Submitted:
03 July 2025
Posted:
04 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material, Location, and Growing Conditions
2.2. Methods
2.2.1. Physicochemical Analysis
2.2.2. Sugar and Organic Acids Content
2.2.3. Total Phenolic Content and Total Antioxidant Activity
2.2.4. Individual Phenolic Compounds
2.2.5. Total and Individual Carotenoid Compounds
2.2.6. Ascorbic Acid Content
2.2.7. Analysis of Volatile Aromatic Compounds (VOCs)
2.3. Statistical Analysis
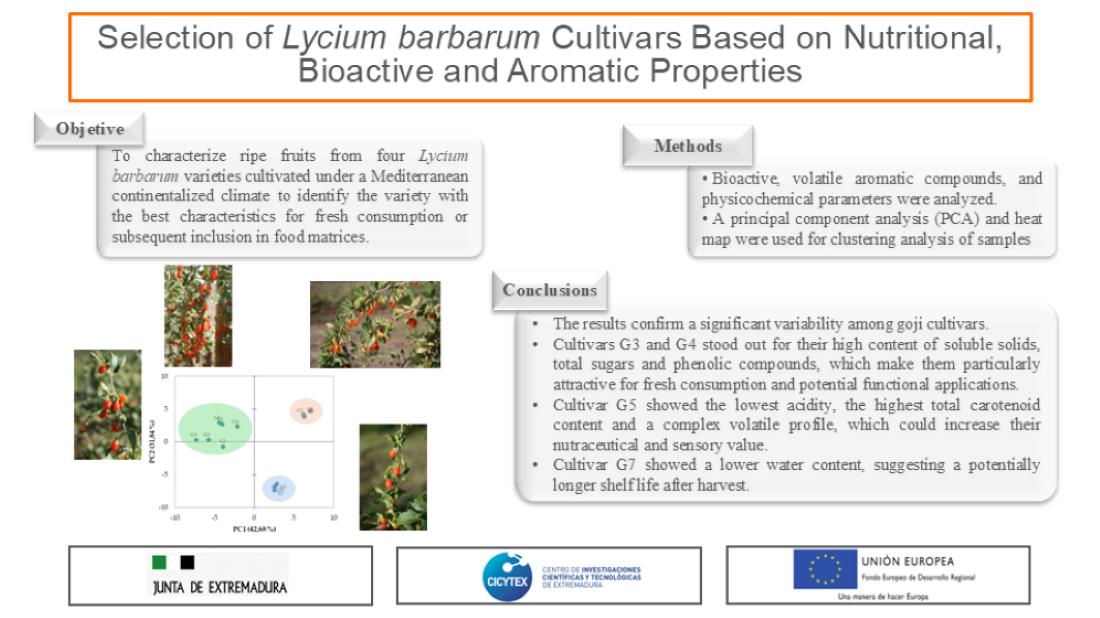
3. Results and Discussion
3.1. Physicochemical Quality Characteristics
3.2. Sugar and Organic Acids Composition
3.3. Bioactive Quality Characteristics
3.3.1. Phenolic Compounds
3.3.2. Carotenoid Compounds
3.3.3. Antioxidant Activity
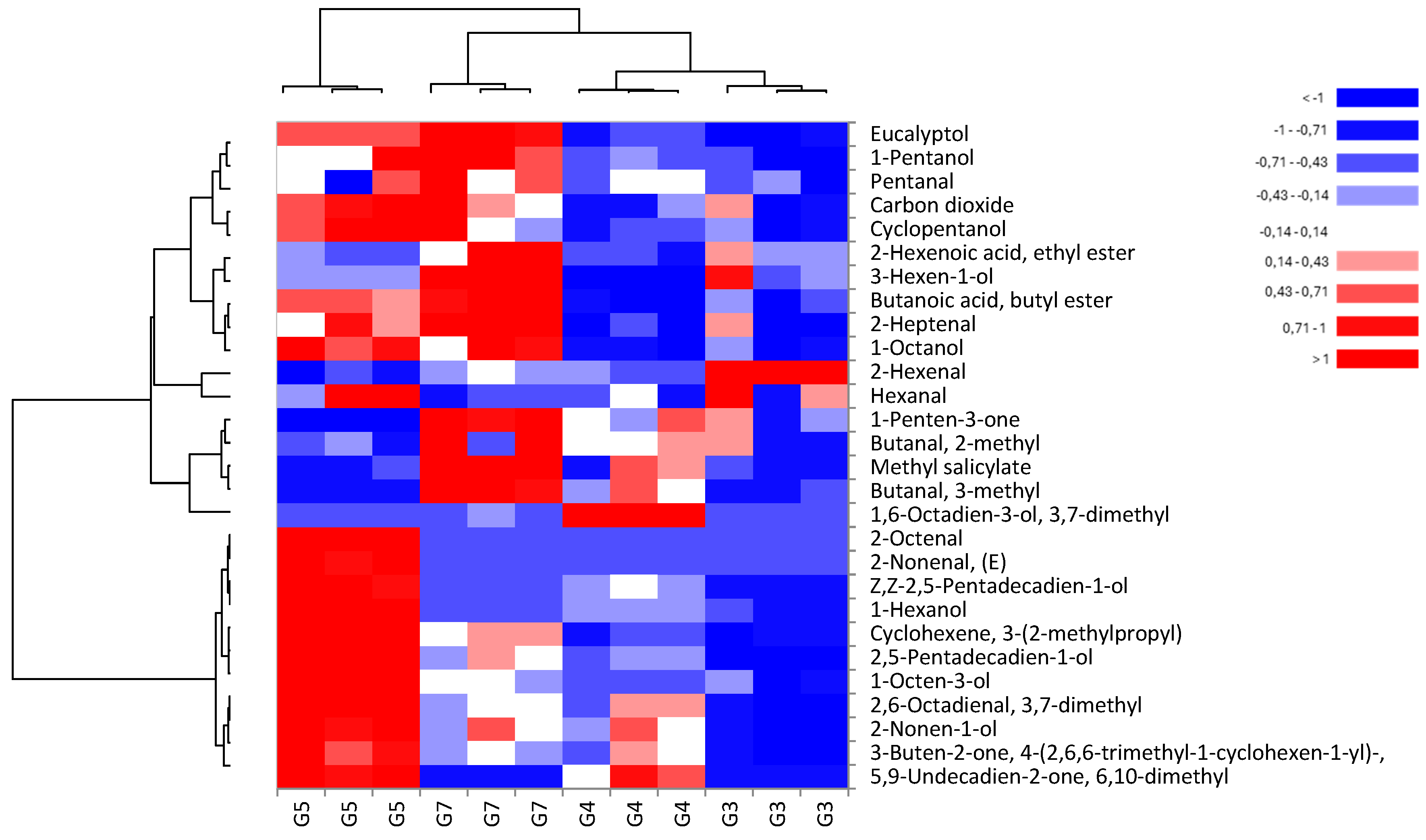
3.4. Volatile Organic Compounds (VOCs)
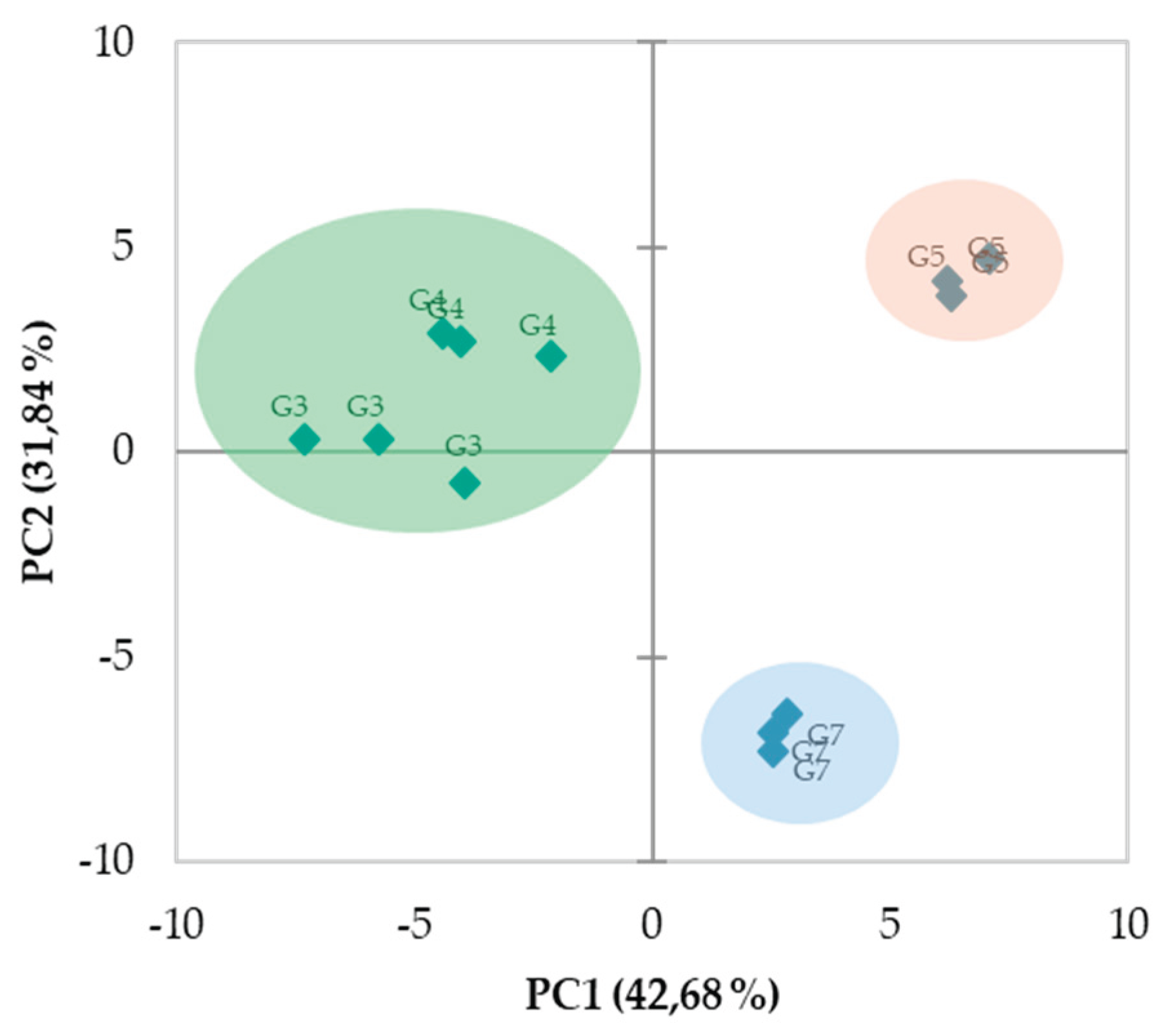
3.5. PCA Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-Car | α-Carotene |
| β-Car | β-carotene |
| β-Crp | β-Cryptoxanthin |
| Cap | Capsanthin |
| AClo | Chlorogenic Acid |
| AnClo | Neoclorogenic Acid |
| ApC | p-coumaroylquinic |
| ApCou | p-coumaric acid |
| GB | Goji Berry |
| LRI | Linear Retention Index |
| PCA | Principal Component Analysis |
| TCC | Total Carotenoid Content |
| TPC | Total Phenolic Compounds |
| Rut | Ruthin |
| t-Fer | t-ferulic acid |
| TSS | Total Soluble Solid |
| TA | Titratable acidity |
| VOC | Volatile Organic Compound |
| Zea | Zeaxanthin |
References
