1. Introduction
Blueberry (
Vaccinium spp.) is a fruit that is highly valued by consumers for its distinctive flavour and aroma. Hailed by the media as a ‘superfood’, due to its high content of health-promoting compounds based on significant amounts of various phytochemicals, blueberries have gained much attention, becoming the second most valued berry in the United States after strawberries (U.S. Department of Agriculture (USDA), Economic Research Service (ERS), 2019). This has led to a 52% increase in global production over the last five years [
1]. In this sense, the inclusion of blueberries in the diet is a relatively easy way to add functionality and increase their commercial value.
Among the compounds to which many of the health benefits of blueberries are attributed are phenolic compounds including both flavonoid and non-flavonoid types, the most abundant of which are anthocyanins. They have been shown to have anti-inflammatory, antioxidant, and vasoprotective effects and, with that, a significant modulatory effect on cellular biomarkers related to oxidative stress and inflammation, which leads to chronic diseases such as type 2 diabetes, neurological decline and cardiovascular disease [
2,
3]. However, the content of phenolic compounds in soft fruit is influenced by many factors, including genetic differences, postharvest storage conditions and the degree of ripeness at harvest [
4,
5]. In this regard, it has been observed that anthocyanin accumulation in overripe blueberries continues after harvest and during postharvest storage, but it can also decrease depending on the oxidative stress to which it is subjected [
6].
An important aspect to consider when marketing blueberries is the loss of quality that can occur due to softening. Fruit softening is a complex horticultural trait that can be caused by numerous factors such as water and turgor loss, cell wall degradation and membrane damage [
7]. In addition, fungal proliferation can also accelerate these processes, as the high levels of sugars and other nutrients and low pH make bacteria less likely to grow, making it easier for yeast and fungi to proliferate. The most common fungi isolated from blueberries belong to the
Botrytis, Alternaria, Fusarium, Penicillium, Cladosporium, Trichoderma and
Aureobasidium families [
8].
Shelf-life is defined as the potential storage time of a product until it becomes unfit for human consumption, or it is rejected by customers, which depends on the quality of the fruit [
9]. Thus, quality parameters that are first perceived by the consumer, such as appearance (freshness) and texture (firmness), are very important, but so are others that determine subsequent purchases which are more related to taste and nutritional value, such as soluble solids content (SSC, mainly sugars), titratable acidity (TA), maturity index (MI: SSC/TA) and nutritional quality [
10]. Although blueberries are classified as a climacteric fruit, they should be harvested as close to commercial ripeness as possible [
8], as they are dependent on the plant for assimilates and do not improve their organoleptic characteristics, especially sweetness, after harvest due to the lack of starch reserves. Therefore, blueberries reach their optimum eating quality if they are left on the plant for a few days after they have turned completely blue. The main reason for the perishability of blueberries is their juicy pulp, their high respiration rate and the fact that they are usually harvested in summer, with high temperatures and humidity, which increases respiration rate and thus the ageing metabolism, resulting in weight loss, softening and decay [
11].
Despite the added value of blueberries, no studies have been carried out on how the fruit arrives at retail markets just before purchase by the consumer, in terms of firmness, physico-chemical parameters, phenolic compounds and fungal growth. Therefore, the aim of this work was to investigate possible differences in quality parameters such as firmness, flavonoid content and fungal contamination between blueberries purchased on the same day from ten different market places, regardless of the supplier. The results showed differences in the quality parameters analysed. While most of the samples show quality parameters in accordance with the standards in terms of pH, SSC and TA, only five of the ten samples show an adequate SSC/TA ratio. A general characteristic of all of them is the low maturity index reached at the point of sale. On the other hand, samples belonging to the same cultivar also show quality differences in terms of the quality parameters between them, suggesting that they have probably experimented with different growing conditions and/or storage that affect the quality when they reach the consumer. Finally, no differences were found between conventionally and organically grown blueberries.
2. Materials and Methods
2.1. Plant Material
Blueberries (
Vaccinium corymbosum L.) were sampled from ten different retail markets located in Madrid (Spain) in May 2021. Nine samples (1-4, 6-10) were of Spanish origin, three of which belonged to cv. Ventura (1-3), of which sample 1 was grown under organic conditions (
Table 1). Sample 4 belonged to cv. Snowchaser and sample number 5, originating from Morocco, belonged to the cv. Royal Blue aroma. For samples 6-10, only the origin is known to be Spain, but no cultivar information could be obtained. Fruit from each retail market was divided into three lots (biological replicates) and each analysed lot consisted of 136 pooled blueberries. Quality attributes of 40 blueberries from each lot were assessed (titratable acid, soluble solids content, pH, and fungal identification) and the mechanical properties of a further 36 blueberries per lot were analysed. In addition, 60 blueberries were randomly selected, frozen in liquid nitrogen and stored at -80 °C for further analysis.
2.2. Quality Assessments
For titratable acidity analysis, 10 g of the homogenised blueberry sample was diluted with 40 mL of deionised water. TA was measured by titration with 0.1 N NaOH to an endpoint of 8.2 using an 862 Compact Titrosampler (Metrohm, Spain) and expressed as % of citric acid (v/w). The soluble solids content was determined using a Mettler AT100 digital refractometer (Mettler, Spain). Finally, the pH of the juice obtained from the homogenised blueberries was measured using a micropH 2000 (Crison, Spain). All the measurements were carried out three times. The maturity index was calculated as the ratio of the above parameters (SSC/TA).
2.3. Mechanical Properties
The measurements of mechanical properties in blueberries were performed by using a TA.HDPlus texturometer (Stable Micro Systems, Ltd., Godalming, UK) equipped with a 30 kg load cell and with the Texture Exponent Software (6.1.13.0 version). A penetration test was performed by using a 2-mm diameter flat cylindrical stainless-steel probe (P/2). The penetration of the fruits was carried out at the berry equator to a penetration distance of 30% of each berry equatorial diameter. Test speed was set at 0.8 mm/s, considering a trigger test force of 0.1 N (10.2 g). During the penetration test, each blueberry was placed over a flat metal plate with the stem-root axis oriented parallel to the surface. Data acquisition was performed with a frequency of 500 points per second.
