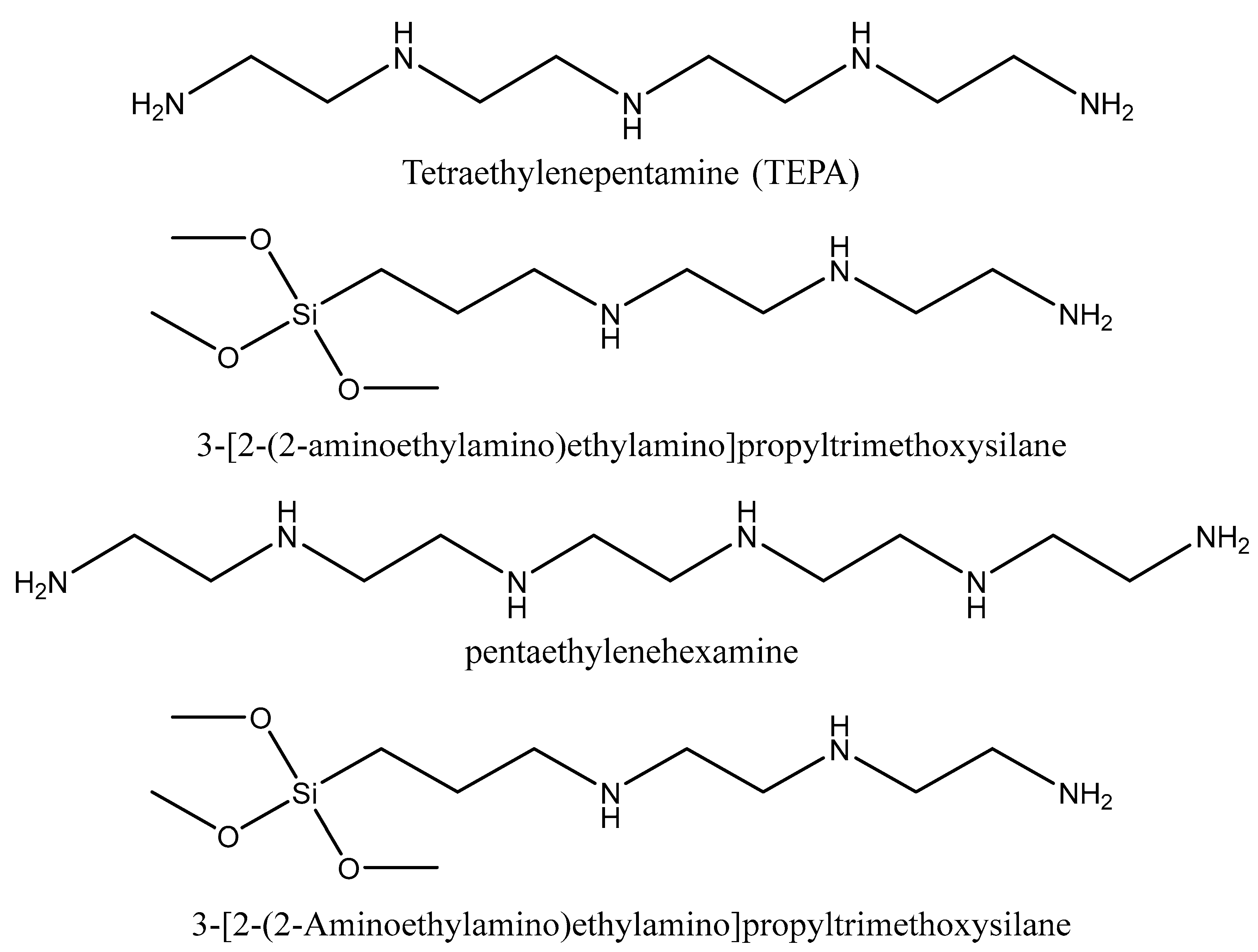
5.1. Synthesis procedures
5.2. Comparison of adsorption capacities
5.3. Sorbent selectivity, regeneration and stability in the cyclic CO2 adsorption –desorption runs
| Synthesis method | Type of silica-based sorbent | Amine type | Regeneration condition | Stability performance | References | ||
| Temperature (°C) | Types of gas flow | No. of cycles (cyclic runs) | Capacity loss (%) | ||||
| Impregnated | MCM-41 | PEHA | 100 | N2 | 15 | Less than 1 | [161] |
| MCM-41 | TEPA +AMP | 100 | N2 for 60 min | 15 | 4.32 | [119] | |
| SBA-15 | PEI-linear | 100 | Ar | 12 | 13.5 | [162] | |
| SBA-15 | Acrylonitrile-modified TEPA | 100 | N2 | 12 | 1.1 | [163] | |
| HMS | PEI-linear | 75 | N2 for 100 min | 4 | 1.6 | [164] | |
| MCF | PEI- branched | 115 | Ar for 20 min | 10 | 32 | [165] | |
| MCF | PEI | 100 | H2 | 10 | 5 | [166] | |
| MCF | Guanidinylated poly(allylamine) | 120 | He | 5 | 17 | [52] | |
| Fumed silica | PEI-linear | 55 | N2 for 15min | 180 | Stable | [167] | |
| MCM-41 | TEPA | 100 | N2 | 10 | 3.43 | [168] | |
| Silica fume | Diisopropanolamine | 50 | N2 | 10 | 7 | [169] | |
| Nano- SiO2 | PEI- branched | 120 | N2 | 30 | 10.5 | [170] | |
| Nano- SiO2 | PEI- branched | 120 | N2 | 30 | 19.4 | [171] | |
| Mesoporous-SiO2 | APTS | 120 | Air for 30 min | 11 | 4.3 | [172] | |
| Porous SiO2 | PEI | 100 | N2 for 30 min | 20 | 5 | [173] | |
| Silica aerogel | TEPA | 75 | Ar for 20 min | 10 | 3.9 | [174] | |
| Porous SiO2 | TEPA | 75 | He for 20 min | 10 | 2 | [175] | |
| SNT | PEI | 110 | N2 for 40 min | 10 | 3.3 | [134] | |
| KCC-1- SiO2 | TEPA | 110 | N2 | 21 | 1.2 | [176] | |
| Mesoporous multilamellar SiO2 |
PEI | 110 | N2 | 10 | 3.7 | [177] | |
| Silica aerogel | TEPA | 80 | Ar for 30 min | 100 | 12 | [176] | |
| Mesoporous SiO2 |
DEA | 90 | N2 | 10 | 12 | [172] | |
| Grafting | SBA-15 | AP | 90 | Vacuum | 10 | 1 | [178] |
| SBA-15 | DEAPTMS | 120 | N2 for 10 min | 100 | 7.2 | [179] | |
| MCM-48 | 2-[2-(3-trimethoxysilyl propylamino) ethylamino] ethylamine |
- | N2 | 20 | Stable | [100] | |
| KIT-6 | APTES | 120 | He | 10 | Stable | [99] | |
| MCF | TRI | 150 | N2 for 30 min | 5 | 1.9 | [180] | |
| HMS | APTS | 110 | N2 for 180 min | 3 | Less than 1 | [181] | |
| MCM-41 | APTS | 105 | N2 for 90 min | 10 | Stable | [117] | |
6. Technical Challenges and Future Trends
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osman, A.I.; Hefny, M.; Maksoud, M.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: a review. Environ. Chem. Lett. 2020, 1-53. [CrossRef]
- Ahn, D. Quantifying the Emissions of Carbon Dioxide (CO2), Carbon Monoxide (Co), and Nitrogen Oxides (Nx) from Human Activities: Top-Down and Bottom-Up Approaches Doctoral dissertation, University of Maryland, College Park, USA, 2021.
