1. Introduction
Up to a half of pT2-3 node-negative prostate cancer (PCa) patients experience biochemical recurrence (BCR) after radical prostatectomy (RP) or radiotherapy [
1]. Detection of responsible lesions in the context of a BCR constitutes a major challenge for conventional imaging modalities as computed tomography (CT) and bone scan.
Choline-based PET/CT has been the traditional imaging modality of choice in restaging patients following BCR [
2]. However, multiple studies have shown low sensitivity and specificity, particularly at low prostate specific antigen (PSA) levels, which can result in delays in salvage therapies [
3,
4]. For several years, and due to these limitations, the development of radionuclides that recognizes prostate-specific membrane antigen (PSMA) ligands has been proposed as an alternative, with higher sensitivity and specificity in BCR PCa patients [
5]. These “top diagnostic” radiotracers have increased the detection rate (DR) of oligometastatic disease (OD) that has driven recent advancements in metastasis-directed treatment strategies of oligometastatic PCa.
18F-DCFPyL [2-(3-(1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid] is a radiofluorinated, small molecule high affinity inhibitor of PSMA [
6]. The current restrictions in its use in our environment explain the dual-tracer diagnostic approach in some cases of BCR, especially in those with a PSA level >2 ng/mL and a previous negative or doubtful choline-labelled PET/CT. Some studies have addressed utility of 68Ga-labelled PSMA ligands and choline-labelled tracers in head to head comparison [
7,
8], although no previous reported experience exists using the newest developed 18F-DCFPyL. On the other hand, if we only use DR to compare both radiotracers, the real diagnostic potential of PSMA-ligands with respect to choline-labelled tracers may be limited. In addition, differences in therapeutic impact have been scarcely assessed [
9].
The Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria summarize standards for study design and reporting of prostate cancer molecular imaging. PROMISE criteria propose a molecular imaging TNM (miTNM) for the interpretation of PSMA ligands PET/CT designed to organize findings in comprehensible categories and to promote the exchange of information among physicians and institutions [
10].
The aim of our study was multiple: (i) to analyse the concordance between 18F-DCFPyL and 18F-Fluorocholine, in head to head comparison, regarding DR and miTNM stage using PROMISE criteria, (ii) to address the predictive value of unfavourable PSA kinetics on miTNM, and (iii) to assess the therapeutic impact of 18F-DCFPyL with respect to 18F-Fluorocholine-PET/CT in patients with BCR PCa.
3. Results
One hundred and thirty-eight patients were enrolled. All the patients’ characteristics are presented in
Table 1.
18F-DCFPyL showed a higher DR than 18F-Fluorocholine, 64.5% (89/138) and 33.3% (46/138), respectively (
Table 2). Both were negative in 44 patients (31.9%) and positive in 41 (29.7%), however, in 20/41 patients, 18F-DCFPyL visualized additional lesions with respect to 18F-Fluorocholine, which entailed miTNM stage change in 17 patients (
Figure 1).
On the other hand, 18F-DCFPyL was positive alone in 48/89 (53.9%) patients, being oligometastatic in 25 (
Figure 2). Five patients were exclusively positive with 18F-Fluorocholine-PET/CT, and thus 18F-DCFPyL down-staged 18F-Fluorocholine results from positive to negative (3 follow-up, 1 biopsy (negative) and 1 ADT). 18F-DCFPyL up-staged 5/21 patients with OD on 18F-Fluorocholine-PET/CT to polimetastatic disease after 18F-DCFPyL.
18F-DCFPyL and 18F-Fluorocholine PET/CTs detected T in 33.3% and 19.6%, respectively, with a moderate concordance. N was observed in 27.5% on 18F-DCFPyL scans and 13.8% on 18F-Fluorocholine. However, the most significant difference was found for M detection, 30.4% and 8.7%, for 18F-DCFPyL and 18F-Fluorocholine respectively (
Table 3).
Regarding first to subsequent BCR, 18F-Choline DR was 35.6% and 27% respectively, and 18F-DCFPyL DR 61.4% and 72.9%. No statistically differences were found in first to subsequent BCR DR neither 18F-Choline or 18F-DCFPyL (p = 0.435 and p = 0.164, respectively). We found weak (k = 0.378, p < 0.001) and poor (k = 0.079, p < 0.467) concordance in first to subsequent BCR DR between 18F-Choline and 18F-DCFPyL.
Correlation between PET/CT results and PSA kinetics
Both 18F-DCFPyL and 18F-Fluorocholine PET/CT DR showed significant associations with PSA groups and PSAvel. No significant association was found with PSAdt. 18F-DCFPyL DR was 81.3% in patients with PSA > 2 ng/ml, higher than patients with 1 < PSA ≤ 2 (58.8%) and PSA ≤ 1 ng/ml (39.1%). It was also higher in patients with PSAvel > 0.2 ng/ml/month (90.5%) compared with those with PSAvel ≤ 0.2 ng/ml/month (53.7%). 18F-Fluorocholine DR was also higher in cases with PSA > 2 ng/ml (46.6%), being 35.3% and 13.04% in patients with 1 < PSA ≤ 2 and PSA ≤ 1 ng/ml respectively, and 52.4% in cases with PSAvel > 0.2 ng/ml/month versus 26.3% with PSAvel ≤ 0.2 ng/ml/month. In addition, mean PSA was statistically different among patients with T and N recurrence on 18F-Fluorocholine-PET/CT (
p = 0.028). Also differences in mean PSA and PSAdt were found between N and M (
p = 0.034), and T and M (
p = 0.031) metabolic disease, respectively, on 18F-DCFPyL-PET/CT (
Table 4).
Using predefined cut-off values of PSA and PSA kinetics values significant association was only found between miTNM and PSA groups on 18F-Fluorocholine-PET/CT (p = 0.033) and not for PSAdt or PSAvel. No statistical association was found between miTNM, PSA or PSA kinetics on 18F-DCFPyL PET/CT. In ROC analysis, only a PSAdt cut-off of 4.09 months showed significant association for the prediction of M stage with 18F-Fluorocholine-PET/CT (66.7% sensitivity, 73.8% specificity, 0.720 AUC, p = 0.012). For 18F-DCFPyL PET/CT the obtained cut-offs in the prediction of M stage were: PSA of 2.41 ng/ml (66.7% sensitivity, 64.4% specificity, 0.675 AUC, p = 0.002), PSAdt of 5.59 months (61.1% sensitivity, 60.8% specificity, 0.679 AUC, p = 0.001) and PSAvel of 0.13 ng/ml/month (66.7% sensitivity, 61.4% specificity, 0.723 AUC, p < 0.001).