From the force-distance curve of each berry, the following mechanical parameters were calculated: the maximum skin-breaking force (N), the distance required to break the skin (mm), the slope of the curve corresponding to skin penetration to break (N/mm) (calculated as the slope of the straight line between the origin and the maximum skin-breaking force), and the work required to break the berry skin (mJ) (calculated as the area under the curve between the origin and the maximum skin-breaking force). The mechanical properties correspond to the average of 36 berries from each of the 10 purchased samples tested.
2.4. Fungi Identification
To identify the different pathogens, 10 fresh blueberries were crushed, and 1 g was diluted with 9 mL of deionised water. Dilutions were then made from 10-2 to 10-6, from which 100 µL were streaked on Petri dishes with Sabouraud Chloramphenicol Agar (SCA) medium (Scharlab, Spain). The plates were left at 22 ºC for 3 days. The different colonies were then isolated and identified by partial sequencing of the 5.8S rRNA and adjacent intergenic regions by using the services of Secugen (Madrid, Spain).
2.5. Total Phenolic and Total Anthocyanins Content
For the extraction of the total phenolic content, 0.2 g of the pulverised blueberry samples stored at -80 ºC were homogenised with 1 mL of a (50:50) solution of methanol acidified (1% HCl)-water (v/v). The samples were then centrifuged at 10,000 x
g for 10 min at room temperature and the supernatants were collected. These steps were repeated to obtain a final volume of 2 mL. The supernatants were filtered through 0.45 μm nylon filters and stored at -20 ºC. The total phenolic content of the extracts was determined by the Folin-Ciocalteu method [
12] and expressed as mg of gallic acid equivalents per 100 g of fresh weight (FW).
Total anthocyanin content was determined by the pH differential method [
13] with modifications as was described by [
14]. The results were expressed as mg of cyanidin-3-glucoside (ε = 26.900 L/mol cm) per 100 g FW.
2.6. Antioxidant Activity (ABTS and FRAP)
The same extract as used for the total phenolic and anthocyanin content was used for the determination of the antioxidant activity. Antioxidant activity was quantified by the ABTS+ method described by [
15] and also following the FRAP method [
16]. A calibration curve was established using a Trolox solution as a standard reference compound (from 0 to 4 mM). Total antioxidant activity was expressed as µmol Trolox Equivalents (TE) per g FW.
2.7. Identification and Quantification of Phenolic Compounds by HPLC-QTOF
These determinations were carried out on the five samples where the cultivar was known. The same extracts were used as for the determination of total phenolic and total anthocyanin content. Aliquots of the extracted phenolic compounds were analysed by high-resolution chromatography with quadrupole mass spectrometer-time of flight (HPLC-QTOF), as described by [
17]. Phenolic peaks were identified by comparison with standards of chlorogenic acid (C
16H
18O
9), coumaric acid (C
9H
8O
3), caffeic acid (C
9H
8O
4) and quercetin 3-glucoside (C
21H
20O
12) in the range of 0.1 to 100 ppm. For the anthocyanins, the peaks were identified against a standard of malvidin 3-glucoside (C
23H
25O
12), cyanidin 3-glucoside (C
21H
21O
11), cyanidin 3-rutinoside (C
27H
31O
15), delphinidin 3-rutinoside (C
27H
31O
16), and pelargonidin 3-glucoside (C
21H
21O
10) at 1-100 ppm. For the remaining compounds without a standard, identification was based on the presence of identical masses and according to their retention times. The software used was MassHunter Data Acquisition B.05.01 and MassHunter Qualitative Analysis B.07.00.
2.8. Statistical Analysis
All the descriptive analyses were performed using the IBM-SPSS statistical program, version 28.0.0 (IBM Corp. Armonk, NY, USA). Differences between blueberries from different markets were determined by a one-way analysis of variance (ANOVA) and the Tukey-b test (p<0.05). Relationships between different analyses were described as Pearson product moment correlation coefficient (r), p<0.01 or p<0.05.
SPSS also has the ability to perform principal component analysis with quantitative or scalar and qualitative or categorical data (CATPCA). Qualitative or categorical characters were treated as unordered variables (multiple nominal), with the number of character states (categories) entered. All analyses used correlation matrices and two dimensions were extracted to produce scatterplots.
3. Results and Discussion
3.1. Quality Assessment of Blueberries
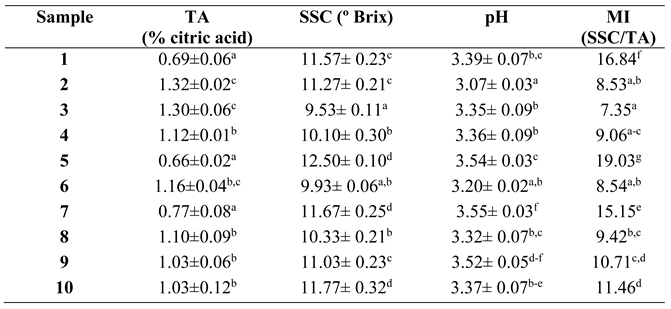
Sugar and organic acids have an important influence on the sensory quality of fruit. A good tasting blueberry should have high sugar and high acidity. Although not all blueberries with high SSC are necessarily good tasting, a low SSC makes it unlikely that they will taste good [
18]. According to [
19], blueberries should contain more than 10% SSC, TA values between 0.3-1.3% citric acid, an SSC/TA ratio of 10-33 and a pH between 2.25 and 4.25 to be of good commercial quality. Based on these quality standards, while the ten samples analysed had acceptable TA and pH, only two samples (3 and 6) had an SSC value below 10 and only five (1, 5, 7, 9 and 10) of the ten samples analysed had a balanced SSC/TA ratio (
Table 1). These included the two organic samples. It should be noted that the five samples that did not have an adequate SSC/TA ratio did not show large differences in terms of SSC but did show differences in TA, which in some samples was almost half of that observed in the samples that met the quality standards. On the other hand, the five samples with an adequate SSC/TA ratio, reached values below 20 in all cases. In the case of samples 1-3 of the Ventura cultivar, only sample 1 of the organic cultivar showed quality parameters according to the standards in terms of SSC/TA ratio. In general, the blueberries analysed from 10 different points of sale showed a low maturity index, considering the recommendations of the quality standards.