- Gunawardene, O.H.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere. 2022, 13(3), .397. [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renewable Sustainable Energy Rev. 2014. 39, 426-443. [CrossRef]
- Omoregbe, O.; Mustapha, A.N.; Steinberger-Wilckens, R.; El-Kharouf, A.; Onyeaka, H. Carbon capture technologies for climate change mitigation: A bibliometric analysis of the scientific discourse during 1998–2018. Energy Reports. 2020. 6, 1200-1212. [CrossRef]
- Lee, S.Y.; Park, S.J.; A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015. 23, 1-11. [CrossRef]
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E.; Adeloju, S. Dynamic modelling and optimisation of flexible operation in post-combustion CO2 capture plants—A review. Comput. Chem. Eng. 2014. 61, 245-265. [CrossRef]
- Cotton, A.; Patchigolla, K.; Oakey, J.E.; Minor and trace element emissions from post-combustion CO2 capture from coal: Experimental and equilibrium calculations. Fuel. 2014. 117, 391-407. [CrossRef]
- Goto, K.; Yogo, K.; Higashii, T.; A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy. 2013.111, 710-720. [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, Z.; Zhang, W.; Zhang, L.; Baena-Moreno, F.M.; Lichtfouse, E. CO2 capture from coalbed methane using membranes: a review. Environ. Chem. Lett. 2020. 18(1), 79-96. [CrossRef]
- Sreenivasalu, B.; Gayatri, D.V.; Sreedhar, I.; Ragharan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [CrossRef]
- Singh, J.; Bhunia, H.; Basu, S.; Development of sulfur-doped carbon monolith derived from phenol-formaldehyde resin for fixed bed CO2 adsorption. Environmental and Innovation. 2020. 20, 101104. [CrossRef]
- Sánchez, J.M.; Maroño, M.; Cillero, D.; Montenegro, L.; Ruiz, E.; Laboratory-and bench-scale studies of a sweet water–gas-shift catalyst for H2 and CO2 production in pre-combustion CO2 capture. Fuel. 2013. 114, 191-198. [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. A new porous material to enhance the kinetics of clathrate process: application to precombustion carbon dioxide capture. Environ. Sci. Technol. 2013. 47(22), 13191-13198. [CrossRef]
- Wienchol P.; Szlȩk A.; Ditaranto M.; Waste-to-energy technology integrated with carbon capture—challenges and opportunities. Energy. 2020. 198:117352. ISSN 0360-5442. [CrossRef]
- Kim, Y.E.; Moon, S.J.; Yoon, Y.I.; Jeong, S.K.; Park, K.T.; Bae, S.T.; Nam, S.C.; Heat of absorption and absorption capacity of CO2 in aqueous solutions of amine containing multiple amino groups. Separation and Purification Technology. 2014. 122, 112-118. [CrossRef]
- Roth, E.A.; Agarwal, S; Gupta, R.K.; Nanoclay-based solid sorbents for CO2 capture. Energy and fuels. 2013. 27(8), 4129-4136. [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. Rsc Advances. 2013. 3(45), 22739-22773. [CrossRef]
- Zhang, X.; He, X.; Gundersen, T. Post-combustion carbon capture with a gas separation membrane: parametric study, capture cost, and exergy analysis. Energy and Fuels.2013. 27(8), 4137-4149. [CrossRef]
- Scholes, C.A.; Ho, M.T.; Wiley, D.E.; Stevens, G.W.; Kentish, S.E. Cost competitive membrane—cryogenic post-combustion carbon capture. Int. J. Greenh. Gas Control., 2013. 17, 341-348. [CrossRef]
- Khraisheh, M.; Mukherjee, S.; Kumar, A.; Al Momani, F.; Walker, G.; Zaworotko, M.J.; An overview on trace CO2 removal by advanced physisorbent materials. Journal of environmental management. 2020. 255, 109874. [CrossRef]
- Anwar, M.N.; Fayyaz, A.; Sohail, N.F.; Khokhar, M.F.; Baqar, M.; Khan, W.D.; Rasool, K.; Rehan, M.; Nizami, A.S. CO2 capture and storage: a way forward for sustainable environment. Journal of environmental management. 2018. 226, 131-144. [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An improved CO2 separation and purification system based on cryogenic separation and distillation theory. Energies. 2014. 7(5), 3484-3502. [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: a review. Ind. Eng. Chem. Res.2012. 51(4), 1438-1463. [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon dioxide capture adsorbents: chemistry and methods. Chem. Sus. Chem, 2017.10(7), 1303-1317. [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chemical reviews. 2012. 112(2), 724-781. [CrossRef]
- Reynolds, A.J.; Verheyen, T.V.; Adeloju, S.B.; Meuleman, E.; Feron, P. Towards commercial scale postcombustion capture of CO2 with monoethanolamine solvent: key considerations for solvent management and environmental impacts. Environ. Sci. Technol. 2012.46(7), 3643-3654. [CrossRef]
- Mumford, K.A.; Wu, Y.; Smith, K.H.; Stevens, G.W. Review of solvent based carbon-dioxide capture technologies. Front Chem Sci Eng. 2015. 9(2), 125-141. [CrossRef]
- Park, J.H.; Celedonio, J.M.; Seo, H.; Park, Y.K. Ko, Y.S. A study on the effect of the amine structure in CO2 dry sorbents on CO2 capture. Catalysis Today. 2016. 265, 68-76. [CrossRef]
- Gunathilake, C.; Gangoda, M.; Jaroniec, M. Mesoporous alumina with amidoxime groups for CO2 sorption at ambient and elevated temperatures. Ind. Eng. Chem. Res. 2016., 55(19), 5598-5607. [CrossRef]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 2015. 49(17), 10728-10735. [CrossRef]
- García-Abuín, A.; Gomez-Diaz, D.; Lopez, A.B.; Navaza, J.M.; Rumbo, A.NMR characterization of carbon dioxide chemical absorption with monoethanolamine, diethanolamine, and triethanolamine. Ind. Eng. Chem. Res. 2013. 52(37), 13432-13438. [CrossRef]
- Kim, Y.E.; Lim, J.A.; Jeong, S.K.; Yoon, Y.I.; Bae, S.T.; Nam, S.C. Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA, and AMP solutions. Bulletin of the Korean Chemical Society. 2013. 34(3),.783-787. [CrossRef]
- Rinprasertmeechai, S.; Chavadej, S.; Rangsunvigit, P.; Kulprathipanja, S. Carbon dioxide removal from flue gas using amine-based hybrid solvent absorption. Int. J. Chem. Eng. 2012. 6, 296-300.
