Submitted:
02 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
1. Materials and Methods
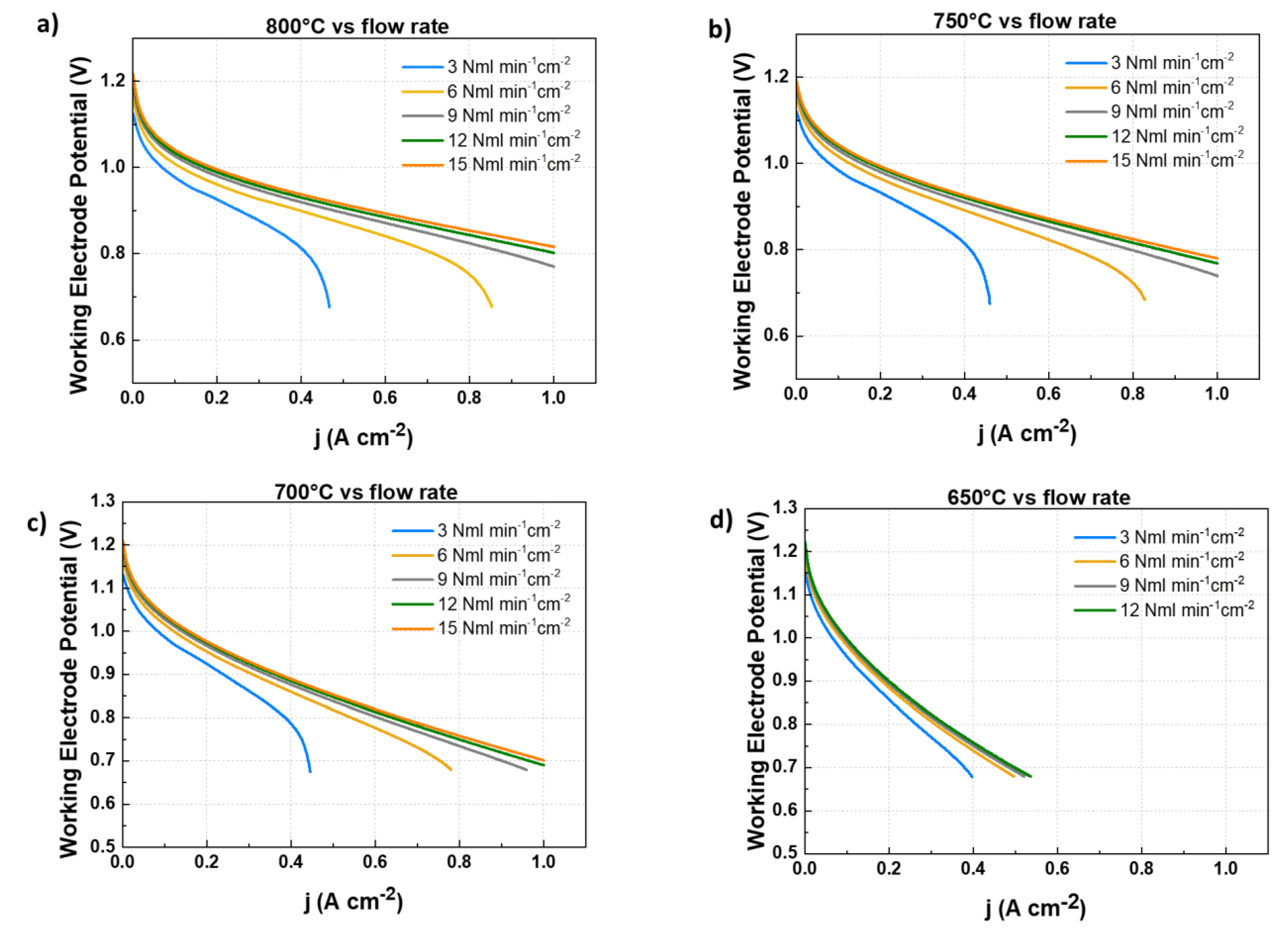
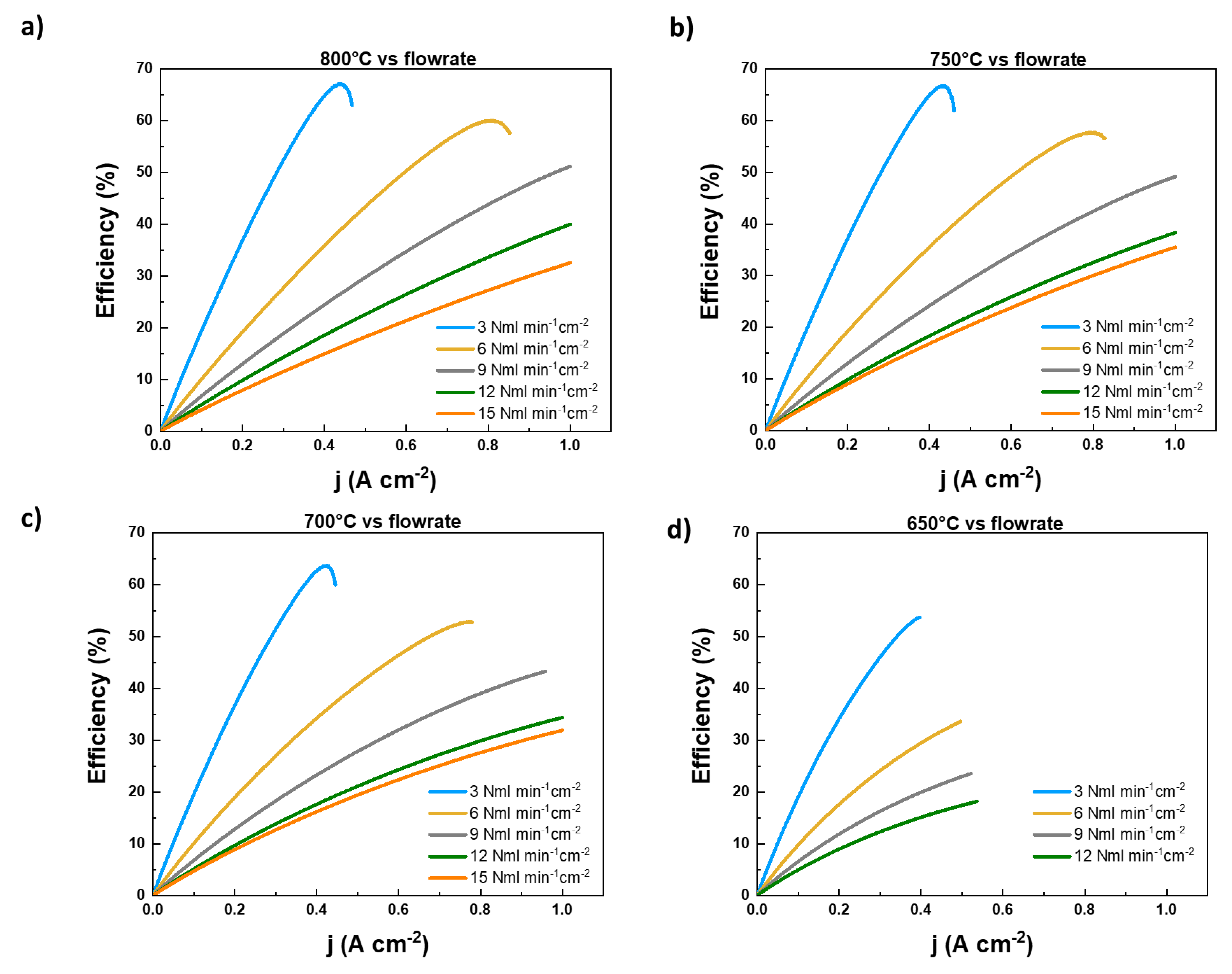
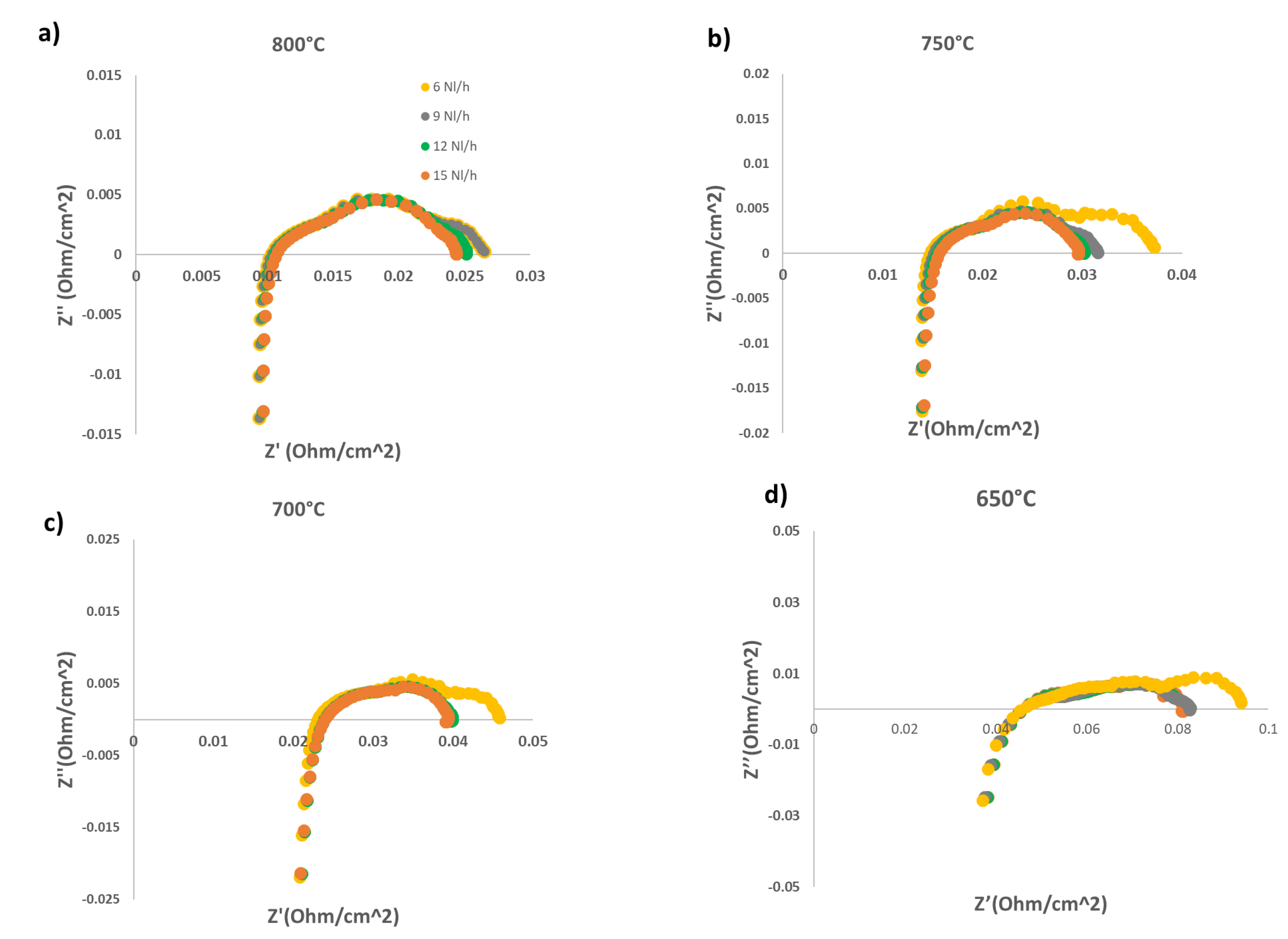
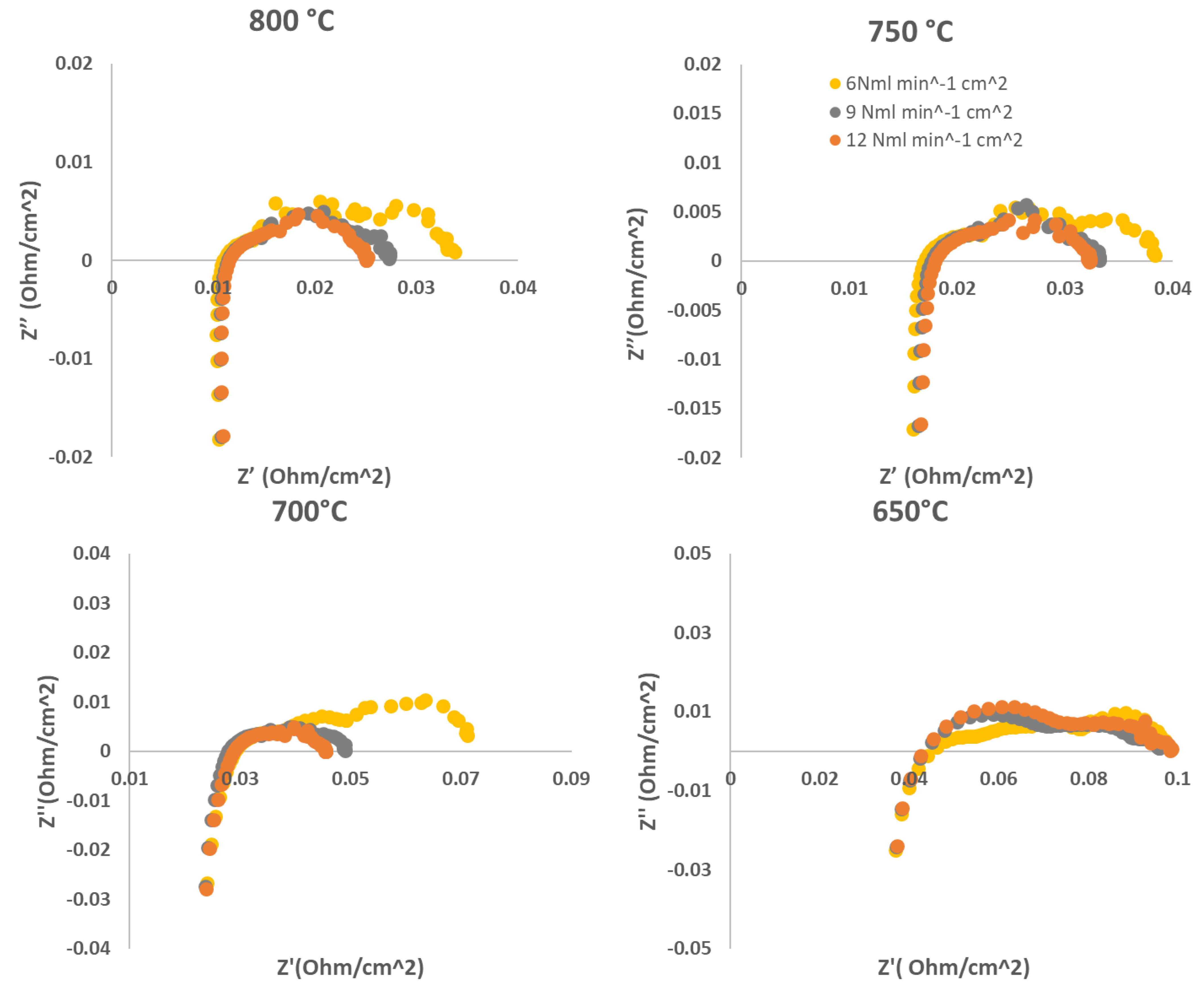
- In the first step, we evaluated the performance of the above described cell fuelled by different hydrogen flowrates; the cell has been tested at four different working temperatures (800°C, 750°C, 700°C and 650°C). For each temperature, different H2 feeding flowrates (3 Nml min-1cm-2, 6 Nml min-1cm-2,, 9 Nml min-1cm-2, 12 Nml min-1cm-2, 15 Nml min-1cm-2) have been considered. The performance of the cell is evaluated acquiring j-V curves and EIS spectra for each combination of temperature and flow rate feeding.