- Antonelli, M.; Donelli, D. Health-Promoting Effects of Goji Berries (Lycium Barbarum): A Literature Overview. Biology and Life Sciences Forum 2024, 40, 1. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Zheng, Y.; Fu, L. Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium Barbarum L.). Applied Sciences 2024, 15, 262. [Google Scholar] [CrossRef]
- FAO What Are Healthy Diets? Joint Statement by the Food and Agriculture Organization of the United Nations and the World Health Organization. 2024.
- Ceccarini, M.R.; Codini, M.; Cataldi, S.; Fioretti, B.; Brecchia, G.; Albi, E.; Beccari, T. Licium Barbarum Cultivated in Italy: Chemical Characterization and Nutritional Evaluation. Italian Journal of Food Science 2022. [Google Scholar] [CrossRef]
- Rubio, R.M.; García, M.R.; Barroso, N.N.; Iñiguez, F.M.S.; Gómez, M.J.R.; Magro, P.C. Classification of Goji Berry (Lycium Barbarum L.) Varieties According to Physicochemical and Bioactive Signature. European Food Research and Technology 2024. [Google Scholar] [CrossRef]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Yiu, G.; Hackman, R.M. Goji Berry Intake Increases Macular Pigment Optical Density in Healthy Adults: A Randomized Pilot Trial. Nutrients 2021, 13, 4409. [Google Scholar] [CrossRef]
- Sun, Q.; Du, M.; Kang, Y.; Zhu, M.J. Prebiotic Effects of Goji Berry in Protection against Inflammatory Bowel Disease. Crit Rev Food Sci Nutr 2023, 63, 5206–5230. [Google Scholar] [CrossRef]
- Yu, J.; Yan, Y.; Zhang, L.; Mi, J.; Yu, L.; Zhang, F.; Lu, L.; Luo, Q.; Li, X.; Zhou, X.; et al. A Comprehensive Review of Goji Berry Processing and Utilization. Food Sci Nutr 2023, 11, 7445–7457. [Google Scholar] [CrossRef]