3.2. Mechanical Parameters
Mechanical properties can be a valuable tool for differentiating the maturity stages of blueberries [
20]. The results of this work showed that there were significant differences both in the shape of the force-distance curves (data not shown) obtained from the analysis of the ten samples and in the values of the different mechanical properties derived from them (
Table 2). Furthermore, the blueberries with the largest mean value of equatorial diameter (17.6 mm) corresponded to sample 3 and showed only significant differences with samples 1, 2 and 10 (16.0, 15.4 mm and 15.1 mm respectively), which were the smallest.
The highest force at skin break (N), which is the force obtained just before the irreversible rupture of the blueberry skin, corresponds to the fruits of samples 7, 8 and 10, although without significant differences with 1 and 9. The lowest values for this parameter correspond to samples 2-6, although only sample 3, with the lowest value, showed differences with sample 2.
The distance (mm) travelled by the probe just before skin break was greatest in the fruit of sample 1 and least in sample 5, with no major differences between the rest of the other samples analysed. The area of the force/deformation curve between the trigger force and the force at the skin break in blueberries was determined and expressed as skin break work (
Table 2). In this case, fruit from samples 1 and 10 had the highest values and again sample 5 had the lowest. Similarly, there were no significant differences between the other samples. It is important to note that our retail sampling involves unknown and uncontrolled variables, such as grower, supply chain and postharvest handling. It is therefore difficult to relate the parameters obtained to the postharvest and/or maturity stage of the fruit. However, we have observed that sample number 5, which presented blueberries with the highest maturity index (SSC/TA:19.03), had the lowest mechanical values described above. In a previous work, Ref. [
20] reported a decrease in skin breakage force and skin breakage energy as an indicator of the progress of ripening in two different blueberry cultivars (Nui and Rahi). These authors go so far as to conclude that these mechanical parameters could be used for commercial or research purposes, with the aim of being used as quality control operations or to evaluate postharvest technological treatments. Although it is true that in our study it has been observed that there are samples with different maturity indexes, with half of them below the quality requirements established for these fruits, we have not been able to reach the same conclusion in terms of mechanical properties. This may be due to the fact that in our study we used a flat cylindrical stainless-steel probe with a diameter of 2 mm, unlike the needle probe used by [
20]. However, other authors have suggested that growing conditions may have a greater influence on skin penetration test results than the stage of ripening stage itself [
21]. However, in contrast to the needle probe with a tip diameter of 0.39 mm and a maximum diameter of 2 mm used in the penetration tests by [
20], Ref. [
21] performed their experiments by using a cylindrical and rounded probe with a lower diameter of 0.16 mm. Our results indicate that, despite the differences in sugar and acid content found in the different samples, there are no major differences in the mechanical properties analysed. Only sample 5, from the Royal Blue Aroma cultivar from Morocco, showed the highest SSC/TA ratio and the lowest values for the different mechanical properties determined. The fact that it is the only sample of different origin may indicate that the time between harvest and marketing is longer and that it may have undergone some postharvest treatment.
3.3. Fungi Identification