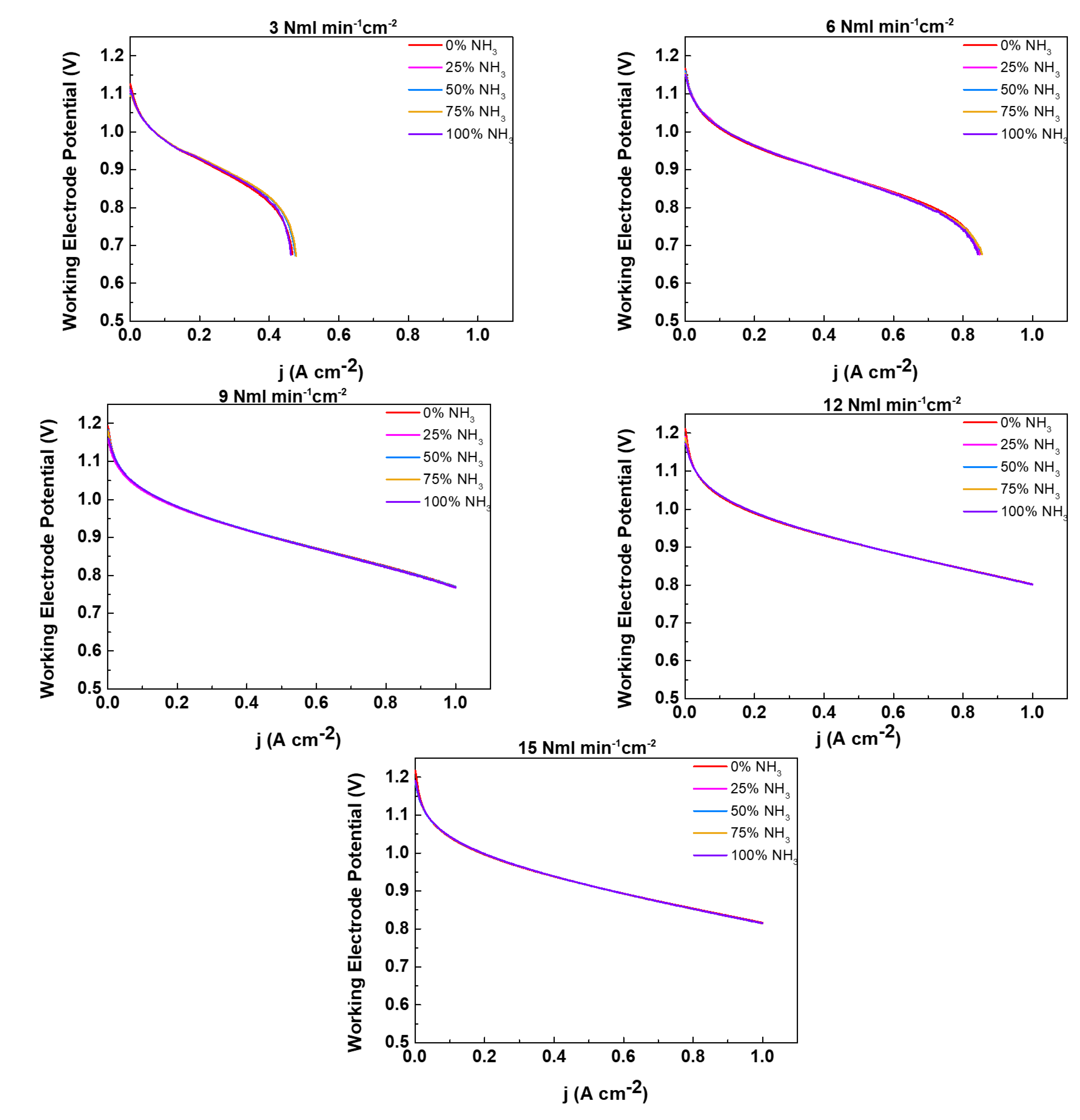
- In the second step, we evaluate how the performance of the cell changes according to fuel feeding; for each of the above mentioned working temperatures and H2 feeding flowrates, the amount of hydrogen is substituted by NH3 at different percentages (0%, 25%, 50%, 75% and 100%). The complete study is composed by j-V curves acquired for each combination of working temperature, gas flowrate and NH3 % . Moreover, EIS spectra have been acquired for each investigated operating condition.
2. Results and discussion
2.1. Evaluation of diluted H2 feeding performance
2.2. NH3 feeding performance evaluation
Conclusions
References
- Hagen, H. Langnickel, X. Sun, ScienceDirect Operation of solid oxide fuel cells with alternative hydrogen carriers. Int. J. Hydrogen Energy. 2019, 44, 18382–18392. [Google Scholar] [CrossRef]
- N. J.J. Dekker, G. Rietveld, Highly efficient conversion of ammonia in electricity by solid oxide fuel cells. J. Fuel Cell Sci. Technol. 2006, 3, 499–502. [Google Scholar] [CrossRef]
- Lucentini, X. Garcia, X. Vendrell, J. Llorca, Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- J. Humphreys, R. Lan, S. Tao, Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- M. A. Abdelkareem, K. Elsaid, T. Wilberforce, M. Kamil, E.T. Sayed, A. Olabi, Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef]
- D. Papurello, S. Silvestri, S. Modena, Biogas trace compounds impact on high-temperature fuel cells short stack performance. Int. J. Hydrogen Energy. 2021, 46, 8792–8801. [Google Scholar] [CrossRef]
- J. Zhou, Z. Wang, M. Han, Z. Sun, K. Sun, Optimization of a 30 kW SOFC combined heat and power system with different cycles and hydrocarbon fuels. Int. J. Hydrogen Energy. 2022, 47, 4109–4119. [Google Scholar] [CrossRef]
- Z. Chen, S. Z. Chen, S. Ristig, M. Poschmann, J. Folke, O. Go, S. Heumann, H. Ruland, Ammonia Decomposition in the Process Chain for a Renewable Hydrogen Supply, (2022) 1413–1425. [CrossRef]