- Fernández-Ríos, A.; Laso, J.; Aldaco, R.; Margallo, M. Superfoods: A Super Impact on Health and the Environment? Curr Opin Environ Sci Health 2023, 31, 100410. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.; Pešić, M.B. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, Vol. 11, Page 248 2022, 11, 248. [Google Scholar] [CrossRef]
- Aguilera, J.M. Berries as Foods: Processing, Products, and Health Implications. Annu Rev Food Sci Technol 2024, 15, 1–26. [Google Scholar] [CrossRef]
- Peng, Q.; Huang, J.; Li, S.; Massou, B.B.; Chen, Z.; Zhu, Q.; Xie, G. Comprehensive Origin Authentication of Wolfberry Pulp (Lycium Barbarum L.) Using Multimodal Sensory Analysis and Chemometrics. Ind Crops Prod 2024, 219, 119023. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and Analysis of Volatile Flavor Compounds in Different Varieties and Origins of Goji Berries Using HS-GC-IMS. LWT 2023, 187, 115322. [Google Scholar] [CrossRef]
- Oğuz, İ.; Oğuz, H.İ.; Ürün, İ.; Attar, Ş.H.; Atasever, S.; Kafkas, N.E. Determination of Aroma and Protein Contents in Organic Lycium Barbarum L. and Lycium Chinense Miller Fruits in Different Ripening Periods. Erwerbs-Obstbau 2023, 65, 1171–1183. [Google Scholar] [CrossRef]
- de Freitas Santos Júnior, A.; Mota, M.D.; de Aragão Tannus, C.; de Souza Dias, F.; de Andrade Santana, D.; Moura, H.F.S.; Magalhães, H.I.F. Phytochemical, Biological and Technological Aspects of Phenolic Bioactives in Goji Berries. Phytochemicals in Goji Berries 2020, 15–37. [Google Scholar] [CrossRef]
- AOAC Moisture in Malt Gravimetric Method. (935.29). In Official Methods of Analysis (17th Ed.); Association of Official Analytical Chemist: Gaithersburg, Maryland, 2000.