Although the samples analysed showed no visible signs of pathogen contamination, it could not be ruled out that the fruits could contain spores of different fungi. A study was therefore carried out to identify them by partial sequencing of the 5.8S rRNA and adjacent intergenic regions.
Five fungi species were identified in the blueberries analysed (
Table 3) corresponding to Aspergillus tubingensis or A. costaricaensis, Sporobolomyces roseus, Cladosporium piniponderosae or C. colombiae, Metschnikowia vanudenii and Penicillium brevicompactum. But not all of them were identified in the 10 samples analysed. Thus, while in samples 1, 6 and 8 none of the 5 fungi identified were determinate, in samples 3 and 7 up to 4 different fungi were identified. Moreover, P. brevicompactum was identified in seven samples, and C. piniponderosae or C. colombiae, were found in five of the ten samples analysed, respectively.
Blueberries, like most fruits, are susceptible to fungal spoilage. Contamination can occur at any stage of the process, from harvesting to consumption, and the more abundant fungal species may differ depending on the place of production [
22]. According to these authors, the most common moulds in blueberries are
Botrytis cinerea (55%) and
Alternaria spp. (46%), followed by
Fusarium, Penicillium, Aureobasidium, Cladosporium and
Trichoderma species. However,
B. cinerea contamination was not present in any of the samples analysed and
Penicillium spp was present in 7 samples. A study analysing organic and conventional fruit showed that fungi that were present on both types of fruit belonged to the genera
Cladosporium,
Penicillium,
Alternaria, and
Aureobasidium [
23]. Moreover, these authors indicated that their presence on the fruit did not depend on the growth conditions of the plant. In this sense, although in our study organic fruit were analysed in two different samples, results showed that in one of them no pathogen was identified, while in the other one almost three different fungi were identified, including
Cladosporium spp. This genus was most frequently found in organically grown fruit (45-84% of all fungi identified) in Brigitte Blue blueberries compared to those of the same cultivar grown under conventional conditions [
23]
3.4. Total Phenolic and Total Anthocyanins Content
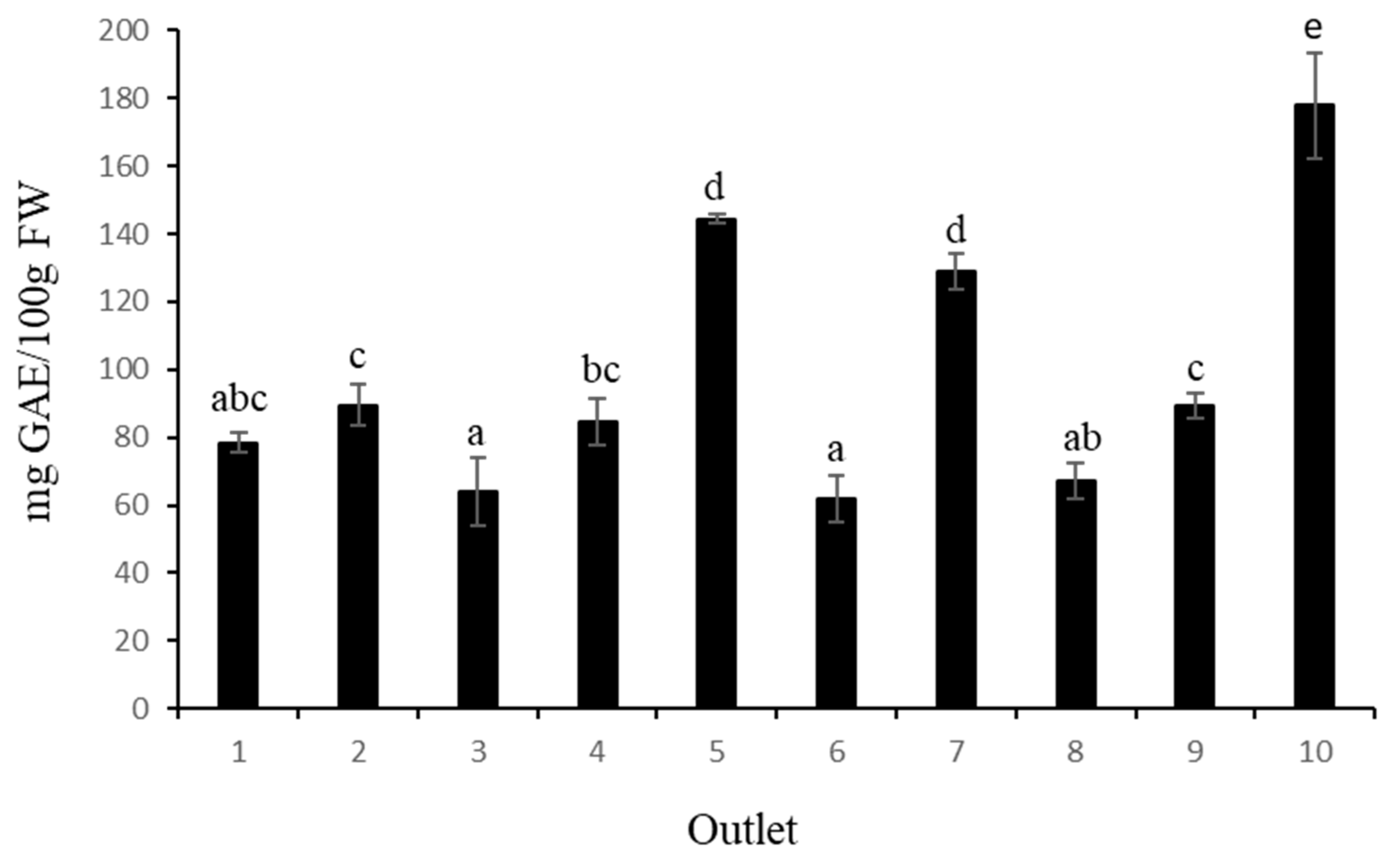
The total phenolic content (TPC) measured in blueberries ranged from 61.94 to 177.94 mg GAE/100 g FW (
Figure 1). Sample number 10 showed the highest TPC with 177.94 mg GAE/100 g FW, followed by samples 5 and 7 (without significant differences between them) and, finally, fruit from samples 1, 3, 6 and 8 showed the lowest TPC values. According to [
24], the mean TPC values for blueberries (cv. Bluecrop) at harvest were 274.48 mg GAE/100 g FW, while Ref. [
25] reported a TPC of 189.90 mg GAE/100 g FW. In general, our results showed that the TPC values obtained in this study were lower than those previously reported. It is known that a decrease in the content of total phenolic content can occur during postharvest storage [
26].
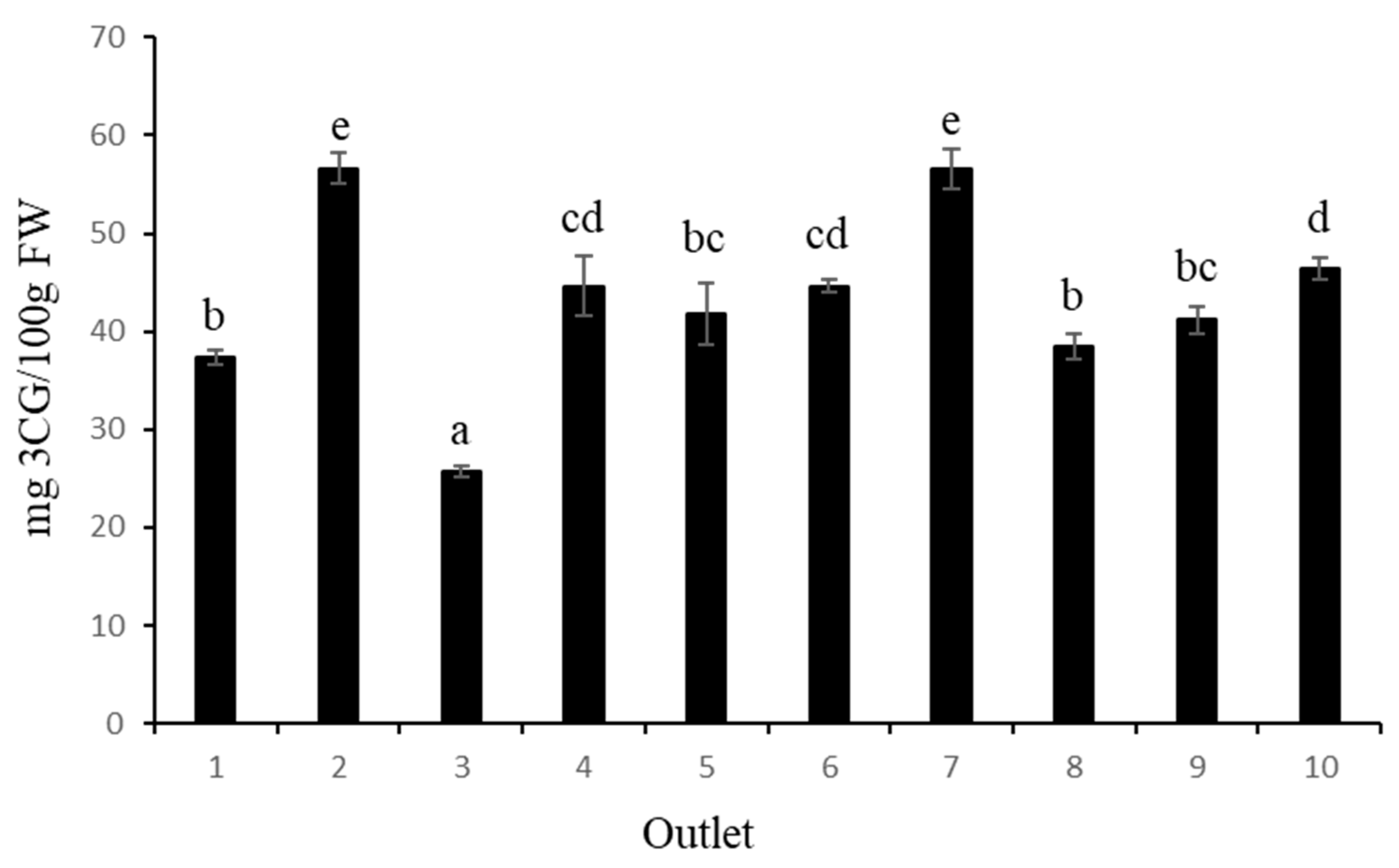
In terms of total anthocyanin content (TAC) (
Figure 2), the highest values were obtained from samples 2 and 7, with 56.66 mg C3G/100 g FW and 56.54 mg C3G/100 g FW, respectively, while fruit from the sample 3 had the lowest value (25.68 mg C3G/100 g FW). In a recent review, Ref. [
27] summarised the results of TAC from different blueberry cultivars and different locations, indicating a high variation among blueberry cultivars for total anthocyanin content, although the method of determination was different. The values ranged from 19.3 to 677.8 mg C3G per 100 g FW. In this sense, genotypes and environmental growing conditions could be the main reasons for the differences in total anthocyanin content between cultivars.