- G. Cinti, U. Desideri, D. Penchini, G. Discepoli, Experimental analysis of SOFC fuelled by ammonia. Fuel Cells. 2014, 14, 221–230. [Google Scholar] [CrossRef]
- S. Mukherjee, S. V. Devaguptapu, A. Sviripa, C.R.F. Lund, G. Wu, Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- S. Hussain, L. Yangping, Review of solid oxide fuel cell materials: cathode, anode, and electrolyte. Energy Transitions. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- N. Mahato, A. Banerjee, A. Gupta, S. Omar, K. Balani, Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- A. J. Jacobson, Materials for solid oxide fuel cells, Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- D. J.L. Brett, A. Atkinson, N.P. Brandon, S.J. Skinner, Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, A. Shipilova, E. Smolyanskiy, S. Rabotkin, V. Semenov, The Properties of Intermediate-Temperature Solid Oxide Fuel Cells with Thin Film Gadolinium-Doped Ceria Electrolyte. Membranes (Basel). 2022, 12, 1–7. [Google Scholar] [CrossRef]
- C. W. Kwon, J.W. Son, J.H. Lee, H.M. Kim, H.W. Lee, K.B. Kim, High-performance micro-solid oxide fuel cells fabricated on nanoporous anodic aluminum oxide templates. Adv. Funct. Mater. 2011, 21, 1154–1159. [Google Scholar] [CrossRef]
- A. A. Solovyev, N.S. Sochugov, S. V. Rabotkin, A. V. Shipilova, I. V. Ionov, A.N. Kovalchuk, A.O. Borduleva, Application of PVD methods to solid oxide fuel cells. Appl. Surf. Sci. 2014, 310, 272–277. [Google Scholar] [CrossRef]
- P. Coddet, H. lin Liao, C. Coddet, A review on high power SOFC electrolyte layer manufacturing using thermal spray and physical vapour deposition technologies. Adv. Manuf. 2014, 2, 212–221. [Google Scholar] [CrossRef]
- Barone, A. Galdi, N. Lampis, L. Maritato, F.M. Granozio, S. Pagano, P. Perna, M. Radovic, U.S. Uccio, Charge density waves enhance the electronic noise of manganites. (2009) 1–6. [CrossRef]