As a result of 18F-DCFPyL-PET/CT, therapeutic management was changed in 40/138 (29%) patients with respect to 18F-Fluorocholine-PET/CT based planning treatment. Escalation was elected in 34 patients: 6 radiotherapy, 5 radiotherapy plus ADT, 6 surgery, 1 prostate cryoablation and 16 ARAT. De-escalation occurred in 6 patients: follow-up in 4 cases (3 18F-DCFPyL negative and 1 with prostatic uptake with both radiotracers and no malignant disease confirmed by biopsy) and ADT instead local treatment in 2 cases. Nevertheless, a potential change in therapeutic management was not achieved because of patient’s comorbidities in 11 patients.
Derived from positive 18F-DCFPyL-PET/CT, 19 patients underwent additional diagnostic procedures to confirm the results: 8 by imaging (3/8 was confirmed) and 11 by histological analysis (8/11 was confirmed) (
Figure 3) (
Figure 4).
18F-DCFPyL-PET/CT was negative in 49/138 patients (7 low, 14 intermediate and 28 high risk). Follow-up was adopted in 29 patients (4 positive 18F-Fluorocholine-PET/CT) and 20 intermediate/high risk patients underwent treatment (12 prostatic fossa radiotherapy, 8 ADT, 1/8 choline-positive). Regarding the false positive, six patients with positive 18F-DCFPyL-PET/CT (2 prostate gland, 3 bone and 1 rectum) had a normal MRI (
Figure 5) (
Figure 6). Ten patients were choline-positive and considered false positive (2 prostate gland, 5 lymph nodes, 2 bone, 1 pelvic mass) due to 18F-DCFPyL PET/CT result, biopsy or clinical follow-up.
For patients who benefited from a treatment change, local treatments were exclusively guided by 18F-DCFPyL in eleven patients. Follow-up showed: PSA decreased in 6 (4 radiotherapy, 1 cryoablation, 1 surgery), PSA increased in 2 surgically treated patients (in one patient an ulterior 18F-DCFPyL-PET/CT revealed an incomplete surgical procedure and in the other one, surgical procedure was performed almost 7 months after 18F-DCFPyL-PET/CT), in 2 patients biochemical progression occurred before treatment decision, and the remaining patient was missed.
Literature search results from PubMed/MEDLINE revealed 17 articles. Reviewing titles and abstracts, 7 articles were excluded: 4 because not in the field of interest of this review and 3 as reviews. Ten articles were selected and retrieved in full-text version [
7,
8,
9,
12,
13,
14,
15,
16,
17,
18]. Data of 1868 patients with BCR PCa who underwent choline-labelled tracers and PSMA ligands PET/CT were eligible for the analysis (systematic review). Methodology and results of the selected papers are summarized on Table 5.
4. Discussion
In BCR, diagnostic impact of PSMA-ligands against choline-labelled tracers is significantly higher in patients with low PSA levels and previous negative/doubtful choline-labelled PET/CT in whom the detection of OD might enable metastasis-directed treatments [
19,
20]. Differences in classification of OD using both tracers exist, with up-staging for the PSMA ligands especially in choline-negative patients [
15]. In our study, patients with previous negative/oligometastatic 18F-Fluorocholine-PET/CT were referred to 18F-DCFPyL expecting a benefit by the detection of more metastases. In fact, we found that 5/21 patients with OD in 18F-Fluorocholine-PET/CT were up-staged to polimetastatic after 18F-DCFPyL, similar to previous reported results [
15]. The absence of consensus about oligometastatic definition can limit diagnostic impact comparison of different radiotracers. In previous studies, OD was defined as M stage with ≤ 5 lesions [
15,
16], whereas other authors considered ≤ 3 as we did in this study [
7]. Chevalme et al. found, using 68Ga-PSMA, OD (1–3 foci) in 31% of the cases with previous negative/doubtful 18F-Fluorocholine [
7]. In our sample, 18F-DCFPyL-PET/CT detected OD in the 52.1% (25/48) of choline-negative cases. In a relevant number of cases, we detected positive lymph nodes and bone lesions which showed divergent findings with both tracers. The majority were only 18F-DCFPyL-positive, probably due to higher lesion/background ratio and sensitivity compared to 18F-Fluorocholine that enables detection of smaller lesions.
Previous authors found a PSMA-ligands DR from 43.8% to 67% in choline-negative cases, greater in those with doubtful findings [
7,
8,
14,
16]. Our results are in accordance with previous, with a 18F-DCFPyL DR of 53.9% in patients with negative/equivocal 18F-Fluorocholine although we did not assess differences between negative and equivocal results based on few cases of the latter (5).
Chevalme et al. found that that DR was lower in first BCR versus previous (63 vs. 72%) [
7]. In our study, no significant differences were found neither 18F-Fluorocholine nor 18F-DCFPyL. On the other hand, DR shows a great association with PSA kinetics with independence of the radiotracer used on PET/CT. However, despite a PSAdt ≤ 6 months has been reported as a strong predictor of positivity of choline-labelled PET/CT [
20], we don’t find a significant association with DR or miTNM for any of the studied radiotracers with their counterparts. This absence of significant association with predefined unfavourable PSA kinetics promotes the interest in exploring other clinical, metabolic and laboratory parameters. In fact, we found that different cut-off values of PSA kinetics were able to predict M stage, especially for 18F-DCFPyL-PET/CT, although with a moderate accuracy.
Regarding disease location on BCR PCa, 18F-DCFPyL-PET/CT detected T in 33.3% of cases, similar to 26% reported by Chevalme et al. [
7], but higher than 11% of Barbaud et al. [
8] explained by higher rate of patients Barbaud et al. included with RP (76%). We observed higher T detection than 18F-Fluorocholine, although with a moderate concordance (k = 0.403,
p < 0.001). Discrimination between benign and malignant intraprostatic tissue is hampered by low specificity of choline-labelled tracers for PCa based on high affinity of this radiotracer by benign hyperplasia [
21,
22]. Lymph node is the most prevalent disease location in BCR PCa, showing PSMA-ligands a DR from 34% to 39% with choline-negative [
7,
8,
15]. Our N disease detection using 18F-DCFPyL was lower (27.5%), with a weak concordance with 18F-Fluorocholine. Previous authors found that 55% of the detected lymph nodes were identified with both tracers. Thus, using PSMA-ligands, increase in DR affecting both the number and locations of lymph nodes is a fact [
15]. However, the most significant difference in our sample was M detection, 30.4% and 8.7%, for 18F-DCFPyL and 18F-Fluorocholine respectively, in accordance with previous studies [
7,
15].