It is also important to note that fruit size has an effect on fruit quality. Polyphenols, especially anthocyanins, are mainly found in the skin of blueberries and, for the same weight, smaller blueberries have a greater skin surface area compared to larger fruit. Although it is true that this statement cannot be extrapolated to all the samples analysed, the fruits of sample 10, with the smallest equatorial diameter (
Table 2), showed the highest values of total phenolics and the third highest value of anthocyanins. The fruits of sample 3, with the largest equatorial diameter of all the samples analysed, showed the lowest values for both determinations.
In any case, our results show variations in anthocyanin and phenolic content among the 10 samples analysed. Finally, it is important to note that any comparison with previous work must take into account all factors affecting phenolic and anthocyanin content, such as cultivar, postharvest treatments, rheological and climatic conditions [
22] which we do not know in our case.
3.5. Antioxidant Activity
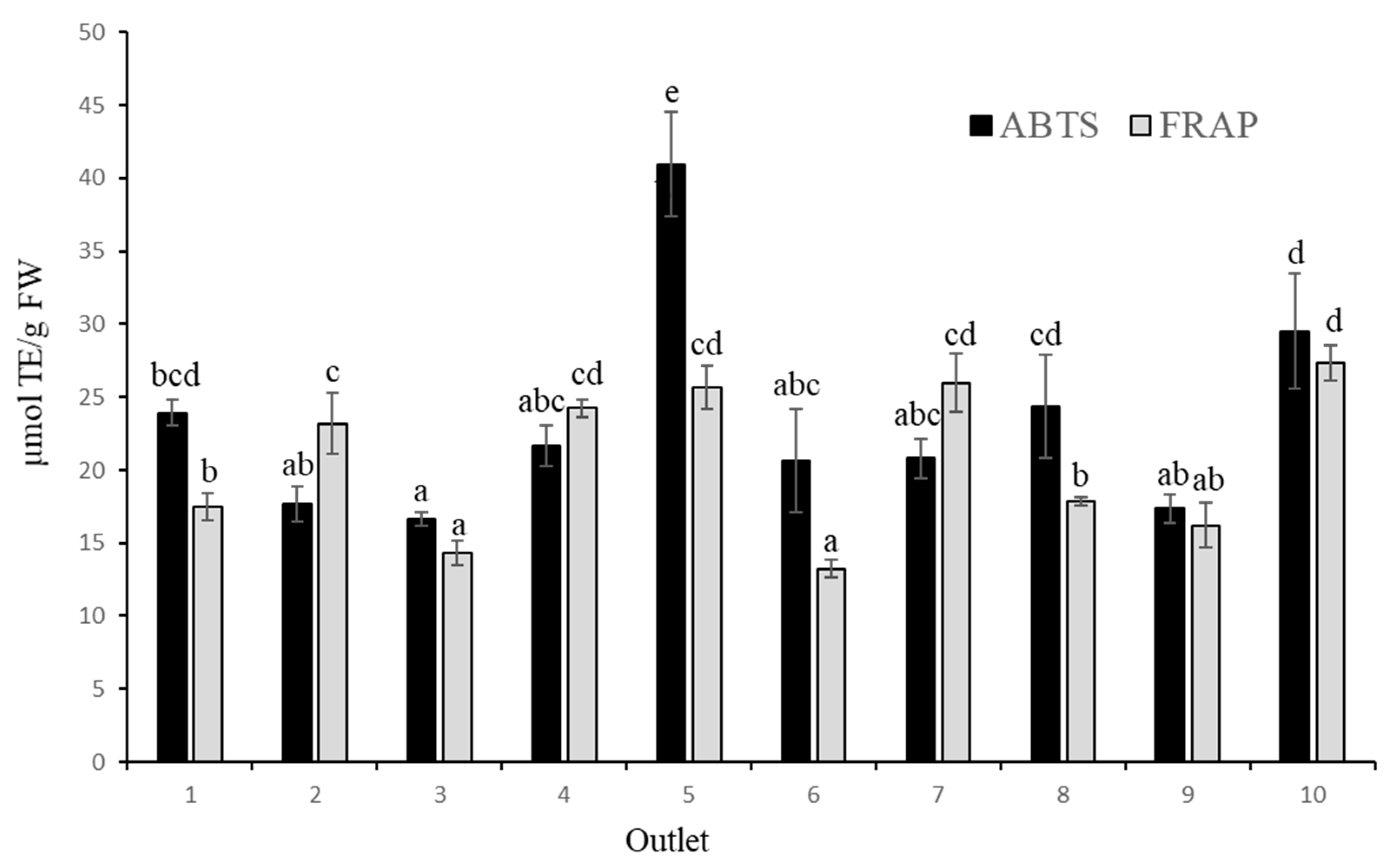
The antioxidant activity determined by the ABTS method (
Figure 3) showed a wide variation, ranging from 16.65 to 40.96 µmol TE/g FW, corresponding to samples 3 and 5, respectively. Using the FRAP method, the results ranged from 13.22 to 27.38 µmol TE/g FW, corresponding to the samples 6 and 10, respectively. The amplitude of both ranges was observed in previous studies [
24,
25,
28]. In general, the antioxidant activity values obtained in this study are similar to those reported by [
24], of 1014-2055 and 699-1740 µmol TE/100 g FW for the ABTS and FRAP methods, respectively.