- P. Orgiani, C. Adamo, C. Barone, A. Galdi, S. Pagano, A.Y. Petrov, O. Quaranta, C. Aruta, R. Ciancio, M. Polichetti, D. Zola, L. Maritato, Epitaxial growth of La0.7 Ba0.3 Mn O3 thin films on MgO substrates: Structural, magnetic, and transport properties. J. Appl. Phys. 2008, 103, 0–8. [Google Scholar] [CrossRef]
- J. L. Makous, L. Maritato, C.M. Falco, J.P. Cronin, G.P. Rajendran, E. V Uhlmann, D.R. Uhlmann, Superconducting and structural properties of sputtered thin films of YBa2Cu3O7−x. Appl. Phys. Lett. 1987, 51, 2164–2166. [Google Scholar] [CrossRef]
- Andreone, A. DiChiara, G. Peluso, M. Santoro, C. Attanasio, L. Maritato, R. Vaglio, Surface impedance measurements of superconducting (NbTi)N films by a ring microstrip resonator technique. J. Appl. Phys. 1993, 73, 4500–4506. [Google Scholar] [CrossRef]
- N. Coppola, P. Polverino, G. Carapella, C. Sacco, A. Galdi, D. Montinaro, L. Maritato, C. Pianese, Optimization of the electrical performances in Solid Oxide Fuel Cells with room temperature sputter deposited Gd0.1ce0.9o1.95 buffer layers by controlling their granularity via the in-air annealing step. Int. J. Hydrogen Energy. 2020, 45, 12997–13008. [Google Scholar] [CrossRef]
- N. Coppola, P. N. Coppola, P. Polverino, G. Carapella, C. Sacco, A. Galdi, A. Ubaldini, V. Vaiano, D. Montinaro, L. Maritato, C. Pianese, Structural and electrical characterization of sputter-deposited Gd0.1Ce0.9O2−δ thin buffer layers at the Y-stabilized zirconia electrolyte interface for IT-solid oxide cells. Catalysts. 8 (2018). [CrossRef]
- N. Coppola, H. Sami, U. Rehman, G. Carapella, P. Polverino, D. Montinaro, F. Martinelli, V. Granata, A. Galdi, L. Maritato, C. Pianese, D. Studi, F. Sa, ScienceDirect Large area solid oxide fuel cells with room temperature sputtered barrier layers : Role of the layer thickness and uniformity in the enhancement of the electrochemical performances and durability. Int. J. Hydrogen Energy. (2023). [CrossRef]