Usually PSMA-ligands spots all choline-positive lesions and discordances are mainly related to choline-negative/PSMA-positive findings [
13,
15]. The explanations of these discordances are contradictory and based on: (i) different metastasis environment with a loss of expression of PSMA that can occur in less than 10% of primary or metastatic prostate tumours [
23]; (ii) tumour progression between scans in cases of a wide time interval [
9]; and (iii) unspecific inflammation can promote choline uptake in lymph nodes, that can explain additionally choline-positive lymph nodes [
24]. PSMA-ligands specificity may range from 36% to 100% [
25,
26], per se as it targets PSMA, a glycoprotein which overexpression seems more characteristic for PCa than up-regulation of choline kinase [
26]. Based on that, we considered findings of PSMA ligands as standard, as previous authors [
15,
17].
Prostatic fossa salvage radiation treatment (SRT) is the current standard of care in men with their first BCR after RP with about half of men achieving a complete biochemical response at 5 years after SRT [
27]. In patients with a previous radical radiotherapy, only brachytherapy is indicated if malignancy is histopathologically confirmed. On the other hand, a second BCR, in patients undergone previous radiotherapy of prostatic fossa, with or without pelvic lymph nodes involved, reduces the potential local treatment options. Therefore, ADT becomes a therapeutic option in patients with BCR without located disease or no indication for any local treatment (SRT or surgery).
Therapeutic impact of PSMA-ligands with respect to choline-labelled PET/CT has been scarcely analysed, ranging from 54% to 74% [
8,
17]. We observed an impact management in 29% of cases although it could be raised to 37% if patients’ comorbidities that limited correct treatment have been included. Escalation was considered when the treatment modification involved changing/adding radiotherapy fields or adding ARAT to the systemic ADT. Thus, men with a previous RP without disease or with disease confined to the prostatic fossa on PET/CT imaging were expected to proceed to SRT or a combination of radiotherapy and ADT if few lesions were defined on 18F-DCFPyL-PET/CT or ADT plus ARAT in case of multiple locations (M stage) in patients with no chemotherapy indication. The therapeutic impact derived from 18F-DCFPyL over 18F-Fluorocholine findings allowed treatment escalation in most of our patients (34/40). However, the assessment of therapeutic impact is controversial, being not only dependent of the accuracy of diagnostic techniques but on other factors as previously received treatments and the comorbidities or physical status of the patients so, although 18F-DCFPyL-PET/CT result could have changed the therapeutic management, this decision was not carried out because of that in some patients of our sample. Diagnostic escalation (additional diagnostic imaging vs. biopsy) to confirm 18F-DCFPyL results is another relevant aspect to be assessed. In our sample, 19 patients were derived to additional diagnostic procedures, only 11 by histological analysis, lower than the 24% reported by Morigi et al. [