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: a review. Sep Sci Technol. 2005. 40(1-3), 321-348. [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon dioxide separation from flue gases: a technological review emphasizing reduction in greenhouse gas emissions. The Scientific World Journal. 2014. 2014. [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem Eng Res Des. 2011. 89(9), 1609-1624. [CrossRef]
- Dave, N.; Do, T.; Puxty, G.; Rowland, R.; Feron, P.H.M.; Attalla, M.I. CO2 capture by aqueous amines and aqueous ammonia–A Comparison. Energy Procedia. 2009. 1(1), 949-954. [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-liquid-based CO2 capture systems: structure, interaction and process. Chemical reviews. 2017. 117(14),.9625-9673. [CrossRef]
- Borhani, T.N.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renewable Sustainable Energy Rev. 2019. 114, 109299. [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renewable Sustainable Energy Rev. 2018. 96, 502-525. [CrossRef]
- Jin, X.; Ge, J.; Zhang, L.; Wu, Z.; Zhu, L.; Xiong, M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics. 2022. 10(7), 87. [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chemical Engineering Journal Advances. 2021. 6, 100091. [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol and Air Quality Research. 2012. 12(5), 745-769. [CrossRef]
- Kumar, S.; Bera, R.; Das, N.; Koh, J. Chitosan-based Zeolite-Y and ZSM-5 porous biocomposites for H2 and CO2 storage. Carbohydr. Polym. 2020. 232, 115808. [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020. 41, 101251. [CrossRef]
- Balashankar, V.S.; Rajendran, A. Process optimization-based screening of zeolites for post-combustion CO2 capture by vacuum swing adsorption. ACS Sustain. Chem. Eng. 2019. 7(21), 17747-17755. [CrossRef]
- Avci, G.; Erucar, I.; Keskin, S. Do new MOFs perform better for CO2 capture and H2 purification? Computational screening of the updated MOF database. ACS Applied Materials & Interfaces. 2020. 12(37), 41567-41579. [CrossRef]
- Modak, A.; Jana, S. Advancement in porous adsorbents for post-combustion CO2 capture. Microporous Mesoporous Mater. 2019. 276, 107-132. [CrossRef]
- Le, M.U.T.; Lee, S.Y.; Park, S.J. Preparation and characterization of PEI-loaded MCM-41 for CO2 capture. Int. J. Hydrog. Energy. 2014. 39(23), 12340-12346. [CrossRef]
- Kim, M.I.; Choi, S.J.; Kim, D.W.; Park, D.W. Catalytic performance of zinc containing ionic liquids immobilized on silica for the synthesis of cyclic carbonates. J Ind Eng Chem. 2014. 20(5), 3102-3107. [CrossRef]
- Alkhabbaz, M.A.; Khunsupat, R.; Jones, C.W. Guanidinylated poly (allylamine) supported on mesoporous silica for CO2 capture from flue gas. Fuel. 2014. 121, 79-85. [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Advances. 2021. 11(21), 12658-12681. [CrossRef]
- Liu, R.S.; Shi, X.D.; Wang, C.T.; Gao, Y.Z.; Xu, S.; Hao, G.P.; Chen, S.; Lu, A.H. Advances in Post-Combustion CO2 Capture by Physical Adsorption: From Materials Innovation to Separation Practice. Chem Sus Chem. 2021. 14(6), 1428-1471. [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; Haszeldine, R.S. Carbon capture and storage update. Energy Environ. Sci. 2014. 7(1), 130-189. [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014.7(1), 232-250. [CrossRef]
- Patel, H.A.; Byun, J.; Yavez, C.T. Carbon dioxide capture adsorbents: Chemistry and Methods. Chem Sus Chem 2017, 10, 1303–1317. [CrossRef]
- Tuci, G.; Iemhoff, A.; Ba, H.; Luconi, L.; Rossin, A.; Papaefthimiou, V.; Palkovits, R.; Artz, J.; Pham-Huu, C.; Giambastiani, G. Playing with covalent triazine framework tiles for improved CO2 adsorption properties and catalytic performance. Beilstein journal of nanotechnology. 2019. 10(1), 1217-1227. [CrossRef]
- Madden, D.G.; O’Nolan, D.; Chen, K.J.; Hua, C.; Kumar, A.; Pham, T.; Forrest, K.A.; Space, B.; Perry, J.J.; Khraisheh, M.; Zaworotko, M.J. Highly selective CO2 removal for one-step liquefied natural gas processing by physisorbents. Chemical Communications. 2019.55(22), 3219-3222. [CrossRef]
- Balsamo, M.; Budinova, T.; Erto, A.; Lancia, A.; Petrova, B.; Petrov, N.; Tsyntsarski, B. CO2 adsorption onto synthetic activated carbon: Kinetic, thermodynamic and regeneration studies. Separation and purification technology. 2013. 116, 214-221. [CrossRef]
- Sreenivasalu, B.; Gayatri, D.V.; Sreedhar, I.; Ragharan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [CrossRef]
- Cherbanski, R.; Komorowska-Durka, M.; Stefanidis, G.D.; Stankiewicz, A.I. Microwave Swing Regeneration Vs Temperature Swing Regeneration Comparison of Desorption Kinetics. Ind. Eng. Chem. Res.2011. 50(14), 8632-8644. [CrossRef]
- Maity, A.; Belgamwar, R.; Polshettiwar, V. Facile synthesis to tune size, textural properties and fiber density of dendritic fibrous nanosilica for applications in catalysis and CO2 capture. Nature protocols. 2019.14(7), 2177-2204. [CrossRef]
- Miricioiu, M.G.; Niculescu, V.C. Fly ash, from recycling to potential raw material for mesoporous silica synthesis. Nanomaterials. 2020. 10(3), 474. [CrossRef]
- Wan, Ying, Dongyuan Zhao. On the controllable soft-templating approach to mesoporous silicates. Chemical reviews. 2007. 107, no. 7 (2007): 2821-2860. [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics.2018. 10(3), 118. [CrossRef]
- Costa, J.A.S.; de Jesus, R.A.; Santos, D.O.; Mano, J.F.; Romao, L.P.; Paranhos, C.M. Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microporous and Mesoporous Materials, 2020. 291, 109698. [CrossRef]
- Brezoiu, A.M.; Deaconu, M.; Nicu, I.; Vasile, E.; Mitran, R.A.; Matei, C.; Berger, D. Heteroatom modified MCM-41-silica carriers for Lomefloxacin delivery systems. Microporous and Mesoporous Materials, 2019. 275, 214-222. [CrossRef]