- Lozano, M.; Vidal-Aragón, M.C.; Hernández, M.T.; Ayuso, M.C.; Bernalte, M.J.; García, J.; Velardo, B. Physicochemical and Nutritional Properties and Volatile Constituents of Six Japanese Plum (Prunus Salicina Lindl.) Cultivars. European Food Research and Technology 2009, 228, 403–410. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Amodio, M.L.; Colelli, G. Quality of Goji Berry Fruit (Lycium Barbarum L.) Stored at Different Temperatures. Foods 2022, 11, 3700. [Google Scholar] [CrossRef]
- Capotorto, I.; Amodio, M.L.; Diaz, M.T.B.; de Chiara, M.L.V.; Colelli, G. Effect of Anti-Browning Solutions on Quality of Fresh-Cut Fennel during Storage. Postharvest Biol Technol 2018, 137, 21–30. [Google Scholar] [CrossRef]
- Savran, A.; Zengin, G.; Aktumsek, A.; Mocan, A.; Glamoćlija, J.; Ćirić, A.; Soković, M. Phenolic Compounds and Biological Effects of Edible Rumex Scutatus and Pseudosempervivum Sempervivum: Potential Sources of Natural Agents with Health Benefits. Food Funct 2016, 7, 3252–3262. [Google Scholar] [CrossRef]
- Manzano Durán, R.; Sánchez, J.E.F.; Velardo-Micharet, B.; Gómez, M.J.R. Multivariate Optimization of Ultrasound-Assisted Extraction for the Determination of Phenolic Compounds in Plums (Prunus Salicina Lindl.) by High-Performance Liquid Chromatography (HPLC). Instrum Sci Technol 2020, 48, 113–127. [Google Scholar] [CrossRef]
- Zacarías-Garcia, J.; Carlos, G.; Gil, J.V.; Navarro, J.L.; Zacarías, L.; Rodrigo, M.J. Juices and By-Products of Red-Fleshed Sweet Oranges: Assessment of Bioactive and Nutritional Compounds. Foods 2023, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Bohoyo-Gil, D.; Dominguez-Valhondo, D.; García-Parra, J.J.; González-Gómez, D. UHPLC as a Suitable Methodology for the Analysis of Carotenoids in Food Matrix. European Food Research and Technology 2012, 235, 1055–1061. [Google Scholar] [CrossRef]
- Campos, F.M.; Ribeiro, S.M.R.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Stringheta, P.C. Optimization of Methodology to Analyze Ascorbic and Dehydroascorbic Acid in Vegetables. Quim Nova 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Quan, J.; An, W.; Zhao, J.; Xi, W. Identification of Characteristic Aroma Volatiles of Ningxia Goji Berries (Lycium Barbarum L.) and Their Developmental Changes. Int J Food Prop 2017, 20, S214–S227. [Google Scholar] [CrossRef]
- Ma, R.H.; Zhang, X.X.; Ni, Z.J.; Thakur, K.; Wang, W.; Yan, Y.M.; Cao, Y.L.; Zhang, J.G.; Rengasamy, K.R.R.; Wei, Z.J. Lycium Barbarum (Goji) as Functional Food: A Review of Its Nutrition, Phytochemical Structure, Biological Features, and Food Industry Prospects. Crit Rev Food Sci Nutr 2023, 63, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, X.; Ali, M.M.; Rizwan, H.M.; Li, B.; Li, H.; Jia, K.; Yang, X.; Ma, S.; Li, S.; et al. Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora Edulis f. Flavicarpa) and Purple (Passiflora Edulis f. Edulis) Passion Fruits. International Journal of Molecular Sciences 2021, 22, 5765. [Google Scholar] [CrossRef]