17]. About half of men who experience BCR after RP and undergo SRT to the prostatic bed, even when there are no significant imaging findings, are currently cured [
28] suggesting that SRT should still be considered despite a negative imaging result [
29]. On the other hand, focused radiotherapy based on PSMA ligands PET/CT, exhibits higher response rates compared to the conventional procedure without metabolic guide, although can not guarantee undetectable PSA in all the cases, that means that PSMA PET/CT still underestimates the extent of the recurrent disease [
8,
29]. In the present analysis, 12 out of 49 patients with a negative 18F-DCFPyL-PET/CT underwent prostatic fossa radiotherapy.
Thus, therapeutic implications of PSMA-ligands can be significant can be significant. Target miss in BCR due to insufficient diagnostic work-up may lead to inadequate definition local disease and to untreated microscopic or macroscopic disease distant from prostatic fossa (N1/M1). The expected result, derived from an earlier and more accurate diagnosis of PSMA ligands PET/CT, is the opportunity for focused management using focal treatments, with a reduction of the introduction of ADT. The possibility of an improved disease control as long as possible before ADT introduction, would reduce the resistance to ADT during their disease, which may limit future therapeutic possibilities [
30,
31].
Regarding limitations, histopathological confirmation of our PET/CT results was not always feasible, although it is a controversial issue and probably neither indicated nor ethical. In addition, 2 months period used between both PET/CT could limit a reliable comparison between both radiotracers in cases of a highly proliferative disease. However, PCa usually presents with slow growth and noticeable changes within this period are very unlikely. With respect to the strengths, this is the first reported experience of 18F-DCFPyL in parallel comparison with 18F-Fluorocholine in a significant sample of patients with BCR PCa.
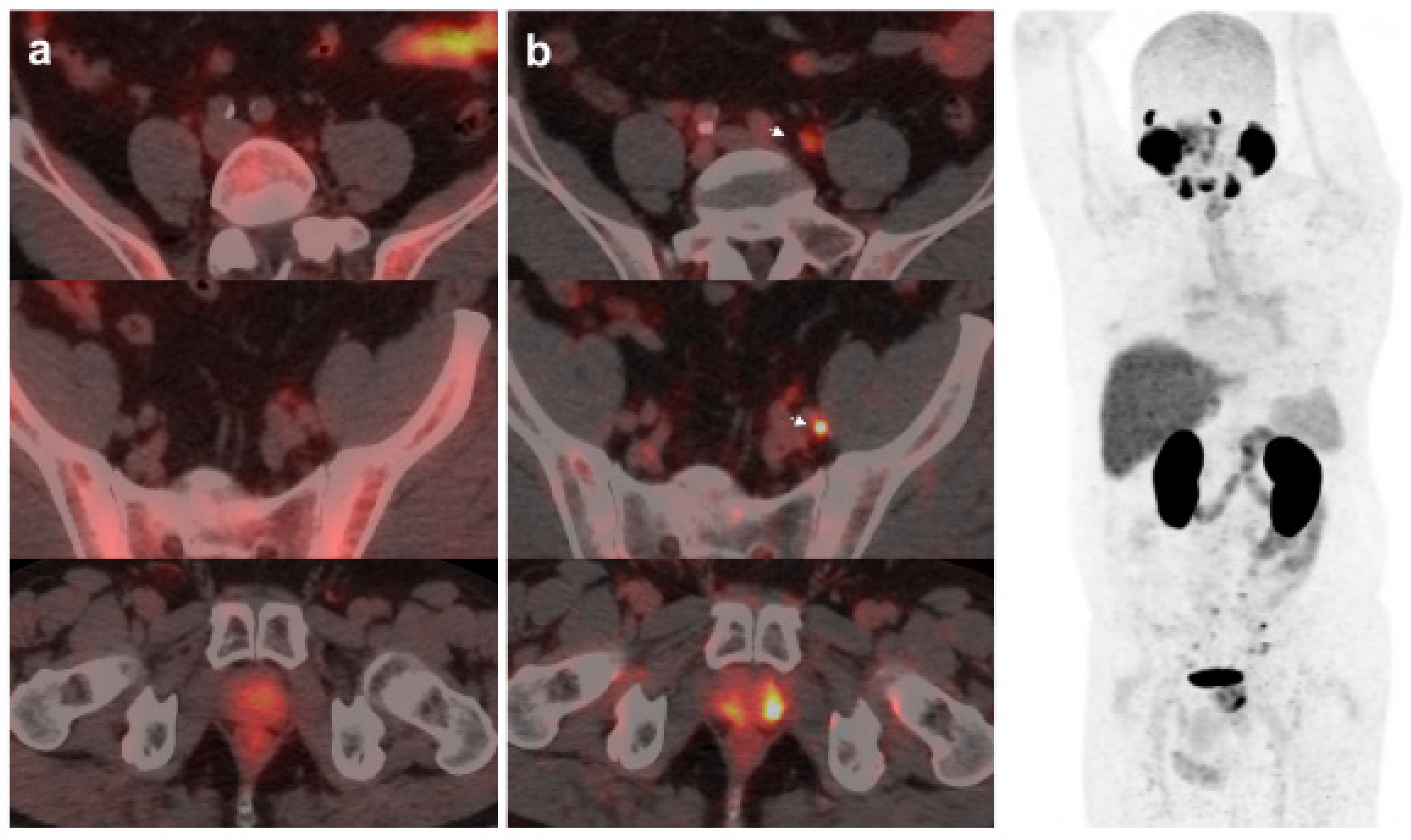
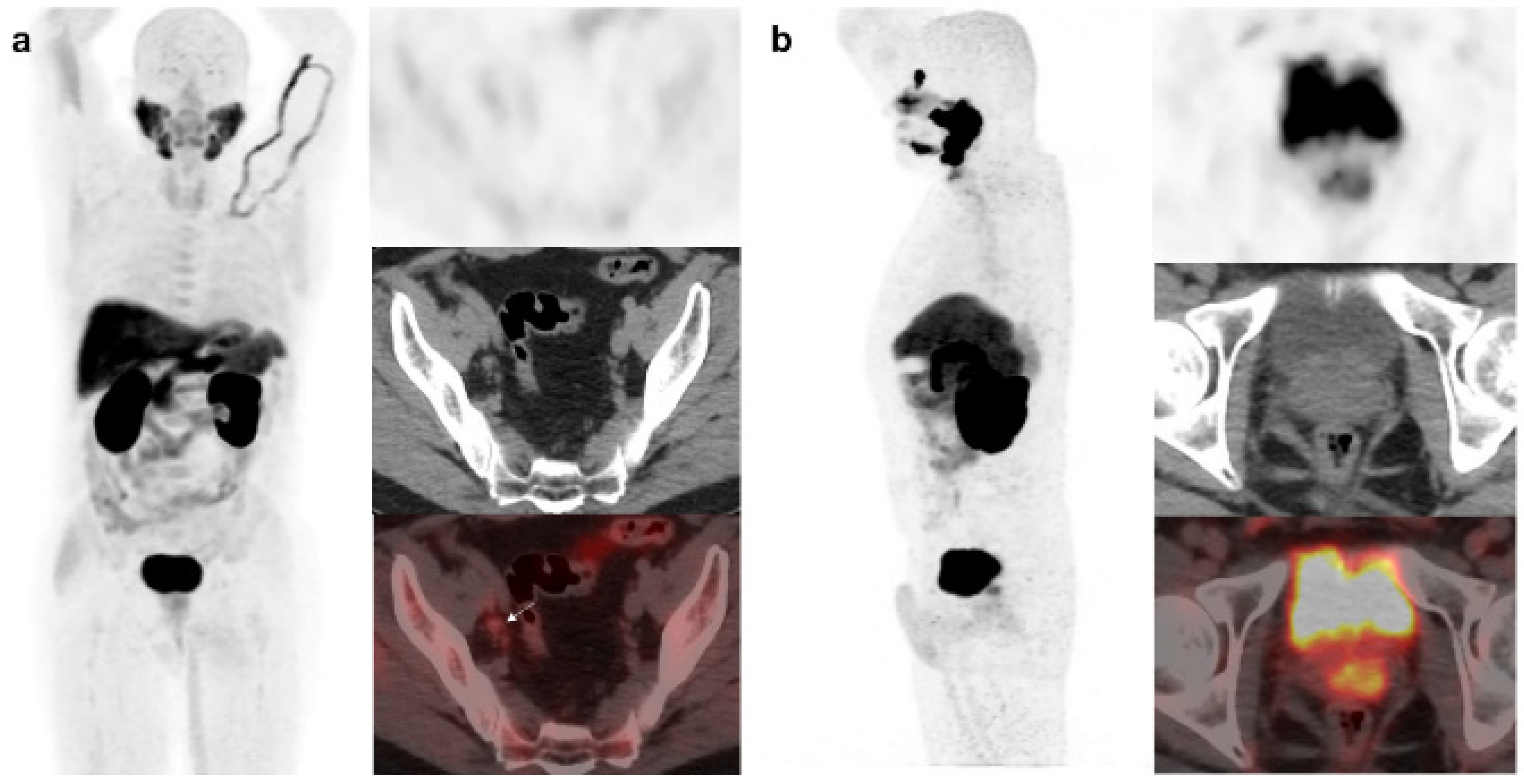
Figure 1.
59-year-old patient. Gleason 7 PCa treated with radiotherapy plus ADT. After ADT withdrawal BCR was detected (PSA 2.44 ng/mL, PSAdt 2.6 mo, PSAvel 0.15 ng/mL/mo). 18F-Fluorocholine (a) demonstrated only prostatic uptake and 18F-DCFPyL-PET/CT (b) showed prostatic tracer uptake and lymph node metastasis (arrows). Time window of sixteen days between both scans. 18F-DCFPyL changed therapeutic management allowing escalation (ADT + Apalutamide).
Figure 1.
59-year-old patient. Gleason 7 PCa treated with radiotherapy plus ADT. After ADT withdrawal BCR was detected (PSA 2.44 ng/mL, PSAdt 2.6 mo, PSAvel 0.15 ng/mL/mo). 18F-Fluorocholine (a) demonstrated only prostatic uptake and 18F-DCFPyL-PET/CT (b) showed prostatic tracer uptake and lymph node metastasis (arrows). Time window of sixteen days between both scans. 18F-DCFPyL changed therapeutic management allowing escalation (ADT + Apalutamide).