- Jesus, R.A.; Rabelo, A.S.; Figueiredo, R.T.; Da Silva, L.C.; Codentino, I.C.; Fantini, M.C.D.A.; Araújo, G.L.B.D.; Araújo, A.A.S.; Mesquita, M.E. Synthesis and application of the MCM-41 and SBA-15 as matrices for in vitro efavirenz release study. J Drug Deliv Sci Technol. 2016, 31, 153-159. [CrossRef]
- Liu, Y.; Li, C.; Peyravi, A.; Sun, Z.; Zhang, G.; Rahmani, K.; Zheng, S.; Hashisho, Z. Mesoporous MCM-41 derived from natural Opoka and its application for organic vapors removal. J. Hazard. Mater. 2021. 408, 124911. [CrossRef]
- Costa, J.A.S.; Paranhos, C.M. Mitigation of silica-rich wastes: an alternative to the synthesis eco-friendly silica-based mesoporous materials. Microporous and Mesoporous Materials, 2020. 309, 110570. [CrossRef]
- Björk, E.M. Synthesizing and characterizing mesoporous silica SBA-15: A hands-on laboratory experiment for undergraduates using various instrumental techniques. J. Chem. Educ.,2017. 94(1), 91-94. [CrossRef]
- Martínez, M.L.; Ponte, M.V.; Beltramone, A.R.; Anunziata, O.A. Synthesis of ordered mesoporous SBA-3 materials using silica gel as silica source. Materials Letters, 2014. 134, 95-98. [CrossRef]
- López-Mendoza, M.A.; Nava, R., Peza-Ledesma, C.; Millán-Malo, B.; Huirache-Acuña, R.; Skewes, P.; Rivera-Muñoz, E.M. Characterization and catalytic performance of Co-Mo-W sulfide catalysts supported on SBA-15 and SBA-16 mechanically mixed. Catalysis Today, 2016. 271, 114-126. [CrossRef]
- Gonzalez, G.; Sagarzazu, A.; Cordova, A.; Gomes, M.E.; Salas, J.; Contreras, L.; Noris-Suarez, K.; Lascano, L. Comparative study of two silica mesoporous materials (SBA-16 and SBA-15) modified with a hydroxyapatite layer for clindamycin controlled delivery. Microporous and Mesoporous Materials, 2018.256, 251-265. [CrossRef]
- Wu, Q.; Li, Y.; Hou, Z.; Xin, J.; Meng, Q.; Han, L.; Xiao, C.; Hu, D.; Duan, A.; Xu, C. Synthesis and characterization of Beta-FDU-12 and the hydrodesulfurization performance of FCC gasoline and diesel. Fuel processing technology, 2018.172, 55-64. [CrossRef]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F.; Nazarpour, R. Disordered mesoporous KIT-1 synthesized by DABCO-based ionic liquid and its characterization. Microporous and Mesoporous Materials, 2016.228, 305-309. [CrossRef]
- Stöber W.; Fink A.; Bohn E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci, 1968. 26, 62-69. [CrossRef]
- Choma, J.; Jamioła, D.; Augustynek, K.; Marszewski, M.; Gao, M.; Jaroniec, M. New opportunities in Stöber synthesis: preparation of microporous and mesoporous carbon spheres. J. Mater. Chem. 2012. 22(25), 12636-12642. [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013. 42(9), 3862-3875. [CrossRef]
- Sakamoto, S.; Yoshikawa, M.; Ozawa, K.; Kuroda, Y.; Shimojima, A.; Kuroda, K. Formation of single-digit nanometer scale silica nanoparticles by evaporation-induced self-assembly. Langmuir. 2018. 34(4), 1711-1717. [CrossRef]
- Sihler, S.; Nguyen, P.L.; Lindén, M.; Ziener, U. Green chemistry in red emulsion: Interface of dye stabilized emulsions as a powerful platform for the formation of sub-20-nm SiO2 nanoparticles. ACS Appl. Mater. Interfaces. 2018. 10(28), 24310-24319. [CrossRef]
- Murray, E.; Born, P.; Weber, A.; Kraus, T. Synthesis of Monodisperse Silica Nanoparticles Dispersable in Non-Polar Solvents. Adv. Eng. Mater. 2010. 12(5), 374-378. [CrossRef]
- Mandal, M.; Kruk, M.Family of single-micelle-templated organosilica hollow nanospheres and nanotubes synthesized through adjustment of organosilica/surfactant ratio. Chem. Mater.2012, 24(1), 123-132. [CrossRef]
- Savic, S.; Vojisavljevic, K.; Počuča-Nešić, M.; Zivojevic, K.; Mladenovic, M.; Knezevic, N. Hard Template Synthesis of Nanomaterials Based on Mesoporous Silica. Metall. Mater. Eng. 2018, 24(4). [CrossRef]
- Zhao, T.; Elzatahry, A.; Li, X.; Zhao, D. Single-micelle-directed synthesis of mesoporous materials. Nat. Rev. Mater. 2019, 4(12), 775-791. [CrossRef]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-dimensional (2D) nanomaterials towards electrochemical nanoarchitectonics in energy-related applications. Bulletin of the Chemical Society of Japan. 2017, 90(6), 627-648. [CrossRef]
- Yamamoto, E.; Kuroda, K. Colloidal mesoporous silica nanoparticles. Bulletin of the chemical society of Japan. 2016, 89(5), 501-539. [CrossRef]
- Kim, H.J.; Yang, H.C.; Chung, D.Y.; Yang, I.H.; Choi, Y.J.; Moon, J.K. Functionalized mesoporous silica membranes for CO2 separation applications. J. Chem. 2015, 2015. [CrossRef]
- Gunathilake, C.; Kalpage, C.; Kadanapitiye, M.; Dassanayake, R.S.; Manchanda, A.S.; Gangoda, M. Facile synthesis and surface characterization of titania-incorporated mesoporous organosilica materials. J. Compos. Sci. 2019. 3(3), 77. [CrossRef]
- Hao, P.; Peng, B.; Shan, B.Q.; Yang, T.Q.; Zhang, K. Comprehensive understanding of the synthesis and formation mechanism of dendritic mesoporous silica nanospheres. Nanoscale Adv. 2020, 2(5), 1792-1810. [CrossRef]
- Panek, R.; Wdowin, M.; Franus, W.; Czarna, D.; Stevens, L.A.; Deng, H.; Liu, J.; Sun, C.; Liu, H.; Snape, C.E. Fly ash-derived MCM-41 as a low-cost silica support for polyethyleneimine in post-combustion CO2 capture. J. CO2 Util.2017, 22, 81-90. [CrossRef]
- Singh, B.; Polshettiwar, V. Solution-phase synthesis of two-dimensional silica nanosheets using soft templates and their applications in CO2 capture. Nanoscale,2019, 11(12), 5365-5376. [CrossRef]
- Li, Y.; Wang, X.; Cao, M. Three-dimensional porous carbon frameworks derived from mangosteen peel waste as promising materials for CO2 capture and supercapacitors. J. CO2 Util. 2018, 27, 204-216. [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S. Development of polyethylenimine-functionalized mesoporous Si-MCM-41 for CO2 adsorption. Fuel Processing Technology, 2017, 167, 622-630. [CrossRef]