- Polat, M.; Mertoglu, K.; Eskimez, I.; Okatan, V. Effects of the Fruiting Period and Growing Seasons on Market Quality in Goji Berry (Lycium Barbarum L.). Folia Horticulturae 2020, 32, 229–239. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; 2010. [Google Scholar]
- Zhao, J.; Li, H.; Xi, W.; An, W.; Niu, L.; Cao, Y.; Wang, H.; Wang, Y.; Yin, Y. Changes in Sugars and Organic Acids in Wolfberry (Lycium Barbarum L.) Fruit during Development and Maturation. Food Chem 2015, 173, 718–724. [Google Scholar] [CrossRef]
- Mutyam, S.; Chilakala, S.; Tallapally, M.; Upadhyayula, V.V.R. Gas Chromatography–Mass Spectrometric Determination of Organic Acids in Fruit Juices by Multiwalled Carbon Nanotube–Based Ion-Pair Dispersive Solid-Phase Extraction and in Situ Butylation. Rapid Communications in Mass Spectrometry 2021, 35, e9165. [Google Scholar] [CrossRef]
- Kafkas, N.E.; Oğuz, H.İ.; Oğuz, İ. Evaluation of Fruit Characteristics of Various Organically-Grown Goji Berry (Lycium Barbarum L., Lycium Chinense Miller) Species during Ripening Stages. Journal of Food Composition and Analysis 2021, 101, 103846. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Compounds Profile, Nutritional Compounds and Bioactive Properties of Lycium Barbarum L.: A Comparative Study with Stems and Fruits. Ind Crops Prod 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Jeepipalli, S.P.K.; Xu, B. Phenolic Profiles and Antioxidant Properties of Goji Berries (Lycium Barbarum). Phytochemicals in Goji Berries 2020, 225–232. [Google Scholar] [CrossRef]
- Poggioni, L.; Romi, M.; Guarnieri, M.; Cai, G.; Cantini, C. Nutraceutical Profile of Goji (Lycium Barbarum L.) Berries in Relation to Environmental Conditions and Harvesting Period. Food Biosci 2022, 49, 101954. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. Bioactive Compounds: Health Benefits and Potential Applications 2019, 33–50. [Google Scholar] [CrossRef]
- Breniere, T.; Fanciullino, A.L.; Dumont, D.; Le Bourvellec, C.; Riva, C.; Borel, P.; Landrier, J.F.; Bertin, N. Effect of Long-Term Deficit Irrigation on Tomato and Goji Berry Quality: From Fruit Composition to in Vitro Bioaccessibility of Carotenoids. Front Plant Sci 2024, 15, 1339536. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Xiao, M.; Zhang, D.; Xie, Z. Characterization of Goji Quality at Different Harvest Stages in Qaidam Basin Based on Transcriptome and Widely Targeted Metabolome. J Food Biochem 2024, 2024, 1139944. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional Constituents and Antioxidant Activities of Eight Chinese Native Goji Genotypes. Food Chem 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Su, Z.; Jia, H.; Sun, M.; Cai, Z.; Shen, Z.; Zhao, B.; Li, J.; Ma, R.; Yu, M.; Yan, J. Integrative Analysis of the Metabolome and Transcriptome Reveals the Molecular Mechanism of Chlorogenic Acid Synthesis in Peach Fruit. Front Nutr 2022, 9, 961626. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, Natural Sources, Dietary Intake and Pharmacokinetic Properties of Ferulic Acid: A Review. Food Chem 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical Characterization, Antioxidant and Antimicrobial Properties of Goji Berries Cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative Studies on Phenolic Profiles, Antioxidant Capacities and Carotenoid Contents of Red Goji Berry (Lycium Barbarum) and Black Goji Berry (Lycium Ruthenicum). Chem Cent J 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Tekin-Cakmak, Z.H.; Kayacan Çakmakoglu, S.; Karasu, S.; Kasapoglu, M.Z.; Avci, E. Effect of Different Drying Techniques on Total Bioactive Compounds and Individual Phenolic Composition in Goji Berries. Processes 2023, 11, 754. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manríquez, D.; Luengwilai, K.; González-Agüero, M. Chapter 1 Aroma Volatiles: Biosynthesis and Mechanisms of Modulation During Fruit Ripening. Adv Bot Res 2009, 50, 1–37. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Forney, C.F.; Song, J. Flavors and Aromas: Chemistry and Biological Functions. Fruit and Vegetable Phytochemicals: Chemistry and Human Health: Second Edition 2017, 1, 515–539. [Google Scholar] [CrossRef]
- Chen, F.; Su, Y.; Zhang, F.; Guo, Y. Low-Temperature Headspace-Trap Gas Chromatography with Mass Spectrometry for the Determination of Trace Volatile Compounds from the Fruit of Lycium Barbarum L. J Sep Sci 2015, 38, 670–676. J Sep Sci 2015, 38, 670–676. [Google Scholar] [CrossRef]
- Endes, Z.; Uslu, N.; Musa Özcan, M.; Fatif, E. Physico-Chemical Properties, Fatty Acid Composition and Mineral Contents of Goji Berry (Lycium Barbarum L.) Fruit. Journal of Agroalimentary Processes and Technologies 2015. [Google Scholar]
- Zhu, H.; Wei, M.; Zhang, Y.; Tao, X. Analysis of Volatile Organic Compounds of Different Types of Peppers (Capsicum Annuum L.) Using Comprehensive Two-Dimensional Gas Chromatography With Time-of-Flight Mass Spectrometry. eFood 2024, 5, e70027. [Google Scholar] [CrossRef]
- Do, S.; Kim, Y.; Yim, J.; Lee, K.G. Analysis of Volatile Compounds, Betaine, and Antioxidant Effect in Goji Berry (Lycium Barbarum L.) Powder Extracted by Various Drying Methods and Extraction Solvents. Curr Res Food Sci 2024, 9, 100798. [Google Scholar] [CrossRef]
| G3 | G4 | G5 | G7 | p | |
| Moisture (g 100 g-1 fw) | 83.44 ± 0.18a | 84.74 ±0.91a | 83.05 ±0.79a | 78.96 ± 0.89b | *** |
| pH | 4.91 ± 0.15b | 5.06 ± 0.12b | 5.29 ± 0.25a | 5.23 ± 0.15a | *** |