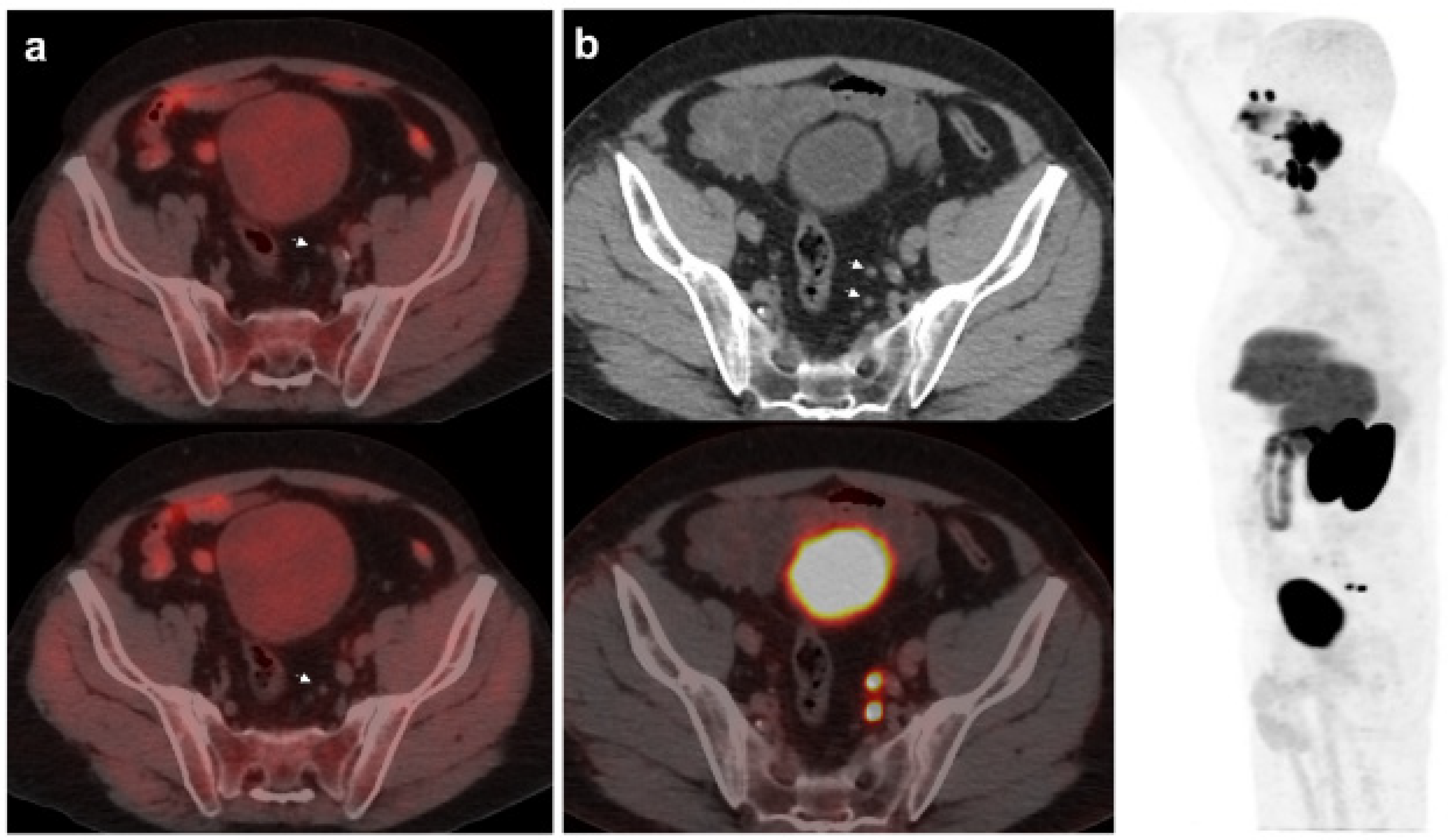
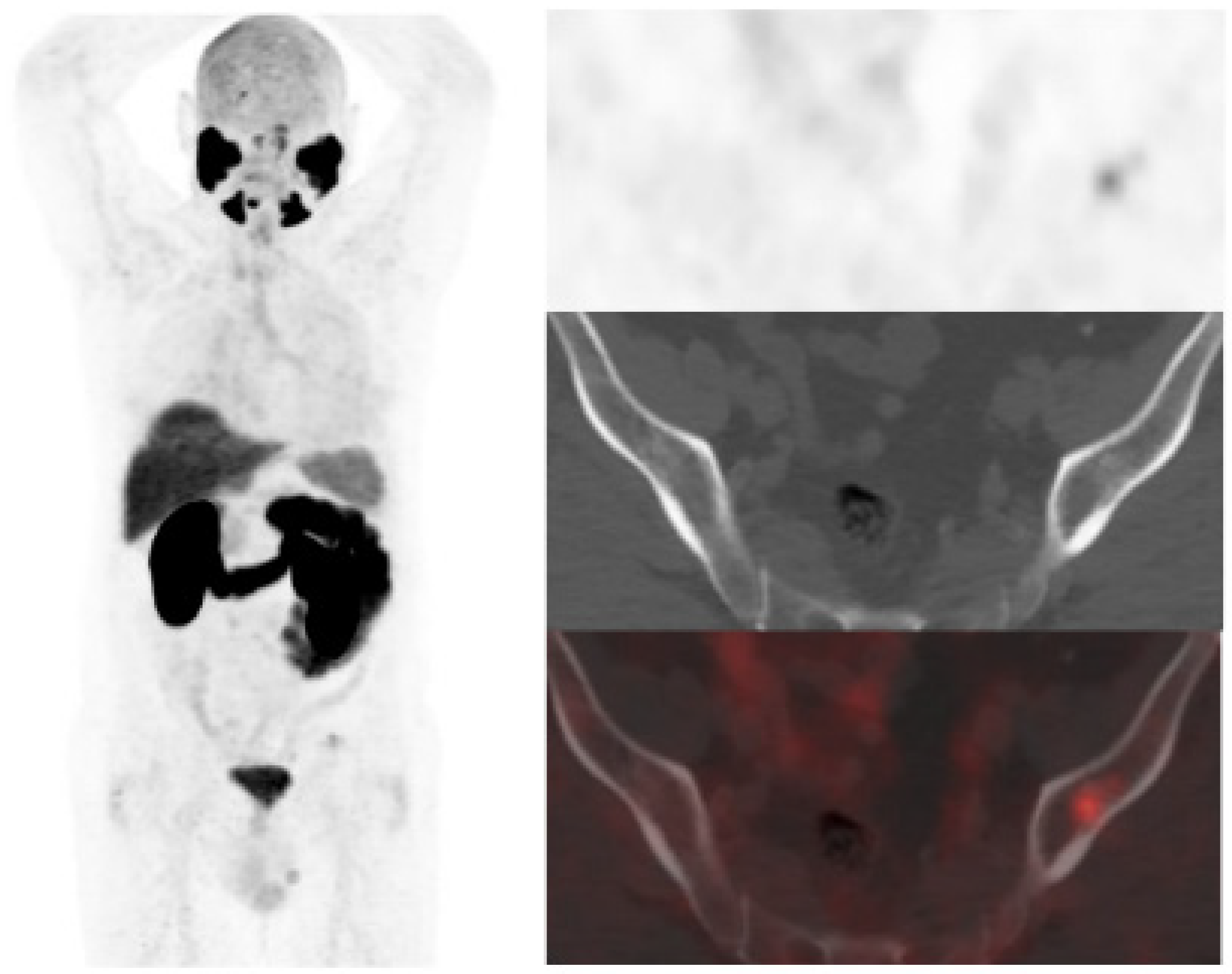
Figure 2.