- Son, W.J.; Choi, J.S.; Ahn, W.S. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous and Mesoporous Materials.2008, 113(1-3), 31-40. [CrossRef]
- Zeleňák, V.; Badaničová, M.; Halamova, D.; Čejka, J.; Zukal, A.; Murafa, N.; Goerigk, G. Amine-modified ordered mesoporous silica: effect of pore size on carbon dioxide capture. Chemical Engineering Journal, 2008, 144(2), 336-342. [CrossRef]
- Lashaki, M.J.; Sayari, A. CO2 capture using triamine-grafted SBA-15: The impact of the support pore structure. Chemical Engineering Journal, 2018, 334, 1260-1269. [CrossRef]
- Kishor, R.; Ghoshal, A.K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption. Chemical Engineering Journal, 2015, 262, 882-890. [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S. CO2 adsorption study on primary, secondary and tertiary amine functionalized Si-MCM-41. Int. J. Greenh. Gas Control.2016, 51, 230-238. [CrossRef]
- Nigar, H.; Garcia-Baños, B.; Peñaranda-Foix, F.L.; Catalá-Civera, J.M.; Mallada, R.; Santamaría, J. Amine-functionalized mesoporous silica: A material capable of CO2 adsorption and fast regeneration by microwave heating. AIChE Journal. 2016, 62(2), 547-555. [CrossRef]
- Heydari-Gorji, A.; Yang, Y.; Sayari, A. Effect of the pore length on CO2 adsorption over amine-modified mesoporous silicas. Energy and Fuels.2011, 25(9), 4206-4210. [CrossRef]
- Gunathilake, C.; Manchanda, A.S.; Ghimire, P.; Kruk, M.; Jaroniec, M. Amine-modified silica nanotubes and nanospheres: synthesis and CO2 sorption properties. Environ. Sci. 2016, 3(4), 806-817. [CrossRef]
- Cabriga, C.K.C.; Clarete, K.V.R.; Zhang, J.A.T.; Pacia, R.M.P.; Ko, Y.S.; Castro, J.C. Evaluation of biochar derived from the slow pyrolysis of rice straw as a potential adsorbent for carbon dioxide. Biomass Convers. Biorefinery 2021. [CrossRef]
- Wang, J.; Yuan, X.; Deng, S.; Zeng, X.; Yu, Z.; Li, S.; Lia, K. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop. Green Chem. 2020, 22, 6836–6845. [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Proc. 2011, 4, 562–567. [CrossRef]
- Moni, P.; Chaves, W.F.; Wilhelm, M.; Rezwan, K. Polysiloxane microspheres encapsulated in carbon allotropes: A promising material for supercapacitor and carbon dioxide capture. Journal of colloid and interface science, 2019, 542, 91-101. [CrossRef]
- Hahn, M.W.; Jelic, J., Berger, E.; Reuter, K.; Jentys, A.; Lercher, J.A. Role of amine functionality for CO2 chemisorption on silica. The Journal of Physical Chemistry B,2016, 120(8), 1988-1995. [CrossRef]
- Chen, C.; Yang, S.T.; Ahn, W.S.; Ryoo, R. Amine-impregnated silica monolith with a hierarchical pore structure: enhancement of CO2 capture capacity. Chemical Communications, 2009, (24), 627-3629. [CrossRef]
- Chen, C.; Son, W.J.; You, K.S.; Ahn, J.W.; Ahn, W.S. Carbon dioxide capture using amine-impregnated HMS having textural mesoporosity. Chemical Engineering Journal, 2010, 161(1-2), 46-52. [CrossRef]
- Chen, C.; Zhang, S.; Row, K.H.; Ahn, W.S. Amine–silica composites for CO2 capture: A short review. Journal of energy chemistry, 2017, 26(5), 868-880. [CrossRef]
- Wei, J.; Liao, L.; Xiao, Y.; Zhang, P.; Shi, Y. Capture of carbon dioxide by amine-impregnated as-synthesized MCM-41. Journal of Environmental Sciences, 2010, 22(10), 1558-1563. [CrossRef]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO2). Adv. Colloid Interface Sci.2010, 153(1-2), 43-57. [CrossRef]
- Li, W.; Choi, S.; Drese, J.H.; Hornbostel, M.; Krishnan, G.; Eisenberger, P.M.; Jones, C.W. Steam-stripping for regeneration of supported amine-based CO2 adsorbents. Chem Sus Chem, 2010, 3(8), 899-903. [CrossRef]
- Bollini, P.; Didas, S.A.; Jones, C.W. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem.2011, 21(39), 15100-15120. [CrossRef]
- Li, Y.; Sun, N.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Huang, W. Grafting of amines on ethanol-extracted SBA-15 for CO2 adsorption. Materials, 2013, 6(3), 981-999. [CrossRef]
- Lopez-Aranguren, P.; Builes, S.; Fraile, J.; Vega, L.F.; Domingo, C. Understanding the performance of new amine-functionalized mesoporous silica materials for CO2 adsorption. Ind. Eng. Chem. Res.2014, 53(40), 15611-15619. [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Perez, E.S. CO2 capture with pore-expanded MCM-41 silica modified with amino groups by double functionalization. Microporous and Mesoporous Materials. 2015. 209, 165-171. [CrossRef]
- Wang, X.; Guo, Q.; Zhao, J.; Chen, L. Mixed amine-modified MCM-41 sorbents for CO2 capture. Int. J. Greenh. Gas Control.2015, 37, 90-98. [CrossRef]
- Jung, H.; Lee, C.H.; Jeon, S.; Jo, D.H.; Huh, J.; Kim, S.H. Effect of amine double-functionalization on CO2 adsorption behaviors of silica gel-supported adsorbents. Adsorption. 2016, 22(8), 1137-1146. [CrossRef]
- Fillerup, E.; Zhang, Z.; Peduzzi, E.; Wang, D.; Guo, J.; Ma, X.; Wang, X.; Song, C. CO Capture from Flue Gas Using Solid Molecular Basket Sorbents. The Pennsylvania State University. 2012. [CrossRef]
- Gargiulo, N.; Peluso, A.; Aprea, P.; Pepe, F.; Caputo, D. CO2 adsorption on polyethylenimine-functionalized SBA-15 mesoporous silica: isotherms and modeling. J. Chem. Eng. Data. 2014. 59(3), 896-902. [CrossRef]
- Heydari-Gorji, A.; Sayari, A.; Thermal, oxidative, and CO2-induced degradation of supported polyethylenimine adsorbents. Ind. Eng. Chem. Res., 2012. 51(19), 6887-6894. [CrossRef]
- Kuwahara, Y.; Kang, D.Y.; Copeland, J.R.; Brunelli, N.A.; Didas, S.A.; Bollini, P; Sievers, C.; Kamegawa, T.; Yamashita, H.; Jones, C.W. Dramatic enhancement of CO2 uptake by poly (ethyleneimine) using zirconosilicate supports. J. Am. Chem. Soc.2012. 134(26), 10757-10760. [CrossRef]