The results showed a positive significant correlation between the assessment of antioxidant activity in the blueberry samples using ABTS and FRAP methods (
Table 3) (r=0.527,
p<0.01). A significant correlation (
p<0.01) was also reported between the antioxidant activity determined by the ABTS or FRAP methods and TPC (ABTS; r=0.644, FRAP; r=0.827). Therefore, the presence of phenolic compounds in fruits contributes significantly to their antioxidant activity [
29,
30]. However, while no correlation was found between TAC and the antioxidant activity determined by the ABTS method, a significant correlation was found with the results obtained by the FRAP method (r=0.623,
p<0.01).
3.6. Identification and Quantification of Phenolic Compounds by HPLC-QTOF
Identification and quantification of anthocyanins, flavonols, flavanols and phenolic acids were carried out on samples of known cultivars (samples 1-5).
Table 4 shows the main phenolic compounds present in blueberries with their chemical formula, exact molecular weight, retention time (min) and method of identification. The individual anthocyanins were identified by comparing the m/z of each anthocyanin molecule and its fragmentation with the value in available published works, as well as by comparison with standard solutions. The identification of the rest of the phenolic compounds (flavanols, flavonols and phenolic acids) was carried out with standard solutions and by generating a formula from the MS spectra, generating a similarity score. Twelve peaks of phenolic compounds were tentatively identified in the analysed blueberry samples.
The different anthocyanins were expressed as their pyranoside forms, as the galactoside and glucoside molecules have exactly the same molecular weight and cannot be distinguished by MS/MS. Also, in the work of [
31], this distinction was made only by the difference in their retention times. However, in our case, as in [
32], some of them were not well-defined peaks and could not be integrated separately. On the other hand, the compounds in the table were ordered according to the elution time, which is consistent with those found in the bibliography [
31,
32,
33], where the general order is delphinidin, cyanidin, petunidin, peonidin and malvidin. Quantification was only carried out for those compounds for which standard solutions were available and which were present in sufficient quantities to be measured. The content of individual anthocyanins and chlorogenic acid in blueberries is therefore given in
Table 5.
The only non-anthocyanin phenolic compound that could be quantified was chlorogenic acid, since caffeic, ferulic, ellagic and gallic acids were below the detection limit. Its content did not differ between the samples, except in the case of sample 5, which had a higher concentration of 59.28 mg/100 g FW, compared to the other samples, which ranged from 30.67 to 35.93 mg/100 g FW. According to [
34], the average chlorogenic acid content of blueberries was 131.18 mg/100 g FW, with a minimum of 64.59 and a maximum of 207.50 mg/100 g FW. These values also agree with those reported by [
31] of 70 mg/100 g FW. Of the samples analysed, only sample 5 was close to the minimum value recorded, while the rest of the samples showed only half of this content.
For the anthocyanins, the quantification was carried out on the arabinoside and pyranoside forms of malvidin, petunidin and delphinidin, as the cyanidin content was too low to quantify (
Table 5). The order of abundance was delphinidin > malvidin > petunidin. This is in agreement with [
31] and [
35], who described the predominant anthocyanin class in American blueberries as delphinidin glycosides. The content of malvidin-3-arabinoside varied between all the samples except those of samples 5 and 1, which also showed the highest values. The lowest value was found in the sample 3, which was one third lower than the other samples. On the other hand, for malvidin-3-pyranoside, fruits of samples 3 and 1 showed the lowest values, with 19.78 and 20.03 mg/100 g FW, respectively, while the highest values belonged to sample 2, with 39.91 mg/100 g FW. For the petunidin-3-arabinoside content, sample 2 showed the highest value (9.52 mg/100 g FW), while sample 3 was devoid of this compound. The same tendency can be observed for petunidin-3-pyranoside, where sample 2 was three times higher than sample 1, which had the lowest value. Finally, the delphinidin derivatives showed the highest content of all anthocyanins. For the rest, sample 2 had the highest content, with 58.07 and 89.65 mg/100 g FW for the arabinoside and pyranoside forms, respectively, followed by sample 4, with 40.62 and 50.50 mg/100g FW.
Overall, of the five samples, sample 5 showed the highest level of chlorogenic acid, which is consistent with the fact that it was the sample with the highest TPC value measured by the pH difference method. It should also be noted that when the levels of the individual anthocyanins identified in the five samples were summed, sample 2 showed the highest levels and sample 3 the lowest, as was also the case with the TACs determined by spectrophotometry. The fact that samples 1, 2 and 3 are from the same cultivar and that they differ in anthocyanin levels may be due to preharvest and postharvest practices, which are unknown to us for these marketed fruits. According to [
36], cultivation practices are one of the main factors influencing the concentration of anthocyanins in fruits, as well as the different types of diseases affecting the plant, soil and climatic conditions, pest control and other agronomic factors. On the other hand, the fact that sample 1 was organically grown did not reflect differences with other conventionally grown samples.