- N. Coppola, P. Polverino, G. Carapella, R. Ciancio, P. Rajak, D. Montinaro, F. Martinelli, L. Maritato, C. Pianese, Large Area Deposition by Radio Frequency Sputtering of Gd0.1Ce0.9O1.95 buffer layers in Solid Oxide Fuel Cells: Structural, Morphological and Electrochemical Investigation. Materials (Basel). 2021, 14, 5826. [Google Scholar] [CrossRef]
- Hagen, J.O. Christensen, B.R. Sudireddy, S. Balomenou, D. Tsiplakides, K.-M. Papazisi, F. Zaravelis, C. Neofytidis, E. Ioannidou, S. Neophytides, D. Niakolas, N. Coppola, L. Maritato, P. Polverino, G. Carapella, C.J. Ferchaud, F. Berkel, J. Vulliet, J. Laurencin, Selection of Highlights of the European Project Next Generation Solid Oxide Fuel Cell and Electrolysis Technology – NewSOC. ECS Trans. 2021, 103, 2205–2216. [Google Scholar] [CrossRef]
- J. Laurencin, M. Hubert, D.F. Sanchez, S. Pylypko, M. Morales, A. Morata, B. Morel, D. Montinaro, F. Lefebvre-Joud, E. Siebert, Degradation mechanism of La0.6Sr0.4Co0.2Fe0.8O3-δ/Gd0.1Ce0.9O2-δ composite electrode operated under solid oxide electrolysis and fuel cell conditions. Electrochim. Acta. 2017, 241, 459–476. [Google Scholar] [CrossRef]
- M. Fardadi, D.F. McLarty, J. Brouwer, F. Jabbari, Enhanced performance of counter flow SOFC with partial internal reformation. Int. J. Hydrogen Energy. 2014, 39, 19753–19766. [Google Scholar] [CrossRef]
- Y. Komatsu, G. Brus, S. Kimijima, J.S. Szmyd, The effect of overpotentials on the transient response of the 300W SOFC cell stack voltage. Appl. Energy. 2014, 115, 352–359. [Google Scholar] [CrossRef]
- G. Brus, K. Miyoshi, H. Iwai, M. Saito, H. Yoshida, Change of an anode’s microstructure morphology during the fuel starvation of an anode-supported solid oxide fuel cell. Int. J. Hydrogen Energy. 2015, 40, 6927–6934. [Google Scholar] [CrossRef]
- N. J.J. Dekker, G. Rietveld, Highly Efficient Conversion of Ammonia in Electricity by Solid Oxide Fuel Cells. J. Fuel Cell Sci. Technol. 2006, 3, 499. [Google Scholar] [CrossRef]
- F. Baldi, L. F. Baldi, L. Wang, M. Pérez-Fortes, F. Maréchal, A cogeneration system based on solid oxide and proton exchange membrane fuel cells with hybrid storage for off-grid applications, Front. Energy Res. 6 (2019). [CrossRef]