| TSS (ºBrix) | 20.27 ± 0.01a | 20.17 ± 0.01a | 18.77 ± 0.05b | 17.73 ± 0.01c | *** |
| TA (%) | 0.42 ± 0.01a | 0.39 ± 0.00a | 0.34 ± 0.00b | 0.40 ± 0.00a | *** |
| RI | 48.80 ± 1.05c | 51.16 ± 0.20b | 54.84 ± 0.65a | 44.46 ± 0.26d | *** |
| G3 | G4 | G5 | G7 | p | |
| Sugars (g 100 g-1 dw) | |||||
| Glucose | 44.10 ± 0.82a | 44.04 ± 1.28a | 26.89 ± 0.92b | 26.36 ± 0.41b | * |
| Fructose | 44.24 ± 0.89ab | 48.77 ± 1.45a | 44.06 ± 2.31ab | 28.99 ± 0.46b | * |
| Total | 88.34 ± 1.67a | 92.81 ± 2.73a | 70.95 ± 3.98b | 55.36 ± 0.87c | *** |
| Organic acids (g 100 g-1 dw) | |||||
| Oxalic | 0.02 ± 0.00a | 0.01 ± 0.00b | 0.01 ± 0.00b | 0.02 ± 0.00a | ** |
| Citric | 1.60 ± 0.12b | 3.57 ± 0.17a | 4.45 ± 0.57a | 0.68 ± 0.02c | *** |
| Tartaric | 1.53 ± 0.10a | 1.52 ± 0.06a | 1.31 ± 0.04b | 1.00 ± 0.04c | *** |
| Malic | 1.73 ± 0.17a | 0.91 ± 0.06b | 0.47 ± 0.18c | 1.09 ± 0.06b | *** |
| Ascorbic | 0.46 ± 0.03a | 0.51 ± 0.03a | 0.38 ± 0.03b | 0.26 ± 0.02c | *** |
| Succinic | 0.96 ± 0.09 | 0.69 ± 0.06 | 0.68 ± 0.04 | 0.65 ± 0.06 | NS |
| Total | 6.29 ± 0.36ab | 7.19 ± 0.25a | 7.31 ± 0.81ab | 3.71 ± 0.18b | * |
| G3 | G4 | G5 | G7 | p | |
| Total phenolic content (TPC) (mg GAE g-1 dw) | 17.86 ± 1.04a | 19.11 ± 2.18a | 14.83 ± 0.97c | 16.79 ± 1.20b | * |
| Individual phenolic compounds (µg phenol g-1 dw) | |||||
| Chlorogenic acid (AClo) | 199.9 ±19.85a | 163.86 ± 17.46a | 213.3 ± 27.4a | 89.85 ± 6.77b | * |
| Neoclorogenic acid (AnClo) | 621.5 ± 84.2c | 890.3 ± 42.6b | 1214.4 ± 47.5a | 287.5 ± 14.0d | *** |
| p-coumaroylquinic (ApC) | 89.74 ± 4.26b | 90.26 ± 9.79b | 209.9 ± 9.8a | 41.28 ± 3.12c | ** |
| p-coumaric acid (ApCou) | n.d.b | n.d.b | 7.03 ± 1.10a | n.d.b | ** |
| t-ferulic acid (t-Fer) | 30.50 ± 3.29b | 40.58 ± 1.89a | 42.92 ± 1.60a | 45.29 ± 5.06a | *** |
| Ruthin (Rut) | n.d.b | n.d.b | 12.67 ± 3.74a | n.d.b | ** |
|
Total carotenoid content (TCC) (mg βCE g-1 dw) |
1.97 ± 0.01ab | 2.45 ± 0.23a | 1.38 ± 0.32b | 1.13 ± 0.09b | ** |
| Individual carotenoid compounds (µg g-1 dw) | |||||
| Capsanthin (Cap) | 147.8 ± 58.3a | 49.02 ± 23.07b | 32.06 ± 4.46b | 95.76 ± 11.48ab | * |
| Zeaxanthin (Zea) | 1398 ± 514.1 | 1722.6 ± 768.7 | 1212.0 ± 268.9 | 1026.7 ± 98.3 | NS |
| β-Cryptoxanthin (β-Crp) | 36.83 ± 11.87 | 31.28 ± 13.24 | 15.01 ± 2.67 | 26.69 ± 2.57 | NS |
| α-carotene (α-Car) | 163.8 ± 49.6a | 149.2 ± 74.2ab | 44.48 ± 9.50b | 74.67 ± 11.01ab | ** |
| β-carotene (β-Car) | 1.47 ± 0.65ab | 2.40 ± 0.57a | 1.17 ± 0.18b | 1.47 ± 0.17ab | * |
| Antioxidant activity (mg TE g-1 dw) | |||||
| DPPH | 12.42 ± 1.11a | 10.04 ± 0.71b | 12.15 ± 0.55a | 12.34 ± 0.71a | * |
| CUPRAC | 102.9 ± 3.0 | 126.0 ± 19.2 | 106.9 ± 10.08 | 104.7 ± 5.2 | NS |
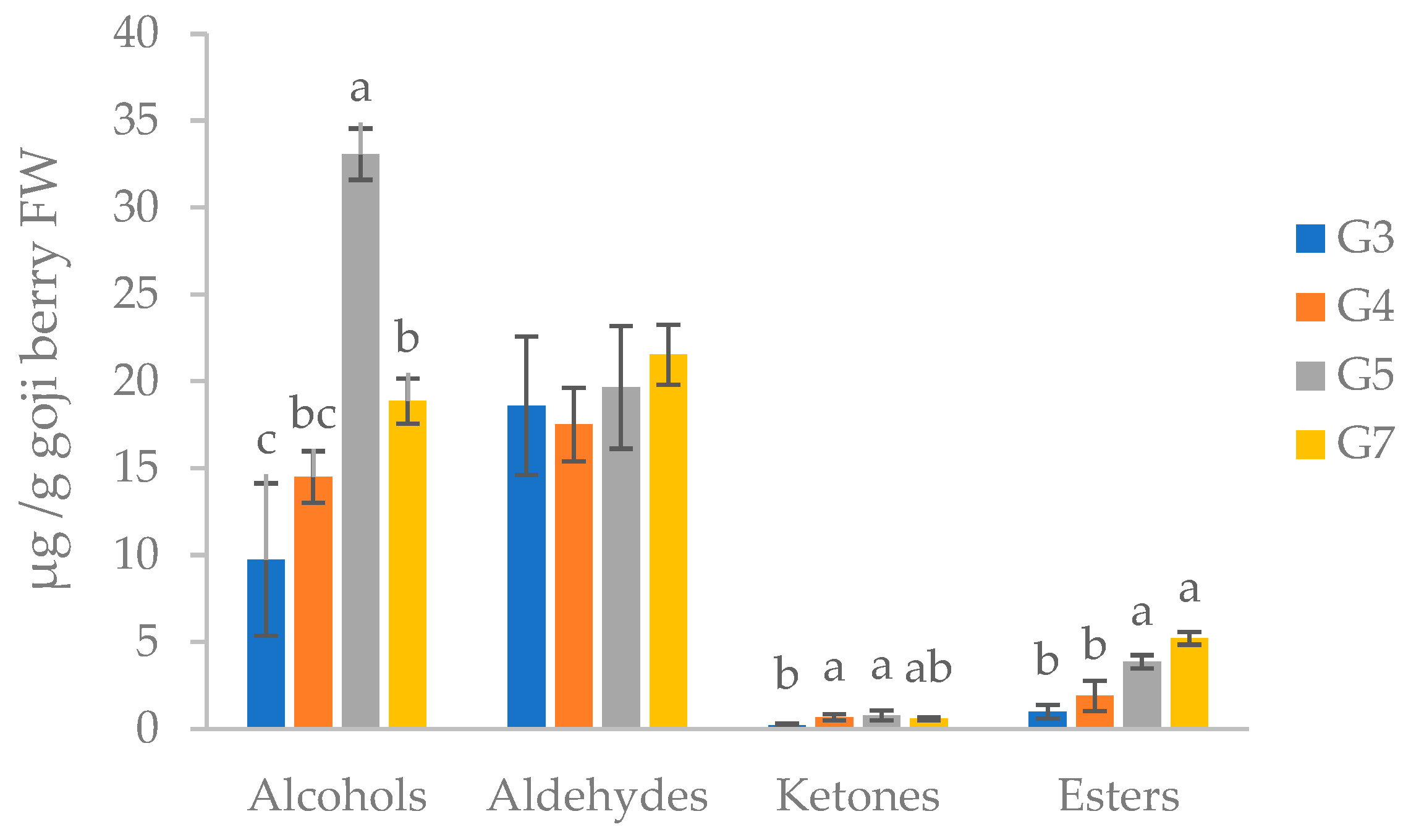
| LRI | Volatile organic compounds | Id. Method | G3 | G4 | G5 | G7 |
| Alcohols | ||||||