3.7. Categorical Principal Components Analysis (CATPCA)
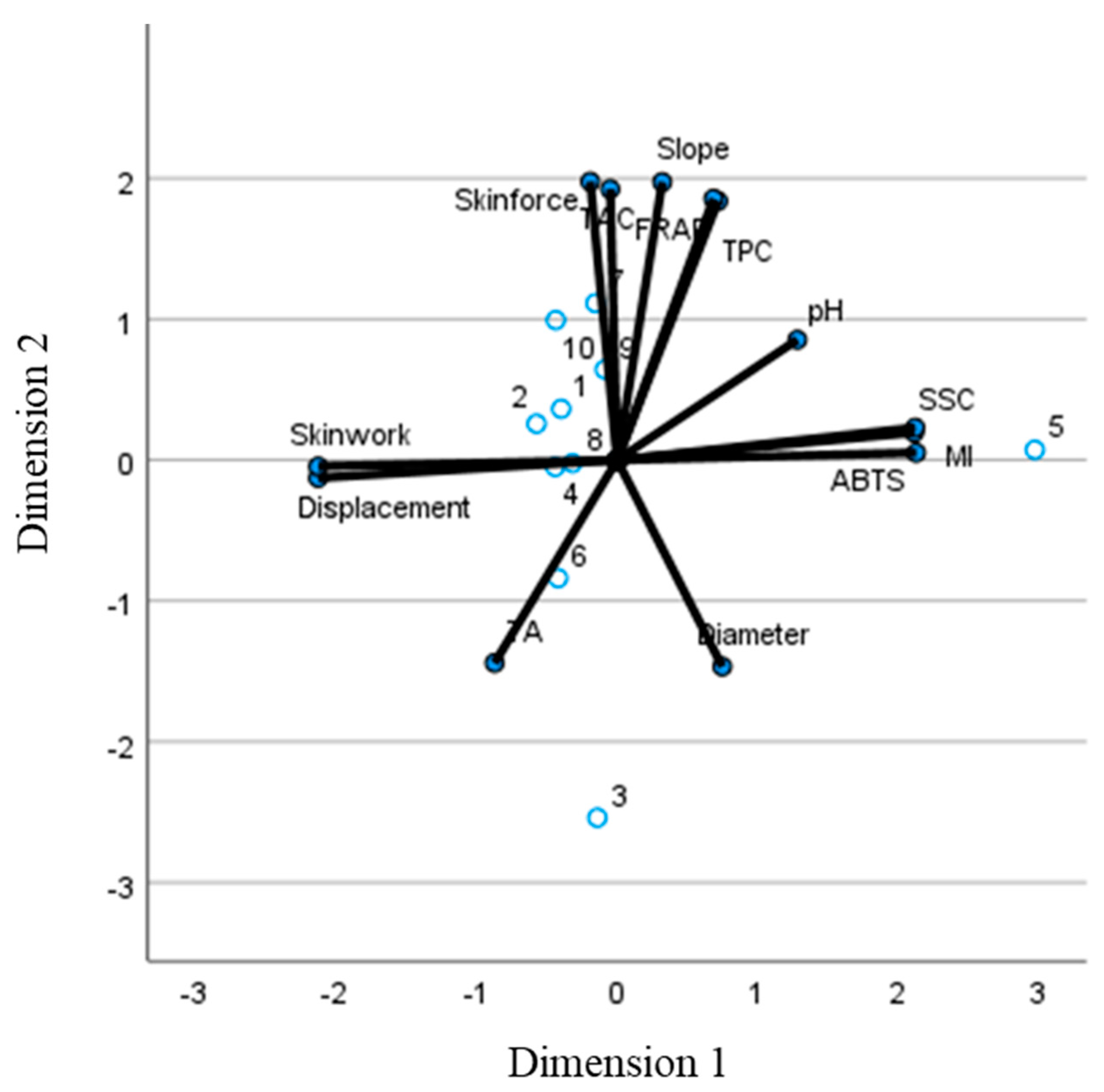
Finally, a CATPCA analysis was performed with the aim of reducing the original set of variables to a smaller set of uncorrelated components that represent most of the information found in the original variables to differentiate the samples. In the CATPCA including all variables (quality and texture), the first dimension (44,27% of the variability) was positively related mainly to SSC, ABTS, MI and the mechanical properties displacement at skin break and skin break work. The second dimension (39,02% of the variability) was positively related to the rest of the variables analysed. With respect to dimension 1, all the samples were closely grouped in the middle (
Figure 4), with sample 5 being the only outlier. This sample had the highest MI, SCC and ABTS values and the lowest values for both mechanical properties affecting this dimension. With respect to dimension 2, most of the samples are clustered around -1/+1, with the exception of sample 3 which is below -2 and samples 7 and 10 which are above +1.
4. Conclusions
The results of this work showed that the blueberries analysed were of acceptable quality according to the standards, at the point of sale; although a common general characteristic of the ten samples analysed from different outlets was the low maturity index values. Although the efforts of producers and marketers to maintain the quality of blueberries have focused on maintaining firmness and the absence of rot, this fact must be taken into account, as the maturity index is the main factor responsible for consumer acceptance of the fruit. Furthermore, no differences in CATPCA results were observed between the organic and conventional samples. Finally, we observed differences between samples of the same cultivar depending on the point of sale, reinforcing the idea that the cultivar is not the only factor influencing the quality of the berries.
Author Contributions
Conceptualization, M.T.S-B. and I.R.; Methodology, M.T.S-B., M.D.A., C.M., M.I.E I.R.; Formal Analysis, M.T.S-B., C.M-A., M.D.A., I.R.; Writing—Original Draft Preparation, I.R.; Writing—Review & Editing, M.T.S-B.; Visualization, C.M. and M.I.E.; Funding Acquisition, M.T.S-B and I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the CICYT project PID2020-113965RB-I00/ AEI/10.13039/501100011033.
Acknowledgments
We are grateful to the Ministry of Science and Innovation for the funding support. This research was supported by the CICYT project PID2020-113965RB-I00/ AEI/10.13039/501100011033.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT Food and Agriculture Organization of the United Nations. Production: Crops. Http://Faostat.Fao.Org. 2019.
- Rodríguez-Daza, M.C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Front Microbiol 2020, 11, 2032. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv Nutr 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T. From Waste Products to Ecochemicals: Fifty Years Research of Plant Secondary Metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From Plant to Health. Phytochem Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic Profile and Antioxidant Activity of Highbush Blueberry (Vaccinium Corymbosum L.) during Fruit Maturation and Ripening. Food Chem 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Dai, H.; Ji, S.; Zhou, X.; Wei, B.; Cheng, S.; Zhang, F.; Wang, S.; Zhou, Q. Postharvest Effects of Sodium Nitroprusside Treatment on Membrane Fatty Acids of Blueberry (Vaccinium Corymbosum, Cv. Bluecrop) Fruit. Sci Hortic 2021, 288. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd Edition - Jorge B Retamales, James F Hancock - Google Livros. 2018, 18–60.