- Oryshchyn, N.F. Harun, D. Tucker, K.M. Bryden, L. Shadle, Fuel utilization effects on system efficiency in solid oxide fuel cell gas turbine hybrid systems. Appl. Energy. 2018, 228, 1953–1965. [Google Scholar] [CrossRef]
- H. C. Yu, F. Zhao, A. V. Virkar, K.Z. Fung, Electrochemical characterization and performance evaluation of intermediate temperature solid oxide fuel cell with La0.75Sr 0.25CuO2.5-δ cathode. J. Power Sources. 2005, 152, 22–26. [Google Scholar] [CrossRef]
- Ahamer, A.K. Opitz, G.M. Rupp, J. Fleig, Revisiting the Temperature Dependent Ionic Conductivity of Yttria Stabilized Zirconia (YSZ). J. Electrochem. Soc. 2017, 164, F790–F803. [Google Scholar] [CrossRef]
- A. L.G. Stern, M., Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar]
- R. Besghaier, L. Dhouibi, M. Jeannin, M.J. Safi, The Synergetic Effect of Flow Velocity and Exposing Time on the Electrochemical Behavior of Cu–Ni 90/10 Alloy in Simulating Conditions of Desalination Plant, Chem. Africa. 2019, 2, 483–495. [Google Scholar] [CrossRef]
- Takahashi, T. Fujitani, Kinetic analysis of decomposition of ammonia over Nickel and Ruthenium catalysts. J. Chem. Eng. Japan. 2016, 49, 22–28. [Google Scholar] [CrossRef]
- A. S. Chellappa, C.M. Fischer, W.J. Thomson, Ammonia decomposition kinetics over Ni-Pt/Al2O3 for PEM fuel cell applications. Appl. Catal. A Gen. 2002, 227, 231–240. [Google Scholar] [CrossRef]
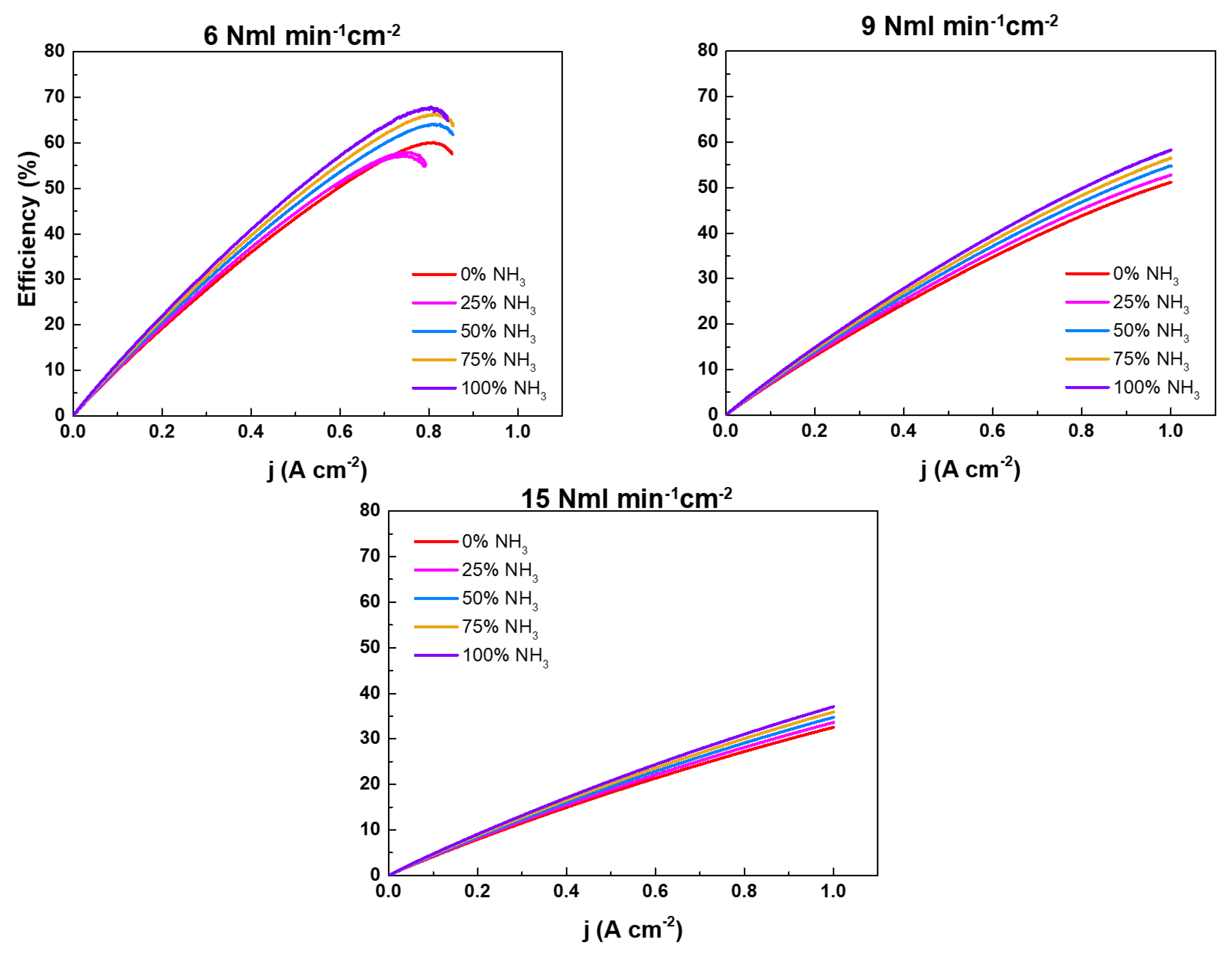
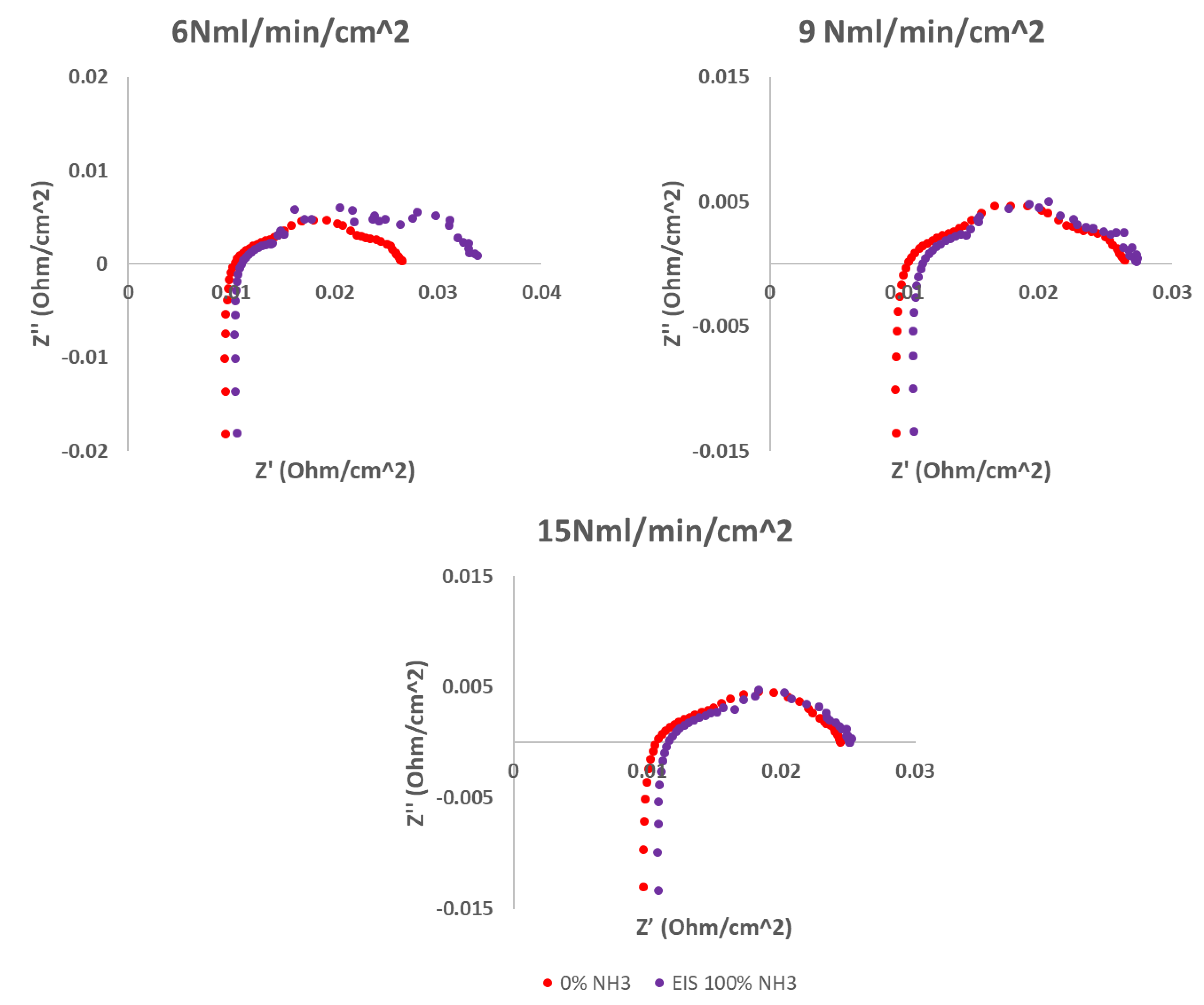
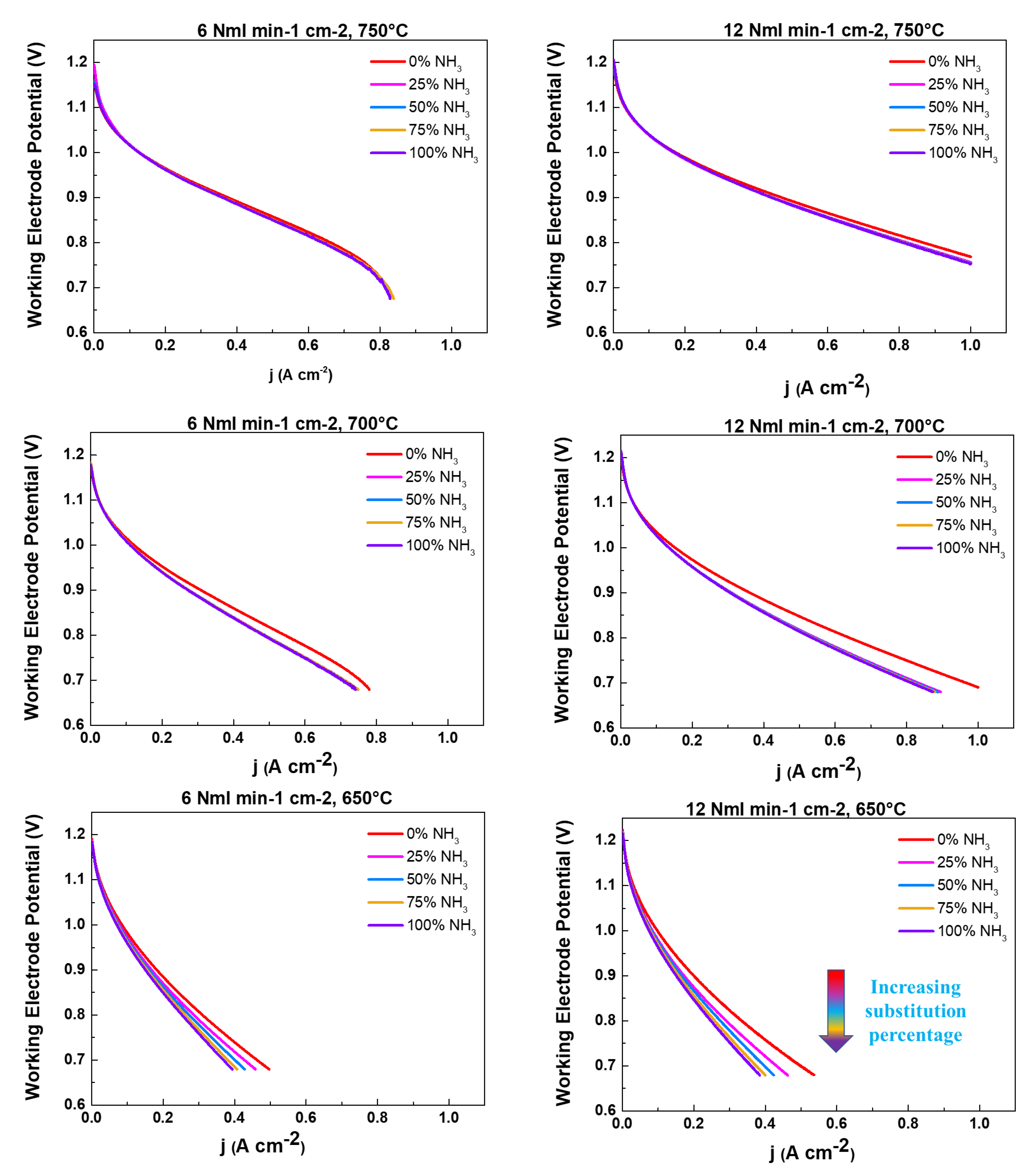
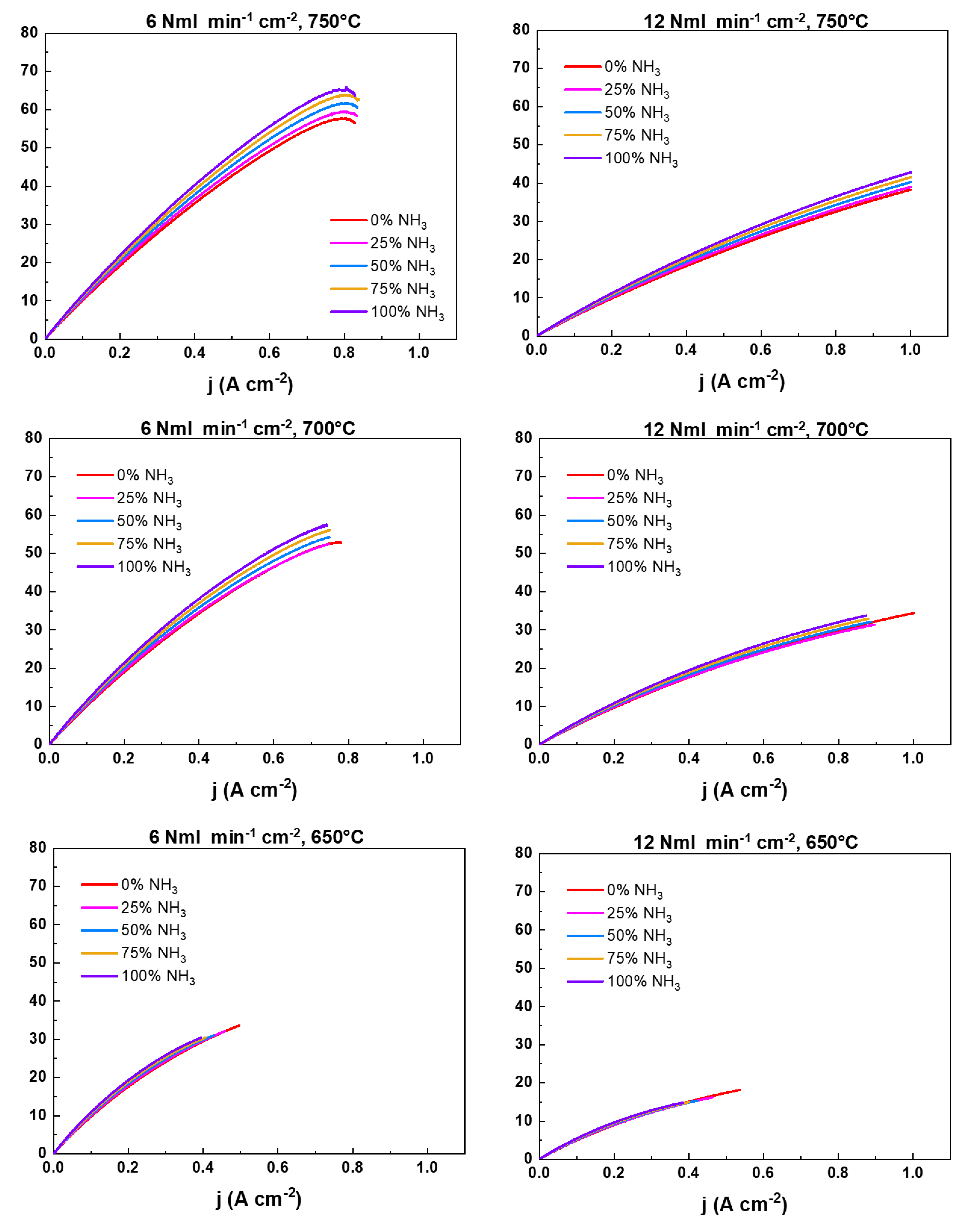
| Working temperatures (°C) | H2 (Nml min-1cm-2) | N2 (Nml min-1cm-2) |
|---|---|---|
| 800, 750, 700, 650 | 15 | 5 |
| 12 | 4 | |
| 9 | 3 | |
| 6 | 2 | |
| 3 | 1 |
| substituted % | NH3 | H2 | N2 |
|---|---|---|---|
| 0 | 0 | 150 | 50 |
| 25 | 25 | 113 | 38 |
| 50 | 50 | 75 | 25 |
| 75 | 75 | 38 | 13 |
| 100 | 100 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