| 660.0 | Cyclopentanol | NIST | 0.14 ± 0.09b | 0.17 ± 0.03b | 0.63 ± 0.12a | 0.40 ± 0.18ab |
| 746.5 | 1-Pentanol | Sigma–Aldrich | 0.27 ± 0.19b | 0.53 ± 0.08ab | 0.89 ± 0.35a | 1.14 ± 0.25a |
| 853.9 | 3-Hexen-1-ol | Sigma–Aldrich | 2.08 ± 0.92ab | 0.77 ± 0.06b | 1.65 ± 0.01b | 3.86 ± 0.39a |
| 870.2 | 1-Hexanol | Sigma–Aldrich | 1.17 ± 0.65d | 3.00 ± 0.32b | 10.49 ± 1.18a | 2.28 ± 0.01c |
| 980.5 | 1-Octen-3-ol | NIST | 5.58 ± 2.34c | 7.00 ± 0.38bc | 16.71 ± 0.84a | 9.34 ± 0.69b |
| 1058.6 | 2,5-Pentadecadien-1-ol | NIST | 0.07 ± 0.04c | 0.37 ± 0.09b | 1.11 ± 0.08a | 0.54 ± 0.15b |
| 1071.4 | 1-Octanol | NIST | 0.30 ± 0.12b | 0.27 ± 0.03b | 0.78 ± 0.13a | 0.75 ± 0.17a |
| 1098.1 | 1,6-Octadien-3-ol, 3,7-dimethyl | NIST | 0.07 ± 0.04b | 2.02 ± 0.49a | 0.07 ± 0.01b | 0.21 ± 0.04a |
| 1103.0 | 2-Nonen-1-ol | NIST | 0.09 ± 0.06c | 0.33 ± 0.08b | 0.54 ± 0.07a | 0.34 ± 0.06b |
| 1300.8 | Z,Z-2,5-Pentadecadien-1-ol | NIST | n.d. | 0.05 ± 0.01b | 0.21 ± 0.06a | 0.02 ± 0.00c |
| Aldehydes | ||||||
| 637.2 | Butanal, 3-methyl | Sigma–Aldrich | 0.63 ± 0.16c | 2.64 ± 1.09b | 0.59 ± 0.20c | 5.48 ± 1.65a |
| 644.1 | Butanal, 2-methyl | Sigma–Aldrich | 0.93 ± 0.45a | 1.36 ± 0.17a | 0.83 ± 0.19a | 2.17 ± 1.05a |
| 672.9 | Pentanal | Sigma–Aldrich | 0.38 ± 0.09b | 0.49 ± 0.05ab | 0.49 ± 0.19ab | 0.74 ± 0.26a |
| 786.1 | Hexanal | Sigma–Aldrich | 14.04 ± 3.20a | 11.88 ± 1.18a | 15.93 ± 3.29a | 11.43 ± 0.71a |
| 849.1 | 2-Hexenal | NIST | 2.20 ± 0.18a | 0.62 ± 0.12c | 0.35 ± 0.14c | 0.87 ± 0.15b |
| 958.8 | 2-Heptenal | NIST | 0.37 ± 0.12b | 0.33 ± 0.04b | 0.53 ± 0.08ab | 0.66 ± 0.03a |
| 973.3 | 2-Nonenal, (E) | NIST | n.d. | n.d. | 0.18 ± 0.06a | n.d. |
| 974.8 | 2-Octenal | NIST | n.d. | n.d. | 0.41 ± 0.04a | n.d. |
| 1098.6 | 2,6-Octadienal, 3,7-dimethyl | NIST | 0.06 ± 0.04c | 0.20 ± 0.05b | 0.33 ± 0.03a | 0.19 ± 0.01b |
| Ketones | ||||||
| 663.1 | 1-Penten-3-one | NIST | 0.18 ± 0.09bc | 0.24 ± 0.10b | n.d. | 0.49±0.10a |
| 1458.3 | 5,9-Undecadien-2-one, 6,10-dimethyl | NIST | n.d. | 0.31 ± 0.11b | 0.52 ± 0.17a | n.d. |
| 1494.1 | 3-Buten-2-one | NIST | 0.02 ± 0.02b | 0.12 ± 0.05b | 0.28 ± 0.10a | 0.11 ± 0.03b |
| Esters | ||||||
| <600.0 | Carbon dioxide | NIST | 0.34 ± 0.17a | 0.37 ± 0.05a | 0.64 ± 0.05a | 0.59 ± 0.13a |
| 989.9 | Cyclohexene, 3-(2-methylpropyl) | NIST | 0.14 ± 0.05b | 0.06 ± 0.03c | 0.08 ± 0.02c | 0.36 ± 0.16a |
| 995.3 | Butanoic acid, butyl ester | NIST | 0.15 ± 0.09b | 0.08 ± 0.04b | 0.37 ± 0.01a | 0.52 ± 0.08a |
| 1194.6 | Methyl salicylate | NIST | 0.24 ± 0.15b | 0.53 ± 0.12b | 2.73 ± 0.34a | 1.38 ± 0.24b |
| 1046.1 | 2-Hexenoic acid, ethyl ester | NIST | 0.14 ± 0.05a | 0.06 ± 0.03b | 0.08 ± 0.02b | 0.36 ± 0.16a |
| Aromatic Compounds | ||||||
| 1027.0 | Eucalyptol | Sigma–Aldrich | 1.43 ± 0.51d | 2.86 ± 0.26c | 5.74 ± 0.14b | 7.47 ± 0.89a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