- Kishor, R.; Ghoshal, A.K. Amine-modified mesoporous silica for CO2 adsorption: the role of structural parameters. Ind. Eng. Chem. Res., 2017. 56(20), 6078-6087. [CrossRef]
- Ko, Y.G.; Lee, H.J.; Oh, H.C.; Choi, U.S. Amines immobilized double-walled silica nanotubes for CO2 capture. J. Hazard. Mater. 2013. 250, 53-60. [CrossRef]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of amine-tethered solid sorbents for direct CO2 capture from the ambient air. Environ. Sci. Technol. 2011. 45(6), 2420-2427. [CrossRef]
- Guo, X.; Ding, L.; Kanamori, K.; Nakanishi, K.; Yang, H. Functionalization of hierarchically porous silica monoliths with polyethyleneimine (PEI) for CO2 adsorption. Microporous and Mesoporous Materials, 2017. 245, 51-57. [CrossRef]
- Liu, Z.; Teng, Y.; Zhang, K.; Chen, H.; Yang, Y. CO2 adsorption performance of different amine-based siliceous MCM-41 materials. J. Energy Chem. 2015. 24(3), 322-330. [CrossRef]
- de Carvalho, L.S.; Silva, E.; Andrade, J.C.; Silva, J.A.; Urbina, M.; Nascimento, P.F.; Carvalho, F.; Ruiz, J.A. 2015. Low-cost mesoporous adsorbents amines-impregnated for CO2 capture. Adsorption. 2015, 21(8), 597-609. [CrossRef]
- López-Aranguren, P.; Builes, S.; Fraile, J.; López-Periago, A.; Vega, L.F.; Domingo, C. Hybrid aminopolymer–silica materials for efficient CO2 adsorption. RSC advances. 2015, 5(127), 104943-104953. [CrossRef]
- Mello, M.R.; Phanon, D.; Silveira, G.Q.; Llewellyn, P.L.; Ronconi, C.M. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture. Microporous and Mesoporous Materials. 2011. 143(1), 174-179. [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S.; Farooq, M. Adsorption behavior of tetraethylenepentamine-functionalized Si-MCM-41 for CO2 adsorption. Chem Eng Res Des. 2017. 122, 33-42. [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interfaces. 2016, 8(27), 17312-17320. [CrossRef]
- Ullah, R.; Atilhan, M.; Aparicio, S.; Canlier, A.; Yavuz, C.T. 2015. Insights of CO2 adsorption performance of amine impregnated mesoporous silica (SBA-15) at wide range pressure and temperature conditions. Int. J. Greenh. Gas Control. 2015, 43, 22-32. [CrossRef]
- Sánchez-Vicente, Y.; Stevens, L.A.; Pando, C.; Torralvo, M.J.; Snape, C.E.; Drage, T.C.; Cabañas, A. 2015. A new sustainable route in supercritical CO2 to functionalize silica SBA-15 with 3-aminopropyltrimethoxysilane as material for carbon capture. J. Chem. Eng.2015, 264, 886-898. [CrossRef]
- Zhao, A.; Samanta, A.; Sarkar, P.; Gupta, R. 2013. Carbon dioxide adsorption on amine-impregnated mesoporous SBA-15 sorbents: experimental and kinetics study. Ind. Eng. Chem. Res. 2013.52(19), 6480-6491. [CrossRef]
- Gholami, M.; Talaie, M.R.; Aghamiri, S.F. Direct synthesis of bi-modal porous structure MCM-41 and its application in CO2 capturing through amine-grafting. Korean Journal of Chemical Engineering. 2014.31(2), 322-326. [CrossRef]
- Loganathan, S.; Tikmani, M.; Ghoshal, A.K. Novel pore-expanded MCM-41 for CO2 capture: synthesis and characterization. Langmuir.2013, 29(10), 3491-3499. [CrossRef]
- Kassab, H.; Maksoud, M.; Aguado, S.; Pera-Titus, M.; Albela, B. Bonneviot, L. Polyethylenimine covalently grafted on mesostructured porous silica for CO2 capture. RSC advances. 2012, 2(6), 2508-2516. [CrossRef]
- Teng, Y.; Li, L.; Xu, G.; Zhang, K.; Li, K. Promoting effect of inorganic alkali on carbon dioxide adsorption in amine-modified MCM-41. Energies. 2016. 9(9), 667. [CrossRef]
- Sharma, P.; Seong, J.K.; Jung, Y.H.; Choi, S.H.; Park, S.D.; Yoon, Y.I. Baek, I.H. Amine modified and pelletized mesoporous materials: Synthesis, textural–mechanical characterization and application in adsorptive separation of carbondioxide. Powder technology. 2012, 219, 86-98. [CrossRef]
- Yan, X.; Komarneni, S.; Yan, Z. CO2 adsorption on Santa Barbara Amorphous-15 (SBA-15) and amine-modified Santa Barbara Amorphous-15 (SBA-15) with and without controlled microporosity. J. Colloid Interface Sci. 2013, 390(1), 217-224. [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. Amino functionalized mesostructured SBA-15 silica for CO2 capture: Exploring the relation between the adsorption capacity and the distribution of amino groups by TEM. Microporous and Mesoporous Materials. 2012. 158, 309-317. [CrossRef]
- Rao, N.; Wang, M.; Shang, Z.; Hou, Y.; Fan, G.; Li, J. CO2 adsorption by amine-functionalized MCM-41: a comparison between impregnation and grafting modification methods. Energy and Fuels. 2018. 32(1), 670-677. [CrossRef]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.B.; Gunathilake, C.; Jaroniec, M. CO2 adsorption on amine-functionalized periodic mesoporous benzenesilicas. ACS applied materials & interfaces. 2015, 7(12), 6792-6802. [CrossRef]
- Dassanayake, R.S.; Gunathilake, C.; Dassanayake, A.C.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized nanocrystalline cellulose–mesoporous silica composites for carbon dioxide sorption at ambient and elevated temperatures. J. Mater. Chem. A. 2017, 5(16), 7462-7473. [CrossRef]
- Gunathilake, C.; Dassanayake, R.S.; Abidi, N.; Jaroniec, M. Amidoxime-functionalized microcrystalline cellulose–mesoporous silica composites for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. A., 2016, 4(13), 4808-4819. [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous calcium oxide–silica and magnesium oxide–silica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A., 2016, 4(28), 10914-10924. [CrossRef]
- Gunathilake, C., Dassanayake, R.S., Kalpage, C.S. and Jaroniec, M. Development of Alumina–Mesoporous Organosilica Hybrid Materials for Carbon Dioxide Adsorption at 25° C. Materials. 2018, 11(11), 2301. [CrossRef]