67-year-old patient. Gleason 7 PCa treated with RP. First BCR treated with prostate fossa radiotherapy. Second BCR (PSA: 0.63 ng/mL, PSAdt 8.6 mo, PSAvel 0.04 ng/mL/mo) scanned with 18F-Fluorocholine (a) and 18F-DCFPyL-PET/CT (b), time window of six days. Lymph nodes metastasis (arrows) were demonstrated only on 18F-DCFPyL scan, changing therapeutic management (escalation). Patient underwent lymph nodes SBRT descending PSA level.
Figure 2.
67-year-old patient. Gleason 7 PCa treated with RP. First BCR treated with prostate fossa radiotherapy. Second BCR (PSA: 0.63 ng/mL, PSAdt 8.6 mo, PSAvel 0.04 ng/mL/mo) scanned with 18F-Fluorocholine (a) and 18F-DCFPyL-PET/CT (b), time window of six days. Lymph nodes metastasis (arrows) were demonstrated only on 18F-DCFPyL scan, changing therapeutic management (escalation). Patient underwent lymph nodes SBRT descending PSA level.
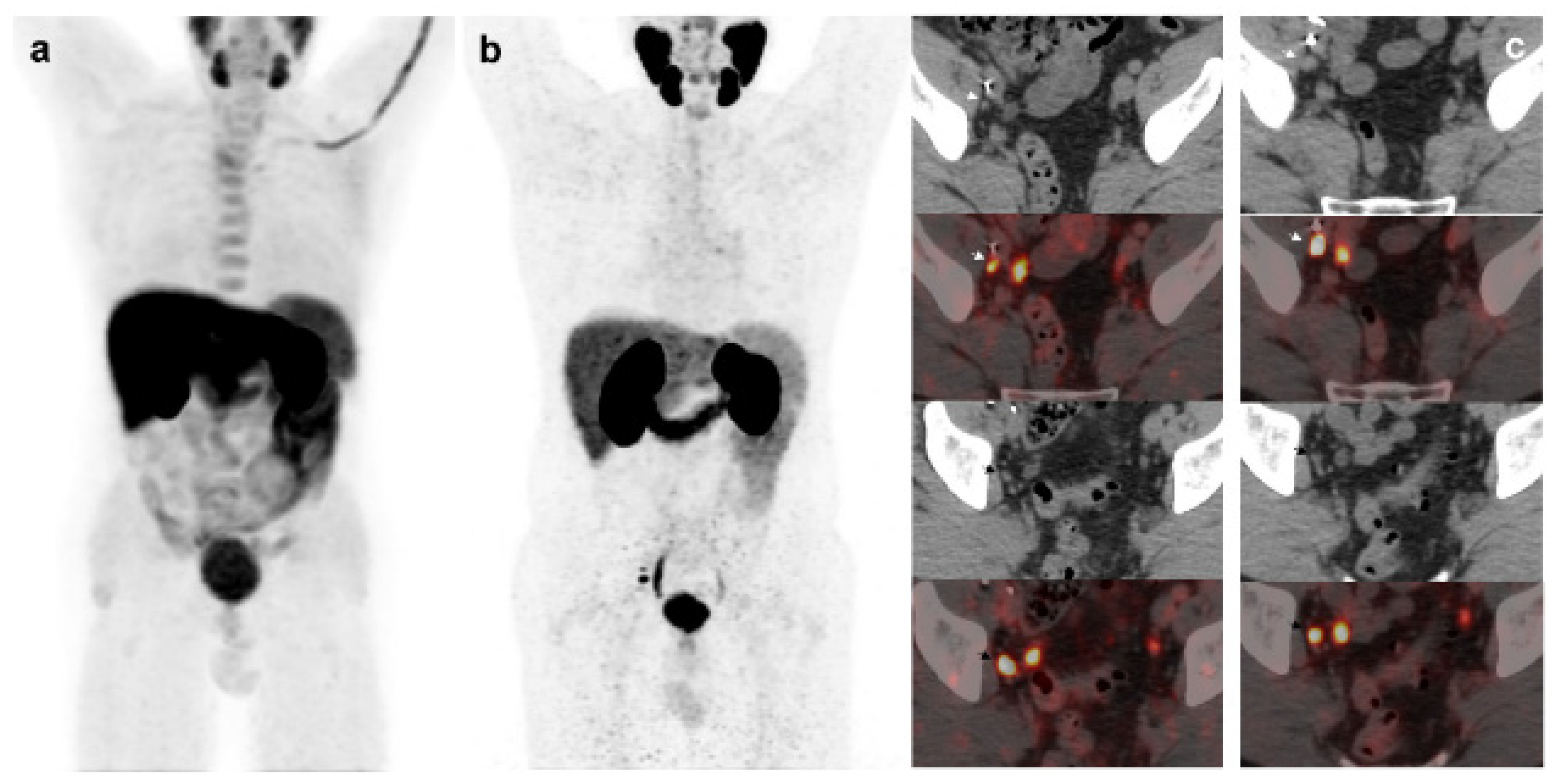
Figure 3.
55-year-old patient. Gleason 8 PCa treated with RP. First BCR treated with prostate fossa radiotherapy. Second BCR (PSA: 0.84 ng/mL, PSAdt 5.99 mo, PSAvel 0.07 ng/mL/mo). 18F-Fluorocholine-PET/CT negative (a). 18F-DCFPyL-PET/CT (b), time window of twenty days, revealed two right external iliac lymph node metastasis (arrows). Lymphadenectomy was decided (escalation), without histopathological confirmation of malignancy. In follow-up PSA progressed (2.07 ng/mL) and an additional 18F-DCFPyL-PET/CT (c) showed exactly same lymph nodes (arrows). SBRT was administered decreasing the PSA level, reclassifying 18F-DCFPyL-PET/CT results as true positive.