- Huynh, N.K.; Wilson, M.D.; Eyles, A.; Stanley, R.A. Recent Advances in Postharvest Technologies to Extend the Shelf Life of Blueberries (Vaccinium Sp.), Raspberries (Rubus Idaeus L.) and Blackberries (Rubus Sp.). J Berry Res 2019, 9, 709–724. [Google Scholar] [CrossRef]
- Kader, A.A. Fruit Maturity, Ripening, and Quality Relationships. Acta Hortic 1999, 485, 203–208. [Google Scholar] [CrossRef]
- Hancock, J.; Callow, P.; Serçe, S.; Hanson, E.; Beaudry, R. Effect of Cultivar, Controlled Atmosphere Storage, and Fruit Ripeness on the Long-Term Storage of Highbush Blueberries. Horttechnology 2008, 18, 199–205. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-phosphotungstic Acid Reagents. Am J Enol Vitic 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Station, O.S.University.A.E.; Wrolstad, R.E. Color and Pigment Analyses in Fruit Products. Oregon State University Station Bulletin, Corvallis, OR 1993, https://ir.library.oregonstate.edu/concern/administrative_report_or_publications/9s1616449.
- Romero, I.; Domínguez, I.; Doménech-Carbó, A.; Gavara, R.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Effect of High Levels of CO2 on the Electrochemical Behavior and the Enzymatic and Non-enzymatic Antioxidant Systems in Black and White Table Grapes Stored at 0 °C. J Sci Food Agric 2019, 99, 6859–6867. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol Med 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Domínguez, I.; Morales-Diaz, N.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Regulation of Flavonoid Biosynthesis Pathway by a Single or Dual Short-Term CO2 Treatment in Black Table Grapes Stored at Low Temperature. Plant Physiol. Biochem. 2020, 156, 30–38. [Google Scholar] [CrossRef]
- Scalzo, J.; Stevenson, D.; Hedderley, D. Polyphenol Compounds and Other Quality Traits in Blueberry Cultivars. J Berry Res 2015, 5, 117–130. [Google Scholar] [CrossRef]
- Beaudry, R. Blueberry quality characteristics and how they can be optimized. Annual Report of the Michigan State Horticultural Society (122nd), 1992, 140-145. https://www.researchgate.net/publication/285702840.
- Rivera, S.; Giongo, L.; Cappai, F.; Kerckhoffs, H.; Sofkova-Bobcheva, S.; Hutchins, D.; East, A. Blueberry Firmness - A Review of the Textural and Mechanical Properties Used in Quality Evaluations. Postharvest Biol Technol 2022, 192. [Google Scholar] [CrossRef]
- Maury, C.; Madieta, E.; Le Moigne, M.; Mehinagic, E.; Siret, R.; Jourjon, F. Development of a Mechanical Texture Test to Evaluate the Ripening Process of Cabernet Franc Grapes. J Texture Stud 2009, 40, 511–535. [Google Scholar] [CrossRef]
- Tănase, E.E.; Popa, V.I.; Popa, M.E.; Geicu-Cristea, M.; Popescu, P.; Drăghici, M.; Miteluț, A.C. Identification Of The Most Relevant Quality Parameters For Berries-A Review. Scientific Bulletin. Series F. Biotechnologies 2016, 20, 222–233, ISSN Online 2285-1372. [Google Scholar]
- Ochmian, I.; Błaszak, M.; Lachowicz, S.; Piwowarczyk, R. The Impact of Cultivation Systems on the Nutritional and Phytochemical Content, and Microbiological Contamination of Highbush Blueberry. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Rodrigues, E.; Poerner, N.; Rockenbach, I.I.; Gonzaga, L.V.; Mendes, C.R.; Fett, R. Phenolic Compounds and Antioxidant Activity of Blueberry Cultivars Grown in Brazil. Food Sci. Technol. 2011, 31, 911–917. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J Agric Food Chem 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Kozos, K.; Ochmian, I.; Chełpiński, P. The Effects of Rapid Chilling and Storage Conditions on the Quality of Brigitta Blue Cultivar Highbush Blueberries (Vaccinium Corymbosum L.). Folia Horticulturae 2014, 26, 147–153. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and Function of Blueberry Anthocyanins: A Review of Recent Advances. J Funct Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Barcia, M.T.; Rodrigues, D.; Da Cruz, P.N.; Hermosín-Gutiérrez, I.; Smith, R.; Godoy, H.T. Antioxidant Activity of Hydrophilic and Lipophilic Extracts of Brazilian Blueberries. Food Chem 2014, 164, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J Agric Food Chem 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Bunea, A.; Ruginǎ, D.O.; Pintea, A.M.; Sconţa, Z.; Bunea, C.I.; Socaciu, C. Comparative Polyphenolic Content and Antioxidant Activities of Some Wild and Cultivated Blueberries from Romania. Not Bot Horti Agrobot Cluj Napoca 2011, 39, 70–76. [Google Scholar] [CrossRef]
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian Bilberries (Vaccinium Myrtillus L.) and Blueberries (Vaccinium Corymbosum L.). J Agric Food Chem 2011, 59, 6998–7004. [Google Scholar] [CrossRef]
- Stein-Chisholm, R.E.; Beaulieu, J.C.; Grimm, C.C.; Lloyd, S.W. LC–MS/MS and UPLC–UV Evaluation of Anthocyanins and Anthocyanidins during Rabbiteye Blueberry Juice Processing. Beverages 2017, 3, 56. [Google Scholar] [CrossRef]
- Lohachoompol, V.; Mulholland, M.; Srzednicki, G.; Craske, J. Determination of Anthocyanins in Various Cultivars of Highbush and Rabbiteye Blueberries. Food Chem 2008, 111, 249–254. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database (Oxford) 2010. [Google Scholar] [CrossRef]
- Taruscio, T.G.; Barney, D.L.; Exon, J. Content and Profile of Flavanoid and Phenolic Acid Compounds in Conjunction with the Antioxidant Capacity for a Variety of Northwest Vaccinium Berries. J. Agric. Food Chem. 2004, 52, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Blueberries and Their Anthocyanins: Factors Affecting Biosynthesis and Properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).