- Gunathilake, C.; Gangoda, M.; Jaroniec, M. 2013. Mesoporous isocyanurate-containing organosilica–alumina composites and their thermal treatment in nitrogen for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. A. 2013, 1(28), 8244-8252. [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous alumina–zirconia–organosilica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A. 2015,3(6), 2707-2716. [CrossRef]
- Choi, W.; Min, K.; Kim, C. Ko, Y.S.; Jeon, J.W.; Seo, H.; Park, Y.K.; Choi, M. Epoxide-functionalization of polyethyleneimine for synthesis of stable carbon dioxide adsorbent in temperature swing adsorption. Nature communications. 2016 7(1), 1-8. [CrossRef]
- Hu, Y.; Liu, W.; Yang, Y.; Qu, M.; Li, H. 2019. CO2 capture by Li4SiO4 sorbents and their applications: current developments and new trends. J. Chem. Eng. 2019. 359, 604-625. [CrossRef]
- Wang, L.; Yang, R.T. Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J. Phys. Chem. C .2011. 115(43), 21264-21272. [CrossRef]
- Rafigh, S.M.; Heydarinasab, A., 2017. Mesoporous chitosan–SiO2 nanoparticles: synthesis, characterization, and CO2 adsorption capacity. ACS Sustain. Chem. Eng. 2017. 5(11), 10379-10386. [CrossRef]
- Yang, J.; Li, J.; Wang, W.; Li, L.; Li, J. Adsorption of CO2, CH4, and N2 on 8-, 10-, and 12-membered ring hydrophobic microporous high-silica zeolites: DDR, silicalite-1, and beta. Ind. Eng. Chem. Res.2013, 52(50), 17856-17864. [CrossRef]
- Zohdi, S.; Anbia, M.; Salehi, S. Improved CO2 adsorption capacity and CO2/CH4 and CO2/N2 selectivity in novel hollow silica particles by modification with multi-walled carbon nanotubes containing amine groups. Polyhedron. 2019, 166, 175-185. [CrossRef]
- Fernandes, J.; Fernandes, A.C.; Echeverría, J.C.; Moriones, P.; Garrido, J.J.; Pires, J. Adsorption of gases and vapours in silica based xerogels. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2019, 561, 128-135. [CrossRef]
- Liu, X.; Gao, F.; Xu, J.; Zhou, L.; Liu, H.; Hu, J. Zeolite@ Mesoporous silica-supported-amine hybrids for the capture of CO2 in the presence of water. Microporous and Mesoporous Materials. 2016, 222, 113-119. [CrossRef]
- Wei, L.; Jing, Y.; Gao, Z.; Wang, Y. 2015. Development of a pentaethylenehexamine-modified solid support adsorbent for CO2 capture from model flue gas. Chinese Journal of Chemical Engineering.2015, 23(2), 366-371. [CrossRef]
- Yan, X.; Zhang, L.; Zhang, Y.; Yang, G.; Yan, Z. Amine-modified SBA-15: effect of pore structure on the performance for CO2 capture. Industrial & Engineering Chemistry Research. 2011,50(6), 3220-3226. [CrossRef]
- Zhang, X.; Qin, H.; Zheng, X.; Wu, W. Development of efficient amine-modified mesoporous silica SBA-15 for CO2 capture. Materials Research Bulletin. 2013, 48(10), 3981-3986. [CrossRef]
- Chen, C.; Son, W.J.; You, K.S.; Ahn, J.W.; Ahn, W.S. Carbon dioxide capture using amine-impregnated HMS having textural mesoporosity. Chemical Engineering Journal. 2010, 161(1-2), 46-52. [CrossRef]
- Subagyono, D.J.; Marshall, M.; Knowles, G.P.; Chaffee, A.L. CO2 adsorption by amine modified siliceous mesostructured cellular foam (MCF) in humidified gas. Microporous and mesoporous materials. 2014, 186, 84-93. [CrossRef]
- Ma, J.; Liu, Q.; Chen, D.; Wen, S.; Wang, T. CO2 adsorption on amine-modified mesoporous silicas. J. Porous Mater. 2014, 21(5), 859-867. [CrossRef]
- Zhang, H.; Goeppert, A.; Prakash, G.S.; Olah, G. Applicability of linear polyethylenimine supported on nano-silica for the adsorption of CO2 from various sources including dry air. RSC Advances. 2015, 5(65), 52550-52562. [CrossRef]
- Wang, X.; Chen, L.; Guo, Q. Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture. Chemical Engineering Journal.2015, 260, 573-581. [CrossRef]
- Liu, J.L.; Lin, R.B. Structural properties and reactivities of amino-modified silica fume solid sorbents for low-temperature CO2 capture. Powder technology. 2013, 241, 188-195. [CrossRef]
- Li, K.; Jiang, J.; Tian, S.; Yan, F.; Chen, X. Polyethyleneimine–nano silica composites: a low-cost and promising adsorbent for CO2 capture. J. Mater. Chem. A. 2015, 3(5), 2166-2175. [CrossRef]
- Li, K.; Jiang, J.; Yan, F.; Tian, S.; Chen, X. The influence of polyethyleneimine type and molecular weight on the CO2 capture performance of PEI-nano silica adsorbents. Applied energy. 2014, 136, 750-755. [CrossRef]
- Quang, D.V.; Hatton, T.A.; Abu-Zahra, M.R. Thermally stable amine-grafted adsorbent prepared by impregnating 3-aminopropyltriethoxysilane on mesoporous silica for CO2 capture. Ind. Eng. Chem. Res.2016, 55(29), 7842-7852. [CrossRef]
- Zeng, W.; Bai, H. High-performance CO2 capture on amine-functionalized hierarchically porous silica nanoparticles prepared by a simple template-free method. Adsorption. 2016,22(2), 117-127. [CrossRef]
- Linneen, N.N.; Pfeffer, R.; Lin, Y.S. CO2 adsorption performance for amine grafted particulate silica aerogels. Chemical Engineering Journal. 2014,254, 190-197. [CrossRef]
- Le, Y.; Guo, D.; Cheng, B.; Yu, J. Amine-functionalized monodispersed porous silica microspheres with enhanced CO2 adsorption performance and good cyclic stability. J. Colloid Interface Sci. 2013, 408, 173-180. [CrossRef]
- Singh, B.; Polshettiwar, V. 2016. Design of CO2 sorbents using functionalized fibrous nanosilica (KCC-1): insights into the effect of the silica morphology (KCC-1 vs. MCM-41). J. Mater. Chem. A. 2016, 4(18), 7005-7019. [CrossRef]
- Zhang, L.; Zhan, N.; Jin, Q.; Liu, H.; Hu, J. Impregnation of polyethylenimine in mesoporous multilamellar silica vesicles for CO2 capture: a kinetic study. Ind. Eng. Chem. Res.2016, 55(20), 5885-5891. [CrossRef]
- Zhou, L.; Fan, J.; Cui, G.; Shang, X.; Tang, Q.; Wang, J.; Fan, M. Highly efficient and reversible CO2 adsorption by amine-grafted platelet SBA-15 with expanded pore diameters and short mesochannels. Green Chemistry. 2014, 16(8),4009-4016. [CrossRef]