Figure 3.
55-year-old patient. Gleason 8 PCa treated with RP. First BCR treated with prostate fossa radiotherapy. Second BCR (PSA: 0.84 ng/mL, PSAdt 5.99 mo, PSAvel 0.07 ng/mL/mo). 18F-Fluorocholine-PET/CT negative (a). 18F-DCFPyL-PET/CT (b), time window of twenty days, revealed two right external iliac lymph node metastasis (arrows). Lymphadenectomy was decided (escalation), without histopathological confirmation of malignancy. In follow-up PSA progressed (2.07 ng/mL) and an additional 18F-DCFPyL-PET/CT (c) showed exactly same lymph nodes (arrows). SBRT was administered decreasing the PSA level, reclassifying 18F-DCFPyL-PET/CT results as true positive.
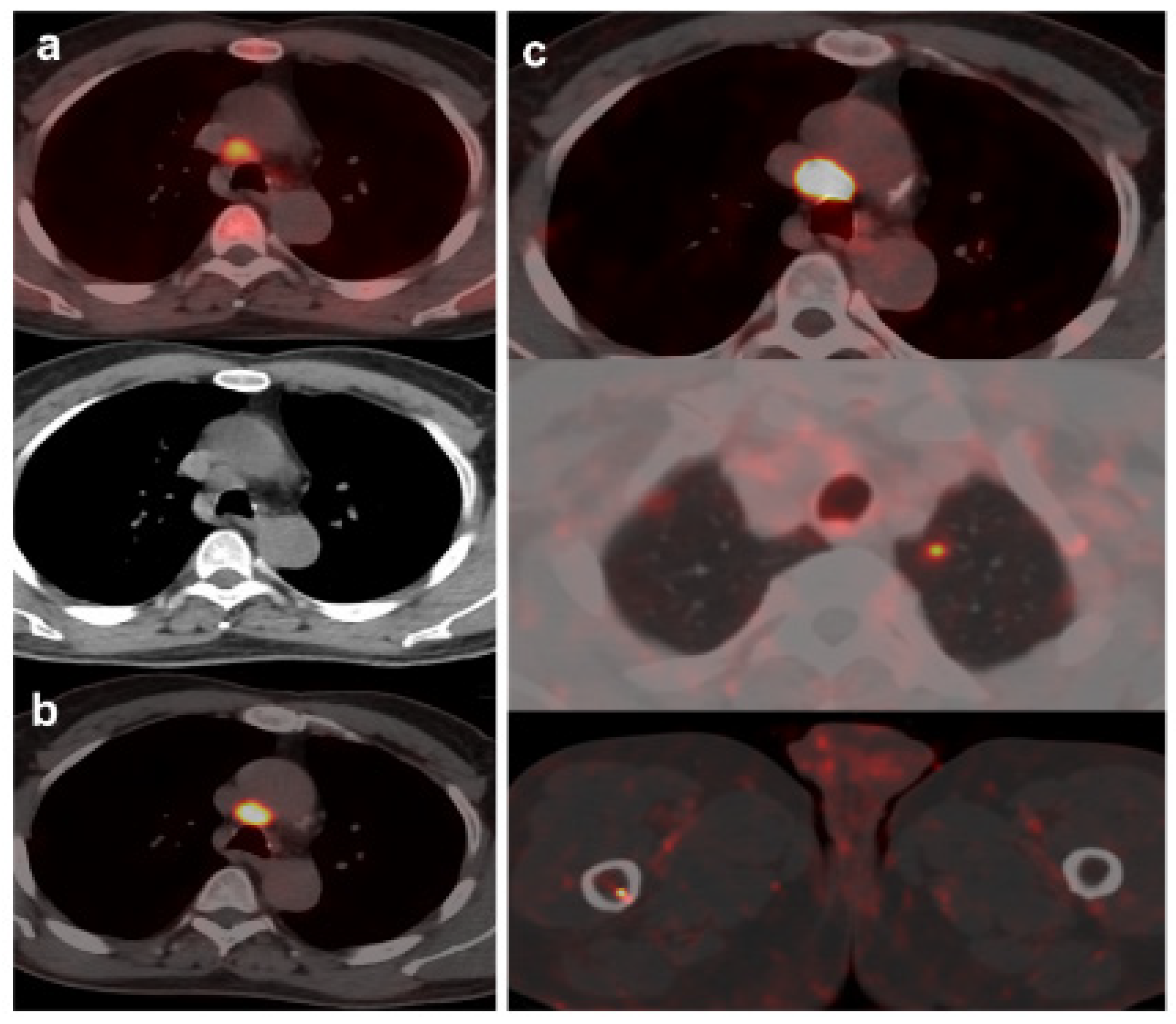
Figure 4.
70-year-old patient. Gleason 9 PCa, treated initially with RP and radiotherapy after his first BCR.Second BCR (PSA: 0.7 ng/mL, PSAdt 5.6 mo, PSAvel 0.05 ng/mL/mo) with 18F-Fluorocholine (a) and 18F-DCFPyL scans showing mediastinal lymph node tracer uptake (b) reported as inflammatory process. Follow-up was decided and PSA level continued increasing. A new 18F-DCFPyL scan (c) was performed 3 months later, showed and increased in size and metabolism of mediastinal lymph node with additional microfoci of radiotracer uptake in lung and bone, suspicious of metastases. An endobronchial ultrasound guided lymph node biopsy confirmed prostatic origin of metastasis. ADT + Apalutamide was initiated (escalation).
Figure 4.
70-year-old patient. Gleason 9 PCa, treated initially with RP and radiotherapy after his first BCR.Second BCR (PSA: 0.7 ng/mL, PSAdt 5.6 mo, PSAvel 0.05 ng/mL/mo) with 18F-Fluorocholine (a) and 18F-DCFPyL scans showing mediastinal lymph node tracer uptake (b) reported as inflammatory process. Follow-up was decided and PSA level continued increasing. A new 18F-DCFPyL scan (c) was performed 3 months later, showed and increased in size and metabolism of mediastinal lymph node with additional microfoci of radiotracer uptake in lung and bone, suspicious of metastases. An endobronchial ultrasound guided lymph node biopsy confirmed prostatic origin of metastasis. ADT + Apalutamide was initiated (escalation).
Figure 5.
BCR in a 71-year-old patient (PSA: 0.26 ng/mL, PSAdt: 1.09 mo, PSAvel: 0.2 ng/mL/mo, PSAdt 1.09 mo, PSAvel 0.2 ng/mL/mo) after RP of PCa (Gleason 6, pT2c). 18F-DCFPyL scan showed a slightuptake on left iliac bone with minimal sclerotic changes. Previous negative 18F-Fluorocholine scan (timewindow of one week). MRI did not confirm malignancy of PSMA uptake (false positive). Prostatic bedradiotherapy was given and PSA level decreased.
Figure 5.
BCR in a 71-year-old patient (PSA: 0.26 ng/mL, PSAdt: 1.09 mo, PSAvel: 0.2 ng/mL/mo, PSAdt 1.09 mo, PSAvel 0.2 ng/mL/mo) after RP of PCa (Gleason 6, pT2c). 18F-DCFPyL scan showed a slightuptake on left iliac bone with minimal sclerotic changes. Previous negative 18F-Fluorocholine scan (timewindow of one week). MRI did not confirm malignancy of PSMA uptake (false positive). Prostatic bedradiotherapy was given and PSA level decreased.
Figure 6.
56-year-old patient. PCa (Gleason 6) treated with braquitherapy. BCR (PSA: 5.4 ng/mL, PSAdt 6.17 mo, PSAvel 0.55 ng/mL/mo). 18F-Fluorocholine (a) showed prostate gland uptake and right external iliac lymph nodes metastasis (arrow). One month later the patient was also scanned with 18F-DCFPyL (b) revealing only prostate gland pathological tracer uptake. Prostate biopsy was negative (false PSMA positive). Follow-up was decided and PSA level keeps oscillating (4–5 ng/mL) with an additional negative 18F-DCFPyL PET/CT one year later.
Figure 6.
56-year-old patient. PCa (Gleason 6) treated with braquitherapy. BCR (PSA: 5.4 ng/mL, PSAdt 6.17 mo, PSAvel 0.55 ng/mL/mo). 18F-Fluorocholine (a) showed prostate gland uptake and right external iliac lymph nodes metastasis (arrow). One month later the patient was also scanned with 18F-DCFPyL (b) revealing only prostate gland pathological tracer uptake. Prostate biopsy was negative (false PSMA positive). Follow-up was decided and PSA level keeps oscillating (4–5 ng/mL) with an additional negative 18F-DCFPyL PET/CT one year later.
Table 1.
Baseline characteristics of 138 study subjects.
Table 1.
Baseline characteristics of 138 study subjects.
| Characteristic |
Value |
| Age (y) |
|
| Mean ± SD |
69.77 ± 7.54 |
| Range |
55–87 |
| Grade group |
|
| 1 |
46 (33.3%) |
| 2 |
39 (28.3%) |
| 3 |
30 (21.7%) |
| 4 |
12 (8.7%) |
| 5 |
11 (8%) |
| D’Amico risk |
|
| Low |
24 (17.4%) |
| Intermediate |
38 (27.5%) |
| High |
76 (55.1%) |
| Primary treatment |
|
| Surgery |
48 (34.8%) |
| Radiotherapy |
60 (43.5%) |
| Both |
30 (21.7%) |
| PSA closest to PET/CTs (ng/ml) |
|
| Mean ± SD |
2.80 ± 4.83 |
| PSA ≤ 1 |
46 (33.4%) |
| 1 < PSA ≤ 2 |
17 (12.3%) |
| PSA > 2 |
75 (54.3%) |
| PSAdt (month) |
|
| Mean ± SD |
7.34 ± 11.74 |
| ≤ 6 |
73 (52.9%) |
| > 6 |
65 (47.1%) |
| PSAvel (ng/ml/month) |
|
| Mean ± SD |
0.26 ± 0.68 |
| ≥ 0.2 |
45 (32.6%) |
| < 0.2 |
93 (67.4%) |
| Biochemical relapse |
|
| First |
100 (72.5%) |
| Second or further |
38 (27.5%) |
Table 2.
Per patient miTNM obtained from 18F-DCFPyL and 18F-Fluorocholine. PET/CT.
Table 2.
Per patient miTNM obtained from 18F-DCFPyL and 18F-Fluorocholine. PET/CT.
| |
18F-DCFPyL |
| (+) |
(-) |
Total |
| T |
18F-Fluorocholine |
(+) |
20 |
7 |
27 |
| (-) |
26 |
85 |
111 |
| Total |
46 |
92 |
138 |
| N1 |
(+) |
4 |
8 |
12 |
| (-) |
15 |
111 |
126 |
| Total |
19 |
119 |
138 |
| N2 |
(+) |
4 |
2 |
6 |
| (-) |
14 |
118 |
132 |
| Total |
18 |
120 |
138 |
| M1a |
(+) |
2 |
1 |
3 |
| (-) |
14 |
121 |
135 |
| Total |
16 |
122 |
138 |
| M1b |
(+) |
5 |
2 |
7 |
| (-) |
16 |
115 |
131 |
| Total |
21 |
117 |
138 |
| M1c |
(+) |
2 |
0 |
2 |
| (-) |
3 |
133 |
136 |
| Total |
5 |
133 |
138 |
Table 3.
Concordance between 18F-DCFPyL and 18F-Fluorocholine miTNM stages.
Table 3.
Concordance between 18F-DCFPyL and 18F-Fluorocholine miTNM stages.
| miTNM |
Kappa (p value) |
| T |
k = 0.403 (p < 0.001) |
| N1 |
k = 0.143 (p = 0.086) |
| N2 |
k = 0.287 (p < 0.001) |
| M1a |
k = 0.181 (p = 0.003) |
| M1b |
k = 0.304 (p < 0.001) |
| M1c |
k = 0.562 (p < 0.001) |
Table 4.
PSA, PSAdt and PSAvel (mean ± SD) in miTNM comparison of 18F-DCFPyL and 18F-Fluorocholine.
Table 4.
PSA, PSAdt and PSAvel (mean ± SD) in miTNM comparison of 18F-DCFPyL and 18F-Fluorocholine.
| |
18F-Fluorocholine |
18F-DCFPyL |
| PSA (ng/ml) |
T |
3.95 ± 1.92 |
3.17 ± 2.16 |
| N |
2.68 ± 2.10 |
2.25 ± 2.14 |
| M |
2.73 ± 1.86 |
4.63 ± 8.67 |
| PSAdt (months) |
T |
5.07 ± 12.13 |
7.56 ± 10.83 |
| N |
6.13 ± 4.23 |
5.87 ± 3.51 |
| M |
9.32 ± 18.42 |
7.34 ± 11.20 |
| PSAvel (ng/ml/month) |
T |
0.45 ± 0.79 |
0.23 ± 0.36 |
| N |
0.28 ± 0.23 |
0.18 ± 0.15 |
| M |
0.34 ± 0.44 |
0.56 ± 1.19 |