- Mittal, N.; Samanta, A.; Sarkar, P.; Gupta, R. Postcombustion CO2 capture using N-(3-trimethoxysilylpropyl) diethylenetriamine-grafted solid adsorbent. Energy Sci. Eng.2015, 3(3), 207-220. [CrossRef]
- Yao, M.; Dong, Y.; Feng, X.; Hu, X.; Jia, A.; Xie, G.; Hu, G.; Lu, J.; Luo, M.; Fan, M. The effect of post-processing conditions on aminosilane functionalizaiton of mesocellular silica foam for post-combustion CO2 capture. Fuel. 2014,123, 66-72. [CrossRef]
- Ko, Y.G.; Lee, H.J.; Kim, J.Y.; Choi, U.S.; 2014. Hierarchically porous aminosilica monolith as a CO2 adsorbent. ACS Appl. Mater. Interfaces.2014. 6(15), 12988-12996. [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the direct capture of CO2 from ambient air. Angewandte Chemie International Edition. 2020. 59(18), 6984-7006. [CrossRef]
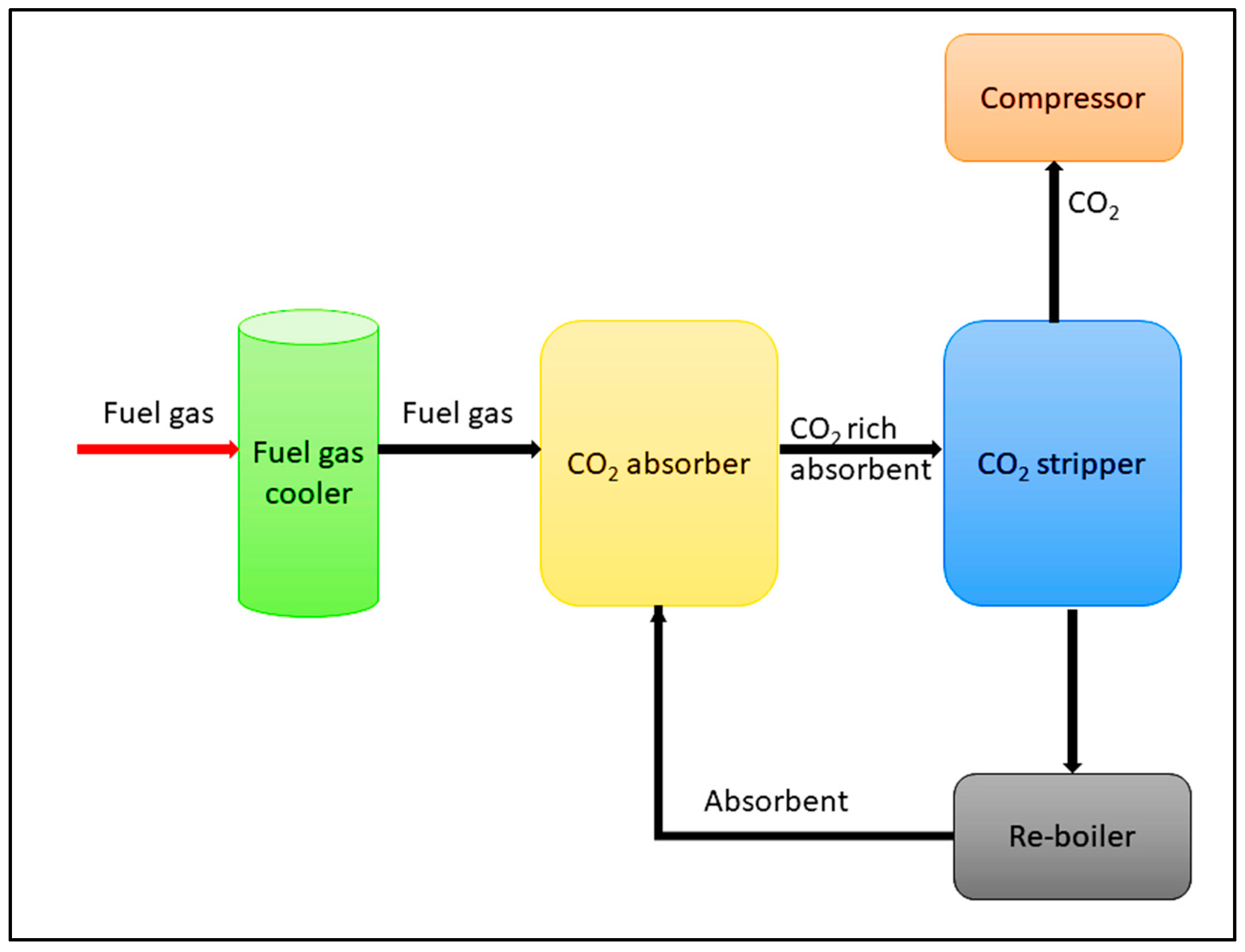
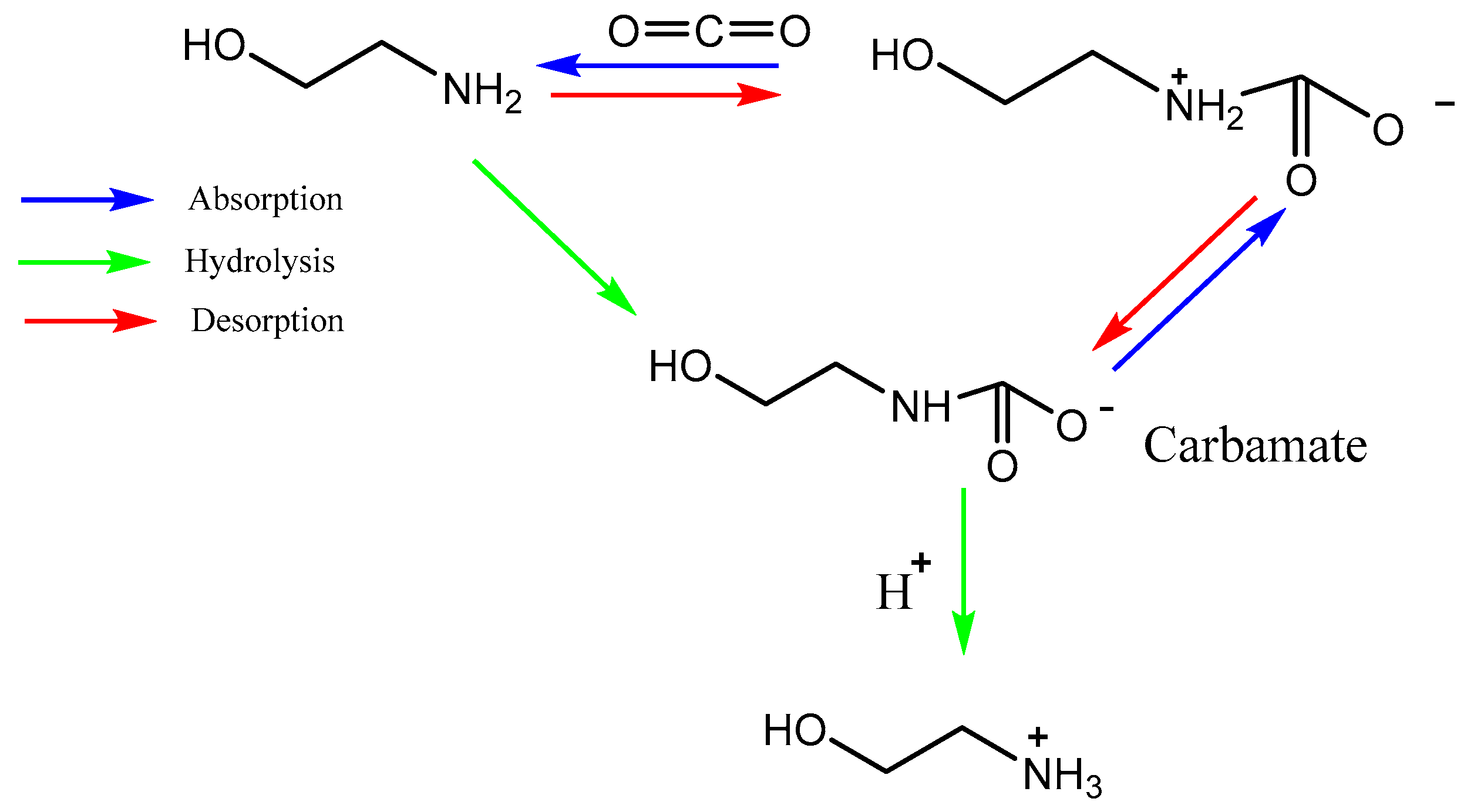
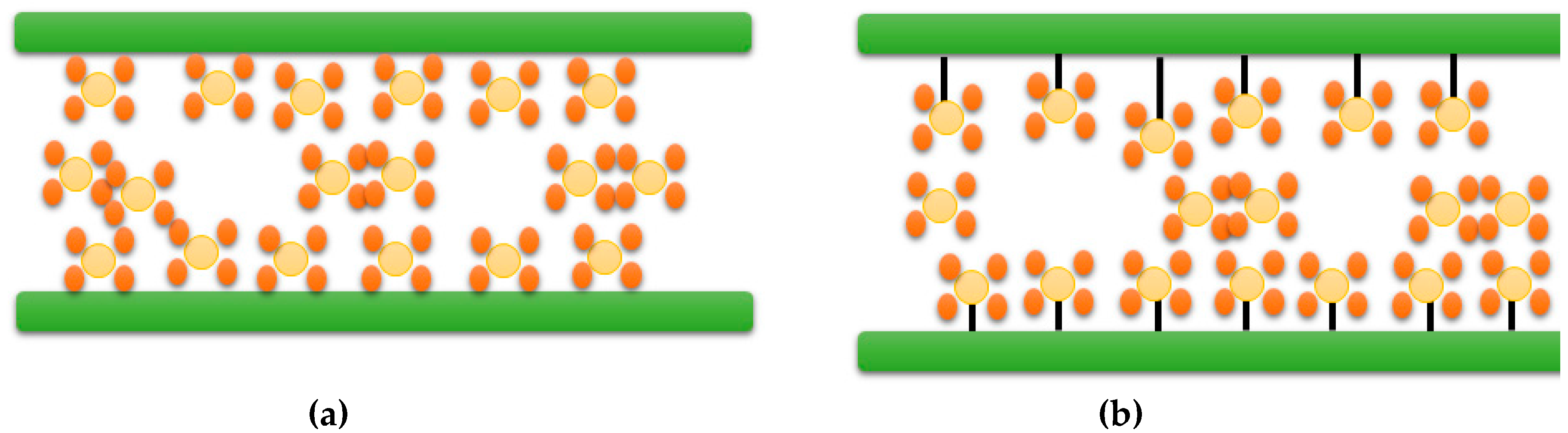
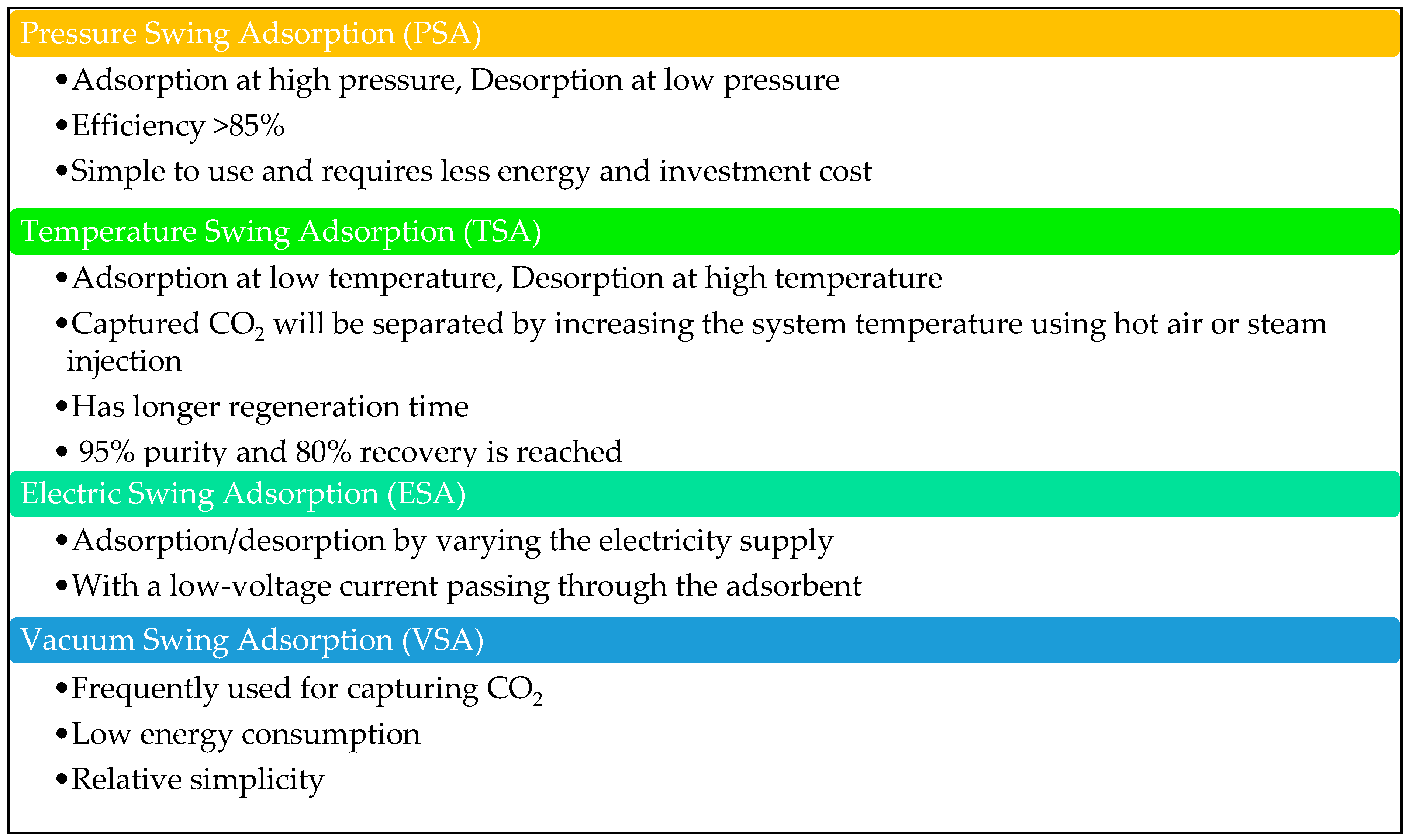
| Type of approach | Details | References |
|---|---|---|
| Improve energy efficiency and promote energy conservation |
|
[4] |
| Increase of usage of low carbon or clean fuels such as natural gas, hydrogen or nuclear power; Substitution for Power generation |
|
[4] |
| Deploy renewable energy |
|
[4] |
| CO2 capture and storage |
|
[4] |
| Technology | Types | Examples | Efficiency (%) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Absorption | Chemical | Amines Caustics |
> 90 | ● Ability to regenerate ● Established method ● Very flexible ● Reacts rapidly ● High absorption capacities |
● High energy requirement for regeneration ● Environmental problems ● High boiling point ● Equipment corrosion |
[21,22] |
| Physical | Selexol Rectisol fluorinated solvents |
|||||
| Adsorption | Chemical | Metal Oxides Si based materials |
>85 | ● Recyclable ● Cost effective ● High stability ● Adjustable catalytic site and pore sizes ● Low energy consumption ● Suitable for separating CO2 from dilute streams |
● High energy cost ● Limited to process feed rates ● Loss of material and pressure drop ● Decreased catalytic efficiency ● Low adsorption capacities |
[6,21] |
| Physical | Carbons Zeolites Si based materials |
|||||
| Membrane-based technologies | Organic Cellulose derivatives Polyamides |
Polyphenyleneoxide, Polydimethylsiloxane |
>80 | ● Simple device ● Easy production process and process flow scheme ● Low energy consumption ● No phase changes ● Capable of maintaining the membrane structure |
● Requires a high-cost module and support materials ● Not suitable for large volumes of emission gases ● Reduced selectivity and separation ● Pressure drops across the membrane ● Less durability |
[6,21] |
| Inorganic | Metallic Ceramics |
|||||
| Cryogenic distillation | ● Low capital investment ● High reliability ● Recovery with high purity of CO2 ● Liquid CO2 production ● Not requiring solvents or other components ● Easily scalable to industrial-scale applications |
● High energy consumption | [6,21,23] |
| Material types | Examples | Advantages | Disadvantages |
|---|---|---|---|
|
Pours silica materials |
M41S SBA-n AMS |
● High specific surface area, Pore volume, and good thermal and mechanical properties | ● High molecular diffusion resistance ● Decreased adsorption capacity at high temperature [42] |
| Zeolites | NaY 13X |
● Low production cost ● Large micropores/mesopores ● Medium CO2 adsorption capacity at room temperature |
● Low CO2 adsorption capacity ● Moisture-sensitivity ● High energy consumption [6,43] |
|
Metal organic frameworks (MOFs) |
M-MOF- 74 IRMOF-6 USO-2-Ni Zn4O(BDC)3 (MOF-5) USO-1-Al(MIL-53) |
● Large specific surface area ● Ease of controlling pore sizes ● High selectivity of CO2 |
● Low CO2 adsorption capacity at the partial pressure ● High production cost ● Complicated synthesis process ● Moisture-sensitivity ● Unstable at high temperature [6] |
|
Alkali-based dry adsorbents |
● Possible adsorption and desorption at a low temperature and wet conditions | ● Low adsorption capability (3–11 wt.%) ● High-temperature reactions ● Requires high temperatures during desorption Complicated operation [6] |
|
|
Metal oxides-based adsorbents |
CaO, MgO | ● Dry chemical adsorbents ● Adsorption/desorption at medium to high temperatures |
● High energy consumption ● High cost for regeneration ● Complicated process [6] |
| Chemisorption | Physisorption | |
|---|---|---|
| Description | ● Chemical reaction occurs between the solid sorbents and CO2 | ● Depends on the physical properties of CO2 and the ability to engage in noncovalent interactions with the solid sorbent |
| Chemical Bonding | ● Covalent Bonding-Occur between functional groups and CO2 in the surface | ● Week Vander-walls forces-London and Dispersion forces, Occur inside pore walls |
| Advantages | ● High selectivity | ● Low recycling energy requirements ● High working capacity ● High selectivity even in wet environments ● Fast |
| Disadvantages | ● High energy required for recycling and the breakage of the chemical bonds ● Slow reactivity |
● Poor selectivity in binary or mixed gas applications |
| References | [55,56] | [57,58,59] |
| Porous SiO2 material | Gas mixture | Selectivity value | Pressure (bar) | Temperature (°C) |
Reference |
|---|---|---|---|---|---|
|
PEI- MCM-41 |
CO2 , N2 and H2 | 25.56 | 1 | 100 | [95] |
| SBA-15 | CO2/N2 | 123 | 1 | 25 | [156] |
| SBA-15 (calcination) | CO2/N2 | 55 | 1 | 25 | [156] |
| Mesoporous chitosan−SiO2 nanoparticles | - | 15.46 | 1 | 25 | [157] |
| hydrophobic microporous high-silica zeolites | CH4:N2 = 50%:50% | 36.5 | 1 | 25 | [158] |
| Hollow silica spherical particles (HSSP) | CO2/N2 | 8.5 | 4 | 25 | [159] |
| microporous silicaxerogel | CO2/CH4 | 60 | 6 | 25 | [160] |
| Silica based xerogels | C2H4/C2H6 | 20 | 6 | 25 | